Abstract
Background
Magnesium (Mg) deficiency is a common problem in diabetic patients. Deficiency of Mg may increase the incidence of diabetes mellitus (DM) and occurrence of diabetic complications. In this study, our aim was to evaluate an association between serum Mg level, glycemic regulation, and diabetic complications.
Material-Methods
In this retrospective study 673 diabetic patients were evaluated. According to Mg levels, the patients were divided into two groups; as normomagnesemic patients and hypomagnesemic patients.
Results
Among the patients, 57.8% were men and 42.2% were women. Mean age was 55.6 years and the mean duration of diabetes was 81 ± 86.9 months. The mean glycosylated hemoglobin (HbA1c) was 9.0 ±2.4 % (4.5-18); mean magnesium level was 1.97 ± 0.25 (1.13 to 3.0) mg / dl. There were 55 patients (8.2%) with diabetic retinopathy and 95 patients (14.1%) with diabetic neuropathy. Five hundred patients (74.3%) had normoalbuminuria; 133 patients (19. 8%) had microalbuminuria (MA) and 40 patients (5.9%) had overt proteinuria. One hundred and seventy one patients (25.4%) had HbA1c levels equal or below 7%; and 502 patients (74.6%) had HbA1c levels above 7%. There was no statistical difference in age or duration of diabetes between the groups formed according to Mg levels. Although there were no differences between the groups for retinopathy and neuropathy, MA was more common in hypomagnesemic patients (p =0.004). HbA1c levels did not differ between the groups (p =0.243). However there was a weak negative correlation between serum Mg and HbA1c levels (r =-0.110, p =0.004) and also between serum Mg and urine protein level (r =-0.127, p =0.018).
Conclusion
Mg depletion is a common problem in patients with DM. It affects both glycemic regulation and the occurence of complications. Also, poor glycemic regulation affects serum Mg levels. Hippokratia 2015; 19 (2):153-157.
Keywords: Hypomagnesemia, poor glycemic control, diabetic complication
Introduction
Magnesium (Mg) has a critical role in the actions of important enzymes and is the fourth most abundant cation in the human body1. It is claimed that there is an inverse relationship between Mg intake and incidence of diabetes mellitus (DM)2. Mg deficiency is common in diabetic patients. The incidence of hypomagnesemia varies between 11 and 47.7%3-7. Compared with the control group, incidence of hypomagnesemia in newly diagnosed diabetes is 10.5-fold and in patients with previously diagnosed diabetes is 8.5-fold more common8.
Microalbuminuria (MA) was first described in diabetic patients in 19829. It was shown to be associated with increased risk of cardiovascular morbidity and mortality in diabetic patients10-12. At the same time, it is accepted as an indicator for the presence of diabetic retinopathy/neuropathy, cardiovascular and peripheral vascular disease and increased mortality11,12. The presence of MA and overt proteinuria in non-insulin dependent diabetes mellitus (NIDDM) is an indicator of poor glycemic control. As well as poor glycemic control; insulin resistance and low Mg level strongly associated with increased the prevalence of MA13.
There have been controversial views on the relationship between MA and Mg deficiency. Some studies demonstrated that MA and overt proteinuria do not affect plasma Mg level4,14.
On the other hand, in other studies a significant reduction in serum Mg level in diabetic cases with MA and proteinuria were reported3,5,15-17. When compared with normomagnesemic people; increased urinary albumin excretion was reported in type 1 DM patients with hypomagnesemia15. A negative correlation between serum Mg and glycosylated hemoglobin (HbA1c) levels was noted4.
The aim of this study was to evaluate whether glycemic regulation and diabetic complications could be changed between normomagnesemic and hypomagnesemic patients.
Material and Methods
Patients
Our study consisted of 673 NIDDM patients, subdivided into three groups according to their 24-hour urinary albumin excretion rate (UAER): normoalbuminuria (<30 mg/day), MA (30-300 mg/day) and overt proteinuria (>300 mg/day). Cases were also stratified by their serum Mg levels: Low Mg level of ≤1.8 mcg/dl and normal range of 1.9-2.6 mcg/dl. All patients were questioned for age, gender, disease duration, medical history, family history, smoking history, usage of alcohol and concomitant diseases.
Measurement
Height, body weight and body mass index (BMI), which was calculated with the formula weight/heightsquare (kg/m2), were recorded for each patient. Fat mass and total body water measured by TANITA device [Body Composition Analyzer Model TBF-300 (Tanita Corporation, Tokyo, Japan)] were also recorded. A venous blood sample was collected from each subject in the morning after 12-hour fasting, to evaluate fasting glucose [hexokinase with enzymatic reference methods, (Roche Diagnostics GmbH, Mannheim, Germany)]; low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), sodium, potassium, magnesium [Colorimetric end point methods with ksilidil blue (Roche Diagnostics GmbH, Mannheim, Germany)], creatinine [kinetic colorimetric assay based on the Jaffe methods (Roche Diagnostics GmbH, Mannheim, Germany)], HbA1c [HPLC-high performance liquid chromatography- Biorad Variant II Turbo (Biorad Medical Diagnostics, California, USA)], glomerular filtration rate (GFR) was calculated with the following equation [(140-age) x weight in kg] / (72 x serum creatinine in mg/dl) for male patients. For female patients, the value was reduced to 85% of that estimated with this equation16.
Diagnosis of diabetic neuropathy was confirmed by a detailed medical history, and neurological examination. Blood pressure and heart rate measurement, pinprick sensation test, perception with monofilaments, vibration and position, and reflexes were performed in all patients. Rarely electrophysiological test were needed.
Diabetic retinopathy was diagnosed with fundus examination, performed by an ophthalmologist.
Exclusion criteria were: patients on drugs that affect Mg levels (diuretics, aminoglycosides, amphotericin B, etc), malabsorption or diarrhea, alcohol consumption, vitamin or mineral supplements in recent past, pregnancy, lactation or sepsis.
Statistical Methods
Statistical analysis was performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics for numeric variables as mean ± standard deviation and median (minimum-maximum) and categorical structure of data was expressed as numbers and percentages. Categorical differences were examined between groups, in terms of structure variables with the Chi-square test. One-way ANOVA test was used for comparison of the normal and low magnesium group. Quantitative variables with normal distribution were compared between two groups using parametric tests, otherwise the Mann-Whitney U test was used. The relationship between two numerical variables was examined using Spearman’s correlation analysis. Results were evaluated in 95% confidence interval, and p value of <0.05 was considered as significant.
Results
In this study, a total of 673 patients with type 2 diabetes mellitus who were recorded in diabetes outpatient clinic of our hospital, were included. Among these patients, 57.8 % (n: 389) were men, and 42.2% (n: 284) were women. Mean age was 55.6 ± 10.4 years and mean duration of diabetes was 81 ± 86.9 months. Mean BMI was 31.48 ± 5.8 kg/m2.
The mean HbA1c was 9.0 ± 2.4 % (range 4.5-18), mean serum creatinine was 0.83 ± 0.22 mg/dl (0.3-1.5), and mean magnesium level was 1.97 ± 0.25 (1.13 to 3.0) mg/dl. Mean glomerular filtration rate (GFR) was 117.88 ± 31.18 ml/minute, mean LDL-C was 127.86 ± 39.3 mg/dl (45-330), mean HDL-C was 42 ± 11.4 mg/dl (26-101), and mean TG was 187.10 ± 129.8 mg/dl (31-710). There were 55 patients (8.2%) with diabetic retinopathy and 95 patients (14.1%) with diabetic neuropathy. Five hundred patients (74.3%) had normoalbuminuria, 133 patients (19. 8%) had MA, and 40 patients (5.9%) had overt proteinuria. A total of 171 patients (25.4%) had an HbA1c levels equal or below 7%, and 502 patients (74.6%) had HbA1c levels above 7%.
According to the serum level of Mg, patients were classified into two groups: low Mg (Group 1, n: 65, 9.7%) and normal Mg group (Group 2, n: 608, 90.3%); there were no statistical differences between two groups in terms of age (p =0.292). Compared with the normomagnesemic group, those in the hypomagnesemic group were more likely to be females (p =0.006). Also, the duration of diabetes was similar in both groups (p =0.058). In both groups BMI (body mass index) and fat mass were similar (p =0.145 and p =0.268) (Table 1).
Table 1. Demographic and laboratory features of the two (low and normal Mg) groups according to serum Mg levels.
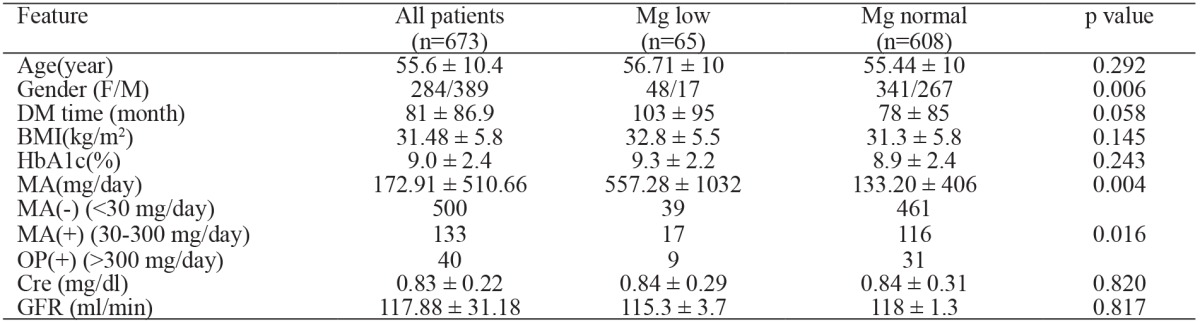
DM: diabetes mellitus, BMI: body mass index, F: female, M: male, HbA1c: glycosylated hemoglobin, Cre: Creatinine, MA: microalbuminuria, OP: overt protenuria, GFR: glomerular filtration rate, p value is refered to two groups: Mg low and Mg normal groups.
There were no correlation between BMI and Mg (r =-0.071, p =0.069) , and between Mg and fat mass (r =-0.068, p =0.116). We did not observe a correlation between Mg and LDL-C (r =-0.049, p =0.207), HDL-C (r =-0.044, p =0.253), or TG (r =-0.027, p =0.487).
There was no difference between the groups for retinopathy and neuropathy (p =0.597 for retinopathy, p =0.297 for neuropathy). However MA was more common in group 1 (p =0.004) (Table 1).
Logistic regression analysis was performed between the two groups, consisted of low and normal Mg levels, according to clinical characteristic and variables. In univariate analysis, a statistical significance was shown between sex, duration of DM and MA (p <0.05). In multivariate analysis a significant and independent relationship was shown between sex and MA (p <0.05) (Table 2).
Table 2. Logistic regression analysis of the two (low and normal Mg) groups according to serum Mg levels.
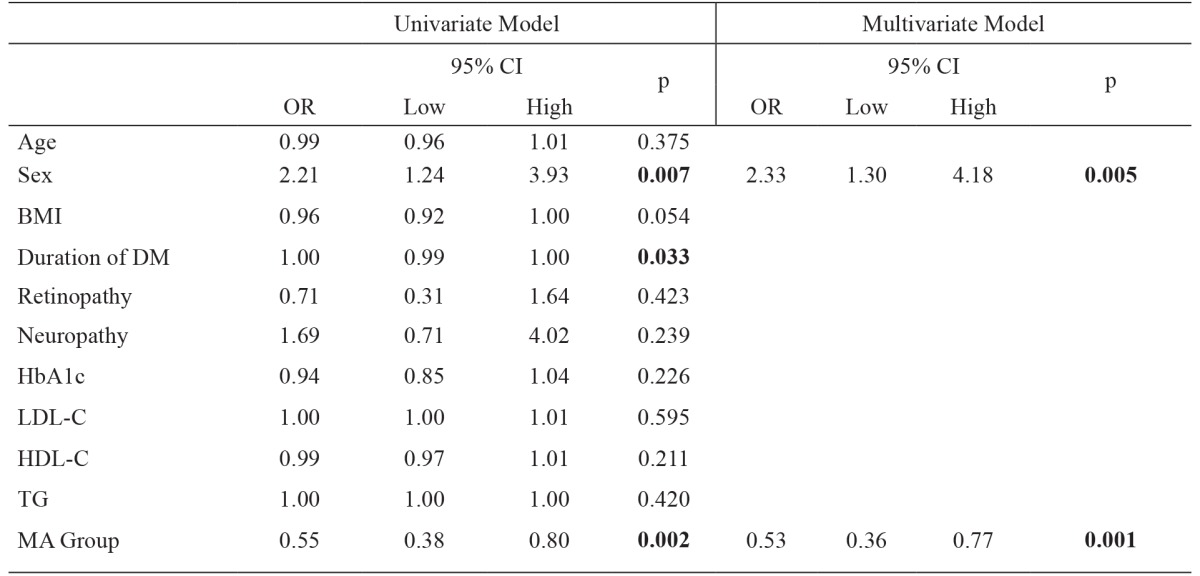
BMI: body mass index, DM: diabetes mellitus, HbA1c: glycosylated hemoglobin, LDL-C: low density lipoprotein cholesterol, HDL-C: high density lipoprotein cholesterol, TG: triglyceride, MA: microalbuminuria, OR: odds ratio.
HbA1c levels did not differ between the groups (p =0.243) (Table 1); however, there was a weak negative correlation between serum Mg and HbA1c levels (r =-0.110, p =0.004) which means that when serum Mg level decreases, HbA1c levels increase. To strengthen the link between HbA1c and Mg, we calculated the median Mg value and found to be 1.97 mg/dl. HbA1c values were higher in the group in which Mg <1.97 mg/dL and were lower in the group in which Mg >1.97 mg/dl (p =0.044). At the same time, there was a weak correlation between serum Mg and urine proteinuria (r =-0.127, p =0.018).
Patients were divided into two groups according to HbA1c levels; one group with HbA1c ≤7% and the other with HbA1c >7%. In the group with HbA1c ≤7%, serum Mg level was lower than in the HbA1c >7% group. Also, MA was more common in the group with HbA1c ≤ 7% (Table 3).
Table 3. Serum magnesium levels and urinary proteinuria according to HbA1c.
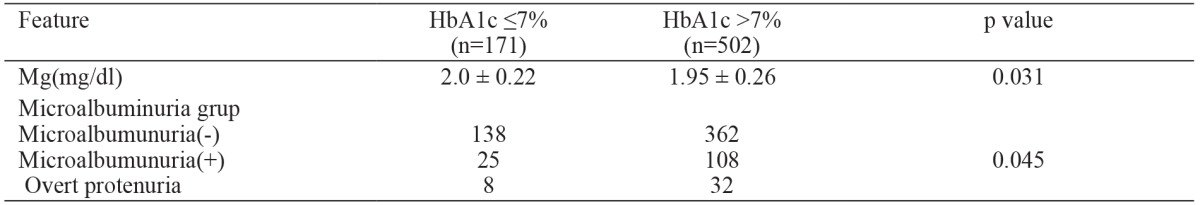
HbA1c: glycosylated hemoglobin, Mg: Magnesium.
Discussion
In this study, we found that MA was common in the hypomagnesemic group compared to the normomagnesemic group regardless of age and duration of diabetes. Also, there was a weak negative correlation between serum Mg and HbA1c levels and between serum Mg and urine proteinuria. Hypomagnesemia is not rare in patients with diabetes mellitus. It may affect both glycemic regulation and nephropathy.
Diabetes mellitus is the most common disorder among endocrine disorders that are associated with hypomagnesemia. So far many studies have shown that Mg levels are lower in diabetic patients2,7,17. According to CARDIA Study (Coronary Artery Risk Development in young Adults) there was an inverse relationship between Mg intake and the incidence of diabetes2.
Mg depletion may cause an insulin-resistant state18,19, poor glycemic control4,15,20 and disordered lipid metabolism in diabetic patients21. Furthermore, poor glycemic control in diabetic patients is a well-known risk factor for Mg depletion20. Significant negative correlation between Mg and fasting plasma glucose, HbA1c and Homa Insulin resistant Index (HOMA-IR) have been shown2,17. Similarly, we found a negative correlation between serum Mg level and HbA1c level. Marhalle et al22 have found that diabetes, dyslipidemia, and hypertension were inversely related with serum Mg levels. But we did not observe any correlation between serum Mg and LDL-C, HDL-C and TG levels.
Some authors have suggested that reduced Mg level could have a role in the pathogenesis of microvascular complications of diabetes23,24. Serum Mg depletion has been reported in diabetic patients who had advanced retinopathy and poor glycemic control25. However we did not find any difference in the presence of retinopathy and neuropathy between the two groups.
The association between MA and depletion of Mg is controversial17,26-28. In a study on type 1 diabetic patients, hypomagnesemia has been associated with poor glycemic control and urine albumin excretion27. Similarly, we also found a negative correlation between Mg level and urine protein excretion. On the other hand, Zargar et al28 suggested that glycemic control and presence of MA did not affect serum Mg levels. Other studies also, have not found any association between Mg and MA in Type 1 and Type 2 diabetes17,28. In another study with adolescents and young adults, serum Mg levels had differed in diabetic patients based on persistent MA level14.
One of the possible mechanisms explaining the relation between MA and Mg deficiency is insulin resistance. Mg can act as a mild calcium antagonist. In patients with Mg deficiency, intracellular calcium is increased. Increased calcium may interrupt response of skelatal muscles and adipocytes to insulin and lead to insulin resistance29. Intracellular Mg plays a role in regulating insulin action, insulin-dependent glucose uptake, and vascular tone. Deficiency of Mg can reduce tyrosine-kinase activity, postreceptorial activity and eventually it may contribute to the development of insulin resistance30,31. On the other hand, insulin deficiency and resistance can effect tubular reabsorption of Mg32. According to other hypotheses, oxidative stress is important in complications of diabetes2. The antioxidative capacity of Mg have also been reported26. Another hypothesis is that by influencing the activity of Na+/K+-ATPase reduction of Mg favors the onset and the progression of diabetic microangiopathy33,34.
In hypomagnesemia endothelial dysfunction occurs due to increased platelet aggregation and vascular calcification35. A relationship between high cholesterol and low serum Mg has been shown in animal studies in which Mg supplementation has also decreased the severity of vascular plaque36. In NIDDM, Mg supplementation reduced serum total cholesterol, and LDL-C and increased HDL-C37. In another study, a negative correlation between serum cholesterol levels and triglyceride levels was reported38. Corica et al39 have found an association of hypomagnesemia with dyslipidemia, high waist circumferences, high blood pressure, MA and overt proteinuria. In another study, no relationship was found between serum lipids and Mg levels3. Also in our study, we did not find any difference in serum lipid levels between hypomagnesemic and normomagnesemic groups. Serum Mg levels is reported to be lower in females than in males but there is not an association between hypomagnesemia and age, BMI, HDL, TG, smoking or alcohol history13. Similarly in our study female gender was more common in the hypomagnesemic group than in the nomomagnesemic group. Furthermore we did not find any assosiation between serum Mg and age, BMI, LDL-C, TG or HDL-C.
Limitation of the current study was the fact that the number of patients in the two groups was not equal. Also, neuropathy was only assessed clinically.
Conclusion
Serum Mg was found to be inversely associated with the prevalence of MA. Hypomagnesemia was found to be associated with poor glycemic control. Large-scale clinical trials are needed in order to determine whether the correction of Mg deficiency could be effective to reduce the incidence of MA and to further elucidate the association between serum Mg and MA.
Conflict of interest
Authors declared no conflict of interest.
References
- 1.Xu J, Xu W, Yao H, Sun W, Zhou Q, Cai L. Associations of serum and urinary magnesium with the pre-diabetes, diabetes and diabetic complications in the Chinese Northeast population. PLoS One. 2013;8:e56750. doi: 10.1371/journal.pone.0056750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim DJ, Xun P, Liu K, Loria C, Yokota K, Jacobs DR, Jr, et al. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care. 2010;33:2604–2610. doi: 10.2337/dc10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corsonello A, Ientile R, Buemi M, Cucinotta D, Mauro VN, Macaione S, et al. Serum ionized magnesium levels in type 2 diabetic patients with microalbuminuria or clinical proteinuria. Am J Nephrol. 2000;20:187–192. doi: 10.1159/000013582. [DOI] [PubMed] [Google Scholar]
- 4.Pickup JC, Chusney GD, Crook MA, Viberti GC. Hypomagnesaemia in IDDM patients with microalbuminuria and clinical proteinuria. Diabetologia. 1994;37:639. doi: 10.1007/BF00403385. [DOI] [PubMed] [Google Scholar]
- 5.Allegra A, Corsonello A, Buemi M, D’Angelo R, di Benedetto A, Bonanzinga S, et al. Plasma, erythrocyte and platelet magnesium levels in type 1 diabetic patients with microalbuminuria and clinical proteinuria. J Trace Elem Med Biol. 1997;11:154–157. doi: 10.1016/S0946-672X(97)80044-2. [DOI] [PubMed] [Google Scholar]
- 6.Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2:366–373. doi: 10.2215/CJN.02960906. [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta A, Sarma D, Saikia UK. Hypomagnesemia in type 2 diabetes mellitus. Indian J Endocrinol Metab. 2012;16:1000–1003. doi: 10.4103/2230-8210.103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons D, Joshi S, Shaw J. Hypomagnesaemia is associated with diabetes: Not pre-diabetes, obesity or the metabolic syndrome. Diabetes Res Clin Pract. 2010;87:261–266. doi: 10.1016/j.diabres.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Viberti GC, Jarrett RJ, Keen H. Microalbuminuria as prediction of nephropathy in diabetics. Lancet. 1982;2:611. doi: 10.1016/s0140-6736(82)90688-2. [DOI] [PubMed] [Google Scholar]
- 10.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 11.Mattock MB, Morrish NJ, Viberti G, Keen H, Fitzgerald AP, Jackson G. Prospective study of microalbuminuria as predictor of mortality in NIDDM. Diabetes. 1992;41:736–741. doi: 10.2337/diab.41.6.736. [DOI] [PubMed] [Google Scholar]
- 12.Niskanen LK, Penttilã I, Parviainen M, Uusitupa MI. Evolution, risk factors, and prognostic implications of albuminuria in NIDDM. Diabetes Care. 1996;19:486–493. doi: 10.2337/diacare.19.5.486. [DOI] [PubMed] [Google Scholar]
- 13.Xu B, Sun J, Deng X, Huang X, Sun W, Xu Y, et al. Low serum magnesium level is associated with microalbuminuria in chinese diabetic patients. Int J Endocrinol. 2013;2013:580685. doi: 10.1155/2013/580685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verrotti A, Basciani F, Carle F, Morgese G, Chiarelli F. Calcium metabolism in adolescents and young adults with type 1 diabetes mellitus without and with persistent microalbuminuria. J Endocrinol Invest. 1999;22:198–202. doi: 10.1007/BF03343541. [DOI] [PubMed] [Google Scholar]
- 15.Arslanoğlu I, Günöz H, Bundak R, Saka N. Hypomagnesaemia in childhood IDDM and risk of nephropathy. Diabetologia. 1995;38:629. doi: 10.1007/BF00400735. [DOI] [PubMed] [Google Scholar]
- 16.Oates JA, Wilkinson GR, Braunwald E, Isselbacher KJ, Petersdorf RG, Wilson JD, et al. Principles of drug therapy. Principles of internal medicine. 11th edition, Mc-Graw-Hill, New York. 1987:342–352. [Google Scholar]
- 17.Sales CH, Pedrosa LF, Lima JG, Lemos TM, Colli C. Influence of magnesium status and magnesium intake on the blood glucose control in patients with type 2 diabetes. Clin Nutr. 2011;30:359–364. doi: 10.1016/j.clnu.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Corica F, Allegra A, Ientile R, Buemi M, Corsonello A, Bonanzinga S, et al. Changes in plasma, erythrocyte, and platelet magnesium levels in normotensive and hypertensive obese subjects during oral glucose tolerance test. Am J Hypertens. 1999;12:128–136. doi: 10.1016/s0895-7061(98)00174-5. [DOI] [PubMed] [Google Scholar]
- 19.Wasada T, Katsumori K, Saeki A, Saito S, Omori Y. Urinary albumin excretion rate is related to insulin resistance in normotensive subjects with impaired glucose tolerance. Diabetes Res Clin Pract. 1997;34:157–162. doi: 10.1016/s0168-8227(96)01348-4. [DOI] [PubMed] [Google Scholar]
- 20.Mather HM, Levin GE. Magnesium status in diabetes. Lancet. 1979;1:924. doi: 10.1016/s0140-6736(79)91400-4. [DOI] [PubMed] [Google Scholar]
- 21.Reverter JL, Sentí M, Rubiés-Prat J, Lucas A, Salinas I, Pizarro E, et al. Relationship between lipoprotein profile and urinary albumin excretion in type II diabetic patients with stable metabolic control. Diabetes Care. 1994;17:189–194. doi: 10.2337/diacare.17.3.189. [DOI] [PubMed] [Google Scholar]
- 22.Mahalle N, Kulkarni MV, Naik SS. Is hypomagnesaemia a coronary risk factor among Indians with coronary artery disease? J Cardiovasc Dis Res. 2012;3:280–286. doi: 10.4103/0975-3583.102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paolisso G, Tirelli A, Coppola L, Verrazzo G, Pizza G, Sgambato S, et al. Magnesium administration reduces platelet hyperaggregability in NIDDM. Diabetes Care. 1989;12:167–168. doi: 10.2337/diacare.12.2.167b. [DOI] [PubMed] [Google Scholar]
- 24.Nadler JL, Malayan S, Luong H, Shaw S, Natarajan RD, Rude RK. Intracellular free magnesium deficiency plays a key role in increased platelet reactivity in type II diabetes mellitus. Diabetes Care. 1992;15:835–841. doi: 10.2337/diacare.15.7.835. [DOI] [PubMed] [Google Scholar]
- 25.Fujii S, Takemura T, Wada M, Akai T, Okuda K. Magnesium levels of plasma, erythrocyte and urine in patients with diabetes mellitus. Horm Metab Res. 1982;14:161–162. doi: 10.1055/s-2007-1018954. [DOI] [PubMed] [Google Scholar]
- 26.Mirrahimi B, Hamishehkar H, Ahmadi A, Mirjalili MR, Aghamohamadi M, Najafi A. The efficacy of magnesium sulfate loading on microalbuminuria following SIRS: One step forward in dosing. Daru. 2012;20:74. doi: 10.1186/2008-2231-20-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galli-Tsinopoulou A, Maggana I, Kyrgios I, Mouzaki K, Grammatikopoulou MG, Stylianou C, et al. Association between magnesium concentration and HbA1c in children and adolescents with type 1 diabetes mellitus. J Diabetes. 2014;6:369–377. doi: 10.1111/1753-0407.12118. [DOI] [PubMed] [Google Scholar]
- 28.Zargar AH, Bashir MI, Masoodi SR, Laway BA, Wani AI, Khan AR, et al. Copper, zinc and magnesium levels in type-1 diabetes mellitus. Saudi Med J. 2002;23:539–542. [PubMed] [Google Scholar]
- 29.McCarty MF. Magnesium may mediate the favorable impact of whole grains on insulin sensitivity by acting as a mild calcium antagonist. Med Hypotheses. 2005;64:619–627. doi: 10.1016/j.mehy.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 30.Barbagallo M, Dominguez LJ. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch Biochem Biophys. 2007;458:40–47. doi: 10.1016/j.abb.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Takaya J, Higashino H, Kobayashi Y. Intracellular magnesium and insulin resistance. Magnes Res. 2004;17:126–136. [PubMed] [Google Scholar]
- 32.Mandon B, Siga E, Chabardes D, Firsov D, Roinel N, De Rouffignac C. Insulin stimulates Na+, Cl-, Ca2+, and Mg2+ transports in TAL of mouse nephron: cross-potentiation with AVP. Am J Physiol. 1993;265:F361–F369. doi: 10.1152/ajprenal.1993.265.3.F361. [DOI] [PubMed] [Google Scholar]
- 33.Ceriello A, Giugliano D, Dello Russo P, Passariello N. Hypomagnesemia in relation to diabetic retinopathy. Diabetes Care. 1982;5:558–559. doi: 10.2337/diacare.5.5.558. [DOI] [PubMed] [Google Scholar]
- 34.Winegrad AI. Banting lecture 1986. Does a common mechanism induce the diverse complications of diabetes? Diabetes. 1987;36:396–406. doi: 10.2337/diab.36.3.396. [DOI] [PubMed] [Google Scholar]
- 35.Rayssiguier Y. Role of magnesium and potassium in the pathogenesis of arteriosclerosis. Magnesium. 1984;3:226–238. [PubMed] [Google Scholar]
- 36.Altura BT, Brust M, Bloom S, Barbour RL, Stempak JG, Altura BM. Magnesium dietary intake modulates blood lipid levels and atherogenesis. Proc Natl Acad Sci USA. 1990;87:1840–1844. doi: 10.1073/pnas.87.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corica F, Allegra A, Di Benedetto A, Giacobbe MS, Romano G, Cucinotta D, et al. Effects of oral magnesium supplementation on plasma lipid concentrations in patients with non-insulin-dependent diabetes mellitus. Magnes Res. 1994;7:43–47. [PubMed] [Google Scholar]
- 38.Haenni A, Ohrvall M, Lithell H. Atherogenic lipid fractions are related to ionized magnesium status. Am J Clin Nutr. 1998;67:202–207. doi: 10.1093/ajcn/67.2.202. [DOI] [PubMed] [Google Scholar]
- 39.Corica F, Corsonello A, Ientile R, Cucinotta D, Di Benedetto A, Perticone F, et al. Serum ionized magnesium levels in relation to metabolic syndrome in type 2 diabetic patients. J Am Coll Nutr. 2006;25:210–215. doi: 10.1080/07315724.2006.10719534. [DOI] [PubMed] [Google Scholar]


