Abstract
Elevated IGF-1/insulin-like growth factor-1 receptor (IGF-1R) autocrine/paracrine signaling in patients with renal cell carcinoma is associated with poor prognosis of the disease independent of their von Hippel-Lindau (VHL) status. Increased expression of IGF-1R in renal cancer cells correlates with their potency of tumor development and progression. The mechanism by which expression of IGF-1R is increased in renal carcinoma is not known. We report that VHL-deficient and VHL-positive renal cancer cells possess significantly decreased levels of mature, pre-, and pri-miR-214 than normal proximal tubular epithelial cells. We identified an miR-214 recognition element in the 3′UTR of IGF-1R mRNA and confirmed its responsiveness to miR-214. Overexpression of miR-214 decreased the IGF-1R protein levels, resulting in the inhibition of Akt kinase activity in both types of renal cancer cells. IGF-1 provoked phosphorylation and inactivation of PRAS40 in an Akt-dependent manner, leading to the activation of mTORC1 signal transduction to increase phosphorylation of S6 kinase and 4EBP-1. Phosphorylation-deficient mutants of PRAS40 and 4EBP-1 significantly inhibited IGF-1R-driven proliferation of renal cancer cells. Expression of miR-214 suppressed IGF-1R-induced phosphorylation of PRAS40, S6 kinase, and 4EBP-1, indicating inhibition of mTORC1 activity. Finally, miR-214 significantly blocked IGF-1R-forced renal cancer cell proliferation, which was reversed by expression of 3′UTR-less IGF-1R and constitutively active mTORC1. Together, our results identify a reciprocal regulation of IGF-1R levels and miR-214 expression in renal cancer cells independent of VHL status. Our data provide evidence for a novel mechanism for IGF-1R-driven renal cancer cell proliferation involving miR-214 and mTORC1.
Keywords: Akt PKB, cancer, cell signaling, microRNA (miRNA), mTOR complex (mTORC)
Introduction
Renal cell carcinoma (RCC)5 accounts for nearly 3% of all malignancies. Among the five histologic subtypes, clear cell renal carcinoma accounts for about 85% of all RCCs (1, 2). About 30% of patients show renal cancer metastasis to lung, liver, bone, and brain at the time of diagnosis, and half of the remaining patients eventually develop metastasis (3–5). Individuals bearing germ line mutation in the von Hippel-Lindau (VHL) tumor suppressor gene located on chromosome 3p have increased risk for clear cell RCC. Inherited forms of RCC occur when the remaining wild type VHL allele is lost.
Apart from inactivated VHL-driven tumorigenesis, IGF-1 signal transduction significantly contributes to the growth of RCC cells in vitro and in vivo in animal models (6, 7). In fact, increased IGF-1 mRNA and protein levels in the kidney are significantly higher in RCC in humans (8, 9). Similarly, IGF-1 receptor (IGF-1R) expression has also been shown to be significantly associated with increased risk of RCC (10, 11). Also, patients with IGF-1R-positive RCC showed significantly reduced survival rates (12, 13). The dimeric IGF-1R shares significantly high homology with insulin receptor. IGF-1R is produced as a single polypeptide, which is cleaved to form the mature α- and β-subunits. The α-protein represents the transmembrane protein with extracellular domain, whereas the β-subunit is exclusively intracellular. The IGF-1 binds to the extracellular domain of α-subunit, resulting in heterotetramerization. Upon ligand binding, conformational change in the juxtamembrane domain induces an increase in tyrosine kinase activity of the β-subunit, which autophosphorylates specific tyrosine residues in the β-subunit. Tyrosine-phosphorylated β-subunit recruits the IRS protein through binding to its N-terminal PTB domain. Receptor-bound IRS protein serves as docking sites for the Src homology 2 domain-containing proteins, which trigger signal transduction to induce tumor growth of RCC mainly by two arms, the Ras/MAPK and phosphatidylinositol 3-kinase/Akt pathways (14, 15). Because of significant homology between IGF-1R and insulin receptor, they can form a hybrid receptor, which binds IGF-1 with an affinity similar to that with IGF-1R heterotetramer alone, and can elicit mitogenic signal transduction in tumor cells (14, 15). Therefore, expression of these receptors in the RCC and availability of the ligands will influence the process of tumorigenesis. Because development of small molecular drugs for inhibition of receptor tyrosine kinases is a field of active research, it is important to consider the therapeutic strategies, which will block both IGF-1R and the hybrid receptors.
MicroRNAs (miRs) are short non-coding RNAs, which silence mRNAs post-transcriptionally in a sequence-specific manner to regulate gene expression. MicroRNAs have emerged to regulate the expression of more than 30% of mRNAs coded by the genome (16, 17). Thus, they contribute to regulation of many physiologic and pathologic processes, including oncogenesis (18). miRNAs are produced as primary transcripts (pri-miRs) by the RNA polymerase II-mediated transcription of inter- as well as intragenic regions of chromosomal DNA (19). pri-miRs are processed in the nucleus by the RNase III activity of Drosha in the microprocessor multiprotein complex to produce short hairpin pre-miRs, which are exported to the cytoplasm by the exportin 5 (19–21). The pre-miRs are then processed by the dicer exonuclease III activity in a complex containing its partner trans-activation-responsive RNA-binding protein to yield RNA duplexes. Unwinding of the duplex RNA generates the guide strand as an ∼22-nucleotide-long mature miRNA (19). Mature miRNA then interacts with the Argonaute 2 to form RNA-dependent silencing complex to bind the 3′UTR of mRNA with imperfect complementarity to induce suppression of translation and degradation.
Expression profiling of miRNAs has been extensively used to understand the progression, development, and invasion of different cancers, including renal cancer (22). Expression of miRNAs has been used to classify the malignant nature of RCC (23). Thus, targeting of specific miRNA(s) may be a therapeutic strategy in RCC. In this study, we identify increased expression of IGF-1R in both VHL-positive and -negative renal cancer cells as compared with the normal proximal tubular epithelial cells. Levels of IGF-1R negatively correlate with the expression of miR-214. We identified a functional miR-214 recognition element in the 3′UTR of IGF-1R mRNA. We report that IGF-1-stimulated Akt kinase and its substrate PRAS40 phosphorylation as well as phosphorylation of mTORC1 substrate 4EBP-1 are necessary for proliferation of renal cancer cells. Finally, we show that miR-214 prevents IGF-1-stimulated renal cancer cell proliferation by targeting IGF-1R and mTORC1.
Experimental Procedures
Materials
Recombinant IGF-1, Nonidet P-40, Na3VO4, phenylmethylsulfonyl fluoride, protease inhibitor mixture, and FLAG and β-actin antibodies were purchased from Sigma. Antibodies for phospho-Akt (Ser-473), phospho-Akt (Thr-308), phospho-GSK3β (Ser-9), phospho-mTOR (Ser-2448), phospho-PRAS40 (Thr-246), phospho-S6 kinase (Thr-389), phospho-4EBP-1 (Thr-37/46), phospho-4EBP-1 (Ser-65), Akt, mTOR, IGF-1R β-subunit, PRAS40, and S6 kinase were obtained from Cell Signaling, Boston. GSK3β and phospho-IGF-1R (Tyr-1165/1166) antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Anti-HA antibody was purchased from Covance, Princeton, NJ. Plasmid isolation kit and RNA isolation reagent were obtained from Qiagen (Valencia, CA) and Invitrogen, respectively. FuGENE-HD was purchased from Roche Applied Science. PVDF membrane was purchased from PerkinElmer Life Sciences. MK-2206 was obtained from Selleck Chemicals, Houston, TX. RT2 real time SYBR Green/ROX PCR Master Mix was obtained from SuperArray Biosciences, Frederick, MD. The primers to detect miR-214, U6 (for normalization), mirVANATM qRT-PCR miRNA detection kit were obtained from Ambion, Austin, TX. Primers to detect human IGF-1R were purchased from Qiagen. Luciferase assay kit was purchased from Promega, Madison, WI. His-TOPO-CMV-miR-214 expression vector was kindly provided by Dr. J. Q. Cheng (H. Lee Moffitt Cancer Center, Tampa, FL). Scramble shRNA vector was obtained from Addgene (plasmid 1864). pMV4EBP-1μ (T35A, T45A, T69A, and S64A) and constitutively active mTOR (containing the following mutations in four amino acids: V2198A, L2216H, L2260P, and I2017T) plasmids have been described previously (24–26). PRAS40 T146A and shPRAS40 plasmids were purchased from Addgene. The 3′UTR-less IGF-1R expression vector was a kind gift from Dr. Douglas Yee (Masonic Cancer Center, University of Minnesota).
Cell Culture
The HK2 normal human proximal tubular epithelial cell has been described previously and grown in DMEM/F-12 (1:1) in the presence of 10% fetal bovine serum (27). The primary human renal proximal tubular epithelial cells (HRPTEC) were purchased from Lonza Inc., Allendale, NJ. These cells were cultured in renal epithelial cell growth medium containing 0.5% serum as suggested by the vendor (Lonza). The ACHN and 786-O renal carcinoma cells were obtained from American Type Culture Collection, Manassas, VA. These cells were grown in RPMI 1640 medium containing 10% fetal bovine serum in the presence of penicillin/streptomycin (28, 29). The A498 and RCC4 renal carcinoma cells were kindly provided by Dr. Karen Block (University of Texas Health Science Center at San Antonio). These cells were grown in DMEM in the presence of 10% fetal bovine serum. At near confluence, the cells were washed and incubated with serum-free medium for 18 h prior to addition of IGF-1 as indicated.
DNA Synthesis and Cell Proliferation Assay
IGF-1 was added to the serum-starved cells at the indicated concentration for 20 h. DNA synthesis was determined by incorporation of [3H]thymidine into trichloroacetic acid-insoluble material as described (30, 31). For proliferation assay, the cells were trypsinized after the indicated incubation period and counted in a hemocytometer as described (32).
Immunoblot Analysis
The cells were lysed in RIPA buffer (20 mm Tris-HCl, pH 7.5, 1% Nonidet P-40, 150 mm NaCl, 5 mm EDTA, 1 mm Na3VO4, 1 mm PMSF, and 0.1% protease inhibitor mixture) at 4 °C for 30 min. The cell debris was pelleted at 10,000 × g for 30 min, and protein concentration was determined for the supernatant with Bio-Rad reagent. Equal amounts of proteins present in the cell extracts were separated by SDS-PAGE. The proteins were transferred to PVDF membrane and immunoblotted with the indicated antibodies as described previously (28, 29).
Secondary Structure Prediction for the MicroRNA Target Site
The 3′UTR of IGF-1R mRNA was analyzed to identify a possible miR-214 recognition element using TargetScan prediction algorithm. The secondary structure for the duplex formation between mature miR-214 and its recognition element in the 3′UTR of IGF-1R mRNA was predicted using the RNA hybrid program (33).
Real Time Quantitative RT-PCR
Total RNA was prepared from cells using TRIzol reagent as described previously (34, 35). 1 μg of RNA was used as template to synthesize cDNA using the mirVana qRT-PCR kit according to the vendor's instruction. qRT-PCR was performed in a real time PCR machine (7900HT, Applied Biosystems). The cycling temperatures and times were as follows: 94 °C for 10 min, followed by 40 cycles at 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s. The primers for detection of pre-miR-214 were as follows: forward primer, 5′-GGCCTGGCTGGACAGAGTTG-3′, and reverse primer, 5′-AGGCTGGGTTGTCATGTGAC-3′. The pri-miR-214 primers are as follows: forward, 5′-ACAGGCTGATTGTATCTGTC-3′, and reverse, 5′-GTAGATGCTATGGTGTGAGG-3′. The cycling temperatures and times for amplifying IGF-1R mRNA were as follows: 94 °C for 10 min, followed by 40 cycles at 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s.
Construction of IGF-1R-3′UTR-Luc Reporter Plasmid
The complementary DNA sequence flanking the miR-214 recognition element in the 3′UTR of IGF-1R mRNA was PCR-amplified using primers containing SpeI and HindIII restriction enzyme sites. The forward and reverse primers were 5′-ACGACTAGTTGAAATTTACAAAGGGCC-3′ and 5′-CATAAGTTGGAGGCAGGGCAGGGAAG-3′, respectively. The DNA fragment was cloned into the pCR2.1 vector. The identity of the PCR product was verified by direct sequencing. Finally, the SpeI/HindIII fragment from the pCR2.1 vector was subcloned into the SpeI/HindIII sites of pMIR-Report luciferase vector downstream of the luciferase cDNA (IGF-1R 3′UTR-Luc) (Fig. 2B).
FIGURE 2.
miR-214 binds to its microRNA recognition element in the IGF-1R 3′UTR. A, sequence complementarity between miR-214 and its target site in the 3′UTR of human IGF-1R. Arrows indicate the mutated bases in the seed binding sequence in the 3′UTR of IGF-1R. B, schematic showing the reporter construct containing the IGF-1R 3′UTR downstream of firefly luciferase gene. The sequence shows the region of binding of miR-214. Underlined sequence shows the seed-binding site. C, expression of miR-214 in the ACHN and 786-O renal cancer cells. The cells were transfected with CMV miR-214 expression vector. Total RNAs were used to detect mature miR-214 by qRT-PCR as described under “Experimental Procedures.” D, specific interaction of miR-214 with its microRNA recognition element in the IGF-1R 3′UTR. Wild type and the miR-214 seed binding mutant IGF-1R 3′UTR (A) luciferase constructs were transfected into ACHN and 786-O cells along with CMV miR-214 expression vector. Luciferase activity was determined in the cell lysates as described under “Experimental Procedures.” Mean of six measurements is shown. *, p < 0.001 versus the vector for the wild type reporter plasmid. E, CMV miR-214 expression plasmid or scramble (Scr) vector was transfected into ACHN, 786-O, A498, and RCC4 renal carcinoma cells. Total RNAs were prepared, and expression of IGF-1R mRNA was detected by real time qRT-PCR as described under “Experimental Procedures.” Mean ± S.E. of four measurements is shown. For ACHN *, p = 0.0006 versus Scr vector. For 786-O, *, p = 0.005 versus Scr vector. For A498 and RCC4, *, p = 0.004 versus Scr vector. F, CMV miR-214 or scramble (Scr) vector was transfected into three wells of ACHN, 786-O, A498, and RCC4 cells. The cell lysates were immunoblotted with IGF-1R and actin antibodies.
Site-directed Mutagenesis
Four bases in the seed sequence of miR-214 recognition element in the IGF-1R 3′UTR-Luc were mutated using QuikChange II site-directed mutagenesis kit as described (36). The mutation was verified by sequencing the DNA. The wild type miR-214 recognition element and its mutated bases are shown in Fig. 2A.
Transient Transfection Assay
Renal cancer cells were transfected with the indicated plasmid DNAs using FuGENE HD as described previously (27–29). For immunoblotting experiments, 16 h post-transfection the cells were serum-starved for 18 h prior to addition of 100 ng/ml IGF-1.
Luciferase Assay
The renal cancer cells were transfected with the reporter plasmid along with the vector and expression plasmid as indicated. The cell lysates were used to determine luciferase activity using a luciferase assay kit as described previously (28, 29). The data are presented as mean of luciferase activity/μg of protein as arbitrary units ± S.E. as described previously (28, 29).
Statistics
Analysis of variance followed by Student-Newman-Keuls analysis was used to determine the significance of the data. Where necessary paired t test was used to analyze data. p value less than 0.05 was considered as significant.
Results
Reduced miR-214 Levels Correlate with High IGF-1R Expression
Enhanced IGF-1R signaling has been reported in RCC in human and in animal models (6–11). Because the expression of IGF-1R may not correlate with the VHL status of renal carcinoma cells, we used VHL-positive ACHN and VHL-negative 786-O clear cell renal carcinoma cell lines. Levels of IGF-1R protein in both these cell lines were high as compared with that in HK2, a normal human proximal tubular epithelial cell line (Fig. 1A, left panel). This elevated expression of IGF-1R was associated with the autophosphorylation of its β-subunit (Fig. 1A, left panel), indicating receptor activation. Two other renal cancer cell lines, A498 and RCC4, also showed increased IGF-1R levels when compared with HK2 cells (Fig. 1A, middle panel). Similarly, when compared with another normal primary human proximal tubular epithelial cell, HRPTEC, the levels of IGF-1R and phospho-IGF-1R were higher (Fig. 1A, right panel). The presence of activated IGF-1R correlated with increased basal DNA synthesis and proliferation in all four RCC cell lines compared with the normal HK2 and HRPTEC cells (Fig. 1, B and C).
FIGURE 1.
Reciprocal expression of IGF-1R and miR-214 in renal cancer cells. A, equal amounts of lysates from normal proximal tubular epithelial HK2, primary HRPTEC and ACHN, 786-O, A498, and RCC4 renal cancer cells were immunoblotted with IGF-1R, phospho-IGF-R, and actin antibodies. B, [3H]thymidine incorporation was determined as described under “Experimental Procedures.” C, proliferation of indicated cells was determined by counting the cells as described under “Experimental Procedures.” D, predicted secondary structures of mature miR-214 and its target in the IGF-1R 3′UTR of human (left) and mouse (right) are shown. Minimum free energy (mfe) for the predicted structures is shown. E–J, total RNAs from HK2, HRPTEC, ACHN, 786-O, A498, and RCC4 cells were used in real time qRT-PCR to detect mature miR-214 (E and H), pre-miR-214 (F and I), and pri-miR-214 (G and J) as described under “Experimental Procedures.” Mean ± S.E. of four measurements is shown. *, p < 0.01 versus HK2 in E–G. *, p < 0.001 versus HK2 or HRPTEC in H–J.
To examine the mechanism of increased expression of IGF-1R in renal cancer cells, we hypothesized that expression of specific miRNA may contribute to its regulation. In fact, 30% of the transcriptome is regulated by miRNAs, and 25% of miRNA recognition elements between human and mouse is highly conserved (16, 17, 37). We used prediction algorithm in TargetScan to mine the 3′UTR of IGF-1R mRNA for the miRNA recognition element. This analysis revealed the presence of an miR-214 recognition element, which is conserved in human and mouse IGF-1R mRNAs. RNA hybrid analysis showed that the predicted minimum free energy (ΔG) for binding of miR-214 with IGF-1R 3′UTR for human and mouse was comparable (human, −26.1 kcal/mol; mouse, −24.7 kcal/mol) (Fig. 1D). Furthermore, the predicted minimum free energies for the binding of seed sequence of miR-214 to IGF-1R 3′UTR of these species were identical and less than −6 kcal/mol. These data support the critical energy requirement for optimal repression of target protein expression (38). Therefore, we examined the expression of mature miR-214 in the normal HK2 and two renal cancer cell lines. Fig. 1E shows a robust reduction in miR-214 expression in ACHN and 786-O cells as compared with that in HK2 cells. Similarly, levels of pre-miR-214 were significantly decreased in the renal cancer cells (Fig. 1F). Because pre-miRs are processed from the primary transcripts (19), we tested the expression of pri-miR-214 in the renal cancer cells. As shown in Fig. 1G, the expression of pri-miR-214 was significantly decreased in the ACHN and 786-O renal carcinoma cells. Similarly, expression of mature, pre-, and pri-miR-214 was significantly lower in A498 and RCC4 when compared with HK2 and HRPTEC normal proximal tubular epithelial cells (Fig. 1, H–J). These data indicate that the expression of miR-214 in the renal cancer cells may be regulated at the transcriptional level.
miR-214 Down-regulates IGF-1R mRNA and Protein Expression
To study the role of miR-214 in IGF-1R expression, we cloned the 3′UTR of IGF-1R mRNA containing the predicted miR-214 recognition element (Fig. 2A) into the pMIR-Report vector downstream of luciferase gene (IGF-1R 3′UTR-Luc) (Fig. 2B). We examined the effect of miR-214 on the reporter activity of IGF-1R 3′UTR-Luc. Transfection of miR-214 into ACHN and 786-O renal carcinoma cells significantly increased the expression of this microRNA (Fig. 2C) and inhibited the luciferase activity of the reporter plasmid in both these cells (Fig. 2D). To determine the specificity of the miR-214 binding to its recognition element in the IGF-1R 3′UTR, we mutated four bases in miR-214 seed-binding site in the reporter construct (Fig. 2A, indicated by arrows). Effect of expression of miR-214 was tested on the luciferase activity. Mutant IGF-1R-Luc reporter construct showed no reduction in luciferase activity when cotransfected with the miR-214 expression vector (Fig. 2D). These results suggest that miR-214 targets the 3′UTR of IGF-1R with significant specificity. Next, we tested the effect of miR-214 on IGF-1R mRNA and protein expression. Expression of miR-214 significantly inhibited both the expression of IGF-1R mRNA and protein in ACHN, 786-O, A498, and RCC4 renal cancer cells (Fig. 2, E and F). These results conclusively demonstrate that miR-214 directly regulates the expression of IGF-1R in the renal carcinoma cells.
miR-214 Regulates IGF-1-stimulated Akt Kinase Activity in Renal Carcinoma Cells
Increased circulating IGF-1 is an independent prognostic marker in patients with renal cell carcinoma (9). Our results above showed that both ACHN and 786-O renal carcinoma cells possess increased levels of IGF-1R than normal proximal tubular epithelial cells (Fig. 1A). To examine the biological function of increased IGF-1R expression in renal cancer cells, we tested the effect of IGF-1 on these cells. IGF-1 increased the tyrosine phosphorylation of IGF-1R β-subunit in ACHN and 786-O renal cancer cells (Fig. 3A). Furthermore, IGF-1 significantly increased the DNA synthesis, which resulted in proliferation of these renal carcinoma cells in a dose-dependent manner (Fig. 3, B and C).
FIGURE 3.
miR-214 regulates IGF-1-stimulated Akt kinase phosphorylation for proliferation of ACHN and 786-O renal cancer cells. A, serum-starved ACHN and 786-O cells were incubated with 100 ng/ml IGF-1 for the indicated periods of time. The cell lysates were immunoblotted with phospho-IGF-1R and IGF-1R antibodies. B and C, ACHN and 786-O cells were incubated with indicated doses of IGF-1. [3H]Thymidine incorporation (B) and cell count (C) were determined as described under “Experimental Procedures.” Mean ± S.E. of four and six measurements is shown. *, p < 0.001 versus control; **, p < 0.001 versus control. D, ACHN and 786-O cells were incubated with 100 ng/ml IGF-1 for indicated periods of time. The cell lysates were immunoblotted with phospho-Akt (Ser-473 and Thr-308) and Akt antibodies. E, ACHN and 786-O cells were treated with 1 μm MK-2206 for 1 h prior to incubation with 100 ng/ml IGF-1 for 15 min. The cell lysates were immunoblotted with indicated antibodies. F and G, ACHN and 786-O cells were treated with 1 μm MK-2206 for 1 h prior to incubation with 100 ng/ml IGF-1. [3H]Thymidine incorporation (F) and cell number (G) were determined as described under “Experimental Procedures.” F, means ± S.E. of six measurements are shown; G, means ± S.E. of four measurements are shown. *, p < 0.001 versus control; **, p < 0.001 versus IGF-treated. H and I, ACHN and 786-O cells were transfected with CMV miR-214 or scramble (Scr) vector. Transfected cells were incubated with 100 ng/ml IGF-1 for 15 min. The cell lysates were immunoblotted with phospho-Akt, Akt (H) and phospho-GSK3β, GSK3β (I) and IGF-1R and actin antibodies as indicated. In parallel dishes transfected with CMV miR-214, expression of miR-214 was determined to demonstrate overexpression of miR-214 (data not shown).
IGF-1 is known to stimulate Akt kinase, which regulates many physiologic responses, including metabolism, survival, and proliferation of normal and cancer cells (39, 40). Incubation of ACHN and 786-O cells with IGF-1 increased phosphorylation of Akt at both Ser-473 and Thr-308 in a time-dependent manner (Fig. 3D). The Akt inhibitor MK-2206 significantly inhibited IGF-1-stimulated phosphorylation of Akt (Fig. 3E). Furthermore, MK-2206 significantly attenuated IGF-1-induced DNA synthesis and proliferation of renal carcinoma cells (Fig. 3, F and G). Next, we tested whether miR-214 affects IGF-1-stimulated Akt phosphorylation. Transfection of miR-214 expression vector into ACHN and 786-O renal cancer cells inhibited phosphorylation of Akt at Ser-473 and Thr-308 in response to IGF-1 (Fig. 3H). To confirm that enhanced phosphorylation of Akt is associated with an increase in its kinase activity, we tested phosphorylation of GSK3β, its substrate. IGF-1 increased phosphorylation of GSK3β in both ACHN and 786-O (Fig. 3I). Expression of miR-214 significantly decreased the phosphorylation of GSK3β in response to IGF-1 (Fig. 3I)). These results demonstrate involvement of miR-214 in activation of Akt kinase by IGF-1R in renal cancer cells.
miR-214 Inhibits IGF-1-stimulated PRAS40 Phosphorylation to Induce Renal Cancer Cell Proliferation
PRAS40 was originally identified as a substrate for Akt, which phosphorylates it at Thr-246 (41). More recently we and others have shown that phosphorylation of PRAS40 regulates protein synthesis leading to an increase in the size of normal renal cells (42–44). However, its role in renal cancer cells has not been investigated. Incubation of the two renal carcinoma cell lines with IGF-1 increased phosphorylation of PRAS40 in a time-dependent manner (Fig. 4A), corresponding to Akt phosphorylation (Fig. 3D). Use of MK-2206 to block Akt kinase activity showed inhibition of IGF-1-induced PRAS40 phosphorylation in ACHN and 786-O cells (Fig. 4B). Because Akt kinase regulates renal cancer cell proliferation (Fig. 3, F and G), we examined the role of PRAS40 phosphorylation in IGF-1-induced renal cancer cell proliferation. We used a mutant of PRAS40 where its Akt phosphorylation site Thr-246 was changed to alanine. Expression of the PRAS40 T246A mutant significantly prevented IGF-1-induced DNA synthesis in both ACHN and 786-O cells (Fig. 4C). We also counted the number of cells to determine cell proliferation directly. PRAS40T246A markedly reduced proliferation of both renal cancer cells (Fig. 4D). Phosphorylation of PRAS40 induces its inactivation (44). Therefore, to mimic its inactivation, we inhibited PRAS40 expression in renal cancer cells using shRNA, which targets PRAS40 mRNA (44). shRNA-mediated down-regulation of PRAS40 modestly but significantly increased DNA synthesis in ACHN and 786-O renal cancer cells, resulting in their proliferation (Fig. 4, E and F). However, inhibition of PRAS40 expression in the presence of IGF-1 was not sufficient to further increase the DNA synthesis as compared with that induced by IGF-1 alone (Fig. 4, E and F).
FIGURE 4.
miR-214 regulates IGF-1-induced phosphorylation of PRAS40, which contributes to proliferation of renal cancer cells. A, serum-starved ACHN and 786-O cells were incubated with 100 ng/ml IGF-1 for indicated periods of time. The cell lysates were immunoblotted with phospho-PRAS40 (Thr-246) and PRAS40 antibodies. B, ACHN and 786-O cells were treated with 1 μm MK-2206 for 1 h prior to incubation with 100 ng/ml IGF-1 for 15 min. The cell lysates were immunoblotted with phospho-PRAS40 (Thr-246) and PRAS40 antibodies. C–F, ACHN and 786-O cells were transfected with PRAS40 T246A mutant (C and D) or shRNA against PRAS40 (E and F). The transfected cells were incubated with 100 ng/ml IGF-1. [3H]Thymidine incorporation (C and E) and cell number (D and F) were determined as described under “Experimental Procedures.” C and E, means ± S.E. of six measurements are shown; D and F, means ± S.E. of three measurements are shown. C and D, *, p < 0.001 versus control; **, p < 0.001 versus IGF-1-stimulated. E, *, p < 0.001 versus control; **, p < 0.05 versus control. F, *, p < 0.001 control; **, p < 0.001 between shPRAS40 and control. C and D, bottom panels show expression of the transfected HA-tagged mutant PRAS40, which results in inhibition of mTORC1 activity as judged by phosphorylation of S6 kinase. E and F, inhibition of expression of PRAS40 by its shRNA is shown, which results in increased phosphorylation of S6 kinase. G, ACHN and 786-O cells were transfected with CMV miR-214 or scramble (Scr) vector. Transfected cells were incubated with 100 ng/ml IGF-1 for 15 min. The cell lysates were immunoblotted with phospho-PRAS40, PRAS40, IGF-1R, and actin antibodies as described. G, expression of miR-214 was measured in parallel dishes transfected with the CMV miR-214 vector (data not shown).
Because miR-214 regulates the phosphorylation and activation of Akt, which in turn phosphorylates and inactivates PRAS40, we examined the effect of miR-214 on PRAS40 phosphorylation. Expression of miR-214 blocked IGF-1-induced phosphorylation of PRAS40 in ACHN and 786-O renal cancer cells (Fig. 4G). These results suggest that miR-214 regulates IGF-1-stimulated phosphorylation of PRAS40 at Thr-246 in renal cancer cells. Furthermore, our data for the first time demonstrate that phosphorylation of PRAS40 in response to IGF-1 contributes to proliferation of renal carcinoma cells.
miR-214 Blocks IGF-1-stimulated mTORC1 Activity
PRAS40 is a negative regulator of the growth factor and nutrient sensor kinase mTORC1. In fact, PRAS40 is a component of the mTORC1 (44). Akt-mediated phosphorylation of PRAS40 at Thr-246 induces its release from mTORC1, resulting in activation of mTORC1 kinase activity (40, 44, 45). Because IGF-1 increased phosphorylation of PRAS40 at Thr-246, we tested the effect of IGF-1 on activation of mTORC1 in ACHN and 786-O renal carcinoma cells. Phosphorylation of S6 kinase at Thr-389 was used as a readout for mTORC1 activity (45, 46). IGF-1 increased phosphorylation of S6 kinase in a time-dependent manner in both renal cancer cells (Fig. 5A). It was reported that mTORC1-activated S6 kinase phosphorylates mTOR at Ser-2448 (47, 48). Therefore, we tested the phosphorylation at this site in response to IGF-1. As shown in Fig. 5B, IGF-1 increased phosphorylation of mTOR in a time-dependent fashion. Expression of miR-214 significantly inhibited the IGF-1-stimulated phosphorylation of S6 kinase and mTOR (Fig. 5, C and D). These results indicate that miR-214 regulates IGF-1-induced activation of mTORC1 in ACHN and 786-O renal tumor cells.
FIGURE 5.
miR-214 regulates IGF-1-induced mTORC1 activation in renal cancer cells. A and B, ACHN and 786-O cells were incubated with 100 ng/ml IGF-1 for indicated periods of time. The cell lysates were immunoblotted with phospho-S6 kinase (Thr-389), S6 kinase, phospho-mTOR (Ser-2448), and mTOR antibodies. C and D, ACHN and 786-O cells were transfected with CMV miR-214 or scramble (Scr) vector. Transfected cells were incubated with 100 ng/ml IGF-1 for 15 min. The cell lysates were immunoblotted with phospho-S6 kinase, S6 kinase (C), and phospho-mTOR and mTOR (D), and IGF-1R and actin antibodies as indicated. Expression of miR-214 was examined in parallel dishes after transfection of CMV miR-214 (data not shown).
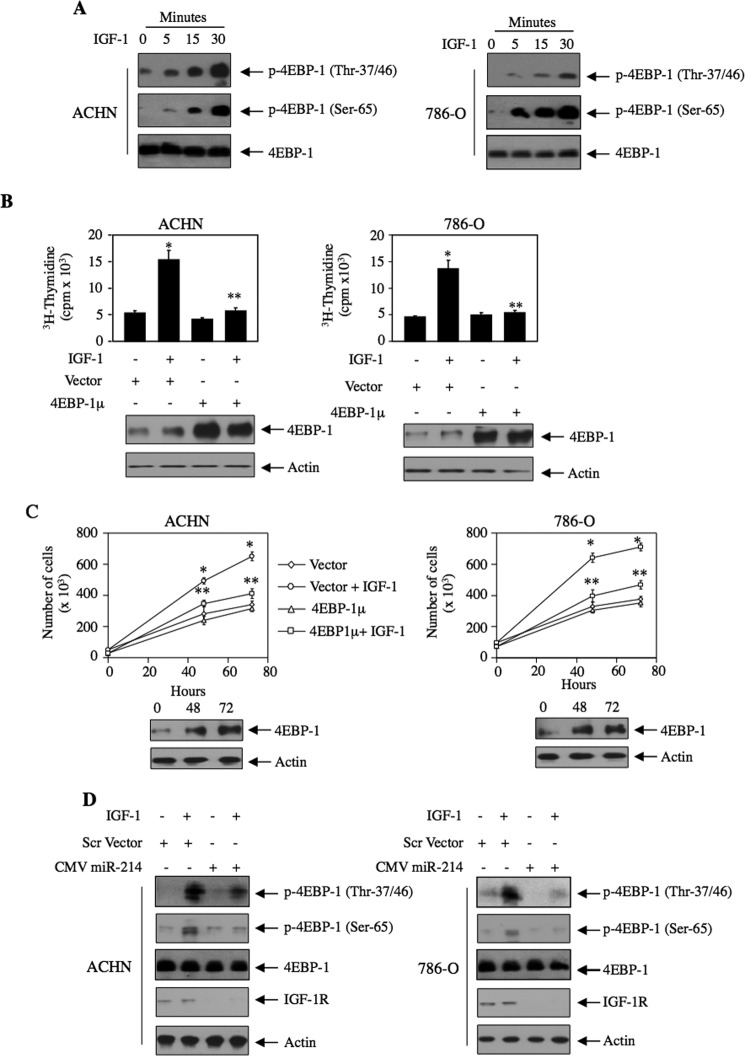
Next, we tested the phosphorylation of another substrate of mTORC1, the mRNA translation initiation repressor 4EBP-1. Incubation of both ACHN and 786-O renal cancer cells with IGF-1 increased phosphorylation of 4EBP-1 at Thr-37/46 and Ser-65, sites known to be phosphorylated by mTORC1, in a time-dependent manner (Fig. 6A). Recently, Dowling et al. (49) has shown that 4EBP-1 as substrate of mTORC1 contributes to proliferation of fibroblasts. We tested its role in proliferation of renal cancer cells using a mutant in which all the phosphorylation sites for mTORC1 are changed to alanine in 4EBP-1 (4EBP-1μ). Expression of 4EBP-1μ inhibited IGF-1-stimulated DNA synthesis in both ACHN and 786-O renal cancer cells (Fig. 6B). Similarly, 4EBP-1μ significantly prevented proliferation of both these cells (Fig. 6C). To examine the role of miR-214 in phosphorylation of 4EBP-1, we transfected miR-214 into these renal tumor cells followed by incubation with IGF-1. Expression of miR-214 markedly blocked the phosphorylation of 4EBP-1 at Thr-37/46 and Ser-65 in response to IGF-1 (Fig. 6D). Together, our data suggest that miR-214 inhibits mTORC1 signal transduction initiated by the IGF-1-induced phosphorylation of PRAS40.
FIGURE 6.
miR-214 regulates IGF-stimulated phosphorylation of 4EBP-1 that contributes to renal cancer cell proliferation. A, ACHN and 786-O renal cancer cells were incubated with 100 ng/ml IGF-1 for indicated periods of time. The cell lysates were immunoblotted with phospho-4EBP-1 (Thr-37/46 and Ser-65) and 4EBP-1 antibodies. B and C, ACHN and 786-O cells were transfected with 4EBP-1μ in which all four mTORC1 phosphorylation sites were mutated to alanine. Transfected cells were incubated with IGF-1. [3H]Thymidine incorporation (B) and cell number (C) were determined as described under “Experimental Procedures.” B, mean ± S.E. of six measurements is shown. *, p < 0.001 versus control; **, p < 0.001 versus IGF-1-treated. C, mean ± S.E. of triplicate measurements is shown. *, p < 0.001 versus control; **, p < 0.01 versus IGF-1-treated at corresponding time. The bottom panels in B and C show the expression of 4EBP-1. D, ACHN and 786-O cells were transfected with CMV miR-214 or scramble (Scr) vector. The transfected cells were incubated with 100 ng/ml IGF-1 for 15 min. The cell lysates were immunoblotted with phospho-4EBP-1, 4EBP-1, IGF-1R, and actin antibodies as indicated. miR-214 expression was examined in parallel dishes transfected with CMV miR-214 (data not shown).
miR-214 Prevents IGF-1-stimulated Renal Cancer Cell Proliferation by Targeting IGF-1R
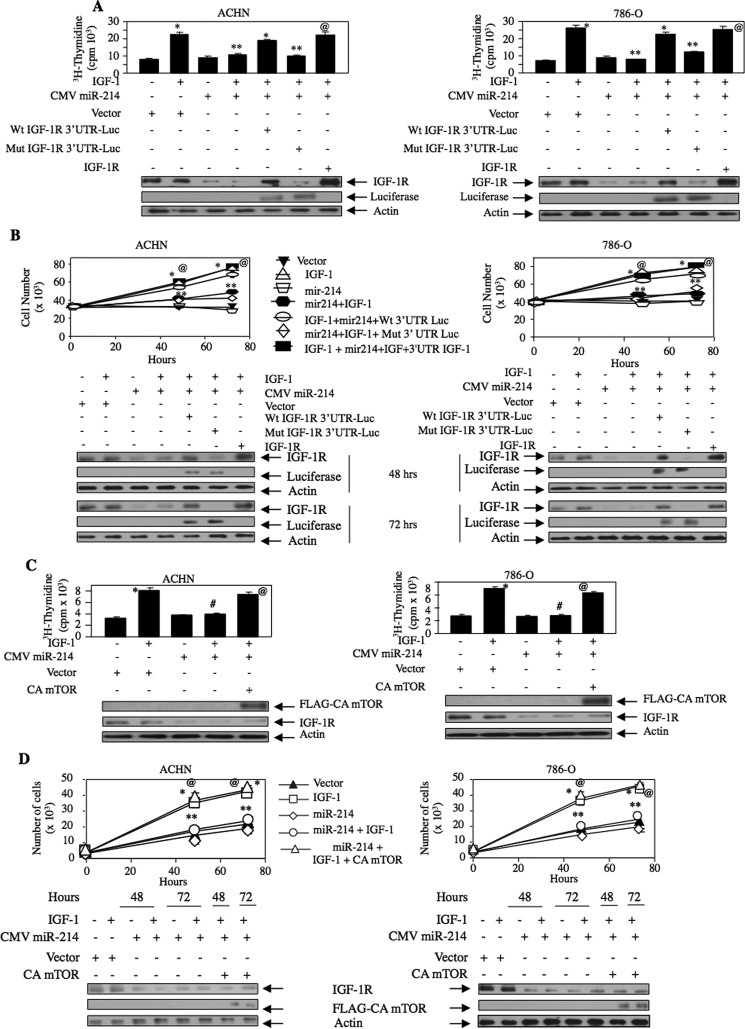
The results described above demonstrate that miR-214 regulates the IGF-1R-stimulated Akt/mTORC1 signaling axis, which contributes to proliferation of renal cancer cells. We tested the hypothesis that miR-214-targeted IGF-1R regulates this proliferation. Expression of miR-214 significantly attenuated IGF-1-induced DNA synthesis and proliferation of ACHN and 786-O renal cancer cells (Fig. 7, A and B). To examine the role of IGF-1R in miR-214 action, we used a plasmid vector that expresses IGF-1R mRNA without its 3′UTR, thus eliminating the inhibitory effect of miR-214. As a control to eliminate the overexpression artifact, we used the wild type and miR-214 recognition element mutant IGF-1R 3′UTR-Luc reporter plasmids (Fig. 2, A and B). Expression of the wild type reporter plasmid quenches the overexpressing miR-214 and thus nullifies the effect of miR-214 on endogenous IGF-1R expression and maintains its level (Fig. 7A, bottom panel, 5th lane). However, expression of the mutant reporter does not quench miR-214. Therefore, overexpressed miR-214 inhibits expression of endogenous IGF-1R (Fig. 7A, bottom, 6th lane). Thus, expression of the wild type reporter plasmid along with miR-214 showed increased DNA synthesis similar to IGF-1 alone (Fig. 7A compare 5th bar with 2nd bar). In contrast, the expression of the mutant reporter along with miR-214 inhibited IGF-1-induced DNA synthesis similar to the effect found with miR-214 (Fig. 7A, compare 6th bar with 4th bar). Importantly, expression of the 3′UTR less IGF-1R significantly reversed the miR-214-mediated inhibition of IGF-1-induced DNA synthesis (Fig. 7A, compare 7th bar with 4th bar). Similarly, expression of IGF-1R significantly blocked the miR-214-induced suppression of IGF-1-induced proliferation of renal carcinoma cells (Fig. 7B). These results suggest that miR-214 targets IGF-1R to regulate IGF-1-stimulated renal cancer cell proliferation.
FIGURE 7.
miR-214-targeted IGF-1R regulates proliferation of renal cancer cells via mTORC1. ACHN and 786-O cells were transfected with CMV miR-214 along with wild type IGF-1R 3′UTR Luc or mutant IGF-1R 3′UTR Luc or 3′UTR less IGF-1R plasmid (A and B). [3H]Thymidine incorporation (A) and for cell number (B) were determined. Mean ± S.E. of triplicate measurements is shown. A, *, p < 0.001 versus unstimulated; **, p < 0.001 versus IGF-1-treated; @, p < 0.001 versus miR-214 plus IGF-1-treated. B, 48 and 72 h, p < 0.001 versus unstimulated; **, p < 0.001 versus IGF-1-treated; @, p < 0.001 versus miR-214 plus IGF-1-treated. Bottom panel shows expression of IGF-1R and luciferase. C and D, ACHN and 786-O cells were transfected with CMV miR-214 and constitutively active mTORC1 plasmid as indicated. The transfected cells were incubated with 100 ng/ml IGF-1. [3H]Thymidine incorporation (C) and cell number (D) were determined as described under “Experimental Procedures.” Mean ± S.E. of four measurements is shown. C, *, p < 0.01 versus control; #, p < 0.01 versus IGF-1-treated; @, p < 0.01 versus miR-214 plus IGF-treated. D, mean ± S.E. of four measurements is shown. *, p < 0.001 versus control; **, p < 0.001 versus IGF-1-treated for corresponding time points; @, p < 0.001 versus miR-214 plus IGF-1-treated. Bottom panels show expression of constitutively active (CA) mTOR and IGF-1R.
We have shown above that miR-214 inhibits the mTORC1 activity in response to IGF-1 (Figs. 5, C and D, and 6D). Therefore, we determined the role of this kinase in miR-214-regulated renal cancer cell proliferation. As expected, miR-214 inhibited IGF-1-induced DNA synthesis and proliferation of ACHN and 786-O renal cancer cells (Fig. 7, C and D). Interestingly, expression of a constitutively active mutant of mTORC1 significantly reversed the miR-214-induced inhibition of IGF-1-stimulated DNA synthesis and proliferation of the two renal carcinoma cell lines (Fig. 7, C and D). These results conclusively indicate a direct role of miR-214 in IGF-1R-mediated mTORC1 activation and proliferation of renal cancer cells by IGF-1.
Discussion
Complex interactions between the transcriptional program and protein expression contribute to the development of clear cell RCC. Inadequate therapeutic options for RCC necessitate identification of specific molecular targets, which can be utilized to prevent progression of this cancer. In this study, we identified IGF-1R as a target of miR-214. We find that reduced expression of miR-214 contributes to the increased IGF-1R expression in renal cancer cells regardless of VHL status. We show that expression of miR-214 inhibits the Akt/mTORC1 axis in the IGF-1R signaling cascade to prevent renal cancer cell proliferation.
Accumulating reports show expression profiling of multiple miRNAs in renal cancer. A recent microarray screen for identification of aberrantly expressed miRNAs in renal cell carcinoma samples showed 38 and 48 miRNAs to be up- and down-regulated, respectively (50). However, experimental validation of only a few miRNAs with their target mRNAs has been reported. VHL deficiency causes increased HIF1α and HIF2α expression of which the latter contributes mainly to the development of sporadic RCC (51). However, HIF-independent function has also been reported in VHL deficiency (52). For example, a recent study in renal cancer cells demonstrated the regulation of various miRNAs in a VHL-dependent manner (53). However, some of these miRNAs were HIF-dependent, although others were not.
IGF-1R can act as an oncogene when expressed in high levels in NIH 3T3 fibroblasts (54). Murine embryonic fibroblasts nullizygous for the IGF-1R gene are incapable of transformation by multiple oncogenes, including Ha-Ras and v-Src (55, 56). Apart from VHL deficiency, IGF-1R-mediated autocrine/paracrine signal transduction has been reported as an independent prognostic factor for development and progression of metastatic RCC (8, 9, 57). In line with these results, we show increased expression of IGF-1R in both VHL-positive ACHN and VHL-negative 786-O, RCC4, and A498 renal cancer cells (Fig. 1A). In breast cancer and in melanoma, amplification of the IGF-1R gene in 15q26 has been reported (58). Transcriptional mechanism is established for increased expression of IGF-1R in many cancers, including renal cancer (59). Ewing sarcoma-WT1 fusion oncoprotein and Sp1 increase transcription of IGF-1R (15, 60, 61). However, tumor suppressor proteins such as p53, PTEN, and BRCA1 inhibit expression of IGF-1R (15, 62). Translational regulation of IGF-1R is also reported. The long 5′UTR of IGF-1R is a target of the RNA-binding protein HuR, which delays cap-dependent translation and inhibits the internal ribosome entry site-containing IGF-1R translational block (63, 64). In contrast, heterogeneous nuclear ribonucleoprotein C has been shown to interact with IGF-1R 5′UTR at the site of HuR binding to promote internal ribosome entry site-mediated translation of IGF-1R (65). Recently, four miRNAs, miR-7, miR-192, miR-215, and miR-145, have been identified, which repress the expression of IGF-1R by post-transcriptional mechanism in tongue squamous cell carcinoma, multiple myeloma, and VHL-deficient RCC (66–68). In this study, we identified miR-214 to target 3′UTR of IGF-1R mRNA (Fig. 2). miR-214 is increased in many cancers, including pancreatic cancer, oral squamous cell carcinoma, and malignant melanoma (69–71). In tongue squamous cell carcinoma and ovarian cancer, enhanced expression of miR-214 is associated with cisplatin resistance (72, 73). Although aberrant expression of many miRNAs has been identified in RCC, we demonstrate significant down-regulation of miR-214 in VHL-positive and VHL-deficient renal cancer cells compared with normal proximal tubular epithelial cells (Fig. 1, E--J). To our knowledge this is the first demonstration in RCC of down-regulation of miR-214, which increases the protein abundance of IGF-1R.
Enhanced phosphorylation of Akt is often detected in samples of RCC in the absence of activating mutation in Akt itself and in its upstream regulator phosphatidylinositol 3-kinase (74, 75). Lack of or reduced PTEN protein levels also increases phosphorylation of Akt, which causes prostate intraepithelial neoplasia, glioblastoma, and endometrial cancer (76). Although mutation in the PTEN gene in RCC has been reported, it is not common, and its expression is also heterogeneous in different renal cancer cells (77, 78). One mechanism of Akt kinase activation in RCC could involve activation of growth factor receptors (14, 15). Our results demonstrate that both ACHN and 786-O renal cancer cells irrespective of their VHL status possess increased levels of IGF-1R compared with the normal kidney epithelial cells (Fig. 1A). This enhanced expression of IGF-1R is associated with increased sensitivity to IGF-1, resulting in activation of Akt kinase, leading to proliferation of renal cancer cells (Fig. 3, A–G). Because increased IGF-1R levels were due to reduced miR-214 (Figs. 1 and 2), exogenous expression of miR-214 inhibited IGF-1-induced Akt kinase phosphorylation and its activation (Fig. 3, H and I).
In the renal cancer cells, we demonstrate that phosphorylation of PRAS40 is mediated by IGF-1R-stimulated Akt kinase (Fig. 4, A and B). Mutation in the Drosophila Lobe protein, the ortholog of mammalian PRAS40, results in hypoactive mTORC1, indicating that Drosophila PRAS40 positively regulates mTORC1 activity (79). In contrast, in mammalian cells, PRAS40 negatively regulates mTORC1 (46). Our data show that phosphorylation of PRAS40 at Thr-246 (Fig. 4A), which results in its inactivation (44), significantly increased the mTORC1 kinase activity as judged by the phosphorylation of S6 kinase and 4EBP-1 (Figs. 5A and 6A). Also, phospho-deficient mutant of PRAS40 blocked IGF-induced proliferation (Fig. 4, C and D). Furthermore, our results demonstrate that PRAS40 negatively regulates IGF-1-induced proliferation of both VHL-positive and -negative renal cancer cells (Fig. 4, E and F). Interestingly, because we found IGF-1-induced Akt activation was sensitive to miR-214, we demonstrated that expression of miR-214 significantly inhibited phosphorylation of PRAS40 in response to IGF-1 (Fig. 4G).
In Drosophila, both S6 kinase and 4EBP-1 regulate cell growth and proliferation (80, 81). However, a recent report established a significant contribution of 4EBP-1 in mammalian cell proliferation. mTORC1-mediated phosphorylation of 4EBP-1 releases eIF4E, which forms the eIF4F complex and regulates translation of a subset of mRNAs necessary for cell proliferation, including VEGF (82–84). Increased VEGF expression in VHL-deficient RCC is mainly mediated by stabilized Hif2α-mediated transcription (51). However, the VEGF protein level is regulated by 4EBP-1-sensitive eIF4E-mediated cap-dependent translation of its mRNA (82, 83). Interestingly, Dowling et al. (49) reported that in 4EBP-deficient murine embryonic fibroblasts, inhibition of mTORC1 had no suppressive effect on the eIF4E-sensitive translation of VEGF mRNA, indicating a significant role of 4EBP in mTORC1-mediated translation of this pro-tumorigenic protein. Furthermore, mTOR inhibitors did not block proliferation of 4EBP-deficient mouse embryo fibroblasts (49). The contribution of 4EBP-1 in the proliferative response of renal cancer cells has not been reported. In ACHN and 786-O renal cancer cells, we demonstrate that the phosphorylation-deficient constitutively active mutant of 4EBP-1 inhibits IGF-1R-stimulated proliferation (Fig. 6, B and C). mTORC1-mediated phosphorylation of 4EBP-1 leads to its inactivation. Thus, inhibition of its phosphorylation promotes its inhibitory activity. We show reduced IGF-1-induced phosphorylation of 4EBP-1 by miR-214, rendering this translational repressor at its active state (Fig. 6D). These data indicate that miR-214-mediated activation of 4EBP-1 may serve as a useful target for prevention of RCC proliferation.
In vitro studies demonstrated that inhibition of mTOR kinase activity blocked expression of Hif1α and Hif2α regardless of cellular oxygen levels (85). Trials using various structural derivatives of rapamycin for a variety of solid tumors, including RCC, have shown limited efficacy. An earlier phase II trial using multiple doses of temsirolimus with metastatic RCC patients showed an overall response rate of 7% (86). In a subsequent phase III trial, temsirolimus was compared with interferon-α alone and in combination. Temsirolimus alone showed significant progression-free and overall survival than that compared with interferon-α alone. But the patients with combination therapy did not show any significant difference when compared with the interferon group alone (2). Furthermore, use of everolimus in a multicenter phase III trial with metastatic RCC patients who progressed on VEGF receptor inhibitors showed a significant progression-free survival versus the placebo arm (87). On the basis of these trials and few others, the Food and Drug Administration approved temsirolimus and everolimus for metastatic RCC (2, 87). However, significant adverse events may limit their therapeutic efficacy (2, 87–89). Furthermore, enhanced mTORC1 activity elicits a negative feedback loop via S6 kinase-mediated phosphorylation of IRS-1 at Ser-307, which inhibits IGF-1R-stimulated phosphorylation of Akt (90, 91). Therefore, inhibition of mTORC1 alone could disrupt this negative feedback loop resulting in increased Akt phosphorylation, which can lead to maintenance of the malignant state. In fact, this feedback loop has been ascribed to be the cause for resistance to mTORC1 inhibitors in many solid tumors.
Because elevated IGF-1R signal transduction causes proliferation and survival of cancer cells, targeting this receptor has been an attractive mode to treat patients with variety of solid tumors. Several clinical trials used IGF-1R-specific antibody therapy. However, due to the presence of hybrid receptors containing IGF-1R and insulin receptor, the down-regulation of the receptor molecules resulted in adverse events in many studies, including hyperglycemia (14). Other preclinical strategies involved development of tyrosine kinase inhibitors. Due to 95% sequence homology in the ATP binding domains of IGF-1R and insulin receptor, it has been a challenge to develop specific tyrosine kinase inhibitors for IGF-1R (14). In this study, we have identified an miRNA, miR-214, which acts as an endogenous inhibitor of IGF-1R protein expression in renal cancer cells. We have shown that miR-214 expression is significantly reduced in VHL-deficient as well as VHL-positive renal cancer cells. Furthermore, expression of miR-214 in renal cancer cells significantly prevents the signal transduction pathways of IGF-1R, including Akt kinase and mTORC1. Finally, we demonstrate that miR-214 significantly blocks IGF-1-induced proliferation of both VHL-positive and -negative renal cancer cells by directly targeting IGF-1R that uses mTORC1 (Fig. 7). Therefore, use of miR-214 may represent an attractive therapy to test in preclinical models of renal cancer. Because mTORC1 inhibitors and tyrosine kinase inhibitors exhibit adverse events, it will be an added benefit to test the efficacy of combinatorial use of miR-214 with low dose mTORC1 inhibitors or with tyrosine kinase inhibitors in preclinical animal models of RCC.
Author Contributions
F. D., N. D., N. G. C., and A. B. performed the experiments. N. G. C. and B. S. K. provided intellectual input and corrected the manuscript. G. G. C. designed the experiments, prepared the figures, and wrote the manuscript.
This work was supported in part by the Veterans Affairs Research Service Merit Review Grant 2 I01 BX000926 and National Institutes of Health Grant RO1 DK50190 (to G. G. C.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We dedicate this paper to Dr. Hanna E. Abboud, who unexpectedly left us on January 7, 2015. His continued encouragement and support were vital for the completion of this work.
- RCC
- renal cell carcinoma
- VHL
- von Hippel-Lindau
- IGF-1R
- insulin-like growth factor-1 receptor
- miR
- microRNA
- HRPTEC
- human proximal tubular epithelial cell
- qRT
- quantitative RT
- mTOR
- mechanistic target of rapamycin
- pri-miR
- primary miR.
References
- 1.Karumanchi S. A., Merchan J., and Sukhatme V. P. (2002) Renal cancer: molecular mechanisms and newer therapeutic options. Curr. Opin. Nephrol. Hypertens. 11, 37–42 [DOI] [PubMed] [Google Scholar]
- 2.Hudes G., Carducci M., Tomczak P., Dutcher J., Figlin R., Kapoor A., Staroslawska E., Sosman J., McDermott D., Bodrogi I., Kovacevic Z., Lesovoy V., Schmidt-Wolf I. G., Barbarash O., Gokmen E., et al. (2007) Temsirolimus, interferon α, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 356, 2271–2281 [DOI] [PubMed] [Google Scholar]
- 3.Rouvière O., Bouvier R., Négrier S., Badet L., and Lyonnet D. (2006) Nonmetastatic renal-cell carcinoma: is it really possible to define rational guidelines for post-treatment follow-up? Nat. Clin. Pract. Oncol. 3, 200–213 [DOI] [PubMed] [Google Scholar]
- 4.McDermott D. F., Regan M. M., Clark J. I., Flaherty L. E., Weiss G. R., Logan T. F., Kirkwood J. M., Gordon M. S., Sosman J. A., Ernstoff M. S., Tretter C. P., Urba W. J., Smith J. W., Margolin K. A., Mier J. W., et al. (2005) Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 23, 133–141 [DOI] [PubMed] [Google Scholar]
- 5.Negrier S., Escudier B., Lasset C., Douillard J. Y., Savary J., Chevreau C., Ravaud A., Mercatello A., Peny J., Mousseau M., Philip T., and Tursz T. (1998) Recombinant human interleukin-2, recombinant human interferon α-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d'Immunotherapie. N. Engl. J. Med. 338, 1272–1278 [DOI] [PubMed] [Google Scholar]
- 6.Rosendahl A., and Forsberg G. (2004) Influence of IGF-IR stimulation or blockade on proliferation of human renal cell carcinoma cell lines. Int. J. Oncol. 25, 1327–1336 [PubMed] [Google Scholar]
- 7.Rosendahl A. H., Holly J. M., Celander M., and Forsberg G. (2008) Systemic IGF-I administration stimulates the in vivo growth of early, but not advanced, renal cell carcinoma. Int. J. Cancer 123, 1286–1291 [DOI] [PubMed] [Google Scholar]
- 8.Cheung C., Vesey D., Cotterill A., Douglas M., Gobe G., Nicol D., and Johnson D. (2005) Altered messenger RNA and protein expressions for insulin-like growth factor family members in clear cell and papillary renal cell carcinomas. Int. J. Urol. 12, 17–28 [DOI] [PubMed] [Google Scholar]
- 9.Rasmuson T., Grankvist K., Jacobsen J., Olsson T., and Ljungberg B. (2004) Serum insulin-like growth factor-1 is an independent predictor of prognosis in patients with renal cell carcinoma. Acta Oncol. 43, 744–748 [DOI] [PubMed] [Google Scholar]
- 10.Parker A. S., Cheville J. C., Blute M. L., Igel T., Lohse C. M., and Cerhan J. R. (2004) Pathologic T1 clear cell renal cell carcinoma: insulin-like growth factor-I receptor expression and disease-specific survival. Cancer 100, 2577–2582 [DOI] [PubMed] [Google Scholar]
- 11.Schips L., Zigeuner R., Ratschek M., Rehak P., Rüschoff J., and Langner C. (2004) Analysis of insulin-like growth factors and insulin-like growth factor I receptor expression in renal cell carcinoma. Am. J. Clin. Pathol. 122, 931–937 [DOI] [PubMed] [Google Scholar]
- 12.Parker A. S., Cheville J. C., Janney C. A., and Cerhan J. R. (2002) High expression levels of insulin-like growth factor-I receptor predict poor survival among women with clear-cell renal cell carcinomas. Hum. Pathol. 33, 801–805 [DOI] [PubMed] [Google Scholar]
- 13.Parker A., Cheville J. C., Lohse C., Cerhan J. R., and Blute M. L. (2003) Expression of insulin-like growth factor I receptor and survival in patients with clear cell renal cell carcinoma. J. Urol. 170, 420–424 [DOI] [PubMed] [Google Scholar]
- 14.Rodon J., DeSantos V., Ferry R. J. Jr., and Kurzrock R. (2008) Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol. Cancer Ther. 7, 2575–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakar S., Leroith D., and Brodt P. (2005) The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: Lessons from animal models. Cytokine Growth Factor Rev. 16, 407–420 [DOI] [PubMed] [Google Scholar]
- 16.Bartel D. P. (2009) MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartel D. P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 18.Couzin J. (2008) MicroRNAs make big impression in disease after disease. Science 319, 1782–1784 [DOI] [PubMed] [Google Scholar]
- 19.Fabian M. R., Sonenberg N., and Filipowicz W. (2010) Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79, 351–379 [DOI] [PubMed] [Google Scholar]
- 20.Grosshans H., and Büssing I. (2010) MicroRNA biogenesis takes another single hit from microsatellite instability. Cancer Cell 18, 295–297 [DOI] [PubMed] [Google Scholar]
- 21.Melo S. A., Moutinho C., Ropero S., Calin G. A., Rossi S., Spizzo R., Fernandez A. F., Davalos V., Villanueva A., Montoya G., Yamamoto H., Schwartz S. Jr., and Esteller M. (2010) A genetic defect in exportin-5 traps precursor microRNAs in the nucleus of cancer cells. Cancer Cell 18, 303–315 [DOI] [PubMed] [Google Scholar]
- 22.Gottardo F., Liu C. G., Ferracin M., Calin G. A., Fassan M., Bassi P., Sevignani C., Byrne D., Negrini M., Pagano F., Gomella L. G., Croce C. M., and Baffa R. (2007) Micro-RNA profiling in kidney and bladder cancers. Urol. Oncol. 25, 387–392 [DOI] [PubMed] [Google Scholar]
- 23.Jung M., Mollenkopf H. J., Grimm C., Wagner I., Albrecht M., Waller T., Pilarsky C., Johannsen M., Stephan C., Lehrach H., Nietfeld W., Rudel T., Jung K., and Kristiansen G. (2009) MicroRNA profiling of clear cell renal cell cancer identifies a robust signature to define renal malignancy. J. Cell. Mol. Med. 13, 3918–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das F., Ghosh-Choudhury N., Mahimainathan L., Venkatesan B., Feliers D., Riley D. J., Kasinath B. S., and Choudhury G. G. (2008) Raptor-rictor axis in TGFβ-induced protein synthesis. Cell. Signal. 20, 409–423 [DOI] [PubMed] [Google Scholar]
- 25.Das F., Ghosh-Choudhury N., Dey N., Mandal C. C., Mahimainathan L., Kasinath B. S., Abboud H. E., and Choudhury G. G. (2012) Unrestrained mammalian target of rapamycin complexes 1 and 2 increase expression of phosphatase and tensin homolog deleted on chromosome 10 to regulate phosphorylation of Akt kinase. J. Biol. Chem. 287, 3808–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohne Y., Takahara T., Hatakeyama R., Matsuzaki T., Noda M., Mizushima N., and Maeda T. (2008) Isolation of hyperactive mutants of mammalian target of rapamycin. J. Biol. Chem. 283, 31861–31870 [DOI] [PubMed] [Google Scholar]
- 27.Das F., Bera A., Ghosh-Choudhury N., Abboud H. E., Kasinath B. S., and Choudhury G. G. (2014) TGFβ-induced deptor suppression recruits mTORC1 and not mTORC2 to enhance collagen I (α2) gene expression. PLoS ONE 9, e109608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bera A., Das F., Ghosh-Choudhury N., Kasinath B. S., Abboud H. E., and Choudhury G. G. (2014) microRNA-21-induced dissociation of PDCD4 from rictor contributes to Akt-IKKβ-mTORC1 axis to regulate renal cancer cell invasion. Exp. Cell Res. 328, 99–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dey N., Das F., Ghosh-Choudhury N., Mandal C. C., Parekh D. J., Block K., Kasinath B. S., Abboud H. E., and Choudhury G. G. (2012) MicroRNA-21 governs TORC1 activation in renal cancer cell proliferation and invasion. PLoS ONE 7, e37366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bera A., Ghosh-Choudhury N., Dey N., Das F., Kasinath B. S., Abboud H. E., and Choudhury G. G. (2013) NFκB-mediated cyclin D1 expression by microRNA-21 influences renal cancer cell proliferation. Cell. Signal. 25, 2575–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choudhury G. G. (2001) Akt serine threonine kinase regulates platelet-derived growth factor-induced DNA synthesis in glomerular mesangial cells: regulation of c-fos AND p27(kip1) gene expression. J. Biol. Chem. 276, 35636–35643 [DOI] [PubMed] [Google Scholar]
- 32.Venkatesan B., Ghosh-Choudhury N., Das F., Mahimainathan L., Kamat A., Kasinath B. S., Abboud H. E., and Choudhury G. G. (2008) Resveratrol inhibits PDGF receptor mitogenic signaling in mesangial cells: role of PTP1B. FASEB J. 22, 3469–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rehmsmeier M., Steffen P., Hochsmann M., and Giegerich R. (2004) Fast and effective prediction of microRNA/target duplexes. RNA 10, 1507–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dey N., Das F., Mariappan M. M., Mandal C. C., Ghosh-Choudhury N., Kasinath B. S., and Choudhury G. G. (2011) MicroRNA-21 orchestrates high glucose-induced signals to TORC1 for renal cell pathology in diabetes. J. Biol. Chem. 286, 25586–25603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandal C. C., Ghosh-Choudhury N., Yoneda T., Choudhury G. G., and Ghosh-Choudhury N. (2011) Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44. J. Biol. Chem. 286, 11314–11327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahimainathan L., Ghosh-Choudhury N., Venkatesan B., Das F., Mandal C. C., Dey N., Habib S. L., Kasinath B. S., Abboud H. E., and Ghosh Choudhury G. (2009) TSC2 deficiency increases PTEN via HIF1α. J. Biol. Chem. 284, 27790–27798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C. I., Shen Y., and Tang T. (2009) Evolution under canalization and the dual roles of microRNAs: a hypothesis. Genome Res. 19, 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doench J. G., and Sharp P. A. (2004) Specificity of microRNA target selection in translational repression. Genes Dev. 18, 504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhaskar P. T., and Hay N. (2007) The two TORCs and Akt. Dev. Cell 12, 487–502 [DOI] [PubMed] [Google Scholar]
- 40.Manning B. D., and Cantley L. C. (2007) AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovacina K. S., Park G. Y., Bae S. S., Guzzetta A. W., Schaefer E., Birnbaum M. J., and Roth R. A. (2003) Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J. Biol. Chem. 278, 10189–10194 [DOI] [PubMed] [Google Scholar]
- 42.Burgos S. A., and Cant J. P. (2010) IGF-1 stimulates protein synthesis by enhanced signaling through mTORC1 in bovine mammary epithelial cells. Domest. Anim. Endocrinol. 38, 211–221 [DOI] [PubMed] [Google Scholar]
- 43.Dey N., Ghosh-Choudhury N., Das F., Li X., Venkatesan B., Barnes J. L., Kasinath B. S., and Ghosh Choudhury G. (2010) PRAS40 acts as a nodal regulator of high glucose-induced TORC1 activation in glomerular mesangial cell hypertrophy. J. Cell. Physiol. 225, 27–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sancak Y., Thoreen C. C., Peterson T. R., Lindquist R. A., Kang S. A., Spooner E., Carr S. A., and Sabatini D. M. (2007) PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell 25, 903–915 [DOI] [PubMed] [Google Scholar]
- 45.Wullschleger S., Loewith R., and Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 46.Zoncu R., Efeyan A., and Sabatini D. M. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiang G. G., and Abraham R. T. (2005) Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 280, 25485–25490 [DOI] [PubMed] [Google Scholar]
- 48.Holz M. K., and Blenis J. (2005) Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J. Biol. Chem. 280, 26089–26093 [DOI] [PubMed] [Google Scholar]
- 49.Dowling R. J., Topisirovic I., Alain T., Bidinosti M., Fonseca B. D., Petroulakis E., Wang X., Larsson O., Selvaraj A., Liu Y., Kozma S. C., Thomas G., and Sonenberg N. (2010) mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 328, 1172–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi Z., Fu Y., Zhao S., Zhang X., and Ma C. (2010) Differential expression of miRNA patterns in renal cell carcinoma and nontumorous tissues. J. Cancer Res. Clin. Oncol. 136, 855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaelin W. G., Jr. (2009) Treatment of kidney cancer: insights provided by the VHL tumor-suppressor protein. Cancer 115, 2262–2272 [DOI] [PubMed] [Google Scholar]
- 52.Young A. P., Schlisio S., Minamishima Y. A., Zhang Q., Li L., Grisanzio C., Signoretti S., and Kaelin W. G. Jr. (2008) VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat. Cell Biol. 10, 361–369 [DOI] [PubMed] [Google Scholar]
- 53.Neal C. S., Michael M. Z., Rawlings L. H., Van der Hoek M. B., and Gleadle J. M. (2010) The VHL-dependent regulation of microRNAs in renal cancer. BMC Med. 8, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaleko M., Rutter W. J., and Miller A. D. (1990) Overexpression of the human insulin-like growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol. Cell. Biol. 10, 464–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sell C., Dumenil G., Deveaud C., Miura M., Coppola D., DeAngelis T., Rubin R., Efstratiadis A., and Baserga R. (1994) Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol. Cell Biol. 14, 3604–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baserga R. (1995) The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 55, 249–252 [PubMed] [Google Scholar]
- 57.Yakar S., Pennisi P., Kim C. H., Zhao H., Toyoshima Y., Gavrilova O., and LeRoith D. (2005) Studies involving the GH-IGF axis: Lessons from IGF-I and IGF-I receptor gene targeting mouse models. J. Endocrinol. Invest. 28, 19–22 [PubMed] [Google Scholar]
- 58.Almeida A., Muleris M., Dutrillaux B., and Malfoy B. (1994) The insulin-like growth factor I receptor gene is the target for the 15q26 amplicon in breast cancer. Genes Chromosomes Cancer 11, 63–65 [DOI] [PubMed] [Google Scholar]
- 59.LeRoith D., and Roberts C. T. Jr. (2003) The insulin-like growth factor system and cancer. Cancer Lett. 195, 127–137 [DOI] [PubMed] [Google Scholar]
- 60.Werner H., Karnieli E., Rauscher F. J., and LeRoith D. (1996) Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc. Natl. Acad. Sci. U.S.A. 93, 8318–8323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Werner H., Re G. G., Drummond I. A., Sukhatme V. P., Rauscher F. J. 3rd, Sens D. A., Garvin A. J., LeRoith D., and Roberts C. T. Jr. (1993) Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc. Natl. Acad. Sci. U.S.A. 90, 5828–5832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maor S. B., Abramovitch S., Erdos M. R., Brody L. C., and Werner H. (2000) BRCA1 suppresses insulin-like growth factor-I receptor promoter activity: potential interaction between BRCA1 and Sp1. Mol. Genet. Metab. 69, 130–136 [DOI] [PubMed] [Google Scholar]
- 63.Meng Z., King P. H., Nabors L. B., Jackson N. L., Chen C. Y., Emanuel P. D., and Blume S. W. (2005) The ELAV RNA-stability factor HuR binds the 5′-untranslated region of the human IGF-IR transcript and differentially represses cap-dependent and IRES-mediated translation. Nucleic Acids Res. 33, 2962–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giraud S., Greco A., Brink M., Diaz J. J., and Delafontaine P. (2001) Translation initiation of the insulin-like growth factor I receptor mRNA is mediated by an internal ribosome entry site. J. Biol. Chem. 276, 5668–5675 [DOI] [PubMed] [Google Scholar]
- 65.Meng Z., Jackson N. L., Choi H., King P. H., Emanuel P. D., and Blume S. W. (2008) Alterations in RNA-binding activities of IRES-regulatory proteins as a mechanism for physiological variability and pathological dysregulation of IGF-IR translational control in human breast tumor cells. J. Cell. Physiol. 217, 172–183 [DOI] [PubMed] [Google Scholar]
- 66.Jiang L., Liu X., Chen Z., Jin Y., Heidbreder C. E., Kolokythas A., Wang A., Dai Y., and Zhou X. (2010) MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells. Biochem. J. 432, 199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gan B., Lim C., Chu G., Hua S., Ding Z., Collins M., Hu J., Jiang S., Fletcher-Sananikone E., Zhuang L., Chang M., Zheng H., Wang Y. A., Kwiatkowski D. J., Kaelin W. G. Jr., et al. (2010) FoxOs enforce a progression checkpoint to constrain mTORC1-activated renal tumorigenesis. Cancer Cell 18, 472–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pichiorri F., Suh S. S., Rocci A., De Luca L., Taccioli C., Santhanam R., Zhou W., Benson D. M. Jr., Hofmainster C., Alder H., Garofalo M., Di Leva G., Volinia S., Lin H. J., Perrotti D., et al. (2010) Down-regulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell 18, 367–381 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Zhang X. J., Ye H., Zeng C. W., He B., Zhang H., and Chen Y. Q. (2010) Dysregulation of miR-15a and miR-214 in human pancreatic cancer. J. Hematol. Oncol. 3, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scapoli L., Palmieri A., Lo Muzio L., Pezzetti F., Rubini C., Girardi A., Farinella F., Mazzotta M., and Carinci F. (2010) MicroRNA expression profiling of oral carcinoma identifies new markers of tumor progression. Int. J. Immunopathol. Pharmacol. 23, 1229–1234 [DOI] [PubMed] [Google Scholar]
- 71.Penna E., Orso F., Cimino D., Tenaglia E., Lembo A., Quaglino E., Poliseno L., Haimovic A., Osella-Abate S., De Pittà C., Pinatel E., Stadler M. B., Provero P., Bernengo M. G., Osman I., and Taverna D. (2011) microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J. 30, 1990–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu Z. W., Zhong L. P., Ji T., Zhang P., Chen W. T., and Zhang C. P. (2010) MicroRNAs contribute to the chemoresistance of cisplatin in tongue squamous cell carcinoma lines. Oral. Oncol. 46, 317–322 [DOI] [PubMed] [Google Scholar]
- 73.Yang H., Kong W., He L., Zhao J. J., O'Donnell J. D., Wang J., Wenham R. M., Coppola D., Kruk P. A., Nicosia S. V., and Cheng J. Q. (2008) MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 68, 425–433 [DOI] [PubMed] [Google Scholar]
- 74.Cho D., Signoretti S., Dabora S., Regan M., Seeley A., Mariotti M., Youmans A., Polivy A., Mandato L., McDermott D., Stanbridge E., and Atkins M. (2007) Potential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinoma. Clin. Genitourin. Cancer 5, 379–385 [DOI] [PubMed] [Google Scholar]
- 75.Qian C. N., Furge K. A., Knol J., Huang D., Chen J., Dykema K. J., Kort E. J., Massie A., Khoo S. K., Vanden Beldt K., Resau J. H., Anema J., Kahnoski R. J., Morreau H., Camparo P., et al. (2009) Activation of the PI3K/AKT pathway induces urothelial carcinoma of the renal pelvis: identification in human tumors and confirmation in animal models. Cancer Res. 69, 8256–8264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cully M., You H., Levine A. J., and Mak T. W. (2006) Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer 6, 184–192 [DOI] [PubMed] [Google Scholar]
- 77.Cancer Genome Atlas Research Network. (2013) Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abou Youssif T., Fahmy M. A., Koumakpayi I. H., Ayala F., Al Marzooqi S., Chen G., Tamboli P., Squire J., Tanguay S., and Sircar K. (2011) The mammalian target of rapamycin pathway is widely activated without PTEN deletion in renal cell carcinoma metastases. Cancer 117, 290–300 [DOI] [PubMed] [Google Scholar]
- 79.Wang Y. H., and Huang M. L. (2009) Reduction of Lobe leads to TORC1 hypoactivation that induces ectopic Jak/STAT signaling to impair Drosophila eye development. Mech. Dev. 126, 781–790 [DOI] [PubMed] [Google Scholar]
- 80.Miron M., Verdú J., Lachance P. E., Birnbaum M. J., Lasko P. F., and Sonenberg N. (2001) The translational inhibitor 4E-BP is an effector of PI(3)K/Akt signalling and cell growth in Drosophila. Nat. Cell Biol. 3, 596–601 [DOI] [PubMed] [Google Scholar]
- 81.Montagne J., Stewart M. J., Stocker H., Hafen E., Kozma S. C., and Thomas G. (1999) Drosophila S6 kinase: a regulator of cell size. Science 285, 2126–2129 [DOI] [PubMed] [Google Scholar]
- 82.De Benedetti A., and Graff J. R. (2004) eIF-4E expression and its role in malignancies and metastases. Oncogene 23, 3189–3199 [DOI] [PubMed] [Google Scholar]
- 83.Graff J. R., Konicek B. W., Carter J. H., and Marcusson E. G. (2008) Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 68, 631–634 [DOI] [PubMed] [Google Scholar]
- 84.Hay N., and Sonenberg N. (2004) Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 85.Del Bufalo D., Ciuffreda L., Trisciuoglio D., Desideri M., Cognetti F., Zupi G., and Milella M. (2006) Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 66, 5549–5554 [DOI] [PubMed] [Google Scholar]
- 86.Atkins M. B., Hidalgo M., Stadler W. M., Logan T. F., Dutcher J. P., Hudes G. R., Park Y., Liou S. H., Marshall B., Boni J. P., Dukart G., and Sherman M. L. (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J. Clin. Oncol. 22, 909–918 [DOI] [PubMed] [Google Scholar]
- 87.Motzer R. J., Escudier B., Oudard S., Hutson T. E., Porta C., Bracarda S., Grünwald V., Thompson J. A., Figlin R. A., Hollaender N., Urbanowitz G., Berg W. J., Kay A., Lebwohl D., and Ravaud A. (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–456 [DOI] [PubMed] [Google Scholar]
- 88.Motzer R. J., Escudier B., Oudard S., Hutson T. E., Porta C., Bracarda S., Grünwald V., Thompson J. A., Figlin R. A., Hollaender N., Kay A., Ravaud A., and RECORD-1 Study Group. (2010) Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 116, 4256–4265 [DOI] [PubMed] [Google Scholar]
- 89.Traynor K. (2009) New oral treatment for kidney cancer approved. Am. J. Health Syst. Pharm. 66, 788. [DOI] [PubMed] [Google Scholar]
- 90.Harrington L. S., Findlay G. M., Gray A., Tolkacheva T., Wigfield S., Rebholz H., Barnett J., Leslie N. R., Cheng S., Shepherd P. R., Gout I., Downes C. P., and Lamb R. F. (2004) The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J. Cell Biol. 166, 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shah O. J., Wang Z., and Hunter T. (2004) Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 14, 1650–1656 [DOI] [PubMed] [Google Scholar]