Sickle cell anemia (SCA) is accompanied by unpredictable episodes of recurrent acute pain during vaso-occlusive crises (VOC), superimposed on chronic pain.1 Pain in SCA can start in infancy and may continue throughout life, leading to sustained activation of the nociceptive mechanisms resulting in poor therapeutic outcomes. Pain is an outcome of nociceptive processing in the central nervous system (CNS), triggered by peripheral nervous system response to exogenous and endogenous stimuli. Activation of transient receptor potential vanilloid 1 (TRPV1) channels on C-fibers, neurogenic inflammation, mast cell activation, systemic inflammation, and oxidative stress in the periphery have been demonstrated in SCA.2,3 However, the extent and mechanisms of CNS involvement remain unknown in SCA. The activation of inflammatory and neuronal cells in the CNS plays a pivotal role in nociception.4 We recently observed that spinal nociceptive neurons are sensitized in sickle mice, suggestive of central sensitization.5 Bidirectional signaling occurs between neurons and immunocompetent cells present in the CNS, including microglia, astrocytes and oligodendrocytes.4 Activated microglia release reactive oxidative species (ROS), inflammatory cytokines, neurotrophic factors, and prostaglandins that excite nociceptive neurons and contribute to the persistence of chronic pain.4 It is therefore likely that the activation of central nociceptive mechanisms contributes to chronic pain in SCA.
Remarkable decreases in inflammation, thiobarbituric acid reactive substances (TBARS, an indicator of oxidative stress), and VOC have been observed in SCA patients receiving coenzyme Q10 (CoQ10).6 Additionally, curcumin reduced markers of oxidative stress in thalassemia patients and also ameliorates pain hypersensitivity in rats with monoarthritis by decreasing spinal neuroinflammation.7,8 Since excess free iron due to hemolysis contributes to oxidative stress and inflammation in SCA, we examined glial activation, inflammation and oxidative stress in the spinal cords of sickle mice and tested the possibility of a synergistic effect of CoQ10 and/or curcumin to ameliorate spinal oxidative stress, glial activation and hyperalgesia.
To examine our hypotheses, we used female transgenic HbSS-BERK sickle mice with murine α and β globin knockouts and expressing human sickle, or normal haemoglobin A (designated sickle or control mice, henceforth, respectively). We bred and characterized these mice by pheno- and genotyping (see Online Supplementary Appendix for details).3,9 These sickle mice have severe hematologic disease, organ damage and tonic hyperalgesia similar to that observed in human SCA.9–11
Sickle mice received either vehicle (olive oil), curcumin (15 mg/kg), CoQ10 (45 mg/kg), or both CoQ10 and curcumin (cotreatment) daily for 4 weeks by gavage. Female mice were used because BERK female mice show more hyperalgesia as compared to males.11 Pain behaviors were evaluated during the proestrous/estrous cycle before treatment and weekly. The mice were tested for mechanical hyperalgesia using paw withdrawal frequency (PWF) in response to von Frey filaments, paw withdrawal latency (PWL) in response to a heat stimulus using a Hargreave’s apparatus; and sensitivity to cold was determined by PWL and PWF per 2 min on a cold plate (see detailed procedures in the Online Supplementary Appendix).11 Spinal cords were harvested after 4 weeks of treatment. Sections were examined by laser scanning confocal microscopy (LSCM) for Iba1, a microglial marker (Wako, Richmond, VA, USA), glial fibrillary acidic protein (GFAP), an astrocyte marker (Abcam, Cambridge, MA, USA), neuropeptide substance P (SP, Abcam) and detection of ROS with dihydroethidium (DHE, Life Technologies, Grand Island, NY, USA).
Spinal glial activation, neuroinflammation and oxidative stress in sickle mice
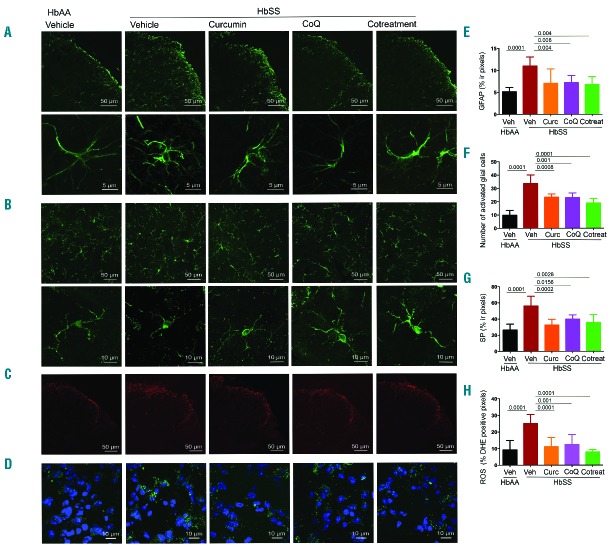
LSCM revealed increased immunoreactivity (ir) for GFAP, Iba1, SP and DHE/ROS staining in the dorsal horn of the spinal cord of sickle mice as compared to control mice, which was suggestive of activation of astrocytic and microglial cells accompanied by neuroinflammation and oxidative stress (Figure 1 A–D). Control spinal cords exhibited a significantly larger proportion of “resting” microglia with small cellular body (somata) and long ramified processes that act as sensors of change in the microenvironment (Figure 1B, lower panel). However, in sickle spinal cords microglial cells show shorter and fewer processes, suggestive of activated microglia (Figure 1B, lower panel). We also observed increased levels of neuropeptide SP-ir and ROS (Figure 1C,D) in the superficial dorsal horn of spinal cords from vehicle-treated sickle mice vs. control mice (P<0.0001; Figure 1G,H).
Figure 1.
Glial activation, neuroinflammation and oxidative stress in spinal cords of sickle mice is ameliorated with curcumin and/or CoQ10. Analysis of activation in glial cells in L4-L5 superficial dorsal horn segments of female sickle mice after daily treatment with curcumin (HbSS Curc), CoQ10 (HbSS CoQ), or both (HbSS Cotreatment) for 4 weeks. Sickle mice (HbSS Vehicle) show increased levels of activated cells as compared to control mice (HbAA Vehicle) which is reduced by all treatments. Representative images of (A) astrocytes (GFAP, upper panel; lower panel showing a magnified field of activated astroglial cells), (B) activated microglia (Iba1 positive cells, upper panel; lower panel shows magnified fields of activated microglial cells, (C) neuropeptide SP, and (D) ROS (green) with DAPI counterstained nuclei (blue). Quantitative analysis of (E) GFAP immunoreactivity, (F) numbers of resting and activated microglial cells, (G) SP immunoreactivity, and (H) ROS levels expressed as percentage of DHE positive pixels. Each image is representative of images from 8 mice per treatment. Scale bars as indicated. Quantitation of fluorescence expressed as the mean value of the pixel density ± SEM of 3 nonadjacent fields per mouse, except (F) activated microglial expressed as number of immunoreactive cells per field. Significance was determined by one-way ANOVA with Bonferroni’s multiple comparison.
Curcumin and/or CoQ10 treatment reduces spinal glial activation, neuroinflammation and oxidative stress
Treatment with curcumin and/or CoQ10 significantly reduced activated astroglial (P<0.01) and microglial cells (P<0.001), SP-ir (P<0.05), and ROS (P<0.05) compared to vehicle-treatment in sickle mice (Figure 1A–H). The reduction in activated astroglia was similar to that found in control mice, while activated microglia remained significantly elevated after treatment as compared to control mice. Thus, both curcumin and CoQ10 are able to reduce SP and ROS expression in sickle mice, and together reduce ROS to levels seen in control mice. Microglial activation is dependent upon MAPK signalling. Previously, we observed increased phosphorylation of MAPK/ERK and p38 MAPK simultaneously with nociceptor sensitization in the spinal cords of these sickle mice as compared to control mice.5 Therefore, it is likely that glial activation contributes to spinal MAPK activation, nociceptor sensitization and chronic hyperalgesia observed in sickle mice.
Astrocyte activation is a result of calcium influx into the astrocytes, which is caused by increased blood flow.12 Previously we observed increased blood flow in the skin of sickle mice as compared to control mice.3 Therefore, it is likely that blood flow may be higher in the spinal cord of sickle mice, contributing to the astrocytic activation observed in our studies. Astrocytes are activated by glutamate, SP and many other neurotransmitters released upon stimulation of hyperexcitable nociceptive neurons in nerve injury. In turn, activated astrocytes release these same neurotransmitters, further augmenting neuronal excitability.4 In the setting of nerve injury or chronic neuropathic pain conditions, astrocyte activation is maintained by microglia-derived inflammatory factors. The increase in concurrent expression of ROS and SP strongly suggests a global constitutive inflammatory state and oxidative stress in the CNS, which may underlie central sensitization leading to chronic pain observed in patients with SCA and sickle mice. Of note, increased circulating levels of SP have been reported in patients with SCA as compared to normal healthy subjects,13 and in the skin and blood of sickle mice as compared to control mice.3 It is plausible that high amounts of spinal SP are released into the periphery due to antidromic release that may occur in central sensitization due to continued neuronal excitation. It is therefore likely that reduced inflammation and oxidative stress orchestrated by ‘nutraceuticals’ and supplements already used widely by consumers may reduce central sensitization and chronic pain.
Curcumin and/or CoQ10 treatment ameliorates mechanical and thermal hyperalgesia in sickle mice
Similar to our previous observations, sickle mice showed increased sensitivity to mechanical and thermal stimuli, suggestive of increased mechanical and thermal hyperalgesia in sickle mice as compared to control mice (Figure 2). Chronic treatment with curcumin and CoQ10, alone or in combination, significantly reduced mechanical and heat hyperalgesia as compared to baseline or vehicle-treated sickle mice (P<0.0001; Figure 2A,B). This decrease started at week 1 irrespective of treatment, but at week 4 cotreatment was slightly more effective than either treatment alone (Figure 2 A,B). Sickle mice exhibited cold hyperalgesia with higher PWF and shorter PWL compared to control mice (P<0.001), which was significantly attenuated after 2 weeks of treatment with curcumin and/or CoQ10 (Figure 2C,D). All treatments of sickle mice presented increased PWL on a cold plate at week 4 in comparison to sickle vehicle group, but it was significantly lower than control mice (P<0.0001), suggesting that cold hyperalgesia is more challenging to treat. Together, these data suggest that curcumin and CoQ10 independently as well as in combination ameliorate chronic hyperalgesia in sickle mice, consistent with their antinociceptive effects observed in other rodent models of hyperalgesia and/or pain in SCA patients.6,8 Therefore, one or the other may be equally effective, perhaps because of their similar mechanism of action of ameliorating oxidative stress, glial activation and SP modulation.
Figure 2.
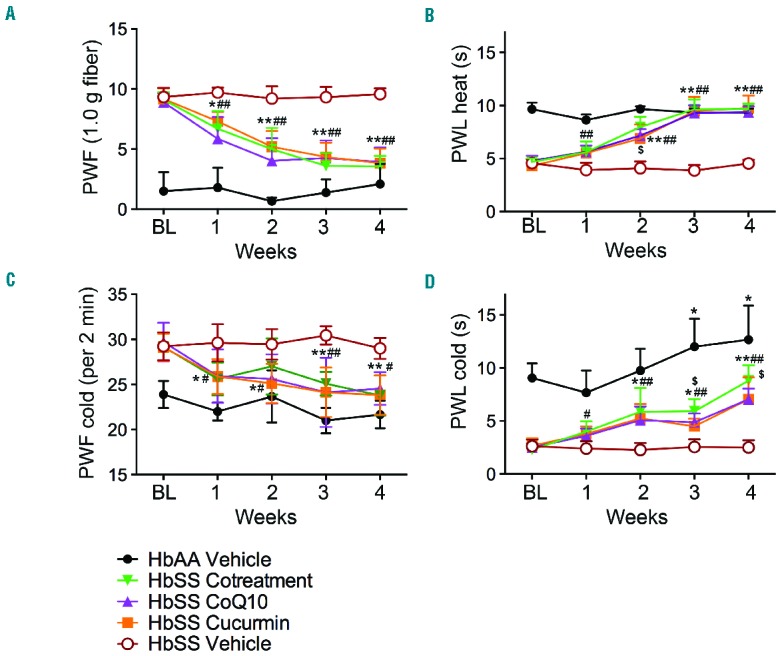
Curcumin and coenzyme Q10 reduce chronic hyperalgesia in sickle mice. Sickle mice (HbSS) were treated with vehicle, curcumin, coenzyme Q10 (CoQ10) or both in combination for 4 weeks, while control mice (HbAA) received vehicle. Pain measures were obtained before starting treatments, Baseline (BL), and weekly after initial treatment. Measures of (A) mechanical hyperalgesia and (B–D) thermal sensitivity to heat and cold are shown. *P<0.05, **P<0.0001 for BL vs. weeks of treatment; #P<0.05, ##p <0.0001 HbSS Vehicle vs. treatments; $P<0.05 HbSS Cotreatment vs. HbSS CoQ10 or HbSS Curcumin. Each value is the mean ± SEM from 6–12 female mice (mean age = 5.6 months old) with 3 observations per mouse. Significance was determined by two-way ANOVA with Bonferroni’s and Student’s t-test.
In clinical trials, curcumin was efficacious in reducing inflammation and oxidative stress and improving the outcomes in several conditions including both rheumatoid arthritis and osteoarthritis.14 Short-term use of theracurcumin with a relatively higher bioavailability than curcumin was effective in reducing pain and symptoms of osteoarthritis in a randomized, double-blind, placebo-controlled prospective study for treating knee osteoarthritis.15 In this study, 8 weeks of theracurcumin treatment significantly reduced knee pain and lowered celecoxib dependence as compared to placebo. Curcumin reduced oxidative stress in thalassaemia patients and decreased iron overload and oxidative stress in the liver and spleen of chronic iron-overloaded rats.7,16 Thus, curcumin may influence the sickle microenvironment that includes large quantities of cell-free iron due to hemolysis and oxidative stress. Treatment with curcumin and/or CoQ10 could be a promising alternative strategy to reduce pain in SCA. Considering the challenges with treating chronic sickle pain and the requirements of high doses of opioids to treat pain, this approach may reduce the requirement of opioid analgesics and/or improve the outcomes of opioid analgesia.
Acknowledgments
We thank Ritu Jha and Susan Thompson for breeding/typing mice; Yann Lamarre, PhD, Marna E. Ericson, PhD and Maureen Reidl, PhD for advice on glial staining.
Footnotes
Funding: this work was supported by NIH RO1 103773 and UO1 HL117664 and Institute for Engineering in Medicine grants to KG. Confocal imaging was performed using the Olympus FluoView 1000 IX2 instrument at the University of Minnesota - University Imaging Centers, http://uic.umn.edu.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Gupta M, Msambichaka L, Ballas SK, Gupta K. Morphine for the treatment of pain in sickle cell disease. Sci World J. 2015;2015:540154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillery CA, Kerstein PC, Vilceanu D, et al. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118(12):3376–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent L, Vang D, Nguyen J, et al. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122(11):1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cataldo G, Rajput S, Gupta K, Simone DA. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain. 2015;156(4):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakur AS, Littaru GP, Moesgaard S, Dan Sindberg C, Khan Y, Singh CM. Hematological parameters and RBC TBARS Level of Q 10 supplemented tribal sickle cell patients: a hospital based study. Indian J Clin Biochem. 2013;28(2):185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalpravidh RW, Siritanaratkul N, Insain P, et al. Improvement in oxidative stress and antioxidant parameters in beta-thalassemia/Hb E patients treated with curcuminoids. Clin Biochem. 2010;43(4–5):424–429. [DOI] [PubMed] [Google Scholar]
- 8.Chen JJ, Dai L, Zhao LX, Zhu X, Cao S, Gao YJ. Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis. Sci Rep. 2015;5:10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paszty C, Brion CM, Manci E, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278(5339):876–878. [DOI] [PubMed] [Google Scholar]
- 10.Cain DM, Vang D, Simone DA, Hebbel RP, Gupta K. Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness. Br J Haematol. 2012;156(4):535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohli DR, Li Y, Khasabov SG, et al. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116(3):456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straub SV, Nelson MT. Astrocytic calcium signaling: the information currency coupling neuronal activity to the cerebral microcirculation. Trends Cardiovasc Med. 2007;17(6):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaels LA, Ohene-Frempong K, Zhao H, Douglas SD. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood. 1998;92(9):3148–3151. [PubMed] [Google Scholar]
- 14.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa Y, Mukai S, Yamada S, et al. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci. 2014;19(6):933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badria FA, Ibrahim AS, Badria AF, Elmarakby AA. Curcumin attenuates iron accumulation and oxidative stress in the liver and spleen of chronic iron-overloaded rats. PLoS One. 2015;10(7):e0134156. [DOI] [PMC free article] [PubMed] [Google Scholar]