ABSTRACT
Glioblastoma multiforme (GBM) represents the most frequent and deadliest primary brain tumor. Aggressive treatment still fails to eliminate deep brain infiltrative and highly resistant tumor cells. Human Vγ9Vδ2 T cells, the major peripheral blood γδ T cell subset, react against a wide array of tumor cells and represent attractive immune effector T cells for the design of antitumor therapies. This study aims at providing a preclinical rationale for immunotherapies in GBM based on stereotaxic administration of allogeneic human Vγ9Vδ2 T cells. The feasibility and the antitumor efficacy of stereotaxic Vγ9Vδ2 T cell injections have been investigated in orthotopic GBM mice model using selected heterogeneous and invasive primary human GBM cells. Allogeneic human Vγ9Vδ2 T cells survive and patrol for several days within the brain parenchyma following adoptive transfer and can successfully eliminate infiltrative GBM primary cells. These striking observations pave the way for optimized stereotaxic antitumor immunotherapies targeting human allogeneic Vγ9Vδ2 T cells in GBM patients.
KEYWORDS: Aminobisphosphonate, human glioblastoma, immunotherapy, mice model, Vγ9Vδ2 T cells
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- BrHPP
bromohydrin pyrophosphate
- CSCs
cancer stem cells
- E:T
effector-to-target ratio
- γδT cells
gamma delta T cells
- GBM
glioblastoma multiforme
- IPP
isopentenyl pyrophosphate
- NBP
aminobisphosphonates
- PAg
phosphoantigens
Introduction
GBM represents the most aggressive glioma (WHO grade IV) with a dismal prognosis (median survival of 9.4 mo and 2 y-mortality > 86%).1 Surgery followed by radiotherapy and temozolomide-based chemotherapy represents the current standard of care for patients and modestly improves their median survival.2 Moreover, rapid tumor relapse often takes place in the vicinity of the resected tumor 3 and could be attributed to a high molecular/cellular heterogeneity in GBM combined with a diffuse and deep invasion of highly radio- and chemoresistant cell subsets,4,5 called cancer stem cells (CSC), which share phenotypic features with normal stem cells.6,7
Chemotherapies remain associated with important toxicities and their efficiency is strongly reduced due to inadequate target drug delivery. In this context, immunotherapies represent effective therapeutic options with minimal toxicities.8 Cellular immunotherapies have been explored 9 and could allow the elimination of deep brain infiltrative tumor cells. GBM-specific tumor antigens (Ag) recognized by αβ T cells,10 stress-induced molecules activating γδ T cells or specific surface molecules that can trigger ADCC have been identified and proposed for effective immunotherapies in GBM.11 Clinical trials have been performed by systemic or intracranial infusion of ex vivo-amplified T lymphocytes from either the GBM tumor, draining lymph nodes, or HLA-mismatched T cells from healthy donors, 12 but have had limited success. Post-resection administrations of selected GBM-reactive cytotoxic T cells in the vicinity of the primary tumor could represent a unique opportunity to deliver concentrated cellular immunotherapy directly to the site of residual malignancy.
Vγ9Vδ2 T cell, characterized by Vδ2 paired to Vγ9 chains TCR,13 represents about 5% of CD3+ cells in peripheral blood and more than 80% of the peripheral γδ T cell population in healthy human.14 Vγ9Vδ2 T cells are rapidly activated during infection and tumor contexts associated with strong functional responses (e.g., proliferation, cytotoxicity, cytokine release).15 The antigenic activation of Vγ9Vδ2 T cells is both a species-specific and cell-to-cell contact-dependent process that requires the engagement of the γδ TCR complex. Most Vγ9Vδ2 T cells are specifically activated by small organic pyrophosphate molecules (phosphoantigens (PAg)) such as isopentenyl pyrophosphate (IPP), which could be produced endogenously as intermediates of the mammalian mevalonate pathway. PAg can accumulate intracellularly upon cell distress induced by transformation or infection events. Aminobisphosphonate (NBP), a pharmacologic inhibitor of the mevalonate pathway, increases IPP levels in cells with elevated pinocytotic activity and/or deregulated metabolism such as tumor cells. NBP efficiently sensitize tumor cells for Vγ9Vδ2 T cell recognition in vitro, a process regulated by both adhesion (e.g., ICAM-1) and NK receptors (e.g., NKG2D) axes. Zoledronate (Zometa®; GMP-grade) is a third generation NBP compound used in humans for the treatment of bone disorders (e.g., metastases from solid tumors).16
The antitumor functions and the physiological roles played by human γδ T cells in vivo have been severely hampered due to: (i) the lack of Vγ9Vδ2 T cell counterparts in non-primate species, (ii) the lack of tumor models in non-human primates, and (iii) the strict species-specificity requirements for antigenic activation.15 Both passive and active immunotherapies targeting γδ T cells in cancer patients have yielded encouraging clinical responses.17 Passive cancer immunotherapies are based on adoptive transfers of PBL-Vγ9Vδ2 T cells previously amplified using both GMP-grade agonist compounds and IL-2, while active immunotherapy aims at directly activating and expanding Vγ9Vδ2 T cells in vivo by using administrations of GMP-grade agonist compounds and IL-2. Under these conditions, most side effects are attributed to the toxicity of IL-2, used at high doses to support the peripheral expansion of γδ T cells.
Our group has recently shown that combined administration of NBP and allogeneic ex vivo-amplified human Vγ9Vδ2 T cells efficiently controls the development of sc tumors in xenografted mice.18 Moreover, NBP-treated human glioma tumor cells are efficiently recognized by Vγ9Vδ2 T cells 19,20 illustrating the practicality of using human γδ T cells as an attractive tool for immunotherapies of GBM. In this study, we have investigated the feasibility and the antitumor efficacy of local allogeneic Vγ9Vδ2 T cell immunotherapies in murine models of orthotopic human GBM tumors using commercial cell line (U-87MG) and highly infiltrative primary GBM cells (GBM-10).
Materials and methods
Expansions of human Vγ9Vδ2 T cells
Human PBMCs were isolated from informed consented healthy blood donors obtained from the Etablissement Français du Sang (Nantes, France). For specific expansions of Vγ9Vδ2 T cells, PBMCs were incubated with 3 µM BrHPP (bromohydrin pyrophosphate), kindly provided by Innate Pharma (Marseille, France) in RPMI supplemented with 10% heat inactivated FCS, 2 mM L-glutamine, 10 mg/mL streptomycin, 100 IU/mL penicillin (all from Gibco, Carlbad, CA) and 100 IU/mL recombinant human IL-2 (rhIL-2) (Chiron, Emeryville, CA). 4 d cultures were supplemented with rhIL-2 (300 IU/mL). Specific amplification of Vδ2+ T cells was estimated by flow cytometry (resting Vγ9Vδ2 T cell lines purity > 85–95% (Fig. S1)).
Immunodeficient mice
NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice (Charles River Laboratories; Wilmington, MA), were bred in the animal facility of the University of Nantes (UTE, SFR F. Bonamy) under SPF status and used at 6–12 weeks of age, accordingly to institutional guidelines (Agreement # 00186.02; Regional ethics committee of the Pays de la Loire (France)).
Human GBM tumor cells
U-87MG cell line (HTB-14™, ATCC, Manassas, VA) was cultured in DMEM low glucose (Gibco) supplemented with 10% heat inactivated FCS, 2 mM L-glutamine, 10 mg/mL streptomycin and 100 IU/mL penicillin. GBM-10 primary culture was grown in defined medium (DEF) containing DMEM/Ham-F12 (Gibco) supplemented with 2 mM L-glutamine, N2 and B27 supplements (Gibco), 2 µg/mL heparin (Sigma-Aldrich, Louis, MO), 20 ng/mL EGF (Peprotech, Rocky Hill, NJ), 25 ng/mL bFGF (Peprotech), 10 mg/mL streptomycin and 100 IU/mL penicillin.
Stereotaxic implantation of human GBM and T cells in mouse
Human GBM cells (104 in 2 µL PBS) were injected using a stereotactic frame (Stoelting,Wood Dale, IL) at 2 mm on the right of the medial suture and 0.5 mm in front of the bregma, depth: 2.5 mm. For in vivo sensitization assay, 0.4 or 1 µg of zoledronate were injected into the tumor bed of 14 d tumor bearing mice. For adoptive T cell transfer assays, 2 × 107 human Vγ9Vδ2 T cells were stereotaxically injected, either in 10 µL sterile PBS or 40 µg/mL zoledronate solution (Zometa®; Novartis, Basel, Switzerland), into the GBM tumor bed, 7 (1 injection) or 7 and 14 d (2 injections) after tumor implantation.
Flow cytometry
For cell surface staining, human GBM cells were incubated with 10 µg/mL of APC-labeled anti-human CD44 mAb (clone G44-26; BD Biosciences, Franklin lakes, NJ), PE-Cy5-labeled anti-human CD90 mAb (clone Thy1/310; Beckman Coulter, Fullerton, CA), PE-labeled anti-human CD133 mAb (clone AC133; Myltenyi Biotec, Bergisch-Gladbach, Germany), AF488-labeled anti-human SSEA1 mAb (clone MC480, BD Biosciences) or associated isotype controls. For intracellular staining, human GBM cells were permeabilized, incubated with anti-human Nestin mAb (clone 10C2, Millipore, Billerica, MA), anti-human Olig2 mAb (clone ab81093, Abcam, Cambridge, UK), anti-human Tuj polyclonal Rabbit Ab (Sigma-Aldrich), anti-human GFAP mAb (clone 52, BD Biosciences) or associated isotype controls, followed with secondary staining with AF488-labeled or AF647-labeled antibodies. Acquisition was performed using a FACSCalibur flow cytometer (BD Biosciences) and the events were analyzed using the FlowJo software (Treestar, Ashland, OR).
Limiting dilution assay (LDA)
For LDA analysis, cells were dissociated and seeded at an initial concentration of 2 × 103 cells / mL from which serial dilutions were performed in 96-well plate. Cells were cultured for 15 d, after which the fraction of wells that did not contain neurospheres for each cell-plating density was calculated as described by Das and colleagues.21
Immunohistochemistry (IHC) analysis
Brains were fixed with 4% paraformaldehyde-PBS, embedded in paraffin wax and serially sectioned. Sections were incubated with 2% BSA and then with polyclonal Rabbit anti-human CD3 Ab (Dako, Agilent Technologies, Santa Clara, CA) or rabbit anti-human MHC class I Ab (clone EPR1394Y; Abcam). Revelation was performed by using polymer histofine rabbit to mouse coupled to HRP (Microm Microtech France, Francheville, France) and DAB detection system (Leica, Wetzlar, Germany). Slides were scanned using the NanoZoomer 2.0 HT (Hamamatsu Photonics K.K., Hamamatsu, Japan).
Isolation and staining of fresh brain cells
For human T cell detection assays, cells collected from the brains, by using the adult brain dissociation kit (Myltenyi Biotec), were labeled with PE-labeled anti-human CD3 mAb (clone UCHT1; Beckman Coulter) and analyzed by flow cytometry. For GBM cells isolations, cells were collected from the brains by using the human tumor dissociation kit (Myltenyi Biotec) followed by a discontinuous 30/70% isotonic Percoll gradient (Sigma-Aldrich)22 and isolated by using anti-human HLA mAb (clone w6/32; BioXCell, West Lebanon, NH) and the “CELLection™ Pan Mouse IgG Kit” (Gibco), accordingly to the manufacturer's instructions. Cell purity was assessed by flow cytometry using APC-labeled mouse anti-human HLA-ABC mAb (clone G46–2.6; BD Biosciences) and routinely rated higher than 85%. Isolated cells were then used for in vitro functional assays.
In vitro functional assays
Cultured or mouse brain isolated human GBM cells were pretreated 16 h with zoledronate (at the indicated concentrations). For CD107a surface mobilization assays, GBM cells were co-cultured 0, 24 or 72 h after zoledronate-sensitization, with Vγ9Vδ2 T cells (E/T ratio: 1/1) in culture medium containing 5 µM monensin (Sigma) and APC-labeled anti-human CD107a mAb (clone H4A3; BD Biosciences) for 4 h. Vγ9Vδ2 T cells were then labeled with FITC-labeled anti-human Vδ2 TCR mAb (IMMU389; Beckman Coulter) and analyzed by flow cytometry. For cytolytic activity assays, GBM cells were incubated 1 h with 51Cr (2,77 µCi / 106 cells), washed and co-cultured 4 h with Vγ9Vδ2 T cells (E/T ratio: 10/1). 51Cr release activity was measured in supernatants using a MicroBeta counter (PerkinElmer, Waltham, MA). Percentage of tumor target cell lysis = (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. Maximum and spontaneous releases were determined by adding 1% Triton X-100 or medium respectively, to 51Cr-labeled tumor target cells in the absence of T cells.
Statistical analysis
Data are expressed as mean ± SEM and were analyzed using GraphPad Prism 6.0 software (GraphPad Software, Inc., San Diego, CA). In vitro dose-response experiments were analyzed by calculating log effective concentration 50% (logEC50) using nonlinear regression and variable slope. Student's t (*p <0.05; **p <0.005; ***p < 0.0005) or log rank tests (see indicated p value) were used to reveal significant differences.
Results
Adoptively-transferred human PBL Vγ9Vδ2 T cells survive and patrol within the brain
We aimed to evaluate whether Vγ9Vδ2 T cells, amplified from PBLs of healthy donors, survived and moved within the brain parenchyma following a stereotaxic injection. Then, IHC analysis of human CD3 expression was performed on sections prepared from the brains of NSG mice previously injected with resting Vγ9Vδ2 T cells (2 × 106 cells). As expected, at day 1, CD3+ T cells were preferentially localized in the brain tissue surrounding the injection site (Fig. 1A). Interestingly, at day 7 those cells were detected not only in the regions adjacent to the injection site (Fig. 1B, upper panel), but also in the second brain hemisphere that did not receive γδ T cell injection (Fig. 1B, lower panel). Cytometry analysis, performed on cells isolated from mice stereotaxically injected with Vγ9Vδ2 T cells (107 cells), showed high frequency values of CD3+ T cells on day 1 (40 ± 8% of total isolated brain cells) which progressively decreased to reach 8% at day 7 (32 ± 3% and 8 ± 2% on day 3 to day 7, respectively) (Fig. 1C). Moreover, CD3+ T cells isolated from the mouse brains could also be activated and expanded, following sorting and antigenic stimulation, indicating that T lymphocytes can survive within the brain (data not shown). Similar results were obtained using γδ T cells prepared from different healthy donor samples but also using human αβ T cells. These results indicate that resting human Vγ9Vδ2 T cells, amplified from PBLs of healthy donors, can survive and patrol within mouse brain parenchyma for several days following their stereotaxic injection.
Figure 1.
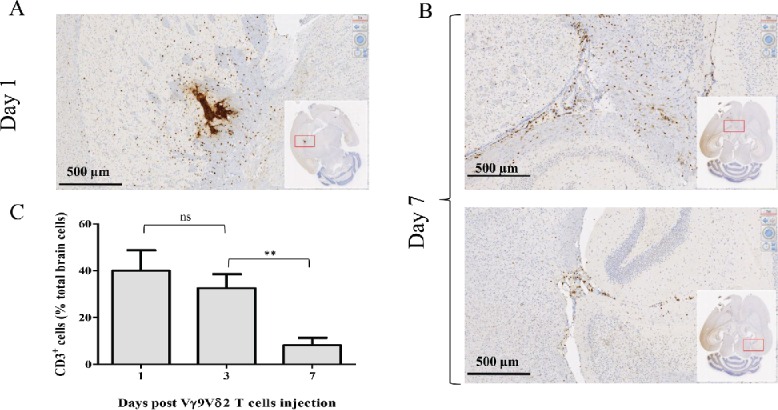
Human Vγ9Vδ2 T cells survive and patrol within the brain of NSG mice. NSG mice underwent intracranial injection of 2 × 106 (A–B) or 107 Vγ9Vδ2 T cells (C). Human-CD3 expression was analyzed by IHC on brain sections on days 1 (A) and 7 (B), or by flow cytometry on brain cells on days 1, 3 and 7 (C). Results are expressed as % of CD3+ cells among total brain cells (mean ± SEM, n = 3 ; ns: not significant, **p <0.005).
Human allogeneic Vγ9Vδ2 T cells react against U-87MG tumor cells
Based on these physiological features and their unique intrinsic characteristics, cytotoxic Vγ9Vδ2 T lymphocytes represent attractive effector candidates for immunotherapies against GBM, especially for the eradication of highly resistant and infiltrative brain tumor cells. The antitumoral reactivity of human allogeneic Vγ9Vδ2 T cells against the prototypical commercially available U-87MG human glioma cell line was analyzed in vitro (51Cr-release assays) (Fig. 2A). As expected, no natural reactivity of allogeneic Vγ9Vδ2 T cells against U-87MG cells was detected and zoledronate pre-treatments of U-87MG induced a significant Vγ9Vδ2 T cells dose-dependent antigenic activation (logEC50 = 0.12 µM). Similar reactivity patterns were established by measuring CD107a expression and IFNγ production in co-culture assays (Fig. S2A and S2B). Blocking assays performed using the antagonist 103.2 mAb,23 indicate that BTN3A/CD277 molecules expressed by these GBM tumor cells mainly contribute to the antigenic reactivity of human Vγ9Vδ2 T cells (Fig. S3). As expected, NK (CD3−CD56+) and αβ T cells, which frequently contaminate the amplified peripheral Vγ9Vδ2 T cells preparations (∼5–10%), were not activated by untreated or zoledronate treated U-87MG (data not shown).
Figure 2.
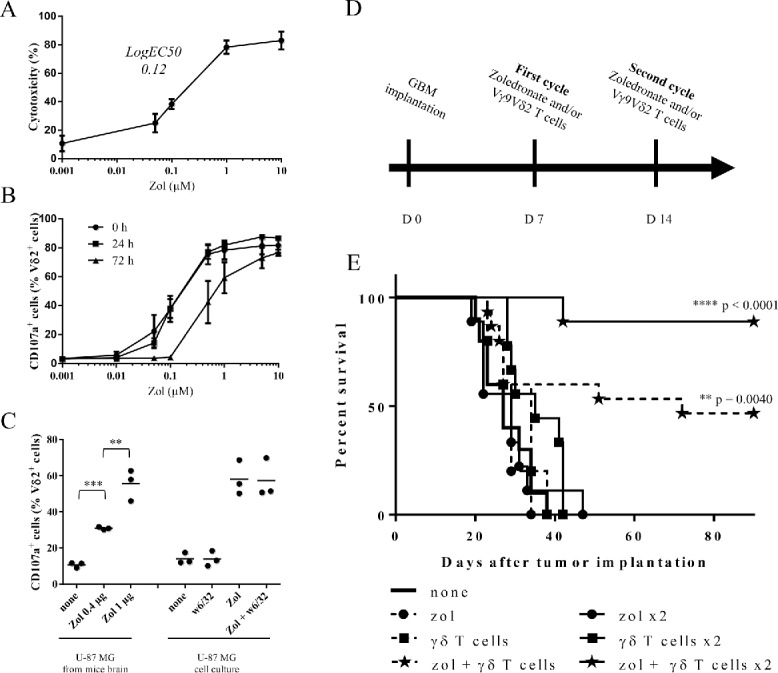
Human allogeneic Vγ9Vδ2 T cells react against U-87MG tumor cells in vitro and eradicate U-87MG cells in vivo. (A–B) U-87MG cells were pretreated overnight with different concentrations of zoledronate. (A) After zoledronate pretreatment, cells were loaded with 51Cr and co-cultured 4 h with Vγ9Vδ2 T cells (E/T ratio: 10/1). 51Cr release was measured in culture supernatants. Results are expressed as % cytotoxicity (mean ± SEM, n = 3). (B) 0, 24 or 72 h after zoledronate pretreatment, U-87MG cells were co-cultured 4 h with Vγ9Vδ2 T cells (E/T ratio: 1/1). Vγ9Vδ2 T cells were analyzed by flow cytometry for CD107a expression. Results are expressed as % of positive cells among Vδ2+ cells (mean ± SEM, n = 3). (C) U-87MG brain tumor-bearing NSG mice were injected at day 14 with zoledronate (0.4 µg or 1 µg). One day later, tumor cells were magnetically isolated using w6/32 mAb and co-cultured 4 h with Vγ9Vδ2 T cells (E/T ratio: 1/1). Vγ9Vδ2 T cells were analyzed by flow cytometry for CD107a expression. U-87MG cells cultured in vitro and pretreated or not with zoledronate (10 µM), in presence or absence of the w6/32 mAb, were used as control. Results are expressed as % of positive cells among Vδ2+ cells (mean ± SEM, n=3; **p <0.005, ***p <0.0005). (D–E) U-87MG brain tumor-bearing NSG mice were treated, or not (— ; n = 10), with single injection (day 7) of zoledronate (----•--- ; n = 5), Vγ9Vδ2 T cells (---▪---; n = 5) or both (---★--- n = 15), or with double injections (days 7 and 14) of zoledronate (—•— ; n = 9), Vγ9Vδ2 T cells (—▪— ; n = 9) or both (—★— ; n = 9). Statistical analysis was performed by log rank-test compared with control (see the indicated p value).
To determine the duration and stability of zoledronate sensitizations, the activation of Vγ9Vδ2 T cells was assessed at increasing time points following U-87MG zoledronate treatments. CD107a expression on Vγ9Vδ2 T cells did not vary during the first 24 h post-treatment, while a marked reduction was measured when GBM tumor cells were sensitized with 0.1, 0.5 or 1 µM of zoledronate 72 h before co-culture (Fig. 2B). No significant modulation was detected for high doses of zoledronate (5 and 10 µM). This indicates that the dose-dependent sensitization step, though transient, can last for several days. We further investigated whether zoledronate efficiently sensitizes human GBM cells in vivo to Vγ9Vδ2 T cell recognition. Two weeks after brain implantation of U-87MG cells, the mice received stereotaxic injections of zoledronate (0.4 µg vs. 1 µg). After 24 h, tumor cells were collected and used in in vitro functional assay. Only U-87MG tumor cells isolated from zoledronate-injected mice strongly activated Vγ9Vδ2 T cells (Fig. 2C). As a control, no effect was observed with the MHC class I-specific mAb (clone w6/32), used for human GBM cells sorting. This indicates that human allogeneic Vγ9Vδ2 T cells can strongly react against zoledronate sensitized human GBM cells and that this reactivity can last for several days.
Adoptively-transferred allogeneic Vγ9Vδ2 T cells eradicate U-87MG cells in vivo
We next investigated whether allogeneic human Vγ9Vδ2 T cells react against human GBM tumor cells in vivo. Following orthotopic implantation of U-87MG cells, mice were treated with either one (day 7) or two cycles (days 7 and 14) of stereotaxic injections of Vγ9Vδ2 T cells and/or zoledronate (Fig. 2D). Untreated mice died within 40 d after tumor grafting (median survival = 27 d) (Fig. 2E) with no significant delay after injection(s) of either zoledronate or Vγ9Vδ2 T cells alone. However, a single combined co-administration of zoledronate and Vγ9Vδ2 T cells already improved the survival of approximately 50% of the treated mice and most of GBM tumor-bearing mice survived after the successive co-administration of zoledronate and Vγ9Vδ2 T cells. Interestingly, no tumor cell was detected in mice brain by IHC at day 90, indicating a complete tumor rejection (data not shown). Then stereotaxic administration(s) of human allogeneic Vγ9Vδ2 T cells efficiently eradicates orthotopic human GBM tumors in vivo and also highlights the opportunity to target allogeneic specific cytotoxic human cells for immunotherapies against GBM.
Primary human GBM cells as cellular tools for the establishment of physiological orthotopic GBM mouse models
One of the main limits of in vivo models established with cell lines is their relative homogeneity associated with the establishment of a compact and poorly invasive tumor while the main clinical features of human GBM is their cellular heterogeneity associated with a very invasive character. An alternative of human GBM cell lines is to use primary cells isolated from human tumor fragments screened, selected 24 and grown under defined media to maintain cellular heterogeneity. Then, primary GBM-10 cells grow as spheres and express high levels of “stemness” markers (e.g., CD133, CD90, CD44) (Fig. 3A). Furthermore, limiting dilution and cell differentiation assays, to determine the presence of CSC, showed that ∼25% of this primary GBM cells are able to give rise to new neurospheres (Fig. 3B). Furthermore, the cells lost the expression of “stemness” markers, such as Nestin, CD133 and SSEA1 upon serum-induced differentiation (Fig. 3C), while expression of the differentiation markers GFAP, TUJ and Olig-2 were significantly increased (Fig. 3D). Finally, stereotaxic implantation in NSG mice of GBM-10 cells led to a disseminated (some tumor cells detected in the second non-injected hemisphere) and slow-growing brain tumors (Fig. 4), as opposed to U-87MG cells that rapidly grow to form a compact well-defined tumor mass. These results indicate that heterogeneous human GBM-10 primary cells, containing a fraction of CSC, can reproduce physiological features of human GBM and represent a tool for establishing a robust orthotopic GBM model in mouse.
Figure 3.
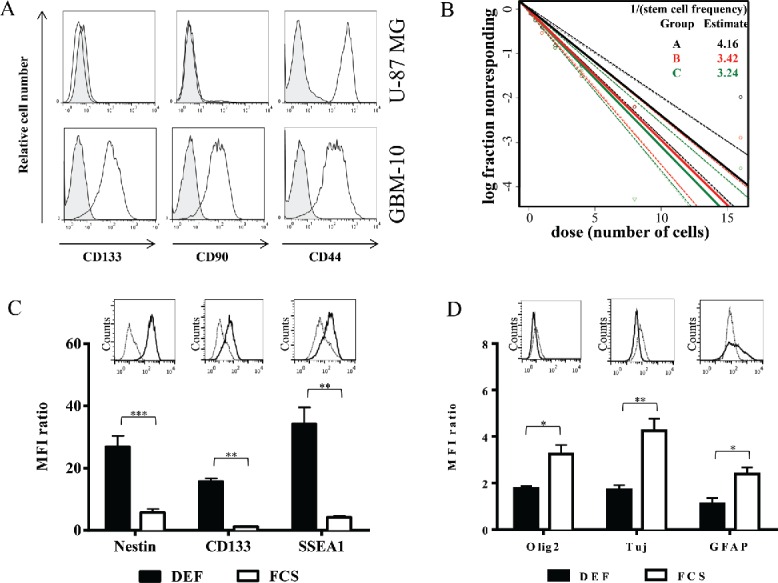
Primary GBM-10 cells as cellular tools for the establishment of a physiological orthotopic human GBM graft model in NSG mice. (A) U-87MG cells and GBM-10 cells were analyzed by flow cytometry for CD133, CD90 and CD44 expression. Gray histograms correspond to isotype control mAbs. (B) GBM-10 cells were seeded with an initial concentration of 2 × 103 cells/mL in 96-well plate. 15 d later the fraction of wells not containing neurospheres for each cell-plating density was calculated. (C–D) GBM-10 primary cells were cultured in DEF or in medium containing FCS and were analyzed by flow cytometry for the Nestin, CD133, SSEA1 (C), Olig2, Tuj and GFAP expression (D). Results are expressed as the median fluorescence intensity (MFI) ratio (MFI test/isotype control) (mean ± SEM, n = 3; *p <0.05, **p <0.005, ***p <0.0005). Inserts show representative histograms from one experiment of three performed.
Figure 4.
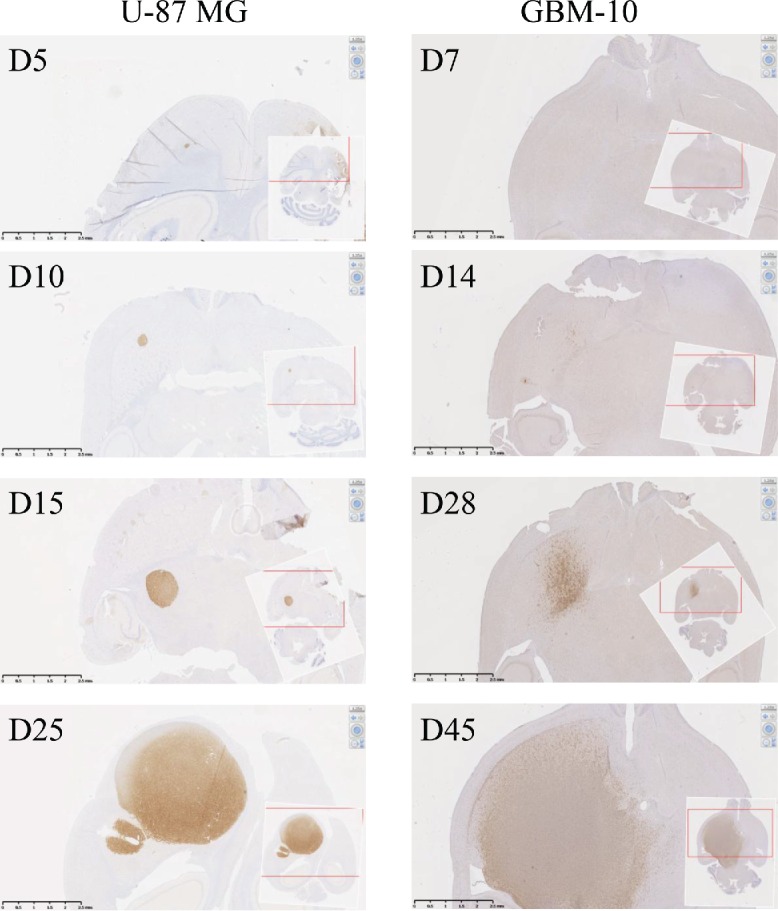
In vivo development of orthotopic human-GBM brain model in NSG mice. Human-MHC class I expression was analyzed by IHC on NSG mouse brain sections performed at days 5, 10, 15 and 25 after U-87MG brain implantation (left pictures) or at days 7, 14, 28 and 45 after GBM-10 implantation (right pictures).
Adoptively-transferred allogeneic human Vγ9Vδ2 T cells efficiently eliminate invasive GBM-10 primary tumor cells
The direct reactivity of allogeneic human Vγ9Vδ2 T cells against primary GBM-10 tumor cells was next assessed in vitro. As for U-87MG cells, Vγ9Vδ2 T cells did not naturally react against GBM-10 tumor cells, while zoledronate triggered a strong and dose-dependent antigenic activation of these effectors (Fig. 5A). Of note, higher doses of zoledronate were required to sensitize primary GBM-10 cells (logEC50 = 6.20 µM and maximal cytotoxicity at 10 µM), as compared to U-87MG cells. Similar reactivity features were established for CD107a expression and IFNγ production (Figs. S2C, S2D and S3). Next, the ability of Vγ9Vδ2 T cells to react against GBM-10 cells was evaluated in vivo. GBM-10 bearing-NSG mice were treated with one (day 7) or two cycles (days 7 and 14) of stereotaxic administrations of allogeneic human Vγ9Vδ2 T cells and/or zoledronate. Untreated mice died within 60 d (median survival = 54.5 d) and no significant variation was measured after single/double stereotaxic injection(s) of either zoledronate or Vγ9Vδ2 T cells alone (Fig. 5B). Importantly, single and also double administrations of zoledronate and Vγ9Vδ2 T cells strongly improved the survival of mice (respectively 20 and 70%). As for U-87MG model, no tumor cell was detected in brain by IHC at day 200, indicating that tumor rejection seemed complete (data not shown). Altogether, these results indicate that stereotaxic administration(s) of allogeneic human Vγ9Vδ2 T cells plus zoledronate efficiently eliminate heterogeneous primary human GBM tumors characterized by “stemness” and invasive properties.
Figure 5.

Adoptively-transferred allogeneic Vγ9Vδ2 T cells efficiently eliminate invasive GBM-10 primary tumor cells (A) GBM-10 cells were pretreated overnight with different concentrations of zoledronate, loaded with 51Cr and then co-cultured 4 h with Vγ9Vδ2 T cells (E/T ratio: 10/1). 51Cr release was measured in culture supernatants and the results are expressed as % cytotoxicity (mean ± SEM, n = 3). (B) Survival curves of GBM-10 brain tumor-bearing NSG mice treated, or not (— ; n = 10), with single injection (day 7) of zoledronate (---•--- ; n = 7), Vγ9Vδ2 T cells (---▪--- ; n = 7) or both (---★--- ; n = 10), or with double injections (days 7 and 14) of zoledronate (—•— ; n = 7), Vγ9Vδ2 T cells (—▪— ; n = 7) or both (—★— ; n = 10). Statistical analysis was performed by log-rank test compared with control (see the indicated p value).
Discussion
Adoptive transfer of tumor-specific T lymphocytes represent promising approaches to efficiently cure malignant infiltrative brain tumors with limited deleterious effect on healthy cells.8 Using orthotopic xenograft mouse models of human GBM cells from either commercially available cell lines or primary GBM cells, our study indicates that stereotaxic administrations of allogeneic ex vivo-amplified human Vγ9Vδ2 T cell effectors plus zoledronate efficiently eradicate brain tumors.
Our results support that cytotoxic human T cells efficiently eliminate most residual tumoral cells which have deeply infiltrated the brain parenchyma, including highly resistant GBM CSC.25 CSC may be involved in tumor recurrence, due to their ability to both infiltrate brain parenchyma and to resist to aggressive chemo- and radiotherapy.26 GBM-10 primary cells, isolated from a fresh biopsy and cultured in defined media maintain the cellular heterogeneity and especially the presence of CSC,24 have been used for establishing new and robust human GBM xenograft mouse model. In contrary to U-87MG cell line triggering a compact and homogenous tumor mass, primary GBM-10 cells are highly infiltrative and exhibiting typical morphological features of human GBM,27 representing new tools for establishing the next-generation animal models of human GBM tumors.
Vγ9Vδ2 T lymphocytes, the most frequent and conserved human peripheral γδ T cell subset in adults,15,28 react against a wide range of tumor cell targets.15 Both active and passive Vγ9Vδ2 T cell immunotherapies have been considered for patients with solid or circulating malignancies.17 While yielding promising results, these phase 0/I trials revealed some issues that will need to be resolved, such as the reduced reactivity of Vγ9Vδ2 T cells against fresh tumors and their possible exhaustion in some patients. Importantly, as for some other non-conventional T cell subsets, the antigenic activation process of Vγ9Vδ2 T cells is TCR- and contact-dependent, involving the expression of key molecules (eg., PAg and butyrophilins),29 but not restricted by MHC class I/II molecules, then eliminating any risk of deleterious direct alloreactive responses toward non-transformed cells.30,31 Therefore, the constitution of clinical allogeneic human Vγ9Vδ2 T cell banks, established from healthy donors, could represent a unique opportunity for designing adoptive transfer antitumor immunotherapies. Administration of effector T cells could be further hampered by the particular immunological status of the central nervous system, notably characterized by the presence of the blood-brain barrier (BBB) and the absence of classical lymphatic drainage system,32,33 then limiting T cell trafficking within the brain parenchyma and representing an additional obstacle to intravenous injections. Clinical trials of adoptive immunotherapy of GBM with human T lymphocytes were based on either intravascular or intracavitary administrations.34-36 However, the survival and activity of T cells within the cerebro-spinal fluid remains unclear as no studies have been performed to describe their behavior or their ability to move and infiltrate the brain parenchyma. Our approach is based on the direct injection of allogeneic amplified γδ T cells in the parenchyma, all around the resection cavity. Such per-operative administrations are already carried out during surgery delivering local chemo- or gene-therapies to GBM patients.37 Supporting this possibility, this study unambiguously shows that a significant fraction of Vγ9Vδ2 T cells survive for several days following a stereotaxic injection into the mouse brain parenchyma and subsequently deeply infiltrate brain tissue. Both in vitro and in vivo assays were carried out with dose-dependent zoledronate sensitizations of a GBM cell line and primary human GBM cells in order to trigger strong Vγ9Vδ2 T cell recognition, as already used for enhancing such reactivities in other oncological contexts.38 Phase I/II trials 39 have shown that zoledronate, indicated for other human pathologies (eg. osteoporosis, multiple myeloma),40 leads to significant IL-2-dependent γδ T cell expansions, correlating to partial or complete clinical responses, thus indicating the feasibility and efficacy of this approach in some cancer patients. Preclinical approaches suggested that NBP-injection could be combined with Vγ9Vδ2 T cells administration in order to induce/enhance their antitumor reactivity.18 As zoledronate does not cross the BBB,41 administrations for GBM immunotherapies required direct brain injections. As expected, stereotaxic administrations of zoledronate and Vγ9Vδ2 T cells did not induced deleterious effects in mice.42 The toxicity of such effective antitumor associations cannot be ruled out in GBM patients and will need to be extensively investigated. Some studies have described that zoledronate, like some other bisphophonate molecules, might induce the depletion of macrophages 43 and act on microglia,44 the main brain myeloid-immune cells. Then, to avoid the use of zoledronate it might be interesting to select particular Vγ9Vδ2 T cells that could naturally and specifically react more efficiently against GBM cells.
In conclusion, this study highlights the feasibility and the antitumor effect of immunotherapies based on local administrations of allogeneic human Vγ9Vδ2 T cells using novel and robust orthotopic human GBM in vivo models. Altogether, these results demonstrate that adoptive allogeneic immunotherapies could represent a promising and effective approach for the treatment of human GBM that remains one of the faster spreading and deadliest cancers.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the staff of University Hospital animal facility of Nantes for animal husbandry and care, the cellular and tissular imaging core facility of Nantes university (MicroPICell) for imaging, and the Cytometry Facility Cytocell from Nantes for their expert technical assistance. We thank also F. Cadeau and R.N. Hellman for the correction of the manuscript.
Funding
This work has been supported by INSERM, CNRS, Université de Nantes, Association pour la Recherche contre le Cancer (#R10139NN), Institut National du Cancer (#V9V2THER, INCa #PLBio2013-201, #PLBio2014-155), Agence Nationale de la Recherche (ANR, #GDSTRESS), Ligue Nationale contre le Cancer (AO InterRegional 2012). This work was realized in the context of the LabEX IGO and the IHU-Cesti programs, supported by the National Research Agency Investissements d'Avenir via the programs ANR-11-LABX-0016-01 and ANR-10-IBHU-005, respectively. The IHU-Cesti project is also supported by Nantes Metropole and the Pays de la Loire Region. The authors declare no competing financial interests. M.B. is currently vice president of the Institut Mérieux (Institut Mérieux, Lyon, F-69002, France) in charge of scientific and medical affairs.
References
- 1.Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet 2012; 379(9830):1984-96; PMID:22510398; http://dx.doi.org/ 10.1016/S0140-6736(11)61346-9 [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K et al.. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009; 10(5):459-66; PMID:19269895; http://dx.doi.org/ 10.1016/S1470-2045(09)70025-7 [DOI] [PubMed] [Google Scholar]
- 3.Buckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo. Clin. Proc. 2007; 82(10):1271-86; PMID:17908533; http://dx.doi.org/ 10.4065/82.10.1271 [DOI] [PubMed] [Google Scholar]
- 4.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444(7120):756-60; PMID:17051156; http://dx.doi.org/ 10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003; 63(18):5821-8; PMID:14522905 [PubMed] [Google Scholar]
- 6.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature 2004; 432(7015):396-401; PMID:15549107; http://dx.doi.org/ 10.1038/nature03128 [DOI] [PubMed] [Google Scholar]
- 7.Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene 2004; 23(58):9392-9400; PMID:15558011; http://dx.doi.org/ 10.1038/sj.onc.1208311 [DOI] [PubMed] [Google Scholar]
- 8.Vauleon E, Avril T, Collet B, Mosser J, Quillien V Overview of cellular immunotherapy for 18 patients with glioblastoma. Clin. Dev. Immunol. 2010; vol. 2010, Article ID 689171, 18 pages; PMID:20953324; http://dx.doi.org/21395383 10.1155/2010/689171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow KH, Gottschalk S. Cellular immunotherapy for high-grade glioma. Immunotherapy 2011; 3(3):423-34; PMID:21395383; http://dx.doi.org/ 10.2217/imt.10.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutoit V, Herold-Mende C, Hilf N, Schoor O, Beckhove P, Bucher J, Dorsch K, Flohr S, Fritsche J, Lewandrowski P et al.. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain 2012; 135(Pt 4):1042-54; PMID:22418738; http://dx.doi.org/ 10.1093/brain/aws042 [DOI] [PubMed] [Google Scholar]
- 11.Gerber DE, Laterra J. Emerging monoclonal antibody therapies for malignant gliomas. Expert Opin. Investig. Drugs. 2007; 16(4):477-94; PMID:17371196; http://dx.doi.org/ 10.1517/13543784.16.4.477 [DOI] [PubMed] [Google Scholar]
- 12.Chung DS, Shin HJ, Hong YK. A new hope in immunotherapy for malignant gliomas: adoptive T cell transfer therapy. J. Immunol. Res. 2014; 2014:326545; PMID:25009822; http://dx.doi.org/ 10.1155/2014/326545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefranc MP, Rabbitts TH. A nomenclature to fit the organization of the human T-cell receptor gamma and delta genes. Res. Immunol. 1990; 141(7):615-8; PMID:2151348; http://dx.doi.org/ 10.1016/0923-2494(90)90068-A [DOI] [PubMed] [Google Scholar]
- 14.Silva-Santos B, Serre K, Norell H. gammadelta T cells in cancer. Nat. Rev. Immunol. 2015; 15(11):683-91; PMID:26449179; http://dx.doi.org/ 10.1038/nri3904 [DOI] [PubMed] [Google Scholar]
- 15.Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr. Opin. Immunol. 2006; 18(5):539-46; PMID:16870417; http://dx.doi.org/ 10.1016/j.coi.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 16.Yeh DC, Chen DR, Chao TY, Chen SC, Wang HC, Rau KM, Feng YH, Chang YC, Lee KD, Ou-Yang F et al.. EORTC QLQ-BM22 quality of life evaluation and pain outcome in patients with bone metastases from breast cancer treated with zoledronic acid. In vivo 2014; 28(5):1001-4; PMID:25189922 [PubMed] [Google Scholar]
- 17.Fournie JJ, Sicard H, Poupot M, Bezombes C, Blanc A, Romagné F, Ysebaert L, Laurent G. What lessons can be learned from gammadelta T cell-based cancer immunotherapy trials? Cell. Mol. Immunol. 2013; 10(1):35-41; PMID:23241899; http://dx.doi.org/ 10.1038/cmi.2012.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santolaria T, Robard M, Leger A, Catros V, Bonneville M, Scotet E. Repeated systemic administrations of both aminobisphosphonates and human Vgamma9Vdelta2 T cells efficiently control tumor development in vivo. J. Immunol. 2013; 191(4):1993-2000; PMID:23836057; http://dx.doi.org/ 10.4049/jimmunol.1300255 [DOI] [PubMed] [Google Scholar]
- 19.Nakazawa T, Nakamura M, Park YS, Motoyama Y, Hironaka Y, Nishimura F, Nakagawa I, Yamada S, Matsuda R, Tamura K et al.. Cytotoxic human peripheral blood-derived gammadeltaT cells kill glioblastoma cell lines: implications for cell-based immunotherapy for patients with glioblastoma. J. Neurooncol. 2014; 116(1):31-9; PMID:24062140 [DOI] [PubMed] [Google Scholar]
- 20.Cimini E, Piacentini P, Sacchi A, Gioia C, Leone S, Lauro GM, Martini F, Agrati C. Zoledronic acid enhances Vdelta2 T-lymphocyte antitumor response to human glioma cell lines. Int. J. Immunopathol. Pharmacol. 2011; 24(1):139-48; PMID:21496396 [DOI] [PubMed] [Google Scholar]
- 21.Das AV, James J, Zhao X, Rahnenfuhrer J, Ahmad I. Identification of c-Kit receptor as a regulator of adult neural stem cells in the mammalian eye: interactions with Notch signaling. Dev. Biol. 2004; 273(1):87-105; PMID:15302600; http://dx.doi.org/ 10.1016/j.ydbio.2004.05.023 [DOI] [PubMed] [Google Scholar]
- 22.Donnou S, Fisson S, Mahe D, Montoni A, Couez D. Identification of new CNS-resident macrophage subpopulation molecular markers for the discrimination with murine systemic macrophages. J. Neuroimmunol. 2005; 169(1-2):39-49; PMID:16169092; http://dx.doi.org/ 10.1016/j.jneuroim.2005.07.016 [DOI] [PubMed] [Google Scholar]
- 23.Harly C, Guillaume Y, Nedellec S, Peigné CM, Mönkkönen H, Mönkkönen J, Li J, Kuball J, Adams EJ, Netzer S et al.. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood 2012; 120(11):2269-79; PMID:22767497; http://dx.doi.org/ 10.1182/blood-2012-05-430470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brocard E, Oizel K, Lalier L, Pecqueur C, Paris F, Vallette FM, Oliver L. Radiation-induced PGE2 sustains human glioma cells growth and survival through EGF signaling. Oncotarget 2015; 6(9):6840-9; PMID:25749386; http://dx.doi.org/ 10.18632/oncotarget.3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho DY, Lin SZ, Yang WK, Lee HC, Hsu DM, Lin HL, Chen CC, Liu CL, Lee WY, Ho LH. Targeting cancer stem cells for treatment of glioblastoma multiforme. Cell Transplant. 2013; 22(4):731-9; PMID:23594862; http://dx.doi.org/ 10.3727/096368912X655136 [DOI] [PubMed] [Google Scholar]
- 26.Thon N, Damianoff K, Hegermann J, Grau S, Krebs B, Schnell O, Tonn JC, Goldbrunner R. Presence of pluripotent CD133+ cells correlates with malignancy of gliomas. Mol. Cell. Neurosci. 2010; 43(1):51-9; PMID:18761091; http://dx.doi.org/ 10.1016/j.mcn.2008.07.022 [DOI] [PubMed] [Google Scholar]
- 27.Silver DJ, Siebzehnrubl FA, Schildts MJ, Yachnis AT, Smith GM, Smith AA, Scheffler B, Reynolds BA, Silver J, Steindler DA. Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment. J Neurosci 2013; 33(39):15603-17; PMID:24068827; http://dx.doi.org/ 10.1523/JNEUROSCI.3004-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Reviews 2007; 215:59-76; PMID:17291279; http://dx.doi.org/ 10.1111/j.1600-065X.2006.00479.x [DOI] [PubMed] [Google Scholar]
- 29.Sandstrom A, Peigne CM, Leger A, Crooks JE, Konczak F, Gesnel MC, Breathnach R, Bonneville M, Scotet E, Adams EJ. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity 2014; 40(4):490-500; PMID:24703779; http://dx.doi.org/ 10.1016/j.immuni.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereboeva L, Harkins L, Wong S, Lamb LS. The safety of allogeneic innate lymphocyte therapy for glioma patients with prior cranial irradiation. Cancer Immunol. Immunother 2015; 64(5):551-62; PMID:25676710; http://dx.doi.org/ 10.1007/s00262-015-1662-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamb LS Jr., Musk P, Ye Z, van Rhee F, Geier SS, Tong JJ, King KM, Henslee-Downey PJ. Human gammadelta(+) T lymphocytes have in vitro graft vs leukemia activity in the absence of an allogeneic response. Bone Marrow Transplant. 2001; 27(6):601-6; PMID:11319589; http://dx.doi.org/ 10.1038/sj.bmt.1702830 [DOI] [PubMed] [Google Scholar]
- 32.Bailey SL, Carpentier PA, McMahon EJ, Begolka WS, Miller SD. Innate and adaptive immune responses of the central nervous system. Critical Rev. Immunol. 2006; 26(2):149-88; PMID:16700651; http://dx.doi.org/ 10.1615/CritRevImmunol.v26.i2.40 [DOI] [PubMed] [Google Scholar]
- 33.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS et al.. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523(7560):337-41; PMID:26030524; http://dx.doi.org/ 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung G, Brandl M, Eisner W, Fraunberger P, Reifenberger G, Schlegel U, Wiestler OD, Reulen HJ, Wilmanns W. Local immunotherapy of glioma patients with a combination of 2 bispecific antibody fragments and resting autologous lymphocytes: evidence for in situ t-cell activation and therapeutic efficacy. Int. J. Cancer 2001; 91(2):225-30; PMID:11146449; http://dx.doi.org/ 10.1002/1097-0215(200002)9999:9999%3c::AID-IJC1038%3e3.3.CO;2-7 [DOI] [PubMed] [Google Scholar]
- 35.Quattrocchi KB, Miller CH, Cush S, Bernard SA, Dull ST, Smith M, Gudeman S, Varia MA. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. . Neuro-oncol. 1999; 45(2):141-57; PMID:10778730 [DOI] [PubMed] [Google Scholar]
- 36.Sloan AE, Dansey R, Zamorano L, Barger G, Hamm C, Diaz F, Baynes R, Wood G. Adoptive immunotherapy in patients with recurrent malignant glioma: preliminary results of using autologous whole-tumor vaccine plus granulocyte-macrophage colony-stimulating factor and adoptive transfer of anti-CD3-activated lymphocytes. Neurosurg. Focus. 2000; 9(6):e9; PMID:16817692; http://dx.doi.org/ 10.3171/foc.2000.9.6.10 [DOI] [PubMed] [Google Scholar]
- 37.Westphal M, Yla-Herttuala S, Martin J, Warnke P, Menei P, Eckland D, Kinley J, Kay R, Ram Z; ASPECT Study Group . Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol 2013; 14(9):823-33; PMID:23850491; http://dx.doi.org/ 10.1016/S1470-2045(13)70274-2 [DOI] [PubMed] [Google Scholar]
- 38.Rogers MJ, Crockett JC, Coxon FP, Monkkonen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 2011; 49(1):34-41; PMID:21111853; http://dx.doi.org/ 10.1016/j.bone.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi H, Tanaka Y, Yagi J, Minato N, Tanabe K. Phase I/II study of adoptive transfer of gammadelta T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol. Immunother. 2011; 60(8):1075-84; PMID:21519826; http://dx.doi.org/ 10.1007/s00262-011-1021-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coleman RE. Risks and benefits of bisphosphonates. Br. J. Cancer 2008; 98(11):1736-40; PMID:18506174; http://dx.doi.org/ 10.1038/sj.bjc.6604382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss HM, Pfaar U, Schweitzer A, Wiegand H, Skerjanec A, Schran H. Biodistribution and plasma protein binding of zoledronic acid. Drug Metab. Dispos. 2008; 36(10):2043-9; PMID:18625688 [DOI] [PubMed] [Google Scholar]
- 42.Kato Y, Tanaka Y, Tanaka H, Yamashita S, Minato N. Requirement of species-specific interactions for the activation of human gamma delta T cells by pamidronate. J. Immunol. 2003; 170(7):3608-13; PMID:12646624; http://dx.doi.org/ 10.4049/jimmunol.170.7.3608 [DOI] [PubMed] [Google Scholar]
- 43.Sabatino R, Antonelli A, Battistelli S, Schwendener R, Magnani M, Rossi L. Macrophage depletion by free bisphosphonates and zoledronate-loaded red blood cells. PloS One 2014; 9(6):e101260; PMID:24968029; http://dx.doi.org/ 10.1371/journal.pone.0101260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rietkotter E, Menck K, Bleckmann A, Farhat K, Schaffrinski M, Schulz M, Hanisch UK, Binder C, Pukrop T. Zoledronic acid inhibits macrophage/microglia-assisted breast cancer cell invasion. Oncotarget 2013; 4(9):1449-60; PMID:24036536; http://dx.doi.org/ 10.18632/oncotarget.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


