Abstract
Manganese homeostasis involves coordinated regulation of specific proteins involved in manganese influx and efflux. However, the proteins that are involved in detoxification/efflux have not been completely resolved nor has the basis by which they select their metal substrate. Here, we compared six proteins, which were reported to be involved in manganese detoxification/efflux, by evaluating their ability to reduce manganese toxicity in chicken DT40 cells, finding that human ZnT10 (hZnT10) was the most significant contributor. A domain swapping and substitution analysis between hZnT10 and the zinc-specific transporter hZnT1 showed that residue Asn43, which corresponds to the His residue constituting the potential intramembranous zinc coordination site in other ZnT transporters, is necessary to impart hZnT10's unique manganese mobilization activity; residues Cys52 and Leu242 in transmembrane domains II and V play a subtler role in controlling the metal specificity of hZnT10. Interestingly, the His → Asn reversion mutant in hZnT1 conferred manganese transport activity and loss of zinc transport activity. These results provide important information about manganese detoxification/efflux mechanisms in vertebrate cells as well as the molecular characterization of hZnT10 as a manganese transporter.
Keywords: manganese, metal homeostasis, substrate specificity, transporter, zinc, ATP13A family protein, SPCA1, efflux, ferroportin
Introduction
Manganese is an essential trace element. Manganese is a key cofactor for a variety of enzymes, including glutamine synthetase, superoxide dismutase 2, decarboxylases, and sugar transferases, and thus is indispensable for central nervous system functions, immune functions, and carbohydrate metabolism (1, 2). At elevated levels, however, manganese is toxic, and exposure to this trace element has been associated with various pathogeneses, including a neurological syndrome called manganism, whose symptoms resemble those of Parkinson disease (3, 4). For these reasons, manganese homeostasis must be tightly controlled at a systemic and cellular level. Much of our current understanding of manganese homeostatic mechanisms is derived from genetic studies of Saccharomyces cerevisiae (5, 6), which reveals that a number of membrane transporter/channel proteins contribute to the control of manganese homeostasis. Based on these results, the manganese import system, i.e. manganese transport in the direction of the cytosol, has been extensively investigated in vertebrate cells (7–9). Two ZIP zinc transporters ZIP8 and ZIP14, known to function as zinc uptake proteins, are involved in manganese uptake from the extracellular site (10, 11). In addition, two Nramp transporters Nramp1 and Nramp2/DMT1 are involved in manganese mobilization into the cytosol. Nramp1 acts to mobilize manganese into the cytosol from phagosomes in macrophages, thereby limiting manganese availability to invading microbes (12), whereas Nramp2/DMT1, a major iron transporter, probably functions in the uptake of manganese into the cytosol of all tissue (7). Manganese can be transported via other membrane proteins, including some types of calcium channels (7, 13), although their contribution to cellular manganese homeostasis remains largely unknown.
In contrast, less is known about manganese transporters/channel proteins that participate in manganese detoxification/efflux (13), and further effort is currently needed to completely understand this important process. This is particularly urgent because these transporters/channel proteins may play a role in an established clinical condition caused by excessive manganese accumulation (3, 8, 14). Of the proteins postulated to be involved in manganese detoxification/efflux, the Golgi-localized secretory pathway Ca2+-ATPase 1 (SPCA1), which is an ortholog of S. cerevisiae Pmr1p, is comparatively well characterized (15–17). Moreover, ATP13A2/PARK9 (hereafter ATP13A2) is involved in manganese detoxification (18) based on the observation that the S. cerevisiae ortholog Ypk9p protects cells from toxicity of manganese and other metals by vacuolar sequestration (19, 20). Importantly, loss-of-function mutations of the ATP13A2 gene were identified to cause an autosomal recessive form of early-onset parkinsonism (Kufor-Rakeb syndrome) (21). Recent significant findings show that loss-of-function mutations of the ZnT10/SLC30A10 gene result in parkinsonism with hypermanganesemia, a syndrome of hepatic cirrhosis, polycythemia, and dystonia (22, 23). ZnT10 is localized to the plasma membrane and is functional in manganese metabolism by effluxing cytosolic manganese (24, 25). ZnT10 is also involved in zinc homeostasis in subcellular localization (26, 27). The finding that loss-of-function mutations of ATP13A2 and ZnT10 genes results in the development of parkinsonism accelerates the necessity to clarify manganese detoxification/efflux mechanisms in cells.
We have previously established chicken DT40 cells as an important model system for studying zinc transporter functions using gene-targeting/re-expression strategies (28–30). This system is useful to explore metal homeostasis and membrane transport protein functions in vertebrate cells because DT40 cells have a similar homeostatic regulation system of metals to that of mammalian cells and allow efficient gene disruption because of their high homologous recombination activity (31). In this study, we investigated transporter/channel proteins that function in detoxification/efflux of manganese using DT40 cells deficient in the SPCA1 gene (SPCA1−/−/− cells) because SPCA1 is involved in manganese resistance via its re-delivery into the Golgi apparatus (16, 17). Using SPCA1−/−/− cells, which showed extreme sensitivity to high manganese concentrations, we compared six human proteins that are involved in manganese detoxification/efflux. Moreover, we investigated how human (h)4 hZnT10, which belongs to the ZnT transporter family, could mobilize manganese by domain swapping/substitution mutational analyses. These studies provide molecular information of the transporters/channels involved in manganese detoxification/efflux in vertebrate cells.
Results
Characterization of DT40 Cells Deficient in the SPCA1 Gene
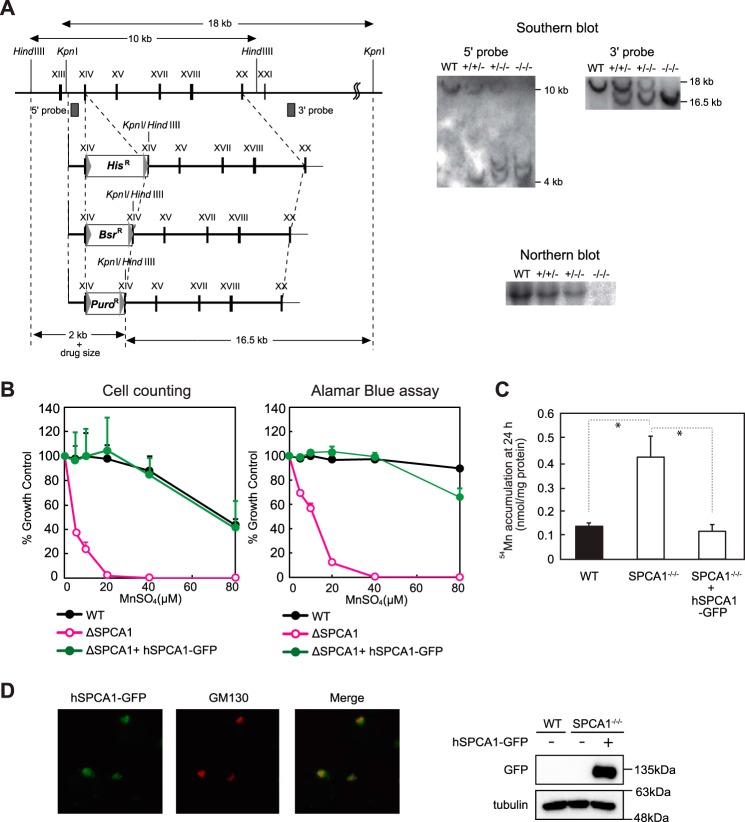
We established DT40 cells deficient in the SPCA1 gene (SPCA1−/−/−) using three KO vectors (Fig. 1A) because DT40 cells have trisomic chromosomes 2. SPCA1−/−/− cells did not show any apparent defects in normal culture, but they showed significantly reduced resistance to high manganese concentrations as expected (Fig. 1B). Here, the cells failed to grow in the presence of 40 μm MnSO4 or higher concentrations. Consistent with this, SPCA1−/−/− cells failed to efflux manganese out of the cells in the manganese retention assay using radioisotope 54Mn (Fig. 1C). Both reduced activities in SPCA1−/−/− cells were completely restored by expression of hSPCA1-GFP (Fig. 1, B and C), indicating that hSPCA1 has a crucial function in manganese homeostasis by controlling its efflux in DT40 cells. hSPCA1-GFP was localized to the Golgi apparatus in SPCA1−/−/−cells (Fig. 1D), indicating that SPCA1 functions by effluxing manganese to the extracellular side via the secretory pathway, as described previously (16, 17).
FIGURE 1.
SPCA1−/−/− cells show significantly reduced resistance to high manganese concentrations. A, targeted disruption of the cSPCA1 gene. Three targeting constructs were designed to disrupt the exon encoding actuator domain. The HisD, Bsr, or Puro drug-resistant marker cassettes were flanked by mutated loxP sites indicated by gray arrowheads. Gray boxes indicate the position of 5′ and 3′ probes. Southern blotting analyses (right upper panels) and Northern blotting analyses (right bottom panel) confirmed the disruption of the SPCA1 gene. B, SPCA1−/−/− cells were significantly sensitive to high manganese concentrations. Cells were grown in the presence of the indicated concentrations of MnSO4 for 2 days, and the number of living cells was counted (left graph) and evaluated by the Alamar Blue assay (right graph). Relative values are plotted as a percentage of living cells without MnSO4 for each group of cells. The growth curves of wild-type (WT), SPCA1−/−/−, and SPCA1−/−/− stably expressing hSPCA1-GFP are shown. Each experiment was performed at least three times. Note that hSPCA1-GFP expression reversed the phenotypes of SPCA1−/−/− cells. C, SPCA1−/−/− cells accumulated high manganese concentrations in the cells. Amounts of manganese in the cells were evaluated by measuring 54Mn accumulated in the cells cultured for 24 h in the presence of 10 μm 54MnCl2. Each value is the mean ± S.D. of three independent experiments (*, p < 0.01). Note that hSPCA1-GFP expression decreased the accumulation of 54Mn in SPCA1−/−/− cells, although the level of accumulated 54Mn in the cells at 24 h does not necessarily reflect steady state levels of cellular manganese. D, subcellular localization of hSPCA1 expressed in SPCA1−/−/− cells. hSPCA1-GFP (green), GM130 (red), and the merged images are shown. Confirmation of stable hSPCA1-GFP expression in SPCA1−/−/− cells by immunoblotting is shown. Ten micrograms of total cellular protein was loaded onto each lane, and the same membrane was used for detection of both hSPCA1 and tubulin. Tubulin is shown as a loading control.
Evaluation of Manganese Detoxification/Efflux Protein Functions in SPCA1−/−/− Cells
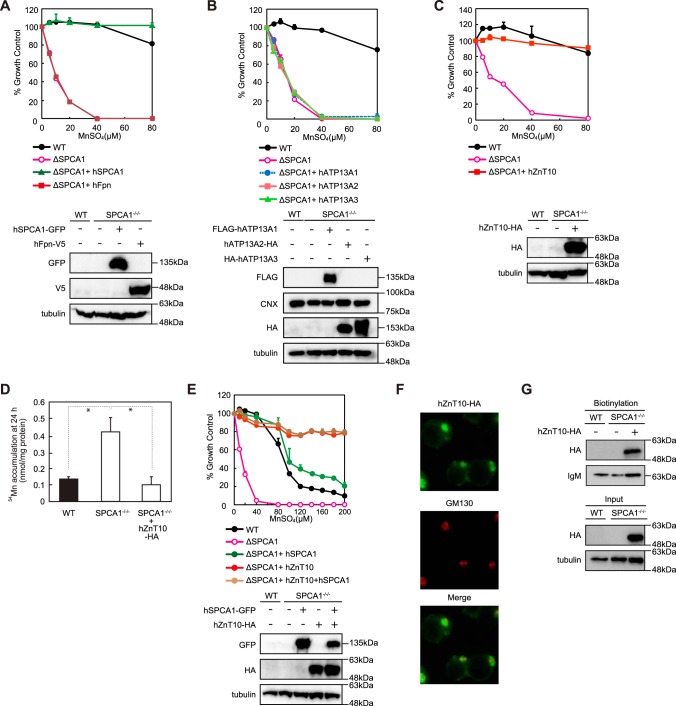
In vertebrates, a number of proteins are involved in manganese detoxification/efflux. We next evaluated these protein functions using SPCA1−/−/− cells by examining restoration of the viability of SPCA1−/−/− cells, stably expressing each of these proteins upon a cytotoxic challenge of increasing manganese concentrations. Specifically, we examined the functions of hFpn, hATP13A1, hATP13A2, hATP13A3, and hZnT10. Fpn locates to the plasma membrane and is indispensable for ferrous iron efflux to the extracellular site, but it has also been shown to mobilize manganese (38, 39). Expression of hFpn, however, failed to confer resistance to high manganese concentrations in SPCA1−/−/− cells, thus indicating that hFpn does not primarily contribute to manganese detoxification/efflux (Fig. 2A). Similarly, we evaluated hATP13A2, which is involved in early-onset parkinsonism, Kufor-Rakeb syndrome (21), and its homologs hATP13A1 and hATP13A3. We also found that hATP13A1–3 showed virtually no effect on the manganese resistance in SPCA1−/−/− cells to high manganese concentrations in the Alamar Blue assays (Fig. 2B). However, a small fraction of the cells cultured at 40 μm MnSO4 appeared morphologically normal by visual observation (data not shown), suggesting that hATP13A1–3 may slightly restore manganese resistance in SPCA1−/−/− cells. We then examined the contribution of hZnT10 to high manganese detoxification. As reported in a complementation assay using an S. cerevisiae pmr1 mutant (23), hZnT10 completely restored the viability of SPCA1−/−/− cells upon high manganese concentrations (Fig. 2C). Consistent with this, hZnT10 decreased accumulated 54Mn in SPCA1−/−/− cells in the manganese retention assay (Fig. 2D). Both results indicate that hZnT10 contributes significantly to manganese detoxification. Co-expression of hZnT10 with hSPCA1 conferred greater resistance to high manganese concentrations than that observed for hSPCA1-only expression, and it restored resistance to a similar level to that of hZnT10-only expression; the cells survived up to a concentration of 200 μm MnSO4 (Fig. 2E). Although we could not examine a reduction of resistance to high manganese concentrations and its restoration by hZnT10 expression in ZnT10-deficient DT40 cells (because DT40 cells do not express ZnT10 mRNA (data not shown)), these results reiterate the key protective function of hZnT10 against high manganese concentrations. hZnT10 was localized mainly to the Golgi apparatus, but weak immunofluorescent signals were detected in the plasma membrane in SPCA1−/−/− cells (Fig. 2F). We closely examined its localization using a surface biotinylation assay with a membrane-impermeable biotinylation reagent and found the biotinylated hZnT10 protein in the cell surface fractions, which confirms that hZnT10 is localized on the cell surface, as reported previously (Fig. 2G) (25). However, the current data cannot be used to infer the relative distribution of hZnT10 between the Golgi apparatus and the plasma membrane because hZnT10 was overexpressed under the control of the strong β-actin promoter (30), which limits our ability to determine whether manganese efflux occurred from the plasma membrane or via a secretory pathway through the Golgi. These results, however, indicate that ZnT10, which is the primary transporter protein in excess manganese detoxification, can efflux manganese on the cell surface to the extracellular site.
FIGURE 2.
Evaluation of the conferment of manganese detoxification using SPCA1−/−/− cells. A, hFpn failed to reverse the phenotypes of SPCA1−/−/− cells. The growth curves of wild-type (WT), SPCA1−/−/−, and SPCA1−/−/− stably expressing hSPCA1-GFP and SPCA1−/−/− stably expressing hFpn-V5 are shown. Confirmation of stable hFpn and hSPCA1 expression in SPCA1−/−/− cells by immunoblotting (lower panels) is shown. B, hATP13A1, hATP13A2, and hATP13A3 had almost no effect on manganese resistance in SPCA1−/−/− cells. The growth curves of wild-type (WT), SPCA1−/−/−, and SPCA1−/−/− stably expressing FLAG-hATP13A1, hATP13A2-HA, or HA-hATP13A3 are shown. Confirmation of stable expression of hATP13A1, hATP13A2, and hATP13A3 in SPCA1−/−/− cells by immunoblotting (lower panels) is shown. C, hZnT10 completely reversed the phenotypes of SPCA1−/−/− cells. The growth curves of wild-type (WT), SPCA1−/−/−, and SPCA1−/−/− stably expressing hZnT10-HA are shown. Confirmation of stable hZnT10 expression in SPCA1−/−/− cells by immunoblotting (lower panels) is shown. D, hZnT10 decreased accumulated 54Mn in SPCA1−/−/− cells. Amounts of accumulated 54Mn in the cells at 24 h were evaluated as in Fig. 1C. Each value is the mean ± S.D. of three independent experiments (*, p < 0.01). E, co-expression of hZnT10 with hSPCA1 conferred more resistance to high manganese concentrations than that of single expression of hSPCA1. The growth curves of wild-type (WT), SPCA1−/−/−, and SPCA1−/−/− stably expressing hZnT10, SPCA1−/−/− stably expressing hSPCA1, and SPCA1−/−/−− stably expressing both hZnT10 and hSPCA1 are shown. Confirmation of stable hZnT10 and hSPCA1 expression in SPCA1−/−/− cells by immunoblotting (lower panels) is shown. Note that co-expression of hZnT10 with hSPCA1 conferred greater resistance to high manganese concentrations compared with that of only hSPCA1 expression. F, immunofluorescence staining of hZnT10 expressed in SPCA1−/−/− cells. hZnT10 (green), GM130 (red), and the merged images are shown. G, cell surface localization of hZnT10 evaluated by the surface biotinylation assay. Cells treated with the biotinylation reagent (sulfo-NHS-SS-biotin) were solubilized, and the biotinylated protein was then captured using streptavidin beads and analyzed by immunoblot analysis. Input refers to aliquots of the biotinylated proteins before avidin capture (i.e. total cell lysate), although biotinylation refers to avidin-captured proteins. Tubulin and IgM were used as loading controls for input and biotinylation, respectively. The representative results of three independent experiments are displayed. A–C and E, cells were grown in the presence of the indicated concentrations of MnSO4 for 2 days, and the numbers of living cells were evaluated by the Alamar Blue assay at least three times. Tubulin and calnexin (CNX) are shown as the loading controls.
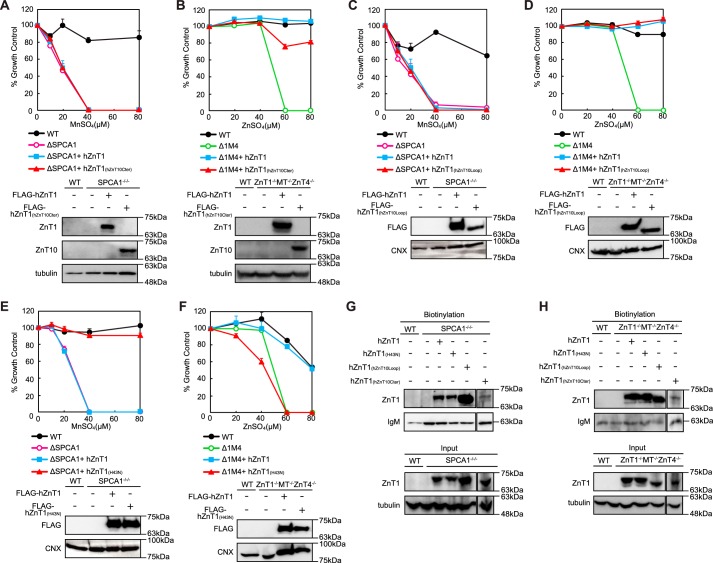
ZnT10 Is Involved in Excess Manganese Detoxification but Not in Excess Zinc Detoxification
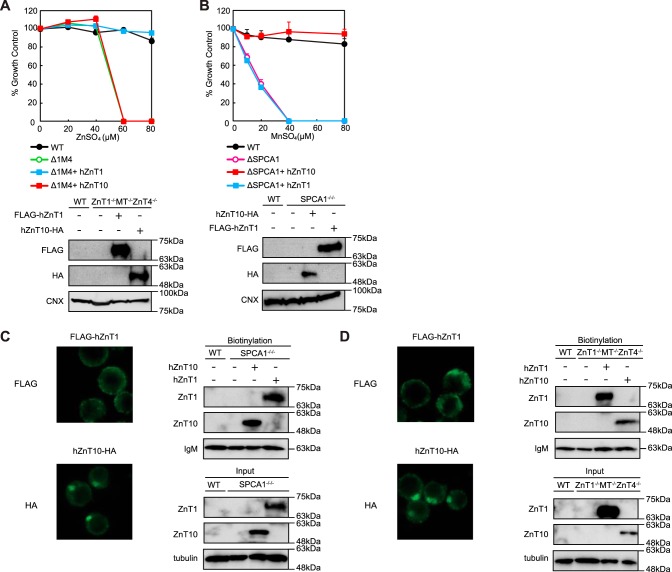
ZnT10 belongs to the zinc transporter family but is involved in manganese mobilization for its detoxification. Consequently, we investigated whether hZnT10 is involved in excess zinc detoxification using ZnT1−/−MT−/−ZnT4−/− cells, which were used previously to evaluate zinc detoxification activity (33, 40). Expression of hZnT10 had almost no effect on the resistance of ZnT1−/−MT−/−ZnT4−/− cells to high zinc concentrations (Fig. 3A). Although the Alamar Blue assays showed no contribution to detoxification at a zinc concentration of 60 μm ZnSO4 or higher, visual observation of the cells cultured in the high zinc conditions showed that a small fraction of the cells appeared still morphologically normal (data not shown). To examine metal transport specificity of hZnT10 in more detail, we compared hZnT10 with that of hZnT1 because ZnT1 and ZnT10 can be subdivided into the same subfamily based on sequence similarities (37% identity between hZnT1 and hZnT10) (41, 42). Expression of hZnT1 reversed the zinc-sensitive phenotype of ZnT1−/−MT−/−ZnT4−/− cells, as described previously (33), but failed to confer manganese resistance in SPCA1−/−/− cells (Fig. 3B). These results clearly showed that expression of hZnT1 or hZnT10 conferred completely different metal resistance in cells. We have not yet examined the cell surface localization of hZnT1 in DT40 cells (30, 33), but close examination by immunofluorescence staining and the surface biotinylation assay showed that hZnT1 was localized to the cell surface in both SPCA1−/−/− (Fig. 3C) and ZnT1−/−MT−/−ZnT4−/− cells (Fig. 3D). The cell surface localization of hZnT10 was confirmed in both cells in parallel studies (Fig. 3, C and D).
FIGURE 3.
ZnT10 is involved in manganese transport rather than zinc transport. A, expression of hZnT10 did not restore zinc resistance of ZnT1−/−MT−/−ZnT4−/− cells. Cells stably expressing hZnT10 or hZnT1 were grown in the presence of the indicated concentrations of ZnSO4 for 2 days, and the numbers of living cells were evaluated by the Alamar Blue assay. Confirmation of stable hZnT10 or hZnT1 expression in ZnT1−/−MT−/−ZnT4−/− cells by immunoblotting (lower panels) is shown. B, expression of hZnT1 failed to confer manganese resistance of SPCA1−/−/− cells. Cells stably expressing hZnT1 or hZnT10 were grown in the presence of the indicated concentrations of MnSO4 for 2 days, and the number of living cells were evaluated by the Alamar Blue assay. Confirmation of stable hZnT10 or hZnT1 expression in SPCA1−/−/− cells by immunoblotting (lower panels) is shown. A and B, Alamar Blue assay was performed at least three times. Calnexin (CNX) is shown as the loading controls. C, plasma membrane localizations of hZnT1 and hZnT10 expressed in SPCA1−/−/−cells is shown. Immunofluorescence staining of both proteins was performed as presented in Fig. 2F). The biotinylation assay was performed as in Fig. 2G. D, plasma membrane localizations of hZnT1 and hZnT10 expressed in ZnT1−/−MT−/−ZnT4−/− cells are shown. Immunofluorescence staining of both proteins was performed as in Fig. 2F. The biotinylation assay was performed as in Fig. 2G. C and D, tubulin and IgM were used as loading controls for input and biotinylation, respectively. The representative results of three independent experiments are presented.
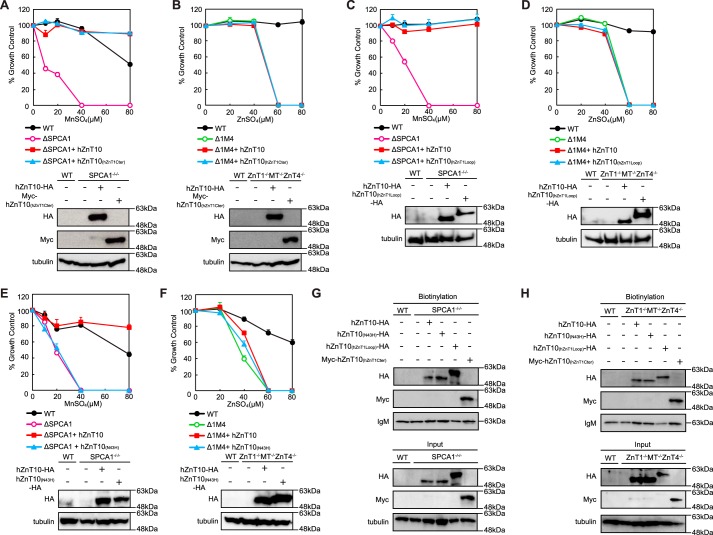
Domain Swapping/Substitution Analysis between hZnT10 and hZnT1
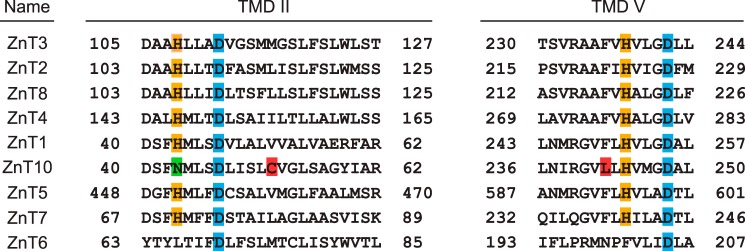
To examine the differences in their metal resistance activity more closely, we constructed domain swapping/substitution mutants between hZnT10 and hZnT1 by considering the unique properties of ZnT10. Specifically, ZnT10 contains an Asn residue instead of a His residue at the conserved position of transmembrane domain (TMD) II, which is thought to form the intramembranous tetrahedral zinc coordination site in other ZnT transporters, including ZnT1 (Fig. 4) (33, 43–48). Moreover, ZnT10 has Arg- and Lys-rich sequences in the cytosolic loop between TMDs III and IV and in the cytosolic C-terminal region, although the cytosolic loop is known to be rich in His residues in other ZnT transporters (49–51). Initially, we expressed the hZnT10 mutants, specifically hZnT10(N43H), hZnT10(hZnT1Cter), or hZnT10(hZnT1Loop) in SPCA1−/−/− or ZnT1−/−MT−/−ZnT4−/− cells, in which the Asn residue in TMD II was substituted with His, the cytosolic C-terminal region, or the cytosolic loop between TMDs III and IV was swapped with corresponding regions of hZnT1. We then examined whether these constructs conferred and altered manganese or zinc resistance in SPCA1−/−/− or ZnT1−/−MT−/−ZnT4−/− cells. Expression of hZnT10(hZnT1Cter) and hZnT10(hZnT1Loop) did not alter manganese resistance in SPCA1−/−/− cells (Fig. 5, A and C) or zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells (Fig. 5, B and D) when compared with that of hZnT10, indicating that the cytosolic C-terminal region or the cytosolic loop between TMDs III and IV of hZnT1 did not impair manganese transport by hZnT10, and the corresponding regions of hZnT10 are not essential for manganese resistance. In contrast, expression of hZnT10(N43H) significantly decreased manganese resistance in SPCA1−/−/− cells (Fig. 5E) and did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells (Fig. 5F). The surface biotinylation assay confirmed that all hZnT10 mutants were localized to the cell surface in both SPCA1−/−/− (Fig. 5G) or ZnT1−/−MT−/−ZnT4−/− cells (Fig. 5H), which excludes the possibility that mislocalization of hZnT10(N43H) in SPCA1−/−/− cells resulted in a decrease in manganese resistance and indicates that the Asn residue in TMD II is extremely important for the manganese transport activity of hZnT10. Taken together, Arg- and Lys-rich sequences in the cytosolic C-terminal region and in the cytosolic loop between TMDs III and IV are not involved in the manganese transport property of hZnT10, whereas the Asn residue in TMD II primarily contributes to manganese transport.
FIGURE 4.
Multiple sequence alignment of TMDs II and V among hZnT transporters. The sequences of TMDs II and V of hZnT transporters were aligned. The sequence order is according to their sequence similarity (41). The conserved His and Asp residues postulated as the zinc-binding site in TMDs II and V are highlighted in orange and blue. Residue Asn43 in TMD II of hZnT10 is highlighted in green. Cys52 and Leu242 residues of hZnT10 that were investigated in Fig. 9 are shown in red. The indicated TMDs of hZnT5 correspond to TMDs XI and XIV.
FIGURE 5.
Asn residue in TMD II of hZnT10 is essential for manganese transport activity. A, expression of hZnT10(hZnT1Cter) did not alter manganese resistance in SPCA1−/−/− cells. B, expression of hZnT10(hZnT1Cter) did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. C, expression of hZnT10(hZnT1Loop) did not alter manganese resistance in SPCA1−/−/− cells. D, expression of hZnT10(hZnT1Loop) did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. E, expression of hZnT10(N43H) significantly decreased manganese resistance in SPCA1−/−/− cells. F, expression of hZnT10(N43H) did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. In A, C, and E or in B, D, and F, cells were grown as in Fig. 3, A and B, and the numbers of living cells were evaluated by the Alamar Blue assay. In A–F, Alamar Blue assay was performed at least three times. Confirmation of stable expression of WT and mutants of hZnT10 in SPCA1−/−/− cells or ZnT1−/−MT−/−ZnT4−/− cells by immunoblotting (lower panels) is shown. Tubulin is shown as a loading control. G, cell surface localization of hZnT10 mutants in SPCA1−/−/− cells was evaluated by the surface biotinylation assay. H, the cell surface localization of hZnT10 mutants in ZnT1−/−MT−/−ZnT4−/− cells was evaluated by the surface biotinylation assay. In G and H, the biotinylation assay was performed as in Fig. 2G. The representative results of three independent experiments are presented.
We then constructed the domain swapping/substitution mutants of hZnT1 with hZnT10 using a similar approach, specifically hZnT1(H43N), hZnT1(hZnT10Cter), and hZnT1(hZnT10Loop), and performed the same experiments. Expression of hZnT1(hZnT10Cter) and hZnT1(hZnT10Loop) did not confer manganese resistance in SPCA1−/−/− cells (Fig. 6, A and C) and did not alter zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells (Fig. 6, B and D), as in the case of hZnT10. Unexpectedly, expression of hZnT1(H43N), however, did confer manganese resistance in SPCA1−/−/− cells (Fig. 6E), and the ability to confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells was lost (Fig. 6F), indicating that the Asn residue in TMD II can confer manganese transport activity to ZnT1. The surface biotinylation assay confirmed that all hZnT1 mutants were localized at the cell surface in both SPCA1−/−/− (Fig. 6G) and ZnT1−/−MT−/−ZnT4−/− cells (Fig. 6H). Taken together, the His-rich clusters in the cytosolic loop between TMDs III and IV of hZnT1 and the cytosolic C-terminal region of hZnT1 are not essential in determining the zinc transport property of hZnT1, although the His residue in TMD II is crucial. Moreover, only substitution of the Asn residue in TMD II is a major determinant of manganese specificity in hZnT1. Moreover, these results reveal that the cytosolic loop between TMDs III and IV and the cytosolic C-terminal region are compatible with each other between hZnT10 and hZnT1 in their manganese or zinc transport activities, despite the presence of several unique differences. The results of Figs. 3, 5, and 6 are summarized in Table 1.
FIGURE 6.
Substitution of Asn residue for His residue in TMD II confers the activity to transport manganese with hZnT1. A, expression of hZnT1(hZnT10Cter) did not confer manganese resistance in SPCA1−/−/− cells. B, expression of hZnT1(hZnT10Cter) did not alter zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. C, expression of hZnT1(hZnT10Loop) did not confer manganese resistance in SPCA1−/−/− cells. D, expression of hZnT1(hZnT10Loop) did not alter zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. E, expression of hZnT1(H43N) did confer manganese resistance in SPCA1−/−/− cells. F, expression of hZnT1(H43N) lost the ability to confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. In A, C, and E or in B, D, and F, cells were grown as in Fig. 3, A and B, and the numbers of living cells were evaluated by the Alamar Blue assay. A–F, Alamar Blue assay was performed at least three times. Confirmation of stable WT and mutants of hZnT1 expression in SPCA1−/−/− cells or ZnT1−/−MT−/−ZnT4−/− cells by immunoblotting (lower panels) is shown. Tubulin and calnexin (CNX) are shown as the loading controls. G, cell surface localization of hZnT1 mutants in SPCA1−/−/− cells was evaluated by the surface biotinylation assay. H, cell surface localization of hZnT1 mutants in ZnT1−/−MT−/−ZnT4−/− cells was evaluated by the surface biotinylation assay. G and H, hZnT1(hZnT10Cter) was detected by an anti-hZnT10 antibody, whereas WT ZnT1 and other hZnT1 mutants were detected by an anti-hZnT1 antibody. The biotinylation assay was performed as in Fig. 2G. The representative results of three independent experiments are displayed.
TABLE 1.
Conferment of manganese and zinc resistance by hZnT10 or hZnT1 and their domain swapped/substituted mutants in SPCA1−/−/− and ZnT1−/−MT−/−ZnT4−/− cells
Relative values presented are evaluations of the results shown in Figs. 3, 5, and 6 as follows. +++, >75% viability compared with that of WT in SPCA1−/−/ − cells at 40 μm MnSO4 or that of WT in ZnT1−/−MT−/−ZnT4−/− cells at 60 μm ZnSO4; ++, +, less growth (25–75%, or ≤25% relative to the viability of each WT); −, no growth.
| Expressed gene | Manganese resistance in SPCA1−/−/− cells | Zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells |
|---|---|---|
| hZnT10 | +++ | −a |
| hZnT1 | − | +++ |
| hZnT10(N43H) | −a | − |
| hZnT1(H43N) | +++ | − |
| hZnT10(hZnT1Cter) | +++ | − |
| hZnT1(hZnT10Cter) | − | ++ |
| hZnT10(hZnT1Loop) | +++ | − |
| hZnT1(hZnT10Loop) | − | +++ |
a A small fraction of the cells appeared morphologically normal by visual observation.
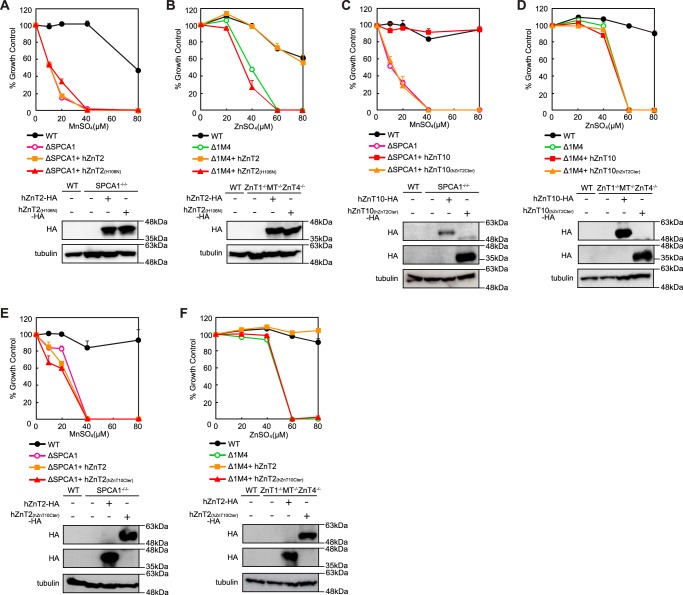
Domain Swapping/Substitution Analysis between hZnT10 and hZnT2
We performed similar swapping/substitution analysis between hZnT10 and hZnT2, using hZnT2(H106N), hZnT10(hZnT2Cter), and hZnT2(hZnT10Cter) mutants. hZnT2 is divided into a different subfamily from hZnT10 and hZnT1 and thus shows only moderate similarity with hZnT10 (28% identity between hZnT10 and hZnT2) (41, 42). hZnT2 expression completely reversed the zinc-sensitive phenotypes of ZnT1−/−MT−/−ZnT4−/− cells (33, 40), but unlike hZnT1(H43N), expression of hZnT2(H106N) failed to confer manganese resistance in SPCA1−/−/− cells (Fig. 7A). However, the hZnT2(H106N) mutant lost the ability to reverse zinc-sensitive phenotypes of ZnT1−/−MT−/−ZnT4−/− cells, as observed for the hZnT1(H43N) mutant (Fig. 7B). Moreover, the swapping of the cytosolic C-terminal region between hZnT10 and hZnT2 caused these mutants to lose manganese and zinc resistance in SPCA1−/−/− cells and ZnT1−/−MT−/−ZnT4−/− cells. Specifically, expression of hZnT10(hZnT2Cter) failed to reverse manganese resistance in SPCA1−/−/− cells and that of hZnT2(hZnT10Cter) failed to reverse zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells (Fig. 7, C and F). In contrast, expression of hZnT10(hZnT2Cter) did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells, whereas that of hZnT2(hZnT10Cter) did not confer manganese resistance in SPCA1−/−/− cells (Fig. 7, D and E). These results suggest that the cytosolic C-terminal region is not compatible between hZnT10 and hZnT2 in their manganese or zinc transport activity.
FIGURE 7.
Domain swapping and substitution analysis between hZnT10 and hZnT2. A, expression of hZnT2(H106N) failed to confer manganese resistance in SPCA1−/−/− cells. B, expression of hZnT2(H106N) lost the ability to confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. C, expression of hZnT10(hZnT2Cter) lost the ability to confer manganese resistance in SPCA1−/−/− cells. D, expression of hZnT10(hZnT2Cter) did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. E, expression of hZnT2(hZnT10Cter) did not confer manganese resistance in SPCA1−/−/− cells. F, expression of hZnT2(hZnT10Cter) lost the ability to confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. In A, C, and E or in B, D, and F, cells were grown as presented in Fig. 3, A and B, and the numbers of living cells were evaluated by the Alamar Blue assay. A–F, Alamar Blue assay was performed at least three times. Confirmation of stable expression of WT and mutants of hZnT2 and hZnT10 in SPCA1−/−/− cells or ZnT1−/−MT−/−ZnT4−/− cells by immunoblotting (lower panels) is shown. Tubulin is shown as the loading control.
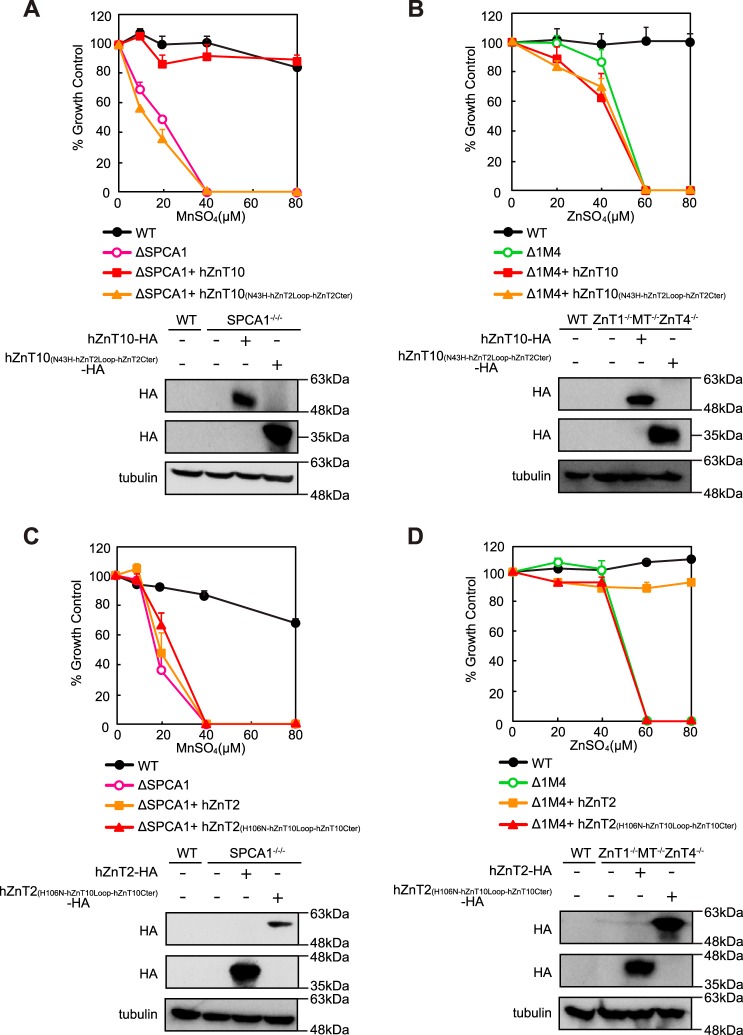
We then investigated this incompatibility using complex domain swapping/substitution mutants between hZnT10 and hZnT2 in which all of the three aforementioned features were swapped and substituted. As observed for each mutant between hZnT10 and hZnT2, expression of hZnT10(N43H-hZnT2Loop-hZnT2Cter) lost the ability to confer manganese resistance in SPCA1−/−/− cells, whereas that of hZnT2(H106N-hZnT10Loop-hZnT10Cter) lost the ability to confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells (Fig. 8, A and D). Moreover, these swapping/substitutions did not have any effects on the metal substrate specificity between hZnT10 and hZnT2. Expression of hZnT10(N43H-hZnT2Loop-hZnT2Cter) did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells, and expression of hZnT2(H106N-hZnT10Loop-hZnT10Cter) did not confer manganese resistance in SPCA1−/−/− cells (Fig. 8, B and C). Taken together, unlike the case of hZnT1 and hZnT10, the abovementioned featured domains are incompatible between hZnT10 and hZnT2. The lower similarity of hZnT2 to hZnT10 than hZnT1 to hZnT10 may endow incompatibility with domain swapping/substitutions between hZnT10 and hZnT2. The results of Figs. 7 and 8 are summarized in Table 2.
FIGURE 8.
Domain swapping and substitution of specific sequences failed to be compatible between hZnT10 and hZnT2. A, expression of hZnT10(N43H-hZnT2Loop-hZnT2Cter) lost the ability to confer manganese resistance in SPCA1−/−/− cells. B, expression of hZnT10(N43H-hZnT2Loop-hZnT2Cter) did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. C, expression of hZnT2(H106N-hZnT10Loop-hZnT10Cter) did not confer manganese resistance in SPCA1−/−/− cells. D, expression of hZnT2(H106N-hZnT10Loop-hZnT10Cter) lost the ability to zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. In A and C or in B and D, cells were grown as presented in Fig. 3, A and B, and the numbers of living cells were evaluated by the Alamar Blue assay. A–D, Alamar Blue assay was performed at least three times. Confirmation of stable expression of WT and mutants of hZnT2 and hZnT10 in SPCA1−/−/−cells or ZnT1−/−MT−/−ZnT4−/− cells by immunoblotting (lower panels) is shown. Tubulin is shown as the loading control.
TABLE 2.
Conferment of manganese and zinc resistance by hZnT10 or hZnT2 and their domain swapped/substituted mutants in SPCA1−/−/− and ZnT1−/−MT−/−ZnT4−/− cells
Relative values presented are evaluations of the results shown in Figs. 7 and 8, as described in Table 1.
| Expressed gene | Manganese resistance in SPCA1−/−/− cells | Zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells |
|---|---|---|
| hZnT10 | +++ | −a |
| hZnT2 | − | +++ |
| hZnT10(N43H) | − | − |
| hZnT2(H106N) | − | − |
| hZnT10(hZnT2Cter) | − | − |
| hZnT2(hZnT10Cter) | − | −a |
| hZnT10(N43H-hZnT2Loop-hZnT2Cter) | − | − |
| hZnT2(H106N-hZnT10Loop-hZnT10Cter) | − | − |
a A small fraction of the cells appeared morphologically normal by visual observation.
Residues Cys52 and Leu242 in the TMDs II and V of hZnT10 Are Involved in the Control of Metal Substrate Specificity in hZnT10
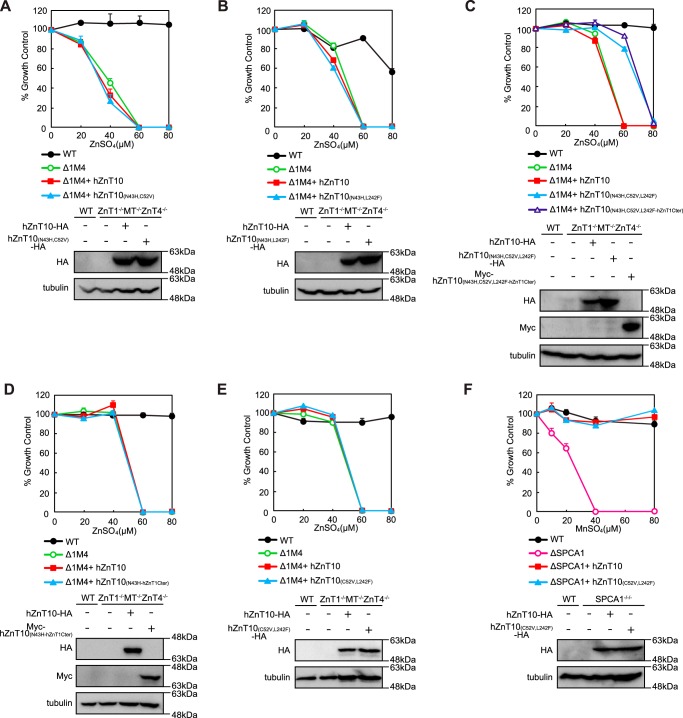
Expression of hZnT10(N43H) failed to reverse zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells despite the fact that reverse substitution of hZnT1 (hZnT1(H43N)) conferred manganese resistance to hZnT1 in the manganese detoxification assay using SPCA1−/−/− cells. This observation indicates that hZnT10 may have a specific mechanism to reduce zinc transport. Finally, we tried to identify specific residues in hZnT10 that are involved in this mechanism. We found that residue Cys52 in TMD II and Leu242 in TMD V are present in hZnT10 but not in other hZnT transporters (Fig. 4) and that both residues are thought to be located on the cytosolic side of the putative intramembranous tetrahedral metal coordination site, based on the structure of a bacterial homolog YiiP (42–46). In other hZnT transporters, these positions are predominantly composed of Val, Ile, and Met in TMD II and Phe in TMD V (Fig. 4). We expressed hZnT10 mutants in ZnT1−/−MT−/−ZnT4−/− cells, in which the Cys and/or Leu residues were substituted with Val and Phe, respectively, in addition to the N43H substitution (hZnT10(N43H,C52V), hZnT10(N43H,L242F) and hZnT10(N43H,C52V,L242F)) (Fig. 9, A–C). Expression of neither hZnT10(N43H,C52V) nor hZnT10(N43H,L242F) reversed zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells (Fig. 9, A and B). However, expression of hZnT10(N43H,C52V,L242F) moderately restored the ability to confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells (Fig. 9C). The cells grew in the presence of 60 μm ZnSO4 but failed to grow in the presence of 80 μm ZnSO4. Moreover, we investigated whether the conferment of zinc resistance by hZnT10(N43H,C52V,L242F) was facilitated by domain swapping of the cytosolic C-terminal region of hZnT1 with the corresponding region (hZnT10(N43H,C52V,L242F-hZnT1Cter)). Swapping of the cytosolic C-terminal region did not lead to significant effects (Fig. 9C). Consistent with this, expression of hZnT10(N43H-hZnT1Cter) did not significantly reverse zinc-sensitive phenotypes of ZnT1−/−MT−/−ZnT4−/− cells (Fig. 9D). Finally, we examined whether substitution of Cys52 and Leu242 with Val and Phe in hZnT10 (hZnT10(C52V,L242F)) may confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells or impair manganese resistance in SPCA1−/−/− cells. However, these mutations did not confer zinc resistance or impair manganese resistance in the respective cell types (Fig. 9, E and F). These results indicate that, in addition to the primary role of Asn43, both Cys52 in TMD II and Leu242 in TMD V of hZnT10 are involved in the regulation of metal substrate specificity in hZnT10.
FIGURE 9.
Residues Cys52 and Leu242 in the TMDs II and IV of hZnT10 are involved in the control of metal substrate specificity in hZnT10. A, expression of hZnT10(N43H,C52V) did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. B, expression of hZnT10(N43H,L242F) did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. C, expression of hZnT10(N43H,C52V,L242F) and hZnT10(N43H,C52V,L242F-hZnT1Cter) partially restored the ability to confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. D, expression of hZnT10(N43H-hZnT1Cter) did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. E, expression of hZnT10(C52V,L242F) did not confer zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells. F, expression of hZnT10(C52V,L242F) did not impair manganese resistance in SPCA1−/−/− cells. In A–E or in F, cells were grown as presented in Fig. 3, A and B, and the numbers of living cells were evaluated by the Alamar Blue assay. The Alamar Blue assay was performed at least three times in A and B and D–F and four times in C. Confirmation of stable expression of hZnT10 mutants in SPCA1−/−/− cells or ZnT1−/−MT−/−ZnT4−/− cells by immunoblotting (lower panels) is shown. Tubulin is shown as the loading control.
Discussion
Genetic studies using S. cerevisiae have contributed significantly to our understanding of manganese metabolism in cells; however, several differences exist between S. cerevisiae and vertebrate cells. For example, Ccc1p functions as a major transporter of iron and manganese that are sequestered into vacuoles and thus detoxifies manganese in S. cerevisiae (52) but is not identified in vertebrate cells. In contrast, Fpn and ZnT10 are expressed in vertebrate cells but are not present in S. cerevisiae. Moreover, in an manganese resistance assay using a pmr1 mutant of S. cerevisiae, extremely high manganese concentrations were required to discriminate manganese resistance (53, 54) when compared with those in vertebrate cells. Thus, manganese metabolism should be studied in vertebrate cells. In this study, we compared six human proteins that are involved in manganese detoxification/efflux by using genetically engineered vertebrate cells, SPCA1−/−/− cells, whose extreme sensitivity to high manganese concentrations enabled us to evaluate their contribution to manganese toxicity without directly measuring manganese mobilization.
The manganese detoxification/efflux functions of the evaluated six proteins were examined in different evaluation cell systems, and thus their contributions have not yet been directly compared with each other. This study is important in this aspect because we evaluated the contribution of each protein in the same cell system using SPCA1−/−/− cells. ATP13A proteins, including ATP13A2, are postulated to contribute to manganese detoxification/efflux in both yeast and vertebrate cells when overexpressed (18–20). However, our results indicate that ATP13A proteins have almost no contribution to manganese detoxification/efflux under high manganese conditions. ATP13A2 is suggested to be involved in intracellular zinc homeostasis (55, 56), but our re-experiments revealed no contribution of hATP13A2 to conferring zinc resistance in ZnT1−/−MT−/−ZnT4−/− cells under high zinc concentrations.5 Fpn is also reported to be involved in manganese mobilization (38, 39). However, our results indicate that hFpn does not contribute to manganese detoxification/efflux in SPCA1−/−/− cells under high manganese concentrations. In contrast to these proteins, SPCA1 and ZnT10 operate to detoxify/efflux manganese via exporting manganese to an extracellular site in vertebrate cells. Our results indicate that ZnT10 is a primary contributor in the detoxification/efflux of manganese, which may reflect that only mutations to the ZnT10 gene have been identified as parkinsonism, although mutations of the SPCA1 gene results in Hailey-Hailey diseases (MIM accession number 169600), which is thought to be related to calcium levels and not to disturbances in manganese metabolism (57).
ZnT10 has received much attention because the loss of ZnT10 function results in parkinsonism with hypermanganesemia (22, 23), despite belonging to ZnT zinc transporters. However, the molecular basis of this condition is missing as well as how ZnT10 recognizes and mobilizes manganese as a transport substrate. Among several characteristic amino acid residues and sequences, we clearly showed that Asn43 in TMD II plays a critical role for ZnT10 function. Interestingly, hZnT1(H43N) converted hZnT1 from zinc mobilization to manganese mobilization. These results confirmed that the position in TMD II is important for determining the transport metal specificity (42, 51), as reported previously using hZnT5 and hZnT8, in which substitution of the His residue with an Asp residue conferred cadmium transport activity in addition to zinc (58). The plant MTP8, which transports manganese but not zinc, has an Asp amino acid at this position. MTP8 has four Asp residues, including the Asp residue in TMDs II and V, which are thought to form an intramembranous tetrahedral metal coordination site (59, 60). Thus, the finding that the Asn residue at this position of TMD II regulates manganese transport ability is unique, and thus clarification of its specific importance should provide insight into understanding the metal coordination properties in TMDs of ZnT transporters and their homologs. The CDF family of proteins are generally divided into three groups based on their phylogenetic relationships as follows: Zn-CDF, Zn/Fe-CDF, and Mn-CDF (51, 61). In this grouping, CDF proteins with the ability to transport manganese, such as MTP8, are classified into the Mn-CDF group, although all of the ZnT transporters are divided into the Zn-CDF family (51, 61, 62). Thus, careful re-grouping should be performed.
This study also revealed that the cytosolic loop between TMDs III and IV and the cytosolic C-terminal region are compatible between hZnT1 and hZnT10. This observation is intriguing because the cytosolic C-terminal portion and the cytosolic loop between TMDs III and IV are thought to form a binuclear zinc-binding site allosterically operating for zinc transport activity in a zinc-regulated fashion (43) and likely contribute to a metal substrate determinant (63, 64). In contrast to the compatibility between hZnT1 and hZnT10, the domain swapping between hZnT10 and hZnT2 was found to be incompatible. This observation suggests that these regions may be functionally and structurally different among ZnT transporters. However, incompatibility between hZnT10 and hZnT2 may result from their different subcellular localizations. Studying and comparing three-dimensional structures of these regions of hZnT10 with those of other hZnT transporters will undoubtedly facilitate answers to this issue.
ZnT10 plays a role in zinc metabolism, locating the early/recycling endosomes or the Golgi apparatus (26, 27, 65). However, we have no data to show that hZnT10 is involved in the detoxification of high zinc concentrations. Importantly, this study does not completely exclude the possibility that ZnT10 has zinc transport activity and contributes to zinc metabolism because this study was performed under conditions of detoxification/efflux against high zinc concentrations (over 60 μm ZnSO4) using ZnT1−/−MT−/−ZnT4−/− cells. In support of this idea, overexpression of hZnT5 and hZnT6 failed to confer resistance to ZnT1−/−MT−/−ZnT4−/− cells in the presence of 60 μm ZnSO4.6 A recent report that the zinc transport function of ZnT10 was accelerated by heterodimer formation with other ZnT transporters, such as ZnT3 (65), suggests that the absence of ZnT transporters in ZnT1−/−MT−/−ZnT4−/− cells may mask the ability of hZnT10 to exert robust zinc transport activity in our evaluation. Because Cys52 and Leu242 residues in the TMDs II and V were found to be involved in the regulation of metal substrate specificity in hZnT10, heterodimer formation may affect the conformation of both residues and accessibility of zinc to the intramembranous metal coordination site, which may also change the affinity of Asn43 toward zinc. Further investigation is required to clarify how ZnT10 contributes to zinc metabolism at the molecular level, as well as its primary function in manganese metabolism.
Because manganese levels are reported to increase in patients of several neurodegenerative diseases (8, 14), manganese detoxification/efflux proteins have potential therapeutic functions because of their ability to decrease manganese accumulation. Thus, the robust manganese detoxification/efflux function of ZnT10 may be important against these pathologies. The present results contribute to providing clues that should facilitate manganese transport activity of ZnT10 in a specific manner, as well as information that aids our understanding of the relationship between manganese and zinc metabolic systems.
Experimental Procedures
Cell Culture and Transfection
Chicken B lymphocyte-derived DT40 cells were maintained in RPMI 1640 medium (Nacalai Tesque, Kyoto, Japan) supplemented with 10% heat-inactivated fetal calf serum (Multiser, Trace Scientific, Melbourne, Australia), 1% chicken serum (Invitrogen), and 50 μm 2-mecaptoethanol (Sigma) at 39.5 °C, as described previously (31). DNA transfection into DT40 cells was carried out using electroporation, as described previously (31). DT40 cells deficient in the SPCA1 gene (SPCA1−/−/− cells) were established using three KO vectors shown in Fig. 1A. Southern and Northern blot analysis was performed as described previously (32). Genomic DNA (20 μg) prepared from DT40 cells or 20 μg of total RNA extracted from the cells using Sepasol I (Nacalai Tesque) was used. Radioimages were obtained using an FLA5000 Bio imaging analyzer (Fujifilm, Tokyo, Japan). More than three independent clones were established per disruptants and transfectants. ZnT1−/−MT−/−ZnT4−/− cells have been established and reported previously (33).
Plasmid Construction
Plasmids used for the expression of C-terminally GFP-tagged hSPCA1 (hSPCA1-GFP) (15), N-terminally FLAG-tagged hATP13A1 (FLAG-hATP13A1), C-terminally HA-tagged hATP13A2 (hATP13A2-HA), N-terminally HA-tagged hATP13A3 (HA-hATP13A3), C-terminally HA tagged hZnT10 (hZnT10-HA), N-terminally Myc-tagged hZnT10 (Myc-hZnT10), N-terminally FLAG-tagged hZnT1 (FLAG-hZnT1), or C-terminally HA tagged hZnT2 (hZnT2-HA) were constructed by inserting each cDNA into pA-Puro or pA-Zeocin vectors (34). The truncated forms of hATP13A1 and hATP13A3 cDNAs were purchased from DNAFORM, and their full-length forms were prepared by ligating them with the missing fragments from RT-PCR-amplified hATP13A1 or hATP13A3 cDNA. The hATP13A2-HA was constructed by replacing a V5-His tag (21) with the HA tag, or the hZnT10-HA was constructed by fusing hZnT10 (23) with the HA tag. The hFpn-V5, which is constructed by fusing hFpn with the V5 tag, was described previously (35). All cDNAs constructed here were sequenced in both directions. The KO vectors used for disruption of the SPCA1 gene were constructed using the amplified genomic DNA fragments with gene-specific primers by KOD-FX polymerase (Toyobo, Osaka Japan), as described previously (31). All plasmids were linearized with appropriate restriction enzymes prior to electroporation.
Cytotoxicity Assay against High Manganese or Zinc Concentrations
DT40 cells were inoculated at a density of 10 × 104 cells/ml in 96-well plates and treated with MnSO4 or ZnSO4 at the indicated concentrations for 2 days. Alamar Blue reagent (AbD Serotec, Ltd., Oxford, UK) was then added to the culture media and incubated for 3–4 h. The absorbance in the medium was measured at 570 and 600 nm using PowerScan 4 (DS Pharma Biomedical, Osaka, Japan), according to the protocol of the manufacturer.
Measurement of Manganese Transport Activity
DT40 cells were cultured in 6-well plates, and 1 μm 54Mn-labeled MnCl2 (PerkinElmer Life Sciences) was added to the culture media in all wells. After 24 h, the cells were washed twice with ice-cold culture medium and suspended in phosphate-buffered saline (PBS) containing 0.05% EDTA. The radioactivity of 54Mn was measured using an auto-well gamma counter (Wizard2; PerkinElmer Life Sciences, Kanagawa, Japan), and data were normalized by the total cellular protein. Data are depicted as mean ± S.D. Statistical significance was determined by the Student's t test and accepted at p < 0.01.
Generation of Anti-hZnT10 and Anti-hZnT1 Monoclonal Antibodies
Fused proteins consisting of the cytosolic C-terminal portion of hZnT10 (95 residues from Leu391 to stop codon) and maltose-binding protein or the cytosolic C-terminal portion of hZnT1 (167 residues from Leu341 to stop codon) and maltose-binding protein were used as antigens. The anti-hZnT10 or anti-hZnT1 monoclonal antibody was produced as described previously (36, 37). Ascites was generated by injection of 1 × 107 hybridoma cells into pristine-primed mice.
Immunoblotting
Immunoblotting was performed as described previously (30). Briefly, the blotted PVDF membrane (Immobilon-P, Millipore Corp., Bedford, MA) was blocked with a solution of 5% skimmed milk and 0.1% Tween 20 in PBS prior to incubation with anti-FLAG M2 (1:3,000, Sigma, F3165), anti-HA HA-11 (1:3,000, Covance, Emeryville, CA, MMS-101P), anti-Myc (1:3,000, Santa Cruz Biotechnology Inc., sc-40), anti-V5 (1:3,000, Nacalai Tesque, 04434–94), anti-ZnT10 (1:3,000), anti-ZnT1 (1:3,000), anti-GFP (1:1,000, Invitrogen, G10362), anti-tubulin, (1:10,000, Sigma, T7816), anti-calnexin (1:10,000, Enzo Life Sciences, ADI-SPA-860), or anti-chicken IgM M4 (1:3,000, Southern Biotech, 8300-01) antibodies in blocking solution. Horseradish peroxidase-conjugated anti-mouse or rabbit secondary antibodies (GE Healthcare, NA931 or NA934) were added at a 1:3,000 dilution for detection. The fluoro-image was obtained using a LAS1000 plus (Fujifilm, Tokyo, Japan) or a LAS 500 (GE Healthcare).
Cell Surface Biotinylation Assay
Cells stably expressing WT or mutant hZnT10 or hZnT1 were washed twice with ice-cold PBS, and then EZ-Link, a sulfo-NHS-SS-biotin reagent (Pierce Protein Biology, ThermoFisher Scientific) was used to biotinylate lysine residues exposed on the extracellular surface. Biotinylated proteins were recovered from streptavidin-coupled beads in 6× SDS sample buffer and then immunoblotted.
Immunofluorescence Staining
Double immunostaining for tagged proteins used in this study and GM130 was performed as described previously (30). Briefly, the cells were stained with an anti-HA tag polyclonal antibody (1:500, MBL, 561), an anti-FLAG tag polyclonal antibody (anti-DDDDK; 1:500, MBL, PM020), anti-GFP (1:50, Invitrogen, G10362), or anti-GM130 (1:100, BD Transduction Laboratories, G65120), followed by goat anti-mouse IgG conjugated to Alexa 594 (Molecular Probes, A11032) or donkey anti-rabbit IgG conjugated to Alexa 488 (Molecular Probes, A21206) as the secondary antibodies. The stained cells were observed using a Zeiss Axioplan 2 microscope equipped with an Olympus digital camera (Metamorph, Olympus, Tokyo, Japan) or a fluorescent microscope FSX100 (Olympus). Images were analyzed using Adobe Photoshop Elements (Adobe Systems Inc., San Jose, CA).
Author Contributions
Y. N. performed the experiments, analyzed, and interpreted the data, and co-wrote the manuscript. N. T., T. T., T. Y., and F. T. performed the experiments, and analyzed the data. H. F. and S. H. performed the manganese retention assay and contributed to the concept. F. O., A. M., and H. N. generated antibodies. K. T., R. R., S. K., and H. M. cloned genes and contributed to the concept. T. K. directed the project, designed and performed the experiments, analyzed and interpreted the data, and wrote the manuscript. All authors reviewed the manuscript.
Acknowledgment
We thank Dr. Christian Kubisch (University Medical Center Hamburg-Eppendorf, Hamburg, Germany) for providing the hATP13A2-V5-His plasmid.
This work was supported by Grants-in-aid for Young Scientists (B), Challenging Exploratory Research and Scientific Research (B), from the Japan Society for the Promotion of Science (KAKENHI) Grants 21780309, 26660086, and 15H04501, the Fuji Foundation for Protein Research, the Salt Science Research Foundation, the Kato Memorial Bioscience Foundation, the Sasakawa Scientific Research Grant from the Japan Science Society, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research (to T. K.). The authors declare that they have no conflicts of interest with the contents of this article.
Y. Nishito, N. Tsuji, and T. Kambe, unpublished data.
Y. Nishito and T. Kambe, unpublished data.
- h
- human
- ZnT
- zinc transporter
- TMD
- transmembrane domain.
References
- 1.Aschner J. L., and Aschner M. (2005) Nutritional aspects of manganese homeostasis. Mol. Aspects Med. 26, 353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood R. J. (2009) Manganese and birth outcome. Nutr. Rev. 67, 416–420 [DOI] [PubMed] [Google Scholar]
- 3.Aschner M., Erikson K. M., Herrero Hernández E., Hernández E. H., and Tjalkens R. (2009) Manganese and its role in Parkinson's disease: from transport to neuropathology. Neuromolecular Med. 11, 252–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucchini R. G., Martin C. J., and Doney B. C. (2009) From manganism to manganese-induced parkinsonism: a conceptual model based on the evolution of exposure. Neuromolecular Med. 11, 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culotta V. C., Yang M., and Hall M. D. (2005) Manganese transport and trafficking: lessons learned from Saccharomyces cerevisiae. Eukaryot. Cell 4, 1159–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddi A. R., Jensen L. T., and Culotta V. C. (2009) Manganese homeostasis in Saccharomyces cerevisiae. Chem. Rev. 109, 4722–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Au C., Benedetto A., and Aschner M. (2008) Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology 29, 569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowman A. B., Kwakye G. F., Herrero Hernández E., and Aschner M. (2011) Role of manganese in neurodegenerative diseases. J. Trace Elem. Med. Biol. 25, 191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujishiro H., Yano Y., Takada Y., Tanihara M., and Himeno S. (2012) Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 4, 700–708 [DOI] [PubMed] [Google Scholar]
- 10.Fujishiro H., Ohashi T., Takuma M., and Himeno S. (2013) Suppression of ZIP8 expression is a common feature of cadmium-resistant and manganese-resistant RBL-2H3 cells. Metallomics 5, 437–444 [DOI] [PubMed] [Google Scholar]
- 11.Jenkitkasemwong S., Wang C. Y., Mackenzie B., and Knutson M. D. (2012) Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 25, 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabado N., Jankowski A., Dougaparsad S., Picard V., Grinstein S., and Gros P. (2000) Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192, 1237–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen P., Chakraborty S., Mukhopadhyay S., Lee E., Paoliello M. M., Bowman A. B., and Aschner M. (2015) Manganese homeostasis in the nervous system. J. Neurochem. 134, 601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hozumi I., Hasegawa T., Honda A., Ozawa K., Hayashi Y., Hashimoto K., Yamada M., Koumura A., Sakurai T., Kimura A., Tanaka Y., Satoh M., and Inuzuka T. (2011) Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J. Neurol. Sci. 303, 95–99 [DOI] [PubMed] [Google Scholar]
- 15.Ton V. K., Mandal D., Vahadji C., and Rao R. (2002) Functional expression in yeast of the human secretory pathway Ca2+, Mn2+-ATPase defective in Hailey-Hailey disease. J. Biol. Chem. 277, 6422–6427 [DOI] [PubMed] [Google Scholar]
- 16.Mukhopadhyay S., and Linstedt A. D. (2011) Identification of a gain-of-function mutation in a Golgi P-type ATPase that enhances Mn2+ efflux and protects against toxicity. Proc. Natl. Acad. Sci. U.S.A. 108, 858–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitch S., Feng M., Muend S., Braiterman L. T., Hubbard A. L., and Rao R. (2011) Vesicular distribution of secretory pathway Ca2+-ATPase isoform 1 and a role in manganese detoxification in liver-derived polarized cells. Biometals 24, 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan J., Zhang T., Jiang L., Chi J., Hu D., Pan Q., Wang D., and Zhang Z. (2011) Regulation of intracellular manganese homeostasis by Kufor-Rakeb syndrome-associated ATP13A2 protein. J. Biol. Chem. 286, 29654–29662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gitler A. D., Chesi A., Geddie M. L., Strathearn K. E., Hamamichi S., Hill K. J., Caldwell K. A., Caldwell G. A., Cooper A. A., Rochet J. C., and Lindquist S. (2009) α-Synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat. Genet. 41, 308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesi A., Kilaru A., Fang X., Cooper A. A., and Gitler A. D. (2012) The role of the Parkinson's disease gene PARK9 in essential cellular pathways and the manganese homeostasis network in yeast. PLoS ONE 7, e34178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez A., Heimbach A., Gründemann J., Stiller B., Hampshire D., Cid L. P., Goebel I., Mubaidin A. F., Wriekat A. L., Roeper J., Al-Din A., Hillmer A. M., Karsak M., Liss B., Woods C. G., et al. (2006) Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 38, 1184–1191 [DOI] [PubMed] [Google Scholar]
- 22.Quadri M., Federico A., Zhao T., Breedveld G. J., Battisti C., Delnooz C., Severijnen L. A., Di Toro Mammarella L., Mignarri A., Monti L., Sanna A., Lu P., Punzo F., Cossu G., et al. (2012) Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet. 90, 467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuschl K., Clayton P. T., Gospe S. M. Jr., Gulab S., Ibrahim S., Singhi P., Aulakh R., Ribeiro R. T., Barsottini O. G., Zaki M. S., Del Rosario M. L., Dyack S., Price V., Rideout A., Gordon K., et al. (2012) Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum. Genet. 90, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng E., Lind P. M., Lindgren C., Ingelsson E., Mahajan A., Morris A., and Lind L. (2015) Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum. Mol. Genet. 24, 4739–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leyva-Illades D., Chen P., Zogzas C. E., Hutchens S., Mercado J. M., Swaim C. D., Morrisett R. A., Bowman A. B., Aschner M., and Mukhopadhyay S. (2014) SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J. Neurosci. 34, 14079–14095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosomworth H. J., Thornton J. K., Coneyworth L. J., Ford D., and Valentine R. A. (2012) Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics 4, 771–779 [DOI] [PubMed] [Google Scholar]
- 27.Patrushev N., Seidel-Rogol B., and Salazar G. (2012) Angiotensin II requires zinc and downregulation of the zinc transporters ZnT3 and ZnT10 to induce senescence of vascular smooth muscle cells. PLoS ONE 7, e33211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki T., Ishihara K., Migaki H., Matsuura W., Kohda A., Okumura K., Nagao M., Yamaguchi-Iwai Y., and Kambe T. (2005) Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J. Biol. Chem. 280, 637–643 [DOI] [PubMed] [Google Scholar]
- 29.Ishihara K., Yamazaki T., Ishida Y., Suzuki T., Oda K., Nagao M., Yamaguchi-Iwai Y., and Kambe T. (2006) Zinc transport complexes contribute to the homeostatic maintenance of secretory pathway function in vertebrate cells. J. Biol. Chem. 281, 17743–17750 [DOI] [PubMed] [Google Scholar]
- 30.Fukunaka A., Kurokawa Y., Teranishi F., Sekler I., Oda K., Ackland M. L., Faundez V., Hiromura M., Masuda S., Nagao M., Enomoto S., and Kambe T. (2011) Tissue nonspecific alkaline phosphatase is activated via a two-step mechanism by zinc transport complexes in the early secretory pathway. J. Biol. Chem. 286, 16363–16373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kambe T. (2014) Methods to evaluate zinc transport into and out of the secretory and endosomal-lysosomal compartments in DT40 cells. Methods Enzymol. 534, 77–92 [DOI] [PubMed] [Google Scholar]
- 32.Matsuura W., Yamazaki T., Yamaguchi-Iwai Y., Masuda S., Nagao M., Andrews G. K., and Kambe T. (2009) SLC39A9 (ZIP9) regulates zinc homeostasis in the secretory pathway: characterization of the ZIP subfamily I protein in vertebrate cells. Biosci. Biotechnol. Biochem. 73, 1142–1148 [DOI] [PubMed] [Google Scholar]
- 33.Fujimoto S., Itsumura N., Tsuji T., Anan Y., Tsuji N., Ogra Y., Kimura T., Miyamae Y., Masuda S., Nagao M., and Kambe T. (2013) Cooperative functions of ZnT1, metallothionein and ZnT4 in the cytoplasm are required for full activation of TNAP in the early secretory pathway. PLoS ONE 8, e77445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki T., Ishihara K., Migaki H., Ishihara K., Nagao M., Yamaguchi-Iwai Y., and Kambe T. (2005) Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J. Biol. Chem. 280, 30956–30962 [DOI] [PubMed] [Google Scholar]
- 35.Kono S., Yoshida K., Tomosugi N., Terada T., Hamaya Y., Kanaoka S., and Miyajima H. (2010) Biological effects of mutant ceruloplasmin on hepcidin-mediated internalization of ferroportin. Biochim. Biophys. Acta 1802, 968–975 [DOI] [PubMed] [Google Scholar]
- 36.Kambe T., Narita H., Yamaguchi-Iwai Y., Hirose J., Amano T., Sugiura N., Sasaki R., Mori K., Iwanaga T., and Nagao M. (2002) Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J. Biol. Chem. 277, 19049–19055 [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto A., Ohkura K., Takahashi M., Kizu K., Narita H., Enomoto S., Miyamae Y., Masuda S., Nagao M., Irie K., Ohigashi H., Andrews G. K., and Kambe T. (2015) Soybean extracts increase cell surface ZIP4 abundance and cellular zinc levels: a potential novel strategy to enhance zinc absorption by ZIP4 targeting. Biochem. J. 472, 183–193 [DOI] [PubMed] [Google Scholar]
- 38.Yin Z., Jiang H., Lee E. S., Ni M., Erikson K. M., Milatovic D., Bowman A. B., and Aschner M. (2010) Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J. Neurochem. 112, 1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madejczyk M. S., and Ballatori N. (2012) The iron transporter ferroportin can also function as a manganese exporter. Biochim. Biophys. Acta 1818, 651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itsumura N., Inamo Y., Okazaki F., Teranishi F., Narita H., Kambe T., and Kodama H. (2013) Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: a novel mechanism for zinc deficiency in a breast-fed infant. PLoS ONE 8, e64045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kambe T., Suzuki T., Nagao M., and Yamaguchi-Iwai Y. (2006) Sequence similarity and functional relationship among eukaryotic ZIP and CDF transporters. Genomics Proteomics Bioinformatics 4, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kambe T. (2012) Molecular architecture and function of ZnT transporters. Curr. Top. Membr. 69, 199–220 [DOI] [PubMed] [Google Scholar]
- 43.Lu M., and Fu D. (2007) Structure of the zinc transporter YiiP. Science 317, 1746–1748 [DOI] [PubMed] [Google Scholar]
- 44.Lu M., Chai J., and Fu D. (2009) Structural basis for autoregulation of the zinc transporter YiiP. Nat. Struct. Mol. Biol. 16, 1063–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta S., Chai J., Cheng J., D'Mello R., Chance M. R., and Fu D. (2014) Visualizing the kinetic power stroke that drives proton-coupled zinc(II) transport. Nature 512, 101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coudray N., Valvo S., Hu M., Lasala R., Kim C., Vink M., Zhou M., Provasi D., Filizola M., Tao J., Fang J., Penczek P. A., Ubarretxena-Belandia I., and Stokes D. L. (2013) Inward-facing conformation of the zinc transporter YiiP revealed by cryoelectron microscopy. Proc. Natl. Acad. Sci. U.S.A. 110, 2140–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shusterman E., Beharier O., Shiri L., Zarivach R., Etzion Y., Campbell C. R., Lee I. H., Okabayashi K., Dinudom A., Cook D. I., Katz A., and Moran A. (2014) ZnT-1 extrudes zinc from mammalian cells functioning as a Zn2+/H+ exchanger. Metallomics 6, 1656–1663 [DOI] [PubMed] [Google Scholar]
- 48.Ohana E., Hoch E., Keasar C., Kambe T., Yifrach O., Hershfinkel M., and Sekler I. (2009) Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 284, 17677–17686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seve M., Chimienti F., Devergnas S., and Favier A. (2004) In silico identification and expression of SLC30 family genes: an expressed sequence tag data mining strategy for the characterization of zinc transporters' tissue expression. BMC Genomics 5, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukada T., and Kambe T. (2011) Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 3, 662–674 [DOI] [PubMed] [Google Scholar]
- 51.Kambe T., Tsuji T., Hashimoto A., and Itsumura N. (2015) The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 95, 749–784 [DOI] [PubMed] [Google Scholar]
- 52.Lapinskas P. J., Lin S. J., and Culotta V. C. (1996) The role of the Saccharomyces cerevisiae CCC1 gene in the homeostasis of manganese ions. Mol. Microbiol. 21, 519–528 [DOI] [PubMed] [Google Scholar]
- 53.Lin S. J., and Culotta V. C. (1996) Suppression of oxidative damage by Saccharomyces cerevisiae ATX2, which encodes a manganese-trafficking protein that localizes to Golgi-like vesicles. Mol. Cell. Biol. 16, 6303–6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei Y., Chen J., Rosas G., Tompkins D. A., Holt P. A., and Rao R. (2000) Phenotypic screening of mutations in Pmr1, the yeast secretory pathway Ca2+/Mn2+-ATPase, reveals residues critical for ion selectivity and transport. J. Biol. Chem. 275, 23927–23932 [DOI] [PubMed] [Google Scholar]
- 55.Park J. S., Koentjoro B., Veivers D., Mackay-Sim A., and Sue C. M. (2014) Parkinson's disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum. Mol. Genet. 23, 2802–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsunemi T., and Krainc D. (2014) Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and α-synuclein accumulation. Hum. Mol. Genet. 23, 2791–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aronchik I., Behne M. J., Leypoldt L., Crumrine D., Epstein E., Ikeda S., Mizoguchi M., Bench G., Pozzan T., and Mauro T. (2003) Actin reorganization is abnormal and cellular ATP is decreased in Hailey-Hailey keratinocytes. J. Invest. Dermatol. 121, 681–687 [DOI] [PubMed] [Google Scholar]
- 58.Hoch E., Lin W., Chai J., Hershfinkel M., Fu D., and Sekler I. (2012) Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc. Natl. Acad. Sci. U.S.A. 109, 7202–7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delhaize E., Kataoka T., Hebb D. M., White R. G., and Ryan P. R. (2003) Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell 15, 1131–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedas P., Schiller Stokholm M., Hegelund J. N., Ladegård A. H., Schjoerring J. K., and Husted S. (2014) Golgi localized barley MTP8 proteins facilitate Mn transport. PLoS ONE 9, e113759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montanini B., Blaudez D., Jeandroz S., Sanders D., and Chalot M. (2007) Phylogenetic and functional analysis of the cation diffusion facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics 8, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gustin J. L., Zanis M. J., and Salt D. E. (2011) Structure and evolution of the plant cation diffusion facilitator family of ion transporters. BMC Evol. Biol. 11, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawachi M., Kobae Y., Mimura T., and Maeshima M. (2008) Deletion of a histidine-rich loop of AtMTP1, a vacuolar Zn2+/H+ antiporter of Arabidopsis thaliana, stimulates the transport activity. J. Biol. Chem. 283, 8374–8383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blindauer C. A., and Schmid R. (2010) Cytosolic metal handling in plants: determinants for zinc specificity in metal transporters and metallothioneins. Metallomics 2, 510–529 [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y., Feresin R. G., Falcon-Perez J. M., and Salazar G. (2016) Differential targeting of SLC30A10/ZnT10 heterodimers to endolysosomal compartments modulates EGF-induced MEK/ERK1/2 activity. Traffic 17, 267–288 [DOI] [PubMed] [Google Scholar]