Abstract
Systemic Candida albicans infection causes high morbidity and mortality and is now the leading cause of nosocomial bloodstream infection in the US. Neutropenia is a major risk factor for poor outcome in infected patients; however, the molecular factors that mediate neutrophil trafficking and effector function during infection are poorly defined. Here, using a mouse model of systemic candidiasis, we found that the neutrophil-selective CXC chemokine receptor Cxcr1 and its ligand, Cxcl5, are highly induced in the Candida-infected kidney, the target organ in the model. To investigate the role of Cxcr1 in antifungal host defense in vivo, we generated Cxcr1−/− mice and analyzed their immune response to Candida. Mice lacking Cxcr1 exhibited decreased survival with enhanced Candida growth in the kidney and renal failure. Surprisingly, increased susceptibility of Cxcr1−/− mice to systemic candidiasis was not due to impaired neutrophil trafficking from the blood into the infected kidney but was the result of defective killing of the fungus by neutrophils that exhibited a cell-intrinsic decrease in degranulation. In humans, the mutant CXCR1 allele CXCR1-T276 results in impaired neutrophil degranulation and fungal killing and was associated with increased risk of disseminated candidiasis in infected patients. Together, our data demonstrate a biological function for mouse Cxcr1 in vivo and indicate that CXCR1-dependent neutrophil effector function is a critical innate protective mechanism of fungal clearance and host survival in systemic candidiasis.
Introduction
Systemic candidiasis, most often caused by the commensal yeast Candida albicans, has emerged as the leading cause of nosocomial bloodstream infection in acutely ill and immunocompromised patient populations in the US (1), with an estimated annual cost that exceeds 2 billion dollars (2,3). Fungal vaccines are not available to prevent disease, and despite the availability of antifungal drugs with good in vitro and preclinical activity against Candida, mortality of affected patients remains 30–40% despite treatment (2,4). Therefore, systemic candidiasis represents an unmet medical condition for which better understanding of the cellular and molecular basis of antifungal immunity is essential to design immune-based strategies for risk stratification, prognostication, prophylaxis and/or treatment of patients.
Neutropenia is a major risk factor for development of candidemia and disseminated candidiasis in patients (2,4). Similarly, neutrophils are indispensable for effective antifungal innate immune responses in the mouse model of systemic candidiasis, as their depletion in the early phase of the infection results in fungal dissemination, uncontrolled fungal proliferation in tissue and increased mortality (4–9).
We previously reported that the chemokine receptor Ccr1 mediates neutrophil recruitment from the blood into the kidney (10), the target organ in the mouse model of the infection (7), during the late phase of systemic candidiasis, when neutrophils promote tissue injury (9,10). However, very little is known about specific molecular factors that mediate early protective trafficking and effector function of neutrophils during systemic candidiasis in vivo. Hence, in the present study we focused on CXCR1, the first chemokine receptor cloned in 1991 (11), which has been shown to mediate chemotaxis as well as both oxidative and non-oxidative cytotoxic antibacterial activity in human neutrophils in vitro (12,13). Consistent with this, a genetic variant of CXCR1 named CXCR1-T276 has been associated with heightened risk of bacterial infection of the kidney in humans (14,15).
Nevertheless, a deeper and broader understanding of the biological role of CXCR1 has been hampered by difficulties identifying and characterizing mouse Cxcr1, which delayed its cloning. Moreover, the human receptor is highly selective for CXCL8/IL-8, a chemokine that is not present in mouse, and only recently mouse Cxcr1 was shown to be a functional receptor for the mouse chemokine Cxcl5/LIX (16). Finally, a Cxcr1 knockout mouse had not been previously available and characterized. Herein, we have developed a Cxcr1-deficient mouse and used it to demonstrate increased susceptibility to systemic candidiasis as a biological phenotype attributable to Cxcr1 deficiency. Importantly, we have also translated this finding to humans by studies with the CXCR1-T276 mutation.
Results
Cxcr1 and its ligand, Cxcl5, are up-regulated after systemic Candida infection in mice
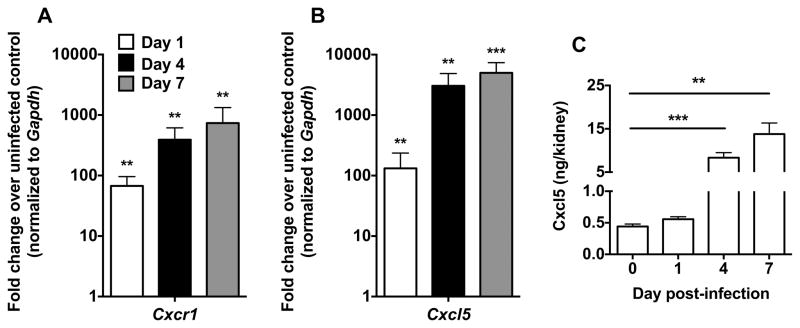
Guided by a broad transcriptional screen of the chemoattractant system in the mouse model of systemic candidiasis (10), we aimed to gain insight into the molecular factors that mediate protective recruitment and effector function of neutrophils in Candida-infected tissues. Thus, we examined the induction of the neutrophil-selective chemokine receptor Cxcr1 and its ligand, Cxcl5, in C57Bl/6 mice infected intravenously with an LD50 inoculum of Candida albicans at early (day 1), intermediate (day 4) and late (day 7) time-points during the course of the infection (7). We focused on the kidney, the major target organ in the mouse model (4,7), and found that mRNA for Cxcr1 was significantly and durably induced after infection; a ~70-fold increase was seen at day 1, whereas the receptor was up-regulated ~400-fold and ~750-fold at days 4 and 7 post-infection relative to the uninfected state, respectively (Fig. 1A). In addition, we observed ~130-fold, ~3500-fold and ~5000-fold inductions of mRNA for Cxcl5 at days 1, 4 and 7 post-infection relative to the uninfected state, respectively (Fig. 1B). Cxcl5 was also significantly induced at the protein level in Candida-infected kidneys (Fig. 1C). Taken together, these results identify Cxcr1 as a candidate control factor in systemic candidiasis.
Figure 1. Systemic candidiasis induces the expression of Cxcr1 and its ligand Cxcl5.
(A) Cxcr1 is induced in Cxcr1+/+ kidneys at days 1, 4 and 7 post-infection. P=0.0023, P=0.0012 and P=0.0022 for Cxcr1 expression at days 1, 4 and 7 versus day 0, respectively (n=5–8; 2 independent experiments; Mann-Whitney test). (B) Cxcl5 is induced in Cxcr1+/+ kidneys at days 1, 4 and 7 post-infection. P=0.01, P=0.0016 and P=0.0004 for Cxcl5 expression at days 1, 4 and 7 versus day 0, respectively (n=5–8; 2 independent experiments; t-test). (C) Cxcl5 is significantly induced at the protein level in Cxcr1+/+ kidneys post-infection (n=6–8; 2 independent experiments). **P=0.0012; Mann-Whitney test; ***P=0.0003; t-test with Welch’s correction. Data represent mean ± SEM.
Cxcr1−/− mice are viable and do not manifest developmental or immune defects at steady state
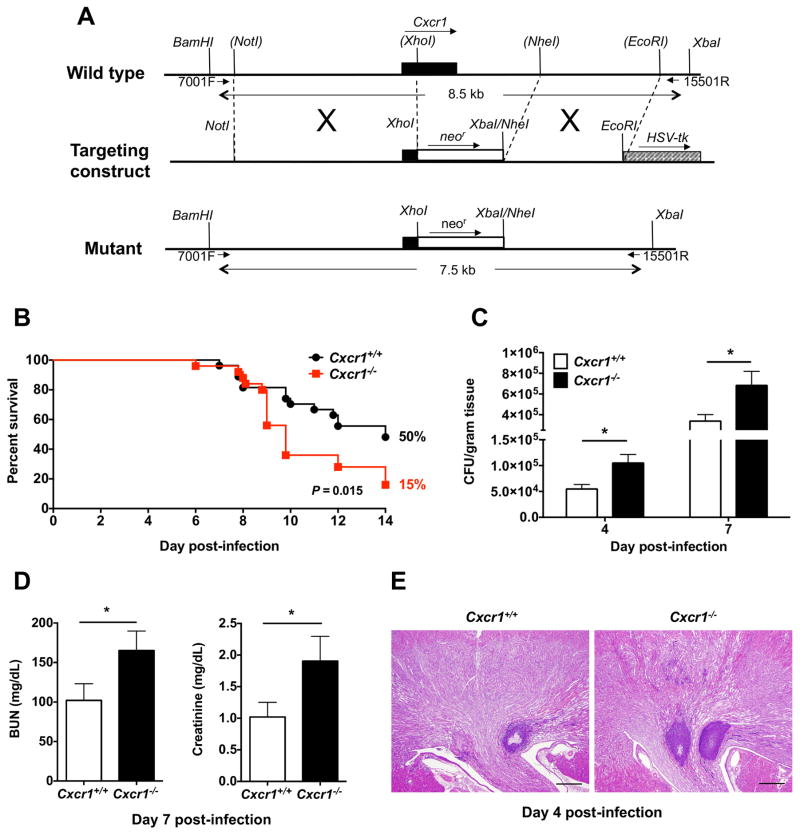
We sought to determine the role of Cxcr1 in host defense during systemic candidiasis in vivo by generating Cxcr1-deficient mice. To inactivate Cxcr1, we replaced a 2.7 kb fragment of the Cxcr1 gene, with a 1.7 kb of neomycin resistance cassette by homologous recombination in 129/Sv embryonic stem cells. The deleted region starts from the middle of the third transmembrane segment predicted from the Cxcr1 sequence (codons 121–351) plus 2,028 bp of the 3′-untranslated region (Fig. 2A). Chimeric mice were made by injecting targeted 129/Sv cell lines into blastocysts from C57Bl/6 mice. The chimeric mice were mated with C57Bl/6 mice to establish heterozygotes, which were interbred to produce Cxcr1+/+, Cxcr1+/−, and Cxcr1−/− littermates. The genotypic frequencies for 241 total progeny of 9 Cxcr1+/− x Cxcr1+/− mating pairs were: 22.4% Cxcr1+/+, 57.6% Cxcr1+/−, and 20% Cxcr1−/−, which is similar to Mendelian expectation for an autosomal gene. Cxcr1−/− mice were viable and fertile, and exhibited normal growth, development, anatomy, behavior, and lifespan compared with Cxcr1+/+ littermates. Importantly, no developmental defects were seen in the kidney at steady state (Fig. S1). Moreover, no abnormalities in the complete blood cell counts or differential white blood cell counts were detected. These mice did not exhibit defects in hemostasis or healing of tail wounds, nor increased susceptibility to spontaneous infection when housed under specific pathogen-free conditions.
Figure 2. Systemic candidiasis results in increased mortality in Cxcr1−/− mice due to enhanced fungal proliferation in the kidney and renal failure.
(A) Schematic showing the generation of Cxcr1−/− mice by homologous recombination. (B) Mortality rates of Cxcr1+/+ and Cxcr1−/− mice after intravenous challenge with Candida (n=25; summary data of 3 independent experiments). P=0.015; Log-rank (Mantel-Cox) test. (C) Fungal burden in the kidneys of Cxcr1+/+ and Cxcr1−/− mice at days 4 (P=0.0135; n=14–15; 3 independent experiments; t-test with Welch’s correction) and 7 (P=0.025; n=30–31; 6 independent experiments; Mann-Whitney test) post-infection (D) Renal function is significantly compromised in Cxcr1−/− mice post-infection. Shown are summary data of serum BUN (P=0.0477; Mann-Whitney test) and creatinine (P=0.0403; Mann-Whitney test) at day 7 post-infection (n=17–18; 3 independent experiments). (E) Histopathology. Representative PAS staining of Cxcr1+/+ and Cxcr1−/− kidney sections at day 4 post-infection. Original magnification, 20x; Bar scale, 500 μm (n=15; 3 independent experiments). All quantitative data represent mean ± SEM.
Cxcl5 is a selective ligand for mouse Cxcr1
It was previously shown that Cxcl5 is a ligand for mouse Cxcr1 in transfected cells in vitro (16). After generating Cxcr1−/− mouse, we aimed to verify that Cxcl5 is indeed a ligand for Cxcr1 in mouse neutrophils. Because Cxcl5 is known to bind Cxcr2, we harvested Cxcr1−/− and Cxcr2−/− mouse neutrophils to examine Cxcl5/Cxcr2 and Cxcl5/Cxcr1 signaling, respectively, using a calcium flux assay. Consistent with Cxcl5 being a known ligand for Cxcr2, Cxcr1−/− neutrophils displayed Cxcl5-dependent calcium flux (Fig. S2). Importantly, Cxcr2−/− neutrophils exhibited dose-dependent Cxcr1-mediated calcium flux upon addition of Cxcl5 (Fig. S2). These data show that, consonant with prior findings in a transfected cell line, Cxcl5 is a functional ligand for mouse Cxcr1.
We then evaluated the selectivity of Cxcl5 as a ligand for mouse Cxcr1 by performing calcium flux assays in Cxcr1−/− and Cxcr2−/− mouse neutrophils using the Cxcr2 ligands Cxcl1/KC and Cxcl2/MIP-2. Consistent with Cxcl1 and Cxcl2 being known ligands for Cxcr2, Cxcr1−/− neutrophils displayed Cxcl1- and Cxcl2-dependent calcium flux (Fig. S3). Importantly, Cxcr2−/− neutrophils did not exhibit significant Cxcr1-mediated calcium flux upon addition of Cxcl1 or Cxcl2, suggesting that Cxcl5 is a selective ligand among ELR+ CXC chemokines for mouse Cxcr1 (Fig. S3).
Cxcr1 is critical for host survival in a mouse model of systemic candidiasis
To determine the impact of Cxcr1 deficiency on susceptibility to systemic candidiasis, we infected Cxcr1−/− mice with an LD50 inoculum (7) and found that their survival was significantly decreased relative to Cxcr1+/+ animals (Fig. 2B). Mortality with this inoculum began around day 7 and only 15% of Cxcr1−/− mice survived through day 14 post-infection compared to 50% of Cxcr1+/+ mice. We examined tissue fungal burden in Cxcr1+/+ and Cxcr1−/− kidneys after infection, which is known to correlate strongly with mortality (4,7). Cxcr1 deficiency resulted in a significant and reproducible ~2-fold increase in renal fungal burden at days 4 and 7 post-infection (Fig. 2C). Accordingly, infected Cxcr1−/− mice developed more severe renal dysfunction (Fig. 2D) and more extensive renal histopathology, including increased number of large kidney abscesses post-infection (Fig. 2E and S4). Specifically, the average number of abscesses per kidney measuring >50 μm in greatest dimension in Cxcr1+/+ mice at day 4 post-infection was 0.73 (SEM, ± 0.27), whereas the number of such abscesses per kidney in Cxcr1−/− mice was 1.37 (SEM, ± 0.2)(P=0.0283; n=14–15; 4 independent experiments; Mann-Whitney test). In contrast, Cxcr1−/− mice did not exhibit greater fungal proliferation (Fig. S5) or histological abnormalities (Fig. S6) in the spleen, liver or brain post-infection. Thus, for the remainder of our studies, we focused on the effects of Cxcr1 in the kidney.
Neutrophils are the predominant Cxcr1-expressing immune cells in the kidney
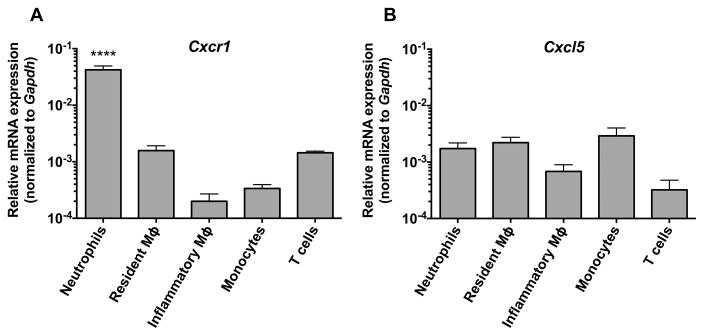
To better understand the biological effects of Cxcr1 in the model, we next examined the expression of the receptor by qRT-PCR in various immune cell types that we FACS-sorted from the kidney at steady state and after Candida infection (Fig. S7). Neutrophils were by far the predominant Cxcr1-expressing cells in the kidney, whereas significantly lower levels of Cxcr1 expression were detected in resident and inflammatory macrophages, Ly6Chi inflammatory monocytes and T cells (Fig. 3A). In parallel, we sought to examine cellular expression of the Cxcr1 ligand, Cxcl5. Cxcl5 was expressed more evenly by all immune cell types tested in the kidney, including neutrophils (Fig. 3B). This finding extends our previous observations that neutrophils serve as major producers of neutrophil-targeted chemokines in the Candida-infected kidney (10). We then examined the expression of Cxcr1 and Cxcl5 in mouse neutrophils harvested at different time points before and after Candida infection and found significant expression of Cxcr1 and Cxcl5 in kidney neutrophils both at steady state and during systemic candidiasis (Fig. S8). Based on these results, we focused our analysis of Cxcr1-dependent protective mechanisms in the model on accumulation and function of neutrophils, the predominant leukocyte subset in the Candida-infected kidney (7).
Figure 3. Neutrophils are the major Cxcr1-expressing immune cells in the kidney during systemic candidiasis whereas Cxcl5 is evenly expressed among various immune cell types.
(A) Relative expression of Cxcr1 and (B) Cxcl5 in resident macrophages (day 0) and in neutrophils, monocytes, inflammatory macrophages and T cells (day 4 post-infection) FACS-sorted from Cxcr1+/+ kidneys (n=3–6; 2 independent experiments). ****P<0.0001 for neutrophil Cxcr1 expression relative to other immune cells by one-way ANOVA with Bonferroni correction. Data represent mean ± SEM.
Cxcr1 is dispensable for neutrophil trafficking from the blood into the Candida-infected kidney
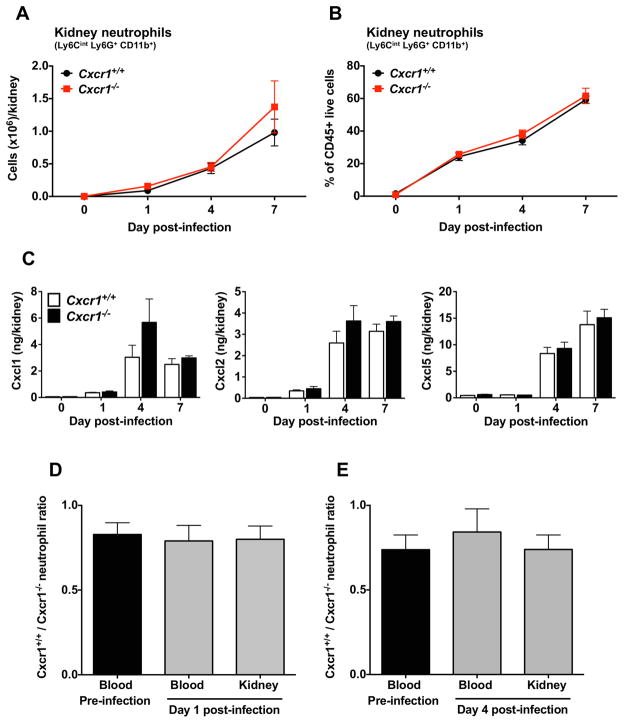
CXCR1 is known to mediate chemotaxis of human neutrophils (12). Therefore, we reasoned that Cxcr1 promotes mouse survival and control of renal fungal proliferation during infection by mediating neutrophil recruitment from the blood into the kidney early in the course of the infection, when neutrophils are protective in the model (9). We used FACS to quantitatively and temporally assess how Cxcr1 affects neutrophil and other leukocyte subset accumulation in the kidney and blood at days 1, 4 and 7 post-Candida infection. We found that Cxcr1 was dispensable for neutrophil accumulation in the kidney, blood and bone marrow at steady state and throughout the course of the infection, as absolute neutrophil numbers (Fig. 4A, S9A and S10A) and percentages of neutrophils within total leukocytes (Fig. 4B, S9B and S10B) were similar in the kidney, blood and bone marrow of Cxcr1+/+ and Cxcr1−/− mice. In agreement, the induction of neutrophil-targeted chemoattractants was Cxcr1-independent in the Candida-infected kidney (Fig. 4C and S11). Besides neutrophils, Cxcr1 deficiency did not impair the renal accumulation of monocytes, macrophages or dendritic cells, which have also been shown to mediate protective immunity in the model (Fig. S12)(17–19). In addition, the induction of other protective pro-inflammatory mediators such as IL-1β, IL-6, Ccl2 and Ccl3 was not impaired in Cxcr1−/− kidneys (Fig. S13)(4,8).
Figure 4. Cxcr1 does not mediate neutrophil trafficking in the kidney during systemic candidiasis.
(A, B) Accumulation of neutrophils in Cxcr1+/+ and Cxcr1−/− kidneys at days 1, 4 and 7 post-infection. (A) Number of neutrophils. (B) Percent of neutrophils within total CD45+ leukocytes (n=6–15; 2–4 independent experiments). (C) Induction of neutrophil-targeted CXC chemokines in Cxcr1+/+ and Cxcr1−/− kidneys after Candida infection (n=6–8; 2 independent experiments). (D,E) Ratio of CD45.1+Cxcr1+/+ and CD45.2+Cxcr1−/− neutrophils in the blood before infection and in the blood and kidney at days 1 (D) and 4 (E) after Candida infection of mixed bone marrow radiation chimeras (n=6; 2 independent experiments). Data represent mean ± SEM.
Equivalent accumulation of neutrophils in infected Cxcr1+/+ and Cxcr1−/− kidneys (Fig. 4A,B) despite greater fungal burden in the knockout kidneys (Fig. 2C) could be interpreted as an abnormal response, reflecting a relative impairment of neutrophil influx to the Cxcr1−/− kidney. To address the potential confounding effects of the differential fungal load in Cxcr1+/+ and Cxcr1−/− kidneys on neutrophil trafficking and to examine the direct role of Cxcr1 on neutrophil recruitment from the blood into the kidney in vivo, we generated mixed bone marrow radiation chimeras. Hence, we adoptively transferred a 1:1 ratio of CD45.1+Cxcr1+/+ and CD45.2+Cxcr1−/− bone marrow cells into lethally irradiated CD45.1+Cxcr1+/+ recipient mice and allowed reconstitution for 8 weeks before Candida infection. We then assessed the relative trafficking of Cxcr1+/+ and Cxcr1−/− neutrophils toward the same kidney milieu of infected mixed bone marrow chimeras using differential congenic marker detection by FACS. We found that the relative frequency of CD45.1+Cxcr1+/+ and CD45.2+Cxcr1−/− neutrophils was unchanged before and after infection and was similar between infected blood and infected kidney, indicating that Cxcr1+/+ and Cxcr1−/− neutrophils exhibited comparable recruitment from the blood into the infected kidney at days 1 and 4 post-infection (Fig. 4D,E). These data collectively show that Cxcr1 is dispensable for neutrophil recruitment from the blood into the kidney during systemic candidiasis.
Because Cxcr2 is another major neutrophil-targeted chemoattactant receptor that binds CXC chemokines (12), and we found it to be expressed on blood and kidney neutrophils of Candida-infected Cxcr1+/+ and Cxcr1−/− mice (Fig. S14), we reasoned that Cxcr2 may drive renal neutrophil recruitment during infection in the absence of Cxcr1. To test this, we treated Cxcr1−/− mice with vehicle or the selective Cxcr2 antagonist SB225002, which has been successfully used to inhibit Cxcr2-dependent neutrophil trafficking in mouse models of infection and inflammation in vivo (20,21). We found that Cxcr2 inhibition did not impair neutrophil accumulation in Candida-infected kidneys of Cxcr1−/− mice (Fig. S15), suggesting that chemoattractant receptors other than Cxcr2 are responsible for neutrophil recruitment into the Candida-infected Cxcr1−/− kidney.
Cxcr1 deficiency impairs the fungal killing capacity of kidney neutrophils and results in decreased Candida hyphal damage in the infected kidney
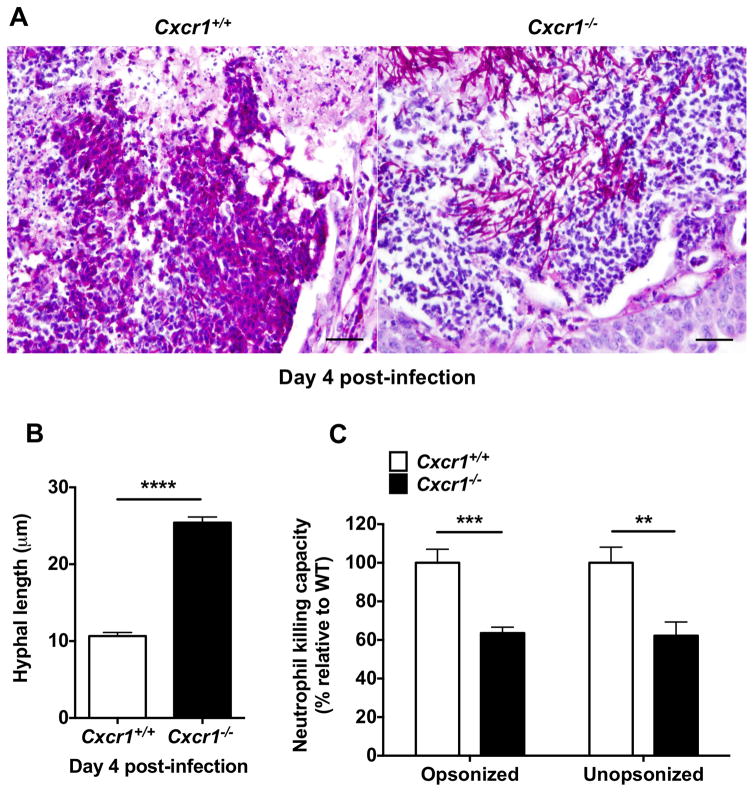
Since Cxcr1 did not mediate neutrophil recruitment in the kidney during systemic candidiasis, we next investigated the role of Cxcr1 on neutrophil effector function in the infected kidney. Chemokine receptors regulate many leukocyte functions apart from directional cell movement, such as cell survival, proliferation, phagocytosis, and killing (13,19,22), and CXCR1 has been reported to promote oxidative and non-oxidative antibacterial effects in human neutrophils (13). Examination of Periodic acid–Schiff (PAS) kidney stains revealed that neutrophilic abscesses in the kidneys of Cxcr1+/+ mice contained degraded Candida hyphal elements consistent with neutrophil antifungal cytotoxic activity, as previously observed in this model (7,10). Strikingly, Candida hyphal elements appeared intact within Candida-infected Cxcr1−/− renal abscesses (Fig. 5A). We quantified the length of >400 randomly picked individual hyphal elements within abscesses from Cxcr1+/+ and Cxcr1−/− kidneys and found significantly longer Candida hyphal elements within Cxcr1−/− abscesses (Fig. 5B), suggesting that Cxcr1−/− neutrophils may be functionally impaired in controlling fungal filamentous growth in the infected kidney.
Figure 5. Cxcr1 deficiency impairs the killing capacity of kidney neutrophils against Candida and results in decreased hyphal damage in the infected kidney.
(A) Representative PAS staining of Cxcr1+/+ and Cxcr1−/− kidney sections (day 4 post-infection) showing degraded hyphal elements within Cxcr1+/+ neutrophil abscesses but intact hyphae within Cxcr1−/− neutrophil abscesses. Original magnification, 400x. Bar scale, 100 μm (B) Candida hyphal length within Cxcr1+/+ and Cxcr1−/− renal neutrophil abscesses at day 4 post-infection (n=452–466 randomly selected hyphal element measurements obtained from 12–13 kidneys per genotype; summary data from 3 independent experiments). ****P<0.0001; Mann-Whitney test. (C) Cxcr1−/− neutrophils MACS-sorted from the kidney at day 4 post-infection have impaired ability to damage opsonized (P=0.0009) and unopsonized (P=0.0034) Candida hyphae ex vivo (n=8; 4 independent experiments; t-test). All quantitative data represent mean ± SEM.
Indeed, using alamarBlue reduction as a measure of fungal inactivation, as previously described (19), Cxcr1−/− neutrophils MACS-sorted from the infected kidney exhibited a significant ~40% defect in their killing capacity of hyphal elements ex vivo (Fig. 5C). We examined fungal killing by kidney neutrophils under both opsonized and unopsonized conditions, since opsonization may be suboptimal within neutrophil abscesses (23), and found that the defect in neutrophil killing was independent of fungal opsonization (Fig. 5C). Similarly, Cxcr1−/− kidney neutrophils exhibited impaired killing against opsonized and unopsonized Candida yeast forms (Fig. S16A). Taken together, these data indicate that Cxcr1 is critical for promoting antifungal killing by neutrophils and inducing Candida hyphal damage within neutrophil abscesses at the site of infection.
Cxcr1 mediates neutrophil degranulation
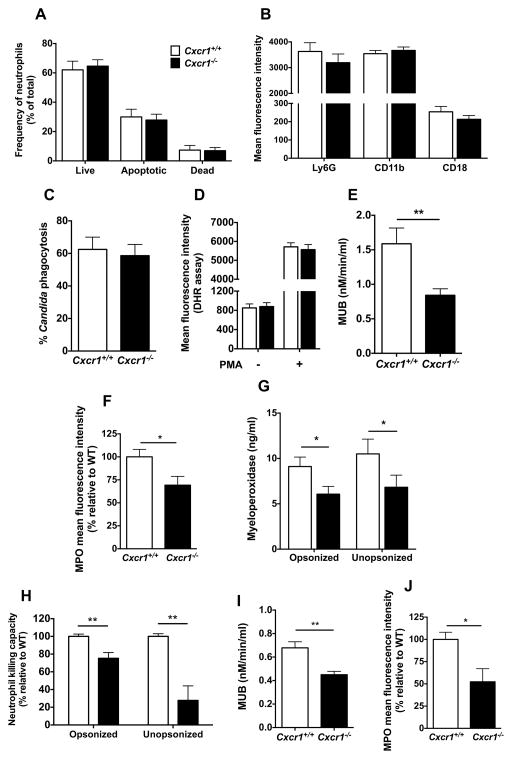
We next investigated the mechanism(s) underlying the decreased neutrophil candidacidal activity in Cxcr1−/− kidneys. We found that Cxcr1 deficiency did not result in defective neutrophil killing by adversely affecting neutrophil survival (Fig. 6A), maturation (Fig. 6B), phagocytosis (Fig. 6C) or oxidative burst (Fig. 6D), indicating that Cxcr1-mediated fungal killing in kidney neutrophils occurs via non-oxidative effector mechanisms.
Figure 6. Cxcr1−/− neutrophils exhibit cell-intrinsic defects in degranulation and non-oxidative Candida killing but no impaired survival, maturation or phagocytosis.
(A) Neutrophil survival. Percentage of live, apoptotic and dead neutrophils in the kidney at day 4 post-infection determined by Annexin V and 7-AAD FACS staining (n=8; 2 independent experiments). (B) Neutrophil maturation. Mean fluorescence intensity of the maturation markers Ly6G, CD11b and CD18 expressed on the surface of kidney neutrophils at day 4 post-infection (n=4–18; 2–5 independent experiments). (C) Neutrophil phagocytosis. Cxcr1+/+ and Cxcr1−/− neutrophils isolated from uninfected bone marrows have similar capacity to internalize dTomato-expressing Candida albicans (n=4; 2 independent experiments). (D) Neutrophil oxidative burst. Mean fluorescence intensity of rhodamine 123 measured by FACS in Cxcr1+/+ and Cxcr1−/− neutrophils MACS-sorted from the kidney at day 4 post-infection with or without additional ex vivo stimulation with PMA (n=4, 2 independent experiments). (E) Neutrophil degranulation. β-methylumbelliferone (MUB) amount measured as a marker of release of granule beta-glucuronidase in Cxcr1+/+ and Cxcr1−/− neutrophils MACS-sorted from the kidney at day 4 post-infection (P=0.004; n=13; 4 independent experiments; Mann-Whitney test). (F) Neutrophil MPO content. Mean fluorescence intensity of MPO assessed by intracellular FACS in Cxcr1+/+ and Cxcr1−/− kidney neutrophils at day 4 post-infection (P=0.0202; n=15; 4 independent experiments; t-test). (G) Neutrophil MPO release. Release of granule MPO from Cxcr1+/+ and Cxcr1−/− neutrophils MACS-sorted from the kidney at day 4 post-infection following ex vivo infection with opsonized (P=0.0308; t-test) or unopsonized (P=0.0384; Mann-Whitney test) Candida hyphae (n=17; 4 independent experiments). (H–J) Cxcr1−/− neutrophils isolated from uninfected bone marrows exhibit defects in Candida hyphal killing (P=0.0032; n=12; 6 independent experiments; t-test with Welch’s correction for opsonized hyphae and P=0.0021; n=9; 5 independent experiments; t-test with Welch’s correction for unopsonized hyphae) (H), degranulation capacity measured as beta-glucuronidase release (P=0.0033; n=6; 3 independent experiments; t-test) (I), and intracellular MPO content (P=0.0207; n=8; 4 independent experiments; Mann-Whitney test) (J) Data represent mean ± SEM.
Filamentous fungal element killing by neutrophils occurs predominantly extracellularly due to the large hyphal size that precludes universal internalization by phagocytes (4,8). We found no defect in neutrophil extracellular trap formation of Cxcr1−/− neutrophils ex vivo (Fig. S17). Hence, we focused our studies on determining whether degranulation was impaired in Cxcr1−/− kidney neutrophils, as chemotactic factors have previously been reported to promote neutrophil degranulation (24–27). Indeed, we found that Cxcr1−/− neutrophils MACS-sorted from the infected kidney had a significant ~50% reduction in degranulation capacity as measured by beta-glucuronidase release (Fig. 6E). Consistent with this finding, intracellular levels of another major neutrophil primary granule protein, myeloperoxidase (MPO), were significantly decreased in Cxcr1−/− kidney neutrophils (Fig. 6F), and Cxcr1−/− neutrophils MACS-sorted from the infected kidney released significantly less MPO upon Candida hyphal challenge ex vivo (Fig. 6G). Collectively, these data show that Cxcr1 mediates degranulation of neutrophils in the Candida-infected kidney.
Cxcr1 deficiency results in a cell-intrinsic defect in degranulation and fungal killing of neutrophils
We next asked whether the impairment in degranulation and fungal killing that we observed in Cxcr1−/− kidney neutrophils was due to a cell-intrinsic neutrophil defect or whether it was caused by kidney-specific alterations in the local immunological milieu of Cxcr1−/− mice. A recent report showed that Syk-dependent production of IL-23p19 by renal mononuclear phagocytes results in secretion of GM-CSF by NK cells, which provides critical “help” for neutrophil maturation and candidacidal activity locally in the Candida-infected kidney (18). Thus, we first examined whether the IL-23p19/GM-CSF axis was impaired in Cxcr1−/− kidneys. We found that mononuclear phagocyte (Fig. S12) and NK cell accumulation (Fig. S18A), as well as mRNA induction for Il-23a and Gmcsf (Fig. S18B,C) were similar in infected Cxcr1+/+ and Cxcr1−/− kidneys. Instead, consistent with a cell-intrinsic defect of Cxcr1−/− neutrophils, we found that neutrophils isolated from the bone marrows of uninfected Cxcr1−/− mice exhibited significantly impaired killing against opsonized and unopsonized Candida yeast and hyphal forms relative to Cxcr1+/+ cells (Fig. 6H and S16B) while oxidative burst was normal (Fig. S19). In addition, Cxcr1−/− neutrophils harvested from uninfected bone marrow exhibited significant decreases in (a) degranulation capacity as measured by beta-glucuronidase release (Fig. 6I), (b) and intracellular MPO levels at steady state, suggestive of a Cxcr1-dependent defect in granulogenesis (Fig. 6J). Therefore, our data collectively show the Cxcr1 deficiency results in degranulation and fungal killing defects in neutrophils that are cell-intrinsic and not caused by an impaired kidney-specific local immunological milieu.
CXCR1-T276 is associated with disseminated candidiasis in humans
In humans, the CXCR1-T276 SNP (rs2234671) caused by a guanine-to-cytosine substitution at nucleotide 827 in exon 2 that changes a serine at position 276 to threonine has been associated with increased susceptibility to bacterial renal infection in two independent cohorts of pediatric patients (14,15), and has been shown to correlate with decreased levels of mRNA and protein expression of CXCR1 in human neutrophils (15,28). To test the hypothesis that the CXCR1-T276 SNP may increase host susceptibility to systemic candidiasis and/or development of adverse outcome after infection, we genotyped 153 candidemic patients and 151 non-infected control patients of mixed European descent from the US (19)(Table S1).
The genotypes were in Hardy-Weinberg equilibrium in both groups. Although there was no difference in the frequencies of patients carrying the mutant CXCR1-T276 allele versus the WT genotype among candidemic and uninfected control subjects (Table S2), candidemic patients carrying the mutant CXCR1-T276 allele were more likely to develop disseminated infection (37.5%) than those carrying the WT allele (16.8%)(Table 1; P=0.0455; OR, 2.97; 95%CI, 0.98–8.99). This association remained significant in multivariate analysis when controlling for other clinical factors. In the final model, presence of the CXCR1-T276 allele (P=0.0242; OR, 3.95; 95%CI, 1.20–13.03), receipt of total parenteral nutrition (P=0.0213; OR, 3.19; 95%CI, 1.19–8.21) and solid organ transplantation (P=0.0073; OR, 4.03; 95%CI, 1.46–11.17) were the only factors independently associated with development of disseminated candidiasis. No association of carrying the CXCR1-T276 SNP was observed with increased mortality among candidemic patients, whereas there was a trend toward development of persistent fungemia in those carrying the mutant allele in multivariate analysis (P=0.1110; OR, 2.79; 95%CI, 0.79–9.82)(Table S3).
Table 1.
Association of the mutant CXCR1-T276 allele with development of disseminated candidiasis after candidemia in patients of mixed European descent (n=153).
| Variable | Univariate analysis P value |
Multivariate analysis OR (95% CI) |
|---|---|---|
| CXCR1-T276 allele (CG + GG) | 0.0455 | 3.95 (1.20–13.03) |
| Male sex | 0.5264 | |
| Immunocompromised state | 0.2882 | |
| Hematopoietic stem cell transplantation | 0.5845* | |
| Solid organ transplantation | 0.0102 | 4.03 (1.46–11.17) |
| Active malignancy | 0.3656 | |
| Solid tumor | 0.5967 | |
| Leukemia | 1.0000* | |
| Lymphoma | 0.3124* | |
| Chemotherapy within past 3 months | 0.6836 | |
| Neutropenia (ANC < 500 cells/mm3) | 0.4837 | |
| Surgery within past 30 days | 0.102 | |
| Receipt of total parenteral nutrition | 0.0891 | 3.19 (1.19–8.21) |
| Dialysis dependent | 1.0000* | |
| Acute renal failure | 0.2652 | |
| Liver disease | 0.1551 | |
| Intensive care unit admission within past 14 days | 0.5278 |
Fisher’s exact test; ANC, absolute neutrophil count
Neutrophils from healthy individuals with the CXCR1-T276 SNP exhibit impaired degranulation and fungal killing capacity
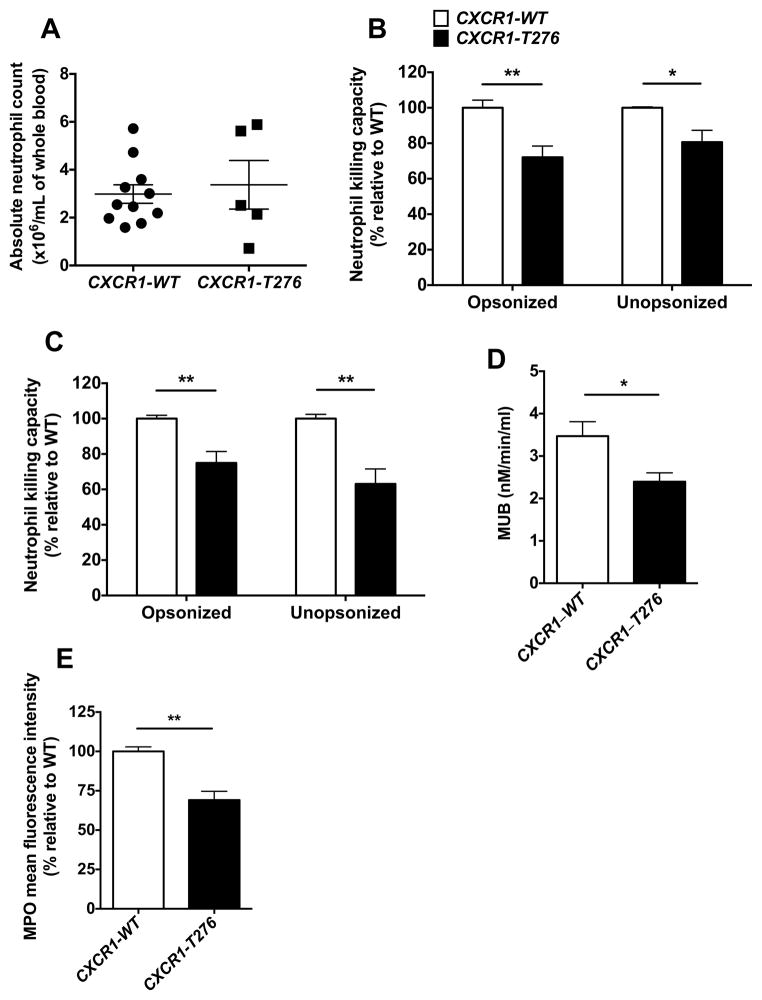
Since the mutant CXCR1-T276 allele was associated with increased susceptibility to disseminated candidiasis in candidemic patients, we next asked whether neutrophils from individuals with the CXCR1-T276 SNP exhibit defective effector antifungal function similar to Cxcr1−/− mouse neutrophils. To investigate this question, we screened >50 healthy donors at the NIH Blood Bank and identified individuals with the CXCR1-WT and CXCR1-T276 alleles, from whom we obtained whole blood to harvest neutrophils for functional studies.
In agreement with the comparable neutrophil numbers in Cxcr1+/+ and Cxcr1−/− mouse blood at steady state, individuals with the CXCR1-T276 allele did not exhibit decreased absolute neutrophil counts relative to CXCR1-WT individuals (Fig. 7A). Remarkably, consistent with our mouse findings, neutrophils from donors with the CXCR1-T276 allele had impaired fungal killing ability against both opsonized and unopsonized yeast and hyphal elements (Fig. 7B,C), exhibited significantly impaired degranulation capacity as measured by beta-glucuronidase release (Fig. 7D), and had decreased intracellular MPO content (Fig. 7E). Therefore, these data collectively show that neutrophils from donors with the mutant CXCR1-T276 allele have impaired degranulation and fungal killing capacity.
Figure 7. The mutant CXCR1-T276 allele is associated with impaired fungal killing and degranulation in human neutrophils.
(A) Healthy donors carrying the CXCR1-T276 allele have similar absolute neutrophil counts with CXCR1-WT donors (n=5–11). (B–E) Neutrophils of healthy donors carrying the CXCR1-T276 allele exhibit impaired ability to damage opsonized and unopsonized Candida yeast (B; P=0.0067 for opsonized and P=0.0332 for unopsonized yeast; n=5–6; 5 independent experiments; t-test with Welch’s correction) and hyphae (C; P=0.0023 for opsonized and P=0.0012 for unopsonized hyphae; n=6–7; 5 independent experiments; Mann-Whitney test) ex vivo, defective degranulation as measured by release of beta glucuronidase (P=0.0381; n=4–6; 3 independent experiments; Mann-Whitney test) (D), and decreased intracellular MPO content (P=0.0012; n=5–6; 3 independent experiments; t-test) (E).
Discussion
In this study, we present a role for the chemokine receptor Cxcr1 in host defense against systemic fungal infection and show that the mutant CXCR1-T276 SNP results in impaired neutrophil function and is associated with susceptibility to systemic candidiasis in humans. The receptor appears to act directly by promoting neutrophil degranulation and fungal killing, which mediates Candida damage in tissue and fungal clearance. Our conclusions are based on detailed analysis of differences in clinical, microbiological, pathological, immunological and molecular parameters between Cxcr1+/+ and Cxcr1−/− mice. Our study reveals a biological function for the mouse chemokine receptor Cxcr1 in vivo and identifies a neutrophil-targeted chemotactic factor that protects against systemic fungal infection in mice and humans.
We focused on Cxcr1 because (a) neutrophils are the major cellular mediators of the innate immune response against systemic candidiasis and their early recruitment into Candida-infected mouse tissues promotes Candida control and host survival (7–9), and because (b) our broad survey of the chemokine system in the mouse model of systemic candidiasis revealed significant induction of Cxcr1, and its ligand Cxcl5, in infected tissues (10). Notably, the functional importance of Cxcr1 for neutrophils had only been suggested through indirect approaches in humans (12,13) and no study had thus far ascribed any biological function for mouse Cxcr1 in vivo; therefore, we generated Cxcr1-deficient mice in order to examine the role of the receptor in antifungal host defense.
We found that Cxcr1 is critical for innate antifungal host defense, as Cxcr1−/− mice have decreased survival and impaired ability to control fungal proliferation in the kidney. We did not find a defect in neutrophil accumulation in Cxcr1−/− mice and our mixed bone marrow chimera experiments revealed no preferential recruitment of Cxcr1+/+ over Cxcr1−/− neutrophils in the Candida-infected kidney, thus collectively ruling out a role of Cxcr1-dependent neutrophil trafficking in the model. Therefore, yet-unknown chemotactic receptors other than Cxcr1 or Ccr1 (10) mediate early protective trafficking of neutrophils from the blood into the Candida-infected kidney. Of interest, Cxcr1+/+ and Cxcr1−/− mice also had similar neutrophil numbers in the blood at steady state, as opposed to the neutrophil expansion reported in Cxcr2-deficient mice (29); this indicates that Cxcr1 and Cxcr2 play differential roles on mouse granulocytopoiesis and neutrophil homeostasis.
Instead of impaired trafficking, Cxcr1-deficient neutrophils appeared unable to cause damage to Candida filamentous elements in vivo in the infected kidney. This prompted us to examine the role of Cxcr1 in fungal killing, as human CXCR1 is known to promote oxidative and non-oxidative killing against bacteria (13). Indeed, we found significant cell-intrinsic defects in neutrophil degranulation and anti-Candida killing in Cxcr1−/− mice. This observation further expands the previously reported contribution of the neutrophil-targeted chemokine receptors CCR1, CXCR2, LTB4R1, FPR1 and C5aR1 (24–27) in neutrophil degranulation. The decreased basal levels of the major neutrophil granule proteins, myeloperoxidase and beta-glucuronidase, in Cxcr1−/− neutrophils suggest that this degranulation defect lies, at least in part, at the level of granulogenesis. Importantly, our data further underscore the pleiotropic “non-conventional” roles that chemotactic factors play in immunity via modulating cellular functions such as survival, proliferation, differentiation, phagocytosis, killing, degranulation and adhesion, besides their well-established mechanistic role in promoting directional cell movement (13,19,22).
Of interest, oxidative burst of Cxcr1−/− neutrophils isolated from Candida-infected kidneys and uninfected bone marrows was intact, suggesting that Cxcr1 likely mediates Candida killing via non-oxidative mechanisms. In fact, in contrast to the mechanistic characterization of signaling cascades that underlie oxidative cytotoxic killing in neutrophils during fungal infection (4,8,30), little is known with regard to the molecular mechanisms of neutrophil non-oxidative fungal cytotoxicity.
Our study has implications beyond the importance of defining the role of Cxcr1 in innate anti-Candida host defense. Our results present a biological function of mouse Cxcr1 in vivo and additional studies will be required to systematically define the spectrum of Cxcr1-dependent effects in non-infectious inflammatory models and in immunity against other pathogens including bacteria, viruses, molds and dimorphic fungi. All mouse immunological studies to date have ascribed identified neutrophil-specific functions of mouse CXC chemokines universally to Cxcr2. Our study shows that mouse Cxcr1 is also a functional receptor in vivo and the differential contribution of CXC chemokine-Cxcr1 versus CXC chemokine-Cxcr2 signaling in neutrophil functions in various models of infection and inflammation merits investigation.
The mutant CXCR1-T276 SNP, which has been associated with increased susceptibility to bacterial renal infection in two independent cohorts of pediatric patients (14,15), provided a unique translational opportunity to extend our mouse findings to humans. Hence, we found that the CXCR1-T276 allele is associated with an increased likelihood for the development of disseminated candidiasis in candidemic patients of mixed European descent. Because the clinical outcome of systemic candidiasis varies substantially among patients with similar clinical and microbiological risk factors, discovery of genetic factors such as CXCR1-T276 and the previously reported CX3CR1-M280 (19) that predispose to disseminated disease could aid in devising individualized prognostication strategies and in identifying candidemic patients in whom intensified diagnostic and therapeutic interventions may be beneficial.
Importantly, we found that neutrophils from individuals carrying the CXCR1-T276 allele exhibit immunological functional defects similar to those identified in Cxcr1−/− neutrophils. These defects may account, at least in part, for the increased patient susceptibility to disseminated candidiasis, and it will be important to define the molecular mechanisms by which the CXCR1-T276 allele impairs CXCR1 signaling and results in defective degranulation and fungal killing. Furthermore, whether similar neutrophil impairments are seen against bacterial pathogens in individuals carrying the CXCR1-T276 allele merits investigation, as it may provide mechanistic insights on the enhanced susceptibility of these individuals to bacterial renal infection (14,15).
Our study has limitations. Unanswered questions remain with regard to the mechanisms by which Cxcl5 mediate Cxcr1-dependent neutrophil effector function, and the molecular mechanisms involved in Cxcr1-mediated neutrophil granulogenesis and non-oxidative fungal killing. In addition, although the control uninfected patient cohort was matched to the candidemic patient cohort with regard to time and hospital ward of enrollment, the two cohorts were not entirely matched at the level of underlying clinical risk factors. Moreover, a matched patient cohort suffering from non-fungal infection was not available for comparison. Like any genetic association study, it is possible that the association of CXCR1-T276 with disseminated candidiasis is due to biases at the population stratification level or linkage with another yet-unknown SNP; thus, this finding requires validation in other cohort studies, which should also incorporate patients from other racial backgrounds.
In conclusion, our study identifies CXCR1 as a crucial factor for innate host defense against systemic fungal infection in mouse and man. Mechanistically, CXCR1 protects from fungal infection by promoting neutrophil degranulation and fungal killing. Thus, genetic variation at CXCR1 is associated with impaired neutrophil effector function and may be useful for risk assessment and prognostication in humans suffering from systemic candidiasis.
Materials and Methods
Study Design
A previous broad screen of the chemokine system showed that Cxcr1 and its ligand were induced in vivo in Candida-infected mouse tissue, suggesting that the receptor may play an important role in antifungal immunity. To investigate this, we generated Cxcr1−/− mice and found that they were highly susceptible to systemic Candida infection as they developed increased mortality and accelerated tissue fungal proliferation. Interestingly, Cxcr1 was dispensable for neutrophil trafficking from the blood into the Candida-infected tissue. Instead, Cxcr1 deficiency resulted in a cell-intrinsic defect in fungal killing and degranulation of mouse neutrophils, which were unable to damage fungal elements in vivo in the infected tissue. The sample size and replicates of experiments for all mouse studies are included in the legends of the corresponding figures. All mice were maintained under specific pathogen–free housing conditions at an American Association for the Accreditation of Laboratory Animal Care–accredited animal facility at the NIAID and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under a protocol approved by the Animal Care and Use Committee of NIAID.
To translate our mouse findings to humans, we examined the impact of carrying the mutant CXCR1-T276 allele on susceptibility of human patients to the infection in a cohort of candidemic and control uninfected individuals from the US enrolled at the Duke University Hospital (DUMC) between 2003 and 2009 (see Supplemental Methods for details). There was no blinding or randomization in the cohort enrollment. The study patients were enrolled after informed consent (or waiver as approved by the Institutional Review Board [IRB]) at DUMC. The study was approved by the IRB at DUMC and was performed in accordance with the Declaration of Helsinki. We found that the presence of the CXCR1-T276 allele was significantly associated with the development of disseminated candidiasis. To examine CXCR1-T276 neutrophil effector function, we screened >50 healthy donors at the NIH Blood Bank and identified individuals carrying the CXCR1-WT or CXCR1-T276 allele. In agreement with the mouse findings, we found that CXCR1-T276 neutrophils had impaired degranulation and fungal killing.
Mouse Model of Systemic Candidiasis and Candida Strains
Cxcr1−/− mice were generated as described in the Supplemental Methods. Cxcr2−/− mice were purchased from The Jackson Laboratories. We used 8–12–week old male Cxcr1+/+ WT, Cxcr1−/− C57Bl/6 and Cxcr2−/− C57Bl/6 mice. CD45.1+ congenic B6.SJL mice were obtained from Taconic Farms.
Candida albicans strain SC5314 was used for all experiments, except for the neutrophil phagocytosis experiments in which the dTomato-expressing CAF2-1 C. albicans strain was used (31). Candida was grown as previously described (7), and ~105 cells were injected intravenously per mouse. Uninfected and infected mice were sacrificed at days 1, 4 or 7 post-infection and the following analyses were performed (see Supplemental Methods for details): tissue fungal burdens; FACS analyses on bone marrow, blood and kidney cells; histopathology; serological analyses of renal function; quantification of cytokines and chemokines by Luminex array; cell sorting; RNA isolation from kidney tissue and bone marrow followed by qRT-PCR analyses; functional analyses of neutrophils isolated from uninfected bone marrow or infected kidney.
Statistics
The mouse experimental data and human neutrophil experimental data were analyzed using unpaired t-test, Mann-Whitney test Mann-Whitney test or one-way ANOVA test as appropriate with GraphPad Prism 6.0 and presented as mean values ± SEM. Statistical significance was defined as P<0.05.
χ2 test was used to compare the frequencies of Candida-infected and non-infected subjects in order to analyze the impact of the CXCR1-T276 SNP on human susceptibility to candidemia. Pearson correlation coefficient or Fisher’s exact test as appropriate were used to perform univariate analyses, and Odds Ratios (ORs) and 95% confidence intervals (CIs) were reported. Variables with P<0.2 were analyzed using multivariable logistic regression by backward elimination. Variables with P<0.05 were retained in the final predictive model and OR and 95% CI were reported for the variables that remained significant in the final multivariable model. SAS software version 9.2 was used for the analysis.
Supplementary Material
Acknowledgments
The authors thank the NIAID animal facilities for animal housing and handling, and the FACS sorting facility for technical assistance with cell sorting. This work was presented in part at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington D.C, September, 2014, and at the Gordon Research Conference on Immunology of Fungal Infections, Galveston, Texas, January, 2015.
Funding: This work was supported by the Division of intramural Research, NIAID, NIH. M.G. Netea was supported by an ERC Consolidator Grant (no. 310372). The funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
List of Supplementary Materials
Fig. S1. Cxcr1 deficiency does not result in developmental renal defects.
Fig. S2. Cxcl5 promotes dose-dependent Cxcr1-mediated calcium flux in mouse neutrophils.
Fig. S3. Cxcl1 and Cxcl2 do not promote significant Cxcr1-dependent calcium flux in mouse neutrophils.
Fig. S4. Systemic candidiasis results in increased renal tissue injury in Cxcr1−/− mice.
Fig. S5. Cxcr1 deficiency does not impair fungal clearance in the liver, spleen or brain after Candida infection.
Fig. S6. Cxcr1 deficiency does not result in histological abnormalities in the liver, brain or spleen after Candida infection.
Fig. S7. Gating strategy for FACS-sorting of hematopoietic cells from kidney.
Fig. S8. Neutrophils up-regulate Cxcr1 but not Cxcl5 after systemic candidiasis.
Fig. S9. Cxcr1 deficiency does not affect the production of neutrophils in the bone marrow after Candida infection.
Fig. S10. Cxcr1 deficiency does not affect the accumulation of neutrophils in the blood after Candida infection.
Fig. S11. Cxcr1 deficiency does not impair the induction of Cxcl5 in the Candida-infected kidney.
Fig. S12. Cxcr1 deficiency does not impair the accumulation of mononuclear phagocytes in the kidney after Candida infection.
Fig. S13. Cxcr1 does not impair the induction of pro-inflammatory cytokines and chemokines in the kidney after Candida infection.
Fig. S14. Cxcr2 is expressed on blood and kidney neutrophils of Cxcr1+/+ and Cxcr1−/− mice at steady state and during systemic candidiasis.
Fig. S15. A selective Cxcr2 antagonist does not decrease neutrophil accumulation in the Candida-infected kidney of Cxcr1−/− mice.
Fig. S16. Cxcr1 deficiency results in a cell-intrinsic defect in killing of Candida yeast forms.
Fig. S17. Cxcr1 deficiency does not impair the formation of neutrophil extracellular traps ex vivo.
Fig. S18. Cxcr1 deficiency does not impair the accumulation of NK cells or the induction of Il23a and Gmcsf in the kidney after Candida infection.
Fig. S19. Cxcr1 deficiency does not impair the production of reactive oxygen species by bone marrow neutrophils at steady state.
Table S1. Demographic and clinical characteristics of the candidemic and control subjects enrolled in the present study.
Table S2. Association of the mutant CXCR1-T276 allele with susceptibility to systemic candidiasis in subjects of mixed European descent (n=304).
Table S3. Association of the mutant CXCR1-T276 allele with development of persistent fungemia in candidemic patients of mixed European descent (n=153).
Data File 1. Excel file with all data values in tabular format.
Footnotes
Author contributions: MS, TJB, MJ, CAR, JKL, NMG, ALC, BGF and MSL performed experiments; MS, TJB, MDJ, MJ, JRP, BDA, CCRL, BJK, MGN and MSL analyzed data; MS, JLG, TJB, PMM and MSL designed experiments; MS, MDJ and MSL performed statistical analyses; MS and MSL wrote the manuscript.
Competing interests: The authors declare no competing interests.
References
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK I. Emerging Infections Program Healthcare-Associated, T. Antimicrobial Use Prevalence Survey. Multistate point-prevalence survey of health care-associated infections. The New England journal of medicine. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clinical infectious diseases. 2005;41:1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 3.Miller LG, Hajjeh RA, Edwards JE., Jr Estimating the cost of nosocomial candidemia in the united states. Clinical infectious diseases. 2001;32:1110. doi: 10.1086/319613. [DOI] [PubMed] [Google Scholar]
- 4.Lionakis MS. New insights into innate immune control of systemic candidiasis. Medical mycology. 2014;52:555–564. doi: 10.1093/mmy/myu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulurija A, Ashman RB, Papadimitriou JM. Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice, and reveals differences between brain and kidney in mechanisms of host resistance. Microbiology. 1996;142(Pt 12):3487–3496. doi: 10.1099/13500872-142-12-3487. [DOI] [PubMed] [Google Scholar]
- 6.Dejima T, Shibata K, Yamada H, Hara H, Iwakura Y, Naito S, Yoshikai Y. Protective role of naturally occurring interleukin-17A-producing gammadelta T cells in the lung at the early stage of systemic candidiasis in mice. Infection and immunity. 2011;79:4503–4510. doi: 10.1128/IAI.05799-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lionakis MS, Lim JK, Lee CC, Murphy PM. Organ-specific innate immune responses in a mouse model of invasive candidiasis. Journal of innate immunity. 2011;3:180–199. doi: 10.1159/000321157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lionakis MS, Netea MG. Candida and host determinants of susceptibility to invasive candidiasis. PLoS pathogens. 2013;9:e1003079. doi: 10.1371/journal.ppat.1003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. Journal of immunology. 1997;158:2356–2362. [PubMed] [Google Scholar]
- 10.Lionakis MS, Fischer BG, Lim JK, Swamydas M, Wan W, Richard Lee CC, Cohen JI, Scheinberg P, Gao JL, Murphy PM. Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis. PLoS pathogens. 2012;8:e1002865. doi: 10.1371/journal.ppat.1002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- 12.Aul R, Patel S, Summerhill S, Kilty I, Plumb J, Singh D. LPS challenge in healthy subjects: an investigation of neutrophil chemotaxis mechanisms involving CXCR1 and CXCR2. International immunopharmacology. 2012;13:225–231. doi: 10.1016/j.intimp.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Hartl D, Latzin P, Hordijk P, Marcos V, Rudolph C, Woischnik M, Krauss-Etschmann S, Koller B, Reinhardt D, Roscher AA, Roos D, Griese M. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nature medicine. 2007;13:1423–1430. doi: 10.1038/nm1690. [DOI] [PubMed] [Google Scholar]
- 14.Javor J, Bucova M, Cervenova O, Kralinsky K, Sadova E, Suchankova M, Liptakova A. Genetic variations of interleukin-8, CXCR1 and CXCR2 genes and risk of acute pyelonephritis in children. International journal of immunogenetics. 2012;39:338–345. doi: 10.1111/j.1744-313X.2012.01096.x. [DOI] [PubMed] [Google Scholar]
- 15.Lundstedt AC, McCarthy S, Gustafsson MC, Godaly G, Jodal U, Karpman D, Leijonhufvud I, Linden C, Martinell J, Ragnarsdottir B, Samuelsson M, Truedsson L, Andersson B, Svanborg C. A genetic basis of susceptibility to acute pyelonephritis. PloS one. 2007;2:e825. doi: 10.1371/journal.pone.0000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, Zeng M, Jackson C, Sullivan L, Sharif-Rodriguez W, Opdenakker G, Van Damme J, Hedrick JA, Lundell D, Lira SA, Hipkin RW. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. The Journal of biological chemistry. 2007;282:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- 17.Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. The Journal of infectious diseases. 2014;209:109–119. doi: 10.1093/infdis/jit413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitney PG, Bar E, Osorio F, Rogers NC, Schraml BU, Deddouche S, LeibundGut-Landmann S, Reis e Sousa C. Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS pathogens. 2014;10:e1004276. doi: 10.1371/journal.ppat.1004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, Wan W, Lee CC, Lim JK, Rivollier A, Yang JC, Laird GM, Wheeler RT, Alexander BD, Perfect JR, Gao JL, Kullberg BJ, Netea MG, Murphy PM. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. The Journal of clinical investigation. 2013;123:5035–5051. doi: 10.1172/JCI71307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwaiz R, Rahman M, Syk I, Zhang E, Thorlacius H. Rac1-dependent secretion of platelet-derived CCL5 regulates neutrophil recruitment via activation of alveolar macrophages in septic lung injury. The Journal of leukocyte biology. 2015 doi: 10.1189/jlb.4A1214-603R. pii: jlb.4A1214–603R. [DOI] [PubMed] [Google Scholar]
- 21.Bento AF, Leite DF, Claudino RF, Hara DB, Leal PC, Calixto JB. The selective nonpeptide CXCR2 antagonist SB225002 ameliorates acute experimental colitis in mice. The Journal of leukocyte biology. 2008;84:1213–1221. doi: 10.1189/jlb.0408231. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Vicuna R, Gomez-Gaviro MV, Dominguez-Luis MJ, Pec MK, Gonzalez-Alvaro I, Alvaro-Gracia JM, Diaz-Gonzalez F. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis and rheumatism. 2004;50:3866–3877. doi: 10.1002/art.20615. [DOI] [PubMed] [Google Scholar]
- 23.Bamberger DM, Herndon BL. Bactericidal capacity of neutrophils in rabbits with experimental acute and chronic abscesses. The Journal of infectious diseases. 1990;162:186–192. doi: 10.1093/infdis/162.1.186. [DOI] [PubMed] [Google Scholar]
- 24.Hunniger K, Bieber K, Martin R, Lehnert T, Figge MT, Loffler J, Guo RF, Riedemann NC, Kurzai O. A second stimulus required for enhanced antifungal activity of human neutrophils in blood is provided by anaphylatoxin C5a. Journal of immunology. 2015;194:1199–1210. doi: 10.4049/jimmunol.1401845. [DOI] [PubMed] [Google Scholar]
- 25.Southgate EL, He RL, Gao JL, Murphy PM, Nanamori M, Ye RD. Identification of formyl peptides from Listeria monocytogenes and Staphylococcus aureus as potent chemoattractants for mouse neutrophils. Journal of immunology. 2008;181:1429–1437. doi: 10.4049/jimmunol.181.2.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaudreault E, Thompson C, Stankova J, Rola-Pleszczynski M. Involvement of BLT1 endocytosis and Yes kinase activation in leukotriene B4-induced neutrophil degranulation. Journal of immunology. 2005;174:3617–3625. doi: 10.4049/jimmunol.174.6.3617. [DOI] [PubMed] [Google Scholar]
- 27.Jan MS, Huang YH, Shieh B, Teng RH, Yan YP, Lee YT, Liao KK, Li C. CC chemokines induce neutrophils to chemotaxis, degranulation, and alpha-defensin release. Journal of acquired immune deficiency syndromes. 2006;41:6–16. doi: 10.1097/01.qai.0000188336.94090.14. [DOI] [PubMed] [Google Scholar]
- 28.Frendeus B, Godaly G, Hang L, Karpman D, Lundstedt AC, Svanborg C. Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. The Journal of experimental medicine. 2000;192:881–890. doi: 10.1084/jem.192.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Utomo A, Cullere X, Choi MM, Milner DA, Jr, Venkatesh D, Yun SH, Mayadas TN. The beta-glucan receptor Dectin-1 activates the integrin Mac-1 in neutrophils via Vav protein signaling to promote Candida albicans clearance. Cell host & microbe. 2011;10:603–615. doi: 10.1016/j.chom.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brothers KM, Gratacap RL, Barker SE, Newman ZR, Norum A, Wheeler RT. NADPH oxidase-driven phagocyte recruitment controls Candida albicans filamentous growth and prevents mortality. PLoS pathogens. 2013;9:e1003634. doi: 10.1371/journal.ppat.1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 33.Gao JL, Murphy PM. Chemokine receptor knockout mice. Methods in molecular biology. 2000;138:259–274. doi: 10.1385/1-59259-058-6:259. [DOI] [PubMed] [Google Scholar]
- 34.Swamydas M, Lionakis MS. Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. J Vis Exp. 2013:e50586. doi: 10.3791/50586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao JL, Guillabert A, Hu J, Le Y, Urizar E, Seligman E, Fang KJ, Yuan X, Imbault V, Communi D, Wang JM, Parmentier M, Murphy PM, Migeotte I. F2L, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating Fpr2, the low-affinity N-formylpeptide receptor. The Journal of immunology. 2007;178:1450–1456. doi: 10.4049/jimmunol.178.3.1450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.