Abstract
The ubiquitin-proteasome system represents the major pathway of selective intracellular protein degradation in eukaryotes. Misfolded proteins represent an important class of substrates for this pathway, and the failure to destroy misfolded proteins is associated with a number of human diseases. The transcription factor Rpn4 mediates a key proteotoxic stress response whose best known function is to control proteasome abundance by a homeostatic feedback mechanism. Here we identify the uncharacterized zinc finger protein Tmc1 as a dynamically regulated stress-responsive protein. Rpn4 induces TMC1 transcription in response to misfolded proteins. However, this response is counteracted by rapid proteasome-dependent degradation of Tmc1, which serves to normalize Tmc1 protein levels after induction. Precise control of Tmc1 levels is needed in vivo to survive multiple stressors related to proteostasis. Thus, Tmc1 represents a novel effector and substrate of the Rpn4 proteotoxic stress response.
Keywords: proteasome, protein degradation, proteostasis, stress response, yeast, Rpn4, Tmc1, arsenite
Introduction
The timely degradation of misfolded proteins represents a problem of both basic and clinical importance. Failure to destroy misfolded proteins is not only toxic to most or all cell types, but is increasingly thought to directly cause or contribute to a large number of human diseases, most notably many neurodegenerative diseases where pathologists have observed the accumulation of insoluble protein aggregates in diseased cells for more than a century (1–3).
The first line of defense against misfolded proteins likely represents the large family of molecular chaperones. These proteins are capable of recognizing misfolded proteins, often through interaction with exposed hydrophobic domains, and in many cases may be able to assist the protein in recovering its normal fold (2). Proteins that cannot be refolded may be subject to degradation.
Most selective intracellular protein degradation in eukaryotes is carried out by the ubiquitin-proteasome system. A protein to be destroyed in this pathway is first covalently labeled with the small protein ubiquitin. This ubiquitin, often present in the form of one or more multiubiquitin chains, is recognized by the proteasome, a 2.5-MDa multisubunit assembly that is the most complex protease ever described (4, 5). It consists of at least 33 integral subunits and reversibly associates with a significant number of additional factors. The proteasome binds the ubiquitinated substrate, unfolds it, removes the ubiquitin targeting signals, and injects the substrate into its central core where its proteolytic active sites render the protein into small polypeptides. These polypeptides can be further broken down by non-proteasomal proteases into free amino acids, which can then be reused in protein synthesis (6).
Increasing evidence suggests that a major aspect of toxicity of the trivalent metalloid arsenic relates to protein misfolding. Arsenic causes the accumulation of high molecular weight ubiquitin conjugates and induces the accumulation of protein aggregates (7–9). Moreover, phenotypic analysis, including genome-wide studies (9–11), has indicated robust phenotypes for trivalent arsenic for mutants in pathways of protein homeostasis. Mutants defective in protein degradation are often sensitive to trivalent arsenic. By contrast, mutants defective in protein synthesis are frequently resistant to this drug. This includes not only mutants in components of the ribosome proper but also mutants in ribosome biogenesis and other pathways that positively regulate protein synthesis (10, 11). This latter finding has been suggested to reflect a preferential role for arsenic in misfolding of newly synthesized proteins (9, 11).
Cells harbor multiple stress responses designed to eliminate the toxicity associated with misfolded proteins, sometimes referred to as proteotoxicity. Perhaps best known among these are the heat shock response and the unfolded protein response. A third important and evolutionarily conserved proteotoxic stress response is mediated by the transcription factor Rpn4 (12–15). Its best characterized function is to carry out the concerted transcriptional regulation of all proteasome genes (13). Each of these proteasome genes contains a conserved promoter element known as the PACE (proteasome associated control element) sequence, typically located within the first several hundred bases upstream of the start codon (13, 16). In addition to its function as a transcription factor, Rpn4 is also a substrate of the proteasome with an extremely short half-life (12). Under conditions that compromise or overwhelm proteasome function, Rpn4 protein is stabilized, leading to increased Rpn4 transcriptional activity that promotes new proteasome synthesis until proteasome function is restored to levels adequate to again carry out rapid Rpn4 degradation. This homeostatic feedback pathway is crucial for cells to survive diverse stresses arising from misfolded proteins, and indeed Rpn4 protein levels are markedly increased after treatment with sodium arsenite (11).
We and others recently identified the uncharacterized protein Tmc1 (formerly Yor052c) on the basis of its sequence similarity to Cuz1, a newly described component of the ubiquitin-proteasome pathway (17, 18). Specifically, Tmc1 and Cuz1 share a conserved but poorly understood zinc binding domain known as the AN1 domain. Here we show that Tmc1 is a highly regulated novel effector of the Rpn4 proteotoxic stress response. In response to misfolded proteins, Tmc1 is strongly induced by Rpn4. Tmc1 is also a substrate of the ubiquitin-proteasome pathway, and its rapid rate of degradation serves to normalize Tmc1 protein levels after a burst of transcriptional induction. Both the absence of Tmc1 and its overexpression cause robust cellular phenotypes associated with proteostasis pathways, suggesting an important role for Tmc1 within the broader ubiquitin-proteasome system.
Results
Dynamic Regulation of Tmc1 Protein in Response to Arsenic
Tmc1 is a relatively uncharacterized 17-kDa protein and represents one of two AN1-type zinc finger proteins in yeast. Cuz1, the other AN1 zinc finger protein, appears to function in protein degradation via direct interaction with the proteasome and Cdc48 (17, 18). Cuz1 protein is specifically induced by trivalent arsenic (11, 17), which is of interest because accumulating evidence suggests that a major aspect of toxicity of trivalent arsenic relates to protein misfolding (9–11, 20).
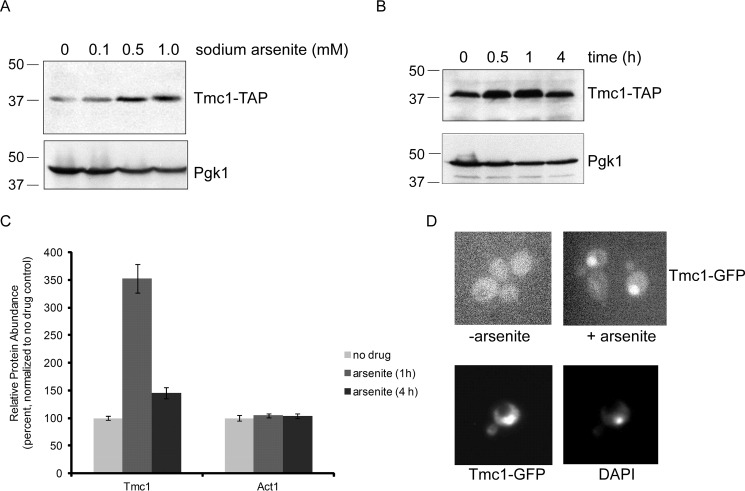
In previous work, the tmc1Δ mutant showed sensitivity to sodium arsenite (see Ref. 18; see also below), suggesting that Tmc1 might also function in a stress response directed at trivalent arsenic toxicity. We began by examining Tmc1 protein levels after treatment with trivalent arsenic. Sampled at 1 h after treatment, we observed a dose-dependent increase in Tmc1 protein in response to sodium arsenite (Fig. 1A). We next looked at the time dependence of this response. Tmc1 protein levels rose dramatically at 0.5 and 1 h after sodium arsenite treatment (Fig. 1B). Surprisingly, Tmc1 levels normalized over time, returning to nearly baseline levels after 4 h (Fig. 1B).
FIGURE 1.
Dynamic regulation of Tmc1 protein levels after arsenic treatment. A, Tmc1-TAP protein levels after treatment with sodium arsenite at the indicated concentrations. Whole cell extracts were prepared and analyzed by SDS-PAGE followed by immunoblot. Upper panel, anti-TAP antibody. Lower panel, anti-Pgk1 antibody (loading control). Molecular weight markers are indicated (kDa). B, Tmc1-TAP protein levels after treatment with sodium arsenite (1 mm) at the indicated time points. Whole cell extracts were prepared and analyzed by SDS-PAGE followed by immunoblot. Upper panel, anti-TAP antibody. Lower panel, anti-Pgk1 antibody (loading control). C, relative protein abundance of Tmc1 at 0, 1, and 4 h after treatment with sodium arsenite (1 mm). The data were generated using a TMT-based mass spectrometry proteomic approach (11). The data shown represent endogenous untagged Tmc1 protein. A control protein, Act1, showed no change. The error bars represent standard deviations from triplicate cultures. In addition, differences between untreated and treated Tmc1 samples were statistically significant by Student's t test (p < 0.01); differences between untreated and treated Act1 samples were not statistically significant. D, induction of Tmc1 after treatment with sodium arsenite (1 mm for 1 h), as visualized by fluorescence microscopy using GFP-tagged Tmc1 protein (upper panels). Staining with the nuclear marker DAPI indicates that induced Tmc1-GFP is mainly nuclear (lower panels).
We recently reported a proteomic characterization of the cellular response to trivalent arsenic (11). Using a tandem mass tag-based (TMT)2 multiplexed mass spectrometry method, we were able to determine the relative protein abundance of nearly 4,600 proteins (of ∼6,000 predicted in Saccharomyces cerevisiae). We used similar conditions as in Fig. 1B: treatment was with 1 mm sodium arsenite, and protein levels were determined at 0, 1, and 4 h. The TMT analysis was highly concordant with the immunoblot results. At 1 h after treatment, Tmc1 levels increased by 3.5-fold, but by 4 h, Tmc1 levels had returned to nearly uninduced levels (Fig. 1C). Act1 was one of several thousand proteins that showed no change in abundance in response to arsenic (Fig. 1C and Ref. 11). The TMT proteomic assay reports on native untagged proteins, indicating that TAP-tagged (Fig. 1, A and B) and native (Fig. 1C) Tmc1 behaved similarly. Thus, Tmc1 protein is dynamically regulated in a dose- and time-dependent manner by trivalent arsenic.
We also visualized Tmc1 induction using a GFP-tagged Tmc1 construct. In the absence of sodium arsenite, Tmc1 levels were difficult to detect above background and showed no specific cellular localization (Fig. 1D). After treatment with sodium arsenite, however, Tmc1-GFP was readily detected. Its localization appeared to be predominantly nuclear, as confirmed by co-localization with the nuclear marker DAPI, although a lesser cytoplasmic component was difficult to exclude (Fig. 1D).
Transcriptional Induction of TMC1 by Rpn4
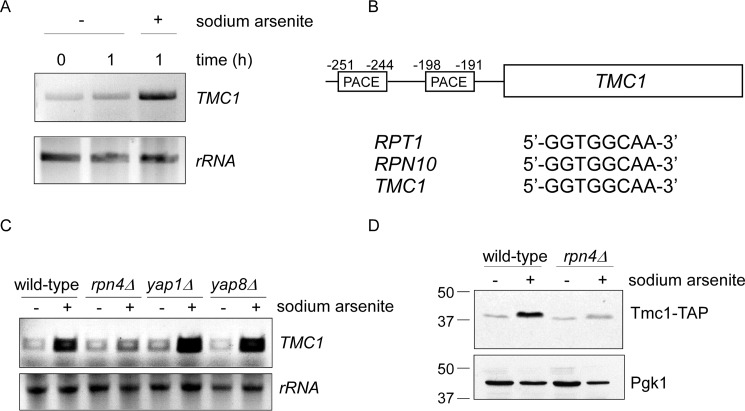
To determine whether increased Tmc1 protein levels after arsenic treatment were due to transcriptional induction, we used RT-PCR. Mock treatment of the cultures for 1 h had no effect on TMC1 RNA levels (Fig. 2A). By contrast, TMC1 RNA levels were significantly increased after treatment with sodium arsenite (Fig. 2A).
FIGURE 2.
Transcriptional induction of TMC1 by Rpn4. A, stress-induced transcription of TMC1 after treatment with sodium arsenite (1 mm) for the indicated times, as determined by RT-PCR analysis (upper panel). Lower panel, ribosomal RNA (loading control). B, schematic diagram of the TMC1 gene with its associated 5′-untranslated region. Two putative PACE motifs are shown with their locations indicated relative to the start codon. Both PACE elements show a canonical sequence (sometimes referred to as a type A motif; Ref. 16), identical to other well established Rpn4 targets such as RPT1 and RPN10. C, RT-PCR analysis of stress-induced transcription of TMC1 in response to sodium arsenite (1 mm for 1 h) in wild-type, rpn4Δ, yap1Δ, and yap8Δ strains, as indicated. Lower panel, ribosomal RNA (loading control). D, stress-induced up-regulation of Tmc1 protein is dependent on Rpn4. Whole cell extracts were prepared and analyzed by SDS-PAGE followed by immunoblot. Upper panel, anti-TAP antibody. Lower panel, anti-Pgk1 antibody (loading control). Sodium arsenite treatment was at 1 mm for 1 h.
The transcription factor Rpn4 typically recognizes an upstream promoter element known as a PACE sequence (13). Previous sequence analyses suggested that TMC1 might harbor two PACE motifs in its promoter (17, 18). They were both canonical PACE motifs, identical to those of well established proteasome subunits like Rpt1 and Rpn10 (Fig. 2B). Moreover, they were located at the typical distance from the start codon (16), further adding support to their potential physiologic importance.
We looked at arsenic-dependent transcript levels of TMC1 from wild-type and rpn4Δ cells. As before, trivalent arsenic strongly induced TMC1 RNA levels; however, this response was largely abrogated in the rpn4Δ mutant (Fig. 2C). As controls, we tested two other transcription factors, Yap1 and Yap8, both of which are known to function in arsenic-induced stress responses (21). In contrast to rpn4Δ, stress-induced transcription of TMC1 appeared to be unaffected in the yap1Δ and yap8Δ mutants (Fig. 2C). Finally, we looked directly at Tmc1 protein levels by immunoblot. Similar to the RT-PCR results, arsenic-induced accumulation of Tmc1 protein was largely abrogated in the rpn4Δ mutant (Fig. 2D). Thus, Tmc1 appears to represent a novel effector of the Rpn4 proteotoxic stress response.
Regulation of Tmc1 Differs from That of Proteasome Subunits
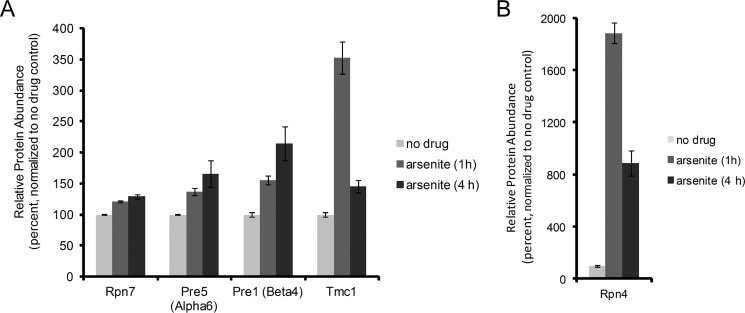
The stress-responsive properties of Tmc1 differed from those of typical proteasome subunits in two aspects. First, the maximum extent of Tmc1 induction was significantly greater than that of typical proteasome subunits (Fig. 3A). Indeed, at the 1-h time point after arsenic treatment, Tmc1 was the 63rd highest induced protein of ∼4,600 proteins detected (see Ref. 11). Second, whereas protein levels of typical proteasome subunits tended to plateau or to continue increasing slightly over time after induction (Fig. 3A), Tmc1 levels normalized over time, as described in Fig. 1. In this regard, the dynamics of the response of Tmc1 protein were actually quite similar to that of its regulator Rpn4 (Fig. 3B and Ref. 11). In fact, unbiased clustering of the TMT data found that Rpn4 was among the top 10 proteins with response profiles similar to Tmc1. Whereas most typical proteasome subunits show long half-lives, likely explaining their stable or slightly increasing protein levels after induction, Rpn4 can be rapidly degraded if sufficient proteasome capacity exists (12). Thus, the normalization of Rpn4 levels over time likely reflects adaptive recovery of proteasome function, which then restores rapid Rpn4 degradation. It was therefore possible that, analogously to Rpn4, normalization of Tmc1 protein levels over time might reflect rapid degradation of the protein.
FIGURE 3.
Differential regulation of Tmc1 and canonical proteasome components by Rpn4. A, relative protein abundance of Tmc1 and the proteasome subunits Rpn7, Pre5 (Alpha6), and Pre1 (Beta4) after treatment with sodium arsenite (1 mm) for the indicated times. B, results for Rpn4. The data were generated using a proteome-wide TMT-based mass spectrometry approach (11). The error bars represent standard deviations from triplicate cultures. In addition, all differences between untreated and treated samples were statistically significant by Student's t test (p < 0.02).
Tmc1 Is a Short-lived Protein and Substrate of the Ubiquitin-Proteasome System
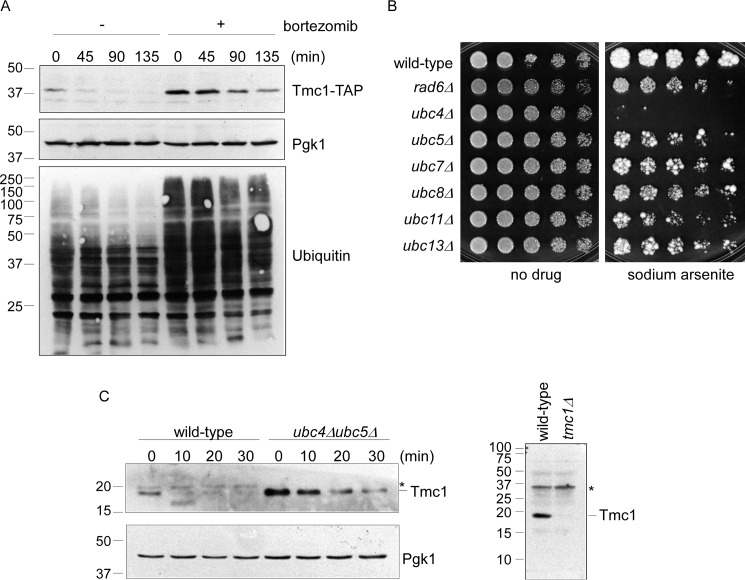
To determine whether Tmc1 protein shows a significant rate of degradation, we conducted cycloheximide chase experiments in logarithmically growing cells (in the absence of arsenic treatment). We found that Tmc1 was indeed turned over at a rapid rate, whereas a control protein, Pgk1, was stable over this time course (Fig. 4A). Note that experiments monitoring TAP-tagged Tmc1 (Fig. 4A) and native untagged Tmc1 (Fig. 4C; also see below) showed similarly short half-lives.
FIGURE 4.
Tmc1 is a short-lived substrate of the ubiquitin-proteasome pathway. A, cycloheximide chase analysis of Tmc1 turnover in the presence or absence of the proteasome inhibitor bortezomib (30 μm). Whole cell extracts were prepared and analyzed by SDS-PAGE followed by immunoblot. Top panel, anti-TAP antibody. Middle panel, anti-Pgk1 antibody (loading control). Bottom panel, anti-ubiquitin antibody, which confirms robust inhibition of the proteasome after bortezomib treatment. B, phenotypic analysis of seven E2 ubiquitinating enzyme mutants. The indicated strains were spotted in 3-fold serial dilutions and grown in the presence or absence of sodium arsenite (1.5 mm) for 1.5–5 days at 30 °C. C, cycloheximide chase analysis of Tmc1 in wild-type cells and a ubc4Δubc5Δ double mutant. Left panels, whole cell extracts were prepared and analyzed by SDS-PAGE followed by immunoblot with anti-Tmc1 and anti-Pgk1 (loading control) antibodies. Note that endogenous (left panel) and TAP-tagged (A) Tmc1 show similarly short half-lives. Right panel, validation of the Tmc1 antibody. Whole cell extracts from wild-type and tmc1Δ strains were prepared and analyzed by SDS-PAGE followed by immunoblot with anti-Tmc1 antibody. Asterisk, nonspecific immunoreactive band.
The ubiquitin-proteasome system represents the major pathway for selective intracellular protein degradation in eukaryotes. To evaluate for a role of the ubiquitin-proteasome system in Tmc1 degradation, we employed the widely used proteasome inhibitor bortezomib. The efficacy of proteasome inhibition was confirmed by immunoblotting for ubiquitin, which shows the accumulation of high molecular weight ubiquitin-immunoreactive material that is characteristic of proteasome inhibition (Fig. 4A). Under these conditions, the turnover of Tmc1 was strongly attenuated (Fig. 4A). The strength of this degradative defect was further indicated by the increase in the steady state abundance of Tmc1 at the zero time point. Thus, Tmc1 is a short-lived protein whose destruction is mediated by the ubiquitin-proteasome system.
Ubiquitin-dependent degradation pathways typically require covalent modification of substrates with ubiquitin by a series of ubiquitinating enzymes known as E1, E2, and E3. Yeast harbor a single E1 enzyme and 11 E2 enzymes (4). We tested 7 of the viable E2 null mutants–rad6Δ, ubc4Δ, ubc5Δ, ubc7Δ, ubc8Δ, ubc11Δ, and ubc13Δ–for survival on arsenic. The ubc4Δ showed marked hypersensitivity to sodium arsenite (Fig. 4B), which is interesting because Ubc4 has been previously implicated in the degradation of misfolded proteins, including misfolded proteins caused by heavy metals (22). Despite extensive sequence similarity and some functional redundancy with its paralog Ubc5, the ubc5Δ mutant did not show a phenotype comparable with that of ubc4Δ (Fig. 4B).
Although the phenotypic sensitivity of the ubc4Δ mutant does not necessarily implicate Ubc4 in Tmc1 degradation, we considered this possibility. Given the sequence similarity (92% identical at the protein level) and functional redundancy between Ubc4 and Ubc5 (22), we focused on a ubc4ubc5Δ double mutant. We observed strong stabilization of Tmc1 in this mutant relative to wild-type (Fig. 4C). Again, the strength of the degradative defect of the ubc4ubc5Δ double mutant is further indicated by the increase in steady state Tmc1 levels at the zero time point. Thus, Tmc1 is a short-lived protein whose degradation is mediated by the proteasome and the E2 ubiquitinating enzymes Ubc4/5.
A Physiologic Role for Tmc1 in Stress Responses
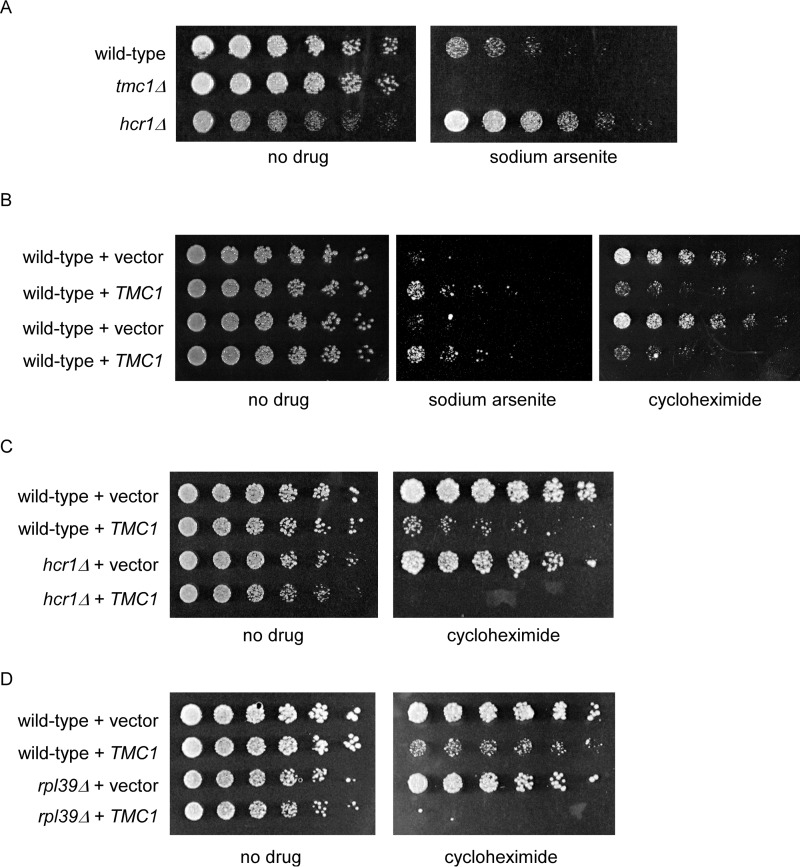
We next sought evidence for a physiologic role for Tmc1 in cells. The tmc1Δ mutant shows sensitivity to trivalent arsenic at relatively high concentrations, as previously reported (Fig. 5A and Ref. 18). Hcr1 is a component of the translation initiation factor eIF3 and positively regulates protein synthesis (23). In contrast to tmc1Δ, the hcr1Δ mutant shows enhanced growth relative to the wild-type strain in the presence of arsenic, even despite its slight basal growth defect on rich media (Fig. 5A). We constructed an overexpression plasmid that expresses TMC1 from the GAPDH promoter. At high concentrations of drug, this plasmid conferred resistance to trivalent arsenic (Fig. 5B). Given that the null mutant shows sensitivity to trivalent arsenic, it seems likely that TMC1 overexpression simply augments its genuine physiologic function, in a sense mimicking the stress-induced expression of Tmc1.
FIGURE 5.
Proteostasis-related phenotypes of Tmc1. A, growth of wild-type, tmc1Δ, and hcr1Δ strains in the presence or absence of sodium arsenite (2 mm), as indicated. The cells were spotted in 3-fold serial dilutions and cultured at 30 °C for 2 days. B, growth of wild-type cells expressing an empty vector or a TMC1 overexpression vector in the presence of sodium arsenite (1.75 mm) or cycloheximide (0.25 μg/ml), as indicated. The cells were spotted in 3-fold serial dilutions and cultured at 30 °C for 2–6 days. Duplicate strains are shown. C, growth of wild-type and hcr1Δ strains expressing an empty vector or a TMC1 overexpression vector in the presence or absence of cycloheximide (0.25 μg/ml), as indicated. The cells were spotted in 3-fold serial dilutions and cultured at 30 °C for 2–7 days. D, growth of wild-type and rpl39Δ strains expressing an empty vector or a TMC1 overexpression vector in the presence or absence of cycloheximide (0.25 μg/ml), as indicated. The cells were spotted in 3-fold serial dilutions and cultured at 30 °C for 2–7 days.
Given the opposing phenotypes of protein degradation and protein synthesis mutants, we tested the effect of Tmc1 overexpression in wild-type cells treated with the ribosome inhibitor cycloheximide. As an inhibitor of the peptidyltransferase site of the ribosome, cycloheximide inhibits protein synthesis but without causing misreading that could result in a significant burden of misfolded proteins. Tmc1 overexpression was rather toxic to cells exposed to cycloheximide (Fig. 5B). This toxic effect was substantially amplified when Tmc1 was expressed in mutants defective in protein synthesis such as hcr1Δ, the eIF3 component mentioned above (Fig. 5C), and rpl39Δ, a component of the ribosome large subunit (Fig. 5D). Although these phenotypes do not indicate the precise mechanistic function of Tmc1, they support an important physiologic role for Tmc1 in proteostasis-related stress response pathways.
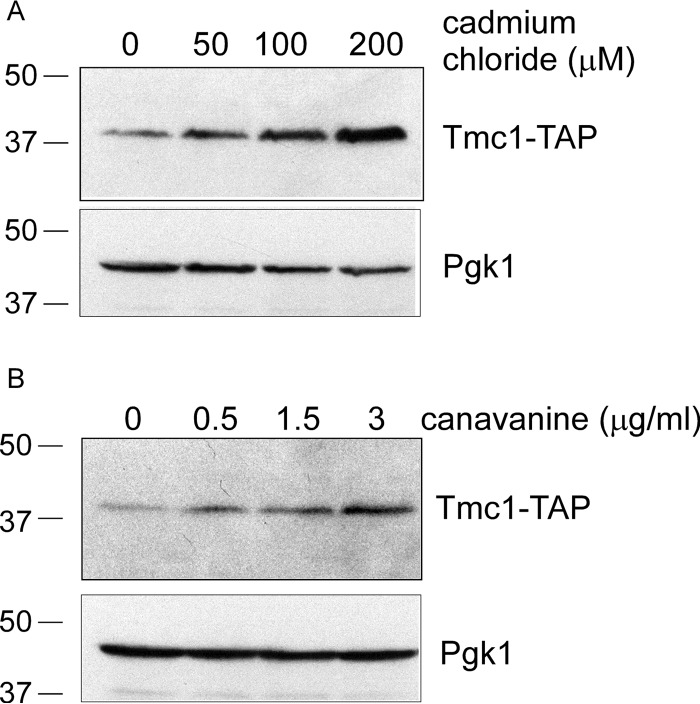
Tmc1 Is Induced by Multiple Causes of Proteotoxic Stress
Finally, we sought to determine whether Tmc1 was specifically responsive to stress induced by trivalent arsenic or whether it might respond to other stresses causing protein misfolding. Thus, we looked at induction of Tmc1 protein levels in response to two well known causes of protein misfolding. The first, cadmium chloride, is a divalent heavy metal whose role in protein misfolding has been established for several decades (24). The second was canavanine, an abnormal amino acid analog of arginine that, when incorporated into nascent proteins, causes them to misfold. We observed robust dose-dependent increases in Tmc1 protein levels in response to both cadmium and canavanine (Fig. 6, A and B). Thus, Tmc1 activity is unlikely to be restricted to trivalent arsenic, a conclusion that is further supported by the fact that Tmc1 transcription is largely controlled by Rpn4, which responds to diverse proteotoxic stresses. However, in contrast to arsenic, we could not detect a significant growth defect of the tmc1Δ mutant when challenged with canavanine (18), suggesting that Tmc1 function may be especially critical under specific proteotoxic conditions.
FIGURE 6.
Diverse proteotoxic stresses induce Tmc1. Tmc1-TAP protein levels after treatment with cadmium chloride (A) or canavanine (B) at the indicated concentrations for 1 h. Whole cell extracts were prepared and analyzed by SDS-PAGE followed by immunoblot. Upper panel, anti-TAP antibody. Lower panel, anti-Pgk1 antibody (loading control).
Discussion
This report presents the first in depth characterization of Tmc1. Our data inform primarily the cellular pathways that regulate Tmc1 function. We have shown that Tmc1 is a novel stress-responsive target of Rpn4, a master regulator of the ubiquitin-proteasome system. Tmc1 transcription is induced in response to multiple proteotoxic insults, and yet this response is rapidly normalized by robust proteasome-mediated degradation of Tmc1. Such dynamic regulation distinguishes Tmc1 from the proteasome, whose subunits are the best established targets of Rpn4 (13, 16). The abundance of typical proteasome subunit proteins tends to plateau or even continue increasing over similar time courses of proteotoxic stress (11). Indeed, the kinetics of the response of Tmc1 seem to more closely mirror those of Rpn4 itself. The significance of this similarity is unclear, but it is worth noting that the degradative pathways of Tmc1 and Rpn4 appear distinct. Although Tmc1 degradation required the E2 enzymes Ubc4/Ubc5, Rpn4 degradation is dependent on Rad6 (25). Additional components of the Rpn4-degradation pathway have been identified, including the E3 enzyme Ubr2 and an accessory factor known as Mub1 (26, 27), as well as a ubiquitin-independent component of degradation (27). Identification of the remaining degradation machinery for Tmc1 will be an important goal for future work.
Although we now understand some aspects of how cells regulate and coordinate Tmc1 function, we have little understanding of the precise mechanistic role of Tmc1. Significant genetic and phenotypic characterization of the toxicity of trivalent arsenic indicates broad relevance for components of proteostasis pathways, i.e. protein synthesis, folding, and degradation (9–11). In vivo phenotypes of Tmc1 could potentially be consistent with either a positive role in protein degradation or a negative role in protein synthesis. Control of Tmc1 transcription by Rpn4 would tend to favor the former possibility. Tmc1 accumulates predominantly in the nucleus upon stress, which may provide a further clue to its function. Given the importance of the Rpn4 proteotoxic stress response, identification of the precise molecular function of Tmc1 is likely to contribute significantly to our understanding of the ubiquitin-proteasome system.
A broader question for future work concerns the breadth and function of the Rpn4 proteotoxic stress response. Although Rpn4 is best known for controlling proteasome abundance via coordinated transcription of both integral proteasome subunits and reversibly associating components (13, 16), bioinformatics analyses have identified potential PACE motifs in the promoters of many other genes (13). Some of these remain within the broader ubiquitin-proteasome family. However, many others have as yet no obvious relationship to this pathway. Moreover, transcriptional profiling experiments indicate many more potential Rpn4 targets, possibly numbering in the hundreds (28). Ultimately, a comprehensive analysis of the function of each Rpn4 effector will be required to fully understand this proteotoxic stress response.
Experimental Procedures
Yeast Strains and Plasmids
Yeast strains are listed in Table 1. Standard techniques were used for strain constructions and transformations. Yeast were cultured at 30 °C. YPD medium consisted of 1% yeast extract, 2% Bacto-peptone, and 2% dextrose. Synthetic medium consisted of 0.7% Difco yeast nitrogen base supplemented with amino acids, adenine, and 2% dextrose. Uracil was omitted from this medium for plasmid selection.
TABLE 1.
Yeast strains
Strains from the Research Genetics Collections (RGC) are available through ThermoFisher Scientific.
| Name | Genotype | Source |
|---|---|---|
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | RGC |
| Tmc1-TAP | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TMC1::TMC1-TAP (HIS3) | RGC |
| Tmc1-GFP | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TMC1::TMC1-GFP (HIS3) | RGC |
| rpn4Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rpn4::KAN | RGC |
| yap1Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 yap1::KAN | RGC |
| yap8Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 yap8::KAN | RGC |
| sGM39 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TMC1::TMC1-TAP (HIS3) rpn4::KAN | This study |
| sGM14 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 TMC1::TMC1-TAP (HIS3) pdr5::KAN | This study |
| rad6Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rad6::KAN | RGC |
| ubc4Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ubc4::KAN | RGC |
| ubc5Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ubc5::KAN | RGC |
| ubc7Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ubc7::KAN | RGC |
| ubc8Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ubc8::KAN | RGC |
| ubc11Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ubc11::KAN | RGC |
| ubc13Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ubc13Δ::KAN | RGC |
| tmc1Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 tmc1::KAN | RGC |
| hcr1Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 hcr1::KAN | RGC |
| sGM2 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 [pKT10] | This study |
| sGM1 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 [pGM1] | This study |
| sJH481 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 hcr1::KAN [pKT10] | This study |
| sJH482 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 hcr1::KAN [pGM1] | This study |
| sGM95 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rpl39::KAN [pKT10] | This study |
| sGM96 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rpl39::KAN [pGM1] | This study |
| sUB62 | MATa lys2-801 leu2-3, 2-112 ura3-52 his3-200 trp1-1 | Ref. 29 |
| sUB453 | MATa lys2-801 leu2-3, 2-112 ura3-52 his3-200 trp1-1 ubc4::HIS3 ubc5::LEU3 | Ref. 30 |
The empty vector pKT10 has been previously described (19). A TMC1 overexpression vector under the control of the GAPDH promoter was constructed by cloning the open reading frame of TMC1 into pKT10 to produce pGM1. The primers used were as follows: 5′-GGCGGTACCATGTCTGATATAAACGAAATC-3′ and 5′-AGCGTCGACTTAAATTTGTATCTTTGGAGC-3′. The construct was verified by sequencing.
Immunoblot Analysis
Whole cell extracts were prepared from logarithmically growing cultures. Cell pellets were obtained by centrifugation at maximum speed (14,000 rpm) in a microcentrifuge, resuspended in 1× Laemmli loading buffer, boiled for 5 min, and analyzed by standard SDS-PAGE followed by immunoblot. For cycloheximide chase analyses, logarithmically growing cells were treated with cycloheximide (100 μg/ml) at time 0, and whole cell extracts were prepared as above at the indicated time points. Proteasome inhibitor treatment was with bortezomib (LC Laboratories, LC-1408) at 30 μm for 1 h prior to acquisition of the zero time point sample. Note that experiments employing proteasome inhibitor were carried out in the pdr5Δ strain background to enhance proteasome inhibition.
The following antibodies were used: anti-rabbit IgG (GE Healthcare, catalog no. NA-934) for TAP tag analysis, anti-Pgk1 (clone 22C5D8; Invitrogen, catalog no. 459250), and anti-ubiquitin (Santa Cruz, catalog no. SC-8017). A polyclonal antibody was raised against Tmc1. To prepare purified recombinant Tmc1 protein to serve as antigen, we cloned the open reading frame of TMC1 into the bacterial expression vector pET45b (EMD Millipore), which provides for an N-terminal His6 tag. The primers used were as follows: 5′-GGCGGTACCATGTCTGATATAAACGAAATC-3′ and 5′-AGCGTCGACTTAAATTTGTATCTTTGGAGC-3′. The plasmid was verified by sequencing.
This plasmid was expressed in the Escherichia coli BL21 (DE3) strain at 30 °C, induced with a final concentration of 1 mm isopropyl β-d-1-thiogalactopyranoside, and then cultured overnight at room temperature. Extracts were prepared by French press, clarified by centrifugation at 16,500 × g for 25 min, and affinity-purified using nickel-nitrilotriacetic acid-agarose (Qiagen). Purified material was eluted with excess imidazole. Eluates were applied to a centrifugal filter with a 3-kDa cutoff (EMD Millipore) and exchanged into PBS. Covance was contracted to prepare the antibody. The antibody was validated by immunoblot analysis of yeast whole cell extracts from wild-type and tmc1Δ cells (Fig. 4C).
Proteomic Analysis
The tandem mass tag-based mass spectrometry proteomic analysis of the cellular response to sodium arsenite was previously described in detail (11). The data presented here represent selected further original analysis of that data set, which encompasses nearly 4,600 yeast proteins.
Fluorescence Microscopy
Logarithmically growing Tmc1-GFP cells were treated with sodium arsenite (1 mm) for 1 h. The nucleic acid dye DAPI (Sigma) was added to a final concentration of 100 ng/ml. Live cells were then analyzed by fluorescence microscopy using an Olympus BX51 microscope outfitted with a 100× oil immersion objective and the appropriate filters.
Reverse Transcription PCR (RT-PCR)
Total RNA was prepared by glass bead lysis in the presence of hot phenol. One hundred μg of RNA were digested with DNase I for 30 min at 37 °C, phenol-extracted, and precipitated. Eight μg of treated RNA were denatured at 65 °C for 10 min and chilled on ice. RT-PCR was performed using the GoScript reverse kit (Promega). One μl of cDNA was subsequently used in standard PCRs to determine the relative abundance of Tmc1 mRNA. The following oligonucleotides for TMC1 were used: 5′-ATGTCTGATATAAACGAAATC-3′ and 5′-TTAAATTTGTATCTTTGGAGC-3′. Note that control reactions in which the reverse transcription step was omitted were performed to ensure that there was no residual contaminating genomic DNA.
Phenotypic Analysis
Overnight cultures in YPD or the appropriate selective media at 30 °C were normalized by culture density and spotted in 3-fold serial dilutions onto plates lacking or containing the indicated drug and cultured at 30 °C.
Author Contributions
J. H. conceived of the project. A. G.-M. and J. H. performed the experiments and wrote the manuscript.
Acknowledgment
We thank Marta Isasa for help with proteomic analysis and for comments on the manuscript. We also thank Meera Bhanu and Miguel Prado.
This work was supported by National Institutes of Health Grant DP5-OD019800 (to J. H.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- TMT
- tandem mass tag-based
- TAP
- tandem affinity purification.
References
- 1.Shulman J. M., De Jager P. L., and Feany M. B. (2011) Parkinson's disease: genetics and pathogenesis. Annu. Rev. Pathol. 6, 193–222 [DOI] [PubMed] [Google Scholar]
- 2.Hipp M. S., Park S. H., and Hartl F. U. (2014) Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 24, 506–514 [DOI] [PubMed] [Google Scholar]
- 3.Labbadia J., and Morimoto R. I. (2015) The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 84, 435–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finley D., Ulrich H. D., Sommer T., and Kaiser P. (2012) The ubiquitin-proteasome system of Saccharomyces cerevisiae. Genetics 192, 319–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomko R. J. Jr., and Hochstrasser M. (2013) Molecular architecture and assembly of the eukaryotic proteasome. Annu. Rev. Biochem. 82, 415–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matyskiela M. E., and Martin A. (2013) Design principles of a universal protein degradation machine. J. Mol. Biol. 425, 199–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkpatrick D. S., Dale K. V., Catania J. M., and Gandolfi A. J. (2003) Low-level arsenite causes accumulation of ubiquitinated proteins in rabbit renal cortical slices and HEK293 cells. Toxicol. Appl. Pharmacol. 186, 101–109 [DOI] [PubMed] [Google Scholar]
- 8.Kim M. N., Choi J., Ryu H. W., and Ryu K. Y. (2015) Disruption of polyubiquitin gene Ubc leads to attenuated resistance against arsenite-induced toxicity in mouse embryonic fibroblasts. Biochim. Biophys. Acta 1853, 996–1009 [DOI] [PubMed] [Google Scholar]
- 9.Jacobson T., Navarrete C., Sharma S. K., Sideri T. C., Ibstedt S., Priya S., Grant C. M., Christen P., Goloubinoff P., and Tamás M. J. (2012) Arsenite interferes with protein folding and triggers formation of protein aggregates in yeast. J. Cell Sci. 125, 5073–5083 [DOI] [PubMed] [Google Scholar]
- 10.Pan X., Reissman S., Douglas N. R., Huang Z., Yuan D. S., Wang X., McCaffery J. M., Frydman J., and Boeke J. D. (2010) Trivalent arsenic inhibits the functions of chaperonin complex. Genetics. 186, 725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra-Moreno A., Isasa M., Bhanu M. K., Waterman D. P., Eapen V. V., Gygi S. P., and Hanna J. (2015) Proteomic analysis identifies ribosome reduction as an effective proteotoxic stress response. J. Biol. Chem. 290, 29695–29706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Y., and Varshavsky A. (2001) RPN4 is a ligand, substrate, and transcriptional regulator of the 26S proteasome: a negative feedback circuit. Proc. Natl. Acad. Sci. U.S.A. 98, 3056–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannhaupt G., Schnall R., Karpov V., Vetter I., and Feldmann H. (1999) Rpn4p acts as a transcription factor by binding to PACE, a nonamer box found upstream of 26S proteasomal and other genes in yeast. FEBS Lett. 450, 27–34 [DOI] [PubMed] [Google Scholar]
- 14.Radhakrishnan S. K., Lee C. S., Young P., Beskow A., Chan J. Y., and Deshaies R. J. (2010) Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol. Cell 38, 17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steffen J., Seeger M., Koch A., and Krüger E. (2010) Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol. Cell 40, 147–158 [DOI] [PubMed] [Google Scholar]
- 16.Leggett D. S., Hanna J., Borodovsky A., Crosas B., Schmidt M., Baker R. T., Walz T., Ploegh H., and Finley D. (2002) Multiple associated proteins regulate proteasome structure and function. Mol. Cell. 10, 495–507 [DOI] [PubMed] [Google Scholar]
- 17.Sá-Moura B., Funakoshi M., Tomko R. J. Jr., Dohmen R. J., Wu Z., Peng J., and Hochstrasser M. (2013) A conserved protein with AN1 zinc finger and ubiquitin-like domains modulates Cdc48 (p97) function in the ubiquitin-proteasome pathway. J. Biol. Chem. 288, 33682–33696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna J., Waterman D., Isasa M., Elsasser S., Shi Y., Gygi S., and Finley D. (2014) Cuz1/Ynl155w, a zinc-dependent ubiquitin-binding protein, protects cells from metalloid-induced proteotoxicity. J. Biol. Chem. 289, 1876–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang G. W., Ishida Y., and Naganuma A. (2006) Identification of F-box proteins that are involved in resistance to methylmercury in Saccharomyces cerevisiae. FEBS Lett. 580, 6813–6818 [DOI] [PubMed] [Google Scholar]
- 20.Zhang X. W., Yan X. J., Zhou Z. R., Yang F. F., Wu Z. Y., Sun H. B., Liang W. X., Song A. X., Lallemand-Breitenbach V., Jeanne M., Zhang Q. Y., Yang H. Y., Huang Q. H., Zhou G. B., Tong J. H., et al. (2010) Arsenic trioxide controls the fate of the PML-RARα oncoprotein by directly binding PML. Science 328, 240–243 [DOI] [PubMed] [Google Scholar]
- 21.Ilina Y., Sloma E., Maciaszczyk-Dziubinska E., Novotny M., Thorsen M., Wysocki R., Tamás M. J. (2008) Characterization of the DNA-binding motif of the arsenic-responsive transcription factor Yap8p. Biochem. J. 415, 467–475 [DOI] [PubMed] [Google Scholar]
- 22.Seufert W., and Jentsch S. (1990) Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J. 9, 543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valásek L., Hasek J., Nielsen K. H., Hinnebusch A. G. (2001) Dual function of eIF3j/Hcr1p in processing 20 S pre-rRNA and translation initiation. J. Biol. Chem. 276, 43351–43360 [DOI] [PubMed] [Google Scholar]
- 24.Jungmann J., Reins H. A, Schobert C., and Jentsch S. (1993) Resistance to cadmium mediated by ubiquitin-dependent proteolysis. Nature 361, 369–371 [DOI] [PubMed] [Google Scholar]
- 25.Wang L., Mao X., Ju D., and Xie Y. (2004) Rpn4 is a physiological substrate of the Ubr2 ubiquitin ligase. J. Biol. Chem. 279, 55218–55223 [DOI] [PubMed] [Google Scholar]
- 26.Ju D., Wang X., Xu H., and Xie Y. (2008) Genome-wide analysis identifies MYND-domain protein Mub1 as an essential factor for Rpn4 ubiquitylation. Mol. Cell Biol. 28, 1404–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju D., and Xie Y. (2004) Proteasomal degradation of RPN4 via two distinct mechanisms, ubiquitin-dependent and -independent. J. Biol. Chem. 279, 23851–23854 [DOI] [PubMed] [Google Scholar]
- 28.Jelinsky S. A., Estep P., Church G. M., and Samson L. D. (2000) Regulatory networks revealed by transcriptional profiling of damaged Saccharomyces cerevisiae cells: Rpn4 links base excision repair with proteasomes. Mol. Cell Biol. 20, 8157–8167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finley D., Ozkaynak E., and Varshavsky A. (1987) The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell 48, 1035–1046 [DOI] [PubMed] [Google Scholar]
- 30.Spence J., Sadis S., Haas A. L., and Finley D. (1995) A ubiquitin mutant with specific defects in DNA repair and multiubiquitination. Mol. Cell Biol. 15, 1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]