Summary
The ability of T cells to respond to a wide array of foreign antigens while avoiding reactivity to self is largely determined by cellular selection of developing T cells in the thymus. While a great deal is known about the cell types and molecules involved in T cell selection in the thymus, our understanding of the spatial and temporal aspects of this process remain relatively poorly understood. Thymocytes are highly motile within the thymus and travel between specialized microenvironments at different phases of their development while interacting with distinct sets of self-peptides and peptide presenting cells. A knowledge of when, where, and how thymocytes encounter self-peptide MHC ligands at different stages of thymic development is key to understanding T cell selection. In the past several years, our laboratory has investigated this topic using two-photon time-lapse microscopy to directly visualize thymocyte migration and signaling events, together with a living thymic slice preparation to provide a synchronized experimental model of T cell selection in situ. Here, we discuss recent advances in our understanding of the temporal and spatial aspects of T cell selection, highlighting our own work, and placing them in the context of work from other groups.
Keywords: thymus, T cell antigen receptor, chemokines, lineage commitment/specification, cell differentiation
Introduction
The selection of a functional and self-tolerant T cell repertoire is coordinated by multiple selection processes that occur during T cell development in the thymus; including positive selection, negative selection, and agonist selection. While positive selection ensures that the T cell repertoire is functional and equipped to make robust responses against foreign antigens, negative selection and agonist selection make significant contributions to enforcing self-tolerance. Distinct thymic microenvironments differ in their ability to support each of these selection events. Thus, the ability of thymocytes to access these discrete microenvironments is a key factor in regulating thymocyte maturation, and ultimately in determining thymocyte fate.
The thymus is comprised of two distinct anatomical sites: the cortex and the medulla. Each of these regions is populated by distinct subsets of thymic resident cells, creating microenvironments that are unique to each site and are specialized to coordinate distinct selection events. For example, expression of a distinct proteasome subunit and unique lysosomal proteases within cortical epithelial cells generate a specialized peptide repertoire to support positive selection. In contrast, a program of promiscuous gene expression in a subset of medullary thymic epithelial cells and ample expression of costimulatory ligands in the medulla make this locale particularly well suited to promoting negative selection (1).
The location of developing T cells within the thymus is tightly linked to their developmental stage. T cell progenitors enter the thymus through blood vessels at the corticomedullary junction and then subsequently localize to the outer regions of the thymic cortex, where they undergo rearrangement of the T cell receptor (TCR) α and β loci. Following successful TCRβ gene rearrangement and preTCR signaling, thymocytes progress to the CD4+CD8+ double positive (DP) stage, migrate to the thymic cortex, and rearrange their TCRα locus (2–4). Cortical DP thymocytes that experience αβTCR signals in response to self-peptides presented by Major Histocompatibility Complex proteins (MHC) can undergo positive selection, resulting in maturation and commitment to either the CD4 or CD8 lineage, depending on whether the selecting MHC was class II or class I, respectively (5). Positive selection also leads to the relocalization of thymocytes from the cortex to the medulla, and the majority of medullary thymocytes exhibit a CD4+CD8− or CD4−CD8+ “single positive” SP phenotype. After several days of further maturation in the medulla, SP thymocyte become competent to leave the thymus as fully functional mature T cells (6).
Two-photon time-lapse imaging studies of living thymic tissue have revealed that developing T cells are highly motile within the 3D environment of the thymus (7, 8). Thymocyte motility is key both to orchestrating migration between different thymic microenvironments at the appropriate developmental stage, and also to shaping the TCR signaling pattern experienced by thymocytes upon encounter with self-peptide MHC complexes in the thymus. In the past several years, our lab has been focused on the inter-relationship between thymocyte motility and T cell repertoire selection in the thymus. Most recently, we have been making extensive use of a thymic slice preparation that greatly facilitates direct visualization of T cell development using 2-photon microscopy, and has also proved to be a powerful system for synchronizing and manipulating T cell development. In this review, we will discuss some of our key findings using this approach, placing them in the context of other advances in the field.
Thymic tissue slices: a versatile model for the study of T cell selection in situ
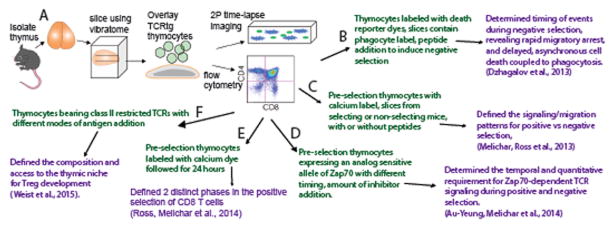
In an effort to develop a tractable experimental system to study T cell development within the 3D tissue environment of the thymus, we and others have turned to a thymic slice model (9, 10) (11) (Figure 1). This approach uses vibratome-cut slices of thymic tissue, and was inspired by decades of successful studies using living brain slices by neurobiologists. When maintained under appropriate conditions, thymic slices retain good viability for several days and faithfully recapitulate many aspects of thymic biology (12). For example, we and others have demonstrated that both human and mouse thymocytes actively migrate into tissue slices and localize to the appropriate thymic location according to their maturation status, such that preselection DP thymocytes accumulate in the cortex and SP thymocytes localize preferentially to the medulla (11, 13). These localization events are dependent on thymocyte responses to chemokine gradients within the slice, as interfering with the expression of chemokine receptors on the overlaid thymocytes, the expression of chemokines within the slice, and pharmacological inhibition of chemokine receptors all resulted in some degree of mislocalization.
Figure 1. Thymic tissue slices provide a robust and versatile system for the study of T cell selection.
A) Thymocytes of defined TCR specificity from TCR transgenic mice are overlaid onto vibratome-cut slices of thymic tissue and cultured for up to three days. Thymocytes migrate to their normal location within the tissue according to chemokine gradients and undergo synchronized development, and can be examined using flow cytometry or 2-photon time-lapse imaging. B–F) Examples of variations of the thymic slice model (in green) and the relevant research advance (in purple). See text and references for details.
In addition to appropriate thymocyte localization, thymic slices also support T cell selection in a manner that closely recapitulates the timing and phenotypic sequences reported from in vivo studies (14–16). By using different starting thymocyte populations expressing defined TCR transgenes, and different thymic slice donors to vary the thymic environment, this approach can be readily adapted to study different aspects of thymic development, including positive selection, negative selection, and agonist selection (Figure 1).
Temporal pattern of TCR signaling during T cell selection in the thymus
One important question that we have addressed using thymic tissue slices is how the temporal pattern of TCR signaling differs during positive and negative selection. Previous studies had addressed this question in vitro, using stimulation of thymocytes with MHC-tetramers loaded with low potency ligands as a mimic of positive selection (17). Intriguingly, low potency ligands induced sustained but low-level signaling events downstream of the TCR, including a sustained rise in intracellular calcium (Figure 2a). Our lab has examined this question in situ by loading preselection thymocytes expressing class I-restricted TCR transgenes (e.g. OT1 or F5 TCRs) with a calcium sensitive reporter dye, and tracking their calcium levels and motility within thymic slices bearing positive selecting ligands. In sharp contrast to the in vitro studies, we observed transient signaling events lasting around 5 minutes, interspersed with periods of ~ 30 minutes of low calcium levels and relatively rapid migration (14) (Figure 2c–d). We also noted that thymocyte encounters with negative selecting ligands led to rapid migratory arrest and sustained increases in intracellular calcium (10) (14) (Figure 2c,d), displaying similar kinetics to that reported for in vitro tetramer stimulation with negative selecting ligands (17) (Figure 2a). It is interesting to note that while preselection thymocytes introduced into positive selecting slices undergo robust positive selection within 2–3 days (14), in vitro stimulation with low potency peptide-MHC tetramers fails to induce positive selection. It is tempting to speculate that the motility of thymocytes within thymic slices allows thymocytes to move away from peptide-MHC bearing thymic epithelial cells, and thus promotes transient TCR signals that support positive selection. Future studies manipulating the temporal pattern of TCR signaling both in vitro and in situ are needed to test this hypothesis.
Figure 2. Temporal patterns of TCR signaling in vitro and in thymic slices.
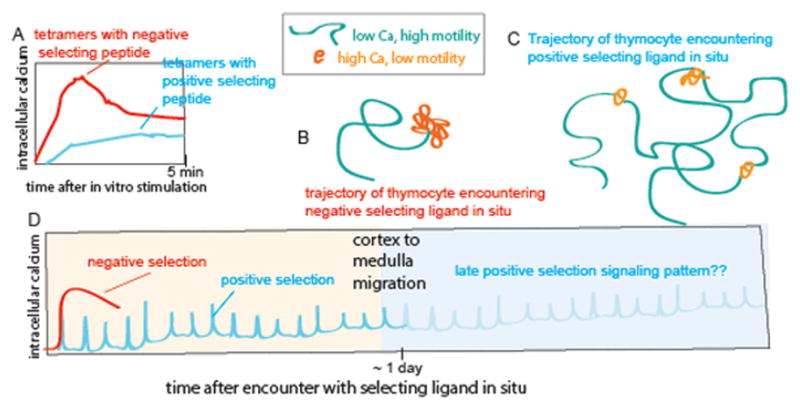
A) Temporal responses to stimulation of pre-selection thymocytes with MHC-tetramers loaded with low or high potency ligands. Adapted from (17). Stimulation with low potency peptide ligands in vitro leads to sustained low-level signaling. B, C) Schematic of representative trajectories of preselection thymocytes within thymic slices during encounters with negative (B) or positive (C) selecting ligands. Green indicates periods of low intracellular calcium and relatively rapid migration, where as orange indicates migratory pauses and elevated intracellular calcium levels. These schematics are based on data from 2-photon microscopy analysis of class I specific (OT1 or F5 TCR transgenic) thymocytes in thymic tissue slices. Using this system we have shown that negative selection correlates with a persistent increase in intracellular calcium, migratory arrest, and thymocyte death within 4–12 hours after the initiation of TCR signaling (10). In contrast, positive selection correlates with serial transient increases in intracellular calcium accompanied by migratory pauses interspersed with periods of rapid migration and low intracellular calcium (14). (D) The pattern of TCR signaling during the first 24 hours of positive selection (cyan) and negative selection (red) inferred from calcium signaling and motility changes in thymic slices. Thymocytes undergo a gradual increase in migratory speed and basal calcium levels throughout the first 24 hours of positive selection, while exhibiting progressively briefer transient signals. At around 24 hours thymocytes change their chemokine receptor expression and migrate from the cortex to the medulla (16). While it has been shown that TCR signaling is required late during positive selection (30, 31), the late signaling pattern has not yet been directly examined (indicated by faint portion of the curve). In addition, while it has also been reported that thymocytes bearing class II specific TCRs also undergo transient signals during positive selection (9), the signaling pattern associated with thymic positive selection on class II MHC has not yet been extensively examined.
Reciprocal changes in sensitivity to cortical versus medullary chemokines accompany T cell maturation
The relocalization of thymocytes from the cortex to the medulla during positive selection is achieved by developmentally regulated expression changes in the receptors for cortical or medullary chemokines (Figure 3). For example, preselection DP thymocytes are negative for the C-C chemokine receptor type 7 (CCR7), but upregulate expression upon positive selection (16, 18), allowing them to respond to the medullary chemokines CCL19 and CCL21 (19, 20). Several studies have demonstrated that CCR7 is essential for appropriate localization of SP thymocytes to the medulla. Specifically, CCR7-deficient SP thymocytes show impaired medullary localization when overlaid onto thymic slices (11). Similarly, we have shown that human SP thymocytes overlaid onto mouse thymic slices deficient in CCL19 and CCL21 also show impaired medullary localization (13). These findings are in agreement with earlier studies demonstrating that both CCR7-deficient and CCL19/21 double-deficient mice show accumulation of SP thymocytes in the cortex, and that premature expression of CCR7 in DP thymocytes causes an accumulation of DP thymocytes in the medulla (19, 21). Together, these studies define the CCR7-CCL19/21 axis as a key mediator of thymocyte migration form the cortex to the medulla following positive selection.
Figure 3. Kinetics of chemokine receptor changes, intrathymic migration, co-receptor down-regulation during positive selection of class II versus class I restricted thymocytes.
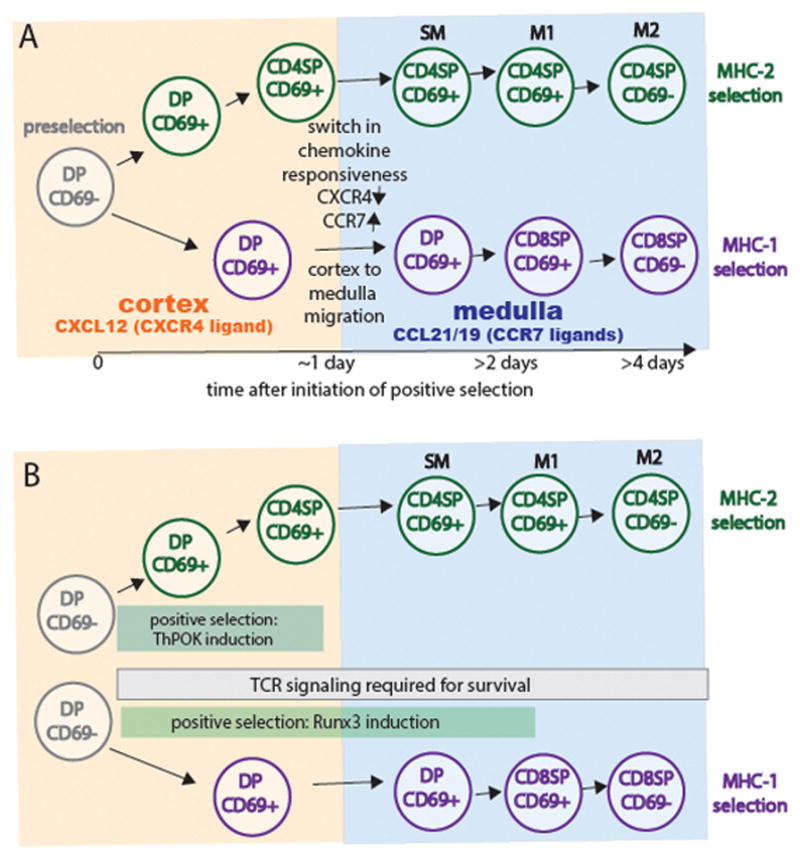
A) Preselection CD4+CD8+ DP thymocyte reside in the cortex and express CXCR4, the receptor for the cortical chemokine, CXCL12. More mature CD4+CD8− and CD4-CD8+ single positive (SP) thymocytes localize to the medulla and express CCR7, the receptor for the medullary chemokines CCL21/CCL19. Thymocytes undergoing selection via class II MHC first down-regulate CD8 and then change their chemokine receptor pattern around 24 hours after the initiation of positive selection. In contrast, although thymocytes undergoing selection via class I MHC also change their chemokine receptor expression around 24 hours, they do not down regulate CD4 until >1 day later. In the medulla, CD4SP thymocytes undergo a TCR independent maturation process (passing through stages termed semimature SM, M1, and M2) that correlate with down regulation of CD24 (HSA), upregulation of Qa2 and MHC class I, and the ability of thymocytes to die or proliferate upon TCR signaling (6, 34). CD8 SP thymocytes appear to progress through similar stages, although this has been less well studied. B) Thymocytes undergoing positive selection via class II MHC upregulate the CD4 defining transcription factor ThPOK and down-regulate CD8 after 24 hours of Zap70 dependent TCR signaling. In contrast, thymocytes undergoing positive selection via class I MHC require Zap70-dependent TCR signaling for an additional day or more in order to upregulate the CD8-defining transcription factor Runx3 and downregulate CD4. Both lineages continue to require TCR signaling to promote cell survival after lineage commitment and co-receptor downregulation (30, 66).
Compared to the medulla, there is a relative dearth of chemokine expression within the thymic cortex. One exception is CXCL12, the ligand for the C-X-C chemokine receptor type 4 (CXCR4), which is expressed at higher levels in the cortex relative to the medulla (20). Interestingly, CXCR4 is high on pre-selection thymocytes and is downregulated following positive selection, a reciprocal pattern to that observed for CCR7 (13, 16, 22). Moreover, positively-selected thymocytes exhibit decreased responsiveness to CXCL12 in vitro (13, 18).Because CXCR4-deficient thymocytes display a block in early T cell development, it has been difficult to assess the role of CXCR4 in controlling the localization of pre-selection DP thymocytes to the cortex (23–25). However, using the thymic slice system, we have demonstrated that treatment of thymic slices with a specific pharmacological inhibitor of CXCR4 abrogates the cortical localization of overlaid human DP thymocytes, indicating that CXCR4 plays an important role in retaining pre-selection DP thymocytes in the cortex (13). Thus, the current data support a model in which the opposing expression of CXCL12 in the cortex and CCR7 ligands in the medulla cooperatively controls the localization and migration of maturing thymocytes during positive selection (Figure 3).
Timing of coreceptor downregulation and migration from the cortex to the medulla during positive selection
While it is often stated that positive selection and the downregulation of CD4 or CD8 is coincident with the migration of thymocytes from the cortex to the medulla, a closer look reveals that this is an oversimplification. Indeed, we have previously reported that while <10% of CD4+CD8+ thymocytes undergoing positive selection on MHC-2 express the medullary chemokine receptor CCR7, 10–30% of CD4+CD8+ thymocytes being selected on MHC-1 show substantial upregulation of CCR7 (26). More recently, we have examined the timing of changes in chemokine receptor expression and the cortex to medulla migration in a synchronized model of positive selection on MHC-1. We showed that preselection OT1 thymocytes undergo a switch in chemokine receptor expression between 12–24 hours after entering positive selecting slices, and accumulate in the medullary regions of thymic slices by 24 hours. However, CD4 downregulation did not occur until 2–3 days after the initiation of positive selection. In agreement with this observation, a recent study using an inducible version of the TCR tyrosine kinase Zap70 to synchronize positive selection, showed that CD8 SP thymocytes appear 2–3 days following the initiation of positive selection. In contrast, CD4 SP thymocytes first appeared just one day following the initiation of positive selection (27), in agreement with earlier BrdU labeling studies (28). The downregulation of CXCR4 and upregulation of CCR7 appear to coincide with CD8 downregulation in class II MHC selected thymocytes, since other studies have demonstrated that a small population of CD4 SP thymocytes retain CXCR4 and lack CCR7 (22, 29). Thus, although the switch in chemokine receptor expression occurs around one day after the initiation of positive selection for both class I and class II-restricted thymocytes, co-receptor downregulation is delayed by at least one more day for class I-restricted thymocytes (Figure 3A).
The delay in coreceptor downregulation during positive selection on class I MHC does not simply reflect a delay in removing co-receptor from the cell surface after positive selection. Rather, it appears to reflect a more fundamental difference in how CD4 and CD8 T cells develop. While one day of Zap70-dependent TCR signaling was sufficient to induce the CD4-defining transcription factor ThPOK, a further two days of signaling were required to fully induce the CD8 defining transcription factor Runx3 (30) and Figure 3B). This timing is consistent with our studies using an analog-sensitive version of Zap70 in thymic slice cultures, in which we found that blocking Zap70-dependent TCR signals at any point before the appearance of CD8SP thymocytes was sufficient to block their development (31). Interestingly, both class I and class II selected thymocytes require TCR signals to promote their continued survival well after the initiation of positive selection(30). This late requirement for TCR-MHC signals may help to eliminate those few “mismatched” thymocytes that adopt a lineage that is incompatible with their TCR specificity (i.e. CD4 cells with TCRs specific for MHC-1 or CD8 cells with TCRs specific for MHC-2).
Altogether, the current data suggest that class I-restricted thymocytes acquire the ability to migrate to the medulla before completing positive selection, whereas for class II-restricted thymocytes, migration to the medulla and the completion of positive selection occur coordinately. This asymmetry between CD4 and CD8 T cell development could give rise to interesting differences regarding the relationship between positive versus negative selection in these two lineages.
Distinct APC populations and peptide display in cortex vs. medulla
As implied from the previous section, the general view that positive selection occurs in the cortex at the DP stage, whereas negative selection occurs in the medulla at the SP stage is an oversimplification. Nevertheless, it is clear that the cortex and medulla exhibit specialized features that promote either positive or negative selection, respectively (Figure 4A). For example, cortical thymic epithelial cells (cTECs) express a unique version of the proteasome, termed the thymoproteosome, which generates cTEC specific peptides for optimal positive selection of CD8 T cells (32). Likewise cTECs express a class II associated protease, L-cathepsin, that generates peptides for positive selection of CD4 T cells (1). Conversely, the peptide display in the medulla may be more akin to that in the periphery, owing to several specialized features that allow for the presentation of peripheral self-antigens. First, the expression of the Autoimmune Regulator (AIRE) within medullary thymic epithelial cells leads to the “ectopic” expression of a broad array of tissue-restricted proteins (1). These tissue-restricted antigens can be presented to thymocytes by the mTECs directly, or transferred to thymic dendritic cells (DCs), which are found at a high density within the thymic medulla. Furthermore, specialized subsets of DCs that import antigens from peripheral sites and/or take up bloodborne antigens localize preferentially within the thymic medulla. As a result of this specialization, thymocytes encounter distinct peptide displays and distinct sets of peptide presenting cells in the cortex versus the medulla (Figure 4A).
Figure 4. Distinct peptide and peptide presenting cells in the cortex versus medulla, and the timing of negative and agonist selection.
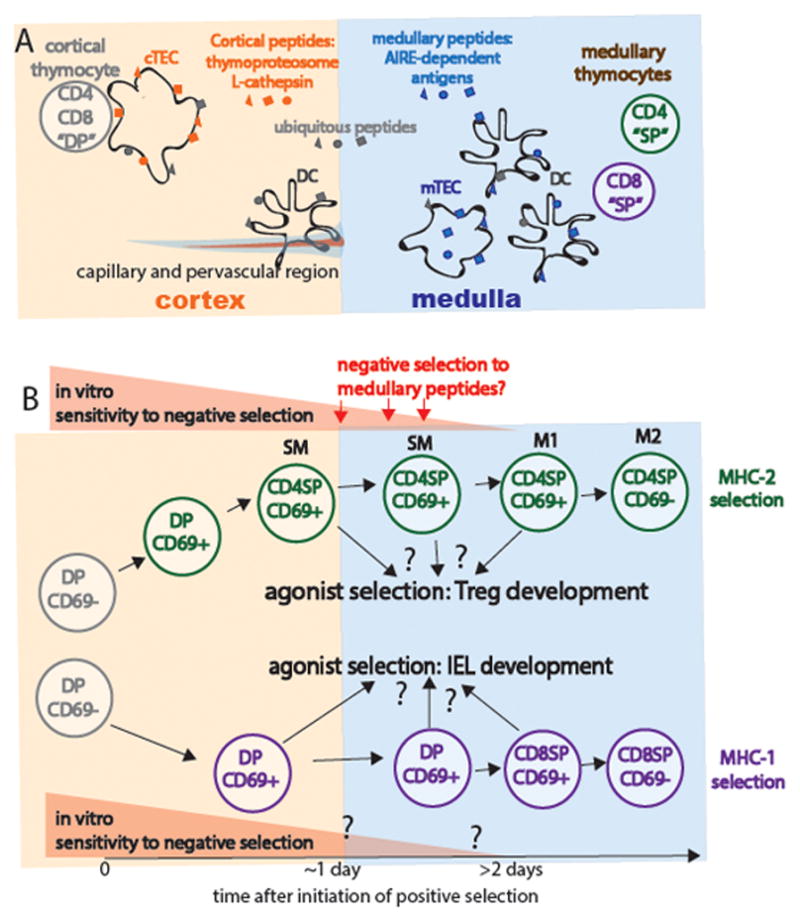
Distinct peptide display and peptide presenting cells in the thymic cortex versus medulla. Cortical thymic epithelial cells (cTECs) express a unique proteasome subunit (the thymoproteasome) and lysosomal protease (L-cathepsin) that allows the generation of a unique peptide repertoire (orange) thought to promote positive selection (reviewed in (67)). In contrast, expression of the transcription factor Aire allows for the expression of tissue restricted antigens (blue) by medullary epithelial cells (mTECs). These tissue-restricted antigens can also be transferred to DCs, which are more prevalent within the medulla. Thus, thymocytes do not have access to tissue-restricted antigens until the positive-selection induced migration to the medulla. Thymocytes are exposed to ubiquitous self-peptides (gray) at all stages of development. Perivascular regions surrounding cortical capillaries have some medullary character, including the presence of the “medullary chemokine CCL21 and dendritic cells (36). B) Timing of negative and agonist selection. Thymocytes gradually lose their susceptibility to negative selection as they progress through progress from DP through CD4 SP stages “semi-mature” SM stage. While SM thymocytes undergo apoptosis upon strong TCR stimulation, M1 and M2 thymocytes become activated and proliferated (6, 34). The susceptibility of class I restricted thymocytes has been less well studied, but appears to follow a similar pattern. Thymocytes that encounter high affinity self-peptide ligands may undergo agonist selection rather than negative selection, giving rise to Tregs or CD8αα IEL.
The distinct APC types in the cortex versus the medulla also differ in their inherent ability to induce negative selection. In line with the idea that cTECs play a primary role in positive selection, cTECs express relatively low levels of the costimulatory molecules B7.1 and B7.2, which are thought to be important for inducing negative selection (33). Accordingly, antigen presentation by cTECs results in relatively inefficient negative selection. In contrast, medullary APCs, namely mTECs and DCs, express relatively high levels of costimulatory molecules and have been shown to effectively mediate negative selection. Thus, encounter of different types of APCs and distinct peptide displays within the cortex versus the medulla can have profound impacts on the timing and location of thymocyte selection processes.
Timing of negative selection
The fact that several features of the medulla are specialized to promote negative selection has shaped the long-standing view that the medulla is an important site for negative selection. In line with this idea, in vitro stimulation of SP thymocytes with strong TCR signal and co-stimulatory ligands triggers apoptosis. In contrast, this same stimulus results in activation and proliferation of mature T cells. For class II-restricted thymocytes, this switch in responsiveness can be traced to a particular developmental transition during the CD4 SP stage delineated by changes in expression of cell surface markers. Using several of these markers to distinguish “semimature” (SM) from “mature” (M1 and M2) CD4 SP thymocytes, Kishimoto and Sprent demonstrated that SM CD4 SP as well as DP thymocytes are susceptible to death when stimulated in vitro through the TCR, whereas more mature CD4 SP thymocytes resemble mature lymph node CD4 T cells in that TCR triggering fails to induce thymocyte death, and instead results in proliferation (6, 34). Thus, at least some SP thymocytes remain competent to undergo negative selection at the stage in which they would be expected to encounter medullary peptides and antigen presenting cells.
Interestingly, much of the specialized machinery for negative selection within the medulla is most concentrated near the corticomedullary junction. For example, the corticomedullary junction contains a dense network of DCs and mature AIRE-expressing mTECs, both of which express ample costimulatory ligands (1). Thus, thymocytes would likely encounter these Aire-dependent antigens soon after they switch their chemokine receptor expression and begin their migration towards the medulla. The positioning of Aire-expressing mTECs at the corticomedullary junction could therefore play a particularly important role in enforcing tolerance to rare self-antigens, as this positioning ensures that thymocytes are exposed to these antigens at a stage in which they are still relatively susceptible to deletion.
On the other hand, DP thymocytes are even more susceptible to death upon TCR stimulation in vitro compared to CD4 SP thymocytes (6, 34). Moreover, preselection DP thymocytes readily undergo negative selection when exposed to cognate antigen both in vitro and in situ suggesting that thymocytes are susceptible to negative selection as soon as they express a functional αβTCR (17) (14). Thus, positive selection is not a prerequisite for negative selection, and these selection events do not seem to be as compartmentalized as originally thought.
Indeed, it is now clear that negative selection can occur efficiently within the cortex. For example, McCaughtry et al demonstrated that negative selection of a class I-restricted TCR transgenic occurs at a DP stage in which the thymocytes lack expression of CCR7. Moreover, thymocytes expressing active caspase 3, which marks apoptotic cells, were detected in the cortex (35).
As mentioned earlier, our in situ time-lapse studies have shown that negative selection correlates with migratory arrest and sustained TCR signaling. Thymocytes in the cortex experienced these signaling interactions predominantly while in contact with DCs, even when the negatively selecting antigen was also presented by cTECs (14). In line with this observation, DCs have been shown to play a critical role in mediating negative selection in the cortex, while cTECs are unable to support efficient negative selection. Several factors could contribute to the enhanced efficacy of DCs in supporting negative selection. DCs express high levels of a variety of costimulatory molecules at the cell surface, including many molecules involved in cellular adhesion. The expression of these molecules could be involved in promoting the longer periods of thymocyte arrest that support negative selection. In line with this idea, we observed that DCs deficient in intercellular adhesion molecule 1 (ICAM-1) were less efficient at inducing the long periods of thymocyte arrest and sustained signaling events characteristic of negative selection (14). Moreover, DCs express higher levels of the costimulatory ligands B7.1 and B7.2, which have been shown to promote negative selection.
The efficiency of negative selection in the cortex is particularly striking given the relative paucity of DCs within the cortex. However, by directly visualizing interactions between DP thymocytes and cortical DCs using 2-photon time-lapse microscopy, we have demonstrated that DP interactions with DCs in the cortex are quite extensive; we estimate that a DP thymocyte can contact 100–400 DCs during its time in the cortex (36). We also observed that DCs within the cortex were intimately associated with the capillary network, and that the “medullary” chemokine CCL21 could be detected within these perivascular regions. Thus, in addition to inducing the migration of positively selected thymocytes towards the medulla, the expression of CCR7 following positive selection could also promote cortical negative selection by attracting thymocytes towards “medullary-like” microenvironments within the cortex, i.e. perivascular regions containing DCs and CCL21 (Figure 4A).
Consistent with findings that negative selection can occur efficiently within the cortex, expression of CCR7 is dispensable for efficient negative selection to ubiquitous self-antigens (19, 35). In contrast, expression of Aire-dependent tissue-restricted antigens is restricted to the thymic medulla (1). Accordingly, mice deficient in CCR7 or CCR7 ligands exhibit tissue-specific autoimmunity (37). Indeed, in the absence of CCR7 or CCR7 ligands, Aire-dependent negative selection of both class I and class II-restricted thymocytes is impaired, highlighting the importance of the CCR7/CCR7L axis in maintaining tolerance to tissue-specific self-antigens (38).
Taken together, the current data suggest that there is an extended “window of opportunity” for thymocyte negative selection, which opens upon the initial expression of the αβTCR, and closes once thymocytes have matured past the semimature SP stage. The first wave of negative selection occurs in the cortex, when thymocytes first encounter ubiquitous self-antigen displayed on cortical DCs. Thymocytes that survive this first wave and successfully undergo positive selection switch their chemokine receptor expression and are subject to a second wave of negative selection as they encounter a new set of self-antigens upon arriving at the medulla or corticomedullary junction. Although their intrinsic susceptibility to negative selection is lower at this stage, encounter with a diverse set of high affinity medullary-specific peptides in the face of increased costimulation can trigger a substantial amount of negative selection.
In line with this model, in vivo studies indicate that negative selection is prevalent within both the DP and the SP populations (29, 39). For example, using a reporter of TCR signaling, Stritesky et al demonstrated an accumulation of polyclonal thymocytes that had experienced high levels of TCR signal in mice deficient in negative selection. This accumulation was apparent within both the DP and SP populations (39). These findings are in agreement with those of another group, who tracked the kinetics of a synchronized wave of thymocyte development in vivo and used mathematical modeling to estimate the extent of thymocyte death versus maturation at each stage of development (40). Interestingly, the greatest amount of negative selection among class I-restricted thymocytes was shown to occur within a subset of DP thymocytes in the first 12–48 hours of positive selection, a timeframe that corresponds with the kinetics that we have observed for chemokine receptor switching and medullary relocalization of positively selected class I-restricted thymocytes in situ (16). As previously mentioned, many of the specialized features that promote negative selection within the medulla are particularly salient at the corticomedullary junction. Thus, it makes sense that the highest levels of deletion are detected within the population of thymocytes expected to be migrating through this region.
Spatial and temporal aspects of agonist selection in the thymus
While strong signals through the TCR can lead to negative selection, under certain conditions these signals can also lead to the development of non-conventional T cell lineages with regulatory functions, a process termed agonist selection (41). There is evidence of overlap among the TCR repertoire of several agonist-selected lineages and thymocytes that undergo negative selection, indicating that the strength of the TCR signal is not the sole factor that dictates thymocyte fate (39, 42, 43). Rather factors such as the timing of phagocytosis (10), access to cytokines, and patterns of thymocyte motility, likely also help to determine whether thymocytes die or undergo agonist selection following strong antigen encounter.
As discussed previously, positive and negative selection are correlated with distinct patterns of thymocyte motility and TCR signaling. The motility and signaling patterns of thymocytes undergoing agonist selection have not yet been characterized, but it is tempting to speculate that these patterns might be distinct from those of thymocytes undergoing negative selection, and could play a role in promoting agonist selection over negative selection. A previous study, in which we characterized the behavior of TCR transgenic thymocytes in the presence of an Aire-dependent cognate antigen in the medulla at steady state, could provide some intriguing hints. In the presence of cognate antigen, thymocytes exhibited slightly lower motility compared to medullary thymocytes expressing positive selecting TCR, and exhibited a highly confined pattern of migration (44). These patterns are in contrast to the thymocyte arrest that we have observed in synchronized models of negative selection. It is tempting to speculate that the patterns of confined migration that we observed might be characteristic of thymocytes undergoing agonist selection. Here, we will discuss recent findings regarding the spatial and temporal aspects of agonist selection in two agonist selected lineages that can arise from thymocytes with conventional MHC specificities: Tregs, and CD8αα IELs.
Thymus-derived regulatory T cells (Tregs)
A well-studied example of agonist selection is the thymic development of regulatory T cells (called Tregs), a population of CD4 T cells that express the transcription factor FoxP3, and play a key role in suppressing inappropriate immune responses. Several studies have outlined a two-step process for Treg development. First, strong TCR signaling induces the upregulation of the high-affinity α-chain of the interleukin 2 (IL-2) receptor (CD25), generating a population of CD25hi FoxP3- thymocytes. Although this population is enriched for thymocytes with the potential to develop into CD25+FoxP3+ Tregs, further commitment to the Treg lineage requires IL-2 induced signaling though the signal transducer STAT5, which results in the expression of FoxP3 (45, 46). Although these two events can occur sequentially, we have recently demonstrated that the spatiotemporal linkage of these two signals results in greatly enhanced Treg development in situ (15). We also provided evidence that DCs within the thymus are a potent source of IL-2 for Treg development (15). Thus, although many types of APCs have been shown to effectively support Treg development, our study suggests that DCs may be particularly efficient at doing so in that they can deliver TCR signals while simultaneously providing a local source of IL-2. Interestingly, existing thymic Tregs limited new Treg development by competing for limiting supplies of IL2. The fate of the Treg progenitors that fail to compete effectively for IL2 is unclear, but these may go on to become conventional auto-reactive T cells, which are held in check by Tregs with related specificities, a strategy that has been termed the “buddy system” (43).
Our data is in line with the view that the majority of thymic Treg develop from CD4SP thymocytes in the medulla (46–48). DC are found at a high density in the medulla, and also express the CD28 ligands B7.1 and B7.2, which should also contribute to their ability to induce Treg development. Furthermore, mTECs can serve as APCs for Aire-dependent antigens, and also express high levels of B7, as well as IL-15 (49), a cytokine that plays a redundant role with IL2 to promote Treg development. Thus, the medullary microenvironment is enriched in cytokines and co-stimulatory molecules that support Treg development.
On the other hand, interfering with cortex to medulla migration by pharmacologic inhibition or genetic deficiency of CCR7 does not significantly reduce the population of CD4 SP FoxP3+ thymocytes, which accumulate in the cortex under these conditions (22, 50). However, the repertoire of the Tregs that develop under these conditions is likely altered and not sufficiently protective, owing to the lack of Tregs specific for Aire-dependent tissue-restricted antigens. Indeed, a paucity of tissue-specific Tregs likely contributes to the organ-specific autoimmunity observed in CCR7-deficient mice (37). Thus, although not directly shown, CCR7 is likely critical for the development of Tregs specific to tissue-specific, but not ubiquitous, self-antigens, in parallel to the previously described requirement for CCR7 in the negative selection of thymocytes to tissue-restricted, but not ubiquitous, self-antigens (37, 38).
As discussed in the previous section, class II-restricted thymocytes are competent to undergo negative selection within a given “window” of development, which closes at the mature CD4 SP stage. Some evidence suggests that a similar window exists during which thymocytes are competent to develop into Tregs. Upon sorting populations of differing maturity within the CD4 SP thymocyte population, Wirnsberger et al demonstrated that immature CD4 SP thymocytes exhibit an enhanced propensity to upregulate FoxP3 in response to TCR stimulation in vitro and in vivo as compared to mature CD4 SP thymocytes (47). Thus, like the ability to undergo negative selection, the ability of CD4 SP thymocytes to develop into Tregs seems to decrease as thymocytes mature. However, in contrast to the ability to undergo negative selection, mature CD4 SP thymocytes do not entirely lose the ability to develop into Tregs. The fact that the window for Treg development appears to extend further than that for negative selection could create a “backup mechanism” to ensure that highly autoreactive thymocytes are excluded from the conventional T cell repertoire despite resistance to negative selection at the later stages of thymic development. Interestingly, the correlation between thymocyte maturation and propensity to develop into Tregs continues during the extrathymic stages of maturation, as recent thymic emigrants show an enhanced ability to develop into Tregs in vivo as compared to fully mature CD4 T cells (51).
TCRab CD8aa intraepithelial lymphocytes
The precursors of αβTCR CD8αα IELs develop in the thymus in response to strong TCR signals, and are therefore also considered an agonist selected lineage (41, 42, 52). Following development in the thymus, these T cells home to the intestinal epithelium, where their exact role(s) remain enigmatic (53). However, CD8αα IELs have been shown to play a protective role in several models of colitis, suggesting that these cells act to promote immune homeostasis and tolerance in the gut (54). In contrast to Tregs, which develop almost exclusively from class II-restricted thymocytes, thymocytes with a wide variety of MHC specificities can give rise to CD8αα IELs. Several groups have recently cloned TCRs from endogenous CD8αα IELs, and identified clones restricted to non-classical MHC molecules, as well as classical MHC I and MHC II molecules (42, 52). Furthermore, it has been demonstrated that both class I and class II-restricted TCR transgenic thymocytes can give rise to CD8αα IELs in the presence of cognate antigen in vivo (55).
The stage(s) during which thymocytes are competent to undergo agonist selection into the CD8αα IEL lineage has been difficult to assess, due to a lack of unambiguous markers for the CD8αα IEL precursors. CD8αα IEL precursors are found within a NK1.1- CD5+ population with low expression of CD4 and CD8 (42, 52). However this population also likely includes conventional αβTCR thymocytes undergoing negative selection Furthermore, given that cells within this population have downregulated both CD4 and CD8, it is impossible to determine what the cell surface phenotype of the thymocyte was prior to its falling within this population. Given the broad range of specificities of CD8αα IELs, it is likely that multiple pathways for CD8αα IEL development exist (Figure 4B).
Nonetheless, several studies have demonstrated that exposure of DP thymocytes to strong agonists in vitro causes these thymocytes to adopt characteristics of CD8αα IELs, indicating that thymocytes might be competent to develop into CD8αα IELs as early as the DP stage (54, 56). Furthermore, one study demonstrated that endogenous superantigen-reactive TCRs were absent from the conventional CD4 and CD8 SP populations, but were present within the DP population, suggesting that the majority of commitment to the CD8αα IEL lineage in vivo occurs at the DP stage (33). Another study suggests that specification of the CD8αα IEL lineage might occur even earlier, among CD4 CD8 double negative thymocytes receiving pre-TCR signals, although continued development required agonist TCR signals (57). On the other hand, class I-restricted thymocytes can give rise to CD8αα IELs even when expression of the cognate antigen is Aire-dependent and therefore restricted to the medulla (55). Thus, class I-restricted thymocytes likely remain competent to undergo agonist selection into the CD8αα IEL lineage at least until a late DP stage at which they would be exposed to medullary antigens (Figure 4B).
Currently, little is understood regarding the cellular and molecular factors that promote CD8αα IEL development. Thus, whether particular thymic microenvironment(s) are particularly effective for driving CD8αα IEL development remains unknown. As a lack of costimulation has been shown to promote CD8αα IEL development, CD8αα IEL development could be most efficient in regions with low expression of costimulatory ligands, namely, the cortex (33). On the other hand, several studies suggest that IL-15 enhances CD8αα IEL development, and IL-15 is detected primarily within the medulla (49). Although costimulatory ligands are more prevalent in the medulla, the higher levels of IL-15 could provide pro-survival signals that serve to limit the extent of negative selection, allowing for CD8αα IEL development in response to medullary antigens. Further studies are needed to delineate the developmental stage(s) at which CD8ααIEL development occurs, and to pinpoint the cell types and microenvironments that support this type of agonist selection.
Functional tuning of TCR responsiveness during thymic selection
How do signals though a single receptor, the αβTCR, lead to so many distinct developmental outcomes? In answering this question, the popular affinity model for thymocyte selection, which dictates that thymocyte fate is determined by the affinity of the TCR for the self peptide-MHC complexes encountered in the thymus, is often invoked. While this model provides a useful starting framework, it does not encompass the dynamic changes in how thymocytes respond to TCR stimulation as they mature. For example, preselection DP are highly sensitive to in vitro stimulation with low potency ligands, and this is thought to allow for positive selection of thymocytes whose TCRs have very low affinity for self-peptide MHC complexes (58–60). As thymocytes mature, they lose the ability to respond to low potency ligands, which may prevent thymocytes from being activated by self-ligands when they encounter them in the periphery. A number of reports have identified the molecular players that contribute to unique ability of preselection thymocytes to detect low potency ligands, and to down-modulate their sensitivity in response to positive selection. These include a voltage gated calcium channel that increases the sensitivity of DP thymocytes to low potency ligands (61), CD5: a surface molecule that is induced by positive selection and negatively regulates TCR signaling (62, 63), miR-181a: microRNA that regulates a family of phosphatases (64), and Themis: an adaptor protein that helps to recruit the tyrosine phosphatase SHP-1 to the TCR complex and prevent thymocytes from over-reacting to low potency ligands (65). Together, these molecules serve to render preselection DP thymocytes highly responsive to low potency (positive selecting) ligands, and also allow thymocytes to dynamically adjust their sensitivity as they mature.
While thymic maturation is associated with the loss of sensitivity to low potency ligands, paradoxically, positive selection and thymocyte maturation are also associated with increased TCR responses. It has been known for some time that surface TCR levels gradually increase during positive selection. Moreover, markers of TCR signaling increase during the late stages of CD8 positive selection, and this is dependent on a TCR-dependent increase in intracellular Zap70 levels throughout positive selection (30). Likewise, a decrease in the expression of the negative regulator CD5 during CD8 positive selection is expected to enhance TCR responsiveness over time.
Dynamic changes in thymocyte motility patterns may also impact their TCR responsiveness. As mentioned earlier, continuous thymocyte migration might be expected to oppose persistent TCR signals, since migration away from sessile peptide-MHC bearing cells would disrupt ongoing TCR signals. In this regard, it is interesting to note that preselection thymocytes migrate in the cortex relatively slowly (around 5 micron/minute), whereas post-selection medullary thymocytes migrate at about twice the speed (10–12 microns/minute) (44). We recently performed a detailed time course analysis of the dynamic behavior and signaling of class I specific (OT1) thymocytes throughout the process of positive selection, revealing a gradual increase in thymocyte speed over the first 24 hours of positive selection (16). These changes were accompanied by a gradual increase in resting intracellular calcium and progressively briefer (2–3 minute) transient signaling events (Figure 2D). It is tempting to speculate that these changes in migratory behavior may contribute to the dynamic changes in TCR responsiveness that accompany positive selection.
Clearly, the change in TCR responsiveness during thymic development cannot be understood solely in terms of increased or decreased TCR sensitivity. Rather, the differences must be understood in relation to the nature of the ligand (e.g. low versus high potency) and the nature of the response (e.g. functional tuning and survival versus negative selection). For example, preselection DP thymocytes may be better equipped to detect low potency ligands than more mature thymocytes, but they respond in a blunted manner that promotes functional tuning rather than negative selection. Moreover, preselection DP thymocytes are highly susceptible to negative selection upon encountering high potency ligands, whereas more mature stages of thymic development are relatively resistant to cell death, and may undergo tuning or agonist selection instead of negative selection. Future studies are needed to further dissect the responses of thymocytes at different stages of development to high and low potency ligands. Ideally, these studies will examine thymocytes within the thymic environment, in order to encompass the impact of cell motility, as well as the specialized APC populations that thymocytes encounter at different stages of their development.
Concluding Remarks
Throughout this review, we have highlighted some of the many open questions and areas where future investigation is needed. What is the temporal pattern of positive selection of CD4 T cells, and how does that differ between positive selection of CD8 T cells? Do differences in the temporal pattern of signaling upon class I versus class II MHC recognition contribute to CD4 versus CD8 lineage commitment? How precisely does the TCR sensitivity and functional response of the thymocyte change during over the course of T cell maturation within 3D thymic environments? Does the frequency/duration of signaling events determine whether TCR signals lead result in positive versus negative selection, or negative versus agonist selection? With recent advances in our ability to synchronize, observe and manipulate T cell selection, the answers to some of these questions may not be far off.
Acknowledgments
Thymus research in the Robey lab is funded by NIH AI064227.
Footnotes
The authors declare no conflict of interest.
References
- 1.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Love PE, Bhandoola A. Signal integration and crosstalk during thymocyte migration and emigration. Nat Rev Immunol. 2011;11:469–477. doi: 10.1038/nri2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladi E, Yin X, Chtanova T, Robey EA. Thymic microenvironments for T cell differentiation and selection. Nat Immunol. 2006;7:338–343. doi: 10.1038/ni1323. [DOI] [PubMed] [Google Scholar]
- 4.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 5.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 6.Hogquist KA, Xing Y, Hsu F-C, Shapiro VS. T Cell Adolescence: Maturation Events Beyond Positive Selection. The Journal of Immunology. 2015;195:1351–1357. doi: 10.4049/jimmunol.1501050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bousso P, Bhakta NR, Lewis RS, Robey E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science. 2002;296:1876–1880. doi: 10.1126/science.1070945. [DOI] [PubMed] [Google Scholar]
- 8.Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3:e160. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 10.Dzhagalov IL, Chen KG, Herzmark P, Robey EA. Elimination of self-reactive T cells in the thymus: a timeline for negative selection. PLoS Biol. 2013;11:e1001566. doi: 10.1371/journal.pbio.1001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrlich LIR, Oh DY, Weissman IL, Lewis RS. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity. 2009;31:986–998. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzhagalov IL, Melichar HJ, Ross JO, Herzmark P, Robey EA. Two-photon imaging of the immune system. Curr Protoc Cytom. 2012;Chapter 12(Unit12.26) doi: 10.1002/0471142956.cy1226s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halkias J, et al. Opposing chemokine gradients control human thymocyte migration in situ. J Clin Invest. 2013;123:2131–2142. doi: 10.1172/JCI67175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melichar HJ, Ross JO, Herzmark P, Hogquist KA, Robey EA. Distinct temporal patterns of T cell receptor signaling during positive versus negative selection in situ. Science Signaling. 2013;6:ra92. doi: 10.1126/scisignal.2004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol. 2015;16:635–641. doi: 10.1038/ni.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross JO, Melichar HJ, Au-Yeung BB, Herzmark P, Weiss A, Robey EA. Distinct phases in the positive selection of CD8+ T cells distinguished by intrathymic migration and T-cell receptor signaling patterns. Proceedings of the National Academy of Sciences. 2014;111:E2550–8. doi: 10.1073/pnas.1408482111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 18.Campbell JJ, Pan J, Butcher EC. Cutting edge: developmental switches in chemokine responses during T cell maturation. J Immunol. 1999;163:2353–2357. [PubMed] [Google Scholar]
- 19.Ueno T, et al. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200:493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith AV, Fallahi M, Nakase H, Gosink M, Young B, Petrie HT. Spatial mapping of thymic stromal microenvironments reveals unique features influencing T lymphoid differentiation. Immunity. 2009;31:999–1009. doi: 10.1016/j.immuni.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwan J, Killeen N. CCR7 directs the migration of thymocytes into the thymic medulla. J Immunol. 2004;172:3999–4007. doi: 10.4049/jimmunol.172.7.3999. [DOI] [PubMed] [Google Scholar]
- 22.Cowan JE, et al. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. Journal of Experimental Medicine. 2013 doi: 10.1084/jem.20122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–4527. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 24.Trampont PC, et al. CXCR4 acts as a costimulator during thymic beta-selection. Vol. 11. Nature Publishing Group; 2010. pp. 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janas ML, Varano G, Gudmundsson K, Noda M, Nagasawa T, Turner M. Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4. Journal of Experimental Medicine. 2010;207:247–261. doi: 10.1084/jem.20091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin X, Ladi E, Chan SW, Li O, Killeen N, Kappes DJ, Robey EA. CCR7 expression in developing thymocytes is linked to the CD4 versus CD8 lineage decision. J Immunol. 2007;179:7358–7364. doi: 10.4049/jimmunol.179.11.7358. [DOI] [PubMed] [Google Scholar]
- 27.Saini M, Sinclair C, Marshall D, Tolaini M, Sakaguchi S, Seddon B. Regulation of Zap70 Expression During Thymocyte Development Enables Temporal Separation of CD4 and CD8 Repertoire Selection at Different Signaling Thresholds. Science Signaling. 2010;3:ra23. doi: 10.1126/scisignal.2000702. [DOI] [PubMed] [Google Scholar]
- 28.Lucas B, Vasseur F, Penit C. Normal sequence of phenotypic transitions in one cohort of 5-bromo-2′-deoxyuridine-pulse-labeled thymocytes. Correlation with T cell receptor expression. J Immunol. 1993;151:4574–4582. [PubMed] [Google Scholar]
- 29.Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-κB. Journal of Experimental Medicine. 2013;210:269–285. doi: 10.1084/jem.20121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinclair C, Ono M, Seddon B. A Zap70-dependent feedback circuit is essential for efficient selection of CD4 lineage thymocytes. Immunol Cell Biol. 2015 doi: 10.1038/icb.2014.107. [DOI] [PubMed] [Google Scholar]
- 31.Au-Yeung BB, et al. Quantitative and temporal requirements revealed for Zap70 catalytic activity during T cell development. Nat Immunol. 2014;15:687–694. doi: 10.1038/ni.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahama Y, Takada K, Murata S, Tanaka K. β5t-containing thymoproteasome: specific expression in thymic cortical epithelial cells and role in positive selection of CD8+ T cells. Curr Opin Immunol. 2012;24:92–98. doi: 10.1016/j.coi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Pobezinsky LA, et al. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nature Publishing Group; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185:263–271. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. Journal of Experimental Medicine. 2008;205:2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ladi E, et al. Thymocyte-dendritic cell interactions near sources of CCR7 ligands in the thymic cortex. J Immunol. 2008;181:7014–7023. doi: 10.4049/jimmunol.181.10.7014. [DOI] [PubMed] [Google Scholar]
- 37.Kurobe H, et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24:165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Nitta T, Nitta S, Lei Y, Lipp M, Takahama Y. CCR7-mediated migration of developing thymocytes to the medulla is essential for negative selection to tissue-restricted antigens. Proceedings of the National Academy of Sciences. 2009;106:17129–17133. doi: 10.1073/pnas.0906956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stritesky GL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proceedings of the National Academy of Sciences. 2013;110:4679–4684. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinclair C, Bains I, Yates AJ, Seddon B. Asymmetric thymocyte death underlies the CD4:CD8 T-cell ratio in the adaptive immune system. Proceedings of the National Academy of Sciences. 2013;110:E2905–14. doi: 10.1073/pnas.1304859110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald BD, Bunker JJ, Ishizuka IE, Jabri B, Bendelac A. Elevated T cell receptor signaling identifies a thymic precursor to the TCRαβ (+)CD4(−)CD8β (−) intraepithelial lymphocyte lineage. Immunity. 2014;41:219–229. doi: 10.1016/j.immuni.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh C-S, Lee H-M, Lio C-WJ. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 44.Le Borgne M, Ladi E, Dzhagalov I, Herzmark P, Liao YF, Chakraborty AK, Robey EA. The impact of negative selection on thymocyte migration in the medulla. Nat Immunol. 2009;10:823–830. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burchill MA, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lio C-WJ, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirnsberger G, Mair F, Klein L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proceedings of the National Academy of Sciences. 2009;106:10278–10283. doi: 10.1073/pnas.0901877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 49.Cui G, et al. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proceedings of the National Academy of Sciences. 2014;111:1915–1920. doi: 10.1073/pnas.1318281111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, Rudensky AY. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proceedings of the National Academy of Sciences. 2008;105:11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paiva RS, Lino AC, Bergman M-L, Caramalho I, Sousa AE, Zelenay S, Demengeot J. Recent thymic emigrants are the preferential precursors of regulatory T cells differentiated in the periphery. Proceedings of the National Academy of Sciences. 2013;110:6494–6499. doi: 10.1073/pnas.1221955110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayans S, et al. αβT cell receptors expressed by CD4(−)CD8αβ(−) intraepithelial T cells drive their fate into a unique lineage with unusual MHC reactivities. Immunity. 2014;41:207–218. doi: 10.1016/j.immuni.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klose CSN, et al. The transcription factor T-bet is induced by IL-15 and thymic agonist selection and controls CD8αα (+) intraepithelial lymphocyte development. Immunity. 2014;41:230–243. doi: 10.1016/j.immuni.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 55.Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, Cheroutre H. Precursors of functional MHC class I- or class II-restricted CD8alphaalpha(+) T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16:355–364. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- 56.Yamagata T, Mathis D, Benoist C. Self-reactivity in thymic double-positive cells commits cells to a CD8 alpha alpha lineage with characteristics of innate immune cells. Nat Immunol. 2004;5:597–605. doi: 10.1038/ni1070. [DOI] [PubMed] [Google Scholar]
- 57.Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA, Cheroutre H. Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity. 2006;25:631–641. doi: 10.1016/j.immuni.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 58.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucas B, Stefanova I, Yasutomo K, Dautigny N, Germain RN. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–376. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- 60.Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nature Publishing Group. 2012;13:121–128. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- 61.Lo W-L, Donermeyer DL, Allen PM. A voltage-gated sodium channel is essential for the positive selection of CD4(+) T cells. Nature Publishing Group. 2012;13:880–887. doi: 10.1038/ni.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azzam HS, et al. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166:5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 64.Li Q-J, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Fu G, et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature. 2013 doi: 10.1038/nature12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sinclair C, Seddon B. Overlapping and asymmetric functions of TCR signaling during thymic selection of CD4 and CD8 lineages. The Journal of Immunology. 2014;192:5151–5159. doi: 10.4049/jimmunol.1303085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson G, Takahama Y. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol. 2012;33:256–263. doi: 10.1016/j.it.2012.03.005. [DOI] [PubMed] [Google Scholar]