ABSTRACT
The loss-of-function mutation of formyl peptide receptor 1 (FPR1) has a negative impact on the progression-free and overall survival of breast cancer patients treated with anthracycline-based adjuvant chemotherapy. This effect may be attributed to the fact that chemotherapy-induced antitumor immunity requires FPR1 and that such anticancer immune responses are responsible for the long-term effects of chemotherapy. Here, we investigated the possible contribution of FPR1 to the efficacy of a combination of mitoxantrone (MTX) and cyclophosphamide (CTX) for the treatment of hormone-induced breast cancer. Breast cancer induced by a combination of medroxyprogesterone acetate (MPA) and 7,12-Dimethylbenz[a]anthracene (DMBA) could be successfully treated with MTX plus CTX in thus far that tumor growth was retarded and overall survival was extended (as compared to vehicle-only treated controls). However, the therapeutic efficacy of the combination therapy was completely abolished when FPR1 receptors were blocked by means of cyclosporin H (CsH). Future genetic studies on neoadjuvant chemotherapy-treated breast cancers are warranted to validate these findings at the clinical level.
KEYWORDS: Annexin A1, chemotherapy, FPR1, immunosurveillance, mammary carcinoma
Abbreviations
- ANXA1
Annexin A1
- CDAMs
cell death-associated molecules
- CsH
Cyclosporin H
- CTX
cyclophosphamide
- DAMPs
danger-associated molecular patterns
- DMBA
7,12-Dimethylbenz[a]anthracene
- FPR1
formyl peptide receptor 1
- MPA
medroxyprogesterone acetate
- MTX
mitoxantrone
- PBS
phosphate buffered saline
- PRRs
pathogen recognition receptors
- TLR
toll like receptor
Introduction
Anticancer immunosurveillance is (one of) the mechanism(s) that reduces the incidence of cancer and that allows the host to control tumor growth, especially at the beginning of oncogenesis, before cancer cells “escape” from immune control by reducing their recognizability by immune effectors (immunoselection) or by actively subverting the immune response (immunosuppression).1 Nonetheless, even in the phase of immune escape, therapeutic measures can be taken to (re)establish immunosurveillance. This has been exemplified by the administration of so-called immune checkpoint blockers,2 which unleash the anticancer immune response from its endogenous brakes.3 Moreover, it appears that most if not all cytotoxic and targeted anticancer agents owe their therapeutic success (especially if calculated in years and decades rather than in weeks and months) to the (re)activation of immunosurveillance.4-8
One of the most frequent scenarios linking the effects of cytotoxic agents and subsequent anticancer immune responses is the following. First, the cytotoxic agent causes the exposure or release of cell death-associated molecules (CDAMs),9 also called danger-associated molecular patterns (DAMPs),10 on or from cancer cells. Such CDAMs/DAMPs are then perceived by pathogen recognition receptors (PRRs) expressed by innate immune effectors to set off the initial phase of the immune response.11-17 Recently, we have found one such pair of CDAMs/DAMPs and PRRs to play a major role in anticancer immune responses against experimental tumors in mice. Indeed, cancer cells that lack the ability to release the CDAM/DAMP Annexin A1 (due to the knockout of the gene coding for Annexin A1, Anxa1) fail to induce an anticancer immune response upon treatment with anthracyclines. Similarly, tumors that have been implanted into mice lacking the AnxA1 receptor, FPR1 (which is mostly expressed by myeloid cells of the innate immune system),18 fail to respond to chemotherapy because Fpr1−/− dendritic cells are unable to approach anthracycline-killed cancer cells and hence cannot cross-present tumor-associated antigens to cytotoxic T lymphocytes.19, 20
The interaction between ANXA1 and FPR1 is important for breast cancer pathogenesis, based on two lines of evidence. First, several reports demonstrated that AnxA1 mRNA and protein levels are reduced in breast cancer as compared to normal tissues.21-23 Moreover, a loss-of-function allele of FPR1 has a negative impact on the metastasis-free and overall survival of breast cancer patients treated with anthracycline-based adjuvant chemotherapy.19 However, no information is available on the role of FPR1 in neoadjuvant breast cancer chemotherapy24 apart from biostatistical analyses indicating that genes whose expression level is influenced by FPR1 may determine the likelihood of complete pathological responses.19 Therefore, we established a model of neoadjuvant chemotherapy in mice bearing hormone-induced breast cancers and evaluated the effects of FPR1 inhibition on treatment efficacy.
Materials and methods
Animals
Mice were maintained (either in the animal facilities of Gustave Roussy Campus Cancer or in the one of the Centre de Recherche des Cordeliers) in specific pathogen-free conditions in a temperature-controlled environment with 12 h light, 12 h dark cycles and received food and water ad libitum. Animal experiments were in compliance with the EU Directive 63/2010 and protocols. Protocols 2012_081, 2013_096 and 2015_026_1114 were approved by the Ethical Committee of the Gustave Roussy Campus Cancer (CEEA IRCIV/IGR no. 26, registered at the French Ministry of Research), while protocols 03941.02 were approved by the Charles Darwin Ethics Committee for Animal Experiments (Paris, France). Six-week-old female wild-type BALB/c mice were obtained from Harlan France (Gannat, France).
Hormone-induced breast cancers
Following a published procedure,14 7-weeks-old female BALB/c mice underwent surgical implantation of slow-release MPA pellets (50 mg, 90 d release; Innovative Research of America, Sarasota, Fl, USA) s.c. into the right flank. 200 μL of 5 mg/mL dimethylbenzantracene (DMBA; Sigma Aldrich, St. Louis, MO, USA) dissolved in corn oil was administered by oral gavage once per week during 6 weeks.
Treatment of established tumors
When the tumor surface reached 25–35 mm2, mice received either 5.17 mg/kg i.p. MTX (Sigma Aldrich, St. Louis, MO, USA) and 50 mg/kg CTX (Sigma Aldrich, St. Louis, MO, USA) together with 30 mg/kg i.p. CsH (Enzo Life Biosciences, Lausen, Switzerland) or an equivalent volume of phosphate buffered saline (PBS). Tumor growth was routinely assessed by means of a caliper. Animals bearing neoplastic lesions that exceeded 20–25% of their body mass were euthanatized. All experiments contained 6 to 10 mice per group and were run at least two times, yielding similar results.
Statistical analysis for in vivo experiments
Tumor growth modeling was carried by linear mixed effect modeling on log pre-processed tumor surfaces. Reported p values are obtained from testing jointly that both tumor growth slopes and intercepts (on log scale) are the same between treatment groups of interests. Relative tumor sizes with respect to PBS control (considered as 100% values) are first computed by extracting the corresponding contrasts from the linear model on the last day of the experiment and then back-transformed to be interpreted as relative change in percentage.25, 26
Results and discussion
A model of breast cancer chemotherapy in mice
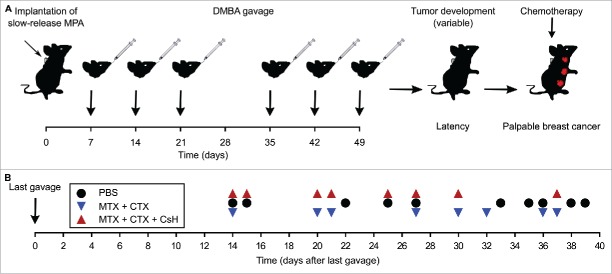
Young female BALB/c mice were implanted with pellets that release the progesterone receptor agonist MPA and then treated orally for 6 weeks with the DNA damaging agent DMBA. This protocol results in the development of one or several breast cancers in >85% of the mice, although the development of cancers occurs in an asynchronous fashion with a variable latency after the last DMBA treatment (Fig. 1A). As soon as the tumors became palpable, the mice were randomized in three different groups that received distinct kinds of treatment (Fig. 1B). As compared to vehicle-only (PBS) treated mice, a mono-therapy with the anthracycline MTX alone had little or no effects (not shown). However, the combination of MTX and CTX27 was able to reduce tumor growth (Fig. 2A) and to increase the survival of mice (Fig. 2B). Hence, we used this combination therapy to evaluate the effects of neutralization of FPR1.
Figure 1.
Characteristics of the breast cancer model used in this study. Breast cancers were induced in young (7-weeks-old) female BALB/c mice by implantation of medroxyprogesterone acetate (MPA)-releasing pellets followed by gavage with the DNA damaging agent 7,12-Dimethylbenz[a]anthracene (DMBA) for the following 6 weeks. The overall scheme of the experiment is shown in (A). Note that the interval between the last DMBA injection and the manifestation of palpable breast cancer lesions is rather variable. This interval is demonstrated for each mouse that was subsequently randomized for assignment to different treatment groups, as shown in (B). PBS, phosphate buffered saline; CsH, cyclosporine H; CTX, cyclophosphamide; MTX, mitoxantrone.
Figure 2.
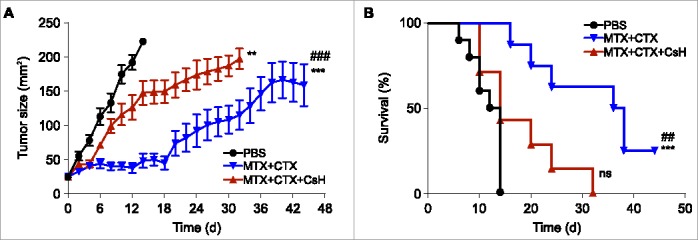
Effects of cyclosporin H on the efficacy of anticancer chemotherapy in a mouse model of breast cancers. (A) Immunocompetent BALB/c wild type (WT) mice bearing palpable hormone-induced mammary cancers received i.p. 5.17 mg/kg mitoxantrone (MTX) and 50 mg/kg cyclophosphamide (CTX) together with 30 mg/kg cyclosporin H (CsH) or an equivalent volume of phosphate buffered saline (PBS) and tumor growth was routinely assessed. Mice were treated when the tumor surface reached 25–35 mm2 (day 0) and tumor growth was routinely assessed starting from the apparition of each tumor. Results from one representative experiment out of two independent ones involving at least four mice/group and yielding similar results are illustrated. Data are represented as means ± SEM over time. **p < 0.01, ***p < 0.001 (Wald test), as compared to PBS-treated tumors; ###p < 0.05 (Wald test), as compared to MTX + CTX + CsH-treated tumors. Kaplan–Meier survival curves are shown in (B). n.s., non-significant, ***p < 0.001 (Log-Rank), as compared to PBS-treated tumors; ##p < 0.01 (Log-Rank), as compared to MTX + CTX+ CsH-treated tumors.
Pharmacological inhibition of FPR1 abolishes chemotherapeutic effects
CsH is considered as a specific FPR1 antagonist, meaning that it can be used to abolish signaling via FPR1 in vivo.28-30 Therefore, we injected CsH concomitantly with the combination regiment (MTX plus CTX) to evaluate the contribution of FPR1 to the efficacy of chemotherapy. CsH largely reverted the beneficial effects of MTX plus CTX on the growth of hormone-induced breast cancers (Fig. 2A). Simultaneously, CsH abrogated the survival advantage of mice with breast cancer that was normally conferred by treatment with MTX plus CTX (Fig. 2B). In conclusion, FPR1-mediated signaling is required for the success of neoadjuvant chemotherapy against breast cancer.
Concluding remarks
To the best of our knowledge, the present report is the first to demonstrate that induced breast cancer responds to chemotherapy (with MTX plus CTX) and that the success of chemotherapy depends on the contribution of the immune system, meaning that immunosuppression by CsH (which acts on FPR1) largely reduces the efficacy of the treatment with respect to tumor growth and host survival.
The animal model that we used for this demonstration resembles locally advanced human breast cancer that is treated with neoadjuvant chemotherapy (i.e. chemotherapy without prior surgical removal of the primary tumor). As mentioned in the Introduction, molecular epidemiological studies performed in the context of adjuvant chemotherapy (i.e. treatment after surgery) of human breast cancer indicate that a loss-of-function allele of FPR1 (single nucleotide polymorphism rs867228 causing nucleotide exchange 1037A>C, leading to the amino acid substitution Glu346Ala) has a negative impact on patient survival.19 In the context of the adjuvant chemotherapy of breast cancer a recessive model could be applied, meaning that the group composed by homozygous and heterozygous carriers of the FPR1 loss-of-function allele exhibited a shorter metastasis-free or overall survival than patients bearing two normal FPR1 alleles. In contrast, in oxaliplatin-treated colorectal cancer, a dominant model had to be applied to detect the effects of the FPR1 loss-of-function allele, meaning that only homozygous carriers of the loss-of-function allele were affected by poor prognosis when compared to all other groups. Indeed, there is a gradient in the functional consequences of FPR1 loss-of-function alleles on the interaction between dendritic cell precursors and dying cancer cells, as detectable in a microfluidic device.19
Based on the aforementioned results, it will be interesting to investigate whether heterozygosity or homozygosity in the FPR1 loss-of-function allele will affect the prognosis of breast cancer patients undergoing neoadjuvant chemotherapy as well. This type of analysis will require careful homogenization of patient cohorts with respect to other PRRs and their loss-of-function alleles. Indeed, toll like receptor (TLR) 4 and TLR3 loss-of-function alleles are epistatic to the FPR1 loss-of function alleles with respect to the outcome of adjuvant breast cancer chemotherapy.19 Hence, molecular epidemiological studies of neoadjuvant breast cancer should simultaneously assess multiple possible immune defects that might affect the efficacy of chemotherapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
G.K. and L.Z. are supported by the Ligue Nationale contre le Cancer (Equipes labellisées), Site de Recherche Intégrée sur le Cancer (IRIC) Socrates, the ISREC Foundation, Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC), the European Commission (ArtForce), a European Research Council Advanced Investigator Grant (to G.K.), Institut Universitaire de France; Fondation pour la Recherche Médicale (FRM), the Institut National du Cancer (INCa), the Fondation de France, the Cancéropôle Ile-de-France, the Fondation Bettencourt-Schueller, the European Research Council (ERC); the LeDucq Foundation; the LabEx Immuno-Oncology and the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). E.E.B. is supported by the Cancéropôle Ile-de-France.
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.org/ 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 2.Chawla A, Philips AV, Alatrash G, Mittendorf E. Immune checkpoints: A therapeutic target in triple negative breast cancer. Oncoimmunology 2014; 3:e28325; PMID:24843833; http://dx.doi.org/ 10.4161/onci.28325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015; 161:205-14; PMID:25860605; http://dx.doi.org/ 10.1016/j.cell.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest 2008; 118:1991-2001; PMID:18523649; http://dx.doi.org/ 10.1172/JCI35180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008; 8:59-73; PMID:18097448; http://dx.doi.org/ 10.1038/nri2216 [DOI] [PubMed] [Google Scholar]
- 6.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39:74-88; PMID:23890065; http://dx.doi.org/ 10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 7.Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N et al.. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014; 3:e955691; PMID:25941621; http://dx.doi.org/ 10.4161/21624011.2014.955691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015 Dec 14;28(6):690-714; PMID:26678337; http://dx.doi.org/10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell 2010; 140:798-804; PMID:20303871; http://dx.doi.org/ 10.1016/j.cell.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 10.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 1994; 12:991-1045; PMID:8011301; http://dx.doi.org/ 10.1146/annurev.iy.12.040194.005015 [DOI] [PubMed] [Google Scholar]
- 11.Obeid M, Tesniere A, Panaretakis T, Tufi R, Joza N, van Endert P, Ghiringhelli F, Apetoh L, Chaput N, Flament C et al.. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev 2007; 220:22-34; PMID:17979837; http://dx.doi.org/ 10.1111/j.1600-065X.2007.00567.x [DOI] [PubMed] [Google Scholar]
- 12.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S et al.. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 2007; 220:47-59; PMID:17979839; http://dx.doi.org/ 10.1111/j.1600-065X.2007.00573.x [DOI] [PubMed] [Google Scholar]
- 13.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E et al.. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15:1170-8; PMID:19767732; http://dx.doi.org/ 10.1038/nm.2028 [DOI] [PubMed] [Google Scholar]
- 14.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O, Niso-Santano M et al.. An immunosurveillance mechanism controls cancer cell ploidy. Science 2012; 337:1678-84; PMID:23019653; http://dx.doi.org/ 10.1126/science.1224922 [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity 2013; 39:211-27; PMID:23973220; http://dx.doi.org/ 10.1016/j.immuni.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 16.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C et al.. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med 2014; 20:1301-9; PMID:25344738; http://dx.doi.org/ 10.1038/nm.3708 [DOI] [PubMed] [Google Scholar]
- 17.Ma Y, Adjemian S, Galluzzi L, Zitvogel L, Kroemer G. Chemokines and chemokine receptors required for optimal responses to anticancer chemotherapy. Oncoimmunology 2014; 3:e27663; PMID:24800170; http://dx.doi.org/ 10.4161/onci.27663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina-Echeverz J, Aranda F, Berraondo P. Myeloid-derived cells are key targets of tumor immunotherapy. Oncoimmunology 2014; 3:e28398; PMID:25050208; http://dx.doi.org/ 10.4161/onci.28398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M et al.. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 2015; 350:972-8; PMID:26516201; http://dx.doi.org/ 10.1126/science.aad0779 [DOI] [PubMed] [Google Scholar]
- 20.Vacchelli E, Ma Y, Baracco EE, Zitvogel L, Kroemer G. Yet another pattern recognition receptor involved in the chemotherapy-induced anticancer immune response: formyl peptide receptor-1. Oncoimmunology 2016; http://dx.doi.org/10.1080/2162402X.2015.1118600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen D, Nooraie F, Elshimali Y, Lonsberry V, He J, Bose S, Chia D, Seligson D, Chang HR, Goodglick L. Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol 2006; 37:1583-91; PMID:16949910; http://dx.doi.org/ 10.1016/j.humpath.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 22.Cao Y, Li Y, Edelweiss M, Arun B, Rosen D, Resetkova E, Wu Y, Liu J, Sahin A, Albarracin CT. Loss of annexin A1 expression in breast cancer progression. Appl Immunohistochem Mol Morphol 2008; 16:530-4; PMID:18776816; http://dx.doi.org/ 10.1097/PAI.0b013e31817432c3 [DOI] [PubMed] [Google Scholar]
- 23.Wang LP, Bi J, Yao C, Xu XD, Li XX, Wang SM, Li ZL, Zhang DY, Wang M, Chang GQ. Annexin A1 expression and its prognostic significance in human breast cancer. Neoplasma 2010; 57:253-9; PMID:20353277; http://dx.doi.org/ 10.4149/neo_2010_03_253 [DOI] [PubMed] [Google Scholar]
- 24.Stoll G, Enot D, Mlecnik B, Galon J, Zitvogel L, Kroemer G. Immune-related gene signatures predict the outcome of neoadjuvant chemotherapy. Oncoimmunology 2014; 3:e27884; PMID:24790795; http://dx.doi.org/ 10.4161/onci.27884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demidenko E. The assessment of tumour response to treatment. J Royal Stat Soc: Series C 2006; 55:365-77; http://dx.doi.org/ 10.1111/j.1467-9876.2006.00541.x [DOI] [Google Scholar]
- 26.Sugar E, Pascoe AJ, Azad N. Reporting of preclinical tumor-graft cancer therapeutic studies. Cancer Biol Ther 2012; 13:1262-8; PMID:22895077; http://dx.doi.org/ 10.4161/cbt.21782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Wakefield LM, Oppenheim JJ. Synergistic antitumor effects of a TGFbeta inhibitor and cyclophosphamide. Oncoimmunology 2014; 3:e28247; PMID:25050195; http://dx.doi.org/ 10.4161/onci.28247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenzel-Seifert K, Hurt CM, Seifert R. High constitutive activity of the human formyl peptide receptor. J Biol Chem 1998; 273:24181-9; PMID:9727041; http://dx.doi.org/ 10.1074/jbc.273.37.24181 [DOI] [PubMed] [Google Scholar]
- 29.Stenfeldt AL, Karlsson J, Wenneras C, Bylund J, Fu H, Dahlgren C. Cyclosporin H, Boc-MLF and Boc-FLFLF are antagonists that preferentially inhibit activity triggered through the formyl peptide receptor. Inflammation 2007; 30:224-9; PMID:17687636; http://dx.doi.org/ 10.1007/s10753-007-9040-4 [DOI] [PubMed] [Google Scholar]
- 30.Qin C, Buxton KD, Pepe S, Cao AH, Venardos K, Love JE, Kaye DM, Yang YH, Morand EF, Ritchie RH. Reperfusion-induced myocardial dysfunction is prevented by endogenous annexin-A1 and its N-terminal-derived peptide Ac-ANX-A1(2-26). Br J Pharmacol 2013; 168:238-52; PMID:22924634; http://dx.doi.org/ 10.1111/j.1476-5381.2012.02176.x [DOI] [PMC free article] [PubMed] [Google Scholar]