Abstract
Obinutuzumab (GA101) is a type II, glycoengineered anti-CD20 monoclonal antibody for the treatment of hematologic malignancies. Obinutuzumab has mechanisms of action that are distinct from those of rituximab, potentially translating into improved clinical efficacy. We present the pharmacokinetic and clinical data from the phase I/II GAUGUIN and phase I GAUDI studies that were used to identify the obinutuzumab dose and regimen undergoing phase III assessment. In phase I (GAUGUIN and GAUDI), non-Hodgkin lymphoma patients received up to a maximum 9 fixed doses (obinutuzumab 50–2000 mg). In GAUGUIN phase II, patients received obinutuzumab 400/400 mg or 1600/800 mg [first dose day (D)1, D8, cycle (C) 1; second dose D1, C2–C8]. The influence of demographic factors on pharmacokinetics and drug exposure on tumor response and toxicity were analyzed using exploratory graphical analyses. Obinutuzumab serum concentrations with 1600/800 mg were compared with a 1000 mg fixed-dose regimen (D1, D8 and D15, C1; D1, C2–C8) using pharmacokinetic modeling simulations. Factors related to CD20-antigenic mass were more influential on obinutuzumab pharmacokinetics with 400/400 versus 1600/800 mg. Higher serum concentrations were observed with 1600/800 versus 400/400 mg, irrespective of CD20-antigenic mass. Tumor shrinkage was greater with 1600/800 versus 400/400 mg; there was no significant increase in adverse events. Fixed dose 1000 mg with an additional C1 infusion resulted in similar serum concentrations to 1600/800 mg in model-based analyses. The obinutuzumab 1000 mg fixed-dose regimen identified in this exploratory analysis was confirmed in a full covariate analysis of a larger dataset, and is undergoing phase III evaluation. GAUGUIN and GAUDI are registered at www.clinicaltrials.gov (clinicaltrials.gov identifier:00517530 and 00825149, respectively).
Introduction
The chimeric anti-CD20 monoclonal antibody rituximab (IgG1k) has revolutionized the treatment of hematologic malignancies. Rituximab plus chemotherapy has become the standard-of-care treatment for follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL) and chronic lymphocytic leukemia (CLL).1–6 Single-agent rituximab is also used as FL maintenance therapy.7,8 Rituximab was the first monoclonal antibody approved for use in malignant diseases. The dose and schedule of rituximab used were mostly developed empirically, without extensive studies to establish dose or dose scheduling, as the substantial clinical benefits achieved with rituximab led to its rapid incorporation into treatment regimens. In phase I studies, indolent non-Hodgkin lymphoma (iNHL) patients received rituximab doses ranging from 10–500 mg/m2.9,10 As no dose-limiting toxicity or clear dose– response relationship was found, the 375 mg/m2 dose was selected for further clinical evaluation.11 This dose was approved in Europe and the US for the treatment of several hematologic cancers.12,13
Pivotal studies have shown large inter-individual variability in rituximab serum concentrations.11,14 As rituximab concentrations correlate with clinical response and progression-free survival (PFS),14–16 an optimized dosing regimen should translate into improved clinical response. Yet personalized, pharmacokinetic (PK)-based maintenance dosing of rituximab 375 mg/m2, administered when serum concentrations decreased to less than 25 μg/mL, yielded no clinical advantage in iNHL.17 However, treatment responders had higher serum concentrations of rituximab than non-responders at one and four months post-treatment, although patient numbers were too small to draw firm conclusions and non-responders still had rituximab serum levels near the target range.17 It is likely that rituximab serum concentrations are the result of a complex interplay of several biological factors that influence antibody behavior (i.e. distribution, catabolism), including histology (CLL vs. NHL), CD20 expression levels on malignant B cells, and tumor burden.11,14,18,19 In vitro studies showed that FcγRIIIa-158VF polymorphisms impact the concentration-effect relationship of rituximab-induced antibody-dependent cell-mediated cytotoxicity (ADCC),20 a critical mechanism of anti-tumor activity.21 As these biological parameters can vary between individuals, the PK of rituximab, and thus disease control, can differ between patients. To optimize dosing, there is a critical need to understand the factors affecting the PK of anti-CD20 monoclonal antibodies, particularly those under clinical development.
Obinutuzumab is a novel, type II, glycoengineered, anti-CD20 monoclonal antibody. Glycoengineering of obinutuzumab has enhanced its binding to the Fc portion of FcγRIIIa expressed by effector cells versus the non-glycoengineered rituximab. The glycoengineered region of obinutuzumab is also expected to reduce the influence of the FcγRIIIa-158VF polymorphism on the in vivo activity of obinutuzumab versus rituximab. Thus, obinutuzumab exhibits enhanced in vitro ADCC versus rituximab.22 As obinutuzumab recognizes a type II epitope on the CD20 antigen, its mechanisms of action are distinct from that of type I anti-CD20 antibodies, such as rituximab.22 For example, the binding of obinutuzumab to CD20 does not induce the translocation of antibody-CD20 complexes into lipid rafts, leading to a lack of complement-dependent cytotoxicity (CDC). Moreover, the binding of obinutuzumab to CD20 leads to increased direct cell death versus rituximab. Obinutuzumab-induced direct cell death is mediated via pathways distinct from classical apoptosis, and may involve actin reorganization and homotypic adhesion.23 Animal studies showed that these mechanistic differences translate into superior efficacy for obinutuzumab versus rituximab.22 Clinically, obinutuzumab monotherapy has exhibited encouraging activity in phase I and II studies in NHL and CLL.24–27 Given the relationship between rituximab serum concentrations and patient outcomes and the mechanistic differences between obinutuzumab and rituximab, early clinical trials assessed the relationship between PK, safety and efficacy with tumor markers. These data were used to explore the relationship between obinutuzumab PK and response, and, in conjunction with PK modeling techniques, to identify an optimized dose and regimen for phase III studies in NHL.
Methods
Patients and study designs
Pharmacokinetic data for analyzing optimal obinutuzumab dose and schedule were derived from iNHL and aggressive NHL (aNHL) patients participating in the phase I/II GAUGUIN and phase Ib GAUDI studies. In GAUGUIN phase I (dose escalation), 21 heavily pre-treated patients with relapsed/refractory CD20-positive iNHL received obinutuzumab monotherapy at a dose of 50/100, 100/200, 200/400, 400/800, 800/1200, 1200/2000, or 1600/800 mg (8 × 21-day cycles; n=3 per cohort). The former dose of each regimen was administered at first infusion (D1, C1) and the latter was infused for the remainder of the regimen (D8, C1; D1, C2–C8).26
The dose selection for GAUGUIN phase II was based on the safety, efficacy and PK results obtained during the phase I dose escalation. During phase II, iNHL (FL) or aNHL [DLBCL, mantle cell lymphoma (MCL)] patients were randomized to obinutuzumab 400/400 mg or 1600/800 mg (former dose of each regimen administered on D1 and D8, C1; latter dose administered on D1, C2–C8; 21-day cycles).24,25
The GAUDI study examined the safety and efficacy of obinutuzumab plus chemotherapy [CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or FC (fludarabine and cyclophosphamide)] in relapsed/refractory FL. Patients (n=56) were randomized to obinutuzumab 400/400 mg plus CHOP, obinutuzumab 400/400 mg plus FC, obinutuzumab 1600/800 mg plus CHOP, or obinutuzumab 1600/800 mg plus FC (n=14 per cohort). The number of cycles received was dictated by the chemotherapy backbone (CHOP: 8 × 21-day cycles; FC: 6 × 28-day cycles). In all cohorts, obinutuzumab was administered on D1 and D8, of C1, and D1 of subsequent cycles.28
In both GAUGUIN and GAUDI, patients had 1 or more bidimensionally measurable lesion (>1.5 cm by computerized tomography scan), life expectancy of more than 12 weeks, and an Eastern Cooperative Oncology Group performance status of 0–2. All patients provided informed consent. The GAUGUIN and GAUDI studies conformed to the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice, and the protocols were approved by local or institutional ethics committees. CONSORT diagrams for GAUGUIN and GAUDI have been published previously.24,25,28
PK assessments and bioanalytical methods
Timings for blood sample collection, bioanalytical methods used and calculation of PK parameters are described in the Online Supplementary Methods.
Tumor response and toxicity assessments
Tumor response was assessed according to International Workshop criteria for NHL.29 Tumor burden was estimated by the sum of product diameters of six specified target lesions.14
Incidences of the grade 3/4 adverse events (AEs), neutropenia and infection were calculated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (v.3.0).
Graphical analyses
Exploratory graphical analyses were conducted to assess the influence of base-line characteristics on obinutuzumab PK, the relationship between obinutuzumab exposure and tumor response, and the relationship between exposure and toxicity (Online Supplementary Methods).
Pharmacokinetic modeling
Pharmacokinetic model development, evaluation and simulations are described fully in the Online Supplementary Methods. In brief, PK model simulations compared obinutuzumab serum concentrations following the 1600/800 mg regimen (D1 and D8, C1; D1, C2–C8) with a new 1000 mg fixed-dose regimen (D1, D8, and D15, C1; D1, C2–C8; 21-day cycle).
Results
Patients’ characteristics
Demographic and base-line disease characteristics of iNHL and aNHL patients participating in phase I and II studies of obinutuzumab have been published24–26,28 and are summarized in Online Supplementary Table S1. Median age ranged from 59.8 years in patients randomized to obinutuzumab plus CHOP in GAUDI to 71.0 years among aNHL patients participating in GAUGUIN phase II. In contrast to GAUGUIN phase I, there were more male than female participants in phase II (43% vs. 63%–68%). Patients in GAUGUIN phase I also had less advanced disease than those in phase II (62% stage III–IV vs. 76%–90%). The recruitment criteria resulted in disease histology and rituximab-refractory status varying across trials. Almost all patients in GAUGUIN and GAUDI had received rituximab, with the median number of any previous anti-lymphoma treatments received by patients ranging from 1–5.
Pharmacokinetics of obinutuzumab monotherapy
In GAUGUIN phase I, patients received up to nine infusions of obinutuzumab monotherapy at doses ranging from 50–2000 mg. Two infusions were administered during cycle 1 to quickly elevate serum concentrations of obinutuzumab. No dose-limiting toxicity was established, and inter-individual variability was observed within each cohort (Figure 1A). As one patient each in the 50/100 mg, 100/200 mg, 200/400 mg, 800/1200 mg, and 1600/800 mg dose groups responded to treatment, no relationship between dose and efficacy was identified.26 These PK data indicated that serum concentrations of obinutuzumab increased with higher doses, and that a dose of at least 400/800 mg was required for CD20 target saturation.26 As a result, two doses of obinutuzumab were selected for evaluation in GAUGUIN phase II: a low-dose cohort receiving 400/400 mg (patients received 400 mg throughout the study: D1 and D8, C1; D1, C2–C8), and a high-dose (1600/800 mg) cohort [patients received 1600 mg of obinutuzumab on D1 and D8, C1, and 800 mg for subsequent cycles (D1, C2–C8)].25
Figure 1.
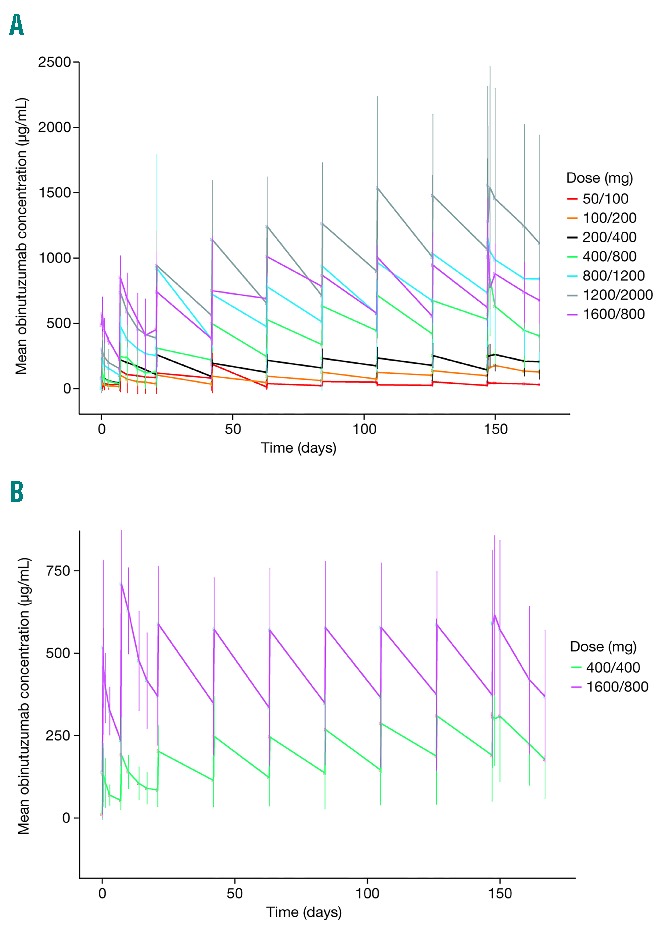
Mean obinutuzumab concentrations. Mean obinutuzumab concentrations (± standard error of mean) observed in (A) phase I and (B) phase II of GAUGUIN.
In the current analysis, AUClast values at cycles 1 and 8 demonstrated that obinutuzumab exposure was higher throughout treatment for the 1600/800 mg versus 400/400 mg dose group (P<0.001 at cycles 1 and 8). Cmax for the 1600/800 mg group on D8 of C1 was comparable to that on D1 of C8, confirming the role of the significant loading dose in achieving steady-state concentrations as early as possible after treatment initiation (Table 1 and Figure 1B).
Table 1.
Obinutuzumab pharmacokinetic parameters of patients with non-Hodgkin lymphoma participating in phase II of the GAUGUIN study.
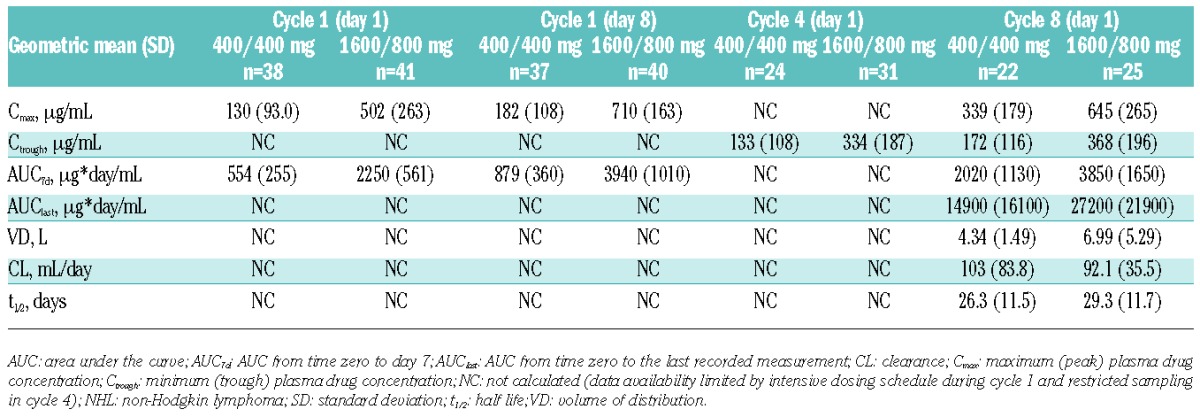
There was clear evidence of a relationship between dose and exposure with obinutuzumab, with statistically significantly higher Cmax and AUC observed in the 1600/800 mg cohort versus the 400/400 mg cohort (both P<0.05) (Table 1).
Factors influencing the pharmacokinetic parameters of obinutuzumab
Demographic and base-line parameters: to explore the influence of different clinical parameters on obinutuzumab PK variability, patients in GAUGUIN phase II were stratified by age (older or younger than the median for each patient cohort), sex, bodyweight, B-cell count before treatment, disease stage (I–II vs. III–IV), and tumor bulk (>5 cm or <5 cm) (Figures 2 and 3). In the low-dose group (400/400 mg), serum concentrations of obinutuzumab were affected by clinical parameters related to CD20-antigenic mass (tumor bulk, disease stage, B-cell count), and varied more for those patients with higher versus lower base-line lymphocyte count, stage, and tumor burden. In contrast, for the high-dose group (1600/800 mg), CD20-antigenic mass had little impact on PK. There was also high inter-individual variation in serum concentration with the low dose of obinutuzumab (400/400 mg) (Figure 2A). However, this inter-individual variation decreased with increasing dose, with an apparent decrease in the elimination process at the highest dose (1600/800 mg) (Figure 2A). This pattern suggests that the CD20 target sites became saturated at the highest dose. In addition, tumor shrinkage was greater among patients in the 1600/800 mg group versus the 400/400 mg group (Figure 2B), further demonstrating that the 1600/800 mg dosing regimen allowed patients to obtain optimal obinutuzumab serum concentrations, which facilitates clinical efficacy.
Figure 2.
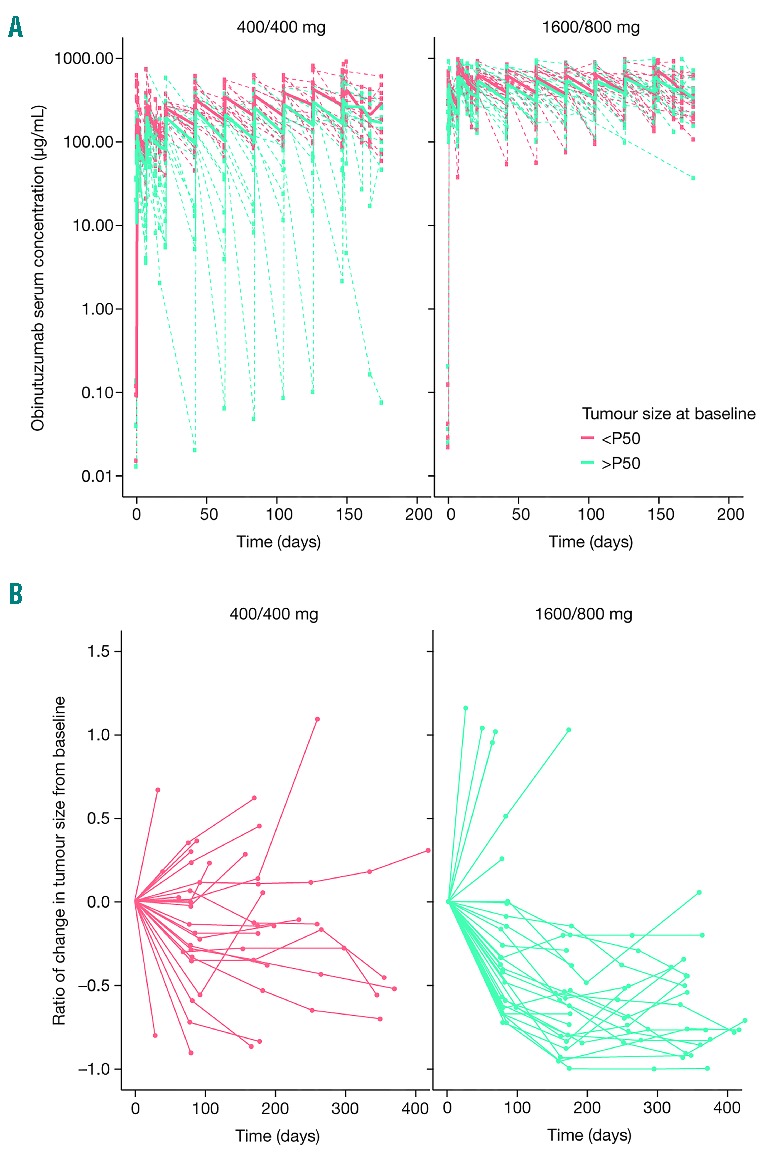
Serum concentrations of obinutuzumab and tumor sizes. (A) Inter-individual serum concentrations of obinutuzumab by tumor burden and dose group in patients with iNHL participating in phase II of GAUGUIN. (B) Inter-individual change in tumor size by dose group in patients with iNHL.
Figure 3.
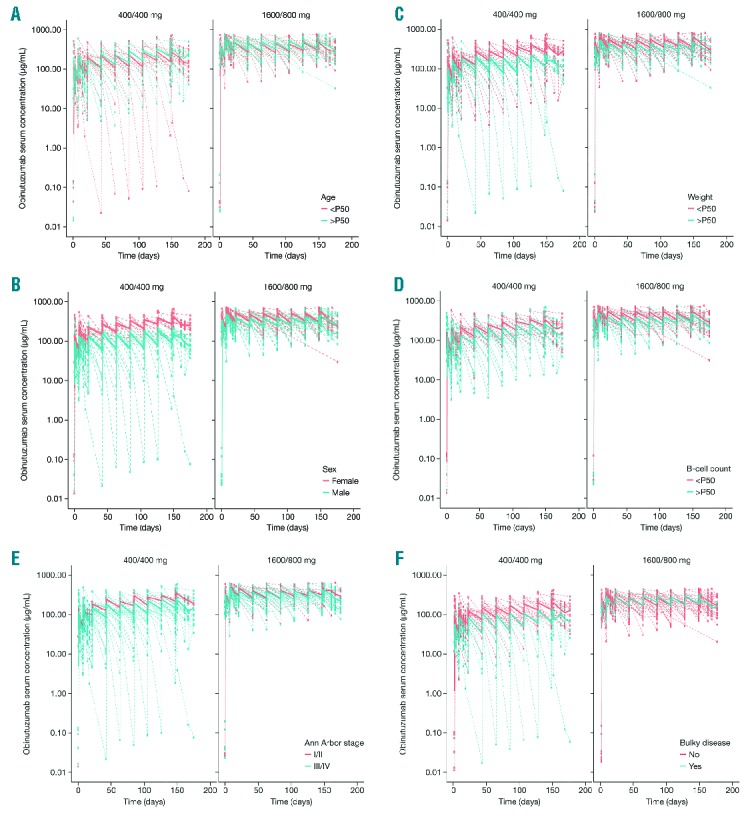
Time-course of obinutuzumab serum concentrations stratified by clinical parameters and dose group in patients with non-Hodgkin lymphoma participating in phase II of GAUGUIN.
With regard to demographics, age did not appear to alter obinutuzumab PK for patients aged 22–85 years (Figure 3A). In the 400/400 mg group, there was a small increase in serum concentrations for women versus men (Figure 3B), for patients with high bodyweight (> median) versus those with low bodyweight (< median) (Figure 3C), for patients with stage I/II versus stage III/IV disease (Figure 3E), and for patients with non-bulky disease versus those with bulky disease (Figure 3F). These differences in serum concentration with sex, weight, disease stage and bulky disease were not observed in the 1600/800 mg group, showing that this dosing regimen conferred high exposure irrespective of patients’ initial demographic characteristics. Trough serum concentrations did not vary according to B-cell count for either dosing regimen (Figure 3D).
NHL subtype: an exploratory graphical analysis demonstrated a high degree of variability in obinutuzumab serum concentrations across different NHL subtypes (Figure 4). MCL patients had lower serum concentrations of obinutuzumab than iNHL or DLBCL patients, possibly due to treatment during the leukemic phase of MCL for some patients. However, it should also be noted that only 4 MCL patients were recruited to GAUGUIN phase II.24 In terms of clinical efficacy, higher tumor response rates were observed in iNHL versus aNHL patients (55% vs. 32%). Although the percentage of responders with MCL is similar to that observed among iNHL patients (50% vs. 55%), only the 4 GAUGUIN MCL patients were included, 2 of whom exhibited partial response (PR).24
Figure 4.
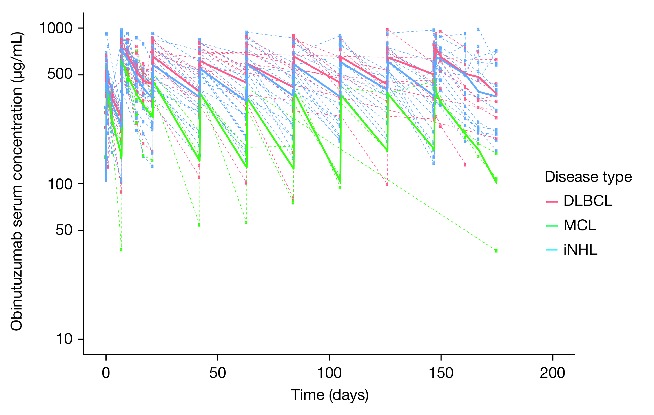
Time course of obinutuzumab serum concentrations in the 1600/800 mg dose group stratified by disease type.
Effect of obinutuzumab exposure on overall response rates and tumor response
Clinical response rates for patients participating in GAUGUIN phase II have been published.24,25 In the cohort of aNHL patients, overall response rate (ORR), measured as end-of-treatment response (ETR), was similar between the two dosing groups: for 400/400 mg, ETR was 24% (80%CI: 12–40) and 2 patients (10%) had a complete response (CR); for 1600/800 mg, ETR was 32% (80%CI: 18–49) and no patient had a CR; difference between groups was not statistically tested.24 In iNHL patients, an apparent improvement in ORR was observed for the higher dose group versus the lower dose group: for 1600/800 mg, ETR was 55% (95%CI: 32–76) and 2 patients (9%) had a CR, whereas for 400/400 mg, ETR was 17% (95%CI: 4–41) and no patient had a CR.25 The difference in ETR between groups in the iNHL patients was 38% (95%CI: 7–68), which was statistically significant.25
An exploratory graphical analysis indicated that patients with higher mean obinutuzumab serum concentrations during the first 5 cycles of treatment may experience greater inhibition of tumor growth. At week 6, tumor size decreased by a median of 17% (95%CI: −81–32) in patients with the lowest mean serum concentrations of obinutuzumab (<160 mg/mL), 38% (95%CI: −72–−5) in patients with serum concentration 160–371 mg/mL and 48% (95%CI: −72–−16) in those with the highest mean concentrations (>371 mg/mL) (Figure 5).
Figure 5.
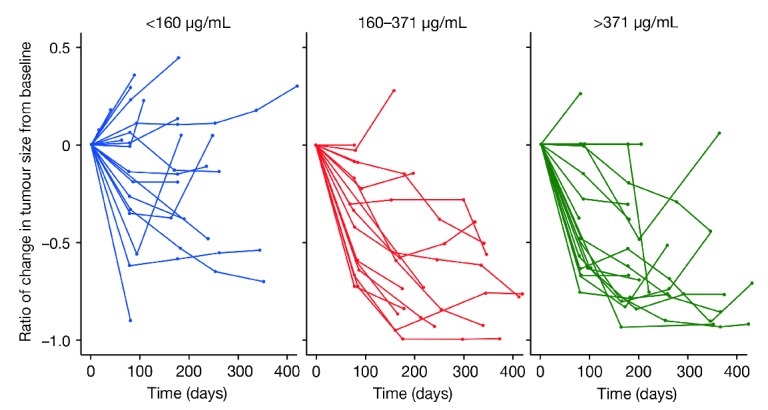
Change in tumor size by mean plasma concentration of obinutuzumab during the first six weeks of treatment in phase I and II of the GAUGUIN study.
Effect of obinutuzumab exposure on toxicity
Previous studies showed that the addition of rituximab to chemotherapy increases the incidence of neutropenia, which is usually transient and manageable.1,3,7 Combined AE data from the GAUGUIN phase II iNHL study and GAUDI study showed that incidence of grade 3 or 4 neutropenia was similar for low- and high-dose groups, i.e. 13 of 46 (28%) and 15 of 50 (30%) for the 400/400 mg and 1600/800 mg groups, respectively. The obinutuzumab doses received by 3 patients in the GAUGUIN phase II aNHL and GAUGUIN phase I studies who had grade 3 or 4 neutropenia were not reported. Exploratory graphical analysis indicated that there was no difference in obinutuzumab exposure between patients who had grade 3/4 neutropenia and those who did not (Online Supplementary Figure S1).
No grade 3/4 infections were observed in GAUGUIN phase I.26 In phase II, 4 iNHL patients (400/400 mg, n=1; 1600/800 mg, n=3) and no aNHL patients experienced grade 3/4 infections.24,25 In the GAUDI study, there were 4 patients with grade 3/4 infections in the 400/400 mg cohort (G-CHOP, n=3; G-FC, n=1) and 7 in the 1600/800 mg cohort (G-CHOP, n=3; G-FC, n=4).28 In total in the GAUGUIN phase II and GAUDI studies, 5 of 67 patients (7%) who received the 400/400 mg dose and 10 of 69 (14%) who received 1600/800 mg experienced grade 3/4 infections. Exploratory graphical analysis showed that there was no difference in obinutuzumab exposure between patients who had grade 3/4 infections and those who did not (Online Supplementary Figure S2).
Pharmacokinetic model for obinutuzumab
A basic structural model of obinutuzumab PK is shown in Online Supplementary Figure S3, and a summary of the final parameter estimates of the time-varying model is shown in Online Supplementary Table S2. The goodness-of-fit diagnostic plots and visual predictive checks did not indicate any model deficiencies (Online Supplementary Figures S4 and S5).
Pharmacokinetic predictions to select an optimal dosing regimen
Based on PK data collected during phases I and II of GAUGUIN, the 1600/800 mg dose of obinutuzumab was identified as likely to produce the highest serum concentrations of obinutuzumab in NHL patients. This decision was also supported by the stronger response in iNHL patients with the 1600/800 mg dose (55%) than with the 400/400 mg dose (17%), coupled with more marked decreases in tumor size at the higher dose and at higher obinutuzumab concentrations (Figures 2B and 5). However, a single dose for all infusions would be significantly more convenient and easier to administer than a bodyweight- or size-adjusted dose. A dose of 1000 mg with three infusions during cycle 1 was then proposed. Under such a regimen, patients would receive a cumulative obinutuzumab dose of 3000 mg in cycle 1, similar to the 3200 mg received during cycle 1 by patients randomized to the 1600/800 mg dose arm of GAUGUIN phase II. PK model-based simulations showed that this new regimen would result in trough serum concentrations of obinutuzumab similar to those observed with the 1600/800 mg dose (Figure 6). Therefore, obinutuzumab 1000 mg administered on D1, D8, and D15 of C1 (21-day cycle) and D1 of subsequent cycles has been selected as the dose and regimen for phase III clinical trial assessment.
Figure 6.
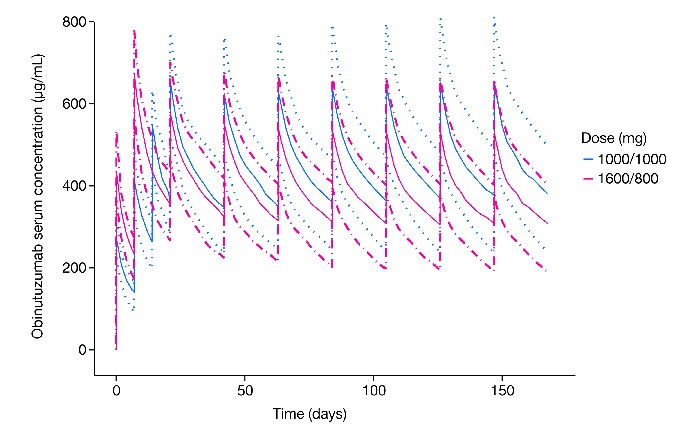
Predicted serum concentrations of obinutuzumab 1000/1000 mg (blue line) versus 1600/800 mg (red line).
Discussion
Obinutuzumab is a humanized, glycoengineered, type II anti-CD20 monoclonal antibody, with demonstrated clinical impact in rituximab-refractory patients with DLBCL, MCL, iNHL and FL.24,25,28 In the randomized, phase III CLL11 study in patients with previously untreated CLL and comorbidities, PFS was significantly longer with obinutuzumab plus chlorambucil (G-Clb) than with rituximab plus chlorambucil (R-Clb).30 The standard rituximab dose and regimen was used (375 mg/m2, D1, C1; 500 mg/m2 D1, C2–C6), while obinutuzumab was administered at a fixed dose of 1000 mg, with additional infusions on D8 and D15 of C1. As a result, the median cumulative antibody dose was increased in the G-Clb arm (8000 mg) versus the R-Clb arm (5106 mg).30 Median cumulative chlorambucil dose was similar between arms (G-Clb: 366 mg; R-Clb: 396 mg). Therefore, the observed differences in efficacy between the study arms may have been influenced by differences in exposure and pharmacokinetics of the two antibodies.
Given differences in the nature of CD20 binding, the biological pathways activated and the underlying mechanism of action, the dose and schedule of obinutuzumab could not be extrapolated from rituximab and the early clinical development program was designed to identify an optimal dose for this new molecule. Consequently, the early clinical studies were undertaken to optimize the dose and schedule of obinutuzumab to achieve the maximal clinical response possible without adversely affecting safety. In this current analysis, PK data from phases I and II clinical studies and a population PK model were used to identify the treatment regimen likely to be clinically effective for the majority of patients with CD20-positive NHL: obinutuzumab 1000 mg administered on D1, D8, and D15 of C1 (21-day cycle) and D1 of C2–C8. With this regimen, target saturation should occur quickly after treatment initiation and serum concentrations of obinutuzumab should be maintained thereafter throughout the treatment course (i.e. steady state). This dose and schedule is anticipated to be clinically effective for the majority of patients with CD20-positive NHL.
At the doses tested clinically, obinutuzumab, like rituximab,9,10 did not display dose-limiting toxicity. In patients treated with low-dose obinutuzumab (400/400 mg), PK data showed an increase in serum concentrations over the treatment duration consistent with the hypothesis that target saturation was not fully achieved in the low-dose cohort. Among patients administered high-dose obinutuzumab (1600/800 mg), steady state was reached quickly, by cycle 2, consistent with the hypothesis of a high degree of target saturation. Clinically, this may translate into improved tumor response rates among patients in the high-dose cohort versus the low-dose cohort.
With regard to patient demographics, weight and sex had an apparent influence on PK, with increased exposure in women or individuals with lower bodyweight. This observation is in agreement with the PK characteristics of antibodies in which clearance usually increases with bodyweight. A fixed-dose strategy with obinutuzumab will, therefore, lead to moderate overexposure in patients with low bodyweight. In a separate detailed PK analysis, bodyweight was shown to not have a clinically significant effect on the PK of obinutuzumab.31
The observation that women with NHL had higher obinutuzumab exposure than men is similar to previous observations with rituximab PK in elderly DLBCL patients.32,33 Elderly females with DLBCL who received rituximab plus CHOP therapy had slower rituximab clearance and improved PFS versus elderly males; however, these trends were not seen for younger patients. This gender difference appears to be more pronounced in elderly patients treated with rituximab plus chemotherapy versus chemotherapy alone.33–36 Therefore, rituximab clearance rates in women appear to decrease with age at a faster rate than in men, resulting in greater exposure to rituximab and subsequently improved outcomes. Results from the SMARTE-R-CHOP and DENSE-R-CHOP trials suggested that increasing the dosing density of rituximab may allow elderly male patients to achieve the same exposure and clinical outcomes as their female counterparts.36,37 In our analysis, female NHL patients receiving the obinutuzumab 400/400 mg dose had higher obinutuzumab exposure than men, indicating a gender difference in obinutuzumab PK. However, women and men had equivalent serum obinutuzumab concentrations at the higher dosing regimen of 1600/800 mg, indicating that optimal exposure is achieved for both genders with this dosing regimen. This observation is supported by findings from the recent PK analysis, where sex did not have a clinically significant effect on the PK of obinutuzumab.31
Our data revealed a relationship between serum concentrations of obinutuzumab and base-line tumor burden: in patients with high base-line tumor burden who received low-dose obinutuzumab (400/400 mg), antibody PK was highly variable. In contrast, the PK of the higher obinutuzumab dose was more homogeneous and independent of base-line tumor burden. Serum concentrations of obinutuzumab are a likely reflection of CD20 saturation, with target saturation yielding higher systemic antibody levels. We, therefore, postulate that CD20 target sites become saturated at increased doses of obinutuzumab.
Stratifying patients by disease stage and B-cell count, exploratory graphical analyses indicated marked differences in trough serum concentrations of obinutuzumab infused at 400/400 mg. For example, trough serum concentrations of antibody were lower in patients with stage III/IV versus stage I/II disease. These differences were not apparent or were considerably less evident in patients receiving the 1600/800 mg dose. Yet PK differences did emerge for the higher dose in patient subgroups defined by disease histology, with higher serum concentrations observed for iNHL versus aNHL patients. Higher clinical response rates were also observed in iNHL versus aNHL patients (55% vs. 32%).24,25 These findings are supported by recent detailed obinutuzumab PK analyses where MCL patients were shown to have higher clearance rates than CLL patients, while those with DLBCL or BCL had the lowest clearance rates.31 These observations are likely due to differences in the levels of circulating target B cells in these diseases, and the relative expression of CD20 receptors on circulating B cells.38–40 Although nearly all of the patients in GAUDI and GAUGUIN had previously received rituximab treatment, this would have had little or no influence on obinutuzumab PK. When obinutuzumab treatment started, concentrations of rituximab in patients previously treated with this antibody would have been minimal. The only possible carry-over effect of rituximab would have been a marginal depletion of the CD20-cell population, which would have allowed a slightly faster saturation of CD20 by obinutuzumab to occur. In those patients who were refractory to rituximab, circulating CD20 cells might still have been depleted, but some CD20 production sites would have been active, leading to relapse. In rituximabnaïve patients, the intensive dosing schedule of obinutuzumab would be expected to rapidly saturate the target and deplete the CD20-cell population.
Because of an intensive dosing schedule during cycle 1 and limited sampling in cycle 4, the data collected did not allow adequate characterization of obinutuzumab elimination using simple non-compartmental analysis (NCA), so PK parameters such as distribution volume and clearance could not be determined. A more intensive sampling schedule during cycle 8 did allow adequate characterization, and pharmacokinetic modeling was, therefore, undertaken as planned, enabling fuller characterization, from which firmer conclusions could be drawn.
The clinical safety of obinutuzumab has been evaluated in a number of clinical studies, including GAUGUIN,24–29,30 and no dose-limiting toxicity has been observed, despite administration of doses of up to 2000 mg. The major safety events observed have been neutropenia, infections and infusion-related reactions, none of which are related to obinutuzumab dose or exposure.31 Therefore, in the context of a large therapeutic window for obinutuzumab, this fixed-dose strategy is an acceptable outcome.
Conclusion
Although the high dose of obinutuzumab (1600/800 mg) has favorable PK properties and demonstrable clinical efficacy, a single fixed-dose regimen would be significantly more convenient in routine clinical practice. PK predictions have shown that a fixed dose of 1000 mg of obinutuzumab with three doses instead of two in cycle 1 would yield a PK profile similar to that measured for the 1600/800 mg dose regimen assessed in GAUGUIN phase II.41 This regimen reflects a loading dose paradigm for obinutuzumab 3000 mg in cycle 1, which is directly comparable to the 3200 mg dose regimen administered in the high-dose group (1600/800 mg) of GAUGUIN. This regimen offers many significant benefits, such as reduced preparation time, less waste, and minimization of errors. Based on in vivo and in silico analyses, obinutuzumab 1000 mg administered in a 21-day cycle on D1, D8, and D15 of C1 and D1 of C2–C8 is anticipated to rapidly saturate CD20 and optimize serum concentrations of antibody, irrespective of patient demographics and disease characteristics. This dose and schedule is now being taken forward for clinical assessment in three phase III studies of obinutuzumab plus chemotherapy in NHL patients.
Acknowledgments
The GAUGUIN and GAUDI studies were funded by F. Hoffmann-La Roche Ltd. Support for third-party medical writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/2/226
Trial registration
The GAUGUIN and GAUDI studies are registered at www.clinicaltrails.gov with the trial identifiers NCT00517530 and NCT00825149, respectively.
References
- 1.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. [DOI] [PubMed] [Google Scholar]
- 2.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus R, Imrie K, Solal-Céligny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–4586. [DOI] [PubMed] [Google Scholar]
- 4.Salles G, Mounier N, de Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood. 2008;112(13):4824–4831. [DOI] [PubMed] [Google Scholar]
- 5.Hallek M, Fischer K, Fingerle-Rowson G, et al. ; International Group of Investigators; German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. [DOI] [PubMed] [Google Scholar]
- 6.Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1756–1765. [DOI] [PubMed] [Google Scholar]
- 7.van Oers MH, Van Glabbeke M, Giurgea L, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28(17): 2853–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51. [DOI] [PubMed] [Google Scholar]
- 9.Maloney DG, Liles TM, Czerwinski DK, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84(8):2457–2466. [PubMed] [Google Scholar]
- 10.Maloney DG, Grillo-López AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90(6):2188–2195. [PubMed] [Google Scholar]
- 11.McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–2833. [DOI] [PubMed] [Google Scholar]
- 12.Rituximab Summary of Product Characteristics, 9 July 2014. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000165/human_med_000897.jsp&mid=WC0b01ac058001d124.
- 13.Rituximab Prescribing Information, revised February 2010. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103705s5311lbl.pdf
- 14.Berinstein NL, Grillo-López AJ, White CA, et al. Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1998;9(9):995–1001. [DOI] [PubMed] [Google Scholar]
- 15.Igarashi T, Kobayashi Y, Ogura M, et al. IDEC-C2B8 Study Group in Japan. Factors affecting toxicity, response and progression-free survival in relapsed patients with indolent B-cell lymphoma and mantle cell lymphoma treated with rituximab: a Japanese phase II study. Ann Oncol. 2002;13(6):928–943. [DOI] [PubMed] [Google Scholar]
- 16.Tobinai K, Igarashi T, Itoh K, et al. ; IDEC-C2B8 Japan Study Group. Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Ann Oncol. 2004;15(5):821–830. [DOI] [PubMed] [Google Scholar]
- 17.Gordan LN, Grow WB, Pusateri A, Douglas V, Mendenhall NP, Lynch JW. Phase II trial of individualized rituximab dosing for patients with CD20-positive lymphoproliferative disorders. J Clin Oncol. 2005;23(6): 1096–1102. [DOI] [PubMed] [Google Scholar]
- 18.Cartron G, Blasco H, Paintaud G, Watier H, Le Guellec C. Pharmacokinetics of rituximab and its clinical use: thought for the best use¿ Crit Rev Oncol Hematol. 2007;62(1):43–52. [DOI] [PubMed] [Google Scholar]
- 19.Daydé D, Ternant D, Ohresser M, et al. Tumor burden influences exposure and response to rituximab: pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20. Blood. 2009;113(16):3765–3772. [DOI] [PubMed] [Google Scholar]
- 20.Dall’Ozzo S, Tartas S, Paintaud G, et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004;64(13):4664–4669. [DOI] [PubMed] [Google Scholar]
- 21.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–758. [DOI] [PubMed] [Google Scholar]
- 22.Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22): 4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alduaij W, Ivanov A, Honeychurch J, et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood. 2011;117(17):4519–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morschhauser F, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large B-cell lymphoma or mantle cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23): 2912–2919. [DOI] [PubMed] [Google Scholar]
- 25.Salles G, Morschhauser F, Solal-Céligny P, et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin’s lymphoma: results of the phase II GAUGUIN study. J Clin Oncol. 2013;31(23):2920–2926. [DOI] [PubMed] [Google Scholar]
- 26.Salles G, Morschhauser F, Lamy T, et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119(22):5126–5132. [DOI] [PubMed] [Google Scholar]
- 27.Sehn LH, Assouline SE, Stewart DA, et al. A phase I study of obinutuzumab induction followed by two years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119(22):5118–5125. [DOI] [PubMed] [Google Scholar]
- 28.Radford J, Davies A, Cartron G, et al. Obinutuzumab (GA101) in combination with FC or CHOP in patients with relapsed or refractory follicular lymphoma: final results of the phase I GAUDI study (BO21000). Blood. 2013;122(7):1137–1143. [DOI] [PubMed] [Google Scholar]
- 29.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244–1253. [DOI] [PubMed] [Google Scholar]
- 30.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110. [DOI] [PubMed] [Google Scholar]
- 31.Gibiansky E, Gibiansky L, Carlile DJ, Jamois C, Buchheit V, Frey N. Population pharmacokinetic analyses of obinutuzumab (GA101) in chronic lymphocytic leukemia (CLL) and non-Hodgkin’s lymphoma (NHL) and exposure-response analyses in CLL. CPT Pharmacometrics Syst Pharmacol. 2014;3:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller C, Murawski N, Wiesen MH, et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012;119(14):3276–3284. [DOI] [PubMed] [Google Scholar]
- 33.Pfreundschuh M, Müller C, Zeynalova S, et al. Suboptimal dosing of rituximab in male and female patients with DLBCL. Blood. 2014;123(5):640–646. [DOI] [PubMed] [Google Scholar]
- 34.Ngo L, Hee SW, Lim LC, et al. Prognostic factors in patients with diffuse large B cell lymphoma: Before and after the introduction of rituximab. Leuk Lymphoma. 2008;49(3):462–469. [DOI] [PubMed] [Google Scholar]
- 35.Riihijärvi S, Taskinen M, Jerkeman M, Leppä S. Male gender is an adverse prognostic factor in B-cell lymphoma patients treated with immunochemotherapy. Eur J Haematol. 2011;86(2):124–128. [DOI] [PubMed] [Google Scholar]
- 36.Pfreundschuh M, Held G, Zeynalova S, et al. Improved outcome of elderly poor-prognosis DLBCL patients with 6×CHOP-14 and 8 applications of rituximab (R) given over an extended period: results of the SMARTE-R-CHOP-14 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood (ASH Annual Meeting Abstracts). 2011; 118(21):(Abstract 592). [Google Scholar]
- 37.Murawski N, Pfreundschuh M, Zeynalova S, et al. Optimization of rituximab for the treatment of DLBCL (I) : Dose-dense rituximab in the DENSE-R-CHOP-14 trial of the DSHNHL. Ann Oncol. 2014;25(9):1800–1806. [DOI] [PubMed] [Google Scholar]
- 38.Ginaldi L, De Martinis M, Matutes E, Farahat N, Morilla R, Catovsky D. Levels of expression of CD19 and CD20 in chronic B cell leukaemias. J Clin Pathol. 1998;51(5): 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suková V, Klabusay M, Coupek P, Brychtová Y, Doubek M, Mayer J. Density expression of the CD20 antigen on population of tumor cells in patients with chronic B-lymphocyte lymphoproliferative diseases. Cas Leuk Cesk. 2006;145(9):712–716. [PubMed] [Google Scholar]
- 40.Prevodnik VK, Lavren ak J, Horvat M, Novakovi BJ. The predictive significance of CD20 expression in B-cell lymphomas. Diagn Pathol. 2011;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morschhauser F, Salles G, Cartron G, et al. Dose selection for Phase III studies of the monoclonal anti-CD20 antibody obinutuzumab (GA101) – a rational approach. Haematologica. 2011;96(8):(Abstract 0935). [Google Scholar]


