Abstract
Differential functions of Rab5 isoforms in endocytosis are not well characterized. Here, we cloned, expressed, and characterized Rab5a and Rab5b from Leishmania and found that both of them are localized in the early endosome. To understand the role of LdRab5 isoforms in different modes of endocytosis in Leishmania, we generated transgenic parasites overexpressing LdRab5a, LdRab5b, or their dominant-positive (LdRab5a:Q93L and LdRab5b:Q80L) or dominant-negative mutants (LdRab5a:N146I and LdRab5b:N133I). Using LdRab5a or its mutants overexpressing parasites, we found that LdRab5a specifically regulates the fluid-phase endocytosis of horseradish peroxidase and also specifically induced the transport of dextran-Texas Red to the lysosomes. In contrast, cells overexpressing LdRab5b or its mutants showed that LdRab5b explicitly controls receptor-mediated endocytosis of hemoglobin, and overexpression of LdRab5b:WT enhanced the transport of internalized Hb to the lysosomes in comparison with control cells. To unequivocally demonstrate the role of Rab5 isoforms in endocytosis in Leishmania, we tried to generate null-mutants of LdRab5a and LdRab5b parasites, but both were lethal indicating their essential functions in parasites. Therefore, we used heterozygous LdRab5a+/− and LdRab5b+/− cells. LdRab5a+/− Leishmania showed 50% inhibition of HRP uptake, but hemoglobin endocytosis was uninterrupted. In contrast, about 50% inhibition of Hb endocytosis was observed in LdRab5b+/− cells without any significant effect on HRP uptake. Finally, we tried to identify putative LdRab5a and LdRab5b effectors. We found that LdRab5b interacts with clathrin heavy chain and hemoglobin receptor. However, LdRab5a failed to interact with the clathrin heavy chain, and interaction with hemoglobin receptor was significantly less. Thus, our results showed that LdRab5a and LdRab5b differentially regulate fluid phase and receptor-mediated endocytosis in Leishmania.
Keywords: endocytosis, hemoglobin, Leishmania, membrane trafficking, Rab
Introduction
Endocytosis is a fundamental process and is required for the uptake of various essential molecules for the maintenance of cells (1, 2). After initial binding with cell surface receptors, endocytic cargos are internalized into common early endosome (EE)3 where they are sorted and targeted to various intracellular destinations (3); however, the mechanism of sorting is not well characterized (4). Moreover, the route of internalization of different ligands also controls the fate of endocytic cargos (5). Therefore, it could be possible that ligand-receptor interaction might activate or modulate the function of certain endocytic Rab-GTPases to dictate its endocytic route as Rab-GTPases are master regulators of intracellular trafficking (6).
Among the large number of Rab proteins, Rab5 is specifically localized to EE and regulates endocytosis (7, 8). Incidentally, three Rab5 isoforms, namely Rab5a, Rab5b, and Rab5c, are present in mammalian cells (9). Initial studies have shown that overexpression of individual Rab5 isoforms stimulates transferrin endocytosis (9), and simultaneous knockdown of all three Rab5 isoforms are required to inhibit insulin-dependent PKB/Akt activation suggesting their functional redundancy (10). However, recent studies have shown that Rab5 isoforms might have different roles in different cell types. For instance, Rab5a has been shown to specifically regulate epidermal growth factor receptor endocytosis in HeLa cells (11, 12), although in osteosarcoma cells, Rab5a interacts with Beclin-1 to regulate autophagosome formation (13). In contrast, Rab5a is also found to regulate trafficking of lysosomal enzymes (14) and induces the transport of intracellular pathogens to lysosomes in macrophages (15). Rab5b is also found to regulate the biogenesis of lysosomes in rat kidney cells (16). However, Rab5c has been shown to enhance β-integrin recycling in EGF-induced cancer cell invasion (17) possibly by increasing cell motility by Rac1 activation (18). Furthermore, expression of Rab5 isoforms can be differentially regulated by diverse cytokines (19, 20). As all isoforms of Rab5 are localized on EE, it is tempting to speculate that Rab5 isoforms might play differential roles in endocytosis of various cargos. However, the role of Rab5 isoforms in regulating various types of endocytosis in a particular cell type has not yet been addressed.
It has been shown that large numbers of putative Rab and SNARE proteins are also present in trypanosomatid parasites indicating the presence of highly conserved protein trafficking pathways in these parasites (21, 22). Previously, we have shown that Leishmania endocytosed hemoglobin (Hb) via receptor-mediated endocytosis (RME) (23) through a specific receptor located in the clathrin-coated flagellar pocket (24, 25). Subsequently, bound hemoglobin is rapidly internalized into discrete Rab5-positive EE (26) and finally targeted to the lysosomal compartment in a Rab7-dependent way (27). However, which Rab5 isoform regulates the Hb endocytosis in Leishmania was not characterized. We have also found that the Rab1-mediated conventional secretory pathway is conserved in Leishmania (28). These results demonstrate that several components of intracellular trafficking machinery are well preserved in Leishmania; thereby, this parasite provides a unique opportunity to determine the intricacies of membrane trafficking and sorting phenomena in an organism. Therefore, we have analyzed the role of Rab5 isoforms in RME and fluid-phase endocytosis (FPE) in Leishmania promastigotes using Hb and HRP as endocytic probes, respectively, and have shown that Rab5 isoforms differentially regulate these two endocytic pathways.
Experimental Procedures
Materials
Unless otherwise stated, all reagents were obtained from Sigma. Platinum High Fidelity Taq polymerase and restriction enzymes were purchased from Invitrogen and Promega Life Science (Madison, WI), respectively. Glutathione-Sepharose 4B beads, protein markers (RPN756 and RPN800), and ECL reagents were obtained from Amersham Biosciences, UK. Dextran-Texas Red (10,000 Da), Alexa Fluor-594 succinimidyl ester, FM4-64, LysoTracker Green, and anti-GFP antibody were obtained from Molecular Probes (Eugene, OR). Geneticin and hygromycin were procured from Gibco BRL (Gaithersburg, MD). The Leishmania expression vectors, pXG-GFP2+ and pXG were kindly provided by Dr. S. M. Beverley (Washington University, St. Louis, MO), and pGL345-Hyg vector was gift from Dr. Jeremy Mottram (University of Glasgow, Glasgow, UK). pNUS-mRFP-nD was a kind gift from Dr. Jean-Paul di Rago (Institut de Biochimie et Génétique Cellulaires, Bordeaux, France). [α-32P]GTP (800 Ci/mmol) was procured from PerkinElmer Life Sciences. All other reagents used were of analytical grade.
Cells
Leishmania donovani (UR6) and L. donovani (Bob strain) promastigotes were obtained from the Indian Institute of Chemical Biology, Kolkata, and Jawaharlal Nehru University, Delhi, India, respectively. Cells were routinely maintained on blood agar slants containing glucose, peptone, sodium chloride, beef heart extract, rabbit blood, and gentamycin as described previously (27). For experiments, cells were cultured in medium M199, pH 7.4, supplemented with 10% FCS, 100 units/ml penicillin, 100 μg/ml streptomycin at 23 °C, and log-phase cells were harvested in phosphate-buffered (10 mm, pH 7.2) saline (0.15 m).
Cloning and Expression of rab5a from L. donovani (Ldrab5a)
To clone Rab5a from Leishmania, a putative Rab5a sequence was identified from a Leishmania major genome with substantial homology with Trypanosoma brucei Rab5a by BLAST search. Accordingly, appropriate forward (5′-GCGGATCCATGAATACCCACCCACCTCAGC-3′) and reverse (5′-GCGAATTCTCAGCAGCAGGTAGACTGGGAG-3′) primers, including start or stop codons, were designed against the putative LdRab5a gene sequence from L. major. These primers were used to amplify the Ldrab5a gene sequence from L. donovani cDNA by RT-PCR. Briefly, PCR was performed in a PerkinElmer Life Sciences thermocycler for 30 cycles (denaturation at 94 °C for 30 s, annealing at 62 °C for 30 s, and extension at 68 °C for 1 min) using High Fidelity Taq. The PCR product was cloned into pGEM-T Easy vector (Promega Life Science) as per the manufacturer's protocol and sequenced using M13 universal primers. After confirming the sequence, the LdRab5a gene product was further subcloned into BamHI/EcoRI sites of the pGEX-4T-2 (Amersham Bioscience) expression vector and transformed into the XL-1 Blue strain of Escherichia coli.
Generation of LdRab5 Mutants
The dominant-positive (Gln to Leu) and dominant-negative (Asn to Ile) mutants of LdRab5a and LdRab5b were generated by the PCR-mediated site-directed mutagenesis approach as described previously (27). Briefly, a mutant primer was designed in such a way that the Gln residue at position 93 changed to an Leu residue in LdRab5a (5′-GAAGCGTTCCAGCCCCGCCG-3′). PCR (30 cycles of denaturation at 94 °C for 30 s, annealing at 62 °C for 30 s, and extension at 68 °C for 30 s) was carried out using this mutant primer as reverse primer and Rab5a:WT primer as forward primer to amplify a megaprimer (290 bp). The amplified megaprimer was gel-purified, and a second PCR was carried out to amplify the full-length LdRba5a:Q93L mutant (∼708 bp) using the megaprimer as a forward primer and a gene-specific reverse primer. Similarly, LdRab5a:N146I mutant was generated using mutated primer (5′-GTGCTCGTCGGAATCAAGAAGGACCTGGGG-3′) as forward primer and Rab5a:WT primer as reverse primer in the first round of PCR. Additionally, LdRab5b:Q80L and LdRab5b:N133I were made by similar methods using (5′-CTTAAAGCGCTCCAGCCCTGCCGTGTC-3′) and (5′-CTGGTAGGCATCAAGAAGGACTTG-3′) as mutated primer, respectively.
The full-length PCR products were subsequently cloned into pGEM-T Easy and sequenced to confirm the respective mutations. Subsequently, mutants were subcloned into pGEX-4T-2 vector and transformed into E. coli. Cells were grown in LB and induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside for 3 h at 37 °C for expression of respective GST fusion proteins using reduced glutathione beads by standard procedure.
Generation of Antibodies against LdRab5a and LdRab5b
LdRab5a and LdRab5b were subcloned into pET28a and expressed as His6 tag proteins. 10 μg of the respective proteins were immunized in BALB/c mice to raise antibodies by a standard method as described previously (26). The specificity of the antibodies against the respective proteins was determined by Western blot analysis using the respective purified proteins.
Preparation of Early Endosomes from L, donovani
Early endosomes containing biotinylated-Hb or avidin-HRP were purified from Leishmania as described previously (26). Briefly, cells were incubated with biotinylated-Hb (2 mg/ml) or avidin-HRP (2 mg/ml) in internalization medium (MEM containing 10 mm HEPES and 5 mm glucose, pH 7.4) for 5 min at 23 °C to label the early endosomal compartment. Cells were washed three times with cold homogenization buffer (HB: 20 mm HEPES, 250 mm sucrose, and 2 mm EGTA, pH 7.2, containing protease inhibitors) and disrupted by release of N2 from a pre-cooled nitrogen cavitation bomb. The unbroken cells, nuclei, and other cell debris were removed by low speed centrifugation at 500 × g for 10 min at 4 °C. The post-nuclear supernatant (0.5 ml) was loaded onto a discontinuous sucrose density gradient formed by layering 0.35 ml of 54%, 1.45 ml of 40%, and 1.45 ml of 30% sucrose in HB. After centrifugation in an MLS 50 rotor (Beckman TL100) at 100,000 × g for 1 h at 4 °C, 50-μl fractions were collected from the top of the gradient as described previously. Early endosomal fractions containing avidin-HRP or biotinylated-Hb were analyzed by Western blot analysis using anti-Rab5a or anti-Rab5b antibodies, respectively.
GTP Binding Assay
GTP binding activity of purified LdRab5a:WT, LdRab5b:WT, and their mutants was detected by a GTP binding assay (26). Briefly, the indicated proteins (2 μg) were blotted onto nitrocellulose membrane, and the membrane was incubated with 1 μCi/ml [α-32P]GTP in 50 mm phosphate buffer, pH 7.5, containing 5 mm MgCl2, 1 mm EGTA, and 0.3% Tween 20 for 3 h at 24 °C. Finally, the membranes were extensively washed to remove unbound radioactivity and visualized by autoradiography.
GTPase Assay
The GTPase activity of the indicated proteins was determined as described previously (26). Briefly, 5 μg of respective protein was immobilized on glutathione beads, and beads were incubated with buffer A (20 mm Tris-HCl, pH 7.8, 100 mm NaCl, 5 mm MgCl2, 1 mm NaH2PO4, and 10 mm β-mercaptoethanol) for 20 min at 25 °C. The bound nucleotide was eluted with 1 m guanidine HCl. Nucleotide-free immobilized protein was then loaded with 2 pmol of [α-32P]GTP (800 Ci/mmol) in 20 μl of buffer A for 10 min at 0 °C. Subsequently, beads were washed and incubated for 1 h at 23 °C to allow the hydrolysis of bound GTP. The beads were washed, incubated in 8 μl of buffer B (0.2% SDS, 2 mm EDTA, 10 mm GDP, 10 mm GTP, pH 7.5), and heated at 70 °C for 2 min to elute the nucleotide from the protein. An aliquot was analyzed using thin layer chromatography and visualized by autoradiography.
Overexpression of LdRab5 Isoforms and Their Mutants in L. donovani Promastigotes
To overexpress LdRab5a, LdRab5b or its mutants in Leishmania promastigotes, the full-length Ldrab5a, Ldrab5b, or its mutant gene was subcloned into pXG vector and pXG-GFP+2 vector to express the proteins without tag or with an N-terminal GFP tag, respectively (29). For subcloning, the respective construct was first linearized by digestion with BamHI and subsequently partially digested with EcoRI followed by ligation with BamHI-EcoRI-digested LdRab5 or its mutants. In addition, Ldrab5a was also subcloned into BglII/XhoI sites of pNUS-mRFP-nD vector to express as RFP fusion protein. Leishmania promastigotes were then transfected with respective constructs, using standard protocol (27). Briefly, Leishmania cells were suspended at a density of 1.0 × 108/ml in HEPES-buffered saline (21 mm HEPES, 137 mm NaCl, 5 mm KCl, 0.7 mm NaH2PO4, and 6 mm glucose, pH 7.4). Cells (0.4 ml) were transferred to pre-cooled electroporation cuvette containing appropriate construct of chilled DNA (40 μg), followed by electroporation using a GenePulser (Bio-Rad) to facilitate DNA uptake by cells. Cells were allowed to recover on ice for 10 min and transferred into drug-free medium for 30 h at 23 °C. Subsequently, stable clones were selected in the presence of G418 antibiotic (50 μg/ml). Overexpression of the respective protein was confirmed by Western blot analysis and by confocal microscopy.
Subcellular Localization of LdRab5a and LdRab5b in L. donovani
To characterize the subcellular localization of LdRab5a and LdRab5b, cells overexpressing GFP-LdRab5a or GFP-LdRab5b proteins were labeled with various compartment-specific markers. The flagellar pocket was labeled by incubating the promastigotes with FM4-64 (30 μm) for 5 min at 4 °C. Similarly, 5 min of internalization of Alexa Fluor-594-labeled hemoglobin (Alexa 594-Hb) and 10 min internalization of dextran-Texas Red at 23 °C were used to mark the EE. Lysosomes were stained with 100 nm LysoTracker Red at 4 °C for 5 min. Overnight serum-starved Leishmania were incubated with 5 μm BODIPY-TR ceramide bound to defatted bovine serum albumin for 1 h at 23 °C to label the Golgi complex. Finally, cells were fixed with 4% paraformaldehyde on ice for 20 min, washed with vPBS (136.9 mm NaCl, 3 mm KCl, 16 mm Na2HPO4, 3 mm KH2PO4, 45.9 mm sucrose, and 10 mm glucose, pH 7.4), and visualized under a Zeiss LSM 510 META confocal microscope.
Fluid Phase Uptake of HRP
HRP was used to measure the fluid-phase endocytosis in Leishmania promastigotes. Log-phase Leishmania promastigotes were harvested, washed, and resuspended in vPBS containing 1 mg/ml BSA at a cell density of 8 × 107cells/ml. Subsequently, 2 × 107cells were incubated with different concentrations of HRP at 23 °C for 60 min. The cells were then washed four times with chilled vPBS containing 10 mg/ml BSA followed by two washes with chilled vPBS to remove uninternalized HRP. Finally, cells were transferred into fresh tubes and lysed by the addition of 50 μl of solubilization buffer (SB: PBS containing 0.5% Triton X-100, 2% methylbenzethonium chloride). An aliquot (10 μl) of the cell lysate was used to measure the uptake of HRP by respective cells using a standard assay procedure (30). Results were expressed as nanograms of HRP per mg of cell protein.
Kinetics of Dextran-Texas Red Trafficking in L. donovani
To measure the kinetics of fluid phase endocytosis, fluorescently labeled dextran-Texas Red was used. Respective Leishmania promastigotes (2 × 107cells/ml) were washed twice with vPBS and resuspended in 250 μl of vPBS containing 150 μg/ml dextran-Texas Red for 10 min. The cell were washed twice with vPBS and incubated for the indicated periods of time at 23 °C. Finally, cells were washed and incubated with 100 nm LysoTracker Green for 5 min on ice. Cells were fixed with 4% paraformaldehyde on ice for 20 min, washed with vPBS, and visualized under a Zeiss LSM 510 META confocal microscope.
Uptake and Degradation of 125I-Hemoglobin by L. donovani Promastigotes
To determine the role of LdRab5a and LdRab5b in Hb endocytosis, uptake and degradation of 125I-Hb by Leishmania promastigotes was measured in cells overexpressing either GFP-LdRab5a or GFP-LdRab5b along with vector-transfected control cells (24). Briefly, cells (2 × 107 cells/ml) were resuspended in vPBS containing 1 mg/ml BSA and incubated with 6 μg/ml 125I-Hb for different time intervals at 23 °C. At respective time intervals, cells were washed to remove the unbound radioactivity, and the cell pellet was dissolved in 0.1 n NaOH. An aliquot was used to determine the cell-associated radioactivity. To determine the degradation of internalized Hb by Leishmania, aliquots of the supernatant from the respective medium were processed for the determination of trichloroacetic acid-soluble non-iodide radioactivity after extraction with chloroform. Results were expressed as nanograms of Hb/mg of cell protein.
Hb endocytosis in LdRab5a+/− and LdRab5b+/− cells was also measured using biotinylated Hb. Briefly, the respective cells were incubated with 6 μg/ml biotinylated Hb for 2 h at 23 °C and washed. Cells were solubilized in SB, and Hb was immunoprecipitated from the cell lysate using anti-Hb antibody. The amount of internalized biotinylated Hb was quantitated using avidin-HRP as described previously (25). Results were expressed as percentage uptake of Hb in indicated cell types considering 100% in the control cells.
Kinetics of Intracellular Trafficking of Hb in L. donovani
Kinetics of intracellular trafficking of Hb in Leishmania was determined as described previously (27). Briefly, respective Leishmania cells (107 cells/ml) were washed twice and resuspended in 250 μl of ice-cold vPBS containing Alexa Fluor-594-labeled Hb (120 μg/ml) and incubated at 23 °C for 5 min to label the early endosomal compartment. Cells were washed three times with cold vPBS to remove unbound Alexa-Hb and resuspended in pre-warmed (23 °C) vPBS for the indicated periods of time. At respective times, cells were transferred onto ice and washed twice with chilled vPBS. The lysosomes of the cells were stained with 100 nm LysoTracker Green for 5 min on ice. Finally, cells were washed with chilled vPBS and visualized under a Zeiss LSM 510 META confocal microscope.
Generation of Rab5a and Rab5b Knock-out L. donovani
Attempts were made to generate LdRab5a and LdRab5b knock-out cells using pGL345-Hyg plasmid as described previously (31). Upstream regions flanking Ldrab5a were PCR-amplified (856 bp) using primers (forward, 5′-GCAAGCTTCCTCCGACTCCGCCTTCCCG-3′, and reverse, 5′-GCGTCGACTTTGTAGGTGATGTCGCGTTTTCGGTG-3′) from L. donovani genomic DNA as template. Similarly, downstream regions flanking Ldrab5a were amplified (1131 bp) using the following primers: forward, 5′-GCCCCGGGTCCCCTCTCCGGACTACCTTCGTG-3′, and reverse, 5′-GCAGATCTTGCTGGCAACATGGCACTGCCTTC-3′; upstream forward, 5′-GCAAGCTTGGCACGAGGAAACCGTGCACATG-3′, and reverse, 5′-GCGTCGACGGTAGTGGCGGATAATCGAAGCGG-3′; and downstream forward, 5′-GCCCCGGGATGGGTGCCACGGGGGTGGT-3′, and reverse, 5′-GCAGATCTAACAGCAACGACAGCTACAGCG-3′ regions flanking Ldrab5b similarly amplified (1118 and 1010 bp) using appropriate primers. The upstream and downstream flanking sequences of LdRab5a or LdRab5b were sequentially cloned into pGL345-Hyg vector in the indicated restriction sites to generate pGL345-LdRab5a-Hyg or pGL345-LdRab5b-Hyg constructs and subsequently digested with HindIII and BglII to prepare LdRab5a-Hyg (5326 bp) or LdRab5b-Hyg (5467 bp) targeting knock-out cassette. In addition, pGL345-LdRab5a-Neo or pGL345-Ld Rab5b-Neo construct was generated by replacing the Hyg cassette with Neo cassette in pGL345-LdRab5a-Hyg or pGL345-LdRab5b-Hyg constructs, respectively. Finally, these constructs were digested with HindIII and BglII to prepare LdRab5a-Neo (5104 bp) or LdRab5b-Neo (5245 bp) targeting knock-out cassette.
Subsequently, Leishmania promastigotes were transfected with 10 μg of the purified LdRab5a-Hyg or LdRab5b-Hyg targeting knock-out cassettes using standard protocol (27). Cells were allowed to recover in drug-free medium for 30 h at 23 °C. Subsequently, cells were plated onto M199 agar plates containing hygromycin (20 μg/ml), and single colonies were isolated. These clones were grown in M199 containing 10% FCS and hygromycin (10 μg/ml) at 23 °C. Cells were harvested and washed, and genomic DNAs were prepared from untransfected LdRab5a-Hyg and LdRab5b-Hyg-transfected cells using genomic DNA purification kit (Promega). Subsequently, the genomic level of rab5a or rab5b was determined from each sample by quantitative PCR using LdRab5a- or LdRab5b-specific primers and SYBR Green Master Mix in a 7500 Fast Real Time PCR System (Applied Biosystems). gapdh was used as an internal control. The results were analyzed by the comparative Ct method (2−ΔΔCt) and are expressed as relative quantity of respective genes in different cell types. The clones from LdRab5a-Hyg- and LdRab5b-Hyg-transfected cells showing 50% reduction in the respective gene level indicated the generation of LdRab5a+/− and LdRab5b+/− cells after the first round of transfection. LdRab5a+/− and LdRab5b+/− cells were further transfected with LdRab5a-Neo and LdRab5b-Neo targeting knock-out cassettes, respectively, using the same protocol to generate LdRab5a and LdRab5b null-mutants. However, no viable cells were obtained after second round of transfection with LdRab5a-Neo or LdRab5b-Neo targeting knock-out cassettes.
Identification of Putative LdRab5a and LdRab5b Interacting Partners by Mass Spectrometry
Equimolar amounts of GST-LdRab5a (5 μg), GST-LdRab5b (5 μg), or GST (2.5 μg) were immobilized on glutathione-Sepharose beads and incubated with 3 mg of Leishmania lysate in 1.5 ml of PBS containing 0.2% Triton X-100 for 2 h at 23 °C. The beads were washed three times with PBS containing 0.1% Triton X-100 followed by three washes with PBS to remove unbound proteins. The bound proteins were resuspended in 100 μl of digestion buffer (50 mm ammonium bicarbonate solution containing 1 mg/ml trypsin gold) and incubated for 20 h at 37 °C. The beads were separated by centrifugation (2 min at 1000 × g), and the supernatant containing digested peptides was acidified using trifluoroacetic acid. The acidified digested peptides were concentrated to 50 μl and desalted using C-18 Zip-Tip by standard protocol. The digested peptides were vacuum-dried, dissolved in solvent A (5% acetonitrile containing 0.1% formic acid), and loaded for reverse phase chromatography using C-18 Picofrit analytical column in a Thermo-Scientific Proxeon Nano LC. Samples were run at a flow rate of 300 nl/min using a linear gradient of solvent B (95% acetonitrile containing 0.1% formic acid): 70 min in 5–40% solvent B, 10 min in 40–80% solvent B, 10 min in 80% solvent B, 5 min in 80–5% solvent B followed by 25 min in 5% solvent B. Mass spectrometry was performed in an Orbitrap Velos mass spectrometer, and data were analyzed using Thermo Proteome Discoverer Software (1.3.0.339 DBV version). Spectra of peptides were queried against L. major Uniprot database containing decoy database using a target false discovery rate of 1% for strict and 5% for relaxed conditions.
Binding of LdRab5a and LdRab5b with Clathrin Heavy Chain and Hb Receptor
To determine the binding of LdRab5a and LdRab5b with clathrin heavy chain (LdCHC) of Leishmania, 5 μg of GST-LdRab5a or GST-LdRab5b was immobilized on glutathione-Sepharose beads and incubated with GFP-LdCHC-overexpressing lysate (4 mg) PBS, pH 7.2, for 2 h at 24 °C. Beads were washed three times with PBS containing 0.1% Triton X-100, followed by three washes with PBS to remove unbound proteins. The proteins were separated on a 10% SDS-PAGE and transferred onto a nitrocellulose membrane. Finally, Western blot analysis was carried out with anti-GFP antibody. Similarly, the experiment was carried out to determine the binding of LdRab5a and LdRab5b with the Hb receptor using anti-HbR antibody.
Statistical Analysis
Statistical analysis was performed using Sigma Plot version 12. Student's two-tailed paired t test or two-tailed Mann-Whitney test was used to determine differences between control and test groups with 95% confidence intervals. p values less than 0.05 were considered to be significant for all analyses. Correlation coefficient of colocalization of different markers in Leishmania was determined using software provided in LSM510 meta confocal microscope.
Results
Cloning, Expression, and Characterization of LdRab5a and LdRab5b Homologs from L. donovani
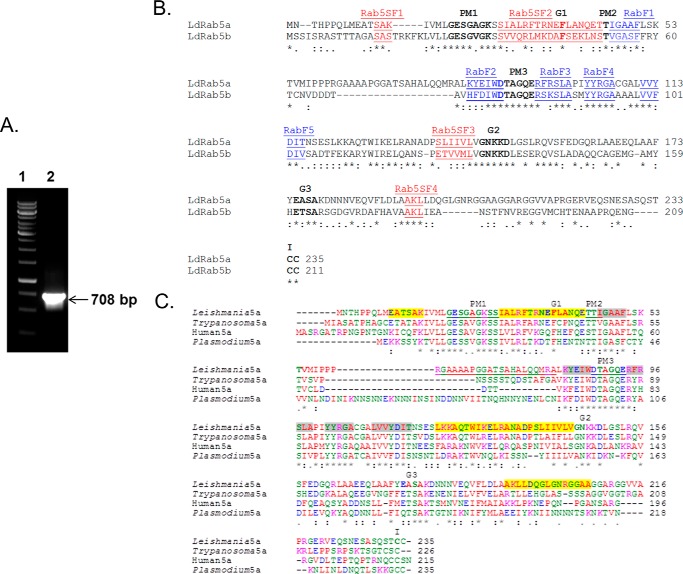
To clone the rab5a homolog from Leishmania, a putative Rab5a-like sequence was identified from the L. major genome, and using appropriate forward and reverse primers, a 708-bp fragment was amplified from Leishmania cDNA by PCR (Fig. 1A). The PCR product was cloned, sequenced, and hypothetically translated into a 235-amino acid sequence (LdRab5a). Comparison of LdRab5a sequence by Clustal W multiple sequence alignment demonstrated that the cloned protein has overall similarities of 75, 59, and 30% with Trypanosoma, human, and Plasmodium Rab5a sequences, respectively (Fig. 1B). Interestingly, the LdRab5a sequence showed an insertion of 21 amino acids (RGAAAAPGGATSAHALQQMRA) in the loop2-β2 region in comparison with human. Previously, we cloned and characterized a Rab5b homolog (LdRab5b) from Leishmania (26), which showed about 74, 61, and 60% similarities with the Trypanosoma, human, and Plasmodium Rab5b sequences, respectively. The comparison of LdRab5a and LdRab5b sequences showed that both proteins contain five distinct Rab family motifs (RabF1-RabF5) and Rab5-specific subfamily motifs (SF1-SF4 regions) indicating these are Rab5 isoforms (32) from Leishmania. Rab5 isoforms in mammalian cells were shown to be more than 80% similar; however, LdRab5a and LdRab5b showed 62% similarity indicating that they are probably functionally more divergent (Fig. 1C).
FIGURE 1.
Cloning of Rab5a homolog from L. donovani. A, amplification of 708-bp (lane 2) fragment of Ldrab5a from L. donovani cDNA by PCR. B, alignment of the amino acid sequence of LdRab5a with Rab5a sequences from different organisms. Residues implicated in guanine phosphate binding (PM1–3), GTP/GDP binding (G1–3), and the isoprenylation motif (I) are marked in bold. The sequence of LdRab5a has been submitted to the GenBankTM database under accession number KP308372. C, comparison of LdRab5a with LdRab5b sequences. Sequence alignment of LdRab5a and LdRab5b shows the presence of highly conserved regions of Rab proteins like the guanine nucleotide-binding regions (G1–G3), phosphate/Mg2+ (PM1–PM3) regions, and the C-terminal isoprenylation motif. Five Rab family (RabF1–RabF5) motifs are marked in blue, and Rab5-specific subfamily motifs (SF1–SF4 regions) are indicated in red.
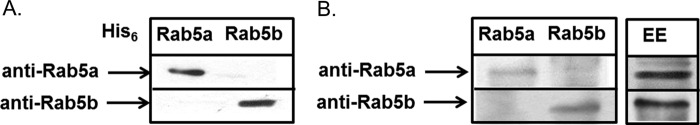
Specific antibodies against LdRab5a and LdRab5b were made, and Western blot analysis showed that antibodies against LdRab5a specifically recognized His6-LdRab5a, whereas anti-Rab5b only detects His6-LdRab5b (Fig. 2A). These antibodies also specifically recognized respective proteins from LdRab5a- or LdRab5b-overexpressing Leishmania lysate (Fig. 2B, left panel). However, these antibodies failed to detect the respective endogenous protein from Leishmania lysate probably due to lower content of endogenous proteins; nevertheless, respective proteins were specifically detected by these antibodies in purified early endosomes from Leishmania as they are enriched in these compartments (Fig. 2B, right panel).
FIGURE 2.
Specificities of anti-LdRab5a and anti-LdRab5b antibodies. A, specificities of anti-LdRab5a and anti-LdRab5b antibodies were determined using purified His6-LdRab5a and His6-LdRab5b proteins by Western blot analysis. B, Western blot analysis was carried out using lysates from LdRab5a- or LdRab5b-overexpressing Leishmania by anti-LdRab5a and anti-LdRab5b antibodies (left panel). Purified endosome containing avidin-HRP or biotinylated-Hbs were probed with anti-LdRab5a or anti-LdRab5b antibodies, respectively (right panel). All results are representative of three independent observations.
Characterization of LdRab5 Isoforms from L. donovani
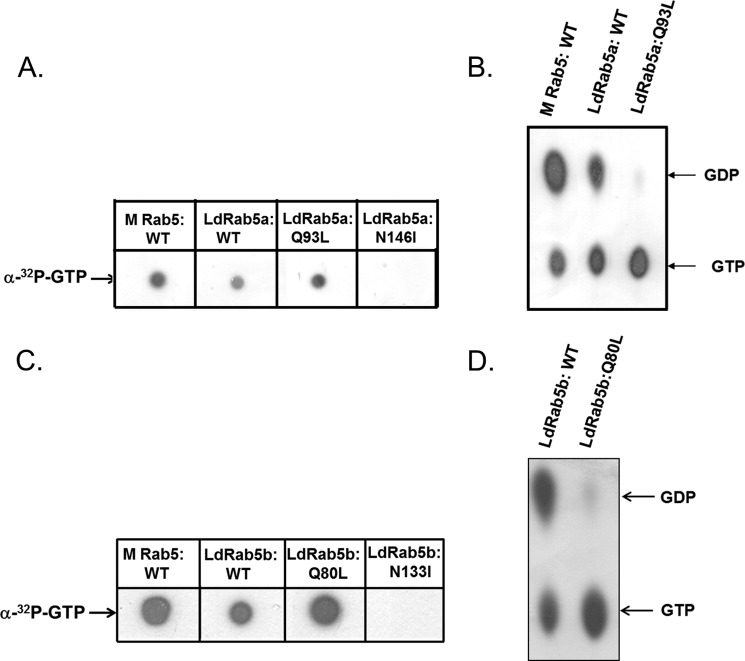
To determine the role of LdRab5 isoforms in endocytosis in Leishmania, dominant-active and dominant-negative mutants of LdRab5 isoforms were generated based on the similar mutations in mammalian Rabs (33). The LdRab5a:N146I mutant was made by substituting isoleucine for asparagine in the NKXD motif, whereas leucine was substituted for glutamine in the WDTAGQE region in LdRab5a:Q93L. Our results demonstrated that both LdRab5a:WT and LdRab5a:Q93L bind with [α-32P]GTP, and no GTP-binding was observed with LdRab5a:N146I (Fig. 3A). Analysis of the GTPase activity of these mutants revealed that LdRab5a:WT protein hydrolyze GTP to GDP, whereas LdRab5a:Q93L is unable to hydrolyze the bound GTP to GDP as expected (Fig. 3B). Similarly, our results showed that LdRab5b:WT and LdRab5b:Q80L bind GTP, but LdRab5b:Q80L is unable to hydrolyze the bound GTP (Fig. 3, C and D). No GTP binding was observed with LdRab5b:N133I (Fig. 3C).
FIGURE 3.
Characterization of LdRab5 isoforms and their mutants. GTP binding of the indicated purified proteins was detected using an [α-32P]GTP overlay assay. A, LdRab5a and its mutants. C, LdRab5b and its mutants. GTPase activity of Rab5 isoforms was determined as described under “Experimental Procedures.” B, LdRab5a and its mutants. D, LdRab5b and its mutants. Mammalian Rab5:WT was used as control. Results are representative of three independent observations.
Localization of LdRab5a and LdRab5b in L. donovani
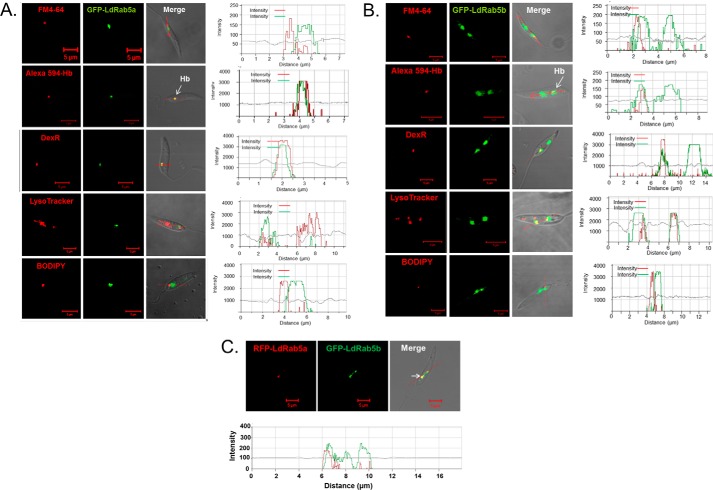
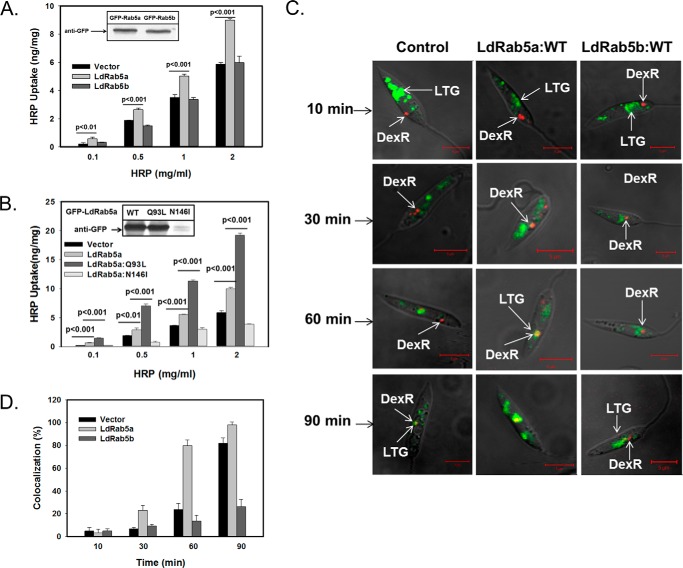
LdRab5a and LdRab5b were overexpressed in L. donovani as GFP fusion proteins and their localizations were determined by confocal microscopy. Our results showed (Fig. 4A) that LdRab5a is predominantly colocalized with 5 min internalized Alexa Fluor-594-conjugated Hb (correlation coefficient 0.80) as well as with 10 min internalization of dextran-Texas Red (correlation coefficient 0.95), which primarily labeled EE. Similarly, LdRab5b was also found to be colocalized with 5 min internalized Alexa Fluor-594-conjugated Hb (correlation coefficient 0.94) and 10 min internalization of dextran-Texas Red (correlation coefficient 0.84) (Fig. 4B). Conversely, LdRab5a and LdRab5b were found to be clearly separated from Texas Red-conjugated BODIPY-ceramide-labeled Golgi (correlation coefficient 0.10 and 0.04) and FM4-64 labeled flagellar pocket (correlation coefficient 0.12 and 0.21). No colocalization of LdRab5a was observed with the LysoTracker Red-labeled late/lysosomal compartment (correlation coefficient 0.20). However, LdRab5b also showed partial colocalization (correlation coefficient 0.79) with LysoTracker Red-labeled perinuclear late compartment (Fig. 4B). In addition, both RFP-LdRab5a and GFP-LdRab5b were found to be colocalized (correlation coefficient 0.96) in the early endosomal compartment in Leishmania (Fig. 4C).
FIGURE 4.
Intracellular localization of LdRab5a and LdRab5b in L. donovani. L. donovani overexpressing GFP-LdRab5a (A) and GFP-LdRab5b (B) were stained with various compartment-specific markers, like FM4-64 (16 μm) for flagellar pocket, 5-min internalized Alexa Fluor-594 Hb, and 10-min internalization of dextran-Texas Red for early endosome, LysoTracker Red (100 nm) for lysosome, and Bodipy-TR ceramide (500 nm) for Golgi. Finally, cells were visualized under a confocal microscope. Results are representative of three independent observations. Arrow indicates colocalization. Red arrow indicates the intensities of various fluorescence probes in different regions. C, overexpression of RFP-LdRab5a and GFP-LdRab5b in Leishmania to determine the colocalization. All results are representative of three independent observations.
LdRab5a Specifically Regulates Fluid-phase Endocytosis in L. donovani
To study the role of LdRab5a or LdRab5b in FPE in Leishmania, cells overexpressing LdRab5a or LdRab5b (Fig. 5A, inset) were incubated with different concentrations of HRP for 1 h at 23 °C and washed, and HRP activity associated with the cells was measured. Interestingly, overexpression of GFP-LdRab5a specifically stimulated HRP uptake about 1.5-fold in comparison with the control cells (Fig. 5A). However, no effect on HRP uptake was observed in GFP-LdRab5b-overexpressing cells compared with control cells (Fig. 5A). In addition, results presented in Fig. 5B show that HRP uptake in LdRab5a:Q93L-overexpressing Leishmania was found to be even higher than the cells expressing LdRab5a:WT. In contrast, about 40% inhibition of HRP uptake in comparison with control was observed in cells overexpressing LdRab5a:N146I when cells were incubated with 2 mg/ml HRP. This 40% inhibition of HRP uptake by LdRab5a:N146I-overexpressing Leishmania is very significant as a substantial amount of LdRab5a:N146I protein was found to be degraded (Fig. 5B, inset). These results are supported by the fact that Ser/Asn mutant of Rab5 (dominant-negative) of Trypanosoma is also not stable in parasites and is degraded.
FIGURE 5.
LdRab5a specifically regulates fluid-phase endocytosis in L. donovani. A, HRP was used as a probe to measure the fluid phase uptake in cells overexpressing GFP-LdRab5a or GFP-LdRab5b. The cells (2 × 107) were incubated for 1 h at 23 °C with the indicated concentrations of HRP, washed, and solubilized in solubilization buffer. An aliquot was used to measure the HRP activity from each sample and are expressed as nanograms of HRP uptake by respective cells ± S.D. of three independent experiments. Inset shows the levels of expression of both proteins in respective cells. B, similar experiments were carried out using cells overexpressing GFP-LdRab5a:WT, GFP-LdRab5a:Q93L, or GFP-LdRab5a:N146I. Results are expressed as nanograms of HRP uptake by respective cells ± S.D. of three independent experiments. Inset shows the levels of expression of each protein in respective cells. C, determination of kinetics of fluid phase endocytosis of dextran-Texas-red by L. donovani. Cells were incubated with dextran-Texas-red for 10 min at 23 °C, washed and chased for indicated period of times at 23 °C. The lysosomal compartments of these cells were stained with LysoTracker Green. Finally, cells were visualized under confocal microscope. Yellow indicates colocalization. Results are representative of three independent observations. D, quantitative analysis of percentage of cells showing the colocalization of dextran-Texas-red with LysoTracker Green in different cell types at indicated time points. Results are expressed as mean ± S.D.
To visualize the role of LdRab5a and LdRab5b in FPE in Leishmania, we analyzed the trafficking of dextran-Texas Red conjugate in the cells overexpressing LdRab5a:WT, LdRab5b:WT, or control cells by confocal microscopy. Cells were incubated with dextran-Texas Red conjugate for 10 min at 23 °C to label the early endosomal compartment, washed, and chased for different time periods. Finally, localization of dextran-Texas Red with lysosomes stained with LysoTracker Green was determined as shown previously (34). Our results showed (Fig. 5C) that 10 min post-internalization of dextran-Texas Red is predominantly present in the early endosomal compartment by more than 90% in all cell types. Further analysis revealed that dextran-Texas Red reaches the perinuclear late endosomal compartment by about 30–60 min post-internalization, and complete colocalization of dextran-Texas Red with lysosomes (Fig. 5C, left) was observed at 90 min in control cells (correlation coefficient 0.82). In contrast, most of the internalized dextran-Texas Red was found in LysoTracker Green-labeled lysosomes within 60 min in LdRab5a-overexpressed Leishmania (correlation coefficient 0.94). However, internalized dextran was found to be accumulated in the lysosomes of LdRab5a-overexpressing Leishmania at about 90 min as dextran is not efficiently degraded in the lysosomes. These results demonstrated that LdRab5a not only induces FPE, but it also enhances the kinetics of lysosomal transport in comparison with control cells (Fig. 5C, middle). However, no significant difference in dextran-Texas Red trafficking was observed in LdRab5b-overexpressed cells compared with control cells (Fig. 5C, right). Further quantitative analysis of several micrographs (n = 50) revealed (Fig. 5D) that dextran-Texas Red is colocalized with LysoTracker Green-labeled lysosomes in about 80% of Rab5a-overexpressed cells after 60 min of internalization in comparison with about 25% in control cells and 14% in LdRab5b-overexpressed cells. However, more than 80% of the control cells showed the colocalization of dextran-Texas Red with LysoTracker Green after 90 min of internalization in comparison with only about 26% in LdRab5b-overexpressed cells. Taken together, these results demonstrated that LdRab5a specifically regulates FPE in the parasite.
LdRab5b Specifically Regulates Receptor-mediated Endocytosis of Hb in L. donovani
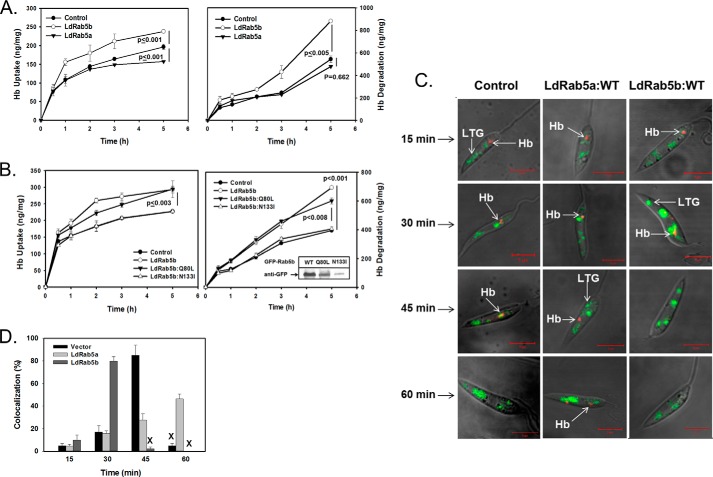
Similar experiments were carried out to determine the role of LdRab5a or LdRab5b in RME in Leishmania promastigotes using Hb as an endocytic probe. In correlation with our previous finding, Hb uptake reached a steady state plateau after 3 h, whereas TCA-soluble radioactivity continued to increase at a linear rate (Fig. 6A), indicating simultaneous uptake and degradation of 125I-Hb by different transgenic Leishmania. Interestingly, our results (Fig. 6A) showed that LdRab5b-overexpressing cells induce almost a 30% increase in 125I-Hb uptake, whereas no significant change in the 125I-Hb uptake is observed in LdRab5a-overexpressing cells in comparison with control cells (Fig. 6A, left). Similarly, LdRab5b-overexpressing cells specifically showed about 2-fold higher degradation of Hb than control cells indicating that overexpression of LdRab5b enhances the degradation of internalized Hb (Fig. 6A, right). In contrast, degradation was found to be almost similar in LdRab5a-overexpressing cells and control cells. In addition, results presented in Fig. 6B showed that overexpression of LdRab5b:WT or LdRab5b:Q80L in Leishmania significantly induces both uptake and degradation of Hb in comparison with control cells. No significant differences in the Hb uptake and degradation were observed in cells overexpressing LdRab5b:N133I mutant in comparison with control cells because most of the mutant protein was degraded inside the cells (Fig. 6B, inset).
FIGURE 6.
LdRab5b specifically regulates the receptor-mediated endocytosis of Hb in L. donovani. A, uptake (left panel) and degradation (right panel) of 125I-Hb was used to measure the receptor-mediated endocytosis of Hb in cells overexpressing GFP-LdRab5a or GFP-LdRab5b. The cells were incubated with 125I-Hb (6 μg) for the indicated times at 23 °C. The uptake and degradation of Hb were measured as mentioned under “Experimental Procedures.” Vector-transfected cells were used as control. Results are expressed as nanograms of Hb per mg of cell protein ± S.D. of three independent experiments. B, similar experiments were carried out using cells overexpressing GFP-LdRab5b:WT, GFP-LdRab5b:Q80L, or GFP-LdRab5b:N133I. Results are expressed as nanograms of Hb per mg of cell protein ± S.D. of three independent experiments. Inset shows the levels of expression of each protein in respective cells. C, determination of the kinetics of receptor-mediated endocytosis of Hb in LdRab5a- or LdRab5b-overexpressing cells. Cells were incubated with Alexa-594-Hb for 5 min at 23 °C, washed, and chased for the indicated period of time at 23 °C. The lysosomal compartments of these cells were stained with LysoTracker Green. Finally, cells were visualized under a confocal microscope. Yellow indicates colocalization. Results are representative of three independent observations. D, quantitative analysis of percentage of cells showing the colocalization of Alexa-594-Hb with LysoTracker Green in different cell types at indicated time points. Results are expressed as mean ± S.D. X indicates the complete degradation of internalized Hb in the respective cells.
To visualize the role of Rab5 isoforms in Hb trafficking in Leishmania, cells were incubated with Alexa 594-Hb for 5 min at 23 °C followed by a chase for different time periods. Subsequently, lysosomes were labeled with LysoTracker Green to determine the kinetics of Hb transport from early endosome to the lysosomes. Our results demonstrated (Fig. 6C, left) that Alexa 594-Hb is internalized into an early compartment near the kinetoplast by 15 min and subsequently moved to a perinuclear late endosomal compartment by about 30 min. Alexa 594-Hb was found to be colocalized with LysoTracker Green-labeled lysosomes at around 45 min (correlation coefficient 0.73), although no Hb was detected by 60 min of Hb internalization in control cells indicating the degradation of internalized Hb. Hb was detected in the perinuclear late endosomal compartment even after 60 min of internalization in Leishmania- overexpressing LdRab5a (Fig. 6C, middle) demonstrating that overexpression of LdRab5a reduces the kinetics of Hb trafficking to the lysosomes in Leishmania. In contrast, Alexa 594-Hb was found to be colocalized with LysoTracker-labeled lysosomes at 30 min (correlation coefficient 0.96), and complete degradation of internalized Hb was detected by 45 min in LdRab5b-overexpressing cells (Fig. 6C, right). Further quantitative analysis of several micrographs (n = 50) showed (Fig. 6D) that Alexa 594-Hb is colocalized with LysoTracker Green-labeled lysosomes in about 80% of LdRab5b-overexpressed cells after 30 min of internalization in comparison with less than 20% in control and LdRab5a-overexpressed cells. However, more than 85% of the control cells showed the colocalization of Alexa 594-Hb with LysoTracker Green after 45 min of internalization, whereas no Hb was detected in more than 95% in LdRab5b-overexpressed cells. In contrast, about 50% of LdRab5a-overexpressed cells showed colocalization after 60 min of Hb internalization, whereas internalized Hb is degraded in more than 95% of the control cells. These results demonstrated that LdRab5b in Leishmania not only induces Hb uptake but also enhances the targeting of internalized Hb to the lysosomes.
Generation and Characterization of Rab5a and Rab5b Knock-out L. donovani
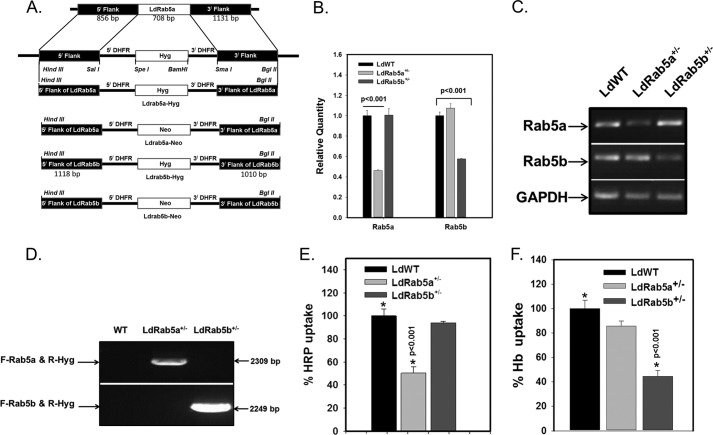
To determine the role of LdRab5a and LdRab5b in various modes of endocytosis in Leishmania, attempts were made to generate Rab5a and Rab5b knock-out Leishmania by targeted gene replacement (31) using appropriate gene knock-out cassettes (Fig. 7A). Essentially, the first copy of the Ldrab5a or Ldrab5b was replaced with the hygromycin resistance gene (HYG, encoding hygromycin phosphotransferase), and single knock-out cells were selected in semisolid medium in the presence of hygromycin. Genomic DNAs were isolated from hygromycin-resistant cells, and quantitative PCR was performed using gene-specific forward and reverse primers. The results presented in Fig. 7B showed about 50% reduction (2−ΔΔCt) in the level of respective Rab5 in single knock-out cells in comparison with WT Leishmania demonstrating successful deletion of the single allele of the indicated gene to generate LdRab5a+/− and LdRab5b+/− cells. These results were further confirmed by RT-PCR (Fig. 7C). These cells were analyzed for correct integration of the hygromycin gene-targeting construct to one of the alleles of Ldrab5a or Ldrab5b by PCR using respective primers. As expected, 2309- and 2249-bp fragments were amplified from LdRab5a+/− and LdRab5b+/− cells, respectively (Fig. 7D). Subsequently, attempts were made to replace the second allele of LdRab5a+/− or LdRab5b+/− cells with the neomycin resistance gene cassettes (Neo, encoding neomycin phosphotransferase). Despite repeated attempts, we failed to obtain viable LdRab5a−/− and LdRab5b−/− cells indicating that functions of both LdRab5a and LdRab5b are possibly essential for parasites. Therefore, LdRab5a+/− and LdRab5b+/− cells were used to determine their role in various modes of endocytosis.
FIGURE 7.
Generation and characterization of Rab5a+/− and Rab5b+/− of Leishmania. A, schematic representation of the generation of LdRab5a or LdRab5b gene knock-out constructs. B, genomic DNA was isolated from the indicated cloned cells, and DNA (35 ng) from respective cells was analyzed by quantitative PCR using LDRab5a- or LdRab5b-specific primers to validate Leishmania:WT, Leishmania:Rab5a+/−, and Leishmania:Rab5b+/−. Amplification of Ld-GAPDH by specific primers (forward, 5′-GAAGTACACGGTGGAGGCTG-3′; reverse, 5′-CGCTGATCACGACCTTCTTC-3′) was used as a control. Results were analyzed with the comparative Ct method (2−ΔΔCt) and are expressed as relative quantity of Rab5a or Rab5b. C, to determine the transcript levels of LdRab5a and LdRab5b, cDNA was prepared from indicated cells, and RT-PCR was carried out using gene-specific primers. GAPDH was used as control. D, to determine the correct integration of LdRab5a-Hyg or LdRab5b-Hyg targeting knock-out cassette to one of the alleles of the LdRab5a or LdRab5b gene, PCR was carried out using forward primer upstream to LdRab5a (forward, 5′-CATCGCAAGCGTCGGTGATGGGC-3′) or LdRab5b (forward, 5′-CCTGTGCAGCCGCGCGCATTG-3′) of the gene-targeting construct and reverse primer (reverse, 5′-CCGCAGGACATATCCACGCCCTC-3′) specific for the hygromycin cassette from genomic DNA isolated from indicated cell types. Results are representative of three independent experiments. E, LdRab5a+/− cells specifically impair the fluid phase HRP uptake. Leishmania:WT, Leishmania:Rab5a+/−, and Leishmania:Rab5b+/− were incubated with 1 mg/ml HRP for 1 h at 23 °C, and HRP uptake by respective cells was measured as described previously. Results are represented as mean ± S.D. of three independent experiments and expressed as relative percentage of HRP uptake by control cells, arbitrarily chosen as 100%. F, LdRab5b+/− cells specifically impair receptor-mediated uptake of Hb. Leishmania:WT, Leishmania:Rab5a+/−, and Leishmania:Rab5b+/− were incubated with 6 μg/ml biotinylated Hb for 2 h at 23 °C, and Hb uptake by respective cells was measured as described under “Experimental Procedures.” Results are represented as mean ± S.D. of three independent experiments and expressed as relative percentage of Hb uptake by control cells, arbitrarily chosen as 100%.
To unequivocally prove the role of LdRab5a and LdRab5b in FPE and RME in Leishmania, we compared the uptake of Hb and HRP in LdRab5a+/− and LdRab5b+/− cells. To determine the role of LdRab5a in FPE Leishmania, we measured the uptake of HRP by these cells and observed about 50% inhibition of HRP uptake in LdRab5a+/− in comparison with control cells (Fig. 7E). No significant change in HRP uptake was detected in LdRab5b+/− cells. Similarly, respective cells were also incubated with 6 μg/ml biotinylated Hb for 2 h at 23 °C and washed, and the amount of Hb present in the cell lysates after immunoprecipitating the internalized Hb with anti-Hb antibody was determined. Our results showed that about 50% of Hb uptake is specifically inhibited in LdRab5b+/− cells in comparison with control cells. No significant change in Hb uptake was observed in LdRab5a+/− cells (Fig. 7F).
Mechanism of Regulation of Various Modes of Endocytosis in L. donovani by Rab5 Isoforms
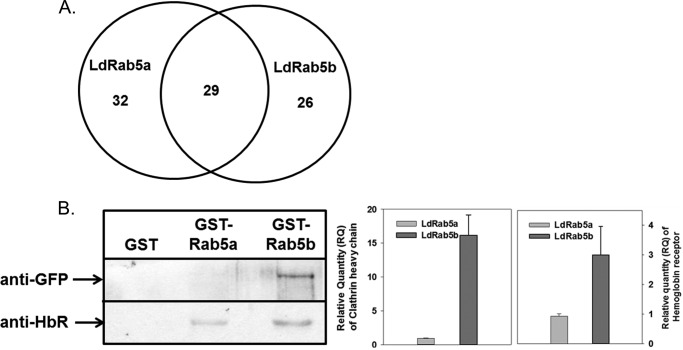
To understand the mechanism of regulation of FPE and RME in Leishmania by LdRab5a and LdRab5b, we tried to identify the putative interacting partners by mass spectrometry. Our interactome analysis showed (Fig. 8A) that 29 proteins might interact with both LdRab5 isoforms, whereas 32 and 26 proteins are specifically associated with LdRab5a and LdRab5b, respectively. However, most of the proteins were found to be unannotated and uncharacterized in Leishmania. Nonetheless, results presented in Table 1 showed some of the putative proteins that were found to be present in LdRab5a and/or LdRab5b interactomes. We found that some enzymes related to glucose metabolism, ATPases, kinases, motor proteins, etc. are present in LdRab5a and LdRab5b interactomes. Most importantly, our results also showed that hexokinase and clathrin heavy chain (LdCHC) are associated with the LdRab5b interactome. These results are consistent with our previous finding that the HbR is a hexokinase (25), and Hb endocytosis a is clathrin-dependent process (23) in Leishmania. Subsequently, we validated these observations using pulldown assay. Our results showed that immobilized GST-LdRab5b specifically binds with LdCHC from GFP-LdCHC-overexpressing Leishmania lysate. Similarly, more pronounced binding of LdRab5b was detected with HbR in comparison with LdRab5a (Fig. 8B).
FIGURE 8.
Identification of putative effectors of LdRab5 isoforms by mass spectrometry. A, to identify the LdRab5a- and LdRab5b-interacting partners, immobilized GST-LdRab5a or GST-LdRab5b was incubated with Leishmania lysate, and proteins bound with respective LdRab5 were analyzed by mass spectrometry as described under “Experimental Procedures.” Venn diagram shows the number of proteins from Leishmania bind with indicated LdRab5. Results are representative of three independent experiments. B, binding of immobilized GST-LdRab5a and GST-LdRab5b with LdCHC or HbR from GFP-LdCHC-overexpressing Leishmania lysate as described under “Experimental Procedures.” Western blot analysis was carried out using anti-GFP and anti-HbR antibodies. All results are representative of three independent observations.
TABLE 1.
Putative interacting partners for LdRab5a and LdRab5b identified by mass spectrometry
Results are representative of three independent observations and + denotes positive interaction. Accession numbers are from UniProt.
| Accession no. | Putative protein | Rab5a | Rab5b |
|---|---|---|---|
| A4HUJ7 | Nuclear transport factor 2 | + | − |
| E9AGE0V | ATPase subunit F | + | − |
| A4HYX7 | Coatomer subunit β | + | − |
| E9BA99 | Mitogen-activated protein kinase 3 | + | − |
| Q4QD34 | Phosphoglycerate kinase | + | − |
| Q4Q6Z4 | Glyceraldehyde- 3-phosphate dehydrogenase | − | + |
| A4HZB1 | Hexokinase | − | + |
| K4HEE6 | Clathrin heavy chain | − | + |
| Q4Q6S8 | ATP synthase, ϵ chain | − | + |
| A4HLD6 | β-Tubulin | − | + |
| I3VJU6 | Activated protein kinase C receptor | − | + |
| A4HUB4 | Ribosomal protein l35a | − | + |
| A4HX43 | Kinesin-like protein | − | + |
| A4I4E4 | ADP ribosylation factor 3 | − | + |
| A4I2L8 | Acyl carrier protein | − | + |
| A4HSB4 | Fructose-1,6-bisphosphatase | − | + |
| O77008 | Casein kinase | − | + |
| A4HV09 | Pyruvate phosphate dikinase | + | + |
| A4I6P8 | Vacuolar-type proton translocating pyrophosphatase | + | + |
| A4I7I7 | Dynein light chain | + | + |
| A4I9P1 | Elongation factor 1-β | + | + |
Discussion
In receptor-mediated endocytosis and fluid-phase endocytosis (4, 5), the endocytic ligands are internalized into Rab5-positive EE and subsequently are sorted to various intracellular destinations (35) like recycling (e.g. transferrin and low density lipoprotein) or degradation (e.g. epidermal growth factor). However, mechanism of sorting is not well characterized. Endosomal sorting could be more complex requiring several signaling molecules like phosphatidylinositol 3-kinase (36), receptor tyrosine kinase (37), and various effectors. Recent studies have shown that APPL1, a Rab5 effector, regulates the differential sorting of EGF and transferrin from endosomes (38). Moreover, cargo sorting might occur at the plasma membrane depending on the mode of entry as clathrin-coated vesicles are also differentially tethered to the two types of endosomes (39). As all Rab5 isoforms are located on EE, it is tempting to speculate that Rab5 isoforms might have a differential role in regulating various modes of endocytosis.
In this study, we have used the unicellular parasite, Leishmania, because this parasite offers a very good model to study endocytosis and intracellular trafficking (23, 26, 27). Moreover, depletion, overexpression, or generation of the null-mutant of target gene/protein shows the phenotype and affects the viability of the cells and thereby indicates the essential functions of the respective Rabs in an organism. Previously, we have shown that Hb endocytosis is a clathrin-mediated process in Leishmania (23–25) and is essential for parasite survival (40). Therefore, we have used Hb as a receptor-mediated endocytic probe and well characterized fluid-phase markers like HRP or dextran (41, 42) to study the role of Rab5 isoforms in different modes of endocytosis in Leishmania.
We have earlier reported that homotypic fusion between early endosomes is LdRab5-dependent in Leishmania (26). Further characterization reveals that previously reported LdRab5 is the LdRab5b isoform (43). Here, we have cloned, expressed, and characterized the LdRab5a homolog from Leishmania. Most importantly, both Rab5 isoforms of Leishmania contain Rab5-specific subfamily motifs (32) and are predominantly localized in EE labeled with 5-min internalized Hb. Taken together, these results indicate that cloned proteins are bona fide Rab5 isoforms from Leishmania. It is important to mention that Leishmania has two isoforms of Rab5, whereas Drosophila melanogaster and Caenorhabditis elegans have only one Rab5 (44, 45), in contrast to three Rab5 isoforms in mammalian cells. These results indicate that Leishmania might have relatively better endosomal organization than Drosophila. melanogaster and Caenorhabditis elegans. Previous studies have shown that TbRab5a and TbRab5b are predominantly associated near the flagellar pocket of Trypanosoma (43). Subsequently, it has been shown that TbRab5a and TbRab5b are differentially expressed in bloodstream form and procyclic form in Trypanosoma, and both are required for the endocytosis of several ligands like tomato lectin, Lucifer Yellow, and LDL in stage-specific parasites (46, 47). However, the role of Rab5 isoforms in specifically regulating FPE and RME in a particular stage of parasites is not yet depicted. We have recently shown that neither PbRab5a nor PbRab5c can complement for the loss of function of PbRab5b indicating their non-overlapping roles in Plasmodium berghei (48). Therefore, we have overexpressed LdRab5a and LdRab5b in Leishmania to determine their roles in various modes of endocytosis in parasites.
We have generated constitutively active and dominant-negative mutants of LdRab5a and LdRab5b and overexpressed them in Leishmania as GFP fusion proteins to determine their specific functions. Our results have shown that overexpression of GFP-LdRab5a:WT and LdRab5a:Q93L specifically induces the fluid-phase endocytosis of HRP and dextran-Texas Red, although no significant difference in HRP uptake was observed in GFP-LdRab5b-overexpressing Leishmania in comparison with control parasites. HRP uptake is found to be significantly inhibited in LdRab5a:N146I-overexpressing transgenic parasites even though substantial amounts of LdRab5a:N146I protein are degraded in these cells. This is consistent with the observation that the dominant-negative mutant of Rab5 is also degraded in Trypanosoma (46). We have failed to generate LdRab5a null-mutants despite repeated attempts demonstrating that LdRab5a function is essential in the parasite. Therefore, we have used heterozygous LdRab5a+/− cells to study RME and FPE. Interestingly, we have found about 50% inhibition of HRP uptake specifically in LdRab5a+/− cells in comparison with WT:Leishmania. No significant difference in Hb endocytosis is observed in these cells. Taken together, these results demonstrate that LdRab5a specifically regulates FPE in the parasite.
In contrast, LdRab5b:WT- and LdRab5b:Q80L-overexpressing cells show enhanced uptake and degradation of Hb in comparison with control cells, but Hb endocytosis is unaffected in LdRab5a-overexpressing cells. To unequivocally prove the role of LdRab5b in RME in Leishmania, we have tried to generate LdRab5b null-mutant Leishmania. However, we have failed to generate the LdRab5b null-mutant despite repeated attempts indicating that LdRab5b function is also essential in the parasite. Therefore, we have used heterozygous LdRab5b+/− cells to study RME and FPE. Interestingly, we have found about 50% inhibition of Hb uptake specifically in LdRab5b+/− cells in comparison with WT:Leishmania. No significant inhibition of HRP uptake is observed in these cells. Taken together, these results demonstrate that LdRab5b specifically regulates RME of Hb in the parasite.
It has been shown in mammalian cells that depending on receptor-ligand interaction, ligand can be internalized into different populations of endosomes; for example, transferrin is internalized into EEA1-positive conventional EE; TGFβ is internalized into endosome enriched with the FYVE domain-containing protein SARA (49); and EGF receptors are shown to be internalized into APPL1-positive EE (50). This could be due to the interaction with different adaptor proteins (51). Similarly, it has been shown in Trypanosoma that TbRab5a is colocalized with endosomes containing variant surface glycoprotein and transferrin, whereas TbRab5b is specifically present in endosomes containing ISG100 speculating that different cargo in Trypanosoma might enter into different compartments (46). However, mechanism of sorting is not clearly established.
It will be interesting to define how these two modes of endocytosis are specifically regulated by Rab5 isoforms in Leishmania. It is well established that Rab5 regulates endocytic trafficking by interacting with several effectors in mammalian cells, e.g. guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), etc. Surprisingly, at least six different Rab5 GEFs like Rabex5, Gapex-5, Rin1, Rin2, Rin3, and Als2 are known today in mammalian cells (52–55). Similarly, RabGap-5 and RN-Tre are Rab5-associated GAPs (56, 57). Thus, the possibility of one Rab5 isoform associated with one specific GEF, GAP, or effector cannot be ruled out. This is supported by the facts that Rin1 selectively activates Rab5a (12), whereas early endosome antigen 1 (EEA1) interacts with Rab5b (58). Thus, it is tempting to speculate that LdRab5 isoforms might interact with specific effectors to regulate a particular mode of endocytosis in Leishmania. However, effectors of different Rabs in trypanosomatid parasites are not yet characterized. We tried to identify the putative interacting partners by mass spectrometry. Surprisingly, our LdRab5a and LdRab5b interactome data do not show any of the conventional Rab5 effectors reported in mammalian cells like EEA1, Rabex5, Rabaptin5, and Rabenosyn5. Moreover, a search for these conventional Rab5 effectors in the L. major database using mammalian Rab5 effectors as query sequences does not show any homologs of these proteins in Leishmania. However, our LdRab5 interactome data predicted a large number of proteins that might interact with LdRab5a and/or LdRab5b, but most of them are unannotated and uncharacterized. Moreover, signal transduction as well as protein trafficking pathways in Leishmania are not well characterized; therefore, the identification of effector molecules is more challenging in parasites. However, we have found some LdRab5 interactors like dynein, kinesin, and tubulin that are shown to regulate endocytosis and vesicular trafficking (59, 60). Moreover, Rab5 is also reported to transport cargo to the nucleus (61); thus, interaction with a nuclear transport factor is an interesting observation. Interestingly, LdRab5b interactome data suggest that this protein might interact with glycolytic enzymes like hexokinase and glyceraldehyde 3-phosphate dehydrogenase. This is possible as the Hb receptor in Leishmania is a hexokinase (25). In addition, cell surface-associated glyceraldehyde-3-phosphate dehydrogenase is reported to be involved in iron acquisition in Staphylococcus (62), and the ATP synthase subunit is found to be an HDL receptor in hepatocytes (63). Moreover, in correlation with our findings, casein kinase-1 is reported to be a Rab5b effector in Plasmodium (64). Our results have also validated that LdRab5b specifically interacts with LdCHC and HbR.
The possibilities of LdRab5 isoforms interacting with different effectors are also supported by the facts that LdRab5a contains an insertion of 21 amino acids (RGAAAAPGGATSAHALQQMRA) in the loop2-β2 region, so it might interact with specific effector(s) to regulate the FPE in Leishmania. LdCHC of Leishmania specifically contains an insertion of 10 amino acid residues (MAQKQDTDLN) in the distal domain (23) and also interacts with LdRab5b, suggesting that LdRab5b-regulated clathrin-mediated endocytosis might be controlled by different effector(s). Taken together, these results suggest that LdRab5b plays a major role in regulating receptor-mediated endocytosis. However, LdRab5a failed to interact with LdCHC thus possibly driving to fluid-phase endocytosis.
In conclusion, our results represent the first demonstration that Rab5 isoforms differentially regulate fluid-phase and receptor-mediated endocytosis in Leishmania. We speculate that regulation of different modes of endocytosis by specific Rab5 isoforms could be due to their interaction with different effectors. Currently, we are trying to identify and understand the role LdRab5a- and LdRab5b-specific effectors in the regulation of various endocytic pathways in parasites.
Author Contributions
A. M. conceived and coordinated the study and wrote the paper. R. R., J. K. V., and A. K. performed the experiments and analyzed the results. G. L. did the bioinformatics and analyzed the data. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Dr. S. M. Beverley (Washington University, St. Louis, MO) and Dr. Jeremy Mottram (University of Glasgow, Glasgow, Scotland, United Kingdom) for providing plasmids. We also acknowledge the help of Dr. Shanta Sen of mass spectrometer facility at NII, New Delhi, India.
This work was supported in part by CEFIPRA Grant 3303-3, the Department of Biotechnology, and a J. C. Bose Fellowship from the Department of Science and Technology, Government of India (to A. M). The authors declare that they have no conflicts of interest with the contents of this article.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) KP308372.
- EE
- early endosome
- RME
- receptor-mediated endocytosis
- FPE
- fluid-phase endocytosis
- LdCHC
- clathrin heavy chain of Leishmania
- HbR
- Hb receptor
- GEF
- guanine nucleotide exchange factor
- GAP
- GTPase-activating protein.
References
- 1.Conner S. D., and Schmid S. L. (2003) Regulated portals of entry into the cell. Nature 422, 37–44 [DOI] [PubMed] [Google Scholar]
- 2.Doherty G. J., and McMahon H. T. (2009) Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 [DOI] [PubMed] [Google Scholar]
- 3.McMahon H. T., and Boucrot E. (2011) Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533 [DOI] [PubMed] [Google Scholar]
- 4.Platta H. W., and Stenmark H. (2011) Endocytosis and signaling. Cur. Opin. Cell Biol. 23, 393–403 [DOI] [PubMed] [Google Scholar]
- 5.Le Roy C., and Wrana J. L. (2005) Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 6, 112–126 [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer S. R. (2013) Rab GTPase regulation of membrane identity. Curr. Opin. Cell Biol. 25, 414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bucci C., Parton R. G., Mather I. H., Stunnenberg H., Simons K., Hoflack B., and Zerial M. (1992) The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70, 715–728 [DOI] [PubMed] [Google Scholar]
- 8.Mukhopadhyay A., Barbieri A. M., Funato K., Roberts R., and Stahl P. D. (1997) Sequential actions of Rab5 and Rab7 regulate endocytosis in the Xenopus oocyte. J. Cell Biol. 136, 1227–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucci C., Lütcke A., Steele-Mortimer O., Olkkonen V. M., Dupree P., Chiariello M., Bruni C. B., Simons K., and Zerial M. (1995) Co-operative regulation of endocytosis by three Rab5 isoforms. FEBS Lett. 366, 65–71 [DOI] [PubMed] [Google Scholar]
- 10.Su X., Lodhi I. J., Saltiel A. R., and Stahl P. D. (2006) Insulin-stimulated interaction between insulin receptor substrate 1 and p85α and activation of protein kinase B/Akt require Rab5. J. Biol. Chem. 281, 27982–27990 [DOI] [PubMed] [Google Scholar]
- 11.Barbieri M. A., Roberts R. L., Gumusboga A., Highfield H., Alvarez-Dominguez C., Wells A., and Stahl P. D. (2000) Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J. Cell Biol. 151, 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen P. I., Kong C., Su X., and Stahl P. D. (2009) Rab5 isoforms differentially regulate the trafficking and degradation of epidermal growth factor receptors. J. Biol. Chem. 284, 30328–30338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Zhao Y., Hu J., Xiao J., Qu L., Wang Z., Ma D., and Chen Y. (2013) A novel ER-localized transmembrane protein, EMC6, interacts with RAB5A and regulates cell autophagy. Autophagy 9, 150–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenfeld J. L., Moore R. H., Zimmer K. P., Alpizar-Foster E., Dai W., Zarka M. N., and Knoll B. J. (2001) Lysosome proteins are redistributed during expression of a GTP-hydrolysis-defective rab5a. J. Cell Sci. 114, 4499–4508 [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Dominguez C., and Stahl P. D. (1999) Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J. Biol. Chem. 274, 11459–11462 [DOI] [PubMed] [Google Scholar]
- 16.Hirota Y., Kuronita T., Fujita H., and Tanaka Y. (2007) A role for Rab5 activity in the biogenesis of endosomal and lysosomal compartments. Biochem. Biophys. Res. Commun. 364, 40–47 [DOI] [PubMed] [Google Scholar]
- 17.Onodera Y., Nam J. M., Hashimoto A., Norman J. C., Shirato H., Hashimoto S., and Sabe H. (2012) Rab5c promotes AMAP1-PRKD2 complex formation to enhance β1 integrin recycling in EGF-induced cancer invasion. J. Cell Biol. 197, 983–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen P. I., Schauer K., Kong C., Harding A. R., Goud B., and Stahl P. D. (2014) Rab5 isoforms orchestrate a “division of labor” in the endocytic network; Rab5C modulates Rac-mediated cell motility. PLoS One 9, e90384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez-Dominguez C., and Stahl P. D. (1998) Interferon-γ selectively induces Rab5a synthesis and processing in mononuclear cells. J. Biol. Chem. 273, 33901–33904 [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharya M., Ojha N., Solanki S., Mukhopadhyay C. K., Madan R., Patel N., Krishnamurthy G., Kumar S., Basu S. K., and Mukhopadhyay A. (2006) IL-6 and IL-12 specifically regulate the expression of Rab5 and Rab7 via distinct signaling pathways. EMBO J. 25, 2878–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field M. C., Natesan S. K., Gabernet-Castello C., and Koumandou V. L. (2007) Intracellular trafficking in the trypanosomatids. Traffic 8, 629–639 [DOI] [PubMed] [Google Scholar]
- 22.Murungi E., Barlow L. D., Venkatesh D., Adung'a V. O., Dacks J. B., Field M. C., and Christoffels A. (2014) A comparative analysis of trypanosomatid SNARE proteins. Parasitol. Int. 63, 341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal S., Rastogi R., Gupta D., Patel N., Raje M., and Mukhopadhyay A. (2013) Clathrin-mediated hemoglobin endocytosis is essential for survival of Leishmania. Biochim. Biophys. Acta 1833, 1065–1077 [DOI] [PubMed] [Google Scholar]
- 24.Sengupta S., Tripathi J., Tandon R., Raje M., Roy R. P., Basu S. K., and Mukhopadhyay A. (1999) Hemoglobin endocytosis in Leishmania is mediated through a 46-kDa protein located in the flagellar pocket. J. Biol. Chem. 274, 2758–2765 [DOI] [PubMed] [Google Scholar]
- 25.Krishnamurthy G., Vikram R., Singh S. B., Patel N., Agarwal S., Mukhopadhyay G., Basu S. K., and Mukhopadhyay A. (2005) Hemoglobin receptor in Leishmania is a hexokinase located in the flagellar pocket. J. Biol. Chem. 280, 5884–5891 [DOI] [PubMed] [Google Scholar]
- 26.Singh S. B., Tandon R., Krishnamurthy G., Vikram R., Sharma N., Basu S. K., and Mukhopadhyay A. (2003) Rab5-mediated endosome-endosome fusion regulates hemoglobin endocytosis in Leishmania donovani. EMBO J. 22, 5712–5722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel N., Singh S. B., Basu S. K., and Mukhopadhyay A. (2008) Leishmania requires Rab7-mediated degradation of endocytosed hemoglobin for their growth. Proc. Natl. Acad. Sci. U.S.A. 105, 3980–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahl S., Parashar S., Malhotra H., Raje M., and Mukhopadhyay A. (2015) Functional characterization of monomeric GTPase Rab1 in the secretory pathway of Leishmania. J. Biol. Chem. 290, 29993–30005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha D. S., Schwarz J. K., Turco S. J., and Beverley S. M. (1996) Use of the green fluorescent protein as a marker in transfected Leishmania. Mol. Biochem. Parasitol. 77, 57–64 [DOI] [PubMed] [Google Scholar]
- 30.Gruenberg J., and Howell K. E. (1989) Membrane traffic in endocytosis: insights from cell-free assays. Annu. Rev. Cell Biol. 5, 453–481 [DOI] [PubMed] [Google Scholar]
- 31.Williams R. A., Smith T. K., Cull B., Mottram J. C., and Coombs G. H. (2012) ATG5 is essential for ATG8-dependent autophagy and mitochondrial homeostasis in Leishmania major. PLoS Pathog. 8, e1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira-Leal J. B., and Seabra M. C. (2000) The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 301, 1077–1087 [DOI] [PubMed] [Google Scholar]
- 33.Li G., and Stahl P. D. (1993) Structure-function relationship of the small GTPase rab5. J. Biol. Chem. 268, 24475–24480 [PubMed] [Google Scholar]
- 34.Zheng Z., Butler K. D., Tweten R. K., and Mensa-Wilmot K. (2004) Endosomes, glycosomes, and glycosylphosphatidylinositol catabolism in Leishmania major. J. Biol. Chem. 279, 42106–42113 [DOI] [PubMed] [Google Scholar]
- 35.Jovic M., Sharma M., Rahajeng J., and Caplan S. (2010) The early endosome: a busy sorting station for proteins at the crossroads. Histol. Histopathol. 25, 99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo L., Wall A. A., Yeo J. C., Condon N. D., Norwood S. J., Schoenwaelder S., Chen K. W., Jackson S., Jenkins B. J., Hartland E. L., Schroder K., Collins B. M., Sweet M. J., and Stow J. L. (2014) Rab8a interacts directly with PI3Kgamma to modulate TLR4-driven PI3K and mTOR signalling. Nat. Commun. 5, 4407. [DOI] [PubMed] [Google Scholar]
- 37.Ménard L., Parker P. J., and Kermorgant S. (2014) Receptor tyrosine kinase c-Met controls the cytoskeleton from different endosomes via different pathways. Nat. Commun. 5, 3907. [DOI] [PubMed] [Google Scholar]
- 38.Kalaidzidis I., Miaczynska M., Brewińska-Olchowik M., Hupalowska A., Ferguson C., Parton R. G., Kalaidzidis Y., and Zerial M. (2015) APPL endosomes are not obligatory endocytic intermediates but act as stable cargo-sorting compartments. J. Cell Biol. 211, 123–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perini E. D., Schaefer R., Stöter M., Kalaidzidis Y., and Zerial M. (2014) Mammalian CORVET is required for fusion and conversion of distinct early endosome subpopulations. Traffic 15, 1366–1389 [DOI] [PubMed] [Google Scholar]
- 40.Guha R., Gupta D., Rastogi R., Vikram R., Krishnamurthy G., Bimal S., Roy S., and Mukhopadhyay A. (2013) Vaccination with Leishmania hemoglobin receptor-encoding DNA protects against visceral leishmaniasis. Sci. Transl. Med. 5, 202ra121. [DOI] [PubMed] [Google Scholar]
- 41.Grab D. J., Webster P., and Lonsdale-Eccles J. D. (1998) Analysis of trypanosomal endocytic organelles using preparative free-flow electrophoresis. Electrophoresis 19, 1162–1170 [DOI] [PubMed] [Google Scholar]
- 42.Engstler M., Thilo L., Weise F., Grünfelder C. G., Schwarz H., Boshart M., and Overath P. (2004) Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J. Cell Sci. 117, 1105–1115 [DOI] [PubMed] [Google Scholar]
- 43.Field H., Farjah M., Pal A., Gull K., and Field M. C. (1998) Complexity of trypanosomatid endocytosis pathways revealed by Rab4 and Rab5 isoforms in Trypanosoma brucei. J. Biol. Chem. 273, 32102–32110 [DOI] [PubMed] [Google Scholar]
- 44.Grant B., and Hirsh D. (1999) Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10, 4311–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wucherpfennig T., Wilsch-Bräuninger M., and González-Gaitán M. (2003) Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 161, 609–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal A., Hall B. S., Nesbeth D. N., Field H. I., and Field M. C. (2002) Differential endocytic functions of Trypanosoma brucei Rab5 isoforms reveal a glycosylphosphatidylinositol-specific endosomal pathway. J. Biol. Chem. 277, 9529–9539 [DOI] [PubMed] [Google Scholar]
- 47.Hall B., Allen C. L., Goulding D., and Field M. C. (2004) Both of the Rab5 subfamily small GTPases of Trypanosoma brucei are essential and required for endocytosis. Mol. Biochem. Parasitol. 138, 67–77 [DOI] [PubMed] [Google Scholar]
- 48.Ezougou C. N., Ben-Rached F., Moss D. K., Lin J. W., Black S., Knuepfer E., Green J. L., Khan S. M., Mukhopadhyay A., Janse C. J., Coppens I., Yera H., Holder A. A., and Langsley G. (2014) Plasmodium falciparum Rab5B is an N-terminally myristoylated Rab GTPase that is targeted to the parasite's plasma and food vacuole membranes. PloS one 9, e87695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y. G., Wang Z., Ma J., Zhang L., and Lu Z. (2007) Endofin, a FYVE domain protein, interacts with Smad4 and facilitates transforming growth factor-β signaling. J. Biol. Chem. 282, 9688–9695 [DOI] [PubMed] [Google Scholar]
- 50.Zoncu R., Perera R. M., Balkin D. M., Pirruccello M., Toomre D., and De Camilli P. (2009) A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 136, 1110–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reider A., and Wendland B. (2011) Endocytic adaptors–social networking at the plasma membrane. J. Cell Sci. 124, 1613–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tall G. G., Barbieri M. A., Stahl P. D., and Horazdovsky B. F. (2001) Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell 1, 73–82 [DOI] [PubMed] [Google Scholar]
- 53.Otomo A., Hadano S., Okada T., Mizumura H., Kunita R., Nishijima H., Showguchi-Miyata J., Yanagisawa Y., Kohiki E., Suga E., Yasuda M., Osuga H., Nishimoto T., Narumiya S., and Ikeda J. E. (2003) ALS2, a novel guanine nucleotide exchange factor for the small GTPase Rab5, is implicated in endosomal dynamics. Hum. Mol. Genet. 12, 1671–1687 [DOI] [PubMed] [Google Scholar]
- 54.Kimura T., Sakisaka T., Baba T., Yamada T., and Takai Y. (2006) Involvement of the Ras-Ras-activated Rab5 guanine nucleotide exchange factor RIN2-Rab5 pathway in the hepatocyte growth factor-induced endocytosis of E-cadherin. J. Biol. Chem. 281, 10598–10609 [DOI] [PubMed] [Google Scholar]
- 55.Su X., Kong C., and Stahl P. D. (2007) GAPex-5 mediates ubiquitination, trafficking, and degradation of epidermal growth factor receptor. J. Biol. Chem. 282, 21278–21284 [DOI] [PubMed] [Google Scholar]
- 56.Lanzetti L., Rybin V., Malabarba M. G., Christoforidis S., Scita G., Zerial M., and Di Fiore P. P. (2000) The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature 408, 374–377 [DOI] [PubMed] [Google Scholar]
- 57.Fuchs E., Haas A. K., Spooner R. A., Yoshimura S., Lord J. M., and Barr F. A. (2007) Specific Rab GTPase-activating proteins define the Shiga toxin and epidermal growth factor uptake pathways. J. Cell Biol. 177, 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Callaghan J., Nixon S., Bucci C., Toh B. H., and Stenmark H. (1999) Direct interaction of EEA1 with Rab5b. Eur. J. Biochem. 265, 361–366 [DOI] [PubMed] [Google Scholar]
- 59.Skjeldal F. M., Strunze S., Bergeland T., Walseng E., Gregers T. F., and Bakke O. (2012) The fusion of early endosomes induces molecular-motor-driven tubule formation and fission. J. Cell Sci. 125, 1910–1919 [DOI] [PubMed] [Google Scholar]
- 60.Liu G., Sanghavi P., Bollinger K. E., Perry L., Marshall B., Roon P., Tanaka T., Nakamura A., and Gonsalvez G. B. (2015) Efficient endocytic uptake and maturation in Drosophila oocytes requires dynamitin/p50. Genetics 201, 631–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y., Low L. H., Putz U., Goh C. P., Tan S. S., and Howitt J. (2014) Rab5 and Ndfip1 are involved in Pten ubiquitination and nuclear trafficking. Traffic 15, 749–761 [DOI] [PubMed] [Google Scholar]
- 62.Modun B., Morrissey J., and Williams P. (2000) The staphylococcal transferrin receptor: a glycolytic enzyme with novel functions. Trends Microbiol. 8, 231–237 [DOI] [PubMed] [Google Scholar]
- 63.Martinez L. O., Jacquet S., Esteve J. P., Rolland C., Cabezón E., Champagne E., Pineau T., Georgeaud V., Walker J. E., Tercé F., Collet X., Perret B., and Barbaras R. (2003) Ectopic β-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature 421, 75–79 [DOI] [PubMed] [Google Scholar]
- 64.Rached F. B., Ndjembo-Ezougou C., Chandran S., Talabani H., Yera H., Dandavate V., Bourdoncle P., Meissner M., Tatu U., and Langsley G. (2012) Construction of a Plasmodium falciparum Rab-interactome identifies CK1 and PKA as Rab-effector kinases in malaria parasites. Biol. Cell 104, 34–47 [DOI] [PMC free article] [PubMed] [Google Scholar]