Minimal residual disease (MRD) is a powerful determinant of overall outcome in multiple myeloma (MM). Previous studies have demonstrated that the presence of MRD at the traditional day 100 assessment point following autologous stem cell transplant (ASCT) independently predicts for both progression-free (PFS) and overall survival (OS). This effect on outcome is demonstrable in patients achieving a complete response (CR) and in those with both high-risk and standard-risk cytogenetics.1–4 As a consequence, MRD assessment is currently being considered as a surrogate end point for survival in academic clinical trials and for regulatory drug approval.5,6 Surrogate end points are clearly desirable in MM given the increasing complexity of treatment schedules and continually improving complete response rates and survival, such that trials of up-front therapy require 5–10 years of follow up in order to demonstrate survival differences. Acceptance of MRD as an appropriate end point would also ideally require the demonstration that this effect was independent of the treatment received. We have, therefore, assessed the impact of induction regimen and MRD on outcome in the context of the Medical Research Council (MRC) Myeloma IX trial (ISRCTN68454111).
MRC Myeloma IX was a multi-center, randomized phase III trial with protocol and clinical results previously reported.7,8 All patients provided written informed consent. This analysis involves 397 patients randomly assigned to CTD (cyclophosphamide, thalidomide, and dexamethasone; n=189) or CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone; n=208) for 4–6 cycles, then high-dose melphalan (HDM, 200 mg/m2) and ASCT. Bone marrow (BM) aspirates for MRD assessment were obtained at the end of induction (n=252) and at day 100 post ASCT (n=397). Patients were then randomly assigned to maintenance thalidomide (50–100 mg daily) or no further therapy. Flow cytometry for MRD detection was performed as reported previously. Briefly, we assessed 500,000 cells incubated with 6-color antibody combinations including CD138/CD38/CD45/CD19 with CD56/CD27 in all cases and CD81/CD117 in some cases as required.3 Statistical analyses were landmarked from date of MRD assessment, with a median follow up of 71 months. Fisher’s exact test was used to perform between-group comparisons and survival (OS and PFS) was analyzed using Kaplan-Meier and the log rank tests with a 5% significance level.
We have previously reported that the CTD regimen showed superior categorical response rates to CVAD. Overall response rate was 82.5%: CTD versus 71.2% with CVAD (P<0.0001). Similarly, the CR rate was 13.0% versus 8.1% when assessed at the end of induction and 50.0% versus 37.2% post ASCT (P=0.008 and 0.0005, respectively).7 MRD analysis was performed in a subset of 397 patients and a greater proportion of patients became MRD-negative with CTD than with CVAD both at the end of induction (25% vs. 13%; P=0.0039) and post ASCT (71% vs. 54%; P<0.001).3 When outcome is assessed according to MRD status post ASCT, there is a highly significant outcome advantage for those who are MRD negative (median PFS 28.6 vs. 15.9 months, P<0.001; median OS 80.6 vs. 59.0 months, P=0.018).3 When this effect is also assessed according to the induction therapy received, it is clear that the prognostic effect of MRD negativity is identical in those who received CTD and those who received CVAD. For MRD-negative patients, the median PFS was 28.9 months with CTD versus 28.7 months with CVAD (P=0.54) (Figure 1A) while the median OS was 80.6 months versus not reached (P=0.81) (Figure 1B). Similarly, in MRD-positive patients, outcome was identical with each induction regimen, with a median PFS of 14.9 months with CTD versus 15.9 months for CVAD (P=0.96) (Figure 1A) and median OS 58.7 versus 61.9 months (P=0.91) (Figure 1B). This pattern was also demonstrable when the PFS analysis was restricted to patients achieving a conventional immunofixation-negative complete response (Figure 1C).
Figure 1.
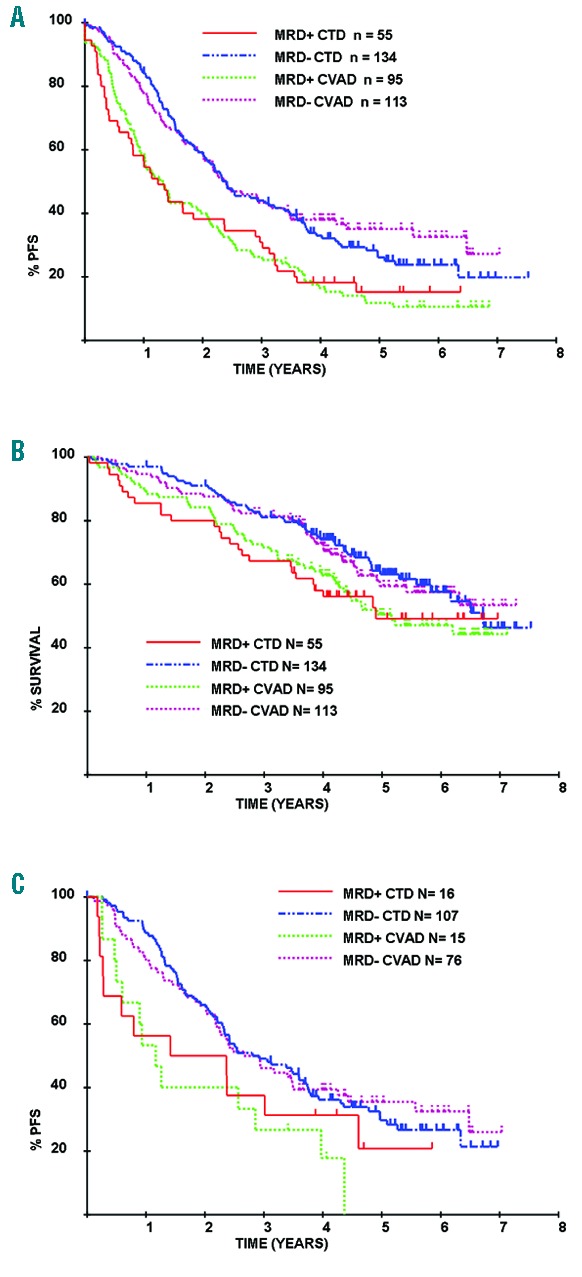
Outcome following autologous stem cell transplant (ASCT) according to day 100 minimal residual disease (MRD) and induction therapy received. The impact of MRD on outcome did not differ according to induction therapy received. For MRD-negative patients the median PFS for CTD was 28.9 months versus 28.7 months for CVAD (P=0.54) (A) while the median OS was 80.6 months and not reached, respectively (P=0.81) (B). Similarly for MRD-positive patients, median PFS for CTD was 14.9 months versus 15.9 months for CVAD (P=0.96) (A) and median OS 58.7 months and 61.9 months, respectively (P=0.91) (B). A similar pattern is also seen when PFS analysis is restricted to complete response (CR) patients (C).
We and others have previously demonstrated that the outcome of patients is also impacted by MRD at the end of induction as well as following ASCT, such that the outcome is best in those MRD-negative at both time points, and worst in those with detectable disease at both time points.1,3 We have also assessed this according to induction regimen and found no significant differences. In those patients who were MRD-negative at both time points, the median PFS was 44.2 months for those receiving CTD and 40.7 months for those receiving CVAD (P=0.84). Similarly, outcomes were identical in those who were MRD-positive post induction and MRD-negative post ASCT (median PFS 25.0 months, CTD; 24.2 months, CVAD; P=0.56) and in those who were positive at both time points (median PFS 13.3 months, CTD; 14.0 months, CVAD; P=0.79) (Figure 2).
Figure 2.
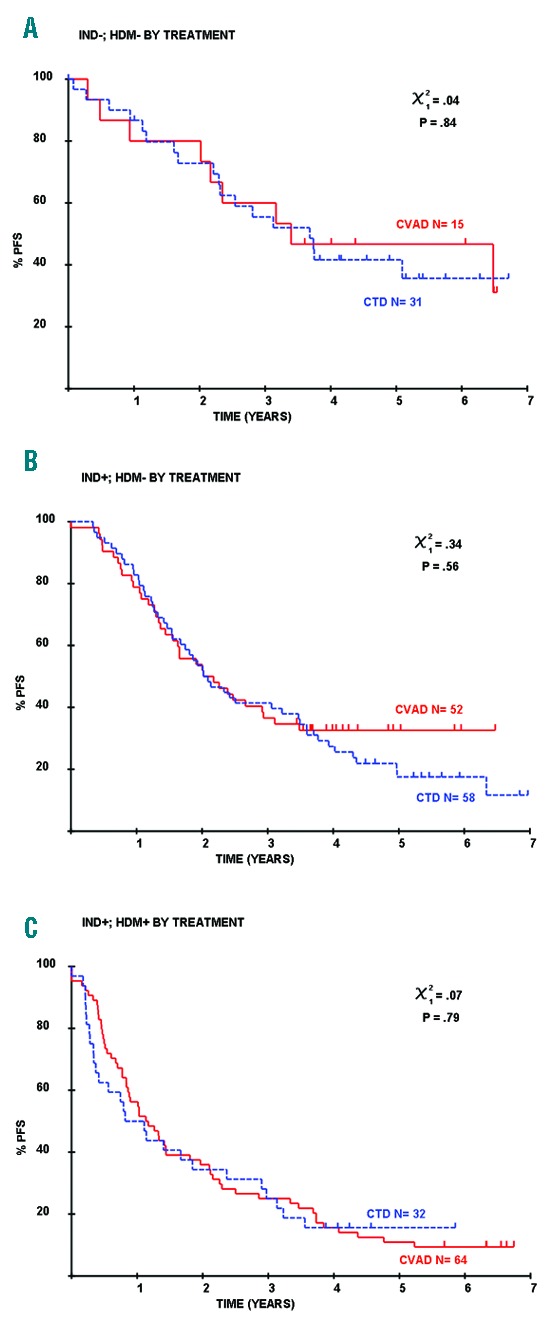
Induction regimens do not impact outcome when considered according to MRD assessed both at the end of induction and following ASCT. The outcome of patients who are MRD-negative at both time points (A), MRD-positive post induction and MRD-negative post ASCT (B) and positive at both time points (C) did not differ according to induction regimen (P=0.84, 0.56 and 0.79, respectively).
The majority of studies of MRD in myeloma have assessed the value of achieving MRD-negativity, typically with a 10−4 threshold, on outcome. Flow cytometry can also provide a quantitative assessment of residual tumor in those with detectable disease. In a recent analysis, we demonstrated that the level of residual disease is also highly informative, such that an approximate 1-year OS benefit is demonstrable for each log of tumor depletion.4 We have also assessed this effect according to induction regimen and note that a greater proportion of patients receiving CVAD had high levels of MRD (<0.1%; CVAD n=140, CTD n=156; >0.1%; CVAD n= 68, CTD n=33; P=0.0005) but no difference in PFS was demonstrable across several logs of detectable disease. For those with MRD more than 1% median, PFS was 9.5 months with CTD and 6.5 months with CVAD (P=0.49). A similar pattern was also demonstrable in MRD-positive patients with lower levels of disease (0.1% – <1% median PFS 13.7 months CTD vs. 13.6 months CVAD, P=0.9; 0.01% – <0.1% median PFS 22.1 months CTD vs. 23.6 months CVAD, P=0.84) (Figure 3).
Figure 3.
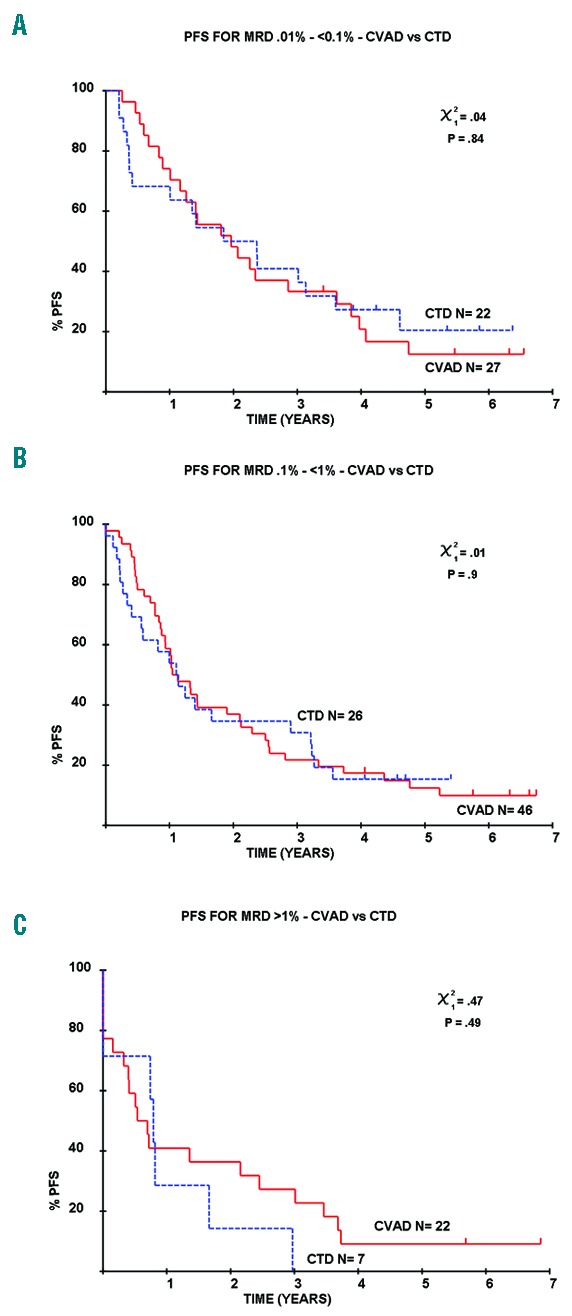
Impact of quantitative level of MRD on outcome. The outcome of MRD-positive patients with MRD levels of 0.01% – <0.1% (A), 0.1% – <1% (B) and greater than 1% (C) did not differ with induction regimen (P=0.84, 0.9 and 0.49, respectively).
We would conclude that these data demonstrate that the prognostic impact of MRD is independent of the induction therapy received. The proportion of patients who become MRD-negative (at the 10−4 level) can vary significantly between regimens, but the impact of MRD-negativity is similar regardless of the induction therapy received. It is possible that differences may emerge with more effective induction regimens as levels of disease may then vary over several logs in those patients who are MRD-negative. However, data from those with quantifiable residual disease suggest that outcome is determined by the level of disease rather than the regimen received. This phenomenon has also been clearly demonstrated in B-cell chronic lymphocytic leukemia (CLL) in the German CLL Study Group CLL8 trial.9 In this study, patients were treated with fludarabine-cyclophosphamide (FC) with/without rituximab (R). MRD-negativity was associated with improved PFS and OS, and although a greater proportion of patients became MRD-negative with FCR, there was no difference between the treatment arms when outcome was assessed according to levels of disease. These data provide further evidence to support the role of MRD as a surrogate end point for survival in clinical trials.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Paiva B, Vidriales MB, Cervero J, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112(10):4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paiva B, Gutierrez NC, Rosinol L, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119(3):687–691. [DOI] [PubMed] [Google Scholar]
- 3.Rawstron AC, Child JA, de Tute RM, et al. Minimal Residual Disease Assessed by Multiparameter Flow Cytometry in Multiple Myeloma: Impact on Outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31(20):2540–2547. [DOI] [PubMed] [Google Scholar]
- 4.Rawstron AC, Gregory WM, de Tute RM, et al. Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. Blood. 2015;125(12):1932–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landgren O, Gormley N, Turley D, et al. Flow cytometry detection of minimal residual disease in multiple myeloma: Lessons learned at FDA-NCI roundtable symposium. Am J Hematol. 2014;89(12):1159–1160. [DOI] [PubMed] [Google Scholar]
- 6.Mailankody S, Korde N, Lesokhin AM, et al. Minimal residual disease in multiple myeloma: bringing the bench to the bedside. Nat Rev Clin Oncol. 2015;12(5):286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan GJ, Davies FE, Gregory WM, et al. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2012;97(3):442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan GJ, Davies FE, Gregory WM, et al. Long-term follow-up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res. 2013;19(21):6030–6038. [DOI] [PubMed] [Google Scholar]
- 9.Böttcher S, Ritgen M, Fischer K, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012; 30(9):980–988. [DOI] [PubMed] [Google Scholar]


