Editor’s Note:
Our author writes that recent data from the United Nations Office of Drugs and Crime indicate that 540 different drugs classified as new psychoactive substances (NPS) have been identified worldwide as of 2014, and this number is expected to rise. His article describes the complexity of the NPS problem, what is known about the molecular mechanisms of action, and the pharmacological effects of NPS. It also highlights some of the considerable challenges in dealing with this emerging issue.
Drug abuse is a persistent public health problem in modern society, and a disturbing new trend is the increased recreational use of so-called “designer drugs,” “legal highs,” or “research chemicals.” These drugs, collectively known as “new psychoactive substances” (NPS), are synthetic alternatives to traditional illegal drugs of abuse.1
Most NPS are manufactured by Asian laboratories and sold to consumers via the Internet or shipped to locations in Europe, the United States (US) and elsewhere, to be packaged for retail sale. NPS are usually marketed as non-drug products to minimize legal scrutiny. They are given innocuous names and labeled “not for human consumption”.
Compared to traditional drugs of abuse, NPS are cheap, easy to obtain, and not detectable by standard toxicology screens. There are popular examples of NPS that appear to mimic traditionally abused drugs—stimulant-like NPS (e.g., “bath salts”), marijuana-like NPS (e.g., “spice”) and LSD-like NPS (e.g., “N-bombs”)—but no controlled clinical laboratory investigations have been carried out to examine the psychoactive effects of these new drugs in humans.
Nevertheless, the adverse side-effects of NPS in humans are well-documented in the medical literature, indicating that these substances pose obvious health risks. There is no quality control in the manufacture and packaging of these products. Adverse effects that have been reported include agitation, panic attacks, hallucinations, psychosis, violent behaviors, increased heart rate, elevated body temperature, and seizures—some of these ending in death.2 In the US, an alarming spike in toxic exposures and fatalities associated with the abuse of synthetic marijuana-like drugs has occurred during the past year, illustrating the severity and scope of the problem.3
Information freely available on the Internet has facilitated the current rise in availability and use of NPS.4 Scientific articles published in online databases (e.g., PubMed) provide step-by-step recipes for the syntheses of psychoactive compounds, most of which were originally developed as potential medicines or research tools. Such synthetic schemes can be exploited by skilled and corrupt individuals, or businesses, to produce bulk quantities of NPS that are marketed and sold to consumers via the worldwide web. In most countries, adolescents cannot legally buy cigarettes or alcohol, but they can easily purchase powerful psychoactive drugs from websites. Online user forums contain detailed “trip reports” which describe the doses, preferred routes of administration, and expected psychological effects for various NPS, so that users can fine-tune their drug-taking experiences.5
As governments have passed legislation to ban specific problematic NPS, chemists involved with the manufacture of these substances have consulted the scientific or patent literatures and quickly created novel “replacement” drugs to stay one step ahead of law enforcement. The sheer number of new drugs now is staggering. Recent data from the United Nations Office of Drugs and Crime indicate that 540 different NPS have been identified worldwide as of 2014, and of course this number is expected to rise.6
Stimulant-like NPS Interact with Monoamine Transporters
The first stimulant-like NPS to appear in the US were so-called “bath salts” products, which flooded the recreational drug marketplace during late 2010. By early 2011, there was a dramatic rise in reports of bath salts intoxications to poison control centers, and an influx of patients admitted to emergency departments with toxic exposures.7,8 Bath salts products consist of powders or crystals that are meant to be administered intra-nasally or orally to produce their psychoactive effects. While low doses of bath salts induce typical stimulant effects such as increased energy and mood elevation, high doses or binge use can cause severe symptoms including hallucinations, psychosis, increased heart rate, high blood pressure and hyperthermia, often accompanied by combative or violent behaviors.2,8 Deaths from bath salts overdose have been reported.2,8
Bath salts typically take the form of a white or brown crystalline powder and are sold in small plastic or foil packages labeled “not for human consumption.” Sometimes also marketed as “plant food”—or, more recently, as “jewelry cleaner” or “phone screen cleaner”—they are sold online and in drug paraphernalia stores under a variety of brand names, such as “Ivory Wave,” “Bloom,” “Cloud Nine,” “Lunar Wave,” “Vanilla Sky,” “White Lightning,” and “Scarface.”
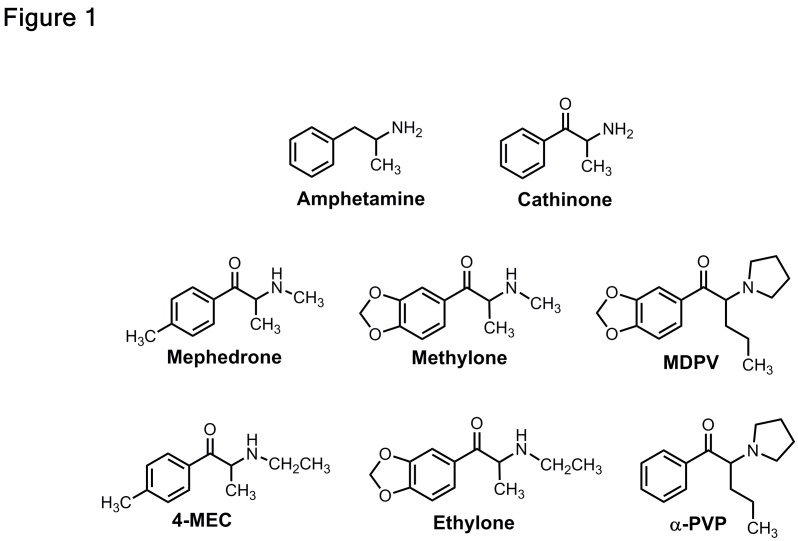
Forensic analysis of bath salts products in 2010 and 2011 revealed the presence of three main synthetic cathinones: methylenedioxypyrovalerone (MDPV), mephedrone, and methylone.8 These compounds are structurally-related to the parent compound cathinone, an amphetamine-like stimulant found in the khat plant, Catha edulis. Legislation passed in 2013 placed these three synthetic cathiones into permanent Schedule I control, making the drugs illegal in the US.9,10 Figure 1 depicts the chemical structures of bath salts cathinones compared to amphetamine. Reports of bath salts exposures have subsided in recent years,7 but MDPV, methylone, and other various structurally-related cathinone analogs shown in Figure 1 are still present in the recreational drug marketplace.11
Figure 1.
Chemical structures of amphetamine, cathinone, and the bath salts cathinones- mephedrone, methylone and MDPV.4-MEC, ethylone and lpha-PVP are replacement analogs that appeared in the recreational drug marketplace after emergency scheduling legislation was enacted in 2011 to ban mephedrone, methylone and MDPV.
Source: Michael Baumann
Like other stimulant drugs of abuse (e.g., cocaine and amphetamine), synthetic cathinones exert their effects by binding to “transporter” proteins on the surface of nerve cells that synthesize the monoamine neurotransmitters dopamine, norepinephrine, and serotonin. These neurotransmitters are released from nerve cells and mediate cell-to-cell chemical signaling.12 The normal role of the transporters is to pull excess amounts of the released monoamine neurotransmitters from the “extracellular” spaces around cells and move them back into the cells that made them (a process called “reuptake”). Thus, monoamine transporters are critical for reducing extracellular concentrations of monoamine neurotransmitters. Drugs that interact with transporters can be divided into two types: 1) cocaine-like inhibitors and 2) amphetamine-like substrates. Inhibitor drugs block neurotransmitter uptake by clogging the transporter channel—much as a sock would clog a vacuum cleaner. Substrate drugs also block the transporter momentarily, but these drugs are small enough that they themselves are translocated through the transporter channel into the cell where they trigger the efflux of neurotransmitter molecules (i.e., transporter-mediated release) into the extracellular space. The releasing action of transporter substrate drugs can be viewed as switching a vacuum cleaner into reverse, causing the dumping of intracellular neurotransmitters into the extracellular medium.
Regardless of molecular mechanism, both types of transporter drugs dramatically increase extracellular concentrations of monoamines, amplifying cell-to-cell chemical signaling in various brain circuits. Elevations in extracellular dopamine are implicated in the pleasurable and addictive properties of stimulants, whereas elevations in norepinephrine are thought to mediate cardiovascular and autonomic effects.
Pharmacologists have examined the biological effects of bath salts cathinones using a variety of methods in laboratory animals. In brain tissue and cultured cells, MDPV is a transporter inhibitor that potently blocks the uptake of dopamine and norepinephrine, with little effect on the uptake of serotonin.13,14 Importantly, MDPV is at least 10-times more potent than cocaine in inhibiting dopamine and norepinephrine uptake. In contrast to MDPV, the transporter substrates mephedrone and methylone evoke the release of dopamine, norepinephrine, and serotonin from nerve cells.14,15 The neurotransmitter-releasing actions of mephedrone and methylone are similar to the effects of the illicit drug MDMA. Consistent with their activities as inhibitors or substrates at dopamine transporters, administration of synthetic cathinones to rats produces dose-related increases in extracellular concentrations of dopamine in the mesolimbic system, a pathway of nerve cells implicated in pleasure and addiction.13,15
All synthetic cathinones investigated to date produce dose-related stimulation of locomotor activity when administered to rats or mice, probably due to enhancement of dopamine transmission. Recently, the addictive potential of synthetic cathinones has been investigated using the rat self-administration model, which is considered the gold standard for testing the abuse liability of drugs due to its high degree of predictive validity.16
In the self-administration studies, rats fitted with intravenous catheters are placed in chambers equipped with two levers. Presses on the “active” lever result in computer-controlled intravenous drug infusions, whereas presses on the other “inactive” lever have no consequence. Under these circumstances, rats will learn to self-inject MDPV, mephedrone, and methylone, indicating that these drugs have substantial abuse potential.16,17
It is worth noting that MDPV) is much more potent and effective in the self-administration assay when compared to methylone. Additionally, MDPV seems more apt to produce life-threatening adverse effects in humans.8 Even though different cathinones may be found in bath salts products, MDPV was the main compound present in blood and urine from fatal cases of bath salts overdose during the first wave of abuse in the US. It is tempting to speculate that potent inhibition of dopamine uptake by MDPV is responsible for neurological symptoms and hyperthermia in bath salts overdose cases, while inhibition of norepinephrine uptake could underlie elevations in blood pressure and heart rate.
Marijuana-like NPS Interact with Cannabinoid Receptors
Marijuana-like NPS, also known as synthetic cannabinoids, appeared in the US recreational drug marketplace in the early 2000s, and by the end of the decade were being widely used.18,19 The initial products containing synthetic cannabinoids were marketed as “spice,” “K2,” or “herbal incense,” and consisted of plant material laced with psychoactive compounds. Like marijuana, synthetic cannabinoids are usually smoked to produce their psychoactive effects. Low doses of synthetic cannabinoids produce marijuana-like effects, including perceptual distortions and mood elevation, but higher doses or binge use can produce serious adverse effects, including increased heart rate, uncontrolled vomiting, acute kidney injury, panic attacks, hallucinations, psychosis, and seizures.2,3,19 Deaths from synthetic cannabinoid overdose are rare but have occurred.2,3
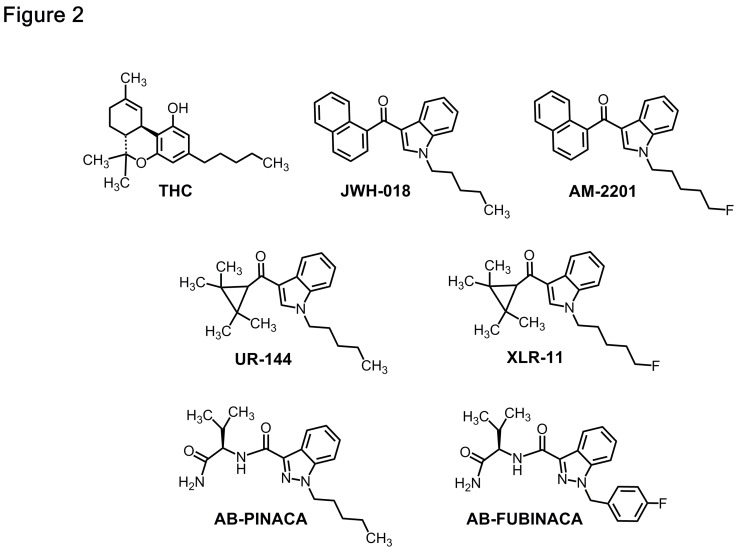
Forensic evaluation of spice and K2 products in 2010 and 2011 identified the active ingredients as the naphthoylindole JWH-018 and its analogs.11Figure 2 shows that JWH-018 and other synthetics are structurally distinct from naturally-occurring tetrahydrocannabinol (THC),9 the active ingredient in marijuana. JWH-018 and related synthetic cannabinoids were originally synthesized by research scientists as tools to study the function of cannabinoid-1 (CB1) and cannabinoid-2 (CB2) receptors, the cell surface receptors where THC exerts it effects.20 Clandestine chemists hijacked the recipes for the manufacture of these synthetic drugs and made them available for misuse by humans.
Figure 2.
Chemical structures of THC, JWH-018, and common synthetic cannabinoids. AM-2201 is an analog of JWH-018 that appeared in the recreational drug marketplace after emergency scheduling legislation was enacted in 2011 to ban JWH-018. UR-144, XLR-11, ABPINACA and AB-FUBINICA represent subsequent generations of replacement cannabinoid drugs that have appeared in the recreational drug marketplace over the past few years.
Source: Michael Baumann
Due to the public health risks posed by synthetic cannabinoids, legislation passed in 2013 placed JWH-018 and several of its analogs into permanent Schedule 1 control, making these substances illegal in the US.9 In response to drug scheduling, many replacement analogs have appeared in the recreational drug marketplace, including cyclopropyl ketone indoles such as UR-144 and XLR-11 during 2013 and 2014, and indazoles such as AB-PINACA and AB-FUBINACA in more recent months (see Figure 2).3,11,20 In contrast to the diminishing reports of bath salts exposures since 2011, poison control data show a sharp increase in intoxications with synthetic cannabinoids in 2015.18
As noted above, JWH-018 and other synthetic cannabinoids bind to and stimulate CB1 and CB2 receptors located on the ends of axons (nerve terminals) that transmit neurochemical messages from one cell to another, but the synthetic drugs are more potent and exert stronger effects than THC.21,22 The high potency of synthetic cannabinoids at CB1 and CB2 receptors may underlie the increased propensity for these drugs to produce adverse effects when compared to marijuana.2,3,19 Early research demonstrated that CB1 receptors are found in the brain, whereas CB2 receptors are found in peripheral organs such as the spleen.23 More recent evidence shows that CB1 and CB2 receptors are both present in the brain, but CB1 receptors are found in much higher amounts in nervous tissue and probably mediate the psychoactive effects of THC and synthetic cannabinoids.
Under normal circumstances, CB1 receptors are stimulated by naturally-occurring cannabinoid compounds (i.e., endocannabinoids) in the brain, such as anandamide and 2-arachidonoyl glycerol.23 CB1 receptors are located on nerve terminals throughout the brain, and activation of these receptors can inhibit the release of excitatory neurotransmitters (e.g., glutamate) or inhibitory neurotransmitters (e.g., GABA).23,24 Thus, CB1 receptors are modulators of the cell-to-cell signaling of other neurotransmitter systems. Endocannabinoids and cannabinoid receptors are implicated in the control of mood, appetite, pain sensation, learning, and memory. As such, drugs that interact with cannabinoid receptors would be predicted to have profound effects on brain function. The physiological role of CB2 receptors in the brain is not well understood, but the activation of CB2 receptors by cannabinoids likely contributes to the overall profile of drug effects. Additionally, it seems feasible that synthetic cannabinoids may have actions that are mediated by non-cannabinoid mechanisms.
Pharmacologists have examined the effects of synthetic cannabinoids in laboratory animals using a variety of methods. The administration of THC to mice is known to produce four characteristic responses: 1) decreased motor activity, 2) reduced body temperature, 3) dulled pain sensation, and 4) a lifeless immobility, known as catalepsy.20 These four responses, collectively known as the “tetrad,” represent the hallmark profile of effects exerted by cannabinoid-type drugs. The effects of THC in the tetrad assay are dose-dependent and reversed by the CB1 receptor blocking drug rimonabant. Not surprisingly, JWH-018 and other synthetic cannabinoids produce the tetrad of effects in mice, but are much more potent than THC.21,22 Furthermore, synthetic cannabinoids can produce very long-lasting effects in the tetrad assay. Metabolism studies have shown that JWH-018 and its analogs are transformed by liver enzymes into several different metabolites.25 Some of these metabolites penetrate into the brain, are potent stimulators of cannabinoid receptors, and have long half-lives. Such findings suggest that the effects of synthetic cannabinoids may be longer-lasting than THC’s due to their more persistent bioactive metabolites.
Unlike the situation with stimulant drugs, THC and synthetic cannabinoids are not readily self-administered by rats or mice.20,26 Consequently, a different behavioral model, known as drug discrimination, is used to assess marijuana-like psychoactive effects in animals.26 In the typical drug discrimination procedure, rats are trained to associate the internal cues produced by THC administration with food rewards. Importantly, drugs are administered by the scientific investigator in this paradigm and not self-injected by the rats. After repeated training sessions, rats learn to “discriminate” the internal cues produced by cannabinoid-type drugs from those produced by vehicle control treatments or other types of psychoactive substances. Studies have shown that JWH-018 and its analogs are recognized as cannabinoid-like in rats trained to discriminate THC from its vehicle,21,26 and similar results have been found with newer synthetics, including UR-144 and XLR-11.22 In studies where more than one synthetic cannabinoid has been evaluated, the rank order of drug potency falls in line with the potency of drugs to activate the CB1 receptor, suggesting the CB1 receptor is responsible for the observed effects.20–23
The Spread of NPS Presents Major Challenges
The growing popularity of NPS presents major challenges for governments, law enforcement, and public health. Controlling the influx of NPS from overseas laboratories is a complex political and economic issue which will require international cooperation among all stakeholders, especially those countries where synthetic drugs are being manufactured.27 Even with reduced production of NPS, monitoring Internet commerce will remain problematic. In the US, drug scheduling legislation has been a primary response to the spread of NPS,9,10 but this approach is often ineffective. For example, after the 2011 emergency scheduling action to ban MDPV and methylone in the US, law enforcement encounters involving methylone increased more than fivefold, and this substance is still present today.11
As mentioned previously, drug scheduling drives the emergence of new chemically-distinct replacement analogs, as clandestine laboratories scramble to stay one step ahead of legislative control.1,4,11 One new analog of MDPV, known as alpha-PVP or “flakka,” is a popular NPS that has wreaked havoc in Florida and other states, causing multiple deaths due to toxic overdose.28 Analogs of methylone, such as ethylone, are now found in tablets sold in the recreational drug marketplace as “molly” (i.e., counterfeit MDMA).29 Finally, drug scheduling hinders the ability of scientists to obtain NPS for study, thereby impeding the very research that is needed to elucidate the effects of these substances as they emerge.30
The abuse of NPS has placed a significant burden on healthcare professionals, especially those providing emergency medical care. Many cases of toxic overdose from bath salts and synthetic cannabinoids are first reported to poison control centers, and subsequently treated in hospital emergency departments.7,18,31 As noted above, symptoms of overdose from NPS include cardiovascular effects such as increased heart rate and blood pressure, and neurological effects such as panic attacks, psychosis, and hallucinations.2,8,19 These symptoms are often accompanied by extreme elevations in body temperature, along with combative or otherwise violent behaviors; thus, subduing and treating such patients can be a harrowing experience for hospital staff. Because the precise substance ingested by a particular patient is usually unknown, targeted treatments or receptor antagonists cannot be administered. Medical treatment is mostly supportive, with benzodiazepines to reduce cardiovascular stimulation and agitation, and whole body cooling to address hyperthermia.31,32
At the present time, most NPS are not detected by routine toxicology screens. So, analytical confirmation of synthetic drug exposure in intoxicated patients is often impossible. The fact that NPS can be used without detection is a primary driving force for the abuse of these substances in individuals subjected to routine drug testing, such as military personnel.33 Sophisticated analytical methods for the detection of NPS and their metabolites are being developed, but such methods are not readily available in most clinical settings.34,35 Unfortunately, forensic toxicologists are faced with the prospect of continually developing new analytical methods to keep pace with the appearance of new replacement NPS.
Widespread abuse of NPS is a complex problem with no simple solutions, and novel drugs continue to emerge at a rapid pace. NPS pose obvious health risks because there is no quality control in their production, their pharmacological effects are poorly understood, and clinical data are limited to cases from emergency room admissions. In general, the pharmacological effects of NPS seem to resemble those of the illicit drugs which they are intended to mimic; but NPS are often much more potent, and “off-target” mechanisms of action have not been established. More basic research in animal models is needed to evaluate the consequences of acute and chronic exposure to various types of NPS. Newly developed analytical methods for detecting NPS must be made widely available to assist in identifying novel substances as they emerge in the recreational drug marketplace. In the interest of public health and safety, better coordination among emergency medical personnel, forensic toxicologists, scientific researchers, law enforcement, and policymakers is essential to foster more effective responses in dealing with this evolving drug-abuse phenomenon.
Footnotes
Michael H. Baumann, Ph.D., is a staff scientist at the National Institute on Drug Abuse, Intramural Research Program, in Baltimore, MD. Baumann’s research focuses on the role of brain dopamine and serotonin systems in mediating the effects of therapeutic and abused stimulant drugs. In 2012, he joined the laboratory of Amy H. Newman, Ph.D., where he established the Designer Drug Research Unit (DDRU). The main goal of the DDRU is to collect, analyze and disseminate the most up-to-date information about the pharmacology and toxicology of newly-emerging designer drugs of abuse, more formally known as new psychoactive substances (NPS). Working with partner organizations such as the Drug Enforcement Administration, the National Drug Early Warning System, and the European Monitoring Centre for Drugs and Drug Addiction, Baumann is kept informed about recent trends in the abuse of NPS. Most recently, his research has characterized the molecular mechanism of action and pharmacological effects for many of the so-called “bath salts” cathinones and their various replacement analogs.
References
- 1.Zawilska JB, Andrzejczak D. Next generation of novel psychoactive substances on the horizon - A complex problem to face. Drug Alcohol Depend. 2015;157:1–17. doi: 10.1016/j.drugalcdep.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 2.Schifano F, Orsolini L, DuccioPapanti G, Corkery JM. Novel psychoactive substances of interest for psychiatry. World Psychiatry. 2015;14:15–26. doi: 10.1002/wps.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trecki J, Gerona RR, Schwartz MD. Synthetic cannabinoid-related illnesses and deaths. New Engl J Med. 2015;373:103–107. doi: 10.1056/NEJMp1505328. [DOI] [PubMed] [Google Scholar]
- 4.Brandt SD, King LA, Evans-Brown M. The new drug phenomenon. Drug Test Analysis. 2014;6:587–597. doi: 10.1002/dta.1686. [DOI] [PubMed] [Google Scholar]
- 5.Erowid Experiences Vault 2015. https://www.erowid.org/experiences/
- 6.United Nations Office of Drugs and Crime World Drug Report. 2015. http://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
- 7.American Association of Poison Control Centers, Bath Salts Alert 2015. http://www.aapcc.org/alerts/bath-salts/
- 8.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 9.Drug Enforcement Administration, Department of Justice. Establishment of drug codes for 26 substances. Final rule. Fed Regist. 2013;78(3):664–6. [PubMed] [Google Scholar]
- 10. http://www.deadiversion.usdoj.gov/fed_regs/rules/2013/fr0412_2.htm.
- 11.Drug Enforcement Administration. Special report: synthetic cannabinoids and cathinones reported in NFLIS, 2010–2013. 2014. http://www.deadiversion.usdoj.gov/nflis/spec_rpt_CathCan_2013.pdf.
- 12.Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. Eur J Pharmacol. 2013;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–62. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–70. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain. Neuropsychopharmacology. 2012;37:1192–203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watterson LR, Olive MF. Synthetic cathinones and their rewarding and reinforcing effects in rodents. Adv Neurosci. 2014 Jun;4:209875. doi: 10.1155/2014/209875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology. 2015 doi: 10.1007/s00213-015-4057-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Association of Poison Control Centers Synthetic Cannabinoids Alert. 2015. http://www.aapcc.org/alerts/synthetic-cannabinoids/
- 19.Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: a case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21:320–356. doi: 10.1111/j.1521-0391.2012.00240.x. [DOI] [PubMed] [Google Scholar]
- 20.Wiley JL, Marusich JA, Huffman JW. Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci. 2014;97:55–63. doi: 10.1016/j.lfs.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiley JL, Marusich JA, Martin BR, Huffman JW. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend. 2012;123:148–153. doi: 10.1016/j.drugalcdep.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. Cannabinoids in disguise: Δ9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145–54. doi: 10.1016/j.neuropharm.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol. 2008;13:147–59. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman AF, Lupica CR. Synaptic targets of Δ9-tetrahydrocannabinol in the central nervous system. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a012237. a012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fantegrossi WE, Moran JH, Radominska-Pandya A, Prather PL. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ(9)-THC: mechanism underlying greater toxicity? Life Sci. 2014;97:45–54. doi: 10.1016/j.lfs.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Järbe TU, Gifford RS. “Herbal incense”: designer drug blends as cannabimimetics and their assessment by drug discrimination and other in vivo bioassays. Life Sci. 2014;97:64–71. doi: 10.1016/j.lfs.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seddon T. Drug policy and global regulatory capitalism: the case of new psychoactive substances (NPS) Int J Drug Policy. 2014;25:1019–1024. doi: 10.1016/j.drugpo.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 28. http://www.sun-sentinel.com/local/broward/fl-flakka-deaths-20150625-story.html.
- 29. https://www.ecstasydata.org/results.php?start=0&search_field=all&s=ethylone.
- 30.Nutt DJ, King LA, Nichols DE. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nat Rev Neurosci. 2013;14:577–585. doi: 10.1038/nrn3530. [DOI] [PubMed] [Google Scholar]
- 31.Musselman ME, Hampton JP. “Not for human consumption”: a review of emerging designer drugs. Pharmacotherapy. 2014;34:745–57. doi: 10.1002/phar.1424. [DOI] [PubMed] [Google Scholar]
- 32.Nelson ME, Bryant SM, Aks SE. Emerging drugs of abuse. Emerg Med Clin North Am. 2014;32:1–28. doi: 10.1016/j.emc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Berry-Cabán CS, Kleinschmidt PE, Rao DS, Jenkins J. Synthetic cannabinoid and cathinone use among US soldiers. US Army Med Dep J. 2012 Oct-Dec;:19–24. [PubMed] [Google Scholar]
- 34.Concheiro M, Anizan S, Ellefsen K, Huestis MA. Simultaneous quantification of 28 synthetic cathinones and metabolites in urine by liquid chromatography-high resolution mass spectrometry. Anal Bioanal Chem. 2013;405:9437–9448. doi: 10.1007/s00216-013-7386-z. [DOI] [PubMed] [Google Scholar]
- 35.Scheidweiler KB, Jarvis MJ, Huestis MA. Nontargeted SWATH acquisition for identifying 47 synthetic cannabinoid metabolites in human urine by liquid chromatography-high-resolution tandem mass spectrometry. Anal Bioanal Chem. 2015;407:883–897. doi: 10.1007/s00216-014-8118-8. [DOI] [PubMed] [Google Scholar]