ABSTRACT
Hippo is a tumor-suppressor pathway that negatively regulates the oncoproteins TAZ and YAP. Moreover, Hippo affects the biology of a variety of non-neoplastic cells in the tumor microenvironment, even including immune cells. We herein assessed the predictive role of TAZ and YAP, assessed by immunohistochemistry, in 50 cervical cancer patients prevalently treated with neoadjuvant chemotherapy. Tumors were classified as positive or negative according to the percentage of tumor-expressing cells and cellular localization. TAZ/YAP were also evaluated in non-neoplastic cells, namely endothelial cells, non-lymphocytic stromal cells and tumor-infiltrating lymphocytes (TILs). TAZ expression in cancer cells (TAZpos) was associated with a reduced pathological complete response (pCR) rate (p = 0.041). Conversely, the expression of TAZ and YAP in TILs (TAZTIL+ and YAPTIL+) seemed to be associated with increased pCRs (p = 0.083 and p = 0.018, respectively). When testing the predictive significance of the concomitant expression of TAZ in cancer cells and its absence in TILs (TAZpos/TAZTIL-), patients with TAZpos/TAZTIL- showed lower pCR rate (p = 0.001), as confirmed in multivariate analysis (TAZpos/TAZTIL-: OR 8.67, 95% CI: 2.31–32.52, p = 0.001). Sensitivity analysis carried out in the 41 patients treated with neoadjuvant chemotherapy yielded comparable results (TAZpos/TAZTIL-: OR 11.0, 95% CI: 2.42–49.91, p = 0.002). Internal validation carried out with two different procedures confirmed the robustness of this model. Overall, we found evidence on the association between TAZ expression in cervical cancer cells and reduced pCR rate. Conversely, the expression of the Hippo transducers in TILs may predict increased treatment efficacy, possibly mirroring the activation of a non-canonical Hippo/MST pathway necessary for T-cells activation and survival.
KEYWORDS: Cervical cancer, Hippo pathway, Hippo transducers, pathological complete response, TAZ, tumor microenvironment; YAP
Abbreviations
- CAFs
cancer-associated fibroblasts
- pCR
pathological complete response
- TAZ
transcriptional co-activator with PDZ-binding motif
- TILs
tumor-infiltrating lymphocytes
- YAP
Yes-associated protein
Introduction
The Hippo pathway is a regulator of tissue growth conserved in the animal kingdom, which plays important regulatory functions during organ development and regeneration.1 Similarly to other embryonic pathways,2 Hippo was later recognized as aberrantly regulated in neoplastic diseases where it acts as a tumor suppressor signal.1
Core components of the Hippo pathway are operatively grouped into a regulatory kinase module and a transcriptional module. The first contains the kinases sterile 20-like kinase 1 (MST1) and 2 (MST2), large tumor suppressor 1 (LATS1) and 2 (LATS2), and the adaptor proteins Salvador homolog 1 (SAV1), MOB kinase activator 1A (MOB1A) and 1B (MOB1B). The transcriptional module is composed by two closely related oncoproteins: the transcriptional co-activator with PDZ-binding motif (TAZ) and the Yes-associated protein (YAP).3
The activation of the Hippo kinase cascade leads to the phosphorylation of TAZ and YAP. This inhibitory phosphorylation promotes nuclear exclusion, cytoplasmic retention and proteasomal degradation of TAZ/YAP.3 When the kinase core module is inactive or its regulatory activity on TAZ/YAP is bypassed by other stimuli, TAZ/YAP translocate to the nucleus where, upon interaction with TEA domain-containing sequence-specific transcription factors (TEAD1 to TEAD4), mediate the transcription of various oncogenes.3
The Hippo pathway also mediates important function in non-neoplastic cells commonly residing in the tumor microenvironment. Hippo transducers were tied to the maintenance of cancer-associated fibroblasts (CAFs).4 In turn, CAFs promote tumor growth, neo-angiogenesis and distant dissemination. Next, TAZ/YAP are required for survival of endothelial cells.5 Finally, a non-canonical, immune-related Hippo/MST pathway has been described as crucial for maintaining immune homeostasis and immunological self-tolerance.6
Activation of TAZ/YAP has been connected to a variety of tumor-promoting functions in a number of neoplastic entities. In breast cancer, TAZ/YAP mediate epithelial-to-mesenchymal transition (EMT), acquisition/retention of cancer stem cell (CSCs) features, chemotherapy resistance and metastatic spread.7 In this setting, exploratory clinical analyses pointed to TAZ/YAP as potential prognostic and predictive biomarkers.8-10
Nevertheless, little is known on the role of the Hippo pathway in cervical cancer.11,12 Recently, YAP activation was found to encourage proliferation and migration of cervical cancer cells through a positive loop involving the epidermal growth factor receptor (EGFR).13 These oncogenic activities were further enforced by the Human Papillomavirus (HPV) E6 protein, which protects YAP from proteasome-dependent degradation.13
As a part of our research agenda focused on the clinical translation of potential CSC-related biomarkers previously identified at the preclinical level, we herein focused our attention on TAZ/YAP in cervical cancer. To this end, TAZ/YAP expression was evaluated in a cohort of 50 cervical cancer patients mostly treated with neoadjuvant chemotherapy, and analyzed for their impact on pCR. Given the involvement of TAZ/YAP in the biology of a variety of cell types cohabiting the tumor microenvironment, TAZ and YAP were assessed both in tumor cells and in non-neoplastic cells, namely endothelial cells, non-lymphocytic stromal cells and TILs.
Results
Out of the 71 patients screened, 50 met the eligibility criteria for this study. Twenty-one patients were not eligible for the following reasons: lack of sufficient biological materials in diagnostic biopsies (n = 11), lack of surgical samples (n = 8) and incomplete clinical data (n = 2). Baseline characteristics of the 50 patients included in this study are summarized in Table 1. In this cohort, the pCR rate was 36% (18 out of 50 patients). Table 2 summarizes the expression of TAZ and YAP in tumor cells, non-lymphocytic stromal cells, endothelial cells and TILs. Representative immunohistochemical staining patterns are illustrated in Fig. 1.
Table 1.
Baseline characteristics and treatment outcome of cervical cancer patients treated with neoadjuvant chemotherapy or chemoradiation (n = 50).
| Characteristics | N (%) |
|---|---|
| Age | |
| Median (min–max) [IQ range] | 47 (27–69) [37.7–57.0] |
| Stage | |
| I B | 12 (24.0) |
| II A | 15 (30.0) |
| II B | 19 (38.0) |
| III A | 2 (4.0) |
| III B | 2 (4.0) |
| Histology | |
| Squamous cell carcinoma | 43 (86.0) |
| Adenocarcinoma | 7 (14.0) |
| Treatment | |
| Chemotherapy | 41 (82.0) |
| Chemoradiation | 9 (18.0) |
| pCR | |
| No | 32 (64.0) |
| Yes | 18 (36.0) |
Table 2.
Expression of TAZ and YAP in cancer cells, non-lymphocytic stromal cells, endothelial cells and TILs.
| N (%) | |
|---|---|
| TAZ (tumor) | |
| Neg | 11 (22.0) |
| Pos | 39 (78.0) |
| TAZ (stroma) | |
| Neg | 25 (50.0) |
| Pos | 25 (50.0) |
| TAZ (endothelium) | |
| Neg | 8 (16.0) |
| Pos | 42 (84.0) |
| TAZ (TILs) | |
| Neg | 43 (86.0) |
| Pos | 7 (14.0) |
| YAP (tumor) | |
| Neg | 18 (36.0) |
| Pos | 32 (64.0) |
| YAP (stroma) | |
| Neg | 13 (26.0) |
| Pos | 37 (74.0) |
| YAP (endothelium) | |
| Neg | 13 (26.0) |
| Pos | 37 (74.0) |
| YAP (TILs) | |
| Neg | 44 (88.0) |
| Pos | 6 (12.0) |
Figure 1.
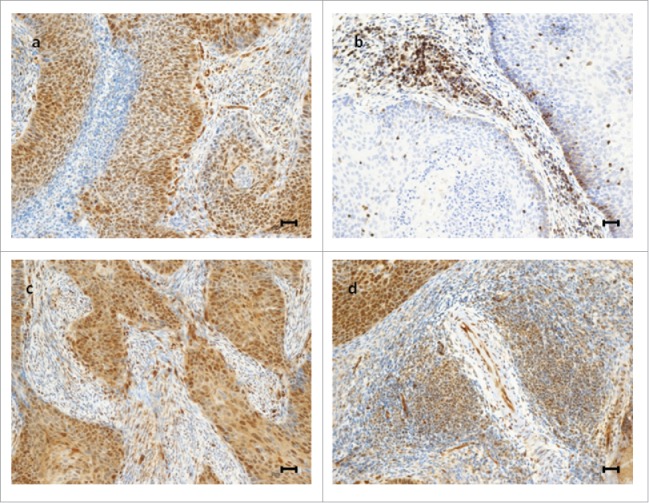
Representative examples of immunohistochemical expression of TAZ and YAP in cervical cancer patients. Upper panel: a tumor expressing TAZ in tumor cells, endothelial cells and non-lymphocytic stromal cells, but not in TILs (A); a tumor expressing TAZ exclusively in TILs (B). Lower panel: a tumor expressing YAP in tumor cells, endothelial cells and non-lymphocytic stromal cells, but not in TILs (C); a tumor expressing YAP in TILs, tumor cells and endothelial cells (D). Scale bar = 30 µm.
As shown in Table 3, the only molecular variable associated with reduced pCR rate was TAZpos (p = 0.041). Conversely, a suggestion for increased pCR rate was noted for TAZTILs+ and YAPTIL+ tumors compared with their negative counterparts (p = 0.083 and p = 0.018 for TAZTILs+ and YAPTIL+, respectively). Univariate and multivariate logistic regression models confirmed the relationship between TAZpos and reduced pCR rate (Table 4). Conversely, TAZ expression in TILs had a protective effect (Table 4).
Table 3.
Association between clinical–molecular variables and pCR (n = 50).
| pCR |
|||
|---|---|---|---|
| No | Yes | Chi squared test | |
| N (%) | N (%) | p value | |
| Age | |||
| ≤ 47 | 19 (73.1) | 7 (26.9) | 0.164 |
| > 47 | 13 (54.2) | 11 (45.8) | |
| Stage | |||
| I B–II A | 17 (63.0) | 10 (37.0) | 0.869 |
| II B–III B | 15 (65.2) | 8 (34.8) | |
| Histology | |||
| Squamous cell carcinoma | 25 (58.1) | 18 (41.9) | 0.040* |
| Adenocarcinoma | 7 (100.0) | 0 (0.0) | |
| Treatment | |||
| Chemotherapy | 27 (65.9) | 14 (34.1) | 0.705* |
| Chemoradiation | 5 (55.6) | 4 (44.4) | |
| TAZ (tumor) | |||
| Neg | 4 (36.4) | 7 (63.6) | 0.041* |
| Pos | 28 (71.8) | 11 (28.2) | |
| TAZ (stroma) | |||
| Neg | 16 (64.0) | 9 (36.0) | 0.999 |
| Pos | 16 (64.0) | 9 (36.0) | |
| TAZ (endothelium) | |||
| Neg | 4 (50.0) | 4 (50.0) | 0.436* |
| Pos | 28 (66.7) | 14 (33.3) | |
| TAZ (TILs) | |||
| Neg | 30 (69.8) | 13 (30.2) | 0.083* |
| Pos | 2 (28.6) | 5 (71.4) | |
| YAP (tumor) | |||
| Neg | 10 (55.6) | 8 (44.4) | 0.351 |
| Pos | 22 (68.8) | 10 (31.2) | |
| YAP (stroma) | |||
| Neg | 7 (53.8) | 6 (46.2) | 0.375 |
| Pos | 25 (67.6) | 12 (32.4) | |
| YAP (endothelium) | |||
| Neg | 8 (61.5) | 5 (38.5) | 0.830 |
| Pos | 24 (64.9) | 13 (35.1) | |
| YAP (TILs) | |||
| Neg | 31 (70.5) | 13 (29.5) | 0.018* |
| Pos | 1 (16.7) | 5 (83.3) | |
Fisher's exact test.
Table 4.
Univariate and multivariate logistic regression models for pCR (n = 50).
| Univariate logistic regression model |
Multivariate logistic regression model§ |
Multivariate logistic regression model# |
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | ||
| Age | > 47 vs. ≤ 47 | 0.43 (0.13–1.42) | 0.168 | 0.73 (0.17–3.09) | 0.674 | ||
| Stage | II B–III B vs. I B– II I A | 1.10 (0.35–3.52) | 0.869 | 2.16 (0.43–10.97) | 0.352 | ||
| Treatment | Chemoradiation vs. Chemotherapy | 0.65 (0.15–2.80) | 0.562 | 0.69 (0.07–6.43) | 0.745 | ||
| TAZ (tumor) | Pos vs. Neg | 4.45 (1.08–18.29) | 0.038 | 7.14 (1.46–34.91) | 0.015 | 6.81 (1.32–35.24) | 0.022 |
| TAZ (TILs) | Pos vs. Neg | 0.17 (0.03–1.01) | 0.052 | 0.11 (0.02–0.80) | 0.029 | 0.09 (0.01–0.82) | 0.033 |
| YAP (TILs) | Pos vs. Neg | 0.08 (0.01–0.79) | 0.030 | 0.11 (0.01–1.22) | 0.072 | 0.10 (0.01–1.67) | 0.109 |
Adjusted for: age, stage and treatment.
Forward stepwise inclusion.
On the basis of the detrimental effect of TAZ expression on cancer cells, and the apparent opposite interaction of the TAZTILs+ and YAPTILs+ phenotypes with pCR, we investigated whether the combination of these molecular endpoints better delineated the category of patients that did not achieve a pCR. As reported in Table S1, both the TAZpos/TAZTILs- and the TAZpos/TAZTILs-/YAPTIL- phenotypes were significantly associated with reduced pCR rate (p = 0.001 and p < 0.001, respectively).
Coherently, the TAZpos/TAZTILs- was the only variable that tested significant at the univariate and multivariate assessment (OR 8.67, 95% CI: 2.31–32.52, p = 0.001), even when adjusting by age, stage and type of treatment (OR 9.13, 95% CI: 2.19–38.09, p = 0.002) (Table 5). The robustness of this model was internally validated with a re-sampling without replacement procedure. The replication rate was 77.5% (155/200 simulations) with statistical significance set at p < 0.01.
Table 5.
Univariate and multivariate logistic regression models evaluating the impact of the TAZpos/TAZTIL- phenotype on pCR (n = 50).
| Univariate logistic regression model |
Multivariate logistic regression model§ |
Multivariate logistic regression model# |
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | ||
| Age at diagnosis | > 47 vs. ≤ 47 | 0.43 (0.13–1.42) | 0.168 | 0.74 (0.18–3.00) | 0.676 | ||
| Stage | II B–III vs. I–II A | 1.10 (0.35–3.52) | 0.869 | 1.86 (0.40–8.61) | 0.425 | ||
| Treatment | Chemoradiation vs. Chemotherapy | 0.65 (0.15–2.80) | 0.562 | 0.39 (0.05–2.82) | 0.352 | ||
| TAZpos/TAZTIL- | Pos vs. Neg | 8.67 (2.31–32.52) | 0.001 | 8.67 (2.31–32.52) | 0.001 | 9.13 (2.19–38.09) | 0.002 |
Adjusted for: age at diagnosis, stage and treatment.
Forward stepwise inclusion.
Comparable results were obtained when the multivariate model was generated by exclusively considering the 41 patients treated with neoadjuvant chemotherapy (OR 11.0, 95% CI: 2.42–49.91, p = 0.002), even when the model was adjusted for clinical variables that were not significant at univariate assessment (OR 15.77, 95% CI: 2.54–98.1, p = 0.003) (Table 6). The replication rate was 69% (138/200 simulations) with statistical significance set at p < 0.01. Moreover, the results of the logistic regression model were confirmed with the bootstrap method (p = 0.004; 95% CI: 1.05–21.09).
Table 6.
Univariate and multivariate logistic regression models for pCR in patients who received chemotherapy (n = 41).
| Univariate logistic regression model |
Multivariate logistic regression model§ |
Multivariate logistic regression model# |
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | ||
| Age at diagnosis | > 47 vs. ≤ 47 | 0.44 (0.12–1.63) | 0.232 | 0.75 (0.14–4.10) | 0.741 | ||
| Stage | II B–III B vs. I B–II A | 1.72 (0.43–6.90) | 0.445 | 4.27 (0.64–28.65) | 0.135 | ||
| TAZpos/TAZTIL- | Pos vs. Neg | 11.0 (2.42–49.91) | 0.002 | 11.0 (2.42–49.91) | 0.002 | 15.77 (2.54–98.1) | 0.003 |
Adjusted for: age and stage.
Forward stepwise inclusion.
Discussion
In the present study, we investigated the relationship between the expression of TAZ/YAP, evaluated both in tumor cells and in the tumor microenvironment, and pCR in a moderately-sized cohort of cervical cancer patients prevalently treated with neoadjuvant chemotherapy. We observed a reduced pCR rate in patients whose tumors expressed TAZ, but not YAP. The predictive ability of TAZ expression in cancer cells was significantly improved when its status in TILs was considered. To our knowledge, this is the first study thoroughly evaluating the expression pattern of both TAZ and YAP in cervical cancer and its supportive environment, and with a clear focus on a clinical outcome.
We acknowledge that the retrospective nature of our study invites caution in the interpretation of the results. Indeed, the findings herein reported are hypothesis-generating and require larger and/or prospective confirmations.
Before discussing our results from a molecular perspective, it is worth anticipating that the use of neoadjuvant chemotherapy for early-stage or locally-advanced cervical cancer patients, and the impact of pCR on long-term survival outcomes, is still controversial.14-18 Nonetheless, in some countries and especially in Europe, great attention is put toward this approach. Consistently, the European Organization for Research and Treatment of Cancer (EORTC) is carrying out a phase III randomized trial, having overall survival as the primary endpoint, comparing neoadjuvant chemotherapy followed by radical hysterectomy vs. chemotherapy plus radiation therapy in FIGO Ib2-IIb cervical cancer patients (ClinicalTrials.gov Identifier: NCT00039338).
From a molecular standpoint, this study raises important points that deserve consideration. First, TAZ and YAP are two homologous transcriptional co-activators. Nevertheless, in our study only TAZ expression was associated with reduced pCR rate. Despite the analogies between TAZ and YAP, in a neoplastic background they might exert partly different functions. For instance, in breast cancer only TAZ was linked to the acquisition/retention of CSC traits,19,20 whereas the molecular output elicited by YAP activation remains ambiguous.7 Indeed, both tumor-promoting and tumor-suppressive functions were reported upon its activation.21-27 Conversely, in the domain of tumor-stroma interplay, YAP function was required for CAFs, whereas TAZ was dispensable for CAF-mediated promotion of cancer-cell invasion.4 Even though YAP seems to elicit tumor-enhancing functions in cervical cancer, in our study only TAZ expression seemed to predict the outcome of interest.
Second, the Hippo pathway is subject to a variety of regulatory forces that act both at the level of core kinases or directly on TAZ/YAP. Beyond a variety of effectors capable of activating MST1/2 or LATS1/2 kinases,28-31 a series of other inputs tune TAZ/YAP activation. These include junctional and apicobasal polarity factors, mechanical forces imposed by the extracellular matrix, G-protein-coupled receptors and Rho GTPases, the actin cytoskeleton, metabolic routes such as the mevalonate pathway and aerobic glycolysis, and the Wnt pathway.5,32-38 Thus, wider pathway analyses, which also involve regulatory branches and target genes, are necessary to provide a clearer picture on how Hippo transducers affect the efficacy of anticancer treatments. On this basis, we have implemented our search for Hippo pathway-related prognostic and predictive biomarkers by taking into account molecular endpoints related to TAZ/YAP activation.
In our opinion, the most intriguing finding of our study relates to the protective role of TAZ and, although to a lower extent, YAP when expressed in TILs. The Hippo/MST pathway was recently recognized as a multifaceted regulator of mammalian adaptive immunity.6 In this context, MST kinases are essential for the development, activation, survival, trafficking and homing of T cells.39-43 Even though the Hippo/MST pathway does not involve the canonical Hippo cascade, we speculate that cytoplasmic expression of TAZ/YAP might be a readout for the activation of the Hippo/MST pathway, thus labeling a subset of lymphocytes particularly reactive against cancer cells. Coherently, TAZ/YAP in TILs had an exclusive cytoplasmic localization. This observation assumes even more relevance when considering the growing interest surrounding TIL status as predictor of therapeutic and survival outcomes.44 When referring to TILs, the attention turns to breast cancer.44 Given that we have already assessed TAZ/YAP in HER2-positive and triple-negative breast cancer patients treated with neoadjuvant chemotherapy, [8, and personal unpublished data] their evaluation in TILs, together with MST kinases, is ongoing and will provide further ground to this hypothesis.
In summary, we observed an inverse relationship between TAZ expression and pCR in a cohort of cervical cancer patients prevalently treated with neoadjuvant chemotherapy. Conversely, the expression of TAZ/YAP in TILs seemed associated with an increased likelihood of achieving a pCR. The concomitant evaluation of TAZ in tumor cells and in TILs provided the most accurate prediction of reduced pCRs. Thus, Hippo pathway-associated biomarkers deserve increased consideration in cervical cancer, and larger studies are warranted.
Patients and methods
Study participants
Fifty histologically confirmed cervical cancer patients who received neoadjuvant chemotherapy (n = 41) or concurrent chemoradiation (n = 9) followed by radical hysterectomy were included in this retrospective analysis. Nine patients treated with chemoradiation were included in this study given that, upon radiological assessment, they were deemed suitable for surgery with radical intent. Analyses were initially carried out in the entire cohort, and then repeated in a sensitivity analysis conducted by excluding those patients who received chemoradiation (n = 9). Neoadjuvant chemotherapy consisted in the TIP regimen (paclitaxel 175 mg/m2 on day 1 + ifosfamide 2500 mg/m2 on days 1 and 2 + cisplatin 50 mg/m2 on day 2 every 21 d for 3 or 4 cycles). Chemoradiation was delivered with the following schedule: intensity-modulated radiation therapy (IMRT) administered over 6 weeks (60 Gy to the tumor and 49–50 Gy to non-metastatic pelvic nodes) plus concomitant weekly single-agent cisplatin at 40 mg/m2/week. Eligibility was determined according to the following criteria: (i) completeness of data related to clinical features and treatment outcomes, (ii) sufficient biological materials for pathological and molecular analyses in diagnostic biopsies, (iii) availability of surgical samples for the evaluation of pCR and (iv) completion of the planned treatment. pCR was defined as a complete disappearance of tumor in the cervix with negative nodes, as suggested by Buda et al.45 This retrospective study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the “Regina Elena” National Cancer Institute of Rome, the coordinating center. Our work was carried out in full compliance with all of the legal requirements pertaining to the institutions involved. Written informed consents were secured before anticancer therapy.
Study procedures
The immunohistochemical assessment of TAZ and YAP was performed in formalin-fixed paraffin-embedded (FFPE) tissues using the monoclonal antibody (MoAb) anti-TAZ (M2-616, BD PharMingen) at the dilution of 1:400 and the MoAb anti-YAP (H-9, Santa Cruz) at the dilution of 1:200.
In cancer cells, TAZ/YAP staining intensity was graded on a 4-grade scale (0: negative, 1+: weak, 2+: moderate, 3+: strong). In order to classify negative (TAZneg, YAPneg) and positive (TAZpos, YAPpos) tumors, we considered cellular localization, staining intensity and percentage of tumor-expressing cells. On this basis, TAZ/YAP positivity was defined as a distinct nuclear immunoreactivity in ≥10% of neoplastic cells.
For the assessment of TAZ/YAP in the tumor microenvironment, the three main cellular components (endothelial cells, non-lymphocytic stromal cells and TILs) were morphologically identified. For each cellular compartment, TAZ/YAP expression was considered positive when cells exhibited a distinct homogeneous/heterogeneous immunoreactivity, irrespectively of the subcellular localization. This was related to the observation that, when expressed, TAZ/YAP were exclusively cytoplasmic in endothelial cells and TILs, and prevalently cytoplasmic in non-lymphocytic stromal cells. In this analysis, we did not include faintly staining cells or positive cells located in the tumor margin or in areas with poor morphology. Two investigators (SB and MM) blinded to treatment outcome independently evaluated immunoreactivity. Discordant cases were discussed and solved at a face-to-face assessment.
Statistical analysis
Descriptive statistics were used to summarize the characteristics of the study participants and the investigated molecular endpoints. The relationships between categorical variables were assessed with the Pearson's Chi-squared test of independence (2-tailed) or the Fisher Exact test, depending upon the size of the groups compared. Univariate logistic regression models helped identify variables potentially impacting pCR. A multivariate logistic regression model was generated using a stepwise regression approach (forward selection) and the related estimates reported as Odds Ratio (OR) and 95% Confident Interval (CI). The enter and remove limits were p = 0.10 and p = 0.15, respectively. A multivariate logistic regression model was also built by adjusting for clinical variables. A sensitivity analysis was carried out by removing the nine patients treated with chemoradiation. We considered statistically significant p values less than 0.05. The consistency of the TAZpos/TAZTILs- model was assessed through an internal validation procedure envisioning re-sampling without replacement. In greater detail, 200 hundred, less-powered datasets were generated by randomly removing ∼20% from the original sample. For each simulation, the multivariate model was repeated and the replication rate was calculated.46 For internal validation, statistical significance was set at p < 0.01. Finally, in order to avoid overfitting bootstrap (re-sampling with replacement) was used as a second procedure for internal validation.47 Five independent procedures, each containing 1.000 bootstrap samples, were applied, and the less optimistic simulation was reported in terms of p value and 95% CI.
Statistical analyses were carried out using SPSS software (SPSS version 21, SPSS Inc., Chicago, IL, USA).
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Tania Merlino for technical assistance.
Funding
This work was supported by Consorzio Interuniversitario Nazionale per la Bio-Oncologia (CINBO) (CN).
References
- 1.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov 2014; 13:63-79; PMID:24336504; http://dx.doi.org/ 10.1038/nrd4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 2015; 12:445-64; PMID:25850553; http://dx.doi.org/ 10.1038/nrclinonc.2015.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 2014; 94:1287-312; PMID:25287865; http://dx.doi.org/ 10.1152/physrev.00005.2014 [DOI] [PubMed] [Google Scholar]
- 4.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G et al.. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 2013; 15:637-46; PMID:23708000; http://dx.doi.org/ 10.1038/ncb2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S et al.. Role of YAP/TAZ in mechanotransduction. Nature. 2011; 474:179-83; PMID:21654799; http://dx.doi.org/ 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 6.Du X, Yu A, Tao W. The non-canonical Hippo/Mst pathway in lymphocyte development and functions. Acta Biochim Biophys Sin (Shanghai) 2015; 47:60-4; PMID:25487919; http://dx.doi.org/ 10.1093/abbs/gmu112 [DOI] [PubMed] [Google Scholar]
- 7.Maugeri-Saccà M, Barba M, Pizzuti L, Vici P, Di Lauro L, Dattilo R, Vitale I, Bartucci M, Mottolese M, De Maria R. The Hippo transducers TAZ and YAP in breast cancer: oncogenic activities and clinical implications. Expert Rev Mol Med 2015; 17:e14; PMID:26136233; http://dx.doi.org/ 10.1017/erm.2015.12 [DOI] [PubMed] [Google Scholar]
- 8.Vici P, Mottolese M, Pizzuti L, Barba M, Sperati F, Terrenato I, Di Benedetto A, Natoli C, Gamucci T, Angelucci D et al.. The Hippo transducer TAZ as a biomarker of pathological complete response in HER2-positive breast cancer patients treated with trastuzumab-based neoadjuvant therapy. Oncotarget 2014; 5:9619-25; PMID:25294813; http://dx.doi.org/ 10.18632/oncotarget.2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehn S, Tobin NP, Sims AH, Stål O, Jirström K, Axelson H, Landberg G. Decreased expression of Yes-associated protein is associated with outcome in the luminal A breast cancer subgroup and with an impaired tamoxifen response. BMC Cancer 2014; 14:119; PMID:24559095; http://dx.doi.org/ 10.1186/1471-2407-14-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Díaz-Martín J, López-García MÁ, Romero-Pérez L, Atienza-Amores MR, Pecero ML, Castilla MÁ, Biscuola M, Santón A, Palacios J. Nuclear TAZ expression associates with the triple-negative phenotype in breast cancer. Endocr Relat Cancer 2015; 22:443-54; PMID:25870251; http://dx.doi.org/ 10.1530/ERC-14-0456 [DOI] [PubMed] [Google Scholar]
- 11.Xiao H, Wu L, Zheng H, Li N, Wan H, Liang G, Zhao Y, Liang J. Expression of Yes-associated protein in cervical squamous epithelium lesions. Int J Gynecol Cancer 2014; 24:1575-82; PMID:25304677; http://dx.doi.org/ 10.1097/IGC.0000000000000259 [DOI] [PubMed] [Google Scholar]
- 12.Liu T, Liu Y, Gao H, Meng F, Yang S, Lou G. Clinical significance of yes-associated protein overexpression in cervical carcinoma: the differential effects based on histotypes. Int J Gynecol Cancer 2013; 23:735-42; PMID:23502453; http://dx.doi.org/ 10.1097/IGC.0b013e31828c8619 [DOI] [PubMed] [Google Scholar]
- 13.He C, Mao D, Hua G, Lv X, Chen X, Angeletti PC, Dong J, Remmenga SW, Rodabaugh KJ, Zhou J et al.. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med 2015; 7:1426-49; PMID:26417066; http://dx.doi.org/ 10.15252/emmm.201404976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HS, Sardi JE, Katsumata N, Ryu HS, Nam JH, Chung HH, Park NH, Song YS, Behtash N, Kamura T et al.. Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: an international collaborative meta-analysis. Eur J Surg Oncol 2013; 39:115-24; PMID:23084091; http://dx.doi.org/ 10.1016/j.ejso.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 15.Landoni F, Sartori E, Maggino T, Zola P, Zanagnolo V, Cosio S, Ferrari F, Piovano E, Gadducci A. Is there a role for postoperative treatment in patients with stage Ib2-IIb cervical cancer treated with neo-adjuvant chemotherapy and radical surgery? An Italian multicenter retrospective study. Gynecol Oncol 2014; 132:611-7; PMID:24342439; http://dx.doi.org/ 10.1016/j.ygyno.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 16.Ye Q, Yuan HX, Chen HL. Responsiveness of neoadjuvant chemotherapy before surgery predicts favorable prognosis for cervical cancer patients: a meta-analysis. J Cancer Res Clin Oncol 2013; 139:1887-98; PMID:24022086; http://dx.doi.org/ 10.1007/s00432-013-1509-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rydzewska L, Tierney J, Vale CL, Symonds PR. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst Rev 2010;(1):CD007406; PMID:20091632; http://dx.doi.org/ 10.1002/14651858.CD007406.pub2 [DOI] [PubMed] [Google Scholar]
- 18.Gadducci A, Sartori E, Maggino T, Zola P, Cosio S, Zizioli V, Lapresa M, Piovano E, Landoni F. Pathological response on surgical samples is an independent prognostic variable for patients with Stage Ib2-IIb cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy: an Italian multicenter retrospective study (CTF Study). Gynecol Oncol 2013; 131:640-4; PMID:24096111; http://dx.doi.org/ 10.1016/j.ygyno.2013.09.029 [DOI] [PubMed] [Google Scholar]
- 19.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A et al.. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011; 147:759-72; PMID:22078877; http://dx.doi.org/ 10.1016/j.cell.2011.09.048 [DOI] [PubMed] [Google Scholar]
- 20.Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi G, Sperati F et al.. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene 2015; 34:681-90; PMID:24531710; http://dx.doi.org/ 10.1038/onc.2014.5 [DOI] [PubMed] [Google Scholar]
- 21.Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, Mantovani F, Damalas A, Citro G, Sacchi A et al.. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell 2005; 18:447-59; PMID:15893728; http://dx.doi.org/ 10.1016/j.molcel.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 22.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 2003; 11:11-23; PMID:12535517; http://dx.doi.org/ 10.1016/S1097-2765(02)00776-1 [DOI] [PubMed] [Google Scholar]
- 23.Yu SJ, Hu JY, Kuang XY, Luo JM, Hou YF, Di GH, Wu J, Shen ZZ, Song HY, Shao ZM. MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer. Clin Cancer Res 2013; 19:1389-99; PMID:23340296; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-1959 [DOI] [PubMed] [Google Scholar]
- 24.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A 2006; 103:12405-10; PMID:16894141; http://dx.doi.org/ 10.1073/pnas.0605579103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol 2009; 11:1444-50; PMID:19935651; http://dx.doi.org/ 10.1038/ncb1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A 2012; 109:E2441-50; PMID:22891335; http://dx.doi.org/ 10.1073/pnas.1212021109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, Gupta S, Liang H, Lin HK, Hung MC et al.. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med 2012; 18:1511-7; PMID:23001183; http://dx.doi.org/ 10.1038/nm.2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell 2010; 18:300-8; PMID:20159599; http://dx.doi.org/ 10.1016/j.devcel.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 2013; 154:1342-55; PMID:24012335; http://dx.doi.org/ 10.1016/j.cell.2013.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell 2011; 21:888-95; PMID:22075147; http://dx.doi.org/ 10.1016/j.devcel.2011.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang HL, Wang S, Yin MX, Dong L, Wang C, Wu W, Lu Y, Feng M, Dai C, Guo X et al.. Par-1 regulates tissue growth by influencing hippo phosphorylation status and hippo-salvador association. PLoS Biol 2013; 11:e1001620; PMID:23940457; http://dx.doi.org/21145499 10.1371/journal.pbio.1001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell 2010; 19:831-44; PMID:21145499; http://dx.doi.org/ 10.1016/j.devcel.2010.11.012 [DOI] [PubMed] [Google Scholar]
- 33.Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem 2011; 286:7018-26; PMID:21224387; http://dx.doi.org/ 10.1074/jbc.C110.212621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR et al.. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 2011; 144:782-95; PMID:21376238; http://dx.doi.org/ 10.1016/j.cell.2011.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H et al.. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012; 150:780-91; PMID:22863277; http://dx.doi.org/ 10.1016/j.cell.2012.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S et al.. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 2014; 16:357-66; PMID:24658687; http://dx.doi.org/ 10.1038/ncb2936 [DOI] [PubMed] [Google Scholar]
- 37.Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo G et al.. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J 2015; 34:1349-70; PMID:25796446; http://dx.doi.org/ 10.15252/embj.201490379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V et al.. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 2014; 158:157-70; PMID:24976009; http://dx.doi.org/ 10.1016/j.cell.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 39.Dong Y, Du X, Ye J, Han M, Xu T, Zhuang Y, Tao W. A cell-intrinsic role for Mst1 in regulating thymocyte egress. J Immunol 2009; 183(6):3865-72; PMID:19692642; http://dx.doi.org/ 10.4049/jimmunol.0900678 [DOI] [PubMed] [Google Scholar]
- 40.Nehme NT, Pachlopnik Schmid J, Debeurme F, André-Schmutz I, Lim A, Nitschke P, Rieux-Laucat F, Lutz P, Picard C, Mahlaoui N et al.. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 2012; 119:3458-68. Dec 14; PMID:22174160; http://dx.doi.org/ 10.1182/blood-2011-09-378364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katagiri K, Katakai T, Ebisuno Y, Ueda Y, Okada T, Kinashi T. Mst1 controls lymphocyte trafficking and interstitial motility within lymph nodes. EMBO J 2009; 28:1319-31; PMID:19339990; http://dx.doi.org/ 10.1038/emboj.2009.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol 2006; 7:919-28; PMID:16892067; http://dx.doi.org/ 10.1038/ni1374 [DOI] [PubMed] [Google Scholar]
- 43.Abdollahpour H, Appaswamy G, Kotlarz D, Diestelhorst J, Beier R, Schäffer AA, Gertz EM, Schambach A, Kreipe HH, Pfeifer D et al.. The phenotype of human STK4 deficiency. Blood 2012; 119:3450-7; PMID:22294732; http://dx.doi.org/ 10.1182/blood-2011-09-378158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F et al.. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015; 26:259-71; PMID:25214542; http://dx.doi.org/ 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buda A, Fossati R, Colombo N, Fei F, Floriani I, Gueli Alletti D, Katsaros D, Landoni F, Lissoni A, Malzoni C et al.. Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the SNAP01 (Studio Neo-Adjuvante Portio) Italian Collaborative Study. J Clin Oncol 2005; 23:4137-45; PMID:15961761; http://dx.doi.org/ 10.1200/JCO.2005.04.172 [DOI] [PubMed] [Google Scholar]
- 46.Bria E, Milella M, Sperduti I, Alessandrini G, Visca P, Corzani F, Giannarelli D, Cerasoli V, Cuppone F, Cecere FL et al.. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung Cancer 2009; 66:365-71; PMID:19327866; http://dx.doi.org/ 10.1016/j.lungcan.2009.02.024 [DOI] [PubMed] [Google Scholar]
- 47.Steyerberg EW, Bleeker SE, Moll HA, Grobbee DE, Moons KG. Internal and external validation of predictive models: a simulation study of bias and precision in small samples. J Clin Epidemiol 2003; 56:441-7; PMID:12812818; http://dx.doi.org/ 10.1016/S0895-4356(03)00047-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


