Abstract
Avian metapneumovirus (aMPV) fusion (F) protein mediates virus-cell membrane fusion to initiate viral infection, which requires F protein binding to its receptor(s) on the host cell surface. However, the receptor(s) for aMPV F protein is still not identified. All known subtype B aMPV (aMPV/B) F proteins contain a conserved Arg-Asp-Asp (RDD) motif, suggesting that the aMPV/B F protein may mediate membrane fusion via the binding of RDD to integrin. When blocked with integrin-specific peptides, aMPV/B F protein fusogenicity and viral replication were significantly reduced. Specifically we identified integrin αv and/or β1-mediated F protein fusogenicity and viral replication using antibody blocking, small interfering RNAs (siRNAs) knockdown, and overexpression. Additionally, overexpression of integrin αv and β1 in aMPV/B non-permissive cells conferred aMPV/B F protein binding and aMPV/B infection. When RDD was altered to RAE (Arg-Ala-Glu), aMPV/B F protein binding and fusogenic activity were profoundly impaired. These results suggest that integrin αvβ1 is a functional receptor for aMPV/B F protein-mediated membrane fusion and virus infection, which will provide new insights on the fusogenic mechanism and pathogenesis of aMPV.
Keywords: fusion protein; infection; integrin; membrane fusion; viral replication; aMPV/B, fusogenicity
Introduction
Avian metapneumovirus (aMPV)3 belongs to the genus Metapneumovirus in the subfamily Pneumovirinae of paramyxoviridae (1, 2). aMPV is a major cause of acute rhinotracheitis in turkeys and is associated with swollen head syndrome in chickens. Thus, aMPV is considered a major threat to the poultry industry (1, 2). Based on phylogenetic analysis, aMPV has been divided into four subtypes: aMPV/A, aMPV/B, aMPV/C, and aMPV/D (3).
The entry of a paramyxovirus into the host cell initially requires fusion of the viral envelope and cellular membrane, which is mediated by the glycoproteins on the viral envelope (4–6). For viruses of the Paramyxovirinae subfamily, such as Newcastle disease virus, fusion (F) protein-mediated membrane fusion requires the assistance of the hemagglutinin-neuraminidase (HN) protein (7–10). However, viruses in the Pneumovirinae subfamily including human respiratory syncytial virus (hRSV), human metapneumovirus (hMPV), and aMPV, the F protein alone in the absence of the small hydrophobic and attachment (G) proteins can mediate membrane fusion and viral infection (11–15), suggesting that the mechanism by which hRSV, hMPV, and aMPV mediate virus-cell membrane fusion might be unique among the Paramyxovirinae subfamily.
The F protein of Paramyxovirinae viruses is activated by a non-F attachment glycoprotein bound to its receptor(s) on the host cell (10, 16). The F protein then undergoes a coordinated series of conformational changes to its most stable form to promote membrane fusion (17–20). Thus, binding of the attachment glycoprotein to the cellular receptor(s) is critical for triggering the fusion process (21, 22). For example, Newcastle disease virus HN protein attaches to sialic acid-containing receptors on the host cell surface and then activates the F protein to induce membrane fusion (23).
For aMPV in Pneumovirinae, the F protein alone can induce membrane fusion (11, 14), suggesting that functions of the aMPV F protein are 2-fold: binding to receptors and mediating fusion. The present study demonstrates that the RDD-binding integrin is a functional receptor that promotes the ability of the aMPV/B F protein to mediate membrane fusion and viral infection.
Experimental Procedures
Cells and Virus Strains
Vero, BHK-21, DF-1, and CHO cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. The cells were grown at 37 °C in a 5% CO2 incubator. The aMPV/B strain aMPV/f was isolated and maintained in our laboratory.
Plasmids and Site-directed Mutagenesis
The F genes of aMPV/A (LAH A strain, GenBankTM AY640317.1), aMPV/B (VCO3/60616 strain, access number: AB548428.1), and aMPV/C (Colorado strain, GenBank AY590688.1) were subcloned into the pCAGGS expression vector with a FLAG tag at the C terminus and designated aMPV/A-F, aMPV/B-F, and aMPV/C-F. The integrin αv and β1 genes from monkey, mouse, and chicken were amplified by RT-PCR and subcloned into the pCAGGS expression vector with a HA tag at the N terminus. The constructs were designated Ma_αv, Ma_β1, Mo_αv, Mo_β1, Ch_αv, and Ch_β1. All F mutants and integrin genes were constructed using an In-Fusion® HD Cloning Kit (Clontech, Mountain View, CA).
Syncytium Formation Assay
This assay was performed to evaluate the fusogenic activity of the F protein as previously described (24, 25). Vero cells grown in 6-well plates were transfected with 1 μg of F gene plasmid per well. Integrin αv and/or β1 were selectively overexpressed, knocked down, or blocked in Vero cells to assess their effects on F protein fusogenic activity. At 48 h post-transfection, syncytia in the transfected cells were measured and photographed using a digital microscope. The areas forming syncytia were compared with the total analyzed areas using the Magnetic Lasso Tool and histogram function of Adobe Photoshop software, and the percentage of cells involved in syncytium formation was calculated to represent fusogenic activity.
Reporter Gene Assay
F protein fusogenic activity was assayed as previously described (6, 26). Vero cells grown in 6-well plates were transfected with 1 μg/well of each plasmid containing the F gene and 0.5 μg/well of luciferase cDNA under control of the T7 promoter. The next morning, the Vero cells were detached from the plates, resuspended, and overlaid onto BHK-21 cells infected with MVA-T7 at a multiplicity of infection (m.o.i.) of 1. Integrin αv and/or β1 were selectively overexpressed, knocked down, or blocked in BHK-21 cells to test their effects on the fusogenic activity of the F protein. At 48 h post-transfection, fusogenic activity was evaluated by quantitating the luciferase activity of the cell lysates using an Envision Multilabel Reader (PerkinElmer Life Sciences).
Fusion Blockade with RGD Peptides
RGD peptides (Selleck Chemicals, Houston, TX) were employed to block integrin as previously described (27). The RGD peptides had no effect on infection by hRSV, which lacks an RGD motif in its analogous F protein, suggesting that RGD peptides do not affect cell culture growth (27). RGE peptides (GRGESP, synthesized by the Beijing Genomics Institute) were added to control cell cultures. Vero or BHK-21 cells were incubated with RGD or RGE peptides for 6 h, followed by a quantitative assay of fusion activity.
Fusion Blockade with Antibody
The assay was performed as previously reported (28). Vero or BHK-21 cells grown in 24-well plates were incubated with 10 μg/ml of integrin antibody specific for α2 (monoclonal antibody, catalogue number MAB228Hu22, Uscn Life Science Inc., Wuhan, China), α3 (polyclonal antibody, catalogue number 100397-T08, Sino Biological Inc., Beijing, China), α6 (polyclonal antibody, catalogue number 100497-T08, Sino Biological Inc.), αv (monoclonal antibody, catalogue number MAB2021Z, lot number: 2569164, Millipore, Billerica, MA), or β1 (monoclonal antibody, catalogue number MAB1959, lot number: 2494042, Millipore) for 6 h, followed by syncytium formation and reporter gene assays.
Viral Replication Analysis in the Presence of Integrin Blockade
Monolayers of Vero, BHK-21, or DF-1 cells grown in 24-well plates were incubated with RGD/RGE peptides or with 10 μg/ml of integrin antibody for 2 h. The treated cells were infected with 20 μl of 106 50% tissue culture infective dose (TCID50)/ml of aMPV/f for 1 h, and the supernatant was collected to determine the aMPV/B titer.
Knockdown of Integrin with siRNAs
Integrin αv- and/or β1-specific or control small interfering RNAs (siRNAs) were transfected into cells. At 6 h post-siRNA treatment, the cells were transfected with a plasmid containing the F gene or infected with 100 μl of 106 TCID50/ml of aMPV/f.
Virus Titer Determination
Supernatant samples from cells infected with aMPV/f at 24, 48, 72, and 96 h post-infection (h.p.i.) were harvested and stored at −80 °C prior to analysis. To determine the titer of aMPV/f, 100 μl of 10-fold serial dilutions of the supernatant samples were cultured with Vero cells in 96-well plates. At 4 days post-infection, syncytium formation was quantified to determine the TCID50.
Biotinylation and Western Blot Assay
This assay was performed to determine the expression level of the proteins on the cell surface as previously described (29, 30). Vero cells in 6-well plates were transfected with 4 μg of DNA encoding FLAG-tagged F proteins per well. At 48 h post-transfection, surface proteins were biotinylated with membrane-impermeable EZ-Link Sulfo-NHS-SS-Biotin (Thermo Scientific, Rockford, IL), and the cells were lysed with cell lysis buffer. The lysates were centrifuged at 12,000 × g for 25 min at 4 °C, and the supernatants were collected. Streptavidin beads (Thermo Scientific) were added to the supernatant to capture the biotinylated surface proteins. Protein samples were analyzed by Western blot with monoclonal anti-FLAG antibody (Sigma). As a reference control for the analysis of F protein and integrin expression on the cell membrane, Na+/K+-ATPase expression on the cell membrane was analyzed using an anti-Na+/K+-ATPase β-1 antibody (monoclonal antibody, catalogue number: 05-382, lot number: 2685213, Millipore). The densitometry was evaluated using Gelpro32 software.
Cell-Cell Binding Assay
A cell-cell binding assay was performed to analyze aMPV/B F protein binding to integrins. aMPV/B F protein and integrins were separately expressed on different cells as previously described (31). Vero cells grown in 6-well plates were transfected with 1 μg of plasmid carrying the F gene (ligand) per well. CHO cells grown in 12-well plates were transfected with 1 μg of plasmid carrying of integrin αv and β1 (target) per well. Transfected Vero cells were detached by adding trypsin and washed with phosphate-buffered saline (PBS) containing 5% FBS. The detached Vero cells were added to the transfected CHO cells and incubated at 4 °C for 2 h. Unbound Vero cells were removed by washing three times with PBS containing 5% FBS. The combined cells were resuspended by pipetting and incubated with a polyclonal anti-FLAG antibody followed by incubation with FITC-conjugated anti-rabbit antibody (Sigma), then analyzed using a LSRII flow cytometer (BD Biosciences). FLAG tag staining of 10,000 cells was analyzed to quantify the ability of F protein to bind to the integrins. Furthermore, to determine whether the RDD motif was the site of aMPV/B F protein binding to the receptor, Vero cells grown in 6-well plates were transfected with 1 μg/well of plasmid carrying the F gene or mutant F genes (ligand), and DF-1 cells grown in 12-well plates were transfected with 1 μg of Ch_αv+β1 (target). The cell-cell binding assay was subsequently performed as described above.
Virus Entry Assay
CHO cells grown in each well of 12-well plates were transfected with 1 μg of integrin αv and β1. At 6 h post-transfection, the CHO cells were incubated with or without antibodies specific for integrin αv and β1 for 1 h and then infected with 20 μl of 106 TCID50/ml of aMPV/f for 1 h. At 0, 1, 2, and 4 h.p.i., the CHO cells were scraped off to extract the RNA. Quantitative RT-PCR was performed according to a previous study (3).
Statistical Analysis
All experiments were repeated three to five times. One-way analysis of variance was employed for statistical analyses (SPSS 13.0). A p value of less than 0.05 was considered statistically significant.
Results
aMPV/B F Protein Carries Conserved RDD Motif
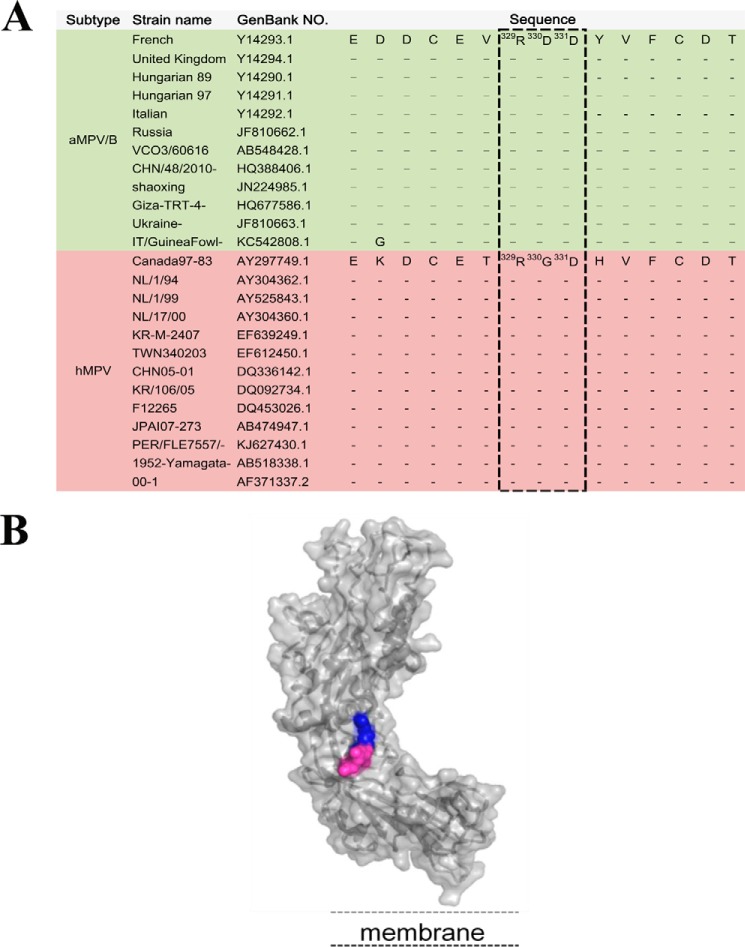
Many viruses such as hMPV utilize RGD-binding integrin as receptors or co-receptors to mediate viral infection (27, 28, 32–38). We compared the full-length protein sequences of the aMPV/B F protein and hMPV F protein and observed that whereas the hMPV F protein contains an RGD motif at residues 329–331, all available aMPV/B F protein sequences contain a conserved RDD motif at the same position (Fig. 1A). Importantly, both the RGD and RDD motifs are predicted to be in solvent-exposed regions on the surfaces of the hMPV and aMPV F proteins, respectively (27, 39) (Fig. 1B). These findings promoted us to investigate whether integrins serve as receptors for aMPV/B F protein-mediated membrane fusion and virus infection.
FIGURE 1.
A conserved integrin-binding motif exists in the aMPV/B and hMPV F proteins. A, F protein sequences from 12 clinical isolates of aMPV/B and 13 clinical isolates of hMPV were aligned. The RDD motif is highlighted in the boxes. B, the three-dimensional structural model represents the conformation of the aMPV/B F protein using the SWISS-MODEL server facilities and based on the atomic coordinates of the structure of the hMPV F protein (Protein Data Bank code 4dag).
Peptide Blockade of Integrin Reduces the Fusogenicity of the aMPV/B F Protein and the Replication of aMPV/B
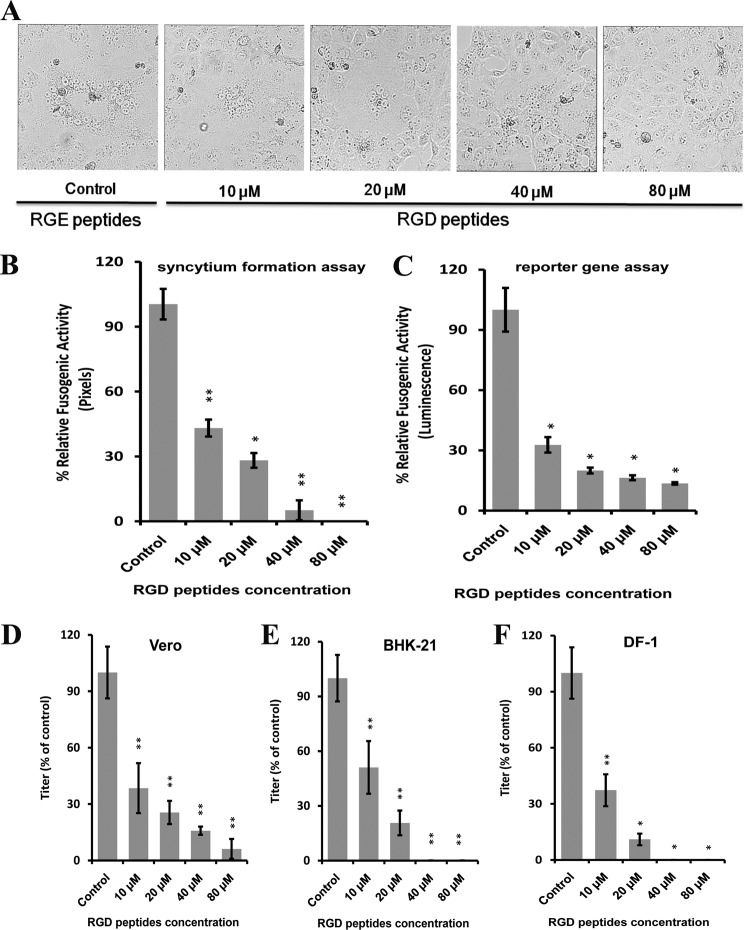
To determine whether the RGD-binding site on integrin is required for aMPV/B F protein-mediated membrane fusion, we incubated Vero and BHK-21 cells with RGD peptides that can block integrin as previously described (27, 40). As shown in Fig. 2, A–C, the fusogenic activity of the aMPV/B F protein was significantly reduced in RGD peptide-treated cells compared with RGE peptide-treated cells. Additionally, the treatment of cells with RGD peptide inhibited aMPV/B F protein fusogenicity in a dose-dependent manner. Moreover, we inoculated Vero, BHK-21, and DF-1 cells with RGD peptide to investigate the effect of integrin on the replication of aMPV/B. At 48 h.p.i., supernatant samples of these infected cells were collected to determine the aMPV/B titer. The replication of aMPV/B was inhibited in RGD peptide-treated cells compared with RGE peptide-treated cells (Fig. 2, D–F). Overall, function-blocking peptides specific for integrin decreased the fusogenicity of the aMPV/B F protein and viral replication.
FIGURE 2.
RGD peptides inhibit the fusogenic activity of the aMPV/B F protein and aMPV/B replication. A, representative micrographs of syncytia formation mediated by the aMPV/B F protein in Vero cells in the presence of different concentrations of RGD peptides (×100 magnification; 8 × 8 cm as shown). RGE peptides (80 μm) were used as the control. B and C, aMPV/B F protein fusogenic activity was determined using syncytium formation and reporter gene assays. RGE peptides (80 μm) were used as the control. D–F, aMPV/B replication in Vero, BHK-21, and DF-1 cells, respectively, in the presence of different concentrations of RGD peptides. The virus titer was measured at 48 h.p.i., and RGE peptides (80 μm) were used as the control. All experiments were repeated three to five times. p < 0.05 and p < 0.005 are indicated by * and **, respectively.
Integrin αv- and β1-specific Antibodies Decrease the Fusogenicity of aMPV/B F Protein and the Replication of aMPV/B
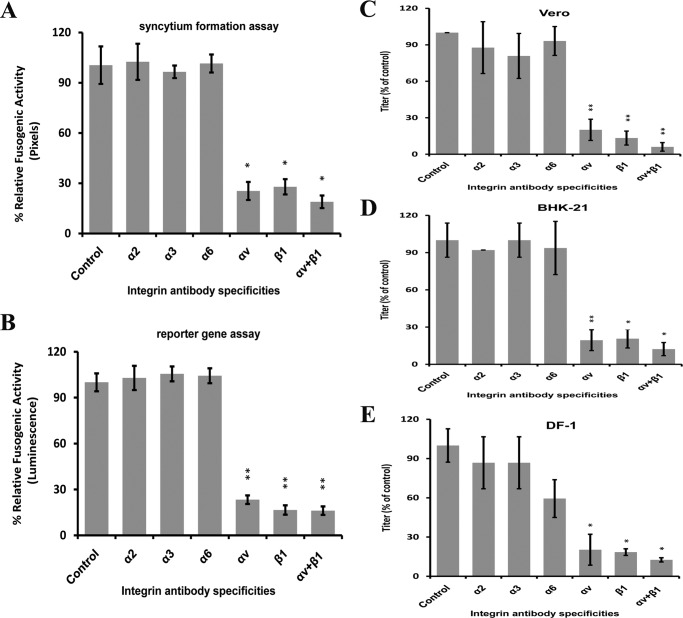
To determine which members of integrin family are responsible for the fusogenic activity of the aMPV/B F protein, we treated Vero and BHK-21 cells with integrin α2-, α3-, α6-, αv-, or β1-specific antibodies. As shown in Fig. 3, A and B, the fusogenicity of the aMPV/B F protein was reduced to ∼30–50% in cells treated with integrin αv- and/or β1-specific antibodies compared with cells treated with other integrin-specific antibodies or control cells. Furthermore, Vero, BHK-21, and DF-1 cells were treated with antibodies for integrin to determine whether integrin is essential for aMPV/B replication. Samples of infected cell supernatants were collected to determine the virus titer at 48 h.p.i. Virus titers were 3–10-fold lower in cells treated with antibodies specific for integrin αv and/or β1 than in untreated cells or in cells treated with antibodies specific for other members of the integrin family. Thus, antibody blocking of integrin αv and β1 inhibited the replication of aMPV/B in Vero, BHK-21, and DF-1 cells (Fig. 3, C–E). Collectively, these results demonstrated that expression of integrin αv and β1 on the cell surface was essential for the infectivity of aMPV/B.
FIGURE 3.
Antibodies specific for αv and β1 integrin block the fusogenic activity of the aMPV/B F protein and aMPV/B replication. A and B, in the presence of integrin-specific antibodies, aMPV/B F protein fusogenic activities were measured by syncytium formation and reporter gene assays. C–E, the replication of aMPV/B in the presence of integrin-specific antibodies in Vero, BHK-21, and DF-1 cells, respectively, was evaluated. The virus titer was measured at 48 h.p.i. All experiments were repeated three to five times. p < 0.05 and p < 0.005 are indicated by * and **, respectively.
Reduced Integrin αv and β1 Expression Inhibits aMPV/B F Protein Fusogenicity and aMPV/B Replication
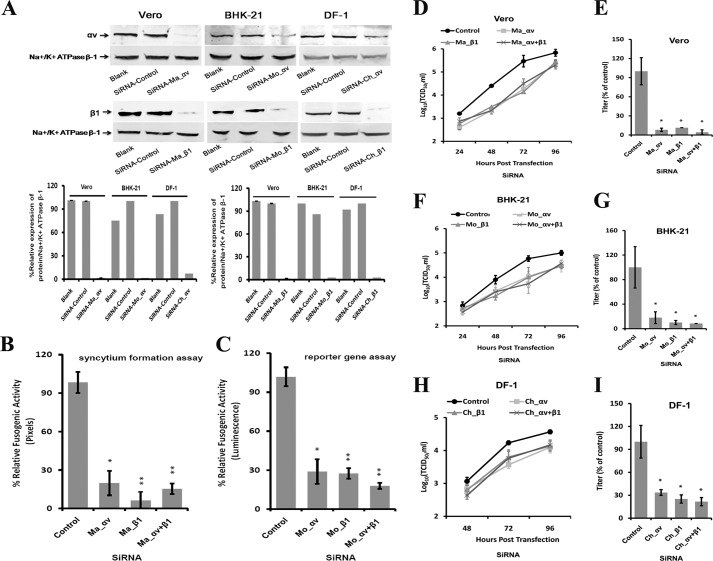
To determine whether reduced integrin αv and/or β1 expression decrease the fusogenic activity of the aMPV/B protein, we transfected Vero and BHK-21 cells with siRNAs targeting integrin αv and/or β1, which reduced the expression of integrin αv and β1 on the cell surface (Fig. 4A). As shown in Fig. 4, B and C, the fusogenic activity of the aMPV/B F protein was reduced in cells treated with integrin-specific siRNAs. Compared with control cells that were not treated with siRNA, siRNA-treated cells exhibited an approximate 3- 16-fold inhibition. We also used siRNA-treated Vero, BHK-21, and DF-1 cells to confirm that integrin αv and β1 were required for aMPV/B replication. As shown in Fig. 4, D, F, and H, lower integrin αv and β1 expression levels inhibited aMPV/B replication in Vero, BHK-21, and DF-1 cells. At 72 h.p.i., siRNAs specific for integrin αv and β1 displayed the greatest inhibition: ∼17-fold in Vero cells, 12-fold in BHK-21 cells, and 5-fold in DF-1 cells (Fig. 4, E, G, and I). Taken together, these results indicated that the cell-surface expression of integrin αv and β1 was correlated with the fusogenic activity of the aMPV/B F protein and the efficiency of aMPV/B replication.
FIGURE 4.
Knockdown of integrin αv- and β1-specific siRNAs inhibits the fusogenic activity of the aMPV/B F protein and the replication of aMPV/B. A, siRNAs targeting monkey integrin αv or β1, mouse integrin αv or β1, and chicken integrin αv or β1 were transfected into Vero, BHK-21, and DF-1 cells, respectively, in 6-well plates. At 48 h post-transfection, the expression of integrin αv or β1 on the cell surface was analyzed by biotinylation and a Western blot assay. B and C, cells were treated with an integrin siRNAs specific for the corresponding species. aMPV/B F protein fusogenic activity was determined using syncytium formation and reporter gene assays. Ma, monkey; Mo, mouse; Ch, chicken. D, F, and H, replication kinetics of aMPV/B in cells treated with siRNAs were evaluated up to 96 h.p.i. E, G, and I, at 72 h.p.i., the supernatant was collected from infected Vero, BHK-21, or DF-1 cells, respectively, to determine the aMPV/B titer and compared with the control. The titer of the supernatant from aMPV/B-infected cells treated with the control siRNAs was set at 100%. All experiments were repeated three to five times. p < 0.05 and p < 0.005 are indicated by * and **, respectively.
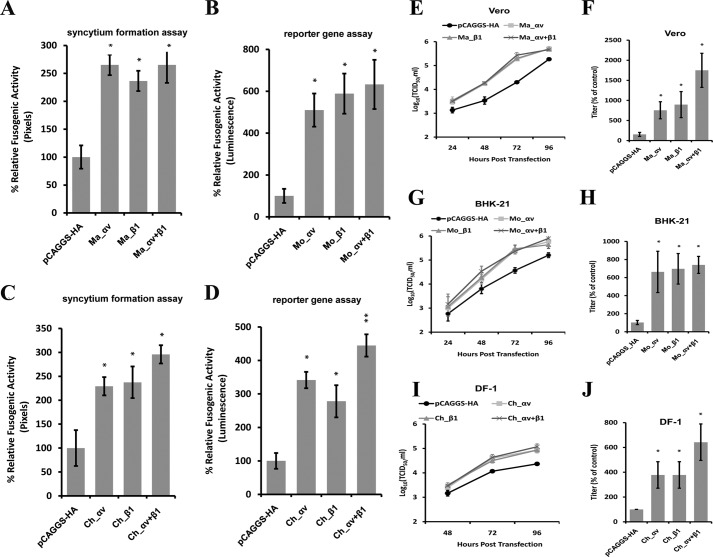
Overexpression of Integrin αv and β1 Enhances aMPV/B F Protein Fusogenicity and Viral Replication
To determine whether overexpression of integrin αv and β1 increases fusogenicity of the aMPV/B F protein, we transiently transfected monkey and mouse integrin αv and/or β1 into Vero and BHK-21 cells. Overexpression of monkey and mouse integrin αv and/or β1 substantially increased the fusogenic activity of the aMPV/B F protein compared with cells transfected with an empty vector (Fig. 5, A and B). Moreover, we further analyzed the effect of the overexpression of chicken αv and/or β1 on the fusogenic activity of the aMPV/B F protein. As shown in Fig. 5, C and D, exogenous expression of chicken αv and/or β1 enhanced the fusogenic activity of the aMPV/B F protein.
FIGURE 5.
Overexpression of integrin αv and β1 enhances the fusogenic activity of the aMPV/B F protein and the replication of aMPV/B. A–D, cells were transfected with plasmid DNAs expressing integrin αv and β1 from different species. aMPV/B F protein fusogenic activities were determined by syncytium formation and reporter gene assays. Ma, monkey; Mo, mouse; Ch, chicken. E, G, and I, the kinetics of aMPV/B replication in different cells overexpressing integrin αv and β1 were determined up to 96 h.p.i. F, H, and J, at 72 h.p.i., the supernatant was collected from infected Vero, BHK-21, or DF-1 cells to determine the virus titer and compared with controls. The virus titer of transfection with the vector pCAGGS was set as 100%. All experiments were repeated three to five times. p < 0.05 and p < 0.005 are indicated by * and **, respectively.
To define the roles of integrin αv and β1 in the replication of aMPV/B, we overexpressed integrin αv and/or β1 in Vero, BHK-21, and DF-1 cells. As shown in Fig. 5, E, G, and I, overexpression of integrin αv and/or β1 enhanced the replication of aMPV/B. At 72 h.p.i., aMPV/B replication was enhanced by ∼11-fold in Vero cells, 8-fold in BHK-21 cells, and 4-fold in DF-1 cells (Fig. 5, F, H, and J). Overall, these results demonstrated that overexpression of integrin αv and β1 resulted in a higher level of aMPV/B F protein fusogenicity and viral replication.
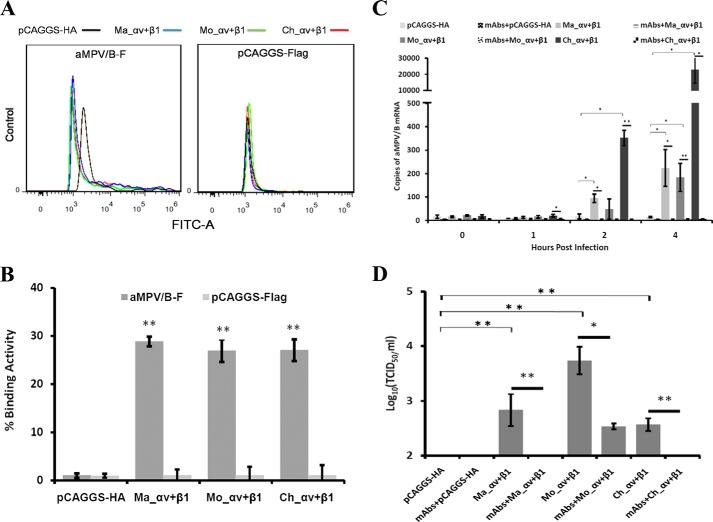
Integrin αv and β1 Expressed on Nonpermissive Cells Confer aMPV/B Infection
Because CHO cells are poorly infected by aMPV/B, we transfected CHO cells with Ma_αv+β1, Mo_αv+β1, or Ch_αv+β1 to determine whether expression of integrin αv and β1 on the cell surface alters the infectivity of aMPV/B. As shown in Fig. 6, A and B, transient overexpression of monkey, mouse, or chicken integrin αv and β1 significantly enhanced the ability of the aMPV/B F protein to bind CHO cells. We excluded the possibility that the integrins bound to other cellular targets by analyzing the activity of expressed integrins binding to an empty vector expressing the FLAG tag (Fig. 6, A and B). Overexpression of monkey, mouse, or chicken integrin αv and β1 increased the efficiency of aMPV/B entry into CHO cells, whereas blocking these integrins with specific antibodies inhibited this effect (Fig. 6C). Moreover, overexpression of monkey, mouse, or chicken integrin αv and β1 improved the replication of aMPV/B in CHO cells, but this effect was suppressed by blocking the integrins with specific antibodies (Fig. 6D). Overall, these results demonstrated that integrin αv and β1 modulation affected aMPV/B F protein binding and aMPV/B productive infection.
FIGURE 6.
Overexpression of integrin αv and β1 enhances the binding activity of the aMPV/B F protein and infection of aMPV/B. A, the binding activity of the aMPV/B F protein and infection with aMPV/B in CHO cells overexpressing the integrins αv and β1 were evaluated using cell-cell binding and virus entry assays. A representative cell-cell binding graph was obtained from flow cytometric analysis. B, the cell-cell binding assay results as a mean percentage from three independent experiments. C, CHO cells that overexpressed integrin αv and/or β1 from different species were treated with corresponding antibodies, then infected with aMPV/B. Virus entry was measured at 0, 1, 2, and 4 h.p.i. D, at 72 h.p.i., the supernatant from infected CHO cells was collected to determine the virus titer. All experiments were repeated three to five times. p < 0.05 and p < 0.005 are indicated by * and **, respectively.
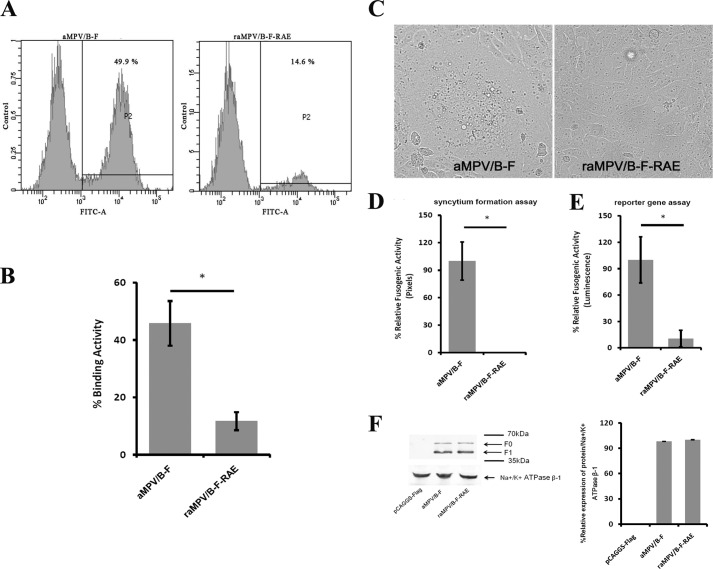
Mutation of the RAE Motif Impairs the Binding and Fusogenicity of the aMPV/B F Protein
To confirm that the RDD motif is the binding site of the aMPV/B F protein to the receptor, we substituted the RDD motif with Arg-Ala-Glu (RAE) as previously described (38) and analyzed the effect of the RAE mutant on the binding and fusogenic activity of the aMPV/B F protein. As shown in Fig. 7, A and B, the RAE mutant eliminated ∼70% of the binding activity compared with the wild-type aMPV/B F protein. Moreover, wild-type aMPV/B F mediated extensive (data not shown) and apparent (Fig. 7C) syncytia in Vero cells, whereas few syncytia were observed in Vero cells expressing a mutant aMPV/B F protein containing the RAE sequence (Fig. 7C). Further quantitative assays revealed that the aMPV/B F protein containing RDD was hyperfusogenic, whereas the fusogenicity of a mutant aMPV/B F protein containing RAE was low (Fig. 7, D and E). We also performed biotinylation and Western blot assays to exclude the possibility that RAE decreased expression of the aMPV/B F protein on the cell surface. As shown in Fig. 7F, expression of the mutant aMPV/B F protein containing RAE was comparable with that of the wild-type aMPV/B F protein. Taken together, these results indicated that the RDD motif was involved in the binding of the aMPV/B F protein to cells and the induction of cell-cell fusion.
FIGURE 7.
Alteration of RDD to RAE inhibits the binding and fusogenic activity of the aMPV/B F protein. A, the aMPV/B F protein RDD motif was mutated to RAE. Vero cells were transfected with either the wild-type aMPV/B F or the RAE mutant. The binding activity of the F proteins was measured in a cell-cell binding assay. A representative cell-cell binding graph was obtained from flow cytometric analysis. B, the cell-cell binding results as the mean percentage from three independent experiments. C, representative micrographs of syncytia formation mediated by the wild-type aMPV/B F protein and the mutant in Vero cells. D and E, the fusogenic activity of the wild-type aMPV/B F protein and the mutant were also measured using syncytium formation and reporter gene assays. F, the surface expression of transfected aMPV/B-F and raMPV/B-F-RAE was confirmed by biotinylation and a Western blot assay. All experiments were repeated three to five times. p < 0.05 and p < 0.005 are indicated by * and **, respectively.
Discussion
aMPV has been considered a major disease threat to the poultry industry (1, 2). The initial step for the entry of aMPV into the host cell is F protein-mediated virus-cell membrane fusion, which is activated by the attachment of the F protein to its receptor(s) on the cell surface (11, 12, 26). In this study, we provide the first evidence that integrin αvβ1 serves as a functional receptor for the aMPV/B F protein, which induces cell-cell fusion and aMPV/B infection. Importantly, our findings indicate a wider role for integrin as a viral receptor.
We previously demonstrated that the aMPV F protein alone can induce cell-cell fusion (41, 42), suggesting that aMPV F protein has the function for binding to its receptor(s). A series of sequence/structure analysis, blockade, knockdown, overexpression, and mutagenesis experiments demonstrated that integrin is a functional receptor for the aMPV/B F protein, which induces cell-cell fusion and aMPV/B infection. Our results indicate that RDD, similar to RGD and RSD, is capable of binding integrin and mediating viral infection. For foot and mouth disease virus (FMDV), Li et al. (43) employed reverse genetics to produce recombinant FMDV containing RDD, RGD, or RSD and determined that the in vitro growth properties of recombinant FMDV containing RDD, RGD, or RSD were similar. They also demonstrated that a non-RGD recombinant FMDV similar to wild-type FMDV containing RGD was virulent in susceptible animals. The role of RDD, RGD, and RSD in regulating aMPV binding, entry, and replication requires further investigation.
By definition, viral attachment to a receptor is a prerequisite for viral infection, and thus the distribution of receptors determines the range of viral infection. Previous reports and our current data indicate that integrin is indeed a functional receptor for both hMPV and aMPV/B (28, 38). However, clinical data indicate that hMPV and aMPV/B have different invasion ranges (44–46). Because the F protein is the primary determinant of metapneumovirus host tropism (47), we speculate that RGD (the conserved motif in the hMPV F protein) and RDD bind integrins of different species with different efficacies. Such differences in receptor binding efficacy may underlie the differences in tropism between hMPV and aMPV/B.
Putative integrin-binding RDD and RSD motifs are present in the aMPV/A and aMPV/C F proteins at sites similar to that of the RDD motif in the aMPV/B F protein at residue positions 329 to 331 (11). We determined whether the RDD-binding integrin is critical for aMPV/A and aMPV/C F protein-mediated cell-cell fusion. An analysis involving a combination of blockade, overexpression, and knockdown demonstrated that integrin αv and β1 are essential for aMPV/A and aMPV/C F protein-induced cell-cell fusion (data not shown). These results indicate that integrin also serves as a receptor for the induction of membrane fusion by the aMPV/A and aMPV/C F proteins. Further research is necessary to determine whether integrin is essential for aMPV/A and aMPV/C infection.
We repeatedly attempted to verify the interactions between the aMPV/B F protein and integrins using various types of assays, but the experimental results were not conclusive. To our knowledge, an interaction of the F protein with a ligand on the cell surface has not been reported for the Pneumovirinae subfamily, including the hMPV F protein. Many reports have indicated that integrin is as a functional receptor in hMPV F protein-mediated cell-cell fusion and virus infection, but direct interactions between the hMPV F protein and integrin have not been described (27, 28, 38). Similarly, the hRSV F protein mediates the innate immune response through Toll-like receptor 4, but interactions between the hRSV F protein and Toll-like receptor 4 have not been described (48, 49). We speculate that aMPV/B F protein binding to integrins occurs rapidly and thus could not be observed under the experimental conditions used in the present study. Analogously, binding of the human immunodeficiency virus (HIV) fusion protein gp120/gp41 to its receptor and subsequent conformational changes occur rapidly (50, 51).
It is believed that the influenza virus hemagglutinin (HA) protein induces membrane fusion within endosomes dependent on low pH (52). The entry of paramyxoviruses into the cell is thought to occur at the cell surface in a low pH-independent manner, a mechanism that requires the binding of the envelope glycoproteins to its receptors to activate membrane fusion of the virus and cell (38). Our prior work has demonstrated that residue His294 is responsible for aMPV/B F protein-mediated cell-cell fusion independent of low pH (42), suggesting that the aMPV/B F protein attachment to integrin leads to cell-surface fusion. Further investigation is needed to determine whether the entry of aMPV/B occurs at the cell surface.
In summary, we have demonstrated that modulation by integrin αvβ1 affects not only aMPV/B F protein-mediated cell-cell fusion but also aMPV/B infection. Our results indicate that the aMPV/B F protein RDD motif binds to integrin on the host cell surface and promotes F protein-mediated membrane fusion. Such binding allows aMPV/B entry into the cell and subsequent aMPV/B infection. This study may shed light on the mechanism by which RDD-binding integrin facilitates aMPV/B infection and the pathogenesis of aMPV.
Author Contributions
B. L. Y. conceived the study, coordinated the work, and drafted the manuscript. X. L. G., Y. Z. L., and Y. Z. performed the experiments and interpreted the results. Y. Q. W., X. L. Q., H. Y. C., C. J. L., Y. P. Z., H. L. G., L. G., and K. L. were involved in the interpretation of the results and critically read the manuscript. B. L. Y., Y. L. G., and X. M. W. were involved in the experimental design. All authors read and approved the final manuscript.
Acknowledgment
We thank Dr. Baoshan Zhang for helpful suggestions.
This work was supported by the fund for the Modern Agro-industry Technology Research System number nycytx-42-G3-01. The authors declare that they have no conflicts of interest with the contents of this article.
- aMPV
- avian metapneumovirus
- F
- fusion
- aMPV/B
- subtype B aMPV
- HN
- hemagglutinin-neuraminidase
- hRSV
- human respiratory syncytial virus
- hMPV
- human metapneumovirus
- G
- attachment
- h.p.i.
- hours post-infection
- TCID50
- 50% tissue culture infective dose
- FMDV
- foot and mouth disease virus.
References
- 1.Cook J. (2000) Avian pneumovirus infections of turkeys and chickens. Vet. J. 160, 118–125 [DOI] [PubMed] [Google Scholar]
- 2.Gough R. (2003) Avian pneumoviruses. in Diseases of Poultry (Saif Y. M., Barnes H. J., Glisson J. R., Fadly A. M., McDougald L. R., and Swaine D. E., eds) pp. 92–99, Iowa State, Ames, IA [Google Scholar]
- 3.Guionie O., Toquin D., Sellal E., Bouley S., Zwingelstein F., Allée C., Bougeard S., Lemière S., and Eterradossi N. (2007) Laboratory evaluation of a quantitative real-time reverse transcription PCR assay for the detection and identification of the four subgroups of avian metapneumovirus. J. Virol. Methods 139, 150–158 [DOI] [PubMed] [Google Scholar]
- 4.Earp L., Delos S., Park H., and White J. (2005) The many mechanisms of viral membrane fusion proteins. in Membrane Trafficking in Viral Replication. pp. 25–66, Springer-Verlag New York Inc., New York: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore J., Jameson B. A., Weiss R. A., and Sattentau Q. (1993) The HIV-cell fusion reaction. Viral Fusion Mechanisms. pp. 233–289, CRC Press, Boca Raton, FL [Google Scholar]
- 6.Nussbaum O., Broder C. C., and Berger E. A. (1994) Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J. Virol. 68, 5411–5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagai S., and Lamb R. A. (1995) Quantitative measurement of paramyxovirus fusion: differences in requirements of glycoproteins between simian virus 5 and human parainfluenza virus 3 or Newcastle disease virus. J. Virol. 69, 6712–6719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutch R. E., Joshi S. B., and Lamb R. A. (1998) Membrane fusion promoted by increasing surface densities of the paramyxovirus F and HN proteins: comparison of fusion reactions mediated by simian virus 5 F, human parainfluenza virus type 3 F, and influenza virus HA. J. Virol. 72, 7745–7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone-Hulslander J., and Morrison T. G. (1997) Detection of an interaction between the HN and F proteins in Newcastle disease virus-infected cells. J. Virol. 71, 6287–6295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talekar A., DeVito I., Salah Z., Palmer S. G., Chattopadhyay A., Rose J. K., Xu R., Wilson I. A., Moscona A., and Porotto M. (2013) Identification of a region in the stalk domain of the Nipah virus receptor binding protein that is critical for fusion activation. J. Virol. 87, 10980–10996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Y., Feng K., Yao X., Cai H., Li J., Mirza A. M., Iorio R. M., and Li J. (2012) Localization of a region in the fusion protein of avian metapneumovirus that modulates cell-cell fusion. J. Virol. 86, 11800–11814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schowalter R. M., Smith S. E., and Dutch R. E. (2006) Characterization of human metapneumovirus F protein-promoted membrane fusion: critical roles for proteolytic processing and low pH. J. Virol. 80, 10931–10941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karron R. A., Buonagurio D. A., Georgiu A. F., Whitehead S. S., Adamus J. E., Clements-Mann M. L., Harris D. O., Randolph V. B., Udem S. A., Murphy B. R., and Sidhu M. S. (1997) Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. U.S.A. 94, 13961–13966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling R., Sinkovic S., Toquin D., Guionie O., Eterradossi N., and Easton A. J. (2008) Deletion of the SH gene from avian metapneumovirus has a greater impact on virus production and immunogenicity in turkeys than deletion of the G gene or M2-2 open reading frame. J. Gen. Virol. 89, 525–533 [DOI] [PubMed] [Google Scholar]
- 15.Biacchesi S., Skiadopoulos M. H., Yang L., Lamirande E. W., Tran K. C., Murphy B. R., Collins P. L., and Buchholz U. J. (2004) Recombinant human metapneumovirus lacking the small hydrophobic SH attachment G glycoprotein: deletion of G yields a promising vaccine candidate. J. Virol. 78, 12877–12887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porotto M., Murrell M., Greengard O., and Moscona A. (2003) Triggering of human parainfluenza virus 3 fusion protein (F) by the hemagglutinin-neuraminidase (HN) protein: an HN mutation diminishes the rate of F activation and fusion. J. Virol. 77, 3647–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bose S., Heath C. M., Shah P. A., Alayyoubi M., Jardetzky T. S., and Lamb R. A. (2013) Mutations in the parainfluenza virus 5 fusion protein reveal domains important for fusion triggering and metastability. J. Virol. 87, 13520–13531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q., Stone J. A., Bradel-Tretheway B., Dabundo J., Benavides Montano J. A., Santos-Montanez J., Biering S. B., Nicola A. V., Iorio R. M., Lu X., and Aguilar H. C. (2013) Unraveling a three-step spatiotemporal mechanism of triggering of receptor-induced Nipah virus fusion and cell entry. Plos Pathog. 9, e1003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith E. C., Smith S. E., Carter J. R., Webb S. R., Gibson K. M., Hellman L. M., Fried M. G., and Dutch R. E. (2013) Trimeric transmembrane domain interactions in paramyxovirus fusion proteins roles in protein folding, stability, and function. J. Biol. Chem. 288, 35726–35735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu R., Palmer S. G., Porotto M., Palermo L. M., Niewiesk S., Wilson I. A., and Moscona A. (2013) Interaction between the hemagglutinin-neuraminidase and fusion glycoproteins of human parainfluenza virus type III regulates viral growth in vivo. Mbio 4, e00803-e008013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bose S., Song A. S., Jardetzky T. S., and Lamb R. A. (2014) Fusion activation through attachment protein stalk domains indicates a conserved core mechanism of paramyxovirus entry into cells. J. Virol. 88, 3925–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jardetzky T. S., and Lamb R. A. (2014) Activation of paramyxovirus membrane fusion and virus entry. Curr. Opin. Virol. 5, 24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaitsev V., von Itzstein M., Groves D., Kiefel M., Takimoto T., Portner A., and Taylor G. (2004) Second sialic acid binding site in Newcastle disease virus hemagglutinin-neuraminidase: implications for fusion. J. Virol. 78, 3733–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avitabile E., Lombardi G., Gianni T., Capri M., and Campadelli-Fiume G. (2004) Coexpression of UL20p and gK inhibits cell-cell fusion mediated by herpes simplex virus glycoproteins gD, gH-gL, and wild-type gB or an endocytosis-defective gB mutant and downmodulates their cell surface expression. J. Virol. 78, 8015–8025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinzler E. R., and Compton T. (2005) Characterization of human cytomegalovirus glycoprotein-induced cell-cell fusion. J. Virol. 79, 7827–7837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herfst S., Mas V., Ver L. S., Wierda R. J., Osterhaus A. D., Fouchier R. A., and Melero J. A. (2008) Low-pH-induced membrane fusion mediated by human metapneumovirus F protein is a rare, strain-dependent phenomenon. J. Virol. 82, 8891–8895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cseke G., Maginnis M. S., Cox R. G., Tollefson S. J., Podsiad A. B., Wright D. W., Dermody T. S., and Williams J. V. (2009) Integrin αvβ1 promotes infection by human metapneumovirus. Proc. Natl. Acad. Sci. U.S.A. 106, 1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Y., Zhang Y., Cai H., Mirza A. M., Iorio R. M., Peeples M. E., Niewiesk S., and Li J. (2014) Roles of the putative integrin-binding motif of the human metapneumovirus fusion (F) protein in cell-cell fusion, viral infectivity, and pathogenesis. J. Virol. 88, 4338–4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirza A. M., Aguilar H. C., Zhu Q., Mahon P. J., Rota P. A., Lee B., and Iorio R. M. (2011) Triggering of the Newcastle disease virus fusion protein by a chimeric attachment protein that binds to Nipah virus receptors. J. Biol. Chem. 286, 17851–17860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schowalter R. M., Chang A., Robach J. G., Buchholz U. J., and Dutch R. E. (2009) Low-pH triggering of human metapneumovirus fusion: essential residues and importance in entry. J. Virol. 83, 1511–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang B., Sun C., Jin S., Cascio M., and Montelaro R. C. (2008) Mapping of equine lentivirus receptor 1 residues critical for equine infectious anemia virus envelope binding. J. Virol. 82, 1204–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van de Walle G. R., Peters S. T., VanderVen B. C., O'Callaghan D. J., and Osterrieder N. (2008) Equine herpesvirus 1 entry via endocytosis is facilitated by αV integrins and an RSD motif in glycoprotein D. J. Virol. 82, 11859–11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Triantafilou K., Triantafilou M., Takada Y., and Fernandez N. (2000) Human parechovirus 1 utilizes integrins αvβ3 and αvβ1 as receptors. J. Virol. 74, 5856–5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gout E., Schoehn G., Fenel D., Lortat-Jacob H., and Fender P. (2010) The adenovirus type 3 dodecahedron's RGD loop comprises an HSPG binding site that influences integrin binding. J. Biomed. Biotechnol. 2010, 541939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothwangl K. B., and Rong L. (2009) Analysis of a conserved RGE/RGD motif in HCV E2 in mediating entry. Virol. J. 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heikkilä O., Susi P., Stanway G., and Hyypiä T. (2009) Integrin αVβ6 is a high-affinity receptor for coxsackievirus A9. J. Gen. Virol. 90, 197–204 [DOI] [PubMed] [Google Scholar]
- 37.Chu J. J., and Ng M.-L. (2004) Interaction of West Nile virus with αvβ3 integrin mediates virus entry into cells. J. Biol. Chem. 279, 54533–54541 [DOI] [PubMed] [Google Scholar]
- 38.Cox R. G., Livesay S. B., Johnson M., Ohi M. D., and Williams J. V. (2012) The human metapneumovirus fusion protein mediates entry via an interaction with RGD-binding integrins. J. Virol. 86, 12148–12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamb R. A., Paterson R. G., and Jardetzky T. S. (2006) Paramyxovirus membrane fusion: lessons from the F and HN atomic structures. Virology 344, 30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pebworth M.-P., Cismas S. A., and Asuri P. (2014) A novel 2.5D culture platform to investigate the role of stiffness gradients on adhesion-independent cell migration. Plos One 9, e110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yun B., Gao Y., Liu Y., Guan X., Wang Y., Qi X., Gao H., Liu C., Cui H., Zhang Y., Gao Y., and Wang X. (2015) Effect of amino acid sequence variations at position 149 on the fusogenic activity of the subtype B avian metapneumovirus fusion protein. Arch. Virol. 160, 2445–2453 [DOI] [PubMed] [Google Scholar]
- 42.Yun B., Guan X., Liu Y., Gao Y., Wang Y., Qi X., Cui H., Liu C., Zhang Y., Gao L., Li K., Gao H., Gao Y., and Wang X. (2015) Trypsin- and low pH-mediated fusogenicity of avian metapneumovirus fusion proteins is determined by residues at positions 100, 101 and 294. Sci. Rep. 5, 15584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li P., Lu Z., Bao H., Li D., King D. P., Sun P., Bai X., Cao W., Gubbins S., Chen Y., Xie B., Guo J., Yin H., and Liu Z. (2011) In vitro and in vivo phenotype of type Asia 1 foot-and-mouth disease viruses utilizing two non-RGD receptor recognition sites. BMC Microbiol. 11, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aung Y. H., Liman M., Neumann U., and Rautenschlein S. (2008) Reproducibility of swollen sinuses in broilers by experimental infection with avian metapneumovirus subtypes A and B of turkey origin and their comparative pathogenesis. Avian Pathol. 37, 65–74 [DOI] [PubMed] [Google Scholar]
- 45.Mase M., Yamaguchi S., Tsukamoto K., Imada T., Imai K., and Nakamura K. (2003) Presence of avian pneumovirus subtypes A and B in Japan. Avian Dis. 47, 481–484 [DOI] [PubMed] [Google Scholar]
- 46.Williams J. V., Harris P. A., Tollefson S. J., Halburnt-Rush L. L., Pingsterhaus J. M., Edwards K. M., Wright P. F., and Crowe J. E. (2004) Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 350, 443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Graaf M., Schrauwen E. J., Herfst S., van Amerongen G., Osterhaus A. D., and Fouchier R. A. (2009) Fusion protein is the main determinant of metapneumovirus host tropism. J. Gen. Virol. 90, 1408–1416 [DOI] [PubMed] [Google Scholar]
- 48.Haynes L. M., Moore D. D., Kurt-Jones E. A., Finberg R. W., Anderson L. J., and Tripp R. A. (2001) Involvement of Toll-like receptor 4 in innate immunity to respiratory syncytial virus. J. Virol. 75, 10730–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurt-Jones E. A., Popova L., Kwinn L., Haynes L. M., Jones L. P., Tripp R. A., Walsh E. E., Freeman M. W., Golenbock D. T., Anderson L. J., and Finberg R. W. (2000) Pattern recognition receptors: TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1, 398–401 [DOI] [PubMed] [Google Scholar]
- 50.Gallo S. A., Reeves J. D., Garg H., Foley B., Doms R. W., and Blumenthal R. (2006) Kinetic studies of HIV-1 and HIV-2 envelope glycoprotein-mediated fusion. Retrovirology 3, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan D. C., and Kim P. S. (1998) HIV entry and its inhibition. Cell 93, 681–684 [DOI] [PubMed] [Google Scholar]
- 52.Carr C. M., and Kim P. S. (1993) A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell 73, 823–832 [DOI] [PubMed] [Google Scholar]