BCR signaling pathway inhibitors such as Syk inhibitors1–3 have shown promise in CLL, a mature B cell cancer where the BCR pathway is constitutively active.4 However, sustained BCR signaling can lead to anergy and apoptosis in a process including IgM downregulation, reduced responsiveness to BCR ligands, high intracellular Ca2+ and activation of BCR pathway kinases.4,5 Several studies suggest that CLL cells are rescued by combined BCR and CD40L triggering; an anti-apoptosis, activation and differentiation signal is mediated by T helper (Th) cells via CD40L (CD40 ligand, CD154) that binds CD40 on the B cell surface.6–10 Thus, CLL patients have CLL-specific Th cells that can support CLL cell proliferation in xenografts,8,9 activated CD40L+ Th cells colocalize with proliferating CLL cells in pseudofollicles,6,7 and CLL cells secrete Th attracting chemokines, thereby recruiting T cell help.6,9 It has been unclear how BCR pathway inhibitors impact the microenvironmental help of CLL cells. Herein we show that CD40L activated the BCR pathway in CLL cells, including Syk and downstream components. CLL cells were very sensitive to the Syk inhibitors that abrogated CD40L induced blastogenesis and proliferation. This suggests that CLL cells require CD40L-dependent activation of the BCR pathway to overcome negative feedback. Hence, CD40 may function as a gatekeeper for the constitutive BCR signals. Treatment with Syk inhibitors may interfere with CLL cell proliferation by blocking CD40L-dependent Syk activation. We also found that normal B cells were not affected by the inhibition, suggesting that treatment could spare the normal B cell compartment.
We stimulated CLL cells (Online Supplementary Table S1) or normal B cells with CD40L using human CD40L+ murine L cell fibroblasts11 for 6 days, and measured proliferation by using the BrdU flow kit (anti-BrdU, 3D4, BD Bioscience). B cell activation was monitored by CD38 expression on gated CD19+ cells (HB7; HIB19, eBioscience). CD40L+ L cells stimulated CLL cell blastogenesis, CD38 expression, generation of CLL cell clusters and proliferation of cells in all patients analyzed (Figure 1A–C and Online Supplementary Figure S1). Blastogenesis and cell clustering were dramatically reduced in the presence of the Syk inhibitor fostamatinib (R406), (Figure 1B). Nevertheless, CLL cells were viable and activated as indicated by the expression of the activation marker CD38 (Figure 1C, Online Supplementary Figure S1). In contrast, both the blastogenesis (9 out of 10 patients) and proliferation responses (10 out of 10 patients) were inhibited in CLL cells (Figure 1D). We extended the analysis to P505-15, a novel and highly selective Syk inhibitor.2 P505-15 inhibited blastogenesis and CLL proliferation (Figure 1D, bottom panel). Remarkably, normal B cells, purified from healthy donors by a negative selection kit (Miltenyi Biotec), were not inhibited in these assays (Figure 1E).
Figure 1.
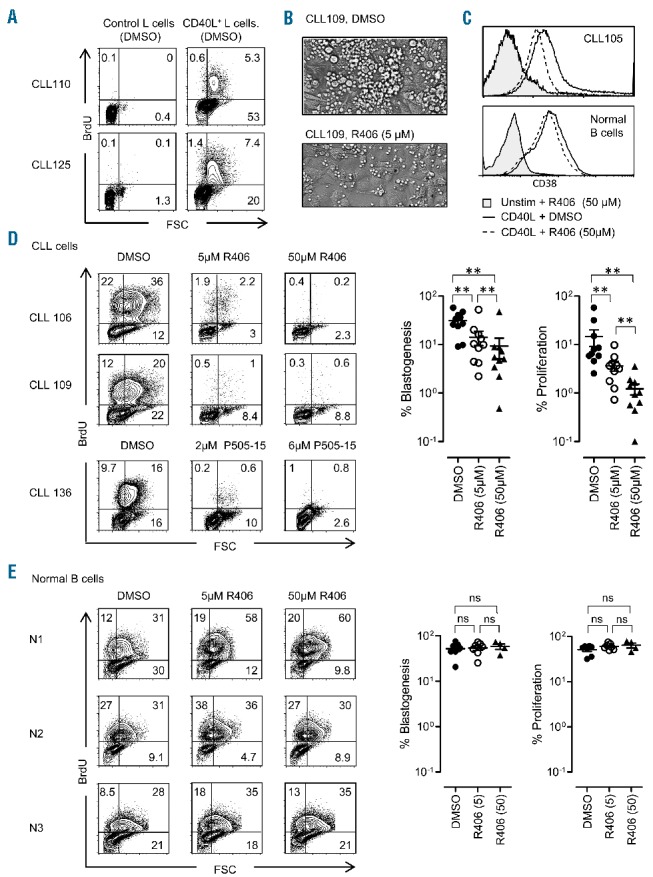
Analysis of Syk inhibition of CD40 signaling in CLL and normal B cells. A–D, CLL cells were stimulated by irradiated CD40L+ or untransfected L cells in the presence of R406, P505-15 (Selleck Chemicals) using relevant in vivo concentrations or DMSO vehicle control. (A) Forward scatter (cell size) vs. BrdU incorporation (anti-BrdU, 3D4, BD Bioscience) analysis (day 5) in the gated CD19+ CLL cells as indicated. (B) Microscopic analysis (day 3) in the absence (top) or presence (bottom) of R406 (5μM). CLL cells were stimulated with irradiated CD40L+ L cells. (C) Expression of the activation marker CD38 on CLL cells (top) or normal negatively selected B cells (Miltenyi Biotec selection kit, bottom) as indicated. (D and E) B cells were stimulated by irradiated CD40L+ L cells. Left: Forward scatter vs. BrdU incorporation analysis (day 5) in the gated CD19+ CLL cells (D), and CD19+ normal B cells (E) as indicated. The distribution of cells with high FSC (blasts, right quadrants) and proliferation (BrdU+, upper quadrants) are shown. Right panel: gated CD19+ CLL cells (D), and CD19+ normal B cells (E), stimulated by CD40L in the presence of DMSO or R406 (5 or 50μM). Blastogenesis (%) was calculated by subtracting unstimulated background: stimulated CLL cells [upper right (UR) + lower right (LR) quadrants] – unstimulated CLL cells [UR+LR]. Proliferation (%): stimulated CLL cells [upper left (UL) + upper right (UR)] quadrants) – unstimulated CLL cells [UL+UR]. Wilcoxon signed-rank test, n=10, P<0.006 for all comparisons in (D); no significant differences in (E) (n=10). See Online Supplementary Table S1 for patient list.
We proceeded to investigate pathway activation with phosphoflow analyses; samples were prepared using phosphoflow reagents from BD Biosciences (San Jose, CA, USA). Permeabilized cells were barcoded with combinations of esters conjugated to Pacific Blue, Alexa Fluor 488 or Pacific Orange (Molecular Probes/Life Technologies) and washed and pooled as described.12 CD19+ barcoded cells (PE-Cy7, SJ25C1, BD Bioscience) were stained with Alexa Fluor 647-conjugated phosphospecific antibodies and acquired on a FACSCanto II, and analyzed as described.12
In agreement with previous reports,4 we found a higher basal activation of the BCR signaling cascade in CLL cells, Syk (pY525/Y526, Cell Signaling Technology), PLCγ2 (pY759, BD Biosciences) and ERK1/2 (pT202/Y204 Cell Signaling Technology, data not shown). After ligation with cross-linking anti-BCR, CLL cells increased the levels of activated Syk (pY525/Y526) within 3 minutes (Figure 2A, upper panel), with kinetics similar to that of normal B cells, but with lower signal amplitude. CLL cells also increased Syk (pY525/526) levels after sCD40L stimulation, showing a delayed response and most evident after 10–30 minutes (Figure 2A, upper panel). This response was higher and significantly different from that seen in normal B cells (Figure 2B). In contrast, sCD40L stimulated NF-κB p65 (pS536) and p38 MAPK (pT180/Y182, both mAbs from Cell Signaling Technology) had similar kinetics in CLL and normal B cells (Figure 2A, lower panels).
Figure 2.
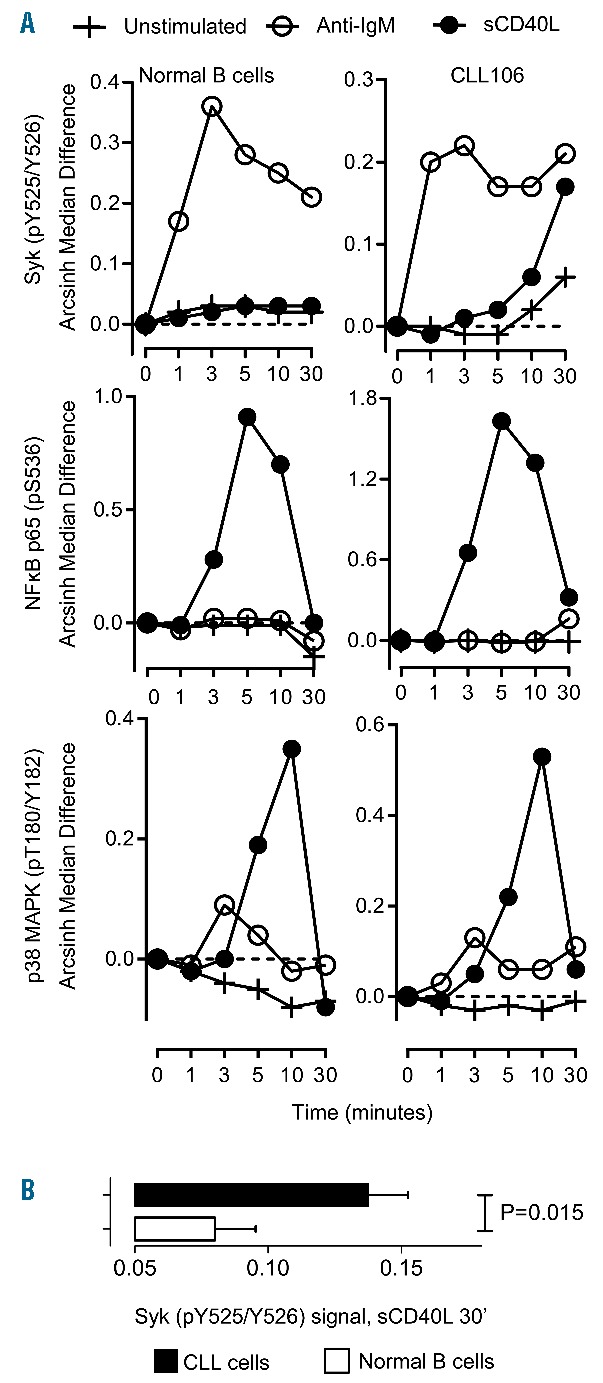
Analysis of Syk activation following CD40 or BCR ligation (A) CLL cells or normal B cells were stimulated with sCD40L (400 ng/mL, ImmunoTools, Germany) or F(ab’)2 anti-human IgM (10 μg/mL, Southern Biotechnology, CA, USA) for the indicated time periods. CD19+ cells were gated, Syk (pY525/Y526), NF-κB p65 (pS536) and p38 MAPK (pT180/Y182) were identified by phosphoflow cytometry, top and bottom panels, respectively. Shown is arcsinh median difference of the fluorescence intensity signals relative to unstimulated sample at time 0 (dotted line) for each time course in representative samples from one CLL patient and one B cell donor. (B) Activated Syk (pY525/Y526), as in (A) in CLL or B cells 30 minutes after stimulation with sCD40L, n=8 CLL patients, n=8 healthy individuals. Mann-Whitney U test, P= 0.015.
The analyses demonstrated that CD40 ligation provided a delayed increase in activated Syk in CLL cells. We next analyzed Syk in CLL cells after 24 hours co-culture with CD40L+ L cells or control L cells. The CD40L induced levels of activated Syk (pY525/526) was higher in these long-duration assays (Figure 3A). The CD40L-dependent Syk activity was markedly and significantly reduced in the presence of both R406 and P505-15 Syk inhibitors (Figure 3A). We proceeded to test levels of phosphorylation-dependent activation of components of the BCR pathway and found that CD40L stimulation increased the activity of Akt (pS473, Cell Signaling Technology), BLNK (pY84, BD Biosciences), Btk/ltk (pY551/Y511, BD Biosciences), ERK1/2 (pT202/Y204) in 5 out of 5 patients (Figure 3B and data not shown), suggesting that the CD40L pathway activation is permissive for constitutively active signals in the BCR pathway. Syk inhibition also reduced the activity of the downstream pathway constituents, although to a lesser degree, with the most pronounced effects seen with P505-15 and proximal constituents, pAkt, pBtk/Itk (Figure 3B).
Figure 3.
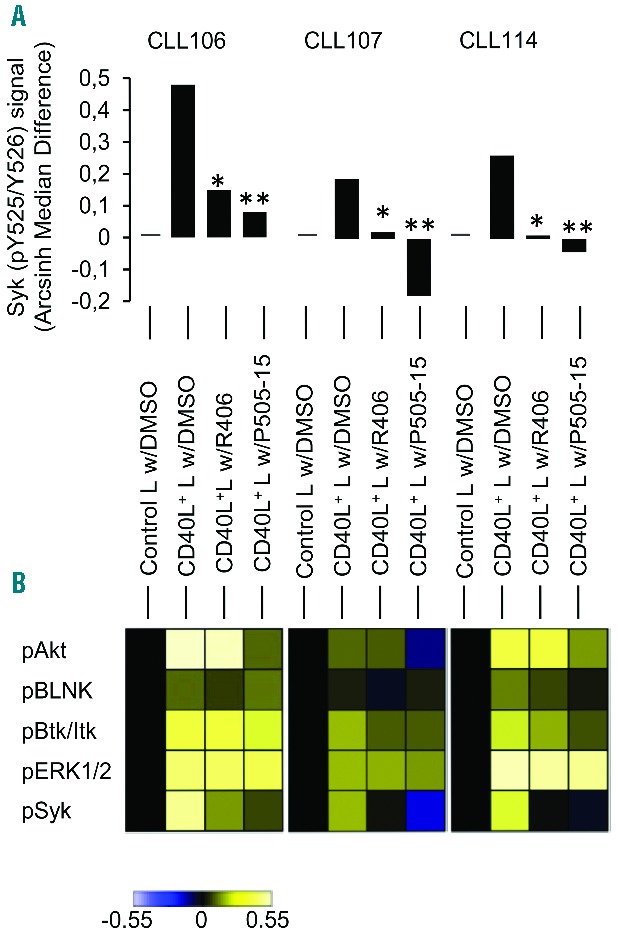
Analysis of Syk inhibitors on BCR pathway activation in CLL cells stimulated by CD40L. (A) CLL cells from three patients were incubated with CD40L+ or untransfected control L cells for 24h. CLL cells were treated with R406 (10 μM) or P505-15 (2 μM) or vehicle (DMSO). CLL cells were then processed and analyzed as in Figure 2. Syk (pY525/Y526) arcsinh median difference is shown. We found a significant difference between Syk activation in DMSO vs. R406 and DMSO vs. P505-15 (paired student’s t-test, P=0.03 and 0.001, respectively). Similar results were obtained with a separate experiment including 2 other patients. (B) Level of phosphorylation of downstream signaling proteins in the BCR pathway from experiment as in (A). Arcsinh median difference is shown for Akt (pS473), BLNK (pY84), Btk/Itk (pY551/Y511), ERK1/2 (pT202/Y204), and Syk (pY525/Y526). Heat map corresponds to −0.55 – +0.55 as indicated in the color bar.
In summary, we herein report that CD40 ligation resulted in increased activity in the BCR pathway of CLL cells; this included phosphorylation of Syk and the downstream components Akt, BLNK, Btk/Itk, and pErk1/2. The CD40L-dependent activation was significantly higher in CLL cells than in normal B cells. CD40L stimulation induced CLL cell blastogenesis and proliferation, and this response was blocked by Syk inhibition in CLL cells but not in B cells. Taken together, the results suggest that CLL cells take advantage of CD40L stimulation by increasing BCR pathway activity, signal integration and CLL cell proliferation. The inhibition of Syk only minimally reduced the CD40L-dependent activation of NF-κB1 and CD38 in CLL cells. We find it likely that the difference from normal B cells is linked to constitutive BCR signaling and anergy in CLL cells.4,5,13–15 Hence, CD40 may act as a gatekeeper for constitutive BCR signals in anergized CLL cells, thereby disinhibiting feedback components. It has been suggested, but not yet demonstrated, that normal anergic B cells would be similarly dependent upon CD40L and sensitive to Syk inhibition. If so, Syk inhibition could play a role in aborting the rescue of anergic autoreactive B cells in autoimmune disease. Although yet to be demonstrated, CD40-dependent Syk activation may be necessary in CLL cells to overcome the negative effects of Lyn/SHP-113 or inositol phosphate phosphatases14,15 to allow full CLL activation and CLL cell proliferation.
Fostamatinib had an objective response in 55% of CLL patients treated with the drug in a phase II trial, the highest response rate in this study that also included other B cell malignancies.1 Other Syk inhibitors including P505-152 are now being developed and tested with promising results.3 Recently the novel Syk inhibitor entospletinib had an objective response of 61% in phase II for relapsed or refractory CLL,3 further trials are under way (NCT01799889, NCT01796470) and also include trials of a combined Syk/JAK inhibitor (NCT01994382). These impressive findings have yet to be followed up in phase III clinical trials. However, the demonstration of marked Syk sensitivity in CLL cells as compared to normal B cells presented herein clearly suggest that further clinical trials are indicated.
Footnotes
Funding: this work was supported by grants from the University of Oslo, Oslo University Hospital Rikshospitalet, the South-Eastern Norway Regional Health Authority, The Swiss National Science Foundation, The Norwegian Cancer Society, The Research Council of Norway, CoE funding 179573/V40 and FriMedBio (204784/V40).
The online version of this paper contains a Supplementary Appendix.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115(13):2578–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spurgeon SE, Coffey G, Fletcher LB, et al. The selective SYK inhibitor P505-15 (PRT062607) inhibits B cell signaling and function in vitro and in vivo and augments the activity of fludarabine in chronic lymphocytic leukemia. The Journal of pharmacology and experimental therapeutics. 2013;344(2):378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharman J, Hawkins M, Kolibaba K, et al. An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2015;125(15):2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minden MD-v, Ubelhart R, Schneider D, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489(7415):309–312. [DOI] [PubMed] [Google Scholar]
- 5.Caligaris-Cappio F. B-chronic lymphocytic leukemia: a malignancy of anti-self B cells. Blood. 1996;87(7):2615–2620. [PubMed] [Google Scholar]
- 6.Ghia P, Strola G, Granziero L, et al. Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L+ T cells by producing CCL22. EurJImmunol. 2002;32(5):1403–1413. [DOI] [PubMed] [Google Scholar]
- 7.Patten PE, Buggins AG, Richards J, et al. CD38 expression in chronic lymphocytic leukemia is regulated by the tumor microenvironment. Blood. 2008;111(10):5173–5181. [DOI] [PubMed] [Google Scholar]
- 8.Os A, Burgler S, Ribes AP, et al. Chronic lymphocytic leukemia cells are activated and proliferate in response to specific T helper cells. Cell Rep. 2013;4(3):566–577. [DOI] [PubMed] [Google Scholar]
- 9.Burgler S, Gimeno A, Parente-Ribes A, et al. Chronic lymphocytic leukemia cells express CD38 in response to Th1 cell-derived IFN-gamma by a T-bet-dependent mechanism. J Immunol. 2015;194(2):827–835. [DOI] [PubMed] [Google Scholar]
- 10.van Kooten C, Banchereau J. CD40-CD40 ligand. Journal of leukocyte biology. 2000;67(1):2–17. [DOI] [PubMed] [Google Scholar]
- 11.Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. The Journal of experimental medicine. 1995;182(5):1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skanland SS, Moltu K, Berge T, Aandahl EM, Tasken K. T-cell co-stimulation through the CD2 and CD28 co-receptors induces distinct signalling responses. The Biochemical journal. 2014;460(3):399–410. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22(1):9–18. [DOI] [PubMed] [Google Scholar]
- 14.Yarkoni Y, Getahun A, Cambier JC. Molecular underpinning of B-cell anergy. Immunological reviews. 2010;237(1):249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contri A, Brunati AM, Trentin L, et al. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. J Clin Invest. 2005;115(2):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]


