Abstract
We previously reported that the xyloside 2-(6-hydroxynaphthyl) β-d-xylopyranoside (XylNapOH), in contrast to 2-naphthyl β-d-xylopyranoside (XylNap), specifically reduces tumor growth both in vitro and in vivo. Although there are indications that this could be mediated by the xyloside-primed glycosaminoglycans (GAGs) and that these differ in composition depending on xyloside and cell type, detailed knowledge regarding a structure-function relationship is lacking. In this study we isolated XylNapOH- and XylNap-primed GAGs from a breast carcinoma cell line, HCC70, and a breast fibroblast cell line, CCD-1095Sk, and demonstrated that both XylNapOH- and XylNap-primed chondroitin sulfate/dermatan sulfate GAGs derived from HCC70 cells had a cytotoxic effect on HCC70 cells and CCD-1095Sk cells. The cytotoxic effect appeared to be mediated by induction of apoptosis and was inhibited in a concentration-dependent manner by the XylNap-primed heparan sulfate GAGs. In contrast, neither the chondroitin sulfate/dermatan sulfate nor the heparan sulfate derived from CCD-1095Sk cells primed on XylNapOH or XylNap had any effect on the growth of HCC70 cells or CCD-105Sk cells. These observations were related to the disaccharide composition of the XylNapOH- and XylNap-primed GAGs, which differed between the two cell lines but was similar when the GAGs were derived from the same cell line. To our knowledge this is the first report on cytotoxic effects mediated by chondroitin sulfate/dermatan sulfate.
Keywords: cancer, chondroitin sulfate, dermatan sulfate, glycosaminoglycan, heparan sulfate, high-performance liquid chromatography (HPLC), structure-function, disaccharide fingerprint, xyloside
Introduction
Proteoglycans (PGs)2 are macromolecules located in the extracellular matrix, associated to the cell surface, or stored in secretory granules of essentially all mammalian cells where they are involved in a variety of biological processes ranging from cellular homeostasis to development and progression of several pathological conditions such as cancer and inflammation (1). PGs consist of a core protein to which one or more linear polysaccharides, glycosaminoglycans (GAGs), are covalently linked. Chondroitin sulfate/dermatan sulfate (CS/DS) and heparin/heparan sulfate (HS) are two classes of GAGs, which are O-linked by xylose to a serine residue of the PG core protein (2, 3). They are composed of alternating N-acetyl-d-galactosamine-uronic acid (GalNAc-UA; where UA is either d-glucuronic acid (GlcUA) or l-iduronic acid (IdoUA)) or N-acetyl-d-glucosamine (GlcNAc)-UA units, respectively. The complete CS/DS and HS chains typically result from extensive processing by class-specific epimerases and sulfotransferases. GlcUA of CS/DS and HS can be epimerized to IdoUA, but complete epimerization is rarely observed; instead, copolymers of GlcUA and IdoUA disaccharides are common. CS/DS can be O-sulfated at position 2 of GlcUA or IdoUA, and at position 4 and 6 of GalNAc. HS can be N-deacetylated/N-sulfated, O-sulfated at position 2 of GlcUA and IdoUA, at position 6 of GlcNAc and, more rarely, at position 3 of GlcNAc. Heparin is generally the more modified version of HS.
GAGs have an immense structural diversity and have been reported to interact with a broad spectrum of biomolecules such as growth factors, selectins, and receptors involved in signaling pathways (1, 4–6). In addition, tumor cells are often associated with abnormalities in GAG expression such as over- or undersulfation or altered chain size (7–9). Because these interactions are often highly dependent on GAG sequence, GAGs are potentially important as diagnostic and therapeutic tools.
β-d-Xylopyranosides, commonly referred to as xylosides, comprise a group of compounds consisting of a xylose residue in β-linkage to an aglycon (10). They can act as substrates for GAG synthesis and can, when exogenously supplied to cells, result in secreted xyloside-primed GAGs as well as in alterations of the endogenous PG expression. Depending on the aglycon structure and the cell type, they may induce synthesis of GAGs with different HS/CS/DS composition. Besides being excellent tools to study the effect of GAGs and PGs on cells, xylosides have been described to affect, for example, coagulation (11), skin regeneration (12), and cell morphology (13, 14) as well as endothelial tube formation (15).
We previously reported that the xyloside 2-(6-hydroxynaphthyl) β-d-xylopyranoside (XylNapOH, Fig. 1), in contrast to 2-naphthyl β-d-xylopyranoside (XylNap, Fig. 1) and most other xylosides, specifically reduces tumor growth both in vitro and in vivo (16–18). The mechanism of action has not been elucidated; however, the xyloside-primed GAGs have been suggested to be involved, possibly as a result of the formation of GAGs with different composition depending on xyloside and cell type. Here, we have isolated XylNapOH- and XylNap-primed GAGs from a breast carcinoma cell line, HCC70, and a breast fibroblast cell line, CCD-1095Sk, and studied the relationship between GAG structure and its effect on cell growth.
FIGURE 1.
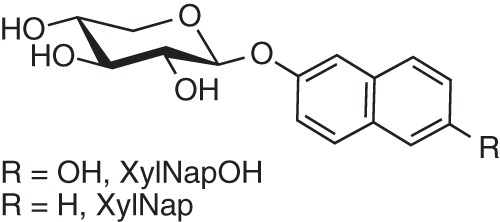
Structure of XylNapOH and XylNap.
Experimental Procedures
Cell Lines
Human breast carcinoma cells, HCC70 (American Type Culture Collection, ATCC), were cultured in RPMI 1640 medium, ATCC modification, supplemented with 10% (v/v) FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin (all from Thermo Scientific). Human breast fibroblasts, CCD-1095Sk (ATCC), were cultured in minimal essential medium with Earle's salts (Biochrom GmbH) supplemented with 10% FBS, 2 mm l-glutamine (Thermo Scientific), 100 units/ml penicillin, and 100 μg/ml streptomycin.
Xyloside Synthesis
XylNapOH and XylNap were synthesized as previously described (10, 18).
Isolation of XylNapOH- and XylNap-primed GAGs
HCC70 cells and CCD-1095Sk cells (passages 5–25) cultured in T75 flasks (Thermo Scientific) to ∼70% confluence were preincubated in DME/F-12 medium supplemented with 10 μg/ml insulin, 25 μg/ml transferrin (all from Sigma), 2 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10 ng/ml EGF (Corning) for 24 h. The cells were then incubated in 15 ml of fresh medium supplemented with 100 μm XylNapOH or XylNap. For radiolabeling, the medium was additionally supplemented with 5 μCi/ml [35S]sulfate (PerkinElmer Life Sciences). After 24, 48, or 72 h of incubation, the cell media were collected and subjected to ion exchange chromatography, hydrophobic interaction chromatography, and precipitation as previously described (19). The precipitate was dissolved in deionized H2O, freeze-dried, and resuspended in deionized H2O before purification of the XylNapOH- and XylNap-primed GAGs on a Superose 12 HR 10/30 column (GE Healthcare) connected to a Thermo Scientific UltiMate 3000 Quaternary Analytical system. The mobile phase consisted of 70% 60 mm NH4OAc, pH 5.6, and 30% MeCN in an isocratic mode at a flow rate of 0.7 ml/min. The XylNapOH- and XylNap-primed GAGs were detected using a FLD-3400RS fluorescence detector (excitation λ = 229 nm and emission λ = 372 nm for XylNapOH and excitation λ = 229 nm and emission λ = 342 nm for XylNap). The fluorescent fractions were collected and pooled, freeze-dried, and quantified using the 1,9-dimethylmethylene blue method (20) using CS A from bovine trachea (Sigma) and HS (Iduron) as standards.
Isolation of PG-GAGs
The procedure was the same as for the isolation of the XylNapOH- and XylNap-primed GAGs, with the following exceptions. The medium used was supplemented with 5 μCi/ml [35S]sulfate only; after 48 h of incubation, the media were subjected only to ion exchange chromatography before precipitation, and the PG-GAG fractions from the HPLC purification were collected based on radioactivity instead of fluorescence. Radioactivity was measured using a liquid scintillation counter (PerkinElmer Life Sciences).
Cell Growth Assay Using the Crystal Violet Method
Confluent HCC70 cells and CCD-1095Sk cells (passages 10–25) were dissociated using TrypLETM Express Enzyme (Thermo Scientific) and seeded in 96-well microculture plates (Greiner Bio-One) at plating densities set to obtain an approximate 2.5-fold increase in cell number after 96 h (1000–5000 cells/well). After 24 h of plating, the cells were allowed to grow in DME/F-12 medium supplemented with 10 μg/ml insulin, 25 μg/ml transferrin, 2 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 10 ng/ml EGF, and increasing concentrations of XylNapOH- or XylNap-primed GAGs from HCC70 cells (1, 2.5, 5, 7.5, and 15 μg/ml) or CCD-1095Sk cells (2.5, 5, 10, 20, and 30 μg/ml), or CS A from bovine trachea, CS B from porcine intestinal mucosa, heparin from porcine intestinal mucosa (all from Sigma), or CS C from shark cartilage (a gift from Dick Heinegård) (2.5, 5, 10, 20, and 30 μg/ml). Untreated cells, blanks only containing medium, and controls with xylosides as references were included. After 24 h or 96 h, the cell density was measured using the crystal violet method as previously described (21). Concurrently with the initiation of each experiment, a plate containing cells at day 0 was fixed and stored at room temperature in Hanks' balanced salt solution, pH 7.4, until staining. After staining, the amount of bound dye was measured by absorbance at 595 nm in a microplate reader. The relative cell number (in % of untreated cells (UT)) was calculated according to Equation 1,
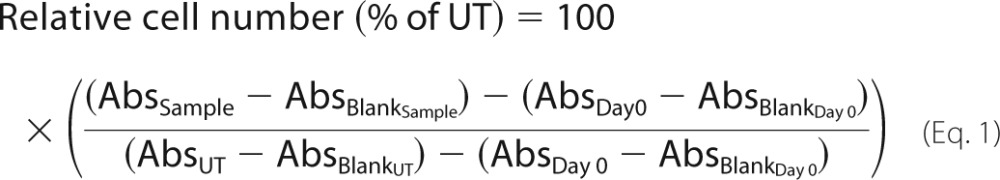 |
where Abs is absorbance (in absorbance units). In the 24 h experiments, day 0 was excluded from the calculation, as there was limited or no cell growth during the 24-h time interval. The inhibitory concentrations of the xyloside-primed GAGs are expressed as IC50 values and calculated after curve-fitting to the data points; Boltzmann sigmoidal fitting was applied where effect was observed, and linear regression was applied where little or no effect was observed.
Xylanase Treatment of XylNapOH- and XylNap-primed GAGs for Cell Growth Assay and Molecular Weight Estimation
To prepare aglycon-lacking XylNapOH-primed GAGs from HCC70 cells for cell growth assay, ∼15 μg of [35S]sulfate-labeled XylNapOH-primed GAGs were treated with 50 milliunits of endo-1,4-β-xylanase (EC 3.2.1.8) from Trichoderma longibrachiatum with endo-β-xylosidase activity (Sigma) in 150 μl of 0.1 m NaOAc, pH 5.0, for 16 h. After treatment, the samples were boiled for 10 min and subsequently centrifuged at 10,000 × g for 10 min before supernatants were dried by centrifugal evaporation. The GAGs were purified on a Superdex Peptide HR 10/30 column (GE Healthcare), run in 0.2 m NH4HCO3 at a flow rate of 0.5 ml/min. The radioactive fractions eluting at a retention time corresponding to that of untreated XylNapOH-primed GAGs were collected and pooled, freeze-dried, and quantified using the 1,9-dimethylmethylene blue method before being used in the cell growth assay (at concentrations of 2.5, 5, and 7.5 μg/ml). For molecular weight estimation, ∼4 μg of XylNapOH- and XylNap-primed GAGs were treated with 20 milliunits of xylanase in 65 μl of the above described buffer for 16 h. After treatment, the samples were worked up as described above. Subsequently, the GAGs were dissolved in deionized H2O, and the free aglycons were removed by centrifugal filtration using filters with a cut-off of 3 kDa (VWR Collection) before size-exclusion chromatography οn a Superose 6 column (GE Healthcare) run in 0.2 m NH4HCO3 at a flow rate of 0.3 ml/min. The elution profiles were compared with those of heparin standards (Iduron and gift from Dilafor).
Determination of Apoptosis of Cells Treated with XylNapOH-primed GAGs from HCC70 Cells
Confluent HCC70 cells and CCD-1095Sk cells (passages 10–25) were dissociated using TrypLETM Express Enzyme and seeded in 4-well glass chamber slides at densities of 1 × 105 cells/well. After 24 h of plating, the cells were preincubated with DME/F-12 medium supplemented with 10 μg/ml insulin, 25 μg/ml transferrin, 2 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10 ng/ml EGF for 24 h before treatment with XylNapOH-primed GAGs (2.5, 5, and 7.5 μg/ml) from HCC70 cells. Untreated cells were included as a control. After 24 h, apoptotic cells were identified using a TUNEL assay (BioVision) performed according to the manufacturer's instructions. Imaging was performed using a Carl Zeiss AxioObserver inverted fluorescence microscope equipped with an EC Plan-NEOFLUAR 20×/0.5 objective. The images were captured using an AxioCam MRm camera and the AxioVision Rel 4.8.2 software (Carl Zeiss).
Disaccharide Fingerprinting of XylNapOH- and XylNap-primed GAGs
Approximately 1 μg of xyloside-primed GAGs was degraded using either chondroitinase ABC (EC 4.2.2.20) (Seikagaku), chondroitinase AC-I and -II (EC 4.2.2.5) (Seikagaku), chondroitinase B (EC 4.2.2.19) (R&D Systems), or heparinase I (EC 4.2.2.7), heparinase II (no EC number), and heparinase III (EC 4.2.2.8) from Flavobacterium heparinum (overexpressed in Escherichia coli, a gift from Jian Liu, University of North Carolina). Chondroitinase ABC degradations were performed in 25 μl of 50 mm NH4OAc, pH 8.0, containing 5 milliunits of enzyme at 37 °C for 16 h. Chondroitinase AC-I and -II degradations were performed in 25 μl of 50 mm NH4OAc, pH 8.0, containing 5 milliunits of each enzyme at 37 °C for 16 h. Chondroitinase B degradations were performed in 25 μl of 50 mm Tris-HCl, pH 7.5 (at 37 °C), 50 mm NaCl, 4 mm CaCl2, 0.1 μg/μl BSA (Sigma) and 2 milliunits of enzyme at 37 °C for 2 h. Heparinase I-III degradations were performed in 25 μl of 20 mm HEPES, pH 7.2, 50 mm NaCl, 4 mm CaCl2, and 2.5 milliunits of each enzyme at 37 °C for 16 h. CS A, CS B, CS C, and heparin were included as controls. Identification and quantification was performed by comparing the HPLC-separated 2-aminoacridone-labeled enzyme-generated disaccharides with 2-aminoacridone-labeled HPLC-separated disaccharide standards (Iduron) as previously described (22). The HS/CS/DS ratios of the XylNapOH- and XylNap-primed GAGs and the PG-GAGs were calculated as the relative proportions (% of total) of HS, IdoUA-containing disaccharides in CS/DS (CS/DSIdoUA), and GlcUA-containing disaccharides in CS/DS (CS/DSGlcUA) according to Equations 2–4, respectively,
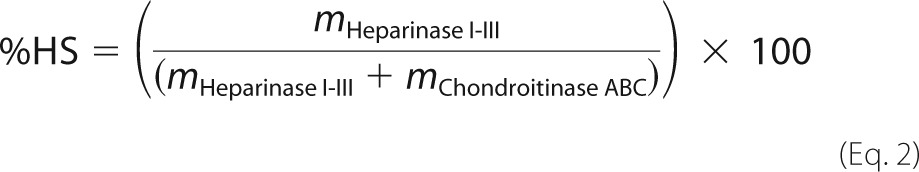 |
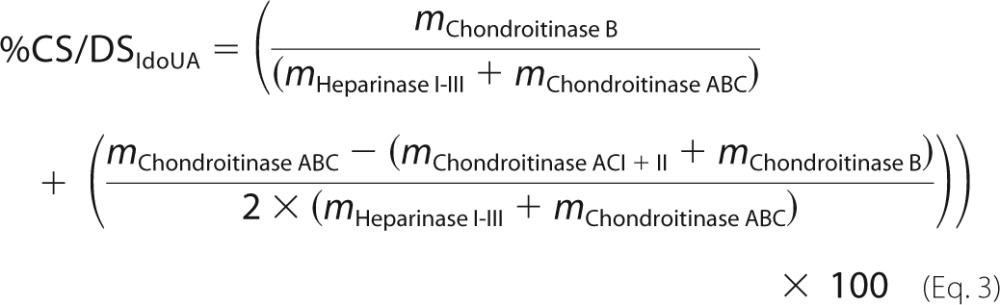 |
where m is mass (in ng) after degradation with the indicated enzymes. Equation 3 was generated based on the cleavage sites of chondroitinase B and chondroitinase AC-I and -II (23).
Enzymatic Degradation of XylNapOH- and XylNap-primed GAGs for Cell Growth Assay
Approximately 15 μg of XylNapOH- and XylNap-primed GAGs from HCC70 cells and CCD-1095Sk cells were degraded using chondroitinase ABC or heparinase I-III. Chondroitinase ABC degradations were performed using 100 milliunits of enzyme, and heparinase I-III degradations were performed using 25 milliunits of heparinase I and 50 milliunits of each heparinase II and III, both in 250 μl of above described buffers at 37 °C for 16 h. After degradation, the samples were worked up as described above followed by purification of the non-degraded GAGs on a Superdex Peptide HR 10/30 column, run in 0.2 m NH4HCO3 at a flow rate of 0.5 ml/min. Fluorescent fractions eluting at a retention time corresponding to that of untreated XylNapOH- and XylNap-primed GAGs were collected and pooled, freeze-dried, and quantified using the 1,9-dimethylmethylene blue method before being used in the cell growth assay (at concentrations of 2.5, 5, and 7.5 μg/ml).
Statistical Analysis
All statistical calculations and curve fitting to data were performed using GraphPad Prism 6.
Results
XylNapOH-primed GAGs from HCC70 Cells Reduce Growth of HCC70 Cells and CCD-1095Sk Cells
XylNapOH- and XylNap-primed GAGs were isolated from HCC70 cells and CCD-1095Sk cells after treatment with 100 μm XylNapOH or XylNap for 48 h. Little or no xyloside-primed GAGs were isolated from the cell extracts, and therefore, only the xyloside-primed GAGs from the culture media were investigated. Very little PG was detected in the culture media after xyloside treatment. The amount of recovered xyloside-primed GAGs depended on the number of cell passages and differed between the cell lines; the amount recovered from the HCC70 cells increased with the number of passages, whereas the amount recovered from the CCD-1095Sk cells decreased with the number of passages. In general, treatment with XylNap resulted in smaller amounts of recovered xyloside-primed GAGs than treatment with XylNapOH. The amounts of XylNapOH- and XylNap-primed GAGs recovered were in the range of 2.5–8 μg/106 HCC70 cells and 10–30 μg/106 CCD-1095Sk cells.
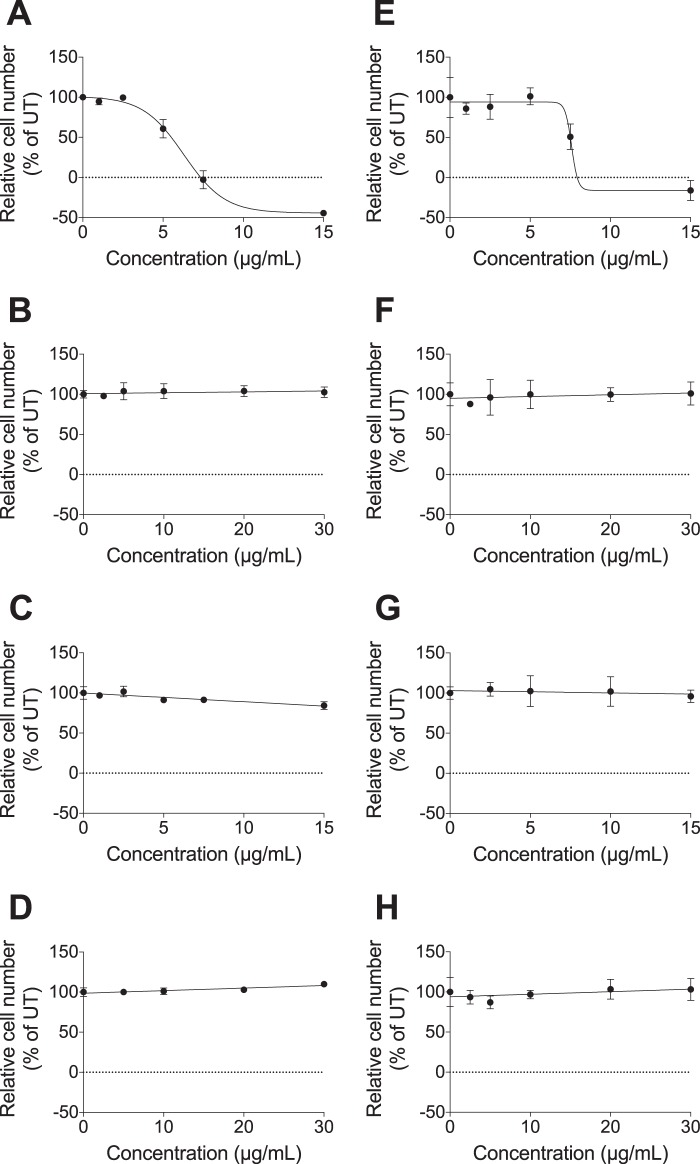
To investigate the effect of the xyloside-primed GAGs on cell growth, HCC70 cells and CCD-1095Sk cells were treated for 96 h with increasing concentrations of XylNapOH- or XylNap-primed GAGs isolated from HCC70 cells or CCD-1095Sk cells. The growth of both HCC70 cells (Fig. 2A) and CCD-1095Sk cells (Fig. 2E) was reduced by treatment with XylNapOH-primed GAGs from HCC70 cells. The effect was concentration-dependent and corresponded to a mean IC50 value of 5.9 ± 2 μg/ml in HCC70 cells and 7.8 ± 0.2 μg/ml in CCD-1095Sk cells. At concentrations above, on average, 7.5 μg/ml and 10 μg/ml, the number of HCC70 cells and CCD1095Sk cells, respectively, was reduced below the initial number of cells, suggesting a cytotoxic effect. XylNap-primed GAGs derived from HCC70 cells had little effect on the growth of HCC70 cells (∼15% reduction at 15 μg/ml of XylNap-primed GAGs from HCC70 cells; Fig. 2C) and none on the growth of CCD-1095Sk cells (Fig. 2G).
FIGURE 2.
XylNapOH-primed GAGs from HCC70 cells reduce growth of HCC70 cells and CCD-1095Sk cells. HCC70 cells and CCD-1095Sk cells were treated with xyloside-primed GAGs at the indicated concentrations for 96 h. A–D, effect on growth of HCC70 cells by XylNapOH-primed GAGs from HCC70 cells (R2 = 0.98) (A), XylNapOH-primed GAGs from CCD-1095Sk cells (B), XylNap-primed GAGs from HCC70 cells (C), and XylNap-primed GAGs from CCD-1095Sk cells (D). E–H, effect on growth of CCD-1095Sk cells by XylNapOH-primed GAGs from HCC70 cells (R2 = 0.88) (E), XylNapOH-primed GAGs from CCD-1095Sk cells (F), XylNap-primed GAGs from HCC70 cells (G), and XylNap-primed GAGs from CCD-1095Sk cells (H). The graphs are representative for each experiment, performed at least in duplicate, in which n = 3–5. The data points are the means ± S.D.
Neither XylNapOH- nor XylNap-primed GAGs derived from CCD-1095Sk cells had any effect on the growth of either of the cell lines despite the fact that the xyloside-primed GAGs were administered at higher concentrations (Fig. 2, B, D, F, and H). The results were consistent across experiments and independent of the number of cell passages before isolation of the xyloside-primed GAGs.
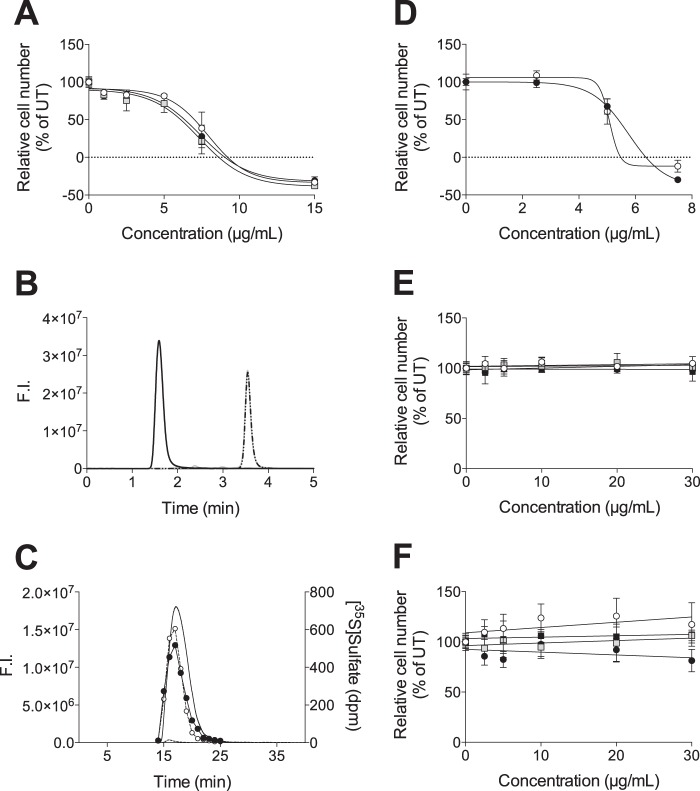
The incubation time with xyloside before GAG isolation is a parameter that potentially could influence the composition of the xyloside-primed GAGs. Therefore, we compared the effect of XylNapOH-primed GAGs isolated from HCC70 cells after 24, 48, and 72 h of incubation on HCC70 cell growth and found it to be similar irrespective of incubation time before isolation (Fig. 3A). This suggests that the composition of the XylNapOH-primed GAGs is constant over time or that the effect on cell growth is insensitive to changes in GAG composition. Alternatively, the xyloside part of the xyloside-primed GAGs could play a role for the effect.
FIGURE 3.
The effect of XylNapOH-primed GAGs from HCC70 cells on cell growth is independent of the incubation time with XylNapOH before GAG isolation and mediated by the GAG part. A, HCC70 cells were treated with XylNapOH-primed GAGs isolated from HCC70 after 24 h (white circles), 48 h (gray squares), and 72 h (black circles) at the indicated concentrations for 96 h (R2 = 0.95, 0.95, and 0.96, respectively). The graphs are representative for each experiment, performed in triplicate, in which n = 3–5. The data points are the means ± S.D. B, chromatogram from reversed phase HPLC on a μRPC C2/C18 (100 × 4.6 mm) column of XylNapOH-primed GAGs from HCC70 cells, untreated (solid black line) or xylanase-treated (gray solid line). After xylanase treatment, the peak with retention time = 1.59 min, corresponding to the XylNapOH-primed GAGs, shifted to retention time = 3.55 min, which co-elutes with 2,6-dihydroxynaphthalene (dashed black line). C, chromatogram from size-exclusion chromatography HPLC on a Superdex Peptide HR 10/30 column; the right y axis displays the fluorescence intensity of untreated (solid line) and xylanase-treated (dashed line) XylNapOH-primed GAGs from HCC70 cells, and the left y axis displays the radioactivity of untreated (black circles, solid line) and xylanase-treated (white circles, dashed line) XylNapOH-primed GAGs from HCC70 cells. The aglycon was retained on the column and is, therefore, not visible on the chromatogram. Excitation λ = 229 nm and emission λ = 372 nm. F.I., fluorescence intensity. D, HCC70 cells were treated with untreated (black circles) and xylanase-treated (white circles) XylNapOH-primed GAGs at the indicated concentrations for 96 h (R2 = 0.99 and 0.96, respectively). HCC70 cells (E) and CCD-1095Sk (F) cells treated with commercially available GAGs at the indicated concentrations for 96 h, CS A (white circles), CS B (gray squares), CS C (black circles), and heparin (black squares). The graphs are representative for each experiment, performed in triplicate, in which n = 3–5. The data points are the means ± S.D.
The GAG Part of XylNapOH-primed GAGs from HCC70 Cells Is Responsible for the Reduction in Cell Growth
To determine whether the GAG part or the xyloside part of the XylNapOH-primed GAGs or a combination of both was responsible for the reduction in cell growth, we repeated the cell growth assay using GAGs that lacked the xyloside part. We used an endo-1,4-β-xylanase from T. longibrachiatum that has an endo-β-xylosidase activity to cleave off the aglycon from the XylNapOH-primed GAGs.
The aglycon, 2,6-dihydroxynaphthlene, of the XylNapOH-primed GAGs is fluorescent but not the GAGs themselves. A comparison of the fluorescence chromatogram of the xylanase-treated XylNapOH-primed GAGs to those of the untreated XylNapOH-primed GAGs and of the aglycon showed that xylanase treatment resulted in a complete shift in retention time of the XylNapOH-primed GAGs to that of the aglycon (Fig. 3B), implying that the cleavage was efficient and occurred specifically between the xylose and the aglycon. In addition, the chromatograms of untreated and xylanase-treated [35S]sulfate-labeled XylNapOH-primed GAGs remained unchanged (Fig. 3C), indicating that the GAG chains were unaffected by the xylanase treatment.
Treatment of HCC70 cells with untreated or xylanase-treated XylNapOH-primed GAGs from HCC70 cells resulted in similar effects on cell growth (Fig. 3D), implying that the GAG part rather than the xyloside part (the aglycon) was responsible for the reduction in cell growth. In comparison, the commercially available GAGs, CS A, CS B, CS C, and heparin, together representing the majority of disaccharide units present in CS/DS and HS (supplemental Tables S1 and S2), had no significant effect on growth on any of the cell lines when administered at the same concentrations as the xyloside-primed GAGs (Fig. 3, E and F), indicating that GAGs in general do not have a growth-reducing effect on HCC70 cells and CCD-1095Sk cells.
The XylNapOH-primed GAGs from HCC70 Cells Induce Apoptosis in HCC70 Cells and CCD-1095Sk Cells
Because the growth-reducing effect of the XylNapOH-primed GAGs appeared to be mediated by a cytotoxic effect, we investigated whether apoptosis was involved. Apoptotic cells, identified using the TUNEL technique, were detected in HCC70 cells and CCD-1095Sk cells treated for 24 h with XylNapOH-primed GAGs from HCC70 cells, whereas they were not detected in untreated cells (Fig. 4A). Because the TUNEL experiments were performed after 24 h of treatment, we performed a cell growth assay after 24 h of treatment. The effect of the XylNapOH-primed GAGs derived from HCC70 cells on cell growth was observed also after 24 h and was similar to that after 96 h (Fig. 4B).
FIGURE 4.
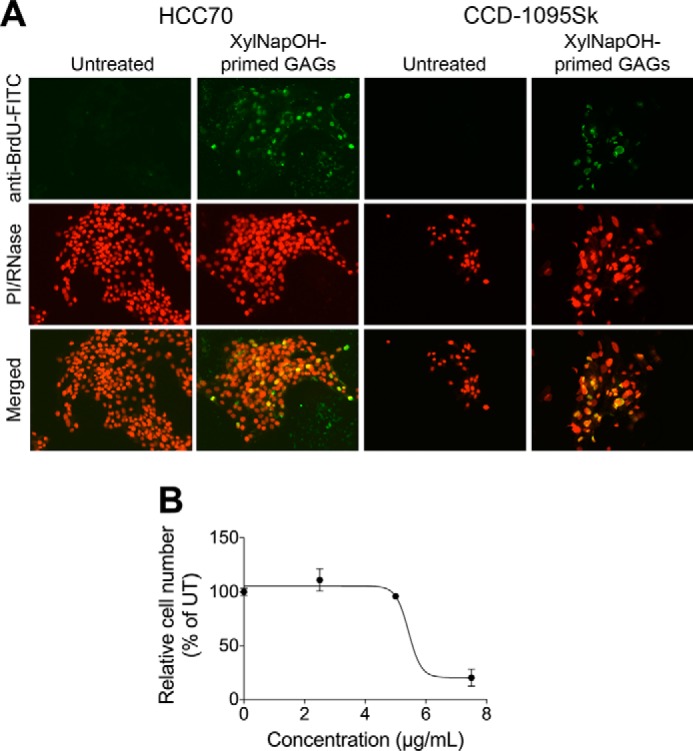
XylNapOH-primed GAGs from HCC70 cells induce apoptosis of HCC70 cells and CCD-1095Sk cells. A, HCC70 cells (left panels) and CCD-1095Sk cells (right panels) untreated or treated with 5 μg/ml XylNapOH-primed GAGs from HCC70 cells for 24 h. DNA strand breaks indicating apoptosis were detected utilizing BrdU and visualized using an anti-BrdU-FITC antibody. Cell nuclei were stained using propidium iodide (PI)/RNase. The merged images displays colocalization of anti-BrdU-FITC-positive cells and cell nuclei. The images are representative for each experiment, performed at least in duplicate. B, HCC70 cells treated with XylNapOH-primed GAGs at the indicated concentrations for 24 h. The experiment was performed in duplicate in which n = 3. The data points are the means ± S.D.
XylNapOH- and XylNap-primed GAGs from HCC70 Cells or CCD-1095Sk Cells Are 22–44 Disaccharide Units in Size
As an initial step in investigating the structure of the XylNapOH- and XylNap-primed GAGs from the two cell lines, their molecular weights were estimated using size-exclusion chromatography HPLC. To prevent interactions between the xyloside-primed GAGs and the column, with peak broadening as a result, [35S]sulfate-labeled xyloside-primed GAGs where the aglycons had been removed by xylanase were used. The peak maxima of the xyloside-primed GAGs on a Superose 6 column were compared with those of heparin with known molecular weights run on the same column (Fig. 5). Although some variability in the peak maxima of the different xyloside-primed GAGs was observed, the molecular weights were estimated to be, on average, 10,000–20,000, which corresponds to ∼22–44 disaccharide units in size (determined using a molecular weight of 459 disaccharide). The molecular weights correspond to IC50 values of 0.3–0.8 μm for the XylNapOH-primed GAGs from HCC70 cells in HCC70 cells and CCD-1095Sk cells. PG-GAGs are commonly in the range of 25–100 kDa in size (24); thus, the XylNapOH- and XylNap-primed GAGs from HCC70 cells and CCD-1095Sk cells were somewhat smaller than PG-GAGs. The similarity in size of the XylNapOH- and XylNap-primed GAGs from HCC70 cells and CCD-1095Sk cells indicates that size is not a determinant for the effect of the XylNapOH-primed GAGs from HCC70 cells. This was further supported by the fact that the CS/DS and HS from HCC70 cells primed on either XylNapOH or XylNap were of similar size as non-degraded XylNapOH- and XylNap-primed GAGs from HCC70 cells (as determined by comparing the sizes of heparinase I-III- or chondroitinase ABC-degraded XylNapOH- and XylNap-primed GAGs from HCC70 cells with those of the non-degraded XylNapOH- and XylNap-primed GAGs from HCC70 cells; data not shown).
FIGURE 5.
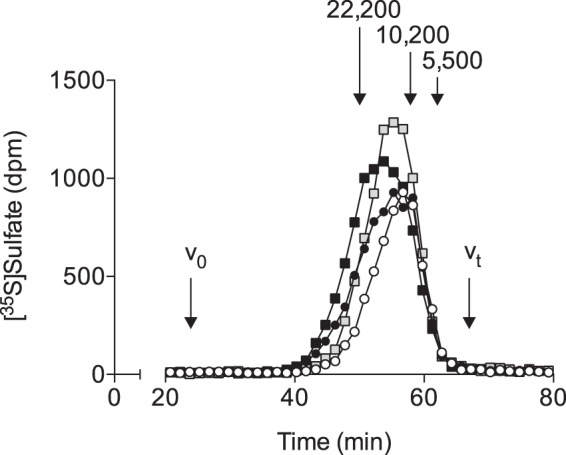
XylNapOH- and XylNap-primed GAGs from HCC70 cells and CCD-1095Sk cells are similar in size. Chromatogram from size-exclusion chromatography HPLC on a Superose 6 HR 10/30 column of XylNapOH-primed GAGs from HCC70 cells (white circles), XylNap-primed GAGs from HCC70 cells (black circles), XylNapOH-primed GAGs from CCD-1095Sk cells (gray squares), and XylNap-primed GAGs from CCD-1095Sk cells (black squares) after treatment with xylanase to prevent interaction between the aglycon and the column. The indicated molecular weights were obtained using heparin standards.
The Disaccharide Composition of the XylNapOH- and XylNap-primed GAGs Is Similar when the GAGs Are Derived from the Same Cell Line but Differs when Derived from Different Cell Lines
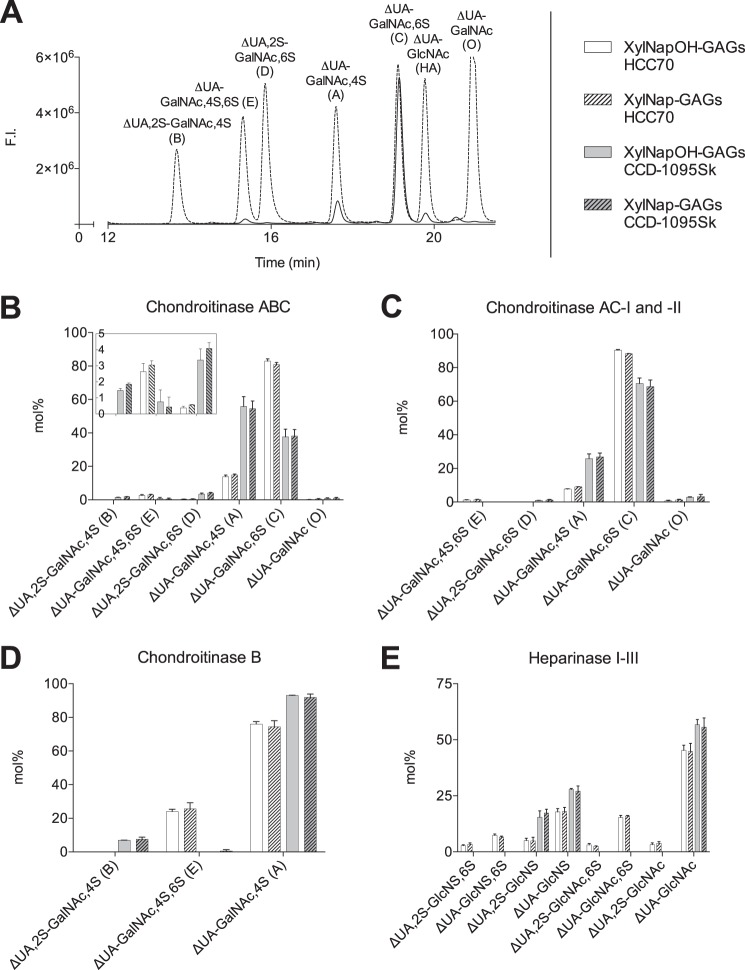
To gain further insight into the GAG structures, disaccharide fingerprinting of the XylNapOH- and XylNap-primed GAGs from the two cell lines was performed. The xyloside-primed GAGs were degraded using chondroitinase ABC, chondroitinase AC-I and -II, chondroitinase B, or heparinase I-III followed by 2-aminoacridone labeling of the disaccharides and separation of these on HPLC (Fig. 6A). The disaccharides were qualitatively and quantitatively characterized by comparing with disaccharide standards. Both the chondroitinase ABC- and heparinase I-III-generated disaccharides varied considerably in composition between the xyloside-primed GAGs isolated from HCC70 cells and CCD-1095Sk cells (Fig. 6, B–E). Interestingly, the disaccharide composition of the xyloside-primed GAGs derived from the same cell line was similar irrespective of whether they were primed on XylNapOH or XylNap. Likewise, the disaccharide composition of the XylNapOH-primed GAGs isolated from HCC70 cells after different incubation times was similar irrespective of incubation time (supplemental Tables S1 and S2).
FIGURE 6.
Disaccharide composition of XylNapOH- and XylNap-primed GAGs from HCC70 cells and CCD-1095Sk cells. A, typical chromatogram from separation on an XBridge BEH Shield RP18 (2.1 × 100 mm, 2.5 μm) column of chondroitinase ABC-degraded and 2-aminoacridone-labeled XylNapOH-primed GAGs from HCC70 cells (solid line) and CS/DS standards (dashed line). The different disaccharides are indicated above the corresponding peaks. ΔUA-GlcNAc (hyaluronic acid (HA)) is indicated but was not investigated in the study. Excitation λ = 425 nm, and emission λ = 525 nm. F.I., fluorescence intensity. The disaccharide data presented in B–E was estimated by the corresponding chromatograms. B–E, disaccharide fingerprint from XylNapOH- and XylNap-primed GAGs from HCC70 cells and CCD-1095Sk cells after enzymatic degradation using chondroitinase ABC (B), chondroitinase AC-I and -II (C), chondroitinase B (D), and heparinase I-III (E). The inset in B displays the data of disaccharides ΔUA,2S-GalNAc,4S (B), ΔUA-GalNAc,4S,6S (E), and ΔUA,2S-GalNAc,6S (D), where the y axis ranges from 0 to 5 mol%. The data are the means of two independent experiments ± S.D. Raw data can be found in supplemental Tables S1 and S2.
The major difference in CS/DS disaccharides between the xyloside-primed GAGs derived from HCC70 cells and CCD-1095Sk cells was in the ΔUA-GalNAc,4S (A unit)/ΔUA-GalNAc,6S (C unit) ratio, which was shifted between the two cell lines: ∼15%/∼82% in the xyloside-primed GAGs from HCC70 cells and ∼56%/∼38% in the xyloside-primed GAGs from CCD-1095Sk cells (Fig. 6B). In addition, ΔUA-GalNAc,4S,6S (E unit) was present in the xyloside-primed GAGs from the HCC70 cells (∼2.8%) but only in trace amounts in those from CCD-1095Sk cells. Instead, ΔUA,2S-GalNAc,4S (B unit) (∼1.7%) and ΔUA,2S-GalNAc,6S (D unit) (∼3.8%) were present in the xyloside-primed GAGs from the CCD-1095Sk cells (Fig. 6B).
Based on the chondroitinase AC-I and -II degradations and chondroitinase B degradations, we concluded that the disaccharide units of the XylNapOH- and XylNap-primed GAGs from HCC70 cells and CCD-1095Sk cells contained both GlcUA and IdoUA (Fig. 6, C and D). For example, the A units and E units of XylNapOH- and XylNap-primed GAGs from HCC70 cells were a mix of GlcUA and IdoUA, as they were detected after chondroitinase AC-I and -II degradation and after chondroitinase B degradation. The B unit of XylNapOH- and XylNap-primed GAGs from CCD-1095Sk cells was detected only after chondroitinase B degradation, whereas the D unit was detected only after chondroitinase AC-I and -II degradation.
All investigated HS disaccharides were detected in the XylNapOH- and XylNap-primed HS from HCC70 cells (Fig. 6E); however, the HS was mainly composed of ΔUA-GlcNAc (∼45%), ΔUA-GlcNS (∼18%), and ΔUA-GlcNAc,6S (∼16%). In contrast, the xyloside-primed HS from CCD-1095Sk cells was composed of only three disaccharides; ΔUA-GlcNAc (∼56%), ΔUA-GlcNS (∼28%), and ΔUA, 2S-GlcNS (∼16%). Taken together, the results indicate that the disaccharide composition of the XylNapOH- and XylNap-primed GAGs is specific and dependent on cell type rather than on xyloside.
The HS/CS/DS Ratios of the XylNapOH- and XylNap-primed GAGs Differ between the Different Cell Lines but Is also Influenced by the Xyloside
The HS/CS/DS ratios of the studied XylNapOH- and XylNap-primed GAGs (Fig. 7) were calculated based on the disaccharide data according to Equations 2–4 under “Experimental Procedures.” The proportion of IdoUA-containing disaccharides in CS/DS (CS/DSIdoUA) calculated using Equation 3 includes the IdoUA-containing disaccharides present both in blocks and as single IdoUA-containing disaccharides, whereas the proportion of IdoUA-containing disaccharides generated by chondroitinase B degradation includes those present in blocks only.
FIGURE 7.
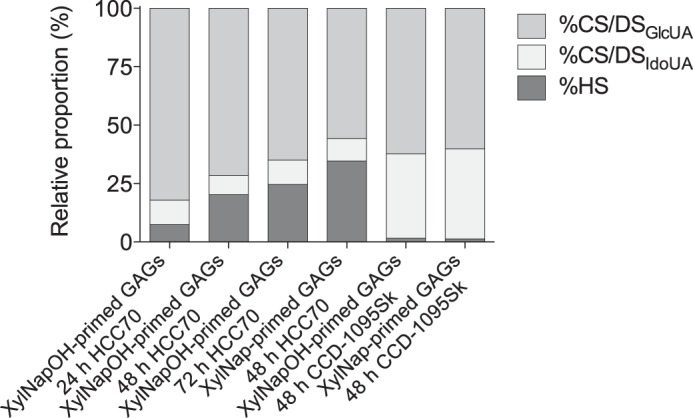
The XylNapOH- and XylNap-primed GAGs from HCC70 cells and CCD-1095Sk cells have different HS/CS/DS ratios. Proportions of CS/DSGlcUA, CS/DSIdoUA, and HS calculated based on the disaccharide data (supplemental Tables S1 and S2). The data are the means of two independent experiments.
Similar to the disaccharide composition, differences in the HS/CS/DS ratios of the xyloside-primed GAGs derived from the two cell lines were observed (Fig. 7). The xyloside-primed GAGs from HCC70 cells were composed of a higher proportion of HS (∼20 and 35% for XylNapOH- and XylNap-primed GAGs, respectively) than those from CCD-1095Sk cells, where HS was almost absent (∼1.4%). Instead, the xyloside-primed GAGs from CCD-1095Sk were composed of a higher proportion of CS/DSIdoUA (∼37%) than the xyloside-primed GAGs from HCC70 cells (∼8.9%). Analogously, the proportion of IdoUA-containing disaccharides generated after chondroitinase B degradation of xyloside-primed GAGs from CCD-1095Sk cells (∼17%) was higher than that generated after chondroitinase B degradation of xyloside-primed GAGs from HCC70 cells (∼1.4%).
Unlike for the disaccharide composition, variability in the HS/CS/DS ratios of the XylNapOH- and XylNap-primed GAGs derived from HCC70 cells was detected. The proportion of HS in the XylNapOH-primed GAGs from HCC70 cells increased with incubation time before isolation, from ∼7.4% after 24 h to ∼20% after 48 h and to ∼25% after 72 h. In the XylNap-primed GAGs isolated from HCC70 cells after 48 h the proportion of HS was ∼35%. Thus, the HS/CS/DS ratio of xyloside-primed GAGs, at least in the HCC70 cells, appears to be influenced not only by the cell type, but also by the xyloside, as previously observed (10, 16).
The Composition of the Xyloside-primed GAGs Does Not Mirror That of the PG-GAGs Derived from the Same Cell Line, but There Are Similarities
To investigate if the xyloside-primed GAGs structurally resembled the endogenous PG-GAGs derived from the same cell line, the PG-GAGs secreted into the culture media of untreated HCC70 cells and CCD-1095Sk cells were studied. The amounts of PG-GAGs recovered after 48 h were 0.7–0.9 μg/106 HCC70 cells and 0.9–1.5 μg/106 CCD-1095Sk cells, which was considerably lower than the amounts recovered after xyloside treatment.
The disaccharide composition of both the CS/DS and the HS of the xyloside-primed GAGs from HCC70 cells was similar to that of the PG-GAGs isolated from HCC70 cells, whereas the HS/CS/DS ratios differed (supplemental Tables S1 and S2). The main difference was in the proportion of HS, which was lower in the xyloside-primed GAGs (∼20 and 35% for XylNapOH- and XylNap-primed GAGs, respectively) than in the PG-GAGs (∼56%). In addition, the proportion of CS/DSIdoUA in the xyloside-primed GAGs (∼8.9%) was slightly higher than that in the PG-GAGs (∼5.0%). Analogously, the proportion of IdoUA-containing disaccharides generated after chondroitinase B degradation of the xyloside-primed GAGs (∼1.4%) was slightly higher than that generated after chondroitinase B degradation of the PG-GAGs (∼0.4%), possibly explaining why the E unit was not observed in the PG-GAGs after chondroitinase B degradation.
The xyloside-primed GAGs from CCD-1095Sk cells differed from the PG-GAGs from CCD-1095Sk cells both in disaccharide composition and in HS/CS/DS ratios (supplemental Tables S1 and S2). The xyloside-primed GAGs were composed of a higher proportion of C units (∼38%) and to a lower proportion of A units (∼56%) than the PG-GAGs from CCD-1095Sk cells (∼14 and ∼81%, respectively). The disaccharides generated after chondroitinase AC-I and -II degradation were the main source of this difference, as the disaccharides generated after chondroitinase B degradation were similar. In addition, fewer HS disaccharides were represented in xyloside-primed HS from CCD-1095Sk cells than in the corresponding PG-HS, possibly as a result of a lower proportion of HS in the xyloside-primed GAGs (∼1.4%) than in the PG-GAGs (∼8.5%). In addition, the proportion of CS/DSIdoUA of the xyloside-primed GAGs (∼37%) was lower than that of the PG-GAGs (∼46%). Analogously, the proportion of IdoUA-containing disaccharides generated after chondroitinase B degradation of the xyloside-primed GAGs (∼17%) was lower than that generated after chondroitinase B degradation of the PG-GAGs (∼36%).
Taken together, these results indicate that the overall GAG composition of the xyloside-primed GAGs does not mirror that of the PG-GAGs of their corresponding cell line. However, some similarities were observed, in particular the disaccharide composition of the GAGs derived from HCC70 cells.
CS/DS from HCC70 Cells Primed on Either XylNapOH or XylNap Is Cytotoxic but Can Be Inhibited by XylNap-primed HS
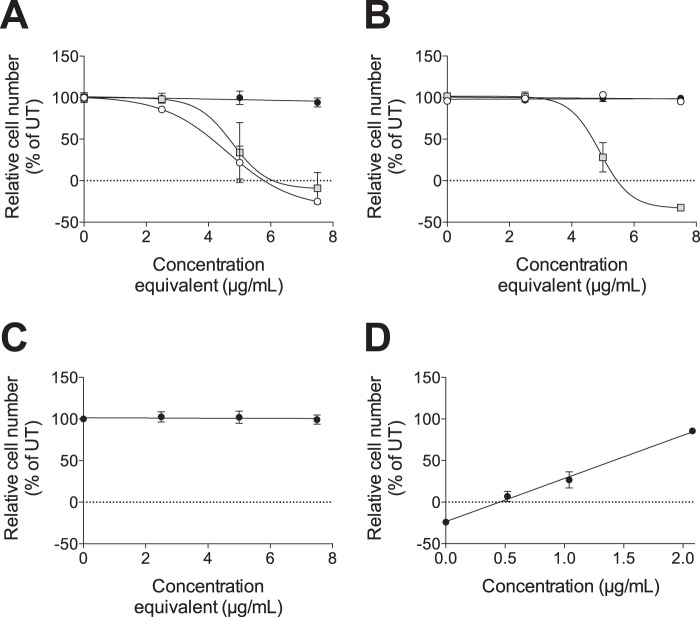
To determine whether CS/DS or HS was responsible for the cytotoxic effect of the XylNapOH-primed GAGs from HCC70 cells, XylNapOH-primed GAGs from HCC70 cells were treated with either heparinase I-III or chondroitinase ABC before treating HCC70 cells. Heparinase I-III treatment of the GAGs, which investigated the effect of the CS/DS, did not alter the cytotoxic effect of the XylNapOH-primed GAGs, whereas chondroitinase ABC treatment of the GAGs, which investigated the effect of HS, resulted in a complete loss of cytotoxic effect (Fig. 8A). This suggests that the XylNapOH-primed CS/DS was responsible for the cytotoxic effect. Because the disaccharide composition of XylNapOH- and XylNap-primed GAGs from HCC70 cells was similar, we performed the same experiment using XylNap-primed GAGs. Interestingly, heparinase I-III-treated XylNap-primed GAGs from HCC70 cells had a similar effect on HCC70 cells as the XylNapOH-GAGs from HCC70 cells (Fig. 8B). The XylNapOH- and XylNap-primed GAGs from CCD-1095Sk cells were included as controls, showing that neither chondroitinase ABC treatment nor heparinase I-III treatment of the xyloside-primed GAGs from CCD-1095Sk cells affected the growth of the cells (data not shown). Taken together, this implies that xyloside-primed CS/DS GAGs from HCC70 cells are cytotoxic irrespective of whether they are primed on XylNapOH or XylNap. In addition, the HS primed on XylNap appeared to inhibit the effect of the CS/DS.
FIGURE 8.
CS/DS from HCC70 cells primed on either XylNapOH or XylNap have a cytotoxic effect, but HS from HCC70 cells primed on XylNap inhibits this effect. HCC70 cells were treated with xyloside-primed GAGs from HCC70 cells for 96 h. A and B, effect on growth by XylNapOH-primed GAGs (A) or XylNap-primed GAGs (B), untreated (white circles) or heparinase I-III-treated (gray squares) or chondroitinase ABC-treated (black circles) (R2 = 0.97, 0.88, and 0.98 for the curves of the untreated and heparinase I-III-treated XylNapOH-primed GAGs and heparinase I-III-treated XylNap-primed GAGs from HCC70 cells, respectively). C, heparinase I-III-treated XylNapOH-primed GAGs together with chondroitinase ABC-treated XylNap-primed GAGs, both at increasing concentrations. The untreated XylNapOH- and XylNap-primed GAGs were administered at the indicated concentrations, whereas the enzyme-treated GAGs were diluted in the same way as the untreated ones before administration. D, fixed concentration (7.5 μg/ml) of heparinase I-III-treated XylNapOH-primed GAGs combined with the indicated concentrations of chondroitinase ABC-treated XylNap-primed GAGs. The graphs are representative for each experiment, performed at least in duplicate, in which n = 3. The data points are the means ± S.D.
To confirm that the XylNap-primed HS from HCC70 cells had an inhibitory effect on the cytotoxic CS/DS, HCC70 cells were treated with a combination of chondroitinase ABC-treated XylNap-primed GAGs and heparinase I-III-treated XylNapOH-primed GAGs, both from HCC70 cells. Increasing concentrations of the two resulted in complete inhibition of the cytotoxic effect of the heparinase I-III-treated XylNapOH-primed GAGs (Fig. 8C). Likewise, a combination of a fixed concentration of the heparinase I-III-treated XylNapOH-primed GAGs (7.5 μg/ml) and increasing concentrations of the chondroitinase ABC-degraded XylNap-treated GAGs (0.5, 1, and 2 μg/ml) resulted in a concentration-dependent inhibition of the cytotoxic effect of the heparinase I-III-treated XylNapOH-primed GAGs (Fig. 8D), thus confirming that the XylNap-primed HS had an inhibitory effect on the cytotoxic CS/DS.
Discussion
We have previously reported a tumor-specific growth-inhibiting effect of the xyloside XylNapOH and suggested that the XylNapOH-primed GAGs could be responsible for this effect. Furthermore, we have observed differences in HS/CS/DS composition between GAGs primed on XylNapOH and GAGs primed on other xylosides, such as XylNap, that lack effect on cell growth. Here, we have characterized the structure-function relationship of XylNapOH- and XylNap-primed GAGs derived from a breast carcinoma cell line, HCC70, and a breast fibroblast cell line, CCD-1095Sk. We demonstrated that XylNapOH-primed GAGs isolated from HCC70 cells reduced growth of HCC70 cells and CCD-1095Sk cells, with IC50 values in the low micromolar range. The growth-reducing effect was a cytotoxic effect, mediated by apoptosis. Importantly, we demonstrated that the GAG part rather than the xyloside part of the XylNapOH-primed GAGs was responsible for the cytotoxic effect. Furthermore, the effect was specific for the XylNapOH-primed GAGs from HCC70 cells, as neither XylNap-primed GAGs from HCC70 cells nor XylNapOH- or XylNap-primed GAGs from CCD-1095Sk cells or commercially available GAGs showed any clear effect on cell growth.
Both HS and CS/DS GAGs have previously been shown to affect growth of cancer cells either after direct administration or as a result of enzymatic treatment of cells. For example, HS fragments generated after heparinase I treatment or heparinase III treatment of tumor cells have been shown to either promote or inhibit tumor growth in vivo, depending on their composition (25). Similarly, treatment of melanoma cells with chondroitinase AC or chondroitinase B has been reported to inhibit growth and migration in vitro (26), and other studies have showed inhibition as well as promotion of cell growth by HS and CS/DS (27–29). Here, we demonstrated that the CS/DS was responsible for the cytotoxic effect of the XylNapOH-primed GAGs from HCC70 cells, whereas the XylNapOH-primed HS did not have any effect on cell growth. Furthermore, the XylNap-primed CS/DS from HCC70 cells had a similar effect, whereas the XylNap-primed HS inhibited the cytotoxic effect of the XylNapOH- or XylNap-primed CS/DS in a concentration-dependent manner, explaining why no cytotoxic effect of the non-degraded XylNap-primed GAGs was observed. Neither the CS/DS nor the HS derived from CCD-1095Sk cells primed on XylNapOH or XylNap had any effect on cell growth, further supporting the notion that the cytotoxic effect was associated with the carcinoma cells.
The fact that HS and CS/DS appear to affect different cells differently indicates that besides possible differences in susceptibility to various GAGs, the composition of the GAGs is important for the biological effect. Thus, we characterized the disaccharide composition of the XylNapOH- and XylNap-primed GAGs. The data revealed considerable differences in disaccharide composition between the xyloside-primed GAGs from HCC70 cells and CCD-1095Sk cells. Interestingly, we found that the disaccharide composition of the xyloside-primed GAGs derived from the same cell line was similar irrespective of whether they were primed on XylNapOH or XylNap. This indicates that the disaccharide composition is specific and cell type-dependent rather than xyloside-dependent. In addition, the results suggest the disaccharide composition as a possible determinant for the biological effect of the GAGs. Interestingly, the disaccharide composition of the xyloside-primed GAGs from HCC70 resembled that of the corresponding PG-GAGs.
The XylNapOH- and XylNap-primed CS/DS derived from HCC70 cells were primarily composed of three disaccharide units: C units, A units, and E units, whereas those derived from CCD-1095Sk cells were primarily composed of A units, C units, D units, and B units. Thus, the prevalence of the E unit, D unit, and B unit distinguished the two. Interestingly, CS E, primarily composed of E units, is involved in some of the few identified functional CS/DS sequences. For example, CS E has been reported to interact with Wnt signaling (5), and L- and P-selectin (6) and to enhance CD44 cleavage (30), all of which could influence tumor progression. However, prevalence of the E unit is unlikely a single determinant for cytotoxicity, not the least because the commercially available CS B contained the E unit (supplemental Table S1), but did not have any effect on cell growth. Thus, the sequential order of the disaccharides, in addition to the disaccharide composition, is likely to be important for the biological effects.
Variations in the HS/CS/DS ratio of xyloside-primed GAGs depending on aglycon have been extensively studied over the years (10, 16, 31, 32). The amount of HS has been of particular interest, as xylosides were initially thought to only induce synthesis of CS. We found that the HS/CS/DS ratios differed between the xyloside-primed GAGs derived from the different cell lines and that the proportion of HS was influenced by the xyloside, which was evident in the HCC70 cells, where the proportion of HS was substantial. This supports previous results showing that the HS/CS/DS ratio is both cell type-dependent and xyloside-dependent.
Although the CS/DS from HCC70 cells primed on both XylNapOH and XylNap appeared structurally and functionally similar, the HS had, despite a similar disaccharide composition, different effects depending on whether it was primed on XylNapOH or XylNap. The XylNap-primed HS inhibited the cytotoxic effect of the XylNapOH- and XylNap-primed CS/DS from HCC70 cells, whereas the XylNapOH-primed HS was inactive. This did not appear to be a result of different proportions of HS in the XylNapOH- and XylNap-primed GAGs (∼20 and ∼35%, respectively), as the proportion of HS primed on XylNapOH increased with increasing incubation time without having any impact on the cytotoxic effect. In addition, the cytotoxic effect of the XylNapOH-primed GAGs remained the same upon HS removal. We speculate that the difference in effect could be due to the sequential order of the disaccharide units; however, this remains to be elucidated.
IdoUA-containing GAGs are associated with an increased structural flexibility compared with equivalent GlcUA-containing GAGs, thereby altering their interaction properties with various biomolecules (33). CS/DSIdoUA was present in the xyloside-primed GAGs from both HCC70 cells and CCD-1095Sk cells; thus, the presence of CS/DSIdoUA does not appear to be a single determinant for effect by the GAGs. However, the proportion could potentially have an impact on the effect, as it differed between the GAGs from HCC70 cells (8.9%) and CCD-1095Sk cells (37%). Interestingly, there was also a considerable difference in the distribution of IdoUA-containing disaccharides between the xyloside-primed GAGs derived from the different cell lines (∼20% versus ∼50% of the CS/DSIdoUA was present in blocks in the xyloside-primed GAGs from HCC70 cell and CCD-1095Sk cells, respectively), another factor that potentially could influence the biological effect.
The data presented here provide new perspectives on xyloside-primed GAGs and their properties. In addition, to our knowledge, this is the first study that demonstrates a cytotoxic effect on cancer cells and normal cells by CS/DS and that this effect can be inhibited by HS.
Author Contributions
A. P. and K. M. designed and coordinated the study. A. P. performed the experiments and analyzed the data. A. P. and E. T. performed the disaccharide fingerprint experiments. A. P., E. T., G. W.-T., A. M., U. E., and K. M. interpreted and reviewed the results. AP wrote the paper, which was reviewed and approved by all of the authors.
Supplementary Material
Acknowledgments
We thank Sophie Manner and Sebastian Clementson for synthesis of the xylosides. Furthermore, we thank Marco Maccarana for valuable discussions.
This work was supported by the Alfred Österlund Foundation, the Crafoord Foundation, the Foundation of the Hedda and John Forssman Fund, Lund University, the Medical Faculty at Lund University, the Swedish Cancer Society, the Swedish Foundation for Strategic Research, and the Swedish Research Council. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Tables S1 and S2.
- PG
- proteoglycan
- HS
- heparan sulfate
- GalNAc
- N-acetyl-d-galactosamine
- UA
- uronic acid
- GlcUA
- d-glucuronic acid
- IdoUA
- l-iduronic acid
- GlcNAc
- N-acetyl-d-glucosamine
- XylNapOH
- 2-(6-hydroxynaphthyl) β-d-xylopyranoside
- XylNap
- 2-naphthyl β-d-xylopyranoside
- GAG
- glycosaminoglycan
- CS/DS
- chondroitin sulfate/dermatan sulfate
- UT
- untreated
- CS/DSIdoUA
- IdoUA-containing disaccharides in CS/DS
- CS/DSGlcUA
- GlcUA-containing disaccharides in CS/DS.
References
- 1.Afratis N., Gialeli C., Nikitovic D., Tsegenidis T., Karousou E., Theocharis A. D., Pavão M. S., Tzanakakis G. N., and Karamanos N. K. (2012) Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 279, 1177–1197 [DOI] [PubMed] [Google Scholar]
- 2.Mikami T., and Kitagawa H. (2013) Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta 1830, 4719–4733 [DOI] [PubMed] [Google Scholar]
- 3.Sugahara K., and Kitagawa H. (2002) Heparin and Heparan Sulfate Biosynthesis. IUBMB Life 54, 163–175 [DOI] [PubMed] [Google Scholar]
- 4.Mizumoto S., Yamada S., and Sugahara K. (2015) Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 34, 35–42 [DOI] [PubMed] [Google Scholar]
- 5.Willis C. M., and Klüppel M. (2012) Inhibition by Chondroitin sulfate E can specify functional Wnt/β-catenin signaling thresholds in NIH3T3 fibroblasts. J. Biol. Chem. 287, 37042–37056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawashima H., Hirose M., Hirose J., Nagakubo D., Plaas A. H., and Miyasaka M. (2000) Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J. Biol. Chem. 275, 35448–35456 [DOI] [PubMed] [Google Scholar]
- 7.Weyers A., Yang B., Yoon D. S., Park J. H., Zhang F., Lee K. B., and Linhardt R. J. (2012) A structural analysis of glycosaminoglycans from lethal and nonlethal breast cancer tissues: toward a novel class of theragnostics for personalized medicine in oncology? OMICS 16, 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joo E. J., Weyers A., Li G., Gasimli L., Li L., Choi W. J., Lee K. B., and Linhardt R. J. (2014) Carbohydrate-containing molecules as potential biomarkers in colon cancer. OMICS 18, 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smetsers T. F., van de Westerlo E. M., ten Dam G. B., Clarijs R., Versteeg E. M., van Geloof W. L., Veerkamp J. H., van Muijen G. N., and van Kuppevelt T. H. (2003) Localization and characterization of melanoma-associated glycosaminoglycans: differential expression of chondroitin and heparan sulfate epitopes in melanoma. Cancer Res. 63, 2965–2970 [PubMed] [Google Scholar]
- 10.Fritz T. A., Lugemwa F. N., Sarkar A. K., and Esko J. D. (1994) Biosynthesis of heparan sulfate on β-d-xylosides depends on aglycone structure. J. Biol. Chem. 269, 300–307 [PubMed] [Google Scholar]
- 11.Toomey J. R., Abboud M. A., Valocik R. E., Koster P. F., Burns-Kurtis C. L., Pillarisetti K., Danoff T. M., and Erhardt J. A. (2006) A comparison of the β-d-xyloside, odiparcil, to warfarin in a rat model of venous thrombosis. J. Thromb. Haemost. 4, 1989–1996 [DOI] [PubMed] [Google Scholar]
- 12.Muto J., Naidu N. N., Yamasaki K., Pineau N., Breton L., and Gallo R. L. (2011) Exogenous addition of a C-xylopyranoside derivative stimulates keratinocyte dermatan sulfate synthesis and promotes migration. PloS ONE 6, e25480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farach M. C., Tang J. P., Decker G. L., and Carson D. D. (1988) Differential effects of p-nitrophenyl-d-xylosides on mouse blastocysts and uterine epithelial cells. Biol. Reprod. 39, 443–455 [DOI] [PubMed] [Google Scholar]
- 14.Margolis R. K., Goossen B., Tekotte H., Hilgenberg L., and Margolis R. U. (1991) Effects of β-xylosides on proteoglycan biosynthesis and morphology of PC12 pheochromocytoma cells and primary cultures of rat cerebellum. J. Cell Sci. 99, 237–246 [DOI] [PubMed] [Google Scholar]
- 15.Raman K., Ninomiya M., Nguyen T. K., Tsuzuki Y., Koketsu M., and Kuberan B. (2011) Novel glycosaminoglycan biosynthetic inhibitors affect tumor-associated angiogenesis. Biochem. Biophys. Res. Commun. 404, 86–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mani K., Belting M., Ellervik U., Falk N., Svensson G., Sandgren S., Cheng F., and Fransson L.-Å. (2004) Tumor attenuation by 2(6-hydroxynaphthyl)-β-d-xylopyranoside requires priming of heparan sulfate and nuclear targeting of the products. Glycobiology 14, 387–397 [DOI] [PubMed] [Google Scholar]
- 17.Nilsson U., Johnsson R., Fransson L.-Å., Ellervik U., and Mani K. (2010) Attenuation of tumor growth by formation of antiproliferative glycosaminoglycans correlates with low acetylation of histone H3. Cancer Res. 70, 3771–3779 [DOI] [PubMed] [Google Scholar]
- 18.Mani K., Havsmark B., Persson S., Kaneda Y., Yamamoto H., Sakurai K., Ashikari S., Habuchi H., Suzuki S., Kimata K., Malmström A., Westergren-Thorsson G., and Fransson L.-Å. (1998) Heparan/chondroitin/dermatan sulfate primer 2-(6-hydroxynaphthyl)-O-β-d-xylopyranoside preferentially inhibits growth of transformed cells. Cancer Res. 58, 1099–1104 [PubMed] [Google Scholar]
- 19.Fransson L.-Å., Karlsson P., and Schmidtchen A. (1992) Effects of cycloheximide, brefeldin A, suramin, heparin, and primaquine on proteoglycan and glycosaminoglycan biosynthesis in human embryonic skin fibroblasts. Biochim. Biophys. Acta 1137, 287–297 [DOI] [PubMed] [Google Scholar]
- 20.Farndale R. W., Sayers C. A., and Barrett A. J. (1982) A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect. Tissue Res. 9, 247–248 [DOI] [PubMed] [Google Scholar]
- 21.Westergren-Thorsson G., Önnervik P.-O., Fransson L.-Å., and Malmström A. (1991) Proliferation of cultured fibroblasts is inhibited by l-iduronate-containing glycosaminoglycans. J. Cell. Physiol. 147, 523–530 [DOI] [PubMed] [Google Scholar]
- 22.Stachtea X. N., Tykesson E., van Kuppevelt T. H., Feinstein R., Malmström A., Reijmers R. M., and Maccarana M. (2015) Dermatan sulfate-free mice display embryological defects and are neonatal lethal despite normal lymphoid and non-lymphoid organogenesis. PloS ONE 10, e0140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linhardt R. J., Avci F. Y., Toida T., Kim Y. S., and Cygler M. (2006) CS lyases: structure, activity, and applications in analysis and the treatment of diseases. Adv. Pharmacol. 53, 187–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnbull J., Powell A., and Guimond S. (2001) Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 11, 75–82 [DOI] [PubMed] [Google Scholar]
- 25.Liu D., Shriver Z., Venkataraman G., El Shabrawi Y., and Sasisekharan R. (2002) Tumor cell surface heparan sulfate as cryptic promoters or inhibitors of tumor growth and metastasis. Proc. Natl. Acad. Sci. U.S.A. 99, 568–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denholm E. M., Lin Y.-Q., and Silver P. J. (2001) Anti-tumor activities of chondroitinase AC and chondroitinase B: inhibition of angiogenesis, proliferation and invasion. Eur. J. Pharmacol. 416, 213–221 [DOI] [PubMed] [Google Scholar]
- 27.Matuoka K., Mitsui Y., and Murota S.-i. (1984) Heparan sulfate enhances growth of transformed human cells. Cell Struct. Funct. 9, 357–367 [DOI] [PubMed] [Google Scholar]
- 28.Syrokou A., Tzanakakis G., Tsegenidis T., Hjerpe A., and Karamanos N. K. (1999) Effects of glycosaminoglycans on proliferation of epithelial and fibroblast human malignant mesothelioma cells: a structure-function relationship. Cell Prolif. 32, 85–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westergren-Thorsson G., Persson S., Isaksson A., Onnervik P. O., Malmström A., and Fransson L. A. (1993) l-iduronate-rich glycosaminoglycans inhibit growth of normal fibroblasts independently of serum or added growth factors. Exp. Cell Res. 206, 93–99 [DOI] [PubMed] [Google Scholar]
- 30.Sugahara K. N., Hirata T., Tanaka T., Ogino S., Takeda M., Terasawa H., Shimada I., Tamura J.-i., ten Dam G. B., van Kuppevelt T. H., and Miyasaka M. (2008) Chondroitin sulfate E fragments enhance CD44 cleavage and CD44-dependent motility in tumor cells. Cancer Res. 68, 7191–7199 [DOI] [PubMed] [Google Scholar]
- 31.Victor X. V., Nguyen T. K., Ethirajan M., Tran V. M., Nguyen K. V., and Kuberan B. (2009) Investigating the elusive mechanism of glycosaminoglycan biosynthesis. J. Biol. Chem. 284, 25842–25853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobsson M., Ellervik U., Belting M., and Mani K. (2006) Selective antiproliferative activity of hydroxynaphthyl-β-d-xylosides. J. Med. Chem. 49, 1932–1938 [DOI] [PubMed] [Google Scholar]
- 33.Ferro D. R., Provasoli A., Ragazzi M., Casu B., Torri G., Bossennec V., Perly B., Sinaÿ P., Petitou M., and Choay J. (1990) Conformer populations of l-iduronic acid residues in glycosaminoglycan sequences. Carbohydr. Res. 195, 157–167 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.