Abstract
Increasing numbers of patients are receiving reduced intensity conditioning regimen allogeneic hematopoietic stem cell transplantation. We hypothesized that the use of bone marrow graft might decrease the risk of graft-versus-host disease compared to peripheral blood after reduced intensity conditioning regimens without compromising graft-versus-leukemia effects. Patients who underwent reduced intensity conditioning regimen allogeneic hematopoietic stem cell transplantation from 2000 to 2012 for acute leukemia, and who were reported to the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation were included in the study. Eight hundred and thirty-seven patients receiving bone marrow grafts were compared with 9011 peripheral blood transplant recipients after reduced intensity conditioning regimen. Median follow up of surviving patients was 27 months. Cumulative incidence of engraftment (neutrophil ≥0.5×109/L at day 60) was lower in bone marrow recipients: 88% versus 95% (P<0.0001). Grade II to IV acute graft-versus-host disease was lower in bone marrow recipients: 19% versus 24% for peripheral blood (P=0.005). In multivariate analysis, after adjusting for differences between both groups, overall survival [Hazard Ratio (HR) 0.90; P=0.05] and leukemia-free survival (HR 0.88; P=0.01) were higher in patients transplanted with peripheral blood compared to bone marrow grafts. Furthermore, peripheral blood graft was also associated with decreased risk of relapse (HR 0.78; P=0.0001). There was no significant difference in non-relapse mortality between recipients of bone marrow and peripheral blood grafts, and chronic graft-versus-host disease was significantly higher after peripheral blood grafts (HR 1.38; P<0.0001). Despite the limitation of a retrospective registry-based study, we found that peripheral blood grafts after reduced intensity conditioning regimens had better overall and leukemia-free survival than bone marrow grafts. However, there is an increase in chronic graft-versus-host disease after peripheral blood grafts. Long-term follow up is needed to clarify whether chronic graft-versus-host disease might increase the risk of late morbidity and mortality.
Introduction
Indications for allogeneic hematopoietic stem cell transplantation (HCT) have changed in the past decade. The most common indication for HCT in 2013 was acute myeloid leukemia (AML), and currently nearly 50% of HCTs are being carried out for acute leukemia including AML and acute lymphoid leukemia (ALL).1,2 Furthermore, following the introduction of reduced intensity conditioning (RIC) regimens, individuals with co-morbidities and those 65 years of age and older are now eligible to undergo HCT.3–5
The use of peripheral blood stem cells (PB) has largely replaced bone marrow (BM) as the main stem cell source for HCT in adults with hematologic malignancies.1 However, limited data are available on the impact of the stem cell source (related or unrelated donor) in the setting of RIC HCT,6–10 including our small previous European Group for Blood and Marrow Transplantation (EBMT) series.9,10 In 8 of 9 published comparative randomized studies, all participants received a myeloablative HCT, using different regimens depending on the underlying disease.6 Only one randomized study by Anasetti et al.7 included RIC regimens in the comparative analysis of BMT versus PBSCT for a variety of hematologic malignancies. Participants predominantly received a myeloablative HCT, with only 20% of the BMT group receiving RIC, and very few were performed for acute leukemia. A study recently published by the Center for International Blood and Marrow Transplant Research (CIBMTR) reported comparative analysis of BMT versus PBSCT after RIC regimen.8 Similar to the report by Anasetti et al.,7 the study population included a variety of hematologic malignancies and the analysis included only 108 with AML patients in the BMT RIC group. Outcomes for unrelated donor allografting have improved over time. This is probably due to improved high-resolution HLA-typing and better matching, and the availability of intensive supportive care.11,12 Moreover, outcomes for unrelated donor HCT after RIC regimens appear comparable to those seen with sibling donor RIC HCT.3,13–15 Here we report on transplant outcomes after related or unrelated donor BMT (n=837) versus PBSCT (n=9011) in the setting of reduced intensity conditioning for the treatment of adults with acute leukemia using data reported to the ALWP of the EBMT from 2000 to 2012.
Methods
Study design and data collection
This was a retrospective multicenter registry analysis. Data were provided and approved for this study by the Acute Leukemia Working Party (ALWP) of the EBMT group registry. The EBMT is a non-profit, scientific society representing more than 600 transplant centers, mainly in Europe. The EBMT promotes all initiatives that aim to improve stem cell transplantation or cellular therapy, which includes registering all activities relating to stem cell transplants. Data are entered, managed, and maintained in a central database that includes all EBMT centers. There are no restrictions regarding centers that can report data except those legal requirements concerning patient consent, data confidentiality and accuracy. Quality control measures include several independent systems: confirmation of validity of the entered data by the reporting team, selective comparison of the survey data with MED-A data sets in the EBMT registry database, cross-checking with the national registries, and regular in-house and external data audits. Since 1990, patients have provided informed consent authorizing the use of their personal information for research purposes. Eligibility criteria for this analysis included adult patients (age >18 years) with acute leukemia receiving HLA-matched or mismatched related or unrelated donor BM or PB transplants after RIC regimens from 2000 to 2012. Two hundred and ninety-four transplant centers reported data on recipients of BM and PB grafts after related or unrelated donor transplantation. We do not have any information about why patients were allocated to a specific graft (BM vs. PB) in the registry, and it is difficult to distinguish between the role of the conditioning approach adopted and the role of a potential effect of the individual center (“center effect”); however, a center effect was not evident in the analysis. All unrelated donors were HLA (-A, -B, -C, DRB1, -DQB1) matched (10/10) or mismatched at one loci. Exclusion criteria included previous allogeneic or cord blood transplantation, and recipients of grafts that were either ex vivo T-cell depleted or CD34 selected. Data were collected on recipient and donor characteristics [age, gender, cytomegalovirus (CMV) serostatus], disease status at transplant, transplant-related factors including conditioning regimen, immunosuppression (in vivo T-cell depletion vs. none), stem cell source (BM or PB), graft-versus-host disease (GvHD) prophylaxis, and outcome variables [acute and chronic GvHD,16,17 relapse, non-relapse mortality (NRM), leukemia-free survival (LFS), overall survival (OS), and causes of death]. Regimens were classified as RIC based on published criteria.18
Statistical analysis
The primary end points of the study were OS and LFS. Secondary end points included relapse incidence (RI), NRM, engraftment, incidence and severity of acute and chronic GvHD. The starting point for time-to-event analysis was date of transplantation. OS was defined as the time to death from any cause. Surviving patients were censored at time of last follow up. LFS was defined as survival without relapse or progression. Patients surviving in continuous CR were censored at the time of last follow up. RI was defined as time to onset of leukemia recurrence. NRM was the competing risk, and patients surviving in continuous complete remission were censored at last contact. NRM was defined as death without relapse/progression (relapse was the competing risk). The two groups were compared according to the stem cell sources (BM vs. PB) using the χ2 test for qualitative variables, whereas the Mann-Whitney test was applied for continuous parameters. Univariate comparisons were made using the log rank test for OS, LFS, and the Gray test for RI, NRM and GvHD cumulative incidences. Multivariate analyses were performed using logistic regression for acute GvHD and Cox proportional hazards model for all other end points (variables tested are provided in Table 1). All factors known as potentially related to the outcome were included in the final model. First-order interactions between the main effect and the other variables were tested in multivariate models. All tests were two-sided. The type I error rate was fixed at 0.05 for determination of factors associated with time to event outcomes. Statistical analyses were performed with SPSS 22.0 (IBM Corp., Armonk, NY, USA) and R 3.1.1 software packages (R Development Core Team, Vienna, Austria).
Table 1.
Patients’ disease and transplant characteristics.
Results
Patients’, disease and transplant characteristics
Details of patients’, disease and transplant characteristics are summarized in Table 1.
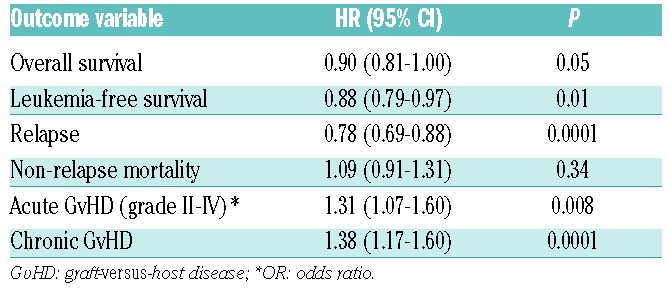
A total of 9848 patients with AL were included in the study: 837 patients received BM and 9011 PB transplants performed between 2000 and 2012; 8777 (89.1%) patients had AML (BM=702, PB=8075) and 1071 (10.9%) ALL (BM=135, PB=936). PBSCT recipients were older with a median age of 57 years (range 18–77) in comparison to 54 years (range 18–77) for the BM group (P<0.0001). Median follow up of surviving patients in the BM group was 29 (IQR, 12–56) months, while that of the PB group was 27 (IQR, 11–54) months (P=0.27). Significantly higher numbers of patients had Karnofsky Performance Status (KPS) score less than 90% (37 vs. 31%; P=0.006) in the BM group. There were more patients with advanced disease in PB compared to the BM group (26 vs. 22%; P=0.002). There were no statistically significant differences in cytogenetic risk categories in AML or ALL groups between patients receiving BM or PB transplantations. The proportion of CMV seropositive recipients and donors were comparable in the BM and PB groups [69% vs. 67% (P=0.26) and 54% vs. 53% (P=0.69), respectively].
Details of transplant characteristics and conditioning regimen are summarized in Table 1. The majority of patients received chemotherapy-based RIC regimens (BM=75%, PB=70%; P=0.002). Among the BM recipients, 388 (46%) received a graft from HLA-identical sibling donor, 220 (26%) matched unrelated donor (MUD) (10/10), 107 (13%) mismatched unrelated donor (MM-URD) (9/10), and 122 (15%) received mismatched-related donor HCT; corresponding numbers in the PB cohort were 4751 (53%), 2894 (32%), 950 (10%), and 416 (5%), respectively. The percentage of patients receiving in vivo T-cell depletion was higher in the PB group (61% vs. 48% in BM; P<0.0001). Patients receiving ex vivo T-cell depletion were excluded from the analysis. The choice of conditioning, graft source and GvHD prophylaxis was dependent on the protocols of the individual centers and the strategies adopted for transplantation.
Engraftment and graft-versus-host disease
Median time to neutrophil recovery (PMN ≥0.5×109/L) was 20 days and 16 days after BM and PB HCT, respectively (P<0.0001). The corresponding day 60 probability of neutrophil recovery (PMN ≥0.5×109/L at day 60) was 88% (95%CI: 86–90) after BM versus 95% (95%CI: 95–96) in the PB group (P<0.0001).
The rates of day 100 grade II-IV acute GvHD were 24% versus 19% (P=0.005) and 10% versus 6% (P=0.003) for grade III-IV GvHD in PB compared with BM transplant recipients.
In multivariate analysis, the factors associated with increased risk of grade II-IV acute GvHD were PB versus BM (OR 1.31; 95%CI: 1.07–1.60; P=0.008; Table 2), active disease (OR 1.33; 95%CI: 1.18–1.51; P<0.0001), poor cytogenetic risk group (OR 1.30; 95%CI: 1.10–1.53; P=0.002), CMV donor seropositivity (OR 1.12; 95%CI: 1.01–1.25; P=0.05), female donor for male recipients (OR 1.19; 95%CI: 1.04–1.35; P=0.01), MUD (10/10) and MM-URD (9/10) allo-SCT compared with HLA-identical donor [OR 1.78; 95%CI: 1.57–2.02 (P<0.0001) and OR 2.30; 95%CI: 1.95–2.71 (P<0.0001), respectively], and absence of in vivo T-depletion (OR 1.46; 95%CI: 1.30–1.64; P<0.0001).
Table 2.
Multivariate analysis adjusted outcomes after transplantation by graft source (peripheral blood vs. bone marrow).
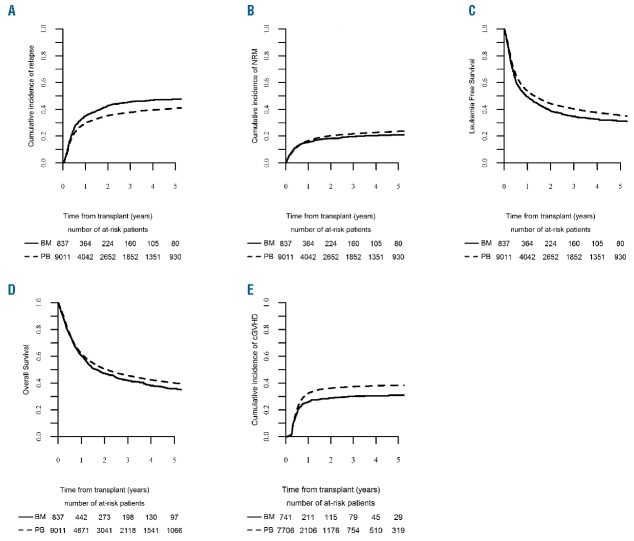
The 2-year incidence of chronic GvHD was higher after PB grafts [36% (95%CI: 35–37) vs. 29% (95%CI: 26–32) in the BM group; P=0.0003] (Table 3 and Figure 1). The severity of chronic GvHD was graded as extensive in 1287 (16.6%) of PBSCT recipients compared with 69 (9.3%) in the BMT group (P<0.0001). Similarly, the GvHD-related mortality was higher after PBSCT compared to BMT (13% vs. 7.4%; P=0.001).
Table 3.
The 2-year adjusted probabilities of transplant outcome after bone marrow versus peripheral blood grafts.
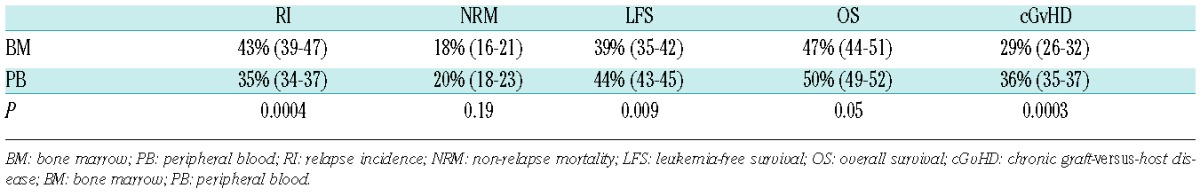
Figure 1.
Long-term outcomes after transplantation by graft type. (A) RI: relapse incidence. (B) NRM: non-relapse mortality. (C) LFS: leukemia-free survival. (D) OS: overall survival. (E) cGvHD: chronic graft-versus-host disease.
In multivariate analysis, chronic GvHD was significantly higher after PB grafts (HR 1.38; 95%CI: 1.17–1.60; P<0.0001) (Table 2). Other factors determining the risk of cGvHD that were independent of graft source included active disease at transplant (HR 1.20, 95%CI: 1.09–1.34; P=0.0004), older age at allo-SCT (HR 1.02, 95%CI: 1.00–1.03; P=0.03), female donor for male recipients (HR 1.18, 95%CI: 1.08–1.30; P=0.0004), MUD (10/10) and MM-URD (9/10) allo-SCT compared with HLA-identical donor (HR 1.12, 95%CI: 1.02–1.23; P=0.02, and HR 1.23, 95%CI: 1.07–1.41; P=0.003, respectively). There was no significant difference in risk of chronic GvHD between HLA-identical and related mismatched donors (HR 0.94, 95%CI: 0.76–1.15; P=0.54). In vivo T-cell depletion was associated with lower risk of chronic GvHD (HR 0.57, 95%CI: 0.53–0.63; P<0.0001) independent of graft type.
Non-relapse mortality
There was no significant difference in 2-year NRM between the BM and PB groups in univariate analysis (18%, 95%CI: 16–21 after BM versus 20%, 95%CI: 18–23 after PB grafts; P=0.19) (Table 3 and Figure 1).
In multivariate analysis, there was no significant difference in NRM between BM and PB grafts (HR 1.09, 95%CI: 0.91–1.31; P=0.34) (Table 2). Other factors associated with higher NRM independent of graft source were CR2 versus CR1 (HR 1.27, 95%CI: 1.11–1.45; P=0.0004), active disease (HR 1.85, 95%CI: 1.65–2.07; P<0.0001), secondary AML (HR 1.32, 95%CI: 1.17–1.48), older age at HCT (HR 1.08, 95%CI: 1.06–1.10; P<0.0001), CMV seropositive recipients (HR 1.34, 95%CI: 1.20–1.50; P<0.0001), CMV seropositive donors (HR 1.13, 95%CI: 1.02–1.25; P=0.02), female donor for a male recipient (HR 1.21, 95%CI: 1.07–1.36; P=0.002), matched-URD (10/10), MM-URD (9/10) and related mismatched donors compared with HLA-identical donor [HR 1.60, 95%CI: 1.42–1.80 (P<0.0001); HR 2.11, 95%CI: 1.81–2.45 (P<0.0001), and HR 2.34 95%CI: 1.94–2.83 (P<0.0001), respectively]. In vivo T-cell depletion was associated with lower NRM (HR 0.79, 95%CI: 0.71–0.87; P<0.0001) independent of graft type.
Relapse
Two-year RI was lower after PB (35%, 95%CI: 34–37) compared to BM transplants (43%, 95%CI: 39–47) (P=0.0004) (Table 3 and Figure 1).
In multivariate analysis, RI was significantly lower after PB grafts (HR 0.78, 95%CI: 0.69–0.88; P=0.0001) (Table 2). Factors associated with higher RI independent of graft source were: diagnosis of ALL versus AML (HR 1.38, 95%CI: 1.23–1.55; P<0.0001), CR2 versus CR1 (HR 1.26, 95%CI: 1.13–1.39), active disease (HR 2.45, 95%CI: 2.26–2.66; P<0.0001), poor cytogenetic risk group (HR 1.51, 95%CI: 1.36–1.68; P<0.0001), in vivo T-cell depletion (HR 1.10, 95% CI, 1.01–1.19; P=0.02), and low-dose total body irradiation (TBI)-based RIC regimens compared to chemotherapy-based regimens (fludarabine combined with busulfan or melphalan) (HR 1.12, 95%CI: 1.03–1.21; P=0.01). Relapse risk was lower for patients receiving MUD (10/10) or MM-URD (9/10) compared with an HLA-identical donor [HR 0.77, 95%CI: 0.71–0.85 (P<0.0001) and HR 0.82, 95%CI: 0.73–0.93 (P=0.002), respectively].
Leukemia-free survival
Two-year LFS was higher after PB (44%, 95%CI: 43–45) compared with BM transplants (39%, 95%CI: 35–42) (P=0.009) (Table 3 and Figure 1).
In multivariate analysis, the only factor associated with superior LFS was the use of PB grafts (HR 0.88, 95%CI: 0.79–0.97; P=0.01) (Table 2).
Factors associated with inferior LFS independent of graft source were diagnosis of ALL versus AML (HR 1.42, 95%CI: 1.29–1.57; P<0.0001), CR2 versus CR1 (HR 1.26, 95%CI: 1.16–1.37), active disease (HR 2.22, 95%CI: 2.07–2.37; P<0.0001), poor cytogenetic risk group (HR 1.35, 95%CI: 1.23–1.47; P<0.0001), secondary AML (HR 1.16, 95%CI: 1.08–1.25), older age at HCT (HR 1.03, 95%CI: 1.02–1.04; P<0.0001), CMV-positive recipients (HR 1.07, 95%CI: 1.00–1.14; P=0.04), MM-URD (9/10) or mismatched-related donor compared with HLA-identical donor [HR 1.16, 95%CI: 1.05–1.27 (P=0.003) and HR 1.26, 95%CI: 1.12–1.43 (P=0.0002), respectively] and low-dose TBI-based RIC regimens compared with chemotherapy-based regimens (fludarabine combined with busulfan or melphalan) (HR 1.08, 95%CI: 1.01–1.15; P=0.02).
Overall survival
Two-year OS was higher after PB grafts (50%, 95%CI: 49–52) compared with BM transplants (47%, 95%CI: 44–51) (P=0.05) (Table 3 and Figure 1). In multivariate analysis, factors associated with superior OS were PB grafts (HR 0.90, 95%CI: 0.81–1.00; P=0.05) (Table 2) and in vivo T-cell depletion (HR 0.93, 95%CI: 0.87–1.00; P=0.04).
Factors associated with inferior OS independent of graft source included a diagnosis of ALL versus AML (HR 1.42, 95%CI: 1.28–1.57; P<0.0001), CR2 versus CR1 (HR 1.28, 95%CI: 1.17–1.39; P<0.0001), active disease at transplant (HR 2.21, 95%CI: 2.06–2.37; P<0.0001), poor cytogenetic risk group (HR 1.27, 95%CI: 1.15–1.39; P<0.0001), secondary AML (HR 1.17, 95%CI: 1.08–1.26; P=0.0001), older age at HCT (by 10 years) (HR 1.04, 95%CI: 1.03–1.06; P<0.0001), CMV-positive recipients (HR 1.12, 95%CI: 1.05–1.20; P=0.0001), female donor for male recipients (HR 1.09, 95%CI: 1.01–1.18; P=0.02), MUD (10/10), MM-URD (9/10) donors and MM-related donor compared with HLA-identical donor [HR 1.09, 95%CI: 1.01–1.18 (P=0.02), HR 1.31, 95%CI: 1.18–1.45 (P<0.0001) and HR 1.44, 95%CI: 1.27–1.64 (P<0.0001), respectively] and low-dose TBI-based RIC regimens compared chemotherapy-based regimens (fludarabine combined with busulfan or melphalan) (HR 1.08, 95%CI: 1.01–1.16; P=0.03).
Impact of graft source on other variables
The primary purpose of our analysis was to explore outcome differences between BM and PB transplantation in patients with acute leukemia after RIC regimens. The effect of stem cell source (BM vs. PB) on survival was independent of disease (interaction test P=0.22). There was no significant interaction between the graft source and disease status at transplant (interaction test P=0.57). Results were similar among HLA-mismatched pairs and recipients with active disease, although this study was not designed to detect potential differences within these subsets.
Discussion
This large, multicenter, registry study shows that the use of PB grafts after RIC gives superior outcome in patients with acute leukemia. Moreover, PBSCT recipients had a lower risk of relapse, most probably due to a stronger graft-versus-leukemia (GvL) effect not just in patients in CR, but, more importantly, in patients with active disease pre-HCT. This finding is of major clinical significance as an increasing number of patients are undergoing RIC HCT for acute leukemia, a significant number of whom receive BM grafts;1 indeed, 8.5% (n=837) of 9848 patients transplanted from 2000 to 2012 at centers reporting to the EBMT received BM grafts. However, the increased risk of chronic GvHD observed in our series after PB grafts is alarming, and long-term follow up is awaited to clarify if excess risk of chronic GvHD among RIC PBSCT recipients translates into continued GvL effect or increased late morbidity and mortality.19
We investigated patient, disease, and transplantation factors affecting survival, LFS, relapse, NRM and GvHD in a well-characterized population of nearly 10,000 adult acute leukemia patients receiving either BMT or PBSCT. Overall, nearly 50% of patients (Figure 1) transplanted after RIC HCT survived beyond two years, with higher survival for AML compared to ALL. Notably, the low-dose TBI-based regimen had inferior outcome (for both patients in CR1 and active diseases) compared to chemotherapy-based RIC regimens. Results were similar among related or unrelated donor groups.
Patients receiving in vivo T-cell depletion (either ATG or alemtuzumab) had a significantly lower risk of chronic GvHD and higher OS despite their higher relapse rate, which was offset by lower NRM. Anti-thymocyte globulin or alemtuzumab with standard GvHD prophylaxis (which has been shown to be effective in lowering GvHD rates as well as its severity) was used for 48% of BM and 61% of PB transplantations in our series. This study was not designed to analyze separately patients receiving low-dose ATG/alemtuzumab, which is commonly used today; however, previous studies have shown that low-dose ATG/alemtuzumab reduced the risk of chronic GvHD and NRM without compromising the GvL effect.20–25
Previous smaller studies demonstrated the non-inferiority of RIC PBSCT compared to RIC BMT for patients with acute leukemia.7–10 Our results differ from the findings in published studies comparing RIC outcomes with BM with PB grafts. This discrepancy may be due to differences in the study populations. Firstly, the improved survival, decreased relapse risk and higher rates of grade II to IV acute GvHD (also grade III-IV) after transplants with PB compared with BM grafts are in contrast to previous reports.6,8,26 The survival rates in the BM and PB groups in our study are comparable to a study from the CIBMTR by Eapen et al.,8 reporting a 3%–5% difference in OS and LFS between the two groups, which was not statistically significant. In our analysis, results were statistically significant, and this may reflect the difference in power between the two studies. Secondly, the cumulative incidence of engraftment and the risk of chronic GvHD were higher after PBSCT compared with BMT, in keeping with other reports.6,7,27,28
Chronic GvHD can impair quality of life and is associated with significant morbidity and mortality among HCT recipients. However, the costs involved, the economic burden and the use of resources to manage long-term complications associated with cGvHD have not been well described. Studies of transplantation costs are complex and difficult to conduct because of the wide variation in transplant methods, conditioning approaches, GvHD prophylaxis regimens and supportive care practices. More research is needed to better understand the costs of cGvHD to patients, centers and the health care system, and to determine whether the lower incidence and severity of cGvHD with BM grafts leads to long-term savings of resources compared to PB grafts. Also, trials aimed at better GvHD prophylaxis that is either drug-mediated or through graft manipulation are needed to reduce chronic GvHD rates after RIC PBSCT.
We acknowledge that there are differences in patients’, disease, and transplantation characteristics between those who received BMT and PBSCT. We have addressed this by performing a carefully controlled well-adjusted analysis that considered patients’, disease, and transplantation characteristics as well as any transplantation center effects in this multicenter registry analysis. In addition, there may be unmeasured and unknown factors that have not been considered, which is an inherent limitation of this type of analysis. However, we believe that the results of this analysis are very important in the absence of available prospective data.
Only through the conduct of well-designed clinical trials will we be able to understand and appreciate the complexities of stem cell source choices and their outcome after RIC HCT. Unfortunately, there are no ongoing trials to compare outcomes after BMT with that after PBSCT following an RIC regimen for acute leukemia. Therefore, in the absence of any prospect of such comparative studies, our data support the use of PB (related or unrelated donor) grafts after RIC for adult patients with acute leukemia in remission or with advanced disease.
Acknowledgments
We thank all European Group for Blood and Marrow Transplantation (EBMT) centers and national registries for contributing patients to the study and data mangers for their superb work. Supplementary information is available at the EBMT Web site. The list of institutions reporting data included in this study is available in the Online Supplementary Appendix.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/2/256
BNS thanks Dr. Katy Rezvani, MD, PhD (Houston, TX, USA), Dr. Agnes Yong, MD, PhD (Adelaide, Australia) and Prof. John Greer (Nashville, TN, USA) for critical reading of the manuscript.
References
- 1.Passweg JR, Baldomero H, Bader P, et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant. 2015;50(4):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR Summary Slides. Available from: http://www.cibmtr.org 2014.
- 3.Reshef R, Porter DL. Reduced-intensity conditioned allogeneic SCT in adults with AML. Bone Marrow Transplant. 2015;50(6):759–769. [DOI] [PubMed] [Google Scholar]
- 4.Bachanova V, Marks DI, Zhang MJ, et al. Ph+ ALL patients in first complete remission have similar survival after reduced intensity and myeloablative allogeneic transplantation: impact of tyrosine kinase inhibitor and minimal residual disease. Leukemia. 2014;28(3):658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner AM, Kim HT, Coughlin E, et al. Outcomes in patients age 70 or older undergoing allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2013;19(9):1374–1380. [DOI] [PubMed] [Google Scholar]
- 6.Holtick U, Albrecht M, Chemnitz JM, et al. Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults. Cochrane Database Syst Rev. 2014;4:CD010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eapen M, Logan BR, Horowitz MM, et al. Bone marrow or peripheral blood for reduced-intensity conditioning unrelated donor transplantation. J Clin Oncol. 2015;33(4):364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagler A, Labopin M, Shimoni A, et al. Mobilized peripheral blood stem cells compared with bone marrow from HLA-identical siblings for reduced-intensity conditioning transplantation in acute myeloid leukemia in complete remission: a retrospective analysis from the Acute Leukemia Working Party of EBMT. Eur J Haematol. 2012;89(3):206–213. [DOI] [PubMed] [Google Scholar]
- 10.Nagler A, Labopin M, Shimoni A, et al. Mobilized peripheral blood stem cells compared with bone marrow as the stem cell source for unrelated donor allogeneic transplantation with reduced-intensity conditioning in patients with acute myeloid leukemia in complete remission: an analysis from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(9):1422–1429. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. [DOI] [PubMed] [Google Scholar]
- 12.Majhail NS, Chitphakdithai P, Logan B, et al. Significant improvement in survival after unrelated donor hematopoietic cell transplantation in the recent era. Biol Blood Marrow Transplant. 2015;21(1):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta V, Tallman MS, He W, et al. Comparable survival after HLA-well-matched unrelated or matched sibling donor transplantation for acute myeloid leukemia in first remission with unfavorable cytogenetics at diagnosis. Blood. 2010;116(11):1839–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks DI, Wang T, Perez WS, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116(3):366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sengsayadeth S, Savani BN, Blaise D, Malard F, Nagler A, Mohty M. Reduced intensity conditioning allogeneic hematopoietic cell transplantation for adult acute myeloid leukemia in complete remission - a review from the Acute Leukemia Working Party of the EBMT. Haematologica. 2015;100(7):859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 17.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. [DOI] [PubMed] [Google Scholar]
- 18.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedrichs B, Tichelli A, Bacigalupo A, et al. Long-term outcome and late effects in patients transplanted with mobilised blood or bone marrow: a randomised trial. Lancet Oncol. 2010;11(4):331–338. [DOI] [PubMed] [Google Scholar]
- 20.Storek J, Mohty M, Boelens JJ. Rabbit Anti-T Cell Globulin in Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015;21(6):959–970. [DOI] [PubMed] [Google Scholar]
- 21.Bacigalupo A, Lamparelli T, Gualandi F, et al. Prophylactic antithymocyte globulin reduces the risk of chronic graft-versus-host disease in alternative-donor bone marrow transplants. Biol Blood Marrow Transplant. 2002;8(12):656–661. [DOI] [PubMed] [Google Scholar]
- 22.Rubio MT, Labopin M, Blaise D, et al. The impact of graft-versus-host disease prophylaxis in reduced-intensity conditioning allogeneic stem cell transplant in acute myeloid leukemia: a study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2015;100(5):683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakraverty R, Orti G, Roughton M, et al. Impact of in vivo alemtuzumab dose before reduced intensity conditioning and HLA-identical sibling stem cell transplantation: pharmacokinetics, GVHD, and immune reconstitution. Blood. 2010;116(16):3080–3088. [DOI] [PubMed] [Google Scholar]
- 24.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–864. [DOI] [PubMed] [Google Scholar]
- 25.Baron F, Labopin M, Blaise D, et al. Impact of in vivo T-cell depletion on outcome of AML patients in first CR given peripheral blood stem cells and reduced-intensity conditioning allo-SCT from a HLA-identical sibling donor: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(3):389–396. [DOI] [PubMed] [Google Scholar]
- 26.Eapen M, Logan BR, Appelbaum FR, et al. Long-term survival after transplantation of unrelated donor peripheral blood or bone marrow hematopoietic cells for hematologic malignancy. Biol Blood Marrow Transplant. 2015;21(1):55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flowers ME, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100(2): 415–419. [DOI] [PubMed] [Google Scholar]
- 28.Mohty M, Kuentz M, Michallet M, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation: long-term results of a randomized study. Blood. 2002;100(9):3128–3134. [DOI] [PubMed] [Google Scholar]