Abstract
Individuals with post-traumatic stress disorder (PTSD) avoid trauma-related stimuli and exhibit blunted hypothalamic-pituitary-adrenal (HPA) axis activation at the time of stress. Our laboratory has established a rodent model of stress that mimics the avoidance symptom cluster of PTSD. Rats are classified as ‘Avoiders’ or ‘Non-Avoiders’ post-stress based on avoidance of a predator-odor paired context. Previously, we demonstrated that Avoiders exhibit an attenuated HPA stress response to predator odor. We hypothesized that corticosterone administration prior to stress would reduce magnitude and incidence of avoidance of a stress-paired context. Furthermore, we predicted that Avoiders would exhibit altered expression of GR signaling machinery elements, such as steroid receptor co-activator (SRC)-1. Male Wistar rats (n = 16) were pre-treated with corticosterone (25 mg/kg) or saline and exposed to predator odor stress paired with a context, and tested for avoidance 24 h later, A second group of corticosterone-naïve rats (n = 24) were stressed (or not stressed), indexed for avoidance 24 h later, and killed 48 h post-odor exposure for analysis of phosphorylated GR, FKBP51, and SRC-1 levels in the paraventricular nucleus (PVN), central amygdala (CeA) and ventral hippocampus (VH), all brain sites that express high quantities of GRs and regulate HPA function. Rats pre-treated with corticosterone exhibited lower magnitude and incidence of avoidance. Predator odor exposure also reduced SRC-1 expression in the PVN and CeA of Avoiders, and increased SRC-1 expression in the VH of Avoiders. SRC-1 expression in PVN, CeA, and VH was predicted by prior avoidance behavior. These results suggest that blunted HPA stress response may contribute to stress-induced neuroadaptations in central SRC-1 levels and behavioral dysfunction in Avoider rats.
Keywords: Central amygdala, paraventricular nucleus, predator odor, ventral hippocampus
Introduction
Post-traumatic stress disorder (PTSD) manifests in a subset of individuals exposed to a traumatic stressor (1). A PTSD diagnosis is defined by presentation of three symptom clusters including intrusive recollection, hyper-arousal, and avoidance of trauma-related stimuli. Important for the present study, not all individuals that are exposed to trauma develop PTSD, a criterion for animal models of this disorder (2).
Stress-related disorders are often accompanied by dysregulation of the hypothalamic-pituitary-adrenal (HPA) stress axis, both at the time of stress and after the development of PTSD (3). While the paraventricular nucleus (PVN) is essential for appropriate initiation and termination of the stress response, the limbic system can also influence the HPA axis (4–6). Limbic forebrain structures such as the central amygdala (CeA) and ventral hippocampus (VH) regulate HPA responses to emotional stress (6). Like the PVN, the CeA and VH are rich in glucocorticoid receptors (GR) (5, 6). Extreme hyper- or hypo-reactivity of the HPA axis can be maladaptive and can contribute to the development of psychiatric disorders that include depression and PTSD (7, 8). Individuals with PTSD exhibit blunted HPA activity immediately after the traumatic event (9, 10), blunted basal 24 h urinary cortisol concentrations collected at 9 am and enhanced negative feedback post-stress (11). Previously, we reported that rats that exhibit persistent (>6 weeks) avoidance of a predator odor-paired context exhibit significantly attenuated ACTH and corticosterone levels immediately following traumatic stress and that low corticosterone at the time of stress is highly predictive of later avoidance (12).
Full GR activation at the transcriptional level requires numerous accessory proteins. For example, the FK506-binding protein-51 (FKBP51; fkbp5 gene) regulates HPA negative feedback by preventing nuclear translocation of the GR complex (13). Humans with PTSD exhibit attenuated peripheral fkbp5 gene expression, suggesting that FKBP51 may represent a promising target for treatment of traumatic stress disorders (13–15). Additionally, transcriptional co-regulators such as steroid receptor co-activator-1 (SRC-1) are involved in gating the transcriptional activity of nuclear receptors. SRC-1 is highly expressed in the brain and regulates GR-mediated corticotropin releasing factor (CRF) gene transcription (16). Finally, GR phosphorylation at various sites regulates GR trafficking and transcriptional activity. GR phosphorylation is initiated by glucocorticoid binding to the receptor, and is also modulated by the activity of various kinases (17, 18): specifically, GR phosphorylation on serine 232 facilitates GR nuclear trafficking and transcriptional activity (18, 19).
The purpose of these studies was two-fold. Because corticosterone/cortisol levels are blunted in Avoider rats and PTSD humans shortly after stress, we first aimed to examine the effect of corticosterone treatment on avoidance of a context paired with predator odor. We hypothesized that administration of corticosterone prior to stress would increase HPA activity at the moment of the stressor and decrease the magnitude and incidence of avoidance of a predator odor-paired chamber. We also wanted to measure the expression of GR elements in the brains of Avoider rats because altered corticosterone levels may affect HPA feedback processes. We hypothesized that predator odor stress would alter expression and/or phosphorylation of GR machinery in a brain region-specific manner in Avoiders relative to Non-Avoiders and unstressed Controls.
Methods
Animals
Adult male Wistar rats (Charles River, Kingston, NY) (250–275 g) were pair-housed and fed a standard rat diet (Purina Rat Chow, Ralston Purina, St. Louis, MO) ad libitum. Rats were exposed to a 12h light/12h dark cycle (lights off at 8am). Rats were handled on a daily basis and given one week to acclimate to the colony room prior to initiation of experimental procedures. A total of 40 animals were used in these studies (Experiment 1 n = 8 vehicle-treated n = 8 corticosterone-treated; Experiment 2: n = 6 controls, n = 8 Non-avoiders, n = 12 Avoiders). Animal procedures were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center (LSUHSC) and were in accordance with the National Institute of Health guidelines.
Conditioned Place Aversion
Rats were exposed to predator odor and indexed based on avoidance of the predator-odor paired context as previously described by our laboratory (20). A conditioned place aversion (CPA) paradigm was utilized in which predator odor was paired with a distinct environment. The conditioning apparatus consisted of two boxes (36 cm length × 30 cm width × 34 cm height) separated by a small triangle. One conditioning chamber had circle visual cues on the walls and circle tactile cues on the floor. The other chamber had striped visual cues on the walls and grid tactile cues on the floor. Guillotine doors between chambers confine animals to individual chambers on neutral and odor conditioning days, but are removed to allow free exploration of both chambers during pre- and post-conditioning tests. Briefly, rats were subjected to a 5-minute pre-test to explore two distinct chambers differing in visual and tactile cues. On the second day, rats were confined to one chamber (neutral environment) for 15 min. On the third day, rats were confined to a second chamber for 15 min with a bobcat (Lynx rufus; Maine Outdoor Solutions; Hermon, Maine) urine-soaked sponge placed under the floor of the chamber. On the fourth day, rats underwent a 5 min video-recorded post-test in which they were allowed to freely explore the two chambers. Following odor exposure, the chambers were cleaned with Quatricide and the room deodorized with Pure Ayre. Control rats were treated identically to odor-exposed rats but the sponges did not contain bobcat urine. Both rats in a single cage experienced the same treatment on the same day. Avoidance was calculated as a difference in time between the postconditioning time spent in odor-paired context and pre-conditioning time spent in odor-paired context. Rats were classified as ‘Avoiders’ if they displayed a >10 sec decrease in time spent in odor-paired chamber (20).
Drugs
In Experiment 1 (see below), 1 hour before predator odor exposure, rats were injected subcutaneously with either saline (NaCl: 0.9%) or corticosterone (25 mg/kg), a dose previously reported to reduce anxiety-like behavior following stress (21). Corticosterone acetate (Sigma Aldrich, St. Louis, MO) was prepared by sonication in 1% Tween 80 solution in normal saline and injected in a volume of 2 ml/kg.
Western Blot
We measured individual levels of total GR, phosphorylated GR (serine 232), FKBP51, and SRC-1 in brain regions involved in HPA regulation including the CeA (AP: −1.92, ML: 4.4, DV: 8.2), PVN (AP: −1.72, ML: 0.0, DV: 8.5), and VH (AP: −5.04, ML: −5.2, DV: 7.2). Rats were sacrificed under light isoflurane anesthesia followed by decapitation. Brains were quickly excised and flash frozen in cold isopentane. Regional brain tissue punches were obtained from frozen coronal brain slices (500μm) using 14–17 gauge punch needles guided by The Rat Brain Atlas of Paxinos and Watson (22). Brain punches were homogenized as previously described (Edwards et al., 2013) by sonication in lysis buffer (1% SDS solution containing sucrose, HEPES, EGTA, and EDTA) containing Protease Inhibitor Cocktail and Phosphatase Inhibitor Cocktails II and III (Sigma, St. Louis, MO). Following homogenization, samples were heated for 5 minutes at 100°C and protein content was measured using the Lowry method (Bio-Rad, Hercules, CA). Samples were stored in 10 ug aliquots at −80 °C until Western analysis. Protein samples (10 ug) were subjected to SDS-polyacrylamide gel electrophoresis on 8% acrylamide gels by using a Tris/Glycine/SDS buffer system (Bio-Rad), followed by electrophoretic protein transfer to polyvinylidene difluoride (PVDF) membranes (GE Healthcare, Piscataway, NJ, USA). Membranes were blocked for 1 hour in 5% non-fat milk at room temperature. The following day, membranes were incubated overnight at 4 °C in primary antibody (see Table 1 for primary antibody concentrations) in 2.5% non-fat milk recognizing the glucocorticoid receptor phosphorylated at rat serine 232 (Antibody #4161; Cell Signaling, Danvers, MA, USA), total GR (Antibody #: PA1-511A ThermoFisher, Waltham, MA) FKBP51 (Antibody #: 46002; AbCam; Cambridge, MA) or SRC-1 (SRC-1 (128E7) Rabbit mAb #2191; Cell Signaling Danvers, MA, USA) (23–25). Membranes were washed and labeled with species-specific, peroxidase-conjugated secondary antibody (1:10K; Bio-Rad) for 1 h at room temperature. Blots were then treated with chemilumenscent substrate (SuperSignal West Pico; Thermo Scientific, Rockford, IL, USA) for 30 seconds, and exposed to autoradiography film (Denville Scientific Inc., Metuchen, NJ) for optimum amount of time in a Hypercassette (Amersham Biosciences, Buckinghamshire, England). The exposed autoradiography film was developed using Konica Medical Film Processor, model: SRX-101A. Following chemiluminescence detection (SuperSignal West Pico; Thermo Scientific, Rockford, IL, USA), blots were stripped for 20min at room temperature (Restore; Thermo Scientific) and were re-probed with tubulin (1:100K; Santa Cruz Biotechnology; Santa Cruz, CA) for normalization purposes. Developed films were scanned as tiff files and the protein bands were quantified using Image J.
Table 1.
Primary antibody concentrations for Western Blot analysis of phosphorylated glucocorticoid receptor (P-GR), total glucocorticoid receptor (GR), FKBP51, and steroid receptor co-activator (SRC)-1 in the paraventricular nucleus (PVN), central amygdala (CeA) and ventral hippocampus (VH).
| PVN | CeA | VH | |
|---|---|---|---|
| pGR (Ser 232) | 1:500 | 1:1000 | 1:1000 |
| GR | 1:1000 | 1:1000 | 1:1000 |
| FKBP51 | 1:20,000 | 1:10,000 | 1:20,000 |
| SRC-1 | 1:1000 | 1:1000 | 1:1000 |
Experimental Protocols
All rats were subjected to one week of handling procedures prior to initiating the experimental protocols. Rats were conscious and unrestrained for the duration of experiments. All experimental procedures were conducted at 9 am near the start of the dark cycle.
Experiment 1. Effect of corticosterone pre-treatment on avoidance
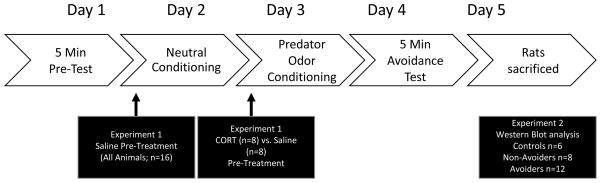
Previously, our laboratory has demonstrated that Avoider rats exhibit blunted levels of corticosterone following predator odor exposure (26). This study aimed to assess whether corticosterone administration prior to predator odor exposure would impact the magnitude and incidence of avoidance (Fig. 1). Rats were subjected to the CPA paradigm described above. On neutral conditioning day, all rats received a vehicle injection one hour prior to conditioning. On the day of predator odor exposure, rats were randomly assigned to “vehicle” (n=8) or “corticosterone” (n=8) groups. Subcutaneous injections of either vehicle or corticosterone were administered one hour prior to predator odor exposure. Rats were indexed for avoidance 24 hours post-stress and classified as ‘Avoiders’ or ‘Non-Avoiders’.
Figure 1.
Schematic of Experimental Design. Rats underwent the conditioned place aversion (CPA) paradigm to index for avoidance of predator odor-paired chamber. In Experiment 1, all rats received a subcutaneous vehicle injection prior to neutral conditioning. Rats were randomized to receive either vehicle or corticosterone pre-treatment prior to odor exposure. In Experiment 2, rats did not receive injections prior to conditioning and were sacrificed at 48 h post-stress for biochemical analysis.
Experiment 2. Effects of predator odor stress on levels of central glucocorticoid receptor (GR)-related proteins
The aim of this study was to characterize the effects of predator odor stress on changes in total GR, phosphorylated GR (pGR), FKBP51, and SRC-1 protein levels in the PVN, CeA, and VH (Fig. 1). Rats (n = 24) were subjected to the CPA paradigm described above. Rats were indexed for avoidance 24 hours post-stress and classified as ‘Avoiders’ or ‘Non-Avoiders’. Forty-eight hours post-stress rats were sacrificed under light isoflurane anesthesia by decapitation. The PVN, CeA, and VH were isolated from coronal brain slices (500μm) using 14–17 gauge punches for subsequent determination of protein levels via Western analysis. Brain punches were analyzed separately for each rat.
Statistical Analysis
Data are shown as mean ± SEM with the number of rats indicated in the figure legends. Data were analyzed using Sigma Stat statistical software. In Experiment 1, avoidance data in Avoiders and Non-Avoiders were analyzed with a t-test. Population distributions were analyzed by Chi-squared test. In Experiment 2, molecular data were analyzed by one-way ANOVA. Pearson’s correlations were used to determine relationships between behavioral and molecular outcomes. Post hoc analysis with Student Neuman–Keuls test was used when appropriate. In all experiments, statistical significance was set at p < 0.05.
RESULTS
Corticosterone pre-treatment decreases avoidance
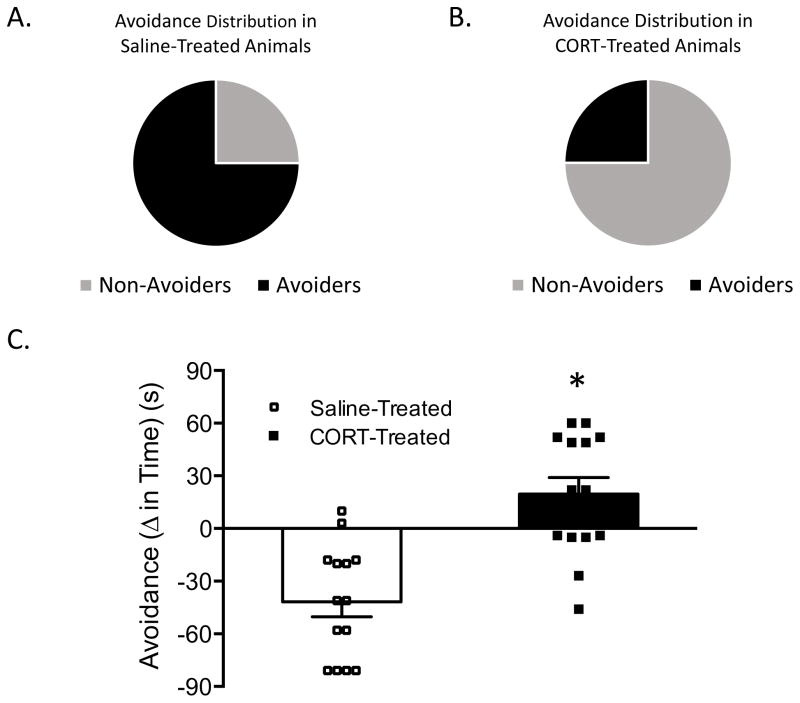
Rats were indexed for avoidance 24 h post-odor exposure. Figure 2 shows the distribution of Non-Avoiders and Avoiders following pretreatment with either saline (Figure 2A) or corticosterone (Figure 2B) 1 h prior to odor exposure. A lower percentage (25%) of rats pre-treated with corticosterone prior to stress were classified as Avoiders, X2 (1, N = 16) =4.00, p = 0.045 relative to saline-treated rats (75% Avoiders). Figure 2C shows that significantly fewer rats pre-treated with corticosterone exhibit significantly less avoidance of the predator odor-paired context when compared to saline-treated rats, t(26) = 4.837, p< 0.001, and that corticosterone-treated Avoiders exhibit less extreme avoidance scores than vehicle-treated Avoiders.
Figure 2.
A. Avoidance distribution in Saline-treated rats. Non-avoiders represented in grey and Avoiders represented in black. B. Avoidance distribution in corticosterone-treated rats. Non-avoiders represented in grey and Avoiders represented in black. C. Avoidance (Δ time preconditioning test to post-conditioning test time spent in predator odor-paired chamber) following pre-treatment with either saline (white bar; n=8) or corticosterone (black bar; n=8). Data are presented as mean ± SEM and were analyzed using a t-test. * indicates p < 0.05 vs. Saline-treated rats.
Predator odor stress decreases SRC-1 protein levels in the PVN
The goal of Experiment 2 was to examine the effect of predator odor stress on total GR, phosphorylated GR (P-GR), FKBP51, and SRC-1 levels in Non-Avoiders and Avoiders in brain regions that receive HPA feedback and regulate HPA axis activity.
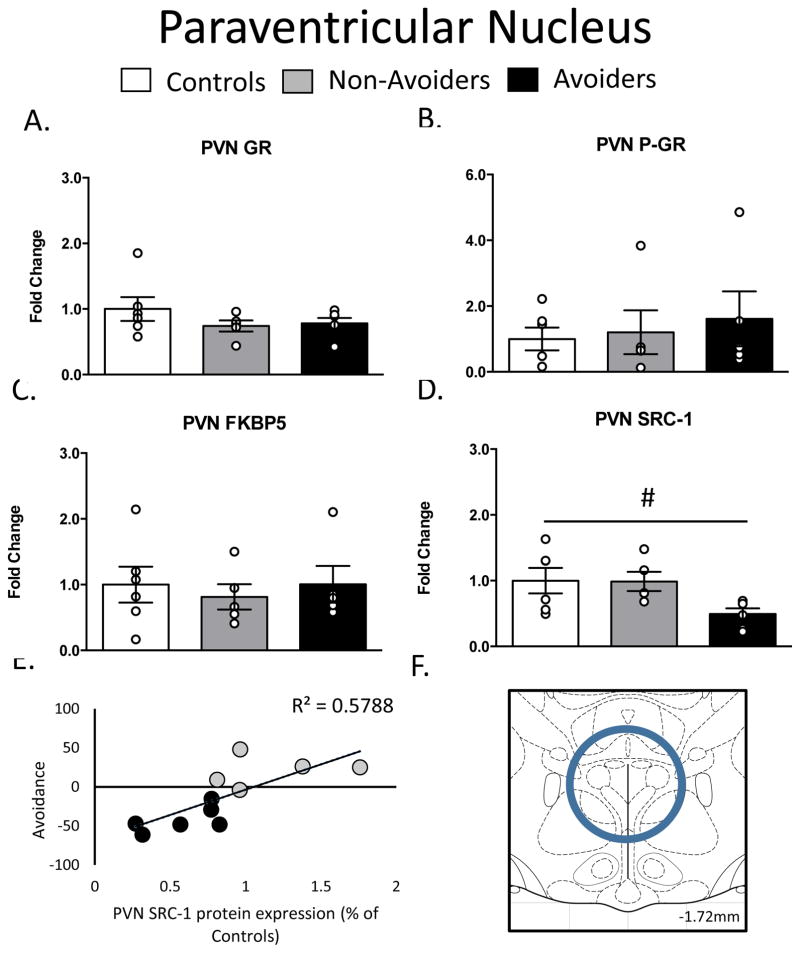
In the PVN, there were no differences in GR, pGR, or FKBP51 protein levels (Figure 3A-3C). As shown in Figure 3D, a one-way ANOVA yielded a significant effect of group (stress history), F(2, 16) = 3.593, p = 0.05, on SRC-1 content in the PVN. There was also a trend (p=0.06) toward a decrease in SRC-1 in Avoiders when compared to Non-Avoiders. This effect was more clearly resolved when we examined individual associations between PVN SRC-1 levels and avoidance of the predator odor stress-paired context. Figure 3E shows that PVN SRC-1 was strongly and negatively correlated with avoidance in all stressed rats (r2 = 0.58; p = 0.006).
Figure 3.
Changes in Glucocorticoid Receptor (GR) machinery in the Paraventricular Nucleus (PVN; AP: −1.72, ML: 0.0, DV: 8.5). A. Total GR (% of Controls), B. pGR (expressed as a ratio of pGR/GR), C. FK506 Binding Protein 51 (FKBP51) (% of Controls) and D. Steroid Receptor Co-Activator (SRC)-1 (% of Controls) measured 48 h post-odor exposure in the PVN of Controls (white bars; n=6), Non-Avoiders (grey bars; n=8) and Avoiders (black bars; n=7). E. Scatter plot for individual rats (Avoider, black dots and Non-Avoider, grey circles; Experiment 2) shows avoidance of predator-paired context versus PVN SRC-1 protein expression 48 h post-stress. Rats that exhibited high avoidance of the predator-paired context 24 h post-odor exposure had lower PVN SRC-1 expression 48 h post-stress. F. Schematic representation of PVN punch dissection. Data are presented as mean ± SEM and were analyzed using a one-way ANOVA. # indicates treatment effect.
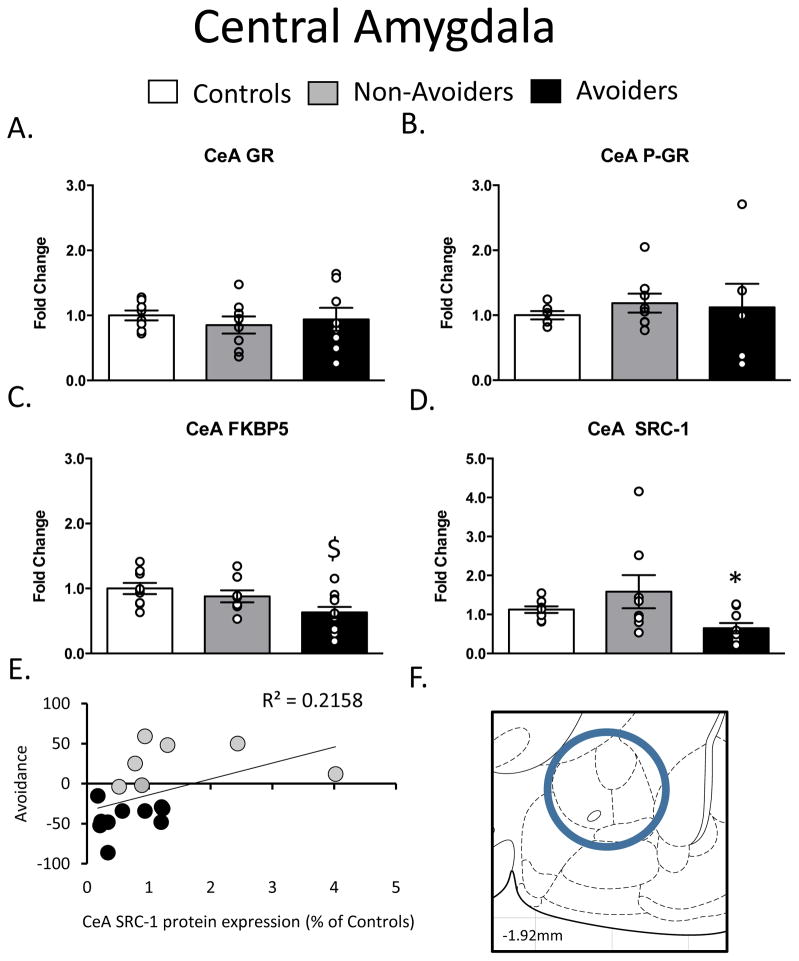
In the CeA, there were no differences in GR or pGR protein levels (Figure 4A–4B). As shown in Figure 4C, a one-way ANOVA yielded a significant effect of group (stress history), F(2, 27) = 5.425, p = 0.01, on FKBP51 protein levels in the CeA. Post-hoc analysis revealed a significant decrease in FKBP51 protein content in Avoiders when compared to control rats (p = 0.01). As shown in Figure 4D, a one-way ANOVA also yielded a significant effect of group (stress history), F(2, 23) = 4.378, p = 0.03, on SRC-1 protein levels in the CeA. Post hoc analysis revealed a significant decrease in SRC-1 protein in Avoiders when compared to Non-Avoiders (p = 0.02). In accordance with this group effect, Figure 4E shows that CeA SRC-1 content was trended to negatively correlated with avoidance in all stressed rats (r2 = 0.22; p = 0.06), an effect just short of statistical significance.
Figure 4.
Changes in Glucocorticoid Receptor (GR) machinery in the Central Amygdala (CeA; AP: −1.92, ML: 4.4, DV: 8.2). A. Total GR (% of Controls), B. pGR (expressed as a ratio of pGR/GR), C. FK506 Binding Protein 51 (FKBP51) (% of Controls) and D. Steroid Receptor Co-Activator (SRC)-1 (% of Controls) measured 48 h post-odor exposure in the CeA of Controls (white bars; n=6), Non-Avoiders (grey bars; n=8) and Avoiders (black bars; n=7). E. Scatter plot for individual rats (Avoider, black dots and Non-Avoider, grey circles; Experiment 2) shows change in preference for predator-paired context versus CeA SRC-1 protein expression 48 h post-stress. Rats that exhibited high avoidance of the predator-paired context 24 h post-odor exposure had lower CeA SRC-1 expression 48 h post-stress. F. Schematic representation of CeA punch dissection. Data are presented as mean ± SEM and analyzed using a one-way ANOVA. $ indicates p<0.05 versus Control rats, * indicates p<0.05 versus Non-Avoiders.
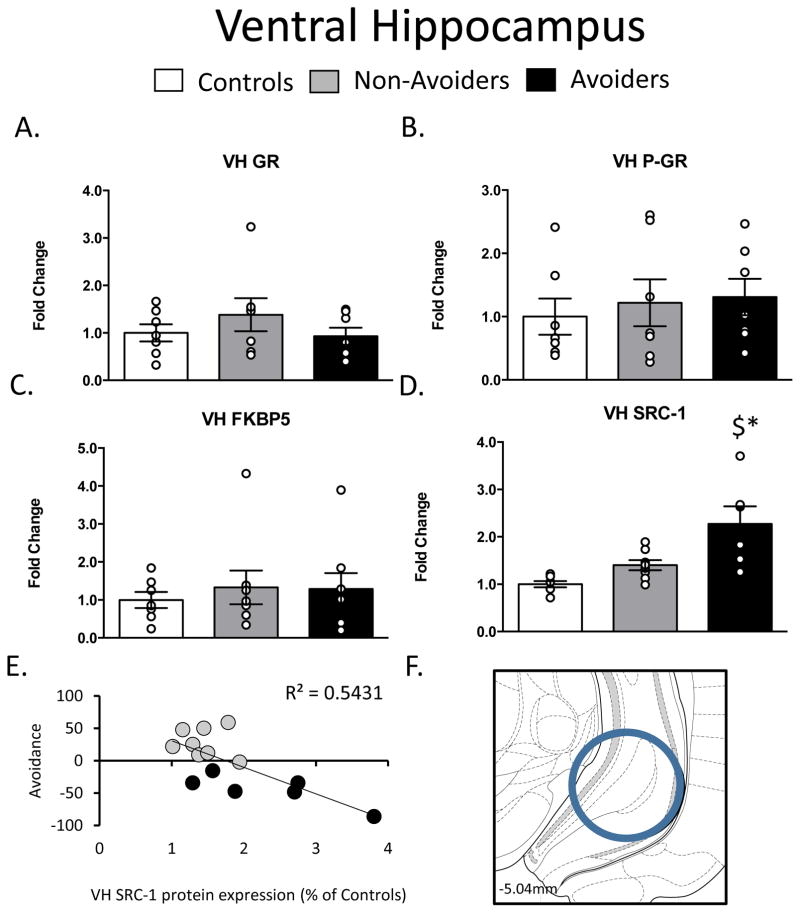
In the VH, there were no differences in GR, pGR, or FKBP51 protein across groups post-stress (Figure 5A–5C). As shown in Figure 5D, a one-way ANOVA yielded a significant effect of group (stress history), F(2, 19) = 7.502, p = 0.005, on SRC-1 protein levels in the VH. Post hoc analysis revealed a significant increase in SRC-1 protein in Avoiders when compared to Control rats (p = 0.004) and Non-Avoiders (p = 0.02).Figure 5E shows that VH SRC-1 content was strongly and positively correlated with avoidance in all stressed rats (r2 = 0.54; p = 0.002).
Figure 5.
Changes in Glucocorticoid Receptor (GR) machinery in the Ventral Hippocampus (VH AP: -5.04, ML: −5.2, DV: 7.2) A. Total GR (% of Controls), B. pGR (expressed as a ratio of pGR/GR), C. FK506 Binding Protein 51 (FKBP51) (% of Controls) and D. Steroid Receptor Co-Activator (SRC)-1 (% of Controls) measured 48 h post-odor exposure in the VH of Controls (white bars; n=6), Non-Avoiders (grey bars; n=8) and Avoiders (black bars; n=7). E. Scatter plot for individual rats (Avoider, black dots and Non-Avoider, grey circles; Experiment 2) shows change in preference for predator-paired context versus VH SRC-1 protein expression 48 h post-stress. Rats that exhibited high avoidance of the predator-paired context 24 h post-odor exposure had higher VH SRC-1 expression 48 h post-stress. F. Schematic representation of VH punch dissection. Data are presented as mean ± SEM and analyzed using a one-way ANOVA. $ indicates p<0.05 versus Control rats.
DISCUSSION
Here, we report that corticosterone administration prior to stress decreases the magnitude and incidence of avoidance of a predator odor-paired chamber. Furthermore, we report that predator odor stress differentially regulates GR machinery in Avoiders relative to Non-Avoiders and unstressed Controls. Previous findings from our laboratory indicate that Avoiders exhibit markedly attenuated circulating ACTH and corticosterone concentrations immediately following predator odor stress (12). Interestingly, blunted ACTH/corticosterone levels at the time of stress predict subsequent avoidance, suggesting that HPA dysregulation may play a role in the later emergence of stress-related symptoms. In the current study, pre-treatment with corticosterone decreased the percentage of rats that met the Avoider criterion and reduced the magnitude of avoidance in rats that continued to show avoidance. Because GR signaling is implicated in stress-related psychiatric disorders, we examined changes in phosphorylated and total GR levels, as well as alterations in other GR co-factors such as FKBP51 and SRC-1, which are important for nuclear translocation and subsequent activation of GR target genes. Our analysis focused on brain regions critical for glucocorticoid regulation of central and systemic stress signaling including the PVN, CeA, and VH. Of the targets investigated, we observed altered SRC-1 levels in the PVN, CeA, and VH of Avoider rats. These data suggest that attenuated SRC-1 activity in the PVN and CeA and augmented SRC-1 function in the VH may contribute to stress-related behaviors. This is especially interesting because SRC-1 regulates CRF gene expression
Prior studies report inconsistent cortisol levels in individuals with PTSD, but the general consensus is that PTSD humans exhibit blunted cortisol response to traumatic stress and lower basal levels following stress (9, 10, 27). Early studies by Mason et al. reported significantly lower 24 hour urinary cortisol levels measured at 9 am in veterans with PTSD relative to veterans without PTSD (28). This study was later confirmed by others examining cortisol changes during the diurnal cycle showing overall lower cortisol levels (during the afternoon (12–8 pm) in women with PTSD (29, 30). Previously, we reported that rats that exhibit persistent avoidance of a predator odor-paired chamber display lower ACTH and corticosterone levels immediately following stress.
Collectively, these data suggest that cortisol-based therapy prior to or following stress exposure may decrease susceptibility to the eventual development of PTSD. Clinical studies have shown that administration of low-dose cortisol following stress reduces symptoms associated with PTSD, including avoidance (31). Additional studies have reported that administration of glucocorticoids as part of a treatment for septic shock decreases the development of PTSD, in particular through decreasing traumatic recollections (11, 31–33). Here, we report that corticosterone pre-treatment prior to predator odor exposure decreases the percentage of rats classified as “Avoiders” and significantly reduces avoidance of the predator odor-paired chamber. Although corticosterone itself can be reinforcing (34) it was previously shown to be incapable of producing a place preference (35). These data suggest that low corticosterone levels in Avoiders post-stress reflect blunted HPA response to stress, although this does not preclude the possibility that negative feedback is also altered in Avoider rats. One potential mechanism for this blunted HPA stress response is altered balance of excitatory and inhibitory inputs to parvocellular neurons in the PVN, a focus of ongoing studies.
Recent evidence suggests that glucocorticoids affect memory consolidation and may be a factor in the decreased avoidance following corticosterone treatment. The effects of stress on memory consolidation largely depend on the timing of the stress exposure and/or glucocorticoid administration. Stress exposure prior to learning can result in both enhanced (36, 37) and impaired (38, 39) memory consolidation. Studies have shown that glucocorticoid administration within a short time frame after learning will facilitate memory formation (39). In our studies, pretreatment with corticosterone decreased avoidance behavior. It is possible that reduced avoidance reflects impaired memory following corticosterone treatment. In addition to timing of stress exposure, other factors such as the timing of glucocorticoid treatment, when testing occurs and how aversive the task is may contribute to the discrepancies among the findings. For example, studies have shown that post-training corticosterone treatment increases memory consolidation in a water-maze spatial task but impairs memory consolidation when the task becomes more aversive (40). Future studies will examine how glucocorticoids affect memory consolidation in Avoiders and Non-Avoiders using traditional fear conditioning procedures.
While the HPA axis is controlled primarily by the PVN, upstream limbic circuitry (e.g., amygdala and hippocampus) involved in emotional processing and memory formation can also influence the HPA stress response (5). For example, electrical stimulation of the amygdala increases circulating corticosterone concentrations, suggesting positive feed-forward regulation of the HPA axis (6, 41, 42). Conversely, the hippocampus is a classic negative feedback site that inhibits HPA axis activity (4, 6, 43): hippocampal lesions increase corticosterone levels following stress (43, 44). More specifically, the ventral hippocampus is the primary mediator of hippocampal inhibition of HPA axis activity (6, 43). Because the PVN, CeA, and VH each express high levels of glucocorticoid receptors (6), we examined predator odor stress effects on GR machinery elements of these brain regions in Avoider and Non-Avoider rats.
Individuals with PTSD exhibit enhanced negative feedback as evidenced by greater glucocorticoid suppression following a dexamethasone suppression test (de Kloet et al., 2008). Here, we examined predator odor stress effects on proteins mediating GR function in PVN, CeA, and VH of Avoider and Non-Avoider rats relative to unstressed Controls. FK506-binding protein of molecular weight 51 (FKBP51) is part of a protein complex that controls cellular localization of GRs. Binding of FKBP51 to HSP90 of the GR complex prevents nuclear translocation, GR phosphorylation, and subsequent transcription of target genes (15). Attenuated FKBP51 expression in circulating peripheral blood mononuclear cells is associated with enhanced glucocorticoid-mediated negative feedback, which is consistent with enhanced HPA negative feedback mechanisms in individuals with PTSD (13, 15). Furthermore, four single nucleotide polymorphisms (SNPs) in the fkbp5 gene are associated with risk for developing PTSD. Here, we observed a significant decrease in FKBP51 protein levels in the CeA of Avoider rats when compared to Controls, but no changes in FKBP51 in the PVN or VH.
An additional co-regulator important for GR-mediated gene transcription is SRC-1 (16, 45). SRC-1 is highly expressed in the brain (46). SRC-1 is involved in CRF gene expression in response to glucocorticoids and stress-related stimuli and may play a role in the differential HPA response in Avoiders and Non-Avoiders (16). SRC-1 negatively regulates CRF gene expression in the hypothalamus but positively regulates gene expression in the CeA. This distinction potentially results from differential expression of SRC-1 splice variants, SRC-1a and SRC-1e (16). Studies by de Kloet et al. demonstrate that the PVN, CeA, and VH all contain both variants, but in different amounts. The PVN has high expression of SRC-1a (a negative GR regulator) and very low, or even undetectable, expression of SRC-1e (a positive GR regulator), while the CeA and VH both contain relatively equal expression of each (46). Thus, selective activation of these splice variants may lead to distinct GR-mediated effects on CRF gene expression in these regions, even in the absence of changes in nuclear pGR or GR levels. Here, we show differential protein levels of total SRC-1 in the PVN, CeA, and VH of rats that exhibit avoidance following stress. In the PVN and CeA, we report decreased SRC-1 levels in Avoiders that are negatively correlated with avoidance (in all stressed rats). In contrast, we find increased SRC-1 levels in the VH in Avoiders that are positively correlated with avoidance (in all stressed rats). While the SRC-1 antibody we employed does not distinguish between 1a and 1e isoforms, our results suggest that stress alters central SRC-1 expression in stress-susceptible individuals, perhaps as a result of the initial blunted HPA response to traumatic stress (47). These results are in partial agreement with past findings that acute restraint stress decreases SRC-1 protein levels in the hypothalamus of both male and female rats but increases SRC-1 levels in the hippocampus of female rats only (Lachize et al., 2009). Future studies will examine differences in SRC-1a and SRC-1e isoforms in these brain regions and their respective contributions to post-stress behavioral change in Avoiders and Non-Avoiders.
In summary, the present study reports that corticosterone pre-treatment prior to predator odor stress decreases avoidance associated of a stress-paired context. Additionally, co-activators and co-factors involved in GR translocation and gene transcription are altered in brain regions critical for HPA axis regulation, including the PVN, CeA, and VH. Changes in SRC-1, in particular, in these regions were highly predictive of avoidance behavior, identifying a potential link between abnormal HPA responses to stress and subsequent stress-related behavioral pathology. Future studies will more precisely characterize SRC-1 mediation of post-stress behaviors in Avoiders vs. Non-Avoiders and unstressed controls.
Figure 6.
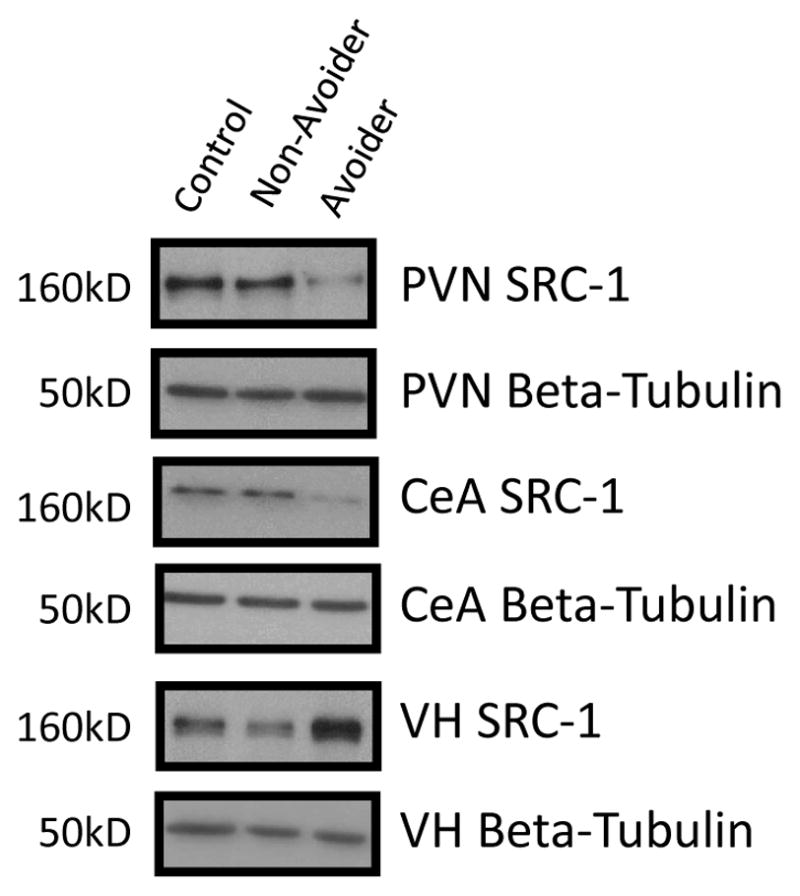
Representative western blot images for steroid receptor co-activator (SRC)-1 levels (molecular weight 160 kDa) in paraventricular nucleus (PVN), Central Amygdala (CeA) and ventral hippocampus (VH) in Control (C; n = 6), Avoider (A; n = 7) and Non-Avoider (NA; n = 8) groups.
Acknowledgments
This research was supported by NIH grants AA018400 (NWG), AA023305 (NWG), AA020839 (SE), and LSUHSC Alcohol and Drug Abuse Center of Excellence pilot funds (AMW). The authors would like to thank Brandon Baiamonte for his assistance with brain dissection.
Footnotes
Conflicts of Interest:
Dr. Gilpin is a consultant for Glauser Life Sciences, Inc.
References
- 1.DSM-5. American Psychiatric Association. Diagnostic and statistical manual of mental disorders Fifth addition. 5. Arlington, VA: 2013. [Google Scholar]
- 2.Whitaker AM, Gilpin NW, Edwards S. Animal models of post-traumatic stress disorder and recent neurobiological insights. Behav Pharmacol. 2014;25(5–6):398–409. doi: 10.1097/FBP.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–95. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174(2):215–24. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 5.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daskalakis NP, Lehrner A, Yehuda R. Endocrine Aspects of Post-traumatic Stress Disorder and Implications for Diagnosis and Treatment. Endocrinol Metab Clin North Am. 2013;42(3):503–13. doi: 10.1016/j.ecl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 8.North CS, Nixon SJ, Shariat S, Mallonee S, McMillen JC, Spitznagel EL, Smith EM. Psychiatric disorders among survivors of the Oklahoma City bombing. JAMA. 1999;282(8):755–62. doi: 10.1001/jama.282.8.755. [DOI] [PubMed] [Google Scholar]
- 9.Daskalakis NP, Lehrner A, Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab Clin North Am. 2013;42(3):503–13. doi: 10.1016/j.ecl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Yehuda R. Neuroendocrine aspects of PTSD. Handb Exp Pharmacol. 2005;(169):371–403. doi: 10.1007/3-540-28082-0_13. [DOI] [PubMed] [Google Scholar]
- 11.Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nerv Ment Dis. 1990;178(6):366–9. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Whitaker AM, Gilpin NW. Blunted Hypothalamo-pituitary Adrenal Axis Response to Predator Odor Predicts High Stress Reactivity. Physiol Behav. 2015 doi: 10.1016/j.physbeh.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–95. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, Müller-Myhsok B, Holsboer F, Buxbaum JD. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66(7):708–11. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Touma C, Gassen NC, Herrmann L, Cheung-Flynn J, Büll DR, Ionescu IA, Heinzmann JM, Knapman A, Siebertz A, Depping AM, Hartmann J, Hausch F, Schmidt MV, Holsboer F, Ising M, Cox MB, Schmidt U, Rein T. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol Psychiatry. 2011;70(10):928–36. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Lachize S, Apostolakis EM, van der Laan S, Tijssen AM, Xu J, de Kloet ER, Meijer OC. Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proc Natl Acad Sci U S A. 2009;106(19):8038–42. doi: 10.1073/pnas.0812062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaaf MJ, Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance. J Steroid Biochem Mol Biol. 2002;83(1–5):37–48. doi: 10.1016/s0960-0760(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 18.Galliher-Beckley AJ, Cidlowski JA. Emerging roles of glucocorticoid receptor phosphorylation in modulating glucocorticoid hormone action in health and disease. IUBMB Life. 2009;61(10):979–86. doi: 10.1002/iub.245. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Dang T, Blind RD, Wang Z, Cavasotto CN, Hittelman AB, Rogatsky I, Logan SK, Garabedian MJ. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol Endocrinol. 2008;22(8):1754–66. doi: 10.1210/me.2007-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry. 2013;3:e296. doi: 10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kozlovsky N, Kaplan Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59(12):1208–18. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The Rat Brain Atlas. 3. 1997. [Google Scholar]
- 23.Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18(3):331–42. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Giraud S, Bienvenu F, Avril S, Gascan H, Heery DM, Coqueret O. Functional interaction of STAT3 transcription factor with the coactivator NcoA/SRC1a. J Biol Chem. 2002;277(10):8004–11. doi: 10.1074/jbc.M111486200. [DOI] [PubMed] [Google Scholar]
- 25.Attwood BK, Bourgognon JM, Patel S, Mucha M, Schiavon E, Skrzypiec AE, Young KW, Shiosaka S, Korostynski M, Piechota M, Przewlocki R, Pawlak R. Neuropsin cleaves EphB2 in the amygdala to control anxiety. Nature. 2011;473(7347):372–5. doi: 10.1038/nature09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitaker AM, Gilpin NW. Blunted hypothalamo-pituitary adrenal axis response to predator odor predicts high stress reactivity. Physiol Behav. 2015;147:16–22. doi: 10.1016/j.physbeh.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yehuda R, Bierer LM, Sarapas C, Makotkine I, Andrew R, Seckl JR. Cortisol metabolic predictors of response to psychotherapy for symptoms of PTSD in survivors of the World Trade Center attacks on September 11, 2001. Psychoneuroendocrinology. 2009;34(9):1304–13. doi: 10.1016/j.psyneuen.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason JW, Wang S, Yehuda R, Riney S, Charney DS, Southwick SM. Psychogenic lowering of urinary cortisol levels linked to increased emotional numbing and a shame-depressive syndrome in combat-related posttraumatic stress disorder. Psychosom Med. 2001;63(3):387–401. doi: 10.1097/00006842-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40(2):79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- 30.Bremner D, Vermetten E, Kelley ME. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. J Nerv Ment Dis. 2007;195(11):919–27. doi: 10.1097/NMD.0b013e3181594ca0. [DOI] [PubMed] [Google Scholar]
- 31.Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161(8):1488–90. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- 32.Yehuda R, Golier J. Is there a rationale for cortisol-based treatments for PTSD? Expert Rev Neurother. 2009;9(8):1113–5. doi: 10.1586/ern.09.79. [DOI] [PubMed] [Google Scholar]
- 33.Schelling G, Roozendaal B, Krauseneck T, Schmoelz M, DE Quervain D, Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Ann N Y Acad Sci. 2006;1071:46–53. doi: 10.1196/annals.1364.005. [DOI] [PubMed] [Google Scholar]
- 34.Piazza PV, Deroche V, Deminière JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A. 1993;90(24):11738–42. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietz D, Wang H, Kabbaj M. Corticosterone fails to produce conditioned place preference or conditioned place aversion in rats. Behav Brain Res. 2007;181(2):287–91. doi: 10.1016/j.bbr.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smeets T, Giesbrecht T, Jelicic M, Merckelbach H. Context-dependent enhancement of declarative memory performance following acute psychosocial stress. Biol Psychol. 2007;76(1–2):116–23. doi: 10.1016/j.biopsycho.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Schwabe L, Bohringer A, Chatterjee M, Schachinger H. Effects of pre-learning stress on memory for neutral, positive and negative words: Different roles of cortisol and autonomic arousal. Neurobiol Learn Mem. 2008;90(1):44–53. doi: 10.1016/j.nlm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16(7):571–6. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- 39.Elzinga BM, Bakker A, Bremner JD. Stress-induced cortisol elevations are associated with impaired delayed, but not immediate recall. Psychiatry Res. 2005;134(3):211–23. doi: 10.1016/j.psychres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learn Mem. 2004;11(2):188–95. doi: 10.1101/lm.61704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 42.Casady RL, Taylor AN. Effect of electrical stimulation of the hippocampus upon corticosteroid levels in the freely-behaving, non-stressed rat. Neuroendocrinology. 1976;20(1):68–78. doi: 10.1159/000122470. [DOI] [PubMed] [Google Scholar]
- 43.Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86(2):449–59. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 44.Buchanan TW, Tranel D, Kirschbaum C. Hippocampal damage abolishes the cortisol response to psychosocial stress in humans. Horm Behav. 2009;56(1):44–50. doi: 10.1016/j.yhbeh.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winnay JN, Xu J, O’Malley BW, Hammer GD. Steroid receptor coactivator-1-deficient mice exhibit altered hypothalamic-pituitary-adrenal axis function. Endocrinology. 2006;147(3):1322–32. doi: 10.1210/en.2005-0751. [DOI] [PubMed] [Google Scholar]
- 46.Meijer OC, Steenbergen PJ, De Kloet ER. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology. 2000;141(6):2192–9. doi: 10.1210/endo.141.6.7489. [DOI] [PubMed] [Google Scholar]
- 47.Lee SK, Kim HJ, Kim JW, Lee JW. Steroid receptor coactivator-1 and its family members differentially regulate transactivation by the tumor suppressor protein p53. Mol Endocrinol. 1999;13(11):1924–33. doi: 10.1210/mend.13.11.0365. [DOI] [PubMed] [Google Scholar]