Abstract
OBJECTIVE
The aim of this study was to systematically review dextrose (d-glucose) prolotherapy efficacy in the treatment of chronic musculoskeletal pain.
DATA SOURCES
Electronic databases PubMed, Healthline, OmniMedicalSearch, Medscape, and EMBASE were searched from 1990 to January 2016.
STUDY SELECTION
Prospectively designed studies that used dextrose as the sole active prolotherapy constituent were selected.
DATA EXTRACTION
Two independent reviewers rated studies for quality of evidence using the Physiotherapy Evidence Database assessment scale for randomized controlled trials (RCTs) and the Downs and Black evaluation tool for non-RCTs, for level of evidence using a modified Sackett scale, and for clinically relevant pain score difference using minimal clinically important change criteria. Study population, methods, and results data were extracted and tabulated.
DATA SYNTHESIS
Fourteen RCTs, 1 case–control study, and 18 case series studies met the inclusion criteria and were evaluated. Pain conditions were clustered into tendinopathies, osteoarthritis (OA), spinal/pelvic, and myofascial pain. The RCTs were high-quality Level 1 evidence (Physiotherapy Evidence Database ≥8) and found dextrose injection superior to controls in Osgood–Schlatter disease, lateral epicondylitis of the elbow, traumatic rotator cuff injury, knee OA, finger OA, and myofascial pain; in biomechanical but not subjective measures in temporal mandibular joint; and comparable in a short-term RCT but superior in a long-term RCT in low back pain. Many observational studies were of high quality and reported consistent positive evidence in multiple studies of tendinopathies, knee OA, sacroiliac pain, and iliac crest pain that received RCT confirmation in separate studies. Eighteen studies combined patient self-rating (subjective) with psychometric, imaging, and/or biomechanical (objective) outcome measurement and found both positive subjective and objective outcomes in 16 studies and positive objective but not subjective outcomes in two studies. All 15 studies solely using subjective or psychometric measures reported positive findings.
CONCLUSION
Use of dextrose prolotherapy is supported for treatment of tendinopathies, knee and finger joint OA, and spinal/pelvic pain due to ligament dysfunction. Efficacy in acute pain, as first-line therapy, and in myofascial pain cannot be determined from the literature.
Keywords: dextrose, prolotherapy, musculoskeletal pain, evidence-based medicine, systematic review, osteoarthritis chronic pain
Background
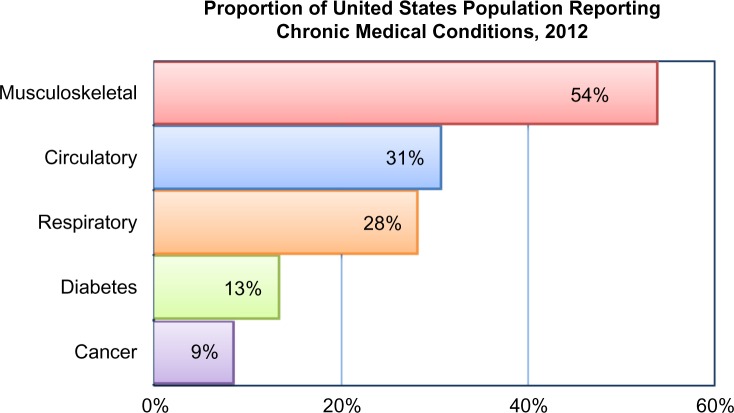
The Institute of Medicine defines chronic pain as pain that persists for a period of three to six months or beyond the time of normal healing.1 Musculoskeletal disorders are the most common source of chronic pain experienced by American adults.2 In 2012, the National Health Interview Survey indicated that half of all adults (aged 18 years and over) reported suffering from a musculoskeletal condition lasting three months or longer, with higher prevalence in women and those in lower income groups.3 In the United States, musculoskeletal conditions are the single most common reason for patients visiting their physicians (Fig. 1). According to the data collected by the Centers for Disease Control and Prevention 2010 National Ambulatory Medical Care Survey, over 97 million American adults visited a physician for musculoskeletal-related complaints or symptoms during that year.4
Figure 1.
Prevalence of musculoskeletal diseases in the United States.
Note: National Center for Health Statistics, National Health Interview Survey, 2012.
Of all the musculoskeletal complaints, cervical and lumbar back pains are the most common symptoms for which adult patients seek medical intervention.3 Of these, one in four individuals is 65 years or older. In 2012, between 12% and 14% of the United States population visited primary care physicians with complaints of back pain. Data indicate that the number of physician visits for pain is increasing. In 2012, more than 52.3 million patients visited a physician with symptoms of back pain, compared to 44.6 million in 2004.3,5 No estimation has been made of the great number of those who seek chiropractic care or physical therapy for treatment of back pain.
Joints of the upper and lower extremities are other common sites of musculoskeletal pain. Between 2002 and 2009, almost 30% of American adults reported recent symptoms of pain, aching, or swelling around a joint.3 The second and third most common sites for chronic musculoskeletal pain reported by adults are knee joints and shoulder joints, which affect 40 million and 19 million people, respectively.3 One in four adults aged 18–64 years report chronic joint pain from multiple joints, and among those 65 years and older, the ratio jumps to more than two in five.3
Not only are musculoskeletal conditions the most common cause of chronic pain, but they also result in significant disability in one out of every two individuals affected. In the United States, 55% of adults with joint pain have difficulty with basic activities, such as movement and sensory, emotional, and mental functioning, or have limitations in complex activities that include full participation in social, occupational, and household functioning.6 In 2014, nearly 18 million adults reported that they were unable to perform at least one daily activity, such as self-care, walking, and rising from a chair due to their musculoskeletal conditions.3 The disabling nature of chronic pain stemming from musculoskeletal conditions can result in isolation, disruption of social activities and relationship involvement, financial hardship, lost productivity, and potential unemployment.5
The economic impact of musculoskeletal conditions in the United States is staggering. In 2011, they cost $796.3 billion, nearly 6% of the annual GDP.3 According to the Institute of Medicine and the United States Bureau of Labor, nearly one million people take time away from work every year to treat and recover from pain or loss of function due to musculoskeletal conditions in the low back or upper extremities. In 2012, one in eight adults of prime working age reported lost work days due to a musculoskeletal condition – totaling 216 million days.7,8 Such injuries are often more severe than the average nonfatal workplace injury or illness and require longer recovery time, entailing an average of nine recovery days compared to an average seven days for all other workplace injuries.8
The predictable aging of the baby boomer cohort is projected to intensify the burden of musculoskeletal disease and disorders. Currently, these disorders account for more than 50% of all chronic conditions in people older than 50 years and are the most common cause of severe, long-term pain and disability in those aged over 65 years.6 The rising obesity epidemic also adds to the burden, and there is a significant positive association between musculoskeletal disorders and obesity. Increased body mass index puts a substantial stress and strain on weight-bearing joints, especially the lower back, hips, and knees, increasing the severity of musculoskeletal disorders. According to the Centers for Disease Control, obese adults receive a diagnosis of arthritis twice as often as nonobese individuals.9 Obese individuals are also particularly at high risk for injuries to upper extremity joints, due to biomechanical compromises linked with higher body weight.10
The increasing prevalence and burden of musculoskeletal conditions has led to an interest in effective nonsurgical solutions, which are more cost efficient and do not have the risks or require the recovery time of surgical approaches. Prolotherapy is one such viable solution.
Prolotherapy
Prolotherapy has been used in clinical practice for more than 80 years to treat various chronic musculoskeletal conditions. Formalized by Dr. George Hackett in the 1950s, prolotherapy is a practical and efficacious therapeutic strategy to treat ligamentous laxity and related musculoskeletal and arthritic conditions.11,12 Interest in prolotherapy has intensified over the past two decades among both physicians and patients,13,14 accompanied by an increasing number of published treatment outcome studies that confirm anecdotal findings that prolotherapy is effective in treating many conditions with few adverse effects, including osteoarthritis (OA),15 musculoskeletal pain,16 joint pain and laxity,16 chronic low back pain,17,18 refractory lateral epicondylosis,19 painful overuse tendinopathy, refractory,16 disabling low back pain,16 and refractory tendinopathies, and OA.20
Prolotherapy is a nonsurgical regenerative injection technique that introduces small amounts of an irritant solution to the site of painful and degenerated tendon insertions (entheses), joints, ligaments, and in adjacent joint spaces during several treatment sessions to promote growth of normal cells and tissues.21–23 Irritant solutions most often contain dextrose (d-glucose), a natural form of glucose normally found in the body, but may also contain combinations of polidocanol, manganese, zinc, human growth hormone, pumice, ozone, glycerin, or phenol.12 In severe cases, autologous cellular solutions may also be needed, such as platelet-rich plasma (PRP), bone marrow, or adipose tissue.24 A major goal of prolotherapy in chronic musculoskeletal conditions is the stimulation of regenerative processes in the joint that will facilitate the restoration of joint stability by augmenting the tensile strength of joint stabilizing structures, such as ligaments, tendons, joint capsules, menisci, and labral tissue.25
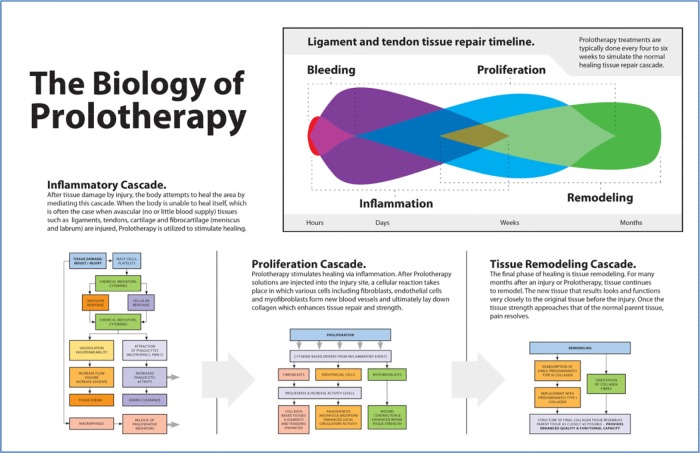
The most common prolotherapy agent used in clinical practice is dextrose, with concentrations ranging from 12.5% to 25%.20 Dextrose is considered to be an ideal proliferant because it is water soluble, a normal constituent of blood chemistry, and can be injected safely into multiple areas and in large quantity. Hypertonic dextrose solutions act by dehydrating cells at the injection site, leading to local tissue trauma, which in turn attracts granulocytes and macrophages and promotes healing. Dextrose proliferant has been approved for injection by United States Food and Drug Administration but not for prolotherapy; thus, it is currently used in prolotherapy as an off-label substance. The mechanism of action behind prolotherapy is not completely understood. However, current theory holds that the injected proliferant mimics the natural healing process of the body by initiating a local inflammatory cascade, which triggers the release of growth factors and collagen deposition. This is accomplished when induced cytokines mediate chemomodulation, which leads to proliferation and strengthening of new connective tissue, joint stability, and a reduction in pain and dysfunction.21,23,25 Figure 2 is a schematic depiction of the application of the therapeutic principle of prolotherapy – encompassing the inflammatory, proliferation, and tissue remodeling phases of the healing and restoration processes of injured ligaments/tendons.
Figure 2.
The biology of prolotherapy. Prolotherapy induces the three stages of healing and restoration: inflammation, proliferation, and tissue remodeling. Reused from: Steilen D, Hauser R, Woldin B, Sawyer S. Chronic Neck Pain: Making the Connection Between Capsular Ligament Laxity and Cervical Instability. The Open Orthopaedics Journal. 2014;8:326–345, under the terms of a CC-BY 2.5 license.
In vitro studies on human fibroblasts and chondrocytes exposure to extracellular dextrose concentrations of only 0.5% have resulted in the proliferation and production of a number of growth factors, several of which are essential to the repair, structural and functional integrity, and growth of tendons, ligaments, and other soft tissues.26,27 These include platelet-derived growth factor,28,29 transforming growth factor β,30 epidermal growth factor,31 basic fibroblast growth factor,32 insulin-like growth factor,33 and connective tissue growth factor.27 In vitro growth factors have been found to promote the expression of types 1 and 3 collagen in tenocytes and are pertinent to the growth of tendon, ligament, and cartilage.32–35
Stimulation of the production of these key growth factors for ligaments, tendons, and cartilage through dextrose prolotherapy could be an inexpensive method of growth stimulation that may prove to be cost effective for the long term.35 When injected into tissue, exogenous dextrose has been found in animal and human studies to stimulate inflammatory response,36 ligament size,37 tendon hypertrophy,38–40 extracellular matrix,39–41 fibroblastic proliferation,39–42 and repair of articular cartilage defects.42,43 When used clinically, dextrose concentrations higher than 10% operate in part through inflammatory mechanisms, while concentrations less than 10% are considered noninflammatory.44
Methods
Objective
The objective of this systematic review is to determine the efficacy of dextrose prolotherapy in chronic musculoskeletal pain.
Search strategy and selection criteria
A systematic review of English and non-English literature published from 1990 to January 2016 was performed using the PubMed, OmniMedicalSearch, Healthline, Medscape, Medline, and EMBASE databases. Keywords included prolotherapy, dextrose, regenerative injection therapy, and musculoskeletal pain. Medical Subject Headings (MeSH) terms included glucose/therapeutic use, intraarticular injections, glucose/administration and dosage, and sclerotherapy. Inclusion criteria were the involvement of human subjects, publication in a peer-reviewed journal, prospective study design, and use of dextrose as the sole prolotherapy proliferant. Exclusion criteria included use of prolotherapy solutions containing P2G, pumice, PRP, bone marrow, lipoaspirate, stem cells, or sodium morrhuatea; retrospective study design; or high velocity manipulation as adjunctive therapy. No lower limit was placed on sample size due to the small overall number of published trials. Non-English studies were considered if they met inclusion criteria, provided an abstract in English, and presented sufficient tabular/graphic data for data abstraction.
Rating the quality of evidence
The selection of instruments to assess the quality of evidence was influenced by the systematic reviews performed by Teasell et al.45 and Krassioukov et al.46, where both controlled and uncontrolled studies were evaluated.
The methodological quality of each study was scored with use of the Physiotherapy Evidence Database (PEDro) tool for randomized controlled trials (RCTs)47 and the Downs and Black (D&B) evaluation tool for non-RCTs.48 The PEDro is an 11-item scale that measures external validity (question 1) and internal validity (questions 2–11). The maximum score is 11; higher scores indicate better methodological quality, with 9–11 excellent, 6–8 good, 4–5 fair, and <4 poor.47,49 The D&B tool contains 27 items that assess reporting, external validity, internal validity (bias), and internal validity (confounding) and has a maximum score of 28.48 In an evaluation of 194 different instruments, the D&B tool was among six tools identified as most suitable for use in systematic reviews for assessing methodological quality in nonrandomized studies50; a further comparison of 18 tools identified the D&B as possessing the best reliability and validity to evaluate the quality of nonrandomized trials.51 For this review, PEDro and D&B scores were obtained by two independent reviewers. Heterogeneity and interrater agreement between reviewers in quality scoring was not formally assessed. The level of evidence of each study was determined with a modification of Sackett’s description of levels of evidence52 as described in Krassioukov et al.46 Accordingly, Sackett’s levels of evidence were collapsed into five categories (Table 1).53
Table 1.
Sackett’s levels of evidence.52
| Level 1 | RCTs with PEDro scores ≥6 |
| Level 2 | RCTs with PEDro scores ≤5, nonrandomized prospective controlled trials, and cohort studies |
| Level 3 | Case-control studies |
| Level 4 | Pre-test/post studies or case series |
| Level 5 | Observational reports, single-subject case reports, or clinical consensus |
Efficacy studies of pain therapy typically assess change in pain intensity from baseline with patient-reported ratings, usually with the visual analog scale (VAS) or numerical rating scale (NRS). Changes in pain score sufficient for clinical relevance can be determined by applying the minimal clinically important change (MCID) criteria.53 Developmental, quantification, and validation studies found that a reduction of three points represents the MCID using VAS,54 a reduction of two points represents the MCID using NRS54 and a decrease of ≤1.5 points with VAS and NRS represents a clinically irrelevant change in pain self-rating.55,56 Studies using VAS or NRS as outcome measurement were dichotomously rated as either MCID or NOT MCID. Study heterogeneity and limited RCTs in each pain subcategory prevented the aggregation of statistical data necessary to perform a meta-analysis. Studies assessing efficacy of dextrose prolotherapy for treatment of OA used the Western Ontario McMaster University Osteoarthritis Index (WOMAC; 100-point scale), which measures pain, stiffness, and functional movement.57
Although the number of published studies on prolotherapy using RCT design demonstrating high levels of evidence are becoming more common, most published research on prolotherapy has been nonrandomized prospective controlled trials, cohort studies, case–control series, case series, or single-subject case reports. Systematic reviews have largely been condition specific15 or have compared different types of injection therapies within the prolotherapy milieu.19 Furthermore, with the exception of one review by Sanderson and Bryant,58 which is limited to dextrose prolotherapy for the management of lower limb tendinopathy and fasciopathy, no recent systematic review has been published that solely addresses the efficacy of dextrose prolotherapy for multiple areas of chronic musculoskeletal pain and includes findings provided not only from RCTs but also those stemming from less rigorous research designs. Although designated lower levels of evidence, the studies discussed here, in addition to those using RCT design, provide useful information that can assist health-care practitioners in clinical decision making.
Results
Search execution and application of the inclusion/exclusion criteria identified 33 studies, including 15 RCTs, 1 case–control study, and 17 case series studies. Studies meeting inclusion were broadly clustered into four musculoskeletal pain conditions based on underlying pathophysiology and/or anatomical pain location. These included 17 studies on tendinopathies,59–76 8 studies on arthritic and degenerative conditions,77–85 7 studies on spinal and pelvic pain,86–92 and 1study on myofascial pain.93 MCID criteria were assessed where applicable.
In the rating of RCTs with the PEDro tool, one item was eliminated in the evaluation of two studies. In the study by Topol et al, item seven was eliminated because the sole outcome measure was a patient-administered psychometric instrument.59 A summary of each reviewed study is shown in Table 2. Except where otherwise stated, all studies utilizing a local anesthesia injection control used an identical agent and concentration contained in the dextrose injection.
Table 2.
Reviewed studies of dextrose prolotherapy in chronic musculoskeletal pain.
| AUTHOR AND YEAR, COUNTRY, DESIGN, EVIDENCE SCORES | POPULATION AND SELECTION | TREATMENT AND EVALUATION | RESULTS |
|---|---|---|---|
| Tendinopathies | |||
| Topol, et al. (2011)59 Argentina Double-blind RCT PEDro 10 Level 1 |
Osgood-Schlatter disease n = 54; athletes aged 9–17 years In clusion: • Anterior knee pain >3 mo. • Replication of pain severity and location to the tibial tuberosity during a single leg squat • Nonresponse to 2 mo. physiotherapy Exclusion: • Pain from patellofemoral crepitus or patellar origin |
Active group: dextrose 12.5%, lidocaine 1% Injection control group: lidocaine 1% Noninjection control group: Usual care (supervised exercise) Injections given at 0, 1, and 2 months (double-blind) At 3 months, subjects not achieving NPPS = 0 were offered monthly dextrose injections as needed (open-label) 9 lidocaine and 8 usual care patients switched to dextrose at 3 months Outcome measure(s): Mean NPPS scores |
6 month follow-up (double-blind): • Greater reduction in pain with dextrose than lidocaine (P = 0.004) and usual care (P < 0.0001) • Greater reduction with lidocaine than usual care (P = 0.024) 12 month follow-up (open-label): • NPPS <4 in 100% of dextrose, 92.3% of lidocaine, and 71.4% of usual care patients (dextrose vs. lidocaine, NS; dextrose vs. usual care, P = 0.008; lidocaine vs. usual care, NS) • NPPS of 0 in 84.2% of dextrose, 46.1% of lidocaine, and 14.2% of usual care patients (dextrose vs. lidocaine, P = 0.024; dextrose vs. usual care, P < 0.0001; lidocaine vs. usual care, P = 0.005) |
| Refai, et al. (2011)60 Double-blind RCT Egypt PEDro: 8 Level 1 |
TMJ n = 12 Inclusion: • Confirmation of painful subluxation or dislocation of the TMJ • Absence of medical condition that could interfere with healing |
Active group: dextrose 10%, mepivacaine 2% Control group: mepivacaine 2% 4 injections in each TMJ, spaced 6 weeks apart Outcome measure(s): • VAS pain score • MMO in cm. between the incisal edges of the upper and lower incisors • Frequency of clicking sound • Frequency of luxation |
MMO in dextrose and control groups: • Baseline: 5.03 and 4.97 (NS) • 6 weeks: 4.72 and 4.93 (NS) • 18 weeks after first injection: 4.35 and 4.93 (P = 0.043) • 3 months after last injection: 4.33 and 4.97 (P = 0.039) Pain: Steady decrease in both groups, but NS Luxation frequency: NS Clicking frequency: NS |
| Zhou et al. (2014)62 Case series China Level 4 |
TMJ n = 45 Inclusion: • Non-neurogenic recurrent dislocation of the TMJ |
Treatment: dextrose 50%, 0.1% lignocaine Outcome measure(s): • Absence of dislocation or subluxation for ≥6 months after treatment |
At ≥ 6 months post treatment 41/45 (91%) no longer had dislocation or subluxation. Of the 41 rehabilitated patients • 26 (63%) required a single injection • 11 (27%) had 2 treatments • 4 (10%) needed a third injection. |
| Yelland et al. (2011)63 | Achilles tendinosis n = 43 |
Treatment: glucose 20%, lgnocaine 0.1%, ropivacain 0.1% Patients randomly selected for: |
At 12 months, proportions achieving the minimum clinically important change for VISA-A • ELE - 73% |
| Double-blind RCT Austrailia Level 1 PEDro10 |
• Eccentric exercises only • Prolotherapy only • Eccentric and prolotherapy Outcome measure(s): • VISA-A |
• Prolotherapy only - 79% • Combined treatment - 86% Mean (95%CI) increases in VISA-A scores at 12 months were: • ELE: 23.7 (15.6 to 31.9) • Prolotherapy only: 27.5 (12.8 to 42.2) • Combined treatment: 41.1 (29.3 to 52.9) At 6 weeks and 12 months - Increases were significantly less for ELE than for combined treatment. Compared with ELE, reductions in stiffness and limitation of activity occurred earlier with prolotherapy and reductions in pain, stiffness and limitation of activity occurred earlier with combined treatment. |
|
| Maxwell (2007)64 Canada Case series Level 4 |
Achilles tendinosis n = 32, mean duration 28.6 months Inclusion: • Failure of conservative therapy • Pain >3 months Exclusion: • Acute tendinitis • Symptoms due to acute trauma, surgery or interventional procedures in past 3 months |
Treatment: dextrose 25% Patients injected every 6 weeks until symptoms resolved or no improvement was shown; mean injections were 4 Outcome measure(s): • VAS pain score • US evaluation |
Mean reduction in pain scores from baseline at 12 months: • At rest: 88.2% (P < 0.0001) • ADL: 84.0% (P < 0.0001) • Physical activity: 78.1% (P < 0.0001) Mean decrease in tendon thickness: 11.7 to 11.1 mm (P < 0.007) at 12 months Tendon neovascularity decreased in 55% |
| Ryan et al. (2010)65 Case series Canada Level 4 |
Achilles tendinosis n = 99 (108 tendons), median duration 21 months Inclusion: • Pain at Achilles tendon insertion or midportion >6 months • Pain directly at posterior border of the calcaneus or along midportion of the tendon 2–6 cm proximal to its insertion • Documented non-response to conservative treatment Tendon locations of tendonosis: • 86 midportion • 22 insertion |
Treatment: dextrose 25% Injection guidance by GS or color Doppler US into abnormal hypoechoic areas and anechoic clefts or foci in the thickened portion of the Achilles tendon 1–3 sites injected per treatment session Patients received a median 5 sessions spaced a mean 5.6 weeks apart Outcome measure(s): • VAS pain score • US evaluation Measurements at baseline, post-test and 28.6 mo. follow-up |
Mean baseline, post-test, and follow-up VAS: Midportion tendonosis (pain improved): • At rest: 34.1, 12.6 (P < 0.001), 3.3 (P < 0.001) • ADL: 50.2, 21.8 (P < 0.001), 9.5 (P < 0.001) • Physical activity: 70.7, 36.7 (P < 0.001), 16.7 (P < 0.001) Insertional tendonosis (pain improved): • At rest: 33.0, 18.0 (NS), 2.7 (P < 0.001) • ADL: 51.3, 29.6 (P < 0.05), 10.0 (P < 0.001) • Physical activity: 69.6, 39.8 (P < 0.01), 17.7 (P < 0.001) Baseline and post-test US findings: • Midportion tendiosis: Size of hypoechoic region (mm2) 81.60 and 52.1 (P < 0.01) • Insertional tendiosis: Intratendinous tear size (mm) 5.3 and 1.6 (P < 0.01) Greater reduction in grades 2 and 3 echotexture and neovascularization severity in midportion vs. insertional patients |
| Lyftogt et al. (2005)66 Case series New Zealand Level 4 |
Achilles tendinopathy n = 16, mean duration 14 months Inclusion: x-ray confirmation |
Treatment: dextrose 20% Outcome measure(s): • VAS pain score |
At 18-week follow-up: • 11/16 pain VAS score of 0 • 14/16 satisfied with therapy |
| Scarpone et al. (2008)67 United States Double-blind RCT Level 1 PEDro10 |
Lateral epicondylitis of the elbow n = 24 Inclusion: ≥6 months duration refractory lateral epicondylosis |
Active group: 50% dextrose/5% sodium morrhuate/4% lidocaine/0.5% sensorcaine/saline Control group: 0.9% saline Three 0.5 mL injections at the supracondylar ridge, lateral epicondyle and annular ligament at baseline, 4 and 8 weeks. Outcome measure(s): • Resting elbow pain (0–10 Likert scale) • Extension and grip strength Each was performed at baseline, 8 and 16 weeks. One-year follow-up included pain assessment and effect of pain on activities of daily living |
Active group vs. Contols: Improved pain scores (4.5 ± 1.7, 3.6 ± 1.2 and 3.5 ± 1.5 versus 5.1 ± 0.8, 3.3 ± 0.9 and 0.5 ± 0.4 at baseline, 8 and 16 weeks, respectively); At 16 weeks, differences were significant compared to baseline scores within and between groups (P<.001). Active group improved extension strength compared to Controls (P < 0.01) and grip strength compared to baseline (P < 0.05) Clinical improvement in Active group maintained at 52 wks. |
| Shin et al. (2002)68 South Korea Case series Level 4 |
Lateral epicondylitis of the elbow n = 84 Inclusion: US confirmation |
Treatment: dextrose 15% Patients received 3 injections spaced 2 months apart Outcome measure(s): VAS pain score |
Mean pain scores at baseline and 6 mo. were 6.79 and 2.95 (P < 0.01) 9 mo. follow-up (n = 71) pain scores same/improved in 80.2%, increased in 19.7% Greater pain reduction in patients without (7.08 to 2.16) vs. with partial tendinous tear (6.9 to 3.67; P < 0.01) |
| Park et al. (2003)69 Case series South Korea Level 4 |
Lateral Epicondylitis of the elbow n = 11 Inclusion: • Partial (n = 11) tear or full thickness but incomplete width tear (n = 1) of common extensor tendons |
Treatment: dextrose 15% Patients received 2–6 injections Outcome measure(s): Change in: • Echogenicity (GS US) • Tendon fibrillar pattern (GS US) • Vascularity (color Doppler US) • Pain (VAS) |
At mean 5.8 month follow-up: VAS decreased a mean 4.5 points 1 tendon–a few echogenic lines in initially anechoic lesion 3 tendons–most of anechoic lesion filled with fibrillar echogenicity except for a small anechogenic focus 2 tendons–initial anechoic lesion became same sized hypoechoic lesion with diffuse fibrillar pattern 6 tendons–initial anechoic lesion smaller, with diffuse fibrillar pattern Hypervascularity in 6 of 11 tendons |
| Ryan et al. (2011)70 Case series Canada Level 4 |
Overuse patellar tendonopathy n = 47, mean duration 21.8 months Inclusion: • Failure of standard-of-care therapy • Confirmation by palpation and US |
Treatment: dextrose 25% Under US guidance, injections given into abnormal hypoechoic areas and anechoic clefts/foci in the thickened portion of the patellar tendon Patients received a median 4 injections 6.4 weeks (mean) apart Outcome measure(s): • VAS pain score • US evaluation |
Mean pain scores at baseline and 45 weeks: • At rest, 38.4 and 18.7 (P < 0.01) • ALD: 51.1 and 25.8 (P < 0.01) • Sports activity: 78.1 and 38.8 (P < 0.01) Change in pain scores during rest, ADL and sport activity correlated with change in echotexture severity (r values 0.306, 0.379 and 0.428, respectively; P < 0.05) General improvement in echotexture and neovascularity severity was found |
| Topol et al. (2008)72 Case series |
Chronic groin pain n = 72 athletes, mean duration |
Treatment: dextrose 12.5% Patients received a mean 2.7 |
Mean scores at baseline and 26 month follow-up (mean): |
| Argentina Level 4 |
11 months Inclusion: • Chronic groin pain from osteitis pubis and/or adductor tendinopathy • nonresponse to conservative therapies |
treatments. Outcome measure(s): • VAS pain score • NPPS assessment of pain-related athletic avoidance |
VAS, 6.47 and 1.18 (P < 0.001) NPPS, 5.13 and 1.06 (P < 0.001) At follow-up, 66/72 (91.6%) had fully resumed sport activities; all but 2 of these were pain-free |
| Topol et al. (2005)73 Case series Argentina Level 4 |
Chronic groin pain n = 24, mean duration 15.5 months Inclusion: • Chronic groin pain from osteitis pubis and adductor tendinopathy |
Treatment: dextrose 12.5% Patients received a mean 2.8 treatments. Outcome measure(s): • VAS pain score • NPPS assessment of pain-related athletic avoidance |
Mean scores at baseline and 17 month follow-up (mean): • VAS, 6.3 and 1.0 (P < 0.001) • NPPS, 5.3 and 0.8 (P < 0.001) 20 of 24 reported an absence of pain at follow-up |
| Bertrand et al. (2016)74 Canada Double-blind RCT Level 1 PEDro10 |
Chronic shoulder pain n = 73 Inclusion: • Examination findings of rotator cuff tendinopathy • US conformation of supraspinatus tendinosis/tear |
Enthesis-Dex group: 25% dextrose, 0.1% lidocaine/saline Entheis-Saline group: 0.1% lidocaine/saline Superficial-Saline group: injection 0.5- to 1-cm depth with 0.1% lidocaine/saline All participants received 3 monthly injections to painful entheses at and concurrent programmed physical therapy. Outcome measure(s): improvement in maximal current shoulder pain ≥2.8 (twice the minimal clinically important difference for VAS pain); USPRS; 0-to-10 satisfaction score (10, completely satisfied) |
At 9-month follow-up the Enthesis-Dextros Group: Maintained greater improvement in pain 59% ≥2.8 VAS compared with Enthesis-Saline (37%; P = .088) and Superficial-Saline (27%; P = .017). Had greater satisfaction: 6.7 ± 3.2 compared with Enthesis-Saline (4.7 ± 4.1; P = .079) and Superficial-Saline (3.9 ± 3.1; P = .003). USPRS findings were not different between groups (P = .734). |
| Lee et al. (2015)75 South Korea Retrospective case controlled series Level 3 |
Chronic shoulder pain n = 151 Inclusion: • Non-traumatic refractory rotator cuff disease • Unresponsive to 3 months of aggressive conservative treatment |
Active group: dextrose 16.5%. Control group: conservative treatment Outcome measure(s): • VAS score of shoulder pain level for the past 1 week; • SPADI score • Isometric strength of the shoulder abductor • AROM of shoulder • Maximal tear size on ultrasonography • Number of analgesic ingestions per day |
Compared with the control group, the active group showed significant improvement at 1-year follow-up in: • VAS score • SPADI score • Isometric strength of shoulder abductor • Shoulder AROM of flexion, abduction, and external rotation |
| Ryan et al. (2009)76 Canada Case series Level 4 |
Plantar fasciitis n = 20, median 21 months duration Inclusion: • Symptoms >6 months • Non-response to conservative treatment Exclusion: acute plantar foot pain, surgery or interventional procedures in last 6 months |
Treatment: dextrose 25% Injections into plantar fascia Injections were given at 6 week intervals; median of 3 treatments per patient Outcome measure(s): VAS pain score |
Mean pain scores at baseline and 18 week follow-up: • At rest, 3.7 and 1.0 (P < 0.001) • With walking, 7.5 and 2.5 (P < 0.001) • With running, 9.2 and 3.9 (P < 0.001) No change in mean pain score from 18 week to 11.8 month (mean) follow-up |
| Osteoarthritis and Degenerative Conditions | |||
| Rabago et al. (2011)77 United States 3-way double-blind RCT PEDro 9 Level 1 |
Knee osteoarthritis n = 89 Inclusion: • ARA criteria moderate-severe knee osteoarthritis • >3 months duration |
Active group: dextrose 15% and dextrose 25% Injection control group: saline Noninjection control group: exercise instruction Injections at 1, 5, and 9 weeks, and weeks 13 and 17 as needed. Extra-articular injections at periarticular tendon and ligament insertions (dextrose 15%), with 1 intra-articular injection (dextrose 25%) through an infero-medial approach. Patients received a mean 4.3 injection sessions Outcome measure(s): • OA-related pain, function and stiffness (WOMAC) • Knee pain severity and frequency (KPS) |
WOMAC composite score: no significant difference between groups WOMAC score, adjusted for gender, age and BMI: greater reduction in mean dextrose (15.32) than saline (7.68) (P < 0.05) and exercise (8.25) (P < 0.05) scores Mean KPS scores in dextrose subjects showed greater improvement per injected knee relative to baseline status (P < 0.001) and compared to both control groups (P < 0.05) |
| Reeves & Hassanein (2000)79 United States Double-blind RCT PEDro 10 Level 1 |
Knee osteoarthritis with/without ACL laxity n = 77 Inclusion: • ≥ grade 2 joint narrowing or ≥ grade 2 osteophytic change in any knee compartment • pain duration ≥6 months |
Active group: 10% dextrose, xylocaine 0.075% Control group: xylocaine 0.075% Tibiofemoral injections Patients received 3 bimonthly injections; dextrose-injected patients then received 3 further bimonthly injections under open-label conditions Outcome measure(s): • VAS pain and swelling scores • Goniometric measurement of joint flexion, • KT1000 measurement of ADD • US |
6 month follow-up (dextrose vs. control): Greater improvement in dextrose vs. control group in pain, swelling, buckling episodes, and knee flexion range (P = 0.015 for all) 12 month follow-up (dextrose vs. baseline): Improvement found in lateral patellofemoral cartilage thickness (P = 0.019) and distal femur width in mm (P = 0.021). Knees w/joint laxity showed improved knee flexion range (+12.8 degrees, P = 0.005) and ADD (57%, P = 0.025). 8/13 dextrose-treated knees with ACL laxity at baseline no longer lax at 1 year |
| Reeves & Hassanein (2003)80 United States Case series Level 4 |
ACL laxity in patients with knee osteoarthritis n = 18 Inclusion: • Laxity with ADD ≥2 mm measured by KT1000 arthrometer • Duration >6 months |
Treatment: dextrose 10% and dextrose 25% Injections of dextrose 10% at months 0, 2, 4, 6, and 10, dextrose 25% at month 12, then dextrose 10% or 25% every 2–4 months through month 36 according to patient preference Outcome measure(s): • VAS pain and swelling scores • Goniometric measurement of joint flexion • KT1000 measurement of ADD |
VAS at baseline and 12 months: • Pain during rest, 2.31 and 1.56 (NS) • With walking, 4.19 and 2.50 (P = 0.004) • With stair use, 5.88 and 4.06 (P = 0.022) • Swelling, 2.75 and 1.31 (NS) VAS at baseline and 36 months: • Pain at rest, 2.31 and 1.25 (NS) • With walking, 4.19 and 2.38 (P = 0.002) • With stair use, 5.88 and 3.82 (P = 0.007) • Swelling, 2.75 and 1.00 (P = 0.017) Biomechanical assessments: Flexion range: 111.88, 125.94 at 12 months (P = 0.001), 122.38 at 36 months (P = 0.002) ADD: 2.88, 1.32 at 12 months (P = 0.023), 0.82 at 36 months (P = 0.002) At 36 months, normal ADD in 10 of 14 knees |
| Dumais et al. (2012)81 Randomized Crossover Study |
Chronic knee osteoarthritis n = 36 Inclusion: • Pain duration ≥6 months • Age ≥18 years |
Treatment: dextrose 20%, lidocaine 0.5% Random assignment: Group A: exercise therapy for 32 weeks in combination with injections inside the |
Group A: • 0–16 weeks - significant change in WOMAC indicating decrease in symptoms (mean ± standard deviation: −21.8 ± 12.5, P < 0.001). |
| Canada Level 1 |
• Able to execute exercises | knee joint on weeks 0, 4, 8, and 12 Group B: exercise therapy for 32 weeks in combination with injections inside the knee joint on weeks 24, 28, and 32 (Group B) Outcome measure(s): Change in WOMAC scores between weeks 0 and 16; and weeks 20 and 36 |
• 20–30 weeks - no significant change in WOMAC scores (−1.2 ± 10.7, P = 0.65). Group B: • 0–16 weeks - no significant change in WOMAC scores (−6.1 ± 13.9, P = 0.11). • 20–30 weeks - significant change in WOMAC indicating decrease in symptoms (−9.3 ± 11.4, P = 0.006). >36 weeks - W OMAC scores improved in both groups by 47.3% (A) and 36.2% (B). The improvement attributable to RIT alone corresponds to a 11.9-point (or 29.5%) decrease in WOMAC scores. |
| Eslamian & Amouzandeh (2015)82 Iran Case series Level 4 |
Moderate knee osteoarthritis n = 24 Inclusion: female |
Treatment: dextrose 20% Injections given at baseline, 4 weeks, 8 weeks Patients were followed for 24 weeks. Outcome Measure(s): • VAS pain scale • AROM • WOMAC Measurements made at baseline, 4, 8, and 24 weeks later. |
At baseline: • Mean AROM (105.41 ± 11.22°) • Mean VAS scale at rest (8.83 ± 1.37) • Mean VAS scale at activity (9.37 ± 1.31) Week 24: • Mean AROM increased by 8° • Mean VAS scale at rest decreased in 45.89% (P < 0.001) • Mean VAS scale at activity decreased in 44.23%, (P < 0.001) • Total WOMAC decreased by 30.5 ± 14.27 points (49.58%) (P < 0.001) Improvements of all parameters were considerable until week 8, and were maintained throughout the study period. |
| Hashemi et al. (2015)83 Iran Double-blind RCT PEDro 9 Level 1 |
Mild to moderate knee osteoarthritis n = 80 Inclusion: Diagnosis of knee osteoarthritis (clinical examination and anteroposterior standing radiography) |
Active Group 1: dextrose 12.5%, lidocaine 1% Active Group 2: 15 g/mL of ozone-oxygen mixture, lidocaine 1% Injections given 3 times at 7 to 10 day intervals. Outcome Measure(s): • VAS pain scale • WOMAC |
Active Groups 1 and 2: • Mean VAS decreased (P < 0.001) • WOMAC increased (P < 0.001 • No significant difference between the two groups |
| Reeves & Hassanein (2000)84 United States Double-blind RCT PEDro 10 Level 1 |
Osteoarthritic finger joints n = 27; average pain duration >4 years Inclusion: • Moderate osteophytosis • Moderate joint space narrowing • Mild osteophytosis plus mild joint space narrowing • Pain duration ≥6 months |
Active group: dextrose 10%, xylocaine 0.075% Control group: xylocaine 0.075% Injections performed 0, 2, and 4 months after enrollment, data obtained 6 months after first injection After 6 months, patients in both groups offered bimonthly dextrose 10% injection Patient attrition: 4/13 active, 3/14 control group Outcome measure(s): • VAS pain scale • Goniometric measurement of joint flexion • X-ray imaging of joint repair |
6 month follow-up: • Greater improvement in dextrose vs. control groups in pain with movement (P = 0.027); other comparisons NS • Greater improvement in flexion range in dextrose vs. control group (P = 0.003) 12 month follow-up: • Dextrose group showed difference from baseline in joint narrowing (P = 0.006) • All other comparisons NS |
| Jahangiri et al. (2014)85 Iran Double-blind RCT PEDro 9 Level 1 |
Osteoarthritic finger joints n = 60 Inclusion: • >40 years of age • history of pain in first carpometacarpal joint >3 months • Pain intensity VAS >30 at baseline • radiographic evidence of OA |
Active group: dextrose 20%, lidocaine 2% Control group: 40 mg methylprednisolone acetate (0.5 ml), lidocaine 2% Outcome measure(s): VAS pain score Hand function and strength of lateral pinch grip Measured at baseline, 2 and 6 months after the treatment |
At 1 and 2 month follow-up results were more favorable among control group than active group participants. At 6 months outcome was more favorable for active group [mean difference (95% CI) in VAS = 1.1 (0.2, 2.0), P = 0.02]. After 6 months of treatment, both study and control groups increased functional level, but study group seemed to be more effective [mean difference (95% CI) in total function score = 1.0 (0.2, 1.8), P = 0.01] |
| Spinal and pelvic pain | |||
| Miller et al. (2006)86 Case series Level 4 |
Discogenic leg pain n = 76, mean duration 39 months Inclusion: • Moderate to severe degenerative disc disease without herniation • Concordant pain reproduction with CT discography • Normal neurological exam • Nonresponse to 6 months of conservative treatment |
Treatment: dextrose 25% Dextrose solution injected into disc space; patients received a mean 3.5 injections into a mean 1.7 discs Outcome measure(s): • Mean NRS pain rating |
n = 37 non-responders (<20% pain reduction); n = 6 temporary (<2 months) responders Of 33 sustained responders, mean (SD) pain scores at baseline, 2 month, and 18-month follow-up: 8.9 (1.4), 2.5 (2.0), and 2.6 (2.2) Mean overall pain improvement: 71% |
| Khan et al. (2008)87 India Case series Level 4 |
Coccygodynia n = 37 Inclusion: • Nonresponse to conservative therapy for >6 months Exclusion: Posttraumatic and post-delivery coccygodynia, sacro-coccygeal subluxation, coccygeal spicule, organic bony pathology on radiograph |
Treatment: dextrose 25% 2 injections into sacro-coccygeal joint 15 days apart; those with VAS pain score >4 given 3rd injection 4 weeks later Outcome measure(s): VAS pain scores |
Baseline pain score: 8.5* Pain score after 1st injection: 3.4 Pain score after 2nd injection: 2.5 n = 7 little-no improvement n = 30 good pain relief *patients w/previous steroid injection (n = 27) had baseline pain score 8.8 |
| Kim WM, et al. (2010)88 South Korea (in English) Double-blind RCT PEDro 9 Level 1 |
Sacroiliac joint pain n = 48 Inclusion: • Pain origin confirmed by pain reduction ≥50% to intraarticular SI joint block with levobupivacaine 0.25% • Following SI block response, non-response to 1 month of medical treatment Exclusion: Cancer, fractures, inflammatory arthritis, infection, fibromyalgia, active litigation |
Active group: dextrose 25%, levobupivacaine 0.25% Control group: triamcinolone 40 mg, levobupivacaine 0.25% Biweekly intraarticular injections into intra-articular SI, up to 3 injections Outcome measure(s): NRS pain scores Oswestry (2 weeks only) |
2-week follow-up: Significant improvement in both groups, no significant difference between active and control 15 month follow-up: Cumulative incidence of ≥50% pain reduction: Dextrose: 58.7% (95% CI 37.9%–79.5%) Steroid: 10.2% (95% CI 6.7%-27.1%) Between-groups difference P < 0.005 |
| Kim HS, et al. (2007)89 South Korea Double-blind RCT Level 2 |
Iliac crest pain syndrome n = 44 |
Active group: dextrose 20%, 1% lidocaine Control group: triamcinolone, lidocaine Weekly injection for 4 weeks Outcome measure(s): • Mean change from baseline in VAS pain • Oswestry • Pressure threshold (algometer, kg/cm2) |
3 month follow-up: Both groups improved in pain, disability and pressure threshold scores (P < 0.05) No significant difference between groups on any measure at any follow-up interval |
| Hooper et al. (2011)90 Canada Case series Level 3 |
Chronic cervical, thoracic or lumbar pain n = 71 litigants (mean pain duration 2.1 years) n = 76 non-litigants (mean pain duration 6.3 years) Inclusion: • Demonstration of laxity on stress testing in spinal, iliolumbar, or sacroiliac ligaments • Pain >6 months • Nonresponse to conventional therapies |
Active treatment: dextrose 20% Injections into the facet capsules of the cervical, thoracic, lumbar spine Patients received weekly injections for up to 3 weeks, and 1 month later if needed Outcome measure(s): • NDI • PSFS • RMDQ |
At baseline, litigants compared to non- litigants: • Higher disability scores (P = 0.001) • More multiple regions affected • More cervical and thoracic regions affected (P < 0.0001) • Shorter mean symptom duration (P < 0.0001) 1-year follow-up: Both litigants and non-litigants improved in all disability scales (P < 0.001). Percentage of litigants vs. non-litigants reporting improvement: • Impression of change scales for symptoms (91/92%) and function (90/90%) • Improved ability to work (76/75%) • Willingness to repeat treatment (91/93%) • Ability to decrease medication (82/81%) • Decreased need for other treatment (80/84%) Litigants showed greater improvement in treatment of the thoracic spine (P < 0.05) |
| Centeno et al. (2005)91 United States Case series Level 4 |
Neck pain n = 6 Inclusion: • ≥50% pain reduction and >2.7 mm absolute cervical translation with 2-day cervical immobilization • Post-MVA cervical instability, neck pain and disability • Pain/disability >6 months • Failure to respond to conservative therapy Exclusion: previous neck injury, connective tissue disease, arthritis or diabetes I or II |
Treatment: dextrose 12.5% Injections targeted instability sites including the spinous processes, lamina, and posterior elements Outcome measure(s): • Mean changes from baseline in VAS pain scores • Radiographic findings |
Pain scores, baseline and 1 month: 5.75 and 3.83 (P = 0.04) Significant correlation between changes in pain scores and translation (rho = 0.88, P = 0.02), Significant correlation between changes in flexion and translation (rho = 0.94, P < 0.01) |
| Lee et al. (2009)92 South Korea Case series Level 4 |
Low back and pelvic pain n = 22, mean duration 39.8 months Inclusion: • Sacroiliac pain confirmed by ≤50% pain reduction with local anesthetic block |
Treatment: Dextrose 25% Injections every other week for 3 weeks Outcome measure(s): • Mean changes from baseline in NRS pain scale • Oswestry |
Mean (range) NRS scores: (P < 0.01) Baseline: 6 (4–8) 10 weeks: 1(0–3) Mean (SD) Oswestry scores (P < 0.01) Baseline: 34.1 (15.5) 10 weeks: 12.6 (9.8) Mean duration of pain reduction ≥50% was 12.2 months |
| Myofascial Pain Syndrome | |||
| Kim MY, et al. (1997)93 South Korea Double-blind RCT Level 2 PEDro 8 |
Myofascial pain syndrome n = 64 |
Active group: dextrose 5% Control group: lidocaine 0.5% Control group: saline Outcome measure(s): • Mean changes from baseline at 7 days in VAS pain score • Pressure threshold (algometer, kg/cm2) |
Change in VAS pain score: Dextrose: 6.87 and 2.39 (P < 0.01) Saline: 6.50 and 3.85 (NS) Lidocaine: 6.95 and 4.05 (NS) Pressure threshold tolerance: Dextrose: 1.79 and 2.49 (P < 0.05) Saline: 1.70 and 1.91 (NS) Lidocaine: 1.75 and 2.07 (NS) |
Abbreviations: ACL, anterior cruciate ligament; ADD, anterior displacement difference; ADL, activities of daily living; AROM, active range of motion; BMI, body mass index; CI, confidence interval; CT, computerized tomography; GS, gray scale; KPS, Knee Pain Scale; MMO, maximum mouth opening; MVA, motor vehicle accident; NDI, Neck Disability Index; NPPS, Nirschl Pain Phase Scale; NRS, Numeric Rating Scale; NS, not significant; OA, osteoarthritis; Oswestry, Oswestry Disability Index; PEDro, Physiotherapy Evidence Database; PRS, Pain Rating Scale; PSFS, Patient Specific Functional Scale; RMDQ, Roland-Morris Disability Questionnaire; SD, standard deviation; SI, sacro-iliac; SPADI, Shoulder Pain and Disability Index; TMJ, temporomandibular joint; US, ultrasound; USPRS, Ultrasound Shoulder Pathology Rating Scale; VAS, Visual Analog Scale; VISA-A, Victorian Institute of Sports Assessment; WOMAC, Western Ontario Macmaster University Osteoarthritis Index.
Tendinopathies
The most robust data supporting the efficacy of prolotherapy for musculoskeletal conditions, compared to control injections, are for chronic, painful overuse tendon conditions.15,16 Independent of location, tendinopathies from repetitive motion, and overuse injury share markedly similar characteristics.70 Cases of tendinopathies in the Achilles tendon,63–65 common elbow extensor,67–69 and patellar tendon70 possess similar histological, sonographic, and clinical features believed to represent an underlying noninflammatory painful degenerative pathophysiology.94 Histopathology of tendon biopsies in patients undergoing surgery for painful tendinopathy has revealed collagen separation, thin, frayed, and fragile tendon fibrils with lengthwise separation from other fibrils, disruption in cross section, and increase in tenocytes with myofibroblastic differentiation (tendon repair cells), proteoglycan ground substance, and neovascularization.
Consensus is growing regarding the efficacy of dextrose prolotherapy as an alternative to surgery for patients with chronic tendinopathy who have persistent pain despite appropriate rehabilitative exercise.95 The efficacy of dextrose injections in tendinopathy is believed to involve the initiation of a healing response secondary to cell membrane perturbation that follows a significant change in osmotic pressure between the extracellular matrix and tendon fibroblasts.94 Granulocytes and platelets gravitate to the inflammatory cytokines and chemotactic factors that are released from the cell membrane, which in turn release prohealing growth factors.35,36,40
Reviewed studies
Osgood–Schlatter disease. Several trials enrolled patients with athletic injury resulting in tendinopathy unresponsive to conservative treatment. In one double-blinded study, young athletes aged 9–17 years with Osgood–Schlatter disease were randomized to dextrose injection, control injection, or to a noninjection (supervised exercise) group. Dextrose prolotherapy patients had substantially greater pain reduction during sport activity than either group at follow-up, with many pain-free during sport involvement. At one year, 84% of the dextrose-treated knees were pain free compared to 46% of the lidocaine-treated knees.59
Temporal mandibular joint syndrome. Few studies have examined the effectiveness of prolotherapy for the treatment of temporal mandibular joint (TMJ) syndrome. One RCT found a significant functional improvement in TMJ patients who underwent dextrose prolotherapy compared to patients in the control group who only received injections of local anesthetic. Pain reduction, however, did not reach significance.60 Another RCT compared patients treated with dextrose prolotherapy and patients given a placebo. For both groups, masticatory efficiency increased, and general pain complaints and joint sounds decreased significantly. There was no significant difference in VAS scores between groups. However, the measurements of the maximum interincisal opening among the treatment group significantly decreased.61 One single case design study in which patients with TMJ were treated with injections of dextrose demonstrated decreased pain, increased quality of life as measured by the VAS, and absence of further dislocation or subluxation for more than six months.62
Achilles tendinopathy. Four studies of Achilles tendinosis were evaluated.63–66 Yelland et al designed a treatment comparison study of eccentric loading exercise versus dextrose injection treatment versus combined exercise and injection to determine the best treatment for Achilles tendinosis. The VISA-A questionnaire (a valid and reliable index of the clinical severity of Achilles tendonopathy that measures the domains of pain, stiffness, function in daily living, and sporting activity) was used in this study. At 12 months, reduction in stiffness and limitation in activity was seen in 73% of the exercise only group, 79% of the injection only group, and 86% of the group treated with a combination of eccentric loading and dextrose injection. However, it is interesting to note that positive results were obtained fastest with prolotherapy alone.63
In another study, Maxwell et al injected a dextrose solution into abnormal areas in the tendon and intrasubstance partial tears (as visualized with ultrasound) in patients suffering from chronic Achilles strain. Using ultrasonographic imaging, significant reductions from baseline were found in the size of hypoechoic region in patients with midportion tendinosis, and in the size of intratendinous tear in patients with tendon thickness. In addition, pain decreased with treatment in 78% of the patients, and ultrasounds showed that the tendons became healthier as demonstrated by fewer discontinuities in the tendon and better organization of the fibers.64
Ryan et al enrolled 99 patients with chronic Achilles tendon symptoms and objective evidence of Achilles degeneration by ultrasound who had failed all previous therapies. Treatment method involved injection inside the tendon with ultrasound guidance into areas of degeneration (hypoechogenicity or tear) with 0.5 mL or less 25% dextrose in 1–3 spots at each treatment. At follow-up, improvement in pain with everyday living improved from 57% at a mean of 28 weeks into treatment to 81% at a mean of 14 months posttreatment. More change was seen by ultrasound at a mean of 28 weeks in the mid-Achilles tendinosis group with significant reductions in the size of hypoechoic regions, intratendinous tears, and neovascularization.65 Lyftogt also found an absence of pain in 78.5% of their small sample of patients with Achilles tendinopathy when treated with prolotherapy.66
Lateral epicondylitis of the elbow. Three studies have demonstrated that lateral epicondylitis of the elbow is responsive to treatment with dextrose prolotherapy.67–69 Scarpone et al conducted a small double-blind RCT with adults with lateral epicondylosis. The treatment group was injected at 0, 1, and 3 months with 0.72% sodium morrhuate, 10.7% dextrose, 0.29% lidocaine, and 0.04% sensorcaine. The treatment group showed significant improvement in pain levels compared with patients given saline injection with the same number of needle punctures and volume (91% versus 33%). In addition, extension strength and grip strength was markedly improved in the treatment group as well.67
Shin et al studied 84 patients with lateral epicondylitis who were treated with dextrose prolotherapy. The pain score was evaluated by using VAS before treatment and one and six months after the third treatment. Ultrasonography was performed on 49 patients who were suspicious of a tendinous tear. Dextrose prolotherapy decreased VAS from 6.79 to 2.95, which reached statistical significance.68 Park et al.69 achieved a significant reduction in pain with VAS from baseline patients with lateral epicondylosis as well with treatment of the lateral epicondyle with 15% dextrose. Evidence of tendon healing was observed via ultrasound imaging, manifesting as diffuse fibrillar patterns in previously anechoic lesions67,68 and areas of hypervascularity.69
Patellar tendinopathy. A case series conducted by Ryan et al examined whether ultrasound-guided injection of hyperosmolar dextrose for treatment of patellar tendinopathy decreased pain scores and normalized the appearance of the patellar tendon on ultrasound. Findings revealed significant reductions in pain at rest and during activity, an overall downgrading of severity in intratendinous tearing and neovascularity as evident with ultrasonography, and a significant correlation between the differences in pain and echotexture severity.70
Plantar fasciitis. Few studies have been conducted examining the effect of prolotherapy on chronic plantar fasciitis. However, Kim and Lee conducted a single-blinded, randomized, controlled study comparing autologous PRP versus dextrose prolotherapy treatments for chronic recalcitrant plantar fasciitis. Patients in both treatment groups received two injections into the plantar fascia under ultrasound guidance at an interval of two weeks. The outcome measures included the pain, disability, and activity limitation subscales measured by means of the Foot Functional Index. Each treatment seems to be effective for chronic recalcitrant PF, expanding the treatment options for patients in whom conservative care has failed. Although PRP treatment resulted better initial improvement in function compared with dextrose prolotherapy treatment, at two- and six-month follow-ups, sustained improvement was comparable in both groups.71
Groin pain. Two uncontrolled trials in athletes with chronic groin pain from osteitis pubis and/or adductor tendinopathy were conducted by Topol et al.72,73 The treatment consisted of monthly injections of 12.5% dextrose with 0.5% lidocaine in abdominal and adductor attachments on the pubis. Therapy yielded substantial reductions in VAS pain and Nirschl Pain Phase Scale (NPPS), a 7-point measure of sports-related symptoms and level of participation, scores and an absence of pain at follow-up in 88.8% and 83.3% of patients, respectively.72 In the second study. Topol et al.73 treated elite rugby and soccer players experiencing chronic groin pain with similar results in pain reduction.
Shoulder joint pain. Dextrose prolotherapy has been shown to reduce pain and disability of traumatic and nontraumatic rotator cuff conditions. A RCT conducted by Bertrand et al.74 revealed that treatment of moderate to severe rotator cuff tendinopathy due to injury with injections of hypertonic dextrose on painful entheses resulted in superior long-term pain improvement and patient satisfaction compared with blinded saline injection over painful entheses, with intermediate results for entheses injection with saline. In a retrospective case–control study, Lee et al.75 demonstrated dextrose prolotherapy improved in pain, disability, isometric strength, and shoulder active range of motion in patients with refractory chronic nontraumatic rotator cuff disease.
Conclusions regarding tendinopathies
Although there is a dearth of studies on treatment with prolotherapy for these two conditions, there is strong Level 1 evidence that dextrose prolotherapy results in substantially reduced pain levels and pain-free resumption of sport activities in Osgood–Schlatter disease59 and functional improvement and pain reduction in TMJ60 Dextrose injections present a low-cost and safe treatment alternative with good long-term evidence for significant reduction of pain from pathology at either the insertion or midportion of the Achilles tendon, at rest and during tendon-loading activities. There is strong Level 4 evidence of statistically and clinically significant reduction in pain from baseline to follow-up in Achilles tendinosis,63,64 and specifically evidence of substantial and comparable pain reduction in patients with midportion or insertion site tendinopathy in Achilles tendinosis,66 and somewhat greater tendon healing in patients with midportion versus insertion site tendinopathy.65 There is strong Level 4 evidence of statistically and clinically significant reduction in pain from baseline to follow-up in dextrose prolotherapy treatments to lateral epicondylitis,67–69 overuse patellar tendinopathy,70 chronic groin pain,72,73 and traumatic and nontraumatic shoulder pains.74,75 Sonographic evidence of tendon repair and healing after dextrose prolotherapy treatments has been shown in Achilles tendinosis,64,65 lateral epicondylitis,68,69 and overuse patellar tendinopathy with a significant correlation between pain reduction and tendon healing in overuse patellar tendinopathy.70 Prolotherapy has also demonstrated a good response in patients with chronic plantar fasciitis reducing pain during rest and activity75; however, further studies including a control group are needed to validate these outcomes.
OA and degenerative conditions
OA is characterized by progressive breakdown of articular cartilage, proteoglycan degradation, and disruption of the collagen network resulting in joint destruction and loss of function.96 In addition to genetic and biochemical factors, several external factors have been associated with OA. These include sudden impact, direct trauma, overuse or repetitive motion injuries, avascular necrosis, corticosteroids, obesity, and ligamentous injury culminating in joint hypermobility and instability.97 Ligament damage resulting in weakness is an important factor in the development of OA as it prevents normal distribution of weight and increases stress on the articular surfaces of the joint causing cartilage injury and joint degeneration. Ligament laxity and joint capsule disruption increases joint hypermobility and also risk of articular cartilage injury due to loss in the stabilization of joint motion by the ligament structure.96–98
Experimental studies have shown the positive effect of hypertonic dextrose in promoting direct intracellular expression of growth factors in tenocytes and fibroblasts.35 Dextrose prolotherapy may also benefit those with knee OA through the stabilization of interarticular ligaments by its positive effect on joint mechanics to promote articular cartilage recovery and improvement in range of motion.35,44
Reviewed studies
Knee OA. A three-arm randomized controlled double-blinded study conducted by Rabago et al found significantly greater improvement in pain reduction, swelling, buckling episodes, and flexion range with dextrose compared with lidocaine injections or exercise. Furthermore, prolotherapy patients showed significantly greater improvement at 52 weeks than control patients.77 In a recently published analysis, Rabago et al.78 reported that most participants have continued to experience progressive improvement of knee pain, function, and stiffness scores at 2.5 years after the initiation of the study.
An RCT conducted by Reeves and Hassanein79 revealed that patients with knee laxity treated with dextrose injections experienced significant improvement in knee flexion range and anterior displacement difference (ADD), with 61.5% exhibiting an absence of laxity as compared with the control group. A long-term open-label continuation of this trial involving the patient subgroup with knee laxity found continuity of effect with significant improvements from baseline in pain during walking and stair use, flexion range, ADD, and a similar proportion of patients with an absence of laxity at follow up.80
Dumais et al conducted a crossover study where participants were randomly assigned to receive exercise therapy for 32 weeks in combination with dextrose injections on weeks 0, 4, 8, and 12 or dextrose injections on weeks 20, 24, 28, and 32. Both groups showed significant reduction of knee OA symptoms as measured by WOMAC scores that were sustained at six-month follow-up.81
Eslamian and Amouzandeh also demonstrated the long-term effects of dextrose prolotherapy in a single-arm prospective study. Significant therapeutic effects of prolotherapy with intraarticular dextrose injection in patients with moderate knee OA were achieved. Pain severity, as measured by WOMAC scores, was reduced at rest and during activity, and articular range of motion was increased. Improvements were still present at six-month follow-up.82
In one RCT by Hashemi et al, the efficacy of dextrose versus ozone as a proliferant was compared in two groups of 40 patients suffering from mild to moderate knee OA. Both groups received intraarticular injections, and the treatment was performed three separate times at 10 days intervals. VAS and WOMAC scores at pretreatment and three months posttreatment were significantly improved for both groups, although were not statistically different between one other.83
Finger and thumb OA. A randomized control trial of patients with thumb and finger joint OA conducted by Reeves and Hassanein found significantly greater improvement among dextrose versus lidocaine patients in pain with movement, flexion motion, and joint narrowing. However, the difference in movement pain was the most impressive, reaching statistical significance with 42% versus 15% improvement.84
In a second randomized control trial of patients with thumb joint OA, Jahangiri et al.85 demonstrated dextrose/lidocaine injections resulted in more favorable VAS scores and improved total function at six months compared to corticosteroid injections, which at one month were offered more pain relief than prolotherapy but without sustained benefit.
Conclusions regarding OA/degenerative conditions
There is strong Level 1 evidence that in patients with knee OA, dextrose prolotherapy results in significant sustained improvement, including reduction of pain and swelling,77–83 fewer buckling episodes, increased knee flexion range, increased lateral patellofemoral cartilage thickness, and decreased ADD and laxity.78 There is Level 1 evidence demonstrating that in patients with osteoarthritic finger and thumb joints, dextrose prolotherapy results in significant improvements in pain with movement, flexion range, and joint narrowing.84,85 There is Level 4 evidence of significant improvements in OA-related pain, stiffness, and function in patients with knee OA81 and significant improvements in pain during rest, walking, and stair use, flexion range, and ADD in OA patients with anterior cruciate ligament (ACL) laxity.79
Spinal and pelvic pain
In approximately 90% of patients, low back pain is mechanical in nature, typically originating from overuse, straining, lifting, or bending that results in ligament sprains, muscle pulls, or disk herniation.99 The popular understanding of back pain is disk herniation as a frequent cause, but to a much greater extent, ligament injury forms the underlying basis.99,100 Ligaments hold the disk in place, and with ligament weakness, the disk is more likely to herniate.101,102
The source of low back and buttock pain as related to the sacroiliac (SI) joint is present in as many as 15%–30% of back pain patients,103,104 and perhaps up to 40% in patients who have had a previous lumbar fusion.105 SI joint dysfunction may also produce pain similar to a herniated lumbar disk along the same sciatic nerve distribution.106,107 Low back pain patients who remain symptomatic despite tailored physiotherapy are believed to possess deficient ligament strength in the posterior elements of the SI joint, resulting in insufficient stability to permit effective muscle recruiting strategies.108 Experimental studies have found prolotherapy effective in stimulating the production of collagen fibers, thus strengthening ligaments.109
Reviewed studies
Discogenic leg pain. Dextrose prolother-apy has been effective in treating patients with coccygodynia pain in both case series studies and RCTs. In a prospective case series with patients experiencing advanced degenerative discogenic leg pain who had failed other treatment, Miller et al.86 found that 43% showed sustained improvement with an overall reduction in NRS pain of 71%. In a case series study, Khan et al.87 found that patients with coccygodynia pain experienced a substantial reduction in pain with mean VAS pain scores dropping from 8.5 at baseline to 2.5 after two dextrose injections spaced 15 days apart. Two RCTs compared the effects of dextrose and steroid injections for low back pain. In patients with SI joint pain, Kim et al.88 found a significantly greater cumulative incidence of pain reduction (≥50%) in dextrose versus steroid-injected patients. In contrast, a trial conducted by Kim et al.89, with brief follow-up in patients with iliac crest pain syndrome found no difference between dextrose and triamcinolone in VAS pain and disability scores; both groups showed significant pain decrease from baseline.
Hooper et al compared treatment outcomes in patients with cervical, thoracic, or lumbar pain who were involved or not involved in pain-related litigation. Both groups showed significant improvement in pain and disability with dextrose prolotherapy.90 Using radiographical imaging and VAS pain scores one month following dextrose injections in six patients with traumatic cervical instability and neck pain, Cenento et al.91 found that patients experienced significant pain reduction and reduction in cervical flexion and extension translation, with a correlation between differences in pain score and translation reduction. Lee et al.92 performed a prospective uncontrolled trial in patients with SI pain and found a mean duration of pain reduction ≥50% of 12.2 months in patients who received dextrose injections into their SI joints.
Conclusions regarding spinal and pelvic pain
There is Level 1 evidence that dextrose prolotherapy results in significantly greater long-term pain reduction than corticosteroid injection in patients with SI joint pain,88 and Level 2 evidence of comparable short-term pain reduction versus corticosteroid injection in patients with SI pain.89 There is strong Level 4 evidence of significant and comparable improvement in pain and disability between patients with chronic cervical, thoracic, or lumbar pain actively involved versus not involved in litigation.90 There is Level 4 evidence of significant pain reduction and association between changes in pain level and radiographical findings in patients with post-motor vehicle accident neck pain and disability,91 significant reduction in pain and disability in patients with low back and pelvic pain,92 and significant pain reduction in patients with coccygodynia.87
Myofascial pain syndrome
The theoretical basis for dextrose prolotherapy in myofascial pain syndrome (MPS) suggests that since MPS is a state of deficient energy metabolism, dextrose injection into myofascial trigger points may stimulate energy production to relieve the associated pain syndrome.93
Reviewed Studies
In an RCT, patients received injections of dextrose 5%, saline solution, or lidocaine 0.5%. At 7 days postinjection, dextrose-treated patients were improved from baseline in pain (VAS) and pressure threshold tolerance (algometer; kg/cm2) (P < 0.05); saline and lidocaine patients did not show improvement from baseline on either measure.93
Conclusions regarding MPS
Improvement in pain following dextrose prolotherapy comes from Level 2 evidence.93
Discussion
This systematic review identified 33 studies evaluating the efficacy of dextrose prolotherapy for chronic musculoskeletal pain. Of the studies reviewed, 14 were RCTs, 1 was a case–control study, and 18 were case series. Fifteen of the 33 studies used subjective VAS/NRS measures only. The remaining 18 studies combined subjective measures with objective measures, including imaging/biomechanical and/or psychometric measures. Collectively, these studies showed relative consistency in treatment outcome – noteworthy considering the overall heterogeneity. Sixteen of the 18 studies reported positive consistency between subjective and objective outcomes; one study found positive subjective and objective outcomes at short-term follow-up and positive objective but not subjective outcomes at long-term follow-up; and one study reported positive outcomes on objective measures only. Both studies analyzing the association between subjective and objective outcomes reported significant correlational data. In aggregate, the studies showed wide variation in patient characteristics, study design, and outcome measurements. Grouping the studies into four musculoskeletal pain conditions based on underlying pathophysiology and/or anatomical pain location provided substantially greater homogeneity within pain groups.
The quality of evidence for the RCTs was very high with those evaluated with the PEDro tool producing scores ≥8. RCT results found that patients randomized to dextrose showed significantly greater improvement in Osgood–Schlatter disease,58 rotator cuff injury,73 knee OA,77,78 osteoarthritic finger and thumb joints,84 and MPS93 than patients randomized to anesthetic injection. Dextrose-treated patients also showed significantly greater improvement compared with patients randomized to saline injections in knee OA82 and MPS93 and with patients randomized to treatment-as-usual in Osgood–Schlatter disease58 and knee OA.82 In an RCT involving patients with TMJ, patients assigned to dextrose injection showed comparable subjective improvement to the anesthetic control group but significantly greater improvement on biomechanical measures.59
In RCTs comparing dextrose to corticosteroid injection, Kim et al.88 found comparable pain reduction when injected into the SI joint, while Kim et al.89 found superior pain reduction with dextrose. This outcome discrepancy is likely explained by the patient follow-up duration of 3 versus 15 months in Kim et al.88 versus Kim et al.89, respectively. A recent meta-analysis concluded that corticosteroid injection for chronic musculoskeletal pain is associated with definite short-term (<8 weeks) benefits and worse intermediate and long-term outcomes compared with other treatment options.108 Two aspects of the Kim et al’s.89 study warrant additional mention: the very high baseline patient pain levels [mean ± SD VAS pain score 8.04 ± 1.17 (dextrose group) and 8.13 ± 1.28 (steroid group) (NS)], and the superiority of dextrose injection in short-term pain relief compared with a standard therapy for moderate–severe musculoskeletal pain.
To replicate the experimental group protocol, eight RCTs utilized local anesthesia injection, four utilized saline, and one study utilized lidocaine/saline as a placebo control conditions in (other than dextrose injection). While in the narrow sense, these control conditions differ from conventional placebo in that they are not completely inert, these conditions are difficult to achieve outside of pharmaceutical trials, and this protocol fulfills a goal of placebo-control in blinding patients and investigators to treatment assignment.109
Prolotherapy for musculoskeletal pain has been in clinical use for decades but only recently has the methodological quality of the published research evolved beyond the minimum level necessary for consideration in the practice of evidence-based medicine.110,111 The Grading of Recommendations Assessment, Development and Evaluation (GRADE) is a widely accepted and utilized criterion for evaluating the evidence quality in treatment recommendations. The types of evidence are hierarchically ranked based on study design, with RCTs at the highest level of clinical study and uncontrolled studies at a much lower level.112,113 This ranking reflects the greater ability of RCTs to minimize the effect of bias and confounding factors on outcome, such that stricter experimental control can produce observed treatment effects that more closely approximate the true treatment effect, and with it a more valid inference of causality.114
Uncontrolled studies possess a greater capacity for distorted outcome reporting than RCTs given the inability to experimentally control many forms of bias and confounding factors.114 However, the GRADE system also recognizes that uncontrolled studies with rigorous methodology, a large treatment effect, consistent evidence from multiple studies, and to the extent possible the ruling out of alternate explanations for positive findings possess strengths that increase the study grade and level of evidence.112,113 It bears mention that several therapies, such as insulin for diabetes and defibrillation for ventricular defibrillation, have been accepted as the standard of care without RCT confirmation of the results from less rigorously designed studies.115,116
The GRADE system also gives consideration to the balance between the health benefits and harms of a therapy.112 The reviewed studies were primarily comprised of patients with moderate to severe levels of pain and functional impairment refractory to established therapies. When outcome consistency, durability of effect, safety, lack of other treatment options, short of surgery or invasive interventional procedures, and low cost are considered, dextrose prolotherapy for chronic musculoskeletal pain surpasses this threshold.
Particular strong points in many of the uncontrolled studies serve to elevate their quality of evidence. For example, many studies combined objective measures, such as sonographic, radiographical, or biomechanical data with subjective assessment. Objective measurements were utilized and revealed positive outcomes in several uncontrolled studies, including Achilles tendinopathy,64,65 lateral epicondylitis of the elbow,67,69 knee OA,79,80,82 finger and thumb OA,84,85 chronic SI/iliolumbar pain,89,90 and myofacial pain.93 The positive findings in tendinopathies, knee OA, SI pain, and iliac crest pain were confirmed by the results of RCTs. The rigorous study design, data reporting, and clinical generalizability found in many of these studies were reflected in their D&B score. Interestingly, while many uncontrolled studies used highly stringent inclusion/exclusion criteria and extensive diagnostic screening for highly specific patient enrollment, others included patients with nonspecific pain, more closely mirroring real-world clinical practice.
Many studies assessed treatment response with VAS or NRS patient self-rating. The reliability and validity of these measures has been questioned based on the subjective nature of pain, and wide variations in patient reporting of baseline pain scores and in applying self-rating to their own pain experience.117 However, no objective pain measurement exists, and VAS/NRS remains the clinical standard in assessing baseline pain level and treatment response.53 Both scales are also widely used in pharmacotherapy efficacy studies; for instance, the pivotal trials of two recently approved opioid formulations for chronic moderate–severe pain, tapentadol ER,118,119 and hydromorphone ER120,121 utilized pain NRS in the assessment of primary study objectives.
Dextrose prolotherapy is a safe therapy, and few adverse events were reported in the reviewed studies. No serious or protracted complications were observed, including nerve damage, pneumothorax, and infection. Although adverse events have the potential for greater severity in the treatment of spinal and intraarticular structures, injection by an experienced prolotherapist can help mitigate this risk.11 Dextrose itself is extremely safe, even with intravenous administration. In a 1998 Food and Drug Administration document, no adverse events had been reported to Abbott Labs for 25% intravenous dextrose solution in 60 years.122,123
Study limitations
The findings in this review may be influenced by publication bias, where studies reporting negative findings are not prepared, submitted, or accepted for publication. Potentially present is reference bias, where the outcomes of references obtained from secondary sources, such as review articles, meta-analyses, and practice guidelines, may be biased in the direction desired by the authors of the review article or practice guideline. It is unlikely that this review possesses reference bias, in that all reviewed studies were obtained from database searches.
Summary
This systematic review evaluated 32 studies on dextrose prolotherapy for chronic musculoskeletal pain. Based on the level of evidence, quality of evidence, and factors directly and indirectly related to evidence quality including the consistency of significant reductions in pain and impairment found within pain groups, between pain groups, across uncontrolled studies between pain groups and conditions, between uncontrolled studies and RCTs in multiple specific pain conditions, between VAS/NRS outcomes and psychometric, biomechanical, and imaging outcomes within studies, the consistent statistically significant improvement in pain and functioning among patients randomized to dextrose versus control groups, the consistent achievement of clinically meaningful reductions in pain level among studies using VAS/NRS outcomes, and the absence of reported side effects other than transient injection site irritation, the following conclusions are made: dextrose prolotherapy is supported in the treatment of tendinopathies in patients who fail conservative therapies; dextrose prolotherapy is supported in the treatment of OA of the knee and finger joints in patients who do not respond to conservative therapies; dextrose prolotherapy is supported in the treatment of spinal and pelvic pain in patients who fail to respond to conservative therapies; the efficacy of dextrose prolotherapy in myofascial pain cannot be determined from a single RCT of brief follow-up duration. With inclusion limited to patients with pain >3–6 months in the reviewed studies, the efficacy of prolotherapy for acute (<3 months) musculoskeletal pain cannot be determined. Overall, dextrose prolotherapy has been demonstrated to be efficacious and should be considered as a treatment for pain and dysfunction associated with chronic musculoskeletal conditions, particularly tendinopaties and OA.
Footnotes
ACADEMIC EDITOR: Chuanju Liu, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 515 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
A study utilizing a proliferate of sodium morrhuate combined with dextrose for the treatment of lateral epicondylitis of the elbow was included; however, studies where sodium morrhaute was the only proliferant used were excluded.67
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Analyzed the data: RAH, JBL, DS-M. Wrote the first draft of the manuscript: JBL. Contributed to the writing of the manuscript: RAH, JBL, DS-M. Agree with manuscript results and conclusions: RAH, JBL, DS-M, DKH. Jointly developed the structure and arguments for the paper: JBL, RAH. Made critical revisions and approved final version: JBL, RAH. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Institute of Medicine . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): IOM; 2011. [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Health . With Chartbook on Trends in the Health of Americans. Hyattsville, MD: National Center for Health Statistics; 2006. (DHHS Publication No. 2006-1232). [Google Scholar]
- 3.National Center for Health Statistics National Health Interview Survey. 2012. [Accessed June 21, 2014]. Available at: http://www.cdc.gov/nchs/nhis.htm.
- 4.Center for Disease Control and Prevention National Ambulatory Medical Care Survey. 2010. [Accessed August 14, 2014]. Available at http://www.cdc.gov/nchs/ahcd.htm.
- 5.United States Bone and Joint Decade . The Burden of Musculoskeletal Diseases in the United States. First ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008. p. 42. [Google Scholar]
- 6.United States Bone and Joint Initiative . The Burden of Musculoskeletal Diseases in the United States. Second ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011. [Google Scholar]
- 7.National Research Council and the Institute of Medicine . Musculoskeletal Disorders and the Workplace: Low Back and Upper Extremities. Panel on Musculoskeletal Disorders and the Workplace. Washington, DC: National Academy Press; 2012. Commission on Behavioral and Social Sciences and Education. [Google Scholar]
- 8.BLS Bureau of Labor Statistics (BLS) News Release. 2012. [Accessed July 12, 2014]. Available at http://www.bls.gov/news.release/archives/osh2_11082012.pdf.
- 9.Anandacoomarasamy A, Caterson I, Sambrook P, Fransen M, March L. The impact of obesity on the musculoskeletal system. Int J Obes (Lond) 2008;32:211–22. doi: 10.1038/sj.ijo.0803715. [DOI] [PubMed] [Google Scholar]
- 10.Faghri PD, Momeni K. Musculoskeletal diseases, overweight and obesity, and aging workforce: how to encounter the problem. J Obes Wt Loss Ther. 2014;S4:e001. [Google Scholar]
- 11.Nair LS. Prolotherapy for tissue repair. Transl Res. 2011;158(3):129–31. doi: 10.1016/j.trsl.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Hackett GS, Hemwall GA, Montgomery GA. Ligament and Tendon Relaxation Treated by Prolotherapy. 5th ed. Oak Park, IL: Gustav A. Hemwall; 1993. [Google Scholar]
- 13.Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65–80. doi: 10.1016/j.pop.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnirring L. Are your patients asking about prolotherapy? Physician Sportsmed. 2000;28(8):15–7. [Google Scholar]
- 15.Kim SR, Stitik TP, Foye PM, Greenwald BD, Campagnolo DI. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: a physiatric perspective. Am J Phys Med Rehabil. 2004;83(5):379–89. doi: 10.1097/01.phm.0000124443.31707.74. [DOI] [PubMed] [Google Scholar]
- 16.Rabago D, Best TM, Beamsley M, Patterson J. A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med. 2005;15(5):376–80. doi: 10.1097/01.jsm.0000173268.05318.a4. [DOI] [PubMed] [Google Scholar]
- 17.Yelland MJ, Del Mar C, Pirozzo S, Schoene ML. Prolotherapy injections for chronic low back pain: a systematic review. Spine (Phila Pa 1976) 2004;29(19):2126–33. doi: 10.1097/01.brs.0000141188.83178.b3. [DOI] [PubMed] [Google Scholar]
- 18.Dagenais S, Yelland MJ, Del Mar C, Schoene ML. Prolotherapy injections for chronic low-back pain. Cochrane Database Syst Rev. 2007 Apr 18;2:CD004059. doi: 10.1002/14651858.CD004059.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Best TM, Rabago D, Zgierska AE, Zeisig E, Ryan M, Crane D. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet-rich plasma. Br J Sports Med. 2009;43(7):471–81. doi: 10.1136/bjsm.2008.052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Distel LM, Best TM. Prolotherapy: a clinical review of its role in treating chronic musculoskeletal pain. PM R. 2011;3(6 suppl 1):S78–81. doi: 10.1016/j.pmrj.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Linetsky FS, Manchikanti L. Regenerative injection therapy for axial pain. Tech Reg Anesth Pain Manage. 2005;9:40–9. [Google Scholar]
- 22.Adams E. Bibliography: Prolotherapy for Musculoskeletal Pain. Boston, MA: Veterans; 0000. [Google Scholar]
- 23.Goswami A. Prolotherapy. J Pain Palliat Care Pharmacother. 2012;26:376–8. doi: 10.3109/15360288.2012.734900. [DOI] [PubMed] [Google Scholar]
- 24.Alderman D, Alexander RW, Harris GR, Astourian PC. Stem cell prolotherapy in regenerative medicine: background, theory and protocols. J Prolotherapy. 2011;3(3):689–708. [Google Scholar]
- 25.DeChellis DM, Cortazzo MH. Regenerative medicine in the field of pain medicine: prolotherapy, platelet-rich plasma therapy, and stem cell therapy-theory and evidence. Tech Reg Anesth Pain Manag. 2011;15(2):74–80. [Google Scholar]
- 26.Reeves KD. Prolotherapy: injection of growth factors or growth factor production stimulants to growth normal cells or tissue. In: Waldman SD, editor. Pain Management. Philadelphia: Elsevier; 2006. pp. 1106–27. [Google Scholar]
- 27.Creaney L, Hamilton B. Growth factor delivery methods of sports injuries: the state of play. Br J Sports Med. 2008;42:314–20. doi: 10.1136/bjsm.2007.040071. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Zapata MJ, Marti-Carvajal A, Sola I. Efficacy and safety of autologous plasma rich in platelets for tissue regeneration: a systematic review. Transfusion. 2009;49:44–56. doi: 10.1111/j.1537-2995.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 29.Oh JH, Ha H, Yu MR, Lee HB. Sequential effects of high glucose on mesangial cell transforming growth factor-beta 1 and fibronectin synthesis. Kidney Int. 1998;54:1872–8. doi: 10.1046/j.1523-1755.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda K, Kawata S, Inui Y, et al. High concentration of glucose increases mitogenic responsiveness to heparin-binding epidermal growth factor-like growth factor in rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:1962–8. doi: 10.1161/01.atv.17.10.1962. [DOI] [PubMed] [Google Scholar]
- 31.Woo SL, Hildebrand K, Watanabe N, et al. Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res. 1999;367S:312–4. doi: 10.1097/00003086-199910001-00030. [DOI] [PubMed] [Google Scholar]
- 32.Tang JB, Xu Y, Ding F, Wang XT. Tendon healing in vitro: promotion of collagen gene expression by bFGF with NF-kB gene activation. J Hand Surg Am. 2003;28:215–20. doi: 10.1053/jhsu.2003.50052. [DOI] [PubMed] [Google Scholar]
- 33.Pugliese G, Pricci F, Locuratolo N, et al. Increased activity of the insulin-like growth factor system in mesangial cells cultured in high glucose conditions: relation to glucose-enhanced extracellular matrix production. Diabetologia. 1996;39:775–84. doi: 10.1007/s001250050510. [DOI] [PubMed] [Google Scholar]
- 34.Reeves KD, Fullerton BD, Topol G. Evidence-based regenerative injection therapy (Prolotherapy) in sports medicine. In: Seidenberg PH, Beutler AI, editors. The Sports Medicine Resource Manual. Amsterdam: Elsevier; 2008. pp. 611–9. [Google Scholar]
- 35.Vora A, Borg-Stein J, Nguyen RT. Regenerative injection therapy for osteoarthritis: fundamental concepts and evidence-based review. PM R. 2012;4:S104S–9. doi: 10.1016/j.pmrj.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez M, Anitua E, Orive G. Platelet-rich therapies in treatment of orthopaedic sport injuries. Sports Med. 2009;39:345–54. doi: 10.2165/00007256-200939050-00002. [DOI] [PubMed] [Google Scholar]
- 37.Tabata Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003;9:S5–15. doi: 10.1089/10763270360696941. [DOI] [PubMed] [Google Scholar]
- 38.Jensen KT, Rabago DP, Best TM, Patterson JJ, Vanderby R., Jr Early inflammatory response of knee ligaments to prolotherapy in a rat model. J Orthop Res. 2008;26:816–23. doi: 10.1002/jor.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen KT, Rabago DP, Best TM, Patterson JJ, Vanderby R., Jr Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347–57. doi: 10.1177/0363546508314431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HJ, Kim SH, Yun DH. The effects of anti-inflammatory drugs on histologic findings of the experimental prolotherapy model. J Korean Acad Rehabil Med. 2006;30:378–84. [Google Scholar]
- 41.Ahn KH, Kim HS, Lee WK. The effect of the prolotherapy on the injured Achilles tendon in a rat model. J Korean Acad Rehabil Med. 2002;26:332–6. [Google Scholar]
- 42.Kim HS, Jeong TS, Wim WS. Comparison of histological changes in aacordance with the level of dextrose-concentration in experimental prolotherapy model. J Korean Acad Rehabil Med. 2003;27:935–40. [Google Scholar]
- 43.Kim SA, Kim EH, Kim SY, et al. The effects of hyperosmolar dextrose and autologous serum injection in the experimental articular defect of rabbit. J Korean Acad Rehabil Med. 2006;30(2):173–8. [Google Scholar]
- 44.Reeves KD. Prolotherapy: basic science, clinical studies, and technique. In: Lennard TA, editor. Pain Procedures in Clinical Practice (ed 2) Philadelphia, PA: Hanley and Belfus; 2000. pp. 172–90. [Google Scholar]
- 45.Teasell RW, Mehta S, Aubut JA, et al. Spinal Cord Injury Rehabilitation Evidence Research Team A systematic review of pharmacologic treatments of pain after spinal cord injury. Arch Phys Med Rehabil. 2010;91(5):816–31. doi: 10.1016/j.apmr.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krassioukov A, Eng JJ, Warburton DE, Teasell R, Spinal Cord Injury Rehabilitation Evidence Research Team A systematic review of the management of orthostatic hypotension after spinal cord injury. Arch Phys Med Rehabil. 2009;90(5):876–85. doi: 10.1016/j.apmr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro) Aust J Physiother. 2002;48:43–9. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 48.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foley NC, Teasell RW, Bhogal SK, Speechley MR. Stroke rehabilitation evidence-based review: methodology. Top Stroke Rehabil. 2003;10:1–7. [PubMed] [Google Scholar]
- 50.Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating nonrandomized intervention studies. Health Technol Assess. 2003;7(27):iii–x. 1–173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 51.Saunders LD, Soomro GM, Buckingham J, Jamtvedt G, Raina P. Assessing the methodological quality of nonrandomized intervention studies. West J Nurs Res. 2003;25:223–37. doi: 10.1177/0193945902250039. [DOI] [PubMed] [Google Scholar]
- 52.Sackett DL, Strauss SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-based Medicine: How to Practice and Teach EBM. Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- 53.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 54.Lee JS, Hobden E, Stiell IG, Wells GA. Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med. 2003;10:1128–30. doi: 10.1111/j.1553-2712.2003.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 55.Kovacs FM, Abraira V, Royuela A, et al. Minimal clinically important change for pain intensity and disability in patients with nonspecific low back pain. Spine. 2007;32:2915–20. doi: 10.1097/BRS.0b013e31815b75ae. [DOI] [PubMed] [Google Scholar]
- 56.Bombardier C, Hayden J, Beaton DE. Minimal clinically important difference: low back pain. Outcome measures. J Rheumatol. 2001;28:431–8. [PubMed] [Google Scholar]
- 57.American College of Rheumatology . Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) – General Description. ACR; 2015. [Accessed March 3, 2015]. Available at http://www.rheumatology.org/practice/clinical/clinicianresearchers/outcomes-instrumentation/WOMAC.asp. [Google Scholar]
- 58.Sanderson LM, Bryant A. Effectiveness and safety of prolotherapy injections for management of lower limb tendinopathy and fasciopathy: a systematic review. J Foot Ankle Res. 2015;8:57. doi: 10.1186/s13047-015-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Topol GA, Podesta LA, Reeves KD, Raya MF, Fullerton BD, Yeh HW. Hyperosmolar dextrose injection for recalcitrant Osgood–Schlatter disease. Pediatrics. 2011;128(5):e1121–8. doi: 10.1542/peds.2010-1931. [DOI] [PubMed] [Google Scholar]
- 60.Refai H, Altahhan O, Elsharkawy R. The efficacy of dextrose prolotherapy for temporomandibular joint hypermobility: a preliminary prospective, randomized, double-blind, placebo-controlled clinical trial. J Oral Maxillofac Surg. 2011;69(12):2962–70. doi: 10.1016/j.joms.2011.02.128. [DOI] [PubMed] [Google Scholar]
- 61.Comert Lilic S, Gungormus M. Is dextrose prolotherapy superior to placebo for the treatment of temporomandibular joint hypermobility? A randomized control trial. Int J Oral Maxillofac Surg. 2016 Feb 1; doi: 10.1016/j.ijom.2016.01.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 62.Zhou H, Hu K, Ding Y. Modified dextrose prolotherapy for recurrent temporomandibular joint dislocation. Br J Oral Maxillofac Surg. 2014;52(1):63–6. doi: 10.1016/j.bjoms.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 63.Yelland MJ, Sweeting KR, Lyftogt JA, Ng SK, Scuffham PA, Evans KA. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45(5):421–8. doi: 10.1136/bjsm.2009.057968. [DOI] [PubMed] [Google Scholar]
- 64.Maxwell NJ, Ryan MB, Taunton JE, Gillies JH, Wong AD. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. AJR Am J Roentgenol. 2007;189:w215–20. doi: 10.2214/AJR.06.1158. [DOI] [PubMed] [Google Scholar]
- 65.Ryan M, Wong A, Taunton J. Favorable outcomes after sonographically guided intratendinous injection of hyperosmolar dextrose for chronic insertional and midportion achilles tendinosis. AJR Am J Roentgenol. 2010;194(4):1047–53. doi: 10.2214/AJR.09.3255. [DOI] [PubMed] [Google Scholar]
- 66.Lyftogt J. Prolotherapy and Achilles tendinopathy: a prospective pilot study of an old treatment. Aust Musculoskel Med J. 2005;10:16–9. [Google Scholar]
- 67.Scarpone M, Rabago DP, Zgierska A, Arbogast G, Snell E. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18(3):248–54. doi: 10.1097/JSM.0b013e318170fc87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shin J, Seo K-M, Kim D-K, Kim B-K, Kang SH. The effect of prolotherapy on lateral epicondylitis of elbow. J Korean Acad Rehabil Med. 2002;26:764–8. [Google Scholar]
- 69.Park JH, Song IS, Lee JB, et al. Ultrasonographic findings of healing of torn tendon in the patients with lateral epicondylitis after prolotherapy. J Korean Soc Med Ultrasound. 2003;22(3):177–83. [Google Scholar]
- 70.Ryan M, Wong A, Rabago D, Lee K, Taunton J. Ultrasound-guided injections of hyperosmolar dextrose for overuse patellar tendinopathy: a pilot study. Br J Sports Med. 2011;45(12):972–7. doi: 10.1136/bjsm.2010.081455. [DOI] [PubMed] [Google Scholar]
- 71.Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the threatment of chronic recalcitrant plantar fasciitis. PM R. 2014;6(2):152–8. doi: 10.1016/j.pmrj.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Topol GA, Reeves KD. Regenerative injection of elite athletes with career-altering chronic groin pain who fail conservative treatment: a consecutive case series. Am J Phys Med Rehabil. 2008;87(11):890–902. doi: 10.1097/PHM.0b013e31818377b6. [DOI] [PubMed] [Google Scholar]
- 73.Topol GA, Reeves KD, Hassanein KM. Efficacy of dextrose prolotherapy in elite male kicking-sport athletes with chronic groin pain. Arch Phys Med Rehabil. 2005;86:697–702. doi: 10.1016/j.apmr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 74.Bertrand H, Reeves KD, Bennett CJ, Bicknell S, Cheng AL. Dextrose prolotherapy versus control injections in painful rotator cuff tendinopathy. Arch Phys Med Rehabil. 2016;97(1):17–25. doi: 10.1016/j.apmr.2015.08.412. [DOI] [PubMed] [Google Scholar]
- 75.Lee DH, Kwack KS, Rah UW, Yoon SH. Prolotherapy for refractory rotator cuff disease: retrospective case-control study of 1-year follow-up. Arch Phys Med Rehabil. 2015;96(11):2027–32. doi: 10.1016/j.apmr.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 76.Ryan MB, Wong AD, Gillies JH, Wong J, Traunton JE. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:303–6. doi: 10.1136/bjsm.2008.050021. [DOI] [PubMed] [Google Scholar]
- 77.Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11(3):229–37. doi: 10.1370/afm.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rabago D, Mundt M, Zgierska A, Grettie J. Hypertonic dextrose injection (prolotherapy) for knee osteoarthritis: long term outcomes. Complement Ther Med. 2015;23(3):388–95. doi: 10.1016/j.ctim.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–79. [PubMed] [Google Scholar]
- 80.Reeves KD, Hassanein KM. Long-term effects of dextrose prolotherapy for anterior cruciate ligament laxity. Altern Ther Health Med. 2003;9(3):58–62. [PubMed] [Google Scholar]
- 81.Dumais R, Benoit C, Dumais A, et al. Effect of regenerative injection therapy on function and pain in patients with knee osteoarthritis a randomized crossover study. Pain Med. 2012;13(8):990–9. doi: 10.1111/j.1526-4637.2012.01422.x. [DOI] [PubMed] [Google Scholar]
- 82.Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with moderate knee osteoarthritis: a single- arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7(2):35–44. doi: 10.1177/1759720X14566618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hashemi M, Parviz J, Mennati S, et al. The effects of prolotherapy with hupertinic dextrose versus prolozone (interaarticular ozone) in patients with knee osteoarthritis. Anesth Pain Med. 2015;5(5):e27858. doi: 10.5812/aapm.27585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reeves KD, Hassanein K. Randomized, prospective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DIP,PIP, and Trapeziometacarpal) joints: evidence of clinical efficacy. J Altern Complement Med. 2000;6:311–20. doi: 10.1089/10755530050120673. [DOI] [PubMed] [Google Scholar]
- 85.Jahangiri A, Moghaddam FR, Najafi S. Hypertonic dextrose versus corticosteroid local injection for the treatment of osteoarthritis in the first carpometacarpal joint: a double-blind randomized clinical trial. J Orthop Sci. 2014;19(5):737–43. doi: 10.1007/s00776-014-0587-2. [DOI] [PubMed] [Google Scholar]
- 86.Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–21. [PubMed] [Google Scholar]
- 87.Khan SA, Kumar A, Varshney MK. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. 2008;16:27–9. doi: 10.1177/230949900801600107. [DOI] [PubMed] [Google Scholar]
- 88.Kim WM, Lee HG, Jeong CW, Kim CM, Yoon MH. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16(12):1285–90. doi: 10.1089/acm.2010.0031. [DOI] [PubMed] [Google Scholar]
- 89.Kim HS, Jung KH, Park IH, et al. Diagnosis and treatment of sacral asymlocation in back pain patients. Korean J Pain. 2007;20:130–7. [Google Scholar]
- 90.Hooper RA, Yelland M, Fonstad P, Southern D. Prospective case series of litigants and non-litigants with chronic spinal pain treated with dextrose prolotherapy. Int Musculoskeletal Med. 2011;33(1):15–20. [Google Scholar]
- 91.Centeno CJ, Elliott J, Elkins WL, Freeman M. Fluoroscopically guided cervical prolotherapy for instability with blinded pre and post radiographic reading. Pain Physician. 2005;8:67–72. [PubMed] [Google Scholar]
- 92.Lee JD, Lee DW, Cheol Won J. Effects of intraarticular prolotherapy on sacroiliac joint pain. Korean J Pain. 2009;22:229–33. [Google Scholar]
- 93.Kim MY, Na YM, Moon JH. Comparison on treatment effects of dextrose water, saline, and lidocaine for trigger point injections. J Korean Acad Rehabil Med. 1997;21:967–73. [Google Scholar]
- 94.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- 95.Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87(7):486–90. [PubMed] [Google Scholar]
- 96.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 97.Wheaton M, Jensen N. The ligament injury connection to osteoarthritis. J Prolotherapy. 2010;2:294–304. [Google Scholar]
- 98.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;(423):7–16. [PubMed] [Google Scholar]
- 99.Borenstein DG. Chronic low back pain. Rheum Dis Clin North Am. 1996;22:439–56. doi: 10.1016/s0889-857x(05)70281-7. [DOI] [PubMed] [Google Scholar]
- 100.Khan KM, Cook JL, Kannus P, Maffuli N, Bonar SF. Time to abandon the ‘tendinitis’ myth. BMJ. 2002;324:626–7. doi: 10.1136/bmj.324.7338.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alderman D. Prolotherapy for musculoskeletal pain. Pract Pain Manage. 2007:10–7. [Google Scholar]
- 102.Ombregt L, Bisschop P, ter Veer HJ. A System of Orthopaedic Medicine. Second ed. London: Churchill Livingstone; 2003. p. 775. [Google Scholar]
- 103.Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318(5):291–300. doi: 10.1056/NEJM198802043180506. [DOI] [PubMed] [Google Scholar]
- 104.Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255–65. doi: 10.5435/00124635-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 105.Carlson SW, Magee S, Carlson WO. An algorithm for the evaluation and treatment of sacroiliac joint dysfunction. S D Med. 2014;67(11):445–9. 451. [PubMed] [Google Scholar]
- 106.Cher D, Polly D, Berven S. Sacroilliac joint pain: burden of disease. Med Devices (Auckl) 2014;7:73–81. doi: 10.2147/MDER.S59437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fortin JD, Vilensky JA, Merkel GJ. Can the sacro-iliac joint cause sciatica? Pain Physician. 2003;6(3):269–71. [PubMed] [Google Scholar]
- 108.Poul-Goudzwaard AL, Vleeming A, Stoeckart R, Snijders CJ, Mens JM. Insufficient lumbopelvic stability: a clinical, anatomical, and biomechanical approach to “a specific” low back pain. Man Ther. 1998;3(1):12–20. doi: 10.1054/math.1998.0311. [DOI] [PubMed] [Google Scholar]
- 109.Fortin JD, Aprill CN, Ponthieux B, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part II: clinical evaluation. Spine. 1994;19(13):1483–9. [PubMed] [Google Scholar]
- 110.Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376(9754):1751–67. doi: 10.1016/S0140-6736(10)61160-9. [DOI] [PubMed] [Google Scholar]
- 111.Manchikanti L, Hirsch JA, Smith HS. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: part 2: randomized controlled trials. Pain Physician. 2008;11(6):717–73. [PubMed] [Google Scholar]
- 112.Atkins D, Best D, Briss PA, et al. GRADE Working Group Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ. GRADE: what is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.The Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration; 2008. [Google Scholar]
- 115.Manchikanti L, Benyamin RM, Falco FJE, Caraway DL, Datta S, Hirsch JA. Guidelines warfare over interventional techniques: is there a lack of discourse or straw man? Pain Physician. 2012;15:E1–26. [PubMed] [Google Scholar]
- 116.Glasziou P, Chalmers I, Rawlins M, Mc- Culloch P. When are randomised trials unnecessary? Picking signal from noise. BMJ. 2007;334:349–51. doi: 10.1136/bmj.39070.527986.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Farrar JT, Portenoy RK, Berlin JA, Kinman J, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88:287–29. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 118.Afilalo M, Etropolski MS, Kuperwasser B, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig. 2010;30(8):489–505. doi: 10.2165/11533440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 119.Buynak R, Shapiro DY, Okamoto A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled Phase III study. Expert Opin Pharmacother. 2010;11(11):1787–804. doi: 10.1517/14656566.2010.497720. [DOI] [PubMed] [Google Scholar]
- 120.Binsfeld H, Szczepanski L, Waechter S, Richarz U, Sabatowski R. A randomized study to demonstrate noninferiority of once-daily OROS(®) hydromorphone with twice-daily sustained-release oxycodone for moderate to severe chronic noncancer pain. Pain Pract. 2010;10(5):404–15. doi: 10.1111/j.1533-2500.2009.00342.x. [DOI] [PubMed] [Google Scholar]
- 121.Hale M, Khan A, Kutch M, Li S. Once-daily OROS hydromorphone ER compared with placebo in opioid-tolerant patients with chronic low back pain. Curr Med Res Opin. 2010;26(6):1505–18. doi: 10.1185/03007995.2010.484723. [DOI] [PubMed] [Google Scholar]
- 122.AbbottLabs FDA Indications for 50% Dextrose. 2004. [Accessed November 12, 2014]. Available at http://www.fda.gov/cder/foi/nda/98/19445-s4-s6.htm.
- 123.AbbottLabs . Approval Documentation for 25% Dextrose Submitted to FDA. Online Documentation. Chicago, IL: Abbott Laboratories; 1998. [Google Scholar]