Recent controversies regarding the prevalence of pulmonary hypertension (PH) in patients with sickle cell disease (SCD) have been raised.1 While the prevalence of PH differed among several SCD cohorts,2–4 the reported range of 6–11% is higher than the general population and those with HIV or scleroderma.1 PH in SCD is often complicated by concurrent left ventricular (LV) diastolic dysfunction and anemia-related changes in hemodynamics. The change in pressure across the pulmonary circulation as reflected by the transpulmonary pressure gradient (TPG) can be measured during right heart catheterization (RHC). TPG is less clouded by anemia-related adaptations than pulmonary vascular resistance (PVR), the more conventional indicator of pulmonary vascular disease. We have shown that elevation in the TPG is associated with increased mortality, but did not explore the relationship of TPG to functional capacity in our previous analyses.4 While the morphologic cardiac manifestations of sickle cell associated cardiomyopathy5–8 and rare left ventricular systolic dysfunction9 have been described, there have been no reports using cardiac magnetic resonance (CMR) imaging in conjunction with invasive hemodynamics to understand the relationship of TPG and right ventricular (RV) remodeling in patients with SCD.
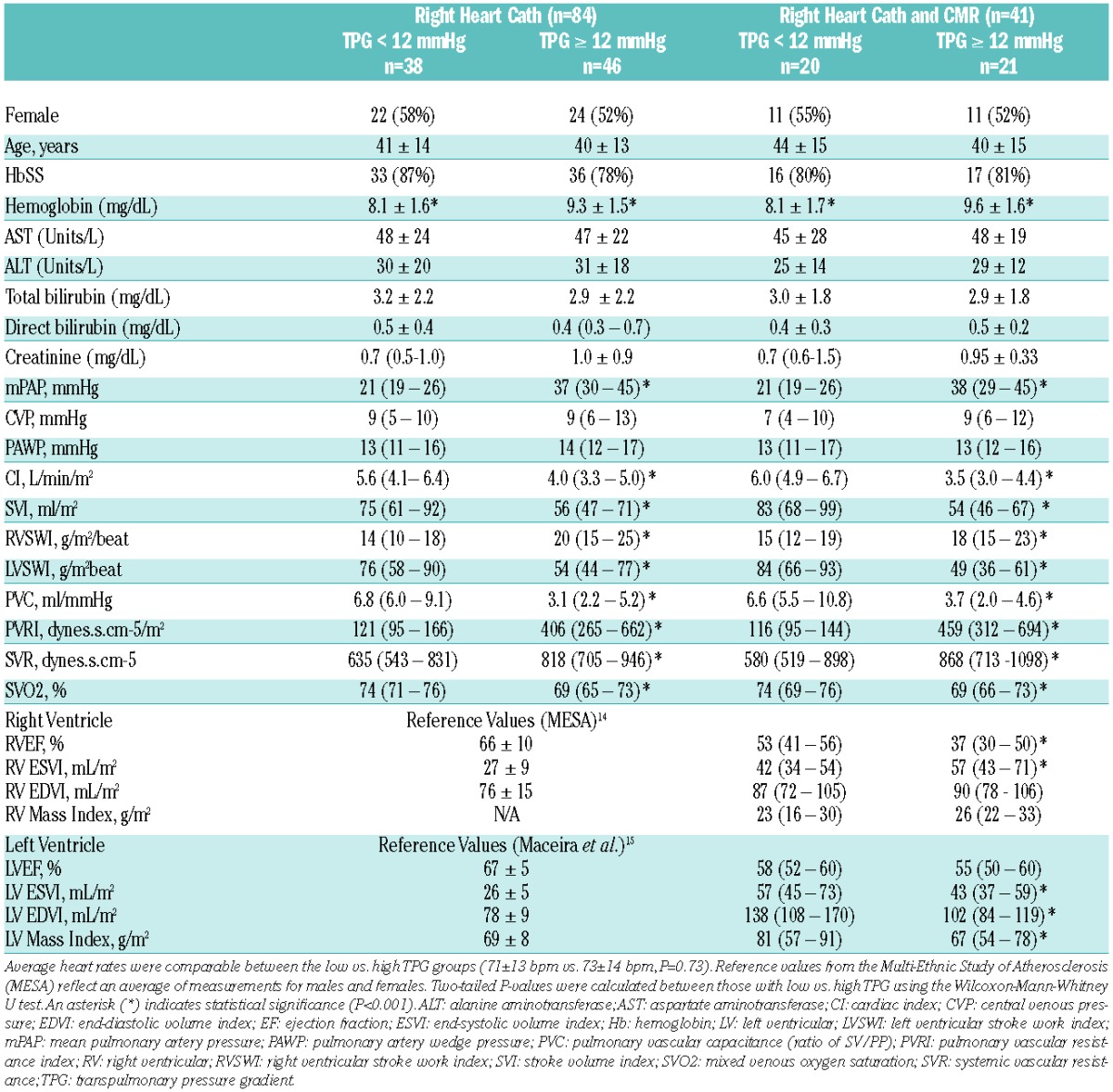
Accordingly, we performed a new hypothesis-generating analysis to assess the relationship of RHC-derived TPG and CMR-derived RV remodeling in patients with SCD. We also investigated the significance of using a TPG threshold and evidence of RV dysfunction to identify patients with overall poor outcomes. Detailed patient enrollment criteria of the Bethesda Sickle Cell Cohort and study procedures have been described.4 Descriptions of procedures and image acquisition are available in the Online Supplementary Methods. Data from 84 consecutive patients with RHC were analyzed; 41 patients underwent CMR within two days of RHC. Patient characteristics are summarized in Table 1. The hemodynamic composition of sickle cell patients with normal vs. elevated TPG are further described in the Online Supplementary Appendix Table S1.
Table 1.
Characteristics of study population.
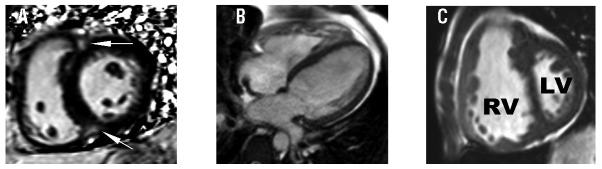
Univariate analyses showed TPG ≥12mmHg and right ventricular ejection fraction (RVEF) <32% were significant predictors of poor survival (Figure 1). In multivariate analysis controlling for age and phenotype, TPG ≥12 mmHg and RVEF <32% remained independently predictive of increased mortality (adjusted hazard ratio [HR] 5.47, 95% CI 1.13–26.42, P=0.035 and adjusted HR 5.11, 95% CI 1.13–23.13, P=0.034, respectively). A TPG ≥12mmHg identified sickle cell patients with a lower than expected cardiac index (P<0.05) (Table 1) and hemodynamics consistent with ventricular interdependence. Patients with TPG ≥12mmHg had a higher prevalence of late gadolinium enhancement (LGE) at the right ventricular insertion points (RVIP) (P=0.030, Figure 2A) and abnormal CMR markers of ventricular remodeling, including increased ventricular mass index (P=0.016, Figure 2B), increased septo-marginal trabeculae (SMT) mass index (P=0.002), and decreased end-diastolic septal-to-LV free wall curvature ratio (P=0.013). LV eccentricity index at end-systole was greater in those with high TPG (P=0.026), indicative of elliptical distortion (Figure 2C).
Figure 1.

Hemodynamic and CMR predictors of mortality in patients with sickle cell disease. (A) Kaplan-Meier survival curve illustrates decreased survival among sickle cell patients with CMR-derived RVEF <32% (n=41). (B) Using a combination of transpulmonary gradient (TPG) and pulmonary artery wedge pressure (PAWP) (n=84) as predictors, Kaplan-Meier survival curves illustrate highest mortality in patients with TPG ≥12mmHg and PAWP ≥15mmHg (52% [14 out of a total of 27 deaths], P=0.001) versus the three remaining groups. Using PAWP ≥15mmHg vs. PAWP <15mmHg alone to dichotomize mortality showed no statistical significance (59% [16 out of 27 deaths] vs. 41% [11 out of 27 deaths], P=0.121). All subjects were censored at 7.9 years. Median followup time was 4.7 years.
Figure 2.
CMR markers of right ventricular remodeling in sickle cell patients with an elevated transpulmonary gradient (TPG). Compared to sickle cell patients with a transpulmonary gradient (TPG) <12mmHg, those with TPG ≥12mmHg have (A) higher frequency of late gadolinium enhancement at the right ventricular insertion point (arrows), (B) higher ventricular mass index, (RV:LV ratio) and (C) higher eccentricity index indicative of elliptical distortion at end-systole consistent with ventricular interdependence. RV: right ventricle; LV: left ventricle.
A significant negative correlation was observed between CMR-derived RVEF and TPG (Spearman r=−0.54, P<0.001). RV dysfunction was also associated with higher New York Heart Association (NYHA) functional classification III/IV (P=0.038), and shorter 6-minute walk distance (6-MWD) (P=0.043). Although the sample size limited our ability to directly compare imaging and hemodynamic data in different combinations of those with and without elevated TPG and PH, we did observe that those with mPAP ≥25mmHg and high TPG also had a lower RVEF (6 out of 18 with RVEF <32%) and higher mortality (10 out of 18 died). We noted that patients with high TPG were limited in their exercise capacity during the 6-MWD. Thirty-three percent of patients in the high TPG group experienced NYHA class III–IV symptoms of dyspnea, angina, and syncope compared to 18% in the low TPG group.
Lastly, a trend for increased mortality was observed in those with an LV EDVI ≤115mL/m2 (HR 3.0, P=0.091), RV EDVI ≥84mL/m2 (HR 2.67, P=0.14), or RV mass index ≥34g/m2 (HR 2.93, P=0.108). An LV EDVI ≤115mL/m2 was associated with increased symptom severity (P=0.047) and decreased 6-MWD (P=0.003). Multivariate analyses of continuous variables, controlling for age and phenotype, showed significant association between LV EDVI, ventricular mass index, and SMT mass index with NYHA III/IV classification and a 6-MWD ≤400 meters (P<0.05).
In this hypothesis-generating analysis, we demonstrated CMR evidence of RV remodeling in those with elevated TPG. We found that a CMR-derived RVEF <32% was associated with decreased survival and higher NYHA classification. A TPG ≥12mmHg effectively categorizes adults with SCD by symptom severity, functional capacity, mortality, and RV function.
Patients with SCD and elevated PA pressure often demonstrate features of either pre- or post-capillary PH, and sometimes both simultaneously,10 but with a distinctive hemodynamic profile: typically borderline elevation in mPAP and PVR along with a higher CO when compared to their idiopathic PAH counterpart.10,11 Despite the borderline elevation in mPAP and PVR, patients with SCD have similar mortality rates to those with PAH. It is accepted that normal resting mPAP is 8–20mmHg; whereas a mPAP ≥25mmHg by RHC is considered to be the threshold for diagnosis of PH. A mPAP between 21–24mmHg is of unclear clinical significance. However, recent data12 suggest that in those with borderline mPAP, an elevated TPG was associated with poor survival. Vachiery et al.13 recently called for a change in terminology when describing the contribution of a pre-capillary component to post-capillary PH, arguing that mechanical components of increased PAWP could trigger pulmonary vasoconstriction and associated changes leading to pulmonary vascular disease, increased RV afterload, and, potentially, RV failure. While not a primary aim in our analysis, we found that TPG performed similarly to the diastolic pulmonary vascular pressure gradient (DPG), a marker of increasing interest. We observed that TPG and DPG are highly correlated with Pearson’s correlation r=0.94, P<0.0001. In our modest cohort of SCD patients, TPG and DPG performed very similarly in predicting mortality (AUC of 0.73 [95% CI 0.61–0.86] for TPG vs. 0.74 [95% CI 0.61–0.87] for DPG, P=0.68) (Online Supplementary Discussion). Herein, rather than classifying sickle cell patients as having pre- or post-capillary PH or no PH, we classified patients as having a high vs. low TPG. This categorization allowed identification of sickle cell patients with lower cardiac index (CI) despite normal LVEF and those with poor functional capacity. Previous categorization by our group using mPAP ≥25mmHg was unable to identify those with lower CI.4
To our knowledge, no previous report has presented CMR evidence of RV remodeling in relation to pulmonary hemodynamics, functional capacity and prognosis in SCD. Several groups have used CMR in small patient populations with SCD and/or thalassemia to describe RV and LV function,7,8 myocardial perfusion, myocardial fibrosis,8 and myocardial iron content.6 Sickle cell associated cardiomyopathy7 was described as 4-chamber dilation with some having myocardial fibrosis, and rarely myocardial iron overload, but no correlative hemodynamics or prognostic data were reported.
We also observed a 4-chamber dilation. However, in patients with high TPG, the indexed LV volumes and mass were lower while the indexed RV volumes and mass were higher with corresponding lower RVEF. Our findings suggest that in patients with SCD and high TPG, the left heart chamber is typically under-filled and CMR findings correlate with RHC findings of higher RVSWI, lower LVSWI, and lower LV cardiac index despite normal LVEF. Along with RV systolic dysfunction, we observed a higher prevalence of LGE at the RVIP in the high TPG group, consistent with prior publications.7,8
Our study has several limitations. Although 84 patients underwent RHC, only 41 of our patients had both RHC and CMR, which is relatively modest, but remains the only published cohort of patients to have both hemodynamic and CMR studies. Due to the small sample size, our findings should be interpreted as hypothesis-generating rather than definitive conclusions. Further, the sample size limitation may have increased type II errors and therefore, our study was only able to detect large differences. Because our subjects were grouped according to TPG rather than by non-PH vs. PH based on conventional definitions of PH, our preliminary findings should be interpreted in the context of those having elevated TPG vs. normal TPG rather than those with PH vs. without PH. Additionally, our grouping based on TPG also introduced a degree of hemodynamic heterogeneity into our study population when one views the population from the conventional definition of PH vs. no PH. Despite the limitations outlined, we were able to demonstrate statistical difference in many novel CMR markers indicative of RV remodeling.
We noted higher hemoglobin in the high TPG group, but the reasons for this may be complex. The higher untransfused hematocrit in SCD can contribute to higher whole blood viscosity, which may in turn contribute to the pressure gradient across the pulmonary circulation. PH in the sickle cell population has been associated with higher hemolytic rate and lower hemoglobin. As a result, clinically symptomatic patients are also more prone to having chronic red cell transfusion therapy prior to RHC and CMR, potentially elevating their hemoglobin and hematocrit at the time of evaluation. These possibilities warrant future investigation.
Our preliminary findings suggest that RV function and morphology may have prognostic implications in patients with SCD. While TPG may not help to pinpoint the specific pathophysiology of PH in SCD, TPG may reflect a composite of complex hemodynamic factors affecting RV function, and therefore poor outcomes were observed in SCD patients with high TPG. Rather than using a single measurement or predictor to assess outcomes in this complex population, perhaps the role of RV adaptation to afterload along with other hemodynamic variables, including the TPG, merit further investigation in a larger cohort to help guide prognosis in patients with SCD.
Acknowledgments
Dr. Arai is the principal investigator on a U.S. Government Cooperative Research and Development Agreement (CRADA) with Siemens.
Footnotes
Funding: this research was supported by the Division of Intramural Research of the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (1ZIAHL006011, 1ZIAHL006015, 1ZIAHL006012, 1ZIAHL004607, 1ZIAHL006137, 1ZIEHL006139, 1ZIDHL006140). ClinicalTrials.gov identifiers: NCT00011648, NCT00081523, NCT00023296, NCT00352430.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Gladwin MT. Prevalence, risk factors and mortality of pulmonary hypertension defined by right heart catheterization in patients with sickle cell disease. Expert Rev Hematol. 2011;4(6):593–596. [DOI] [PubMed] [Google Scholar]
- 2.Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011; 365(1):44–53. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell. Eur Respir J. 2012;39(1):112–118. [DOI] [PubMed] [Google Scholar]
- 4.Mehari A, Alam S, Tian X, et al. Hemodynamic predictors of mortality in adults with sickle cell disease. Am J Respir Crit Care Med. 2013;187(8):840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachdev V, Kato GJ, Gibbs JS, et al. Echocardiographic markers of elevated pulmonary pressure and left ventricular. Circulation. 2011; 124(13):1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westwood MA, Shah F, Anderson LJ, et al. Myocardial tissue characterization and the role of chronic anemia in sickle cell cardiomyopathy. J Magn Reson Imaging. 2007;26(3):564–568. [DOI] [PubMed] [Google Scholar]
- 7.Desai AA, Patel AR, Ahmad H, et al. Mechanistic insights and characterization of sickle cell disease-associated cardiomyopathy. Circ Cardiovasc Imaging. 2014;7(3):430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junqueira FP, Fernandes JL, Cunha GM, et al. Right and left ventricular function and myocardial scarring in adult patients with sickle cell disease: a comprehensive magnetic resonance assessment of hepatic and myocardial iron overload. J Cardiovasc Magn Reson. 2013;15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gladwin MT, Sachdev V. Cardiovascular abnormalities in sickle cell disease. J Am Coll Cardiol. 2012;59(13):1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klings ES. Pulmonary hypertension of sickle cell disease: more than just another lung. Am J Hematol. 2008;83(1):4–5. [DOI] [PubMed] [Google Scholar]
- 11.Leight L, Snider TH, Clifford GO, Hellems HK. Hemodynamic studies in sickle cell anemia. Circulation. 1954;10(5):653–662. [DOI] [PubMed] [Google Scholar]
- 12.Heresi GA, Minai OA, Tonelli AR, et al. Clinical characterization and survival of patients with borderline elevation in pulmonary artery pressure. Pulm Circ. 2013;3(4):916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vachiery JL, Adir Y, Barbera JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100–108. [DOI] [PubMed] [Google Scholar]
- 14.Tandri H, Daya SK, Nasir K, et al. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol. 2006;98(12):1660–1664. [DOI] [PubMed] [Google Scholar]
- 15.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006; 8(3):417–426. [DOI] [PubMed] [Google Scholar]