ABSTRACT
Tumor infiltrating lymphocytes in primary breast cancer (TIL) are acknowledged measures of disease free survival (DFS) in adjuvant and neoadjuvant settings. Little is known about the biology of metastasis infiltrating lymphocytes (mTIL) although the local immunity of the metastatic site may critically influence the infiltrate composite. To address this question, we compared mTIL with their matched TIL in 87 breast cancer patients and their corresponding distant metastasis at four different anatomical locations. Sections of surgical specimen were immunohistochemically analyzed for CD4+, CD8+ and CD20+ lymphocytes in three different tumor compartments: intratumoral lymphocytes (iTIL) defined as lymphocytes in direct contact with breast cancer cells, stromal lymphocytes (sTIL) located within the intratumoral stromal tissue and invasive-margin lymphocytes (imTIL). Overall, we found fewer (p < 0.001) mTIL than TIL. Within the tumor compartments, imTIL were more frequent than sTIL and iTIL both within metastases and the matched primary tumors (PT) (p < 0.001). CD4+ T cells were more numerous than CD8+ T cells and CD20+ B cells (p < 0.001). There was a similar pattern in PT and their corresponding metastasis. Only patients with brain metastases differed from the others displaying less CD20+ B cells at the infiltrative margin of the PT (p < 0.05).
In summary, mTIL were significantly reduced within metastases but still mirrored the infiltrate pattern of the PT, interestingly regardless of the metastatic anatomical locations investigated. Our results suggest that the PT assigns the infiltrating lymphocyte pattern resumed at the metastatic site.
KEYWORDS: Breast cancer, lymphocytes, metastasis, tumor profile
Abbreviations
- CTLs
cytotoxic CD8+ T cells
- DFS
disease free survival
- FFPE
formalin-fixed paraffin-embedded
- HE
hematoxylin and eosin
- imTIL
invasive margin infiltrating intratumoral lymphocytes
- iTIL
intratumoral tumor infiltrating lymphocytes
- mTIL
infiltrating lymphocytes in metastases
- Met
metastatic lesion
- PT
primary tumor
- sTIL
stromal infiltrating intratumoral lymphocytes
- TIL
tumor infiltrating lymphocytes
- TMA
tissue microarray
- Tregs
regulatory T cells
Introduction
Metastatic outgrowth in distant organs is the major cause of breast cancer associated death. In breast cancer, overt metastasis is usually detected after a latency period of 2–20 y after surgical removal and treatment of the PT.1 Yet, tumor dissemination of cancer cells seems to occur even before PT are clinically detectable2,3 most likely due to dormant micrometastatic cells referred to as microscopically undetectable metastatatic tumor cells presumably persisting in a growth arrest phase.4 Among various contributing mechanisms, the role of the immune system, particularly immunosurveillance has been lively discussed.5 In this context, an important role of TIL was recently demonstrated in colorectal cancer. There, the immune contexture of TIL integrated into an immunoscore6 was shown to significantly correlate with DFS and overall survival. Moreover, assessment of the immunoscore proved to be superior with regard to prognosis when compared to the classical TNM staging system suggesting that tumor progression depends on the host immune response.7
Generally, a more favorable prognosis is supported by a cancer-destructive microenvironment consisting of type I immunity such as Th1 CD4+ T cells, cytotoxic CD8+ T cells (CTLs) and pro-inflammatory cytokines while a type II immune environment limits the expansion of CTLs and promotes an immunosuppressive milieu dominated by regulatory T cells (Tregs) and their corresponding cytokines.8-10 Interestingly, the immune profile correlates with the molecular breast cancer subtype. In luminal breast cancers, CTLs were described to be most frequent with only low levels of Tregs being present whereas HER2+ or basal-like subtypes showed the inverse immune profile.11,12 In line with other malignancies, depending on the TIL subsets, TIL were shown to correlate with improved specific survival of breast cancer patients. Particularly a higher frequency of CD8+ TIL correlated with a better prognosis.13,14 TIL are associated with a better neoadjuvant chemotherapy response in Her2+ and triple-negative breast cancers.15-18
These findings have emphasized the importance of evaluating TIL and have supported novel immunomodulatory treatment options. Boosting T cell responses by anti-CTLA4 or anti-PD1 antibodies was shown to improve disease outcome in melanoma or lung cancer.19-21 Immunotherapy is not yet routinely used in breast cancer, but was mainly applied in phase I clinical trials.22
In contrast to primary breast cancer infiltrating lymphocytes (TIL) little is known about the frequency and composition of mTIL. While TIL always face the local immunity of the breast mTIL are confronted with different on-site immunities depending on the anatomical location of metastasis. Experimental studies have proposed innate immunity to play a crucial role in late-stage carcinogenesis and metastasis outgrowth.27-30 Yet, many of these findings are based on metastasis models carrying cancers selected for fast tumor and metastasis growth. Consequently, the human tumor biology may not be properly reflected where the dynamics are slower given often years until metastasis can be detected such that adaptive immunity may dominate even at the site of distant metastasis.
To this end, we characterized frequencies of CD4+, CD8+ and CD20+ infiltrating lymphocyte subsets within three different tumor compartments using 87 primary breast cancer specimen and their corresponding metastatic sites in brain, bone, liver or soft tissue. Our results reveal a comparable distribution and composition but lower frequency of infiltrating lymphocyte subsets within the metastasis when compared to the matched PT. Interestingly, we found this pattern within all metastases regardless of their anatomical location suggesting that the PT shapes the infiltrating immune cell composite rather than the metastases with their local immunity.
Materials and methods
Patients and material cohort
To compare the immunoprofile in primary breast cancer and their corresponding metastases, we searched patients with hematogeneous metastases in the archives of the Institute of Surgical Pathology, University Hospital Zurich in the time period of 1995–2012. There was tissue material of 87 formalin-fixed paraffin-embedded (FFPE) primary breast cancer samples and their corresponding sites of distant metastasis available. Among the metastatic lesions, there were 46 surgical specimens and 41 biopsy specimens. While all PT samples had been characterized for estrogen and progesterone receptor expression at the time of the primary diagnosis, samples from before 2002 had not routinely been assessed for their Her2 expression. Her2 status was retrieved in all but two such cases from a retrospective large tissue microarray (TMA) databank of the Institute of Surgical Pathology. Our study cohort consisted of 87 breast cancer patients suffering from either invasive-ductal or invasive-lobular breast cancer. Four patients were treated with a preoperative chemotherapy, the others underwent adjuvant treatment after surgery according to the time current guidelines and available regiments. Distant metastasis had occurred to either brain, bone, liver or soft tissue (Table 1) the most common sites of metastasis in breast cancer.26 In our cohort, no patient suffered from more than one metastasis at the four investigated anatomical sites. Yet, we cannot exclude that patients exhibited metastases at other but the four investigated locations at the time of metastasis sampling such as lung metastases which may have been monitored by imaging only. Approval of the use of human primary breast cancer samples and metastatic tissue was obtained from the official ethical authorities of the Canton Zurich, Switzerland (ethical approval KER-2012-553). Tissue blocks were cut in multiple 2 μm sections for hematoxylin and eosin (HE) and immunohistochemical stainings.
Table 1.
Clinicopathological data of the 87 breast cancer patients investigated. Clinicopathological data and group distribution of the 87 breast cancer patients with respect to the four anatomical locations at which the corresponding metastasis had occurred. Percentages are shown in relation to the respective metastatic site.
| Site of Metastasis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain (n = 21) |
Bone (n = 24) |
Liver (n = 11) |
Soft tissue (n = 31) |
||||||
| Clinicopathological Parameters | No. | % | No. | % | No. | % | No. | % | |
| Age at initial diagnosis | — | — | — | — | — | — | — | — | |
| Age < 55 | 15 | 71.4% | 9 | 37.5% | 4 | 36.4% | 12 | 38.7% | |
| Age ≥ 55 | 6 | 28.6% | 15 | 62.5% | 7 | 63.6% | 19 | 61.3% | |
| Histological Subtype | |||||||||
| invasive-ductal | 20 | 95.2% | 18 | 75.0% | 11 | 100.0% | 25 | 80.6% | |
| invasive-lobular | 1 | 4.8% | 6 | 25.0% | 0 | 0.0% | 6 | 19.4% | |
| Tumor (T) stage | |||||||||
| T1 | 6 | 28.6% | 6 | 25.0% | 4 | 36.4% | 8 | 25.8% | |
| T2 | 10 | 47.6% | 16 | 66.7% | 6 | 54.5% | 20 | 64.5% | |
| T3 | 4 | 19.0% | 1 | 4.2% | 1 | 9.1% | 2 | 6.5% | |
| T4 | 1 | 4.8% | 1 | 4.2% | 0 | 0.0% | 1 | 3.2% | |
| Nodal (N) stage | |||||||||
| Negative | 5 | 23.8% | 11 | 45.8% | 2 | 18.2% | 12 | 38.7% | |
| Positive | 16 | 76.2% | 13 | 54.2% | 9 | 81.8% | 19 | 61.3% | |
| Tumor grade | |||||||||
| Grade 1 | 0 | 0.0% | 1 | 4.2% | 1 | 9.1% | 3 | 9.7% | |
| Grade 2 | 3 | 14.3% | 12 | 50.0% | 2 | 18.2% | 17 | 54.8% | |
| Grade 3 | 18 | 85.7% | 11 | 45.8% | 8 | 72.7% | 11 | 35.5% | |
| ER status | |||||||||
| Negative | 16 | 76.2% | 2 | 8.3% | 2 | 18.2% | 7 | 22.6% | |
| Positive | 5 | 23.8% | 22 | 91.7% | 9 | 81.8% | 24 | 77.4% | |
| PR status | |||||||||
| Negative | 18 | 85.7% | 7 | 29.2% | 6 | 54.5% | 19 | 61.3% | |
| Positive | 3 | 14.3% | 17 | 70.8% | 5 | 45.5% | 12 | 38.7% | |
| Her2 status | |||||||||
| negative | 8 | 38.1% | 21 | 87.5% | 8 | 72.8% | 27 | 87.1% | |
| positive | 13 | 61.9% | 2 | 8.4% | 3 | 27.2% | 3 | 9.7% | |
| Unknown | 0 | 0 | 1 | 4.1% | 0 | 0 | 1 | 3.2% | |
Immunohistochemistry
Immunohistochemical stainings of tissue sections were performed using automated immunostainers (Ventana Medical Systems, Tucson, AZ, USA or Leica BOND-III, Leica Microsystems, Heerbrugg, Switzerland) utilizing the antibodies monoclonal rabbit-anti-human CD4+-antibody (Ventana, clone SP35, antibody prediluted), monoclonal mouse-anti-human CD8+-antibody (Dako, Baar, Switzerland, clone C8/144B, antibody dilution: 1:100) and monoclonal mouse-anti-human CD20-antibody (Ventana, clone L26, antibody pre-diluted) with pretreatments according to the respective manufactures' instructions. Antibody detections were performed with either Ultra View-AP Kit (substrate: New-Fuchsin) on Ventana Benchmark or Refine-HRP-Kit on BondMax Benchmark, Leica.
Evaluation of tumor infiltrating lymphocytes
To accurately assess tumor infiltrating lymphocytes, we used large tissue sections. We evaluated the areas of interest at first on newly stained HE sections and determined the area based on the morphology. The immunohistochemical sections were corresponding to the HE areas. We distinguished three different anato-pathological compartments on the HE sections both within the PT and the metastatic lesions and assessed them separately, since TIL may have different functions and relevance toward clinical outcome or treatment response depending on their location. We evaluated them separately as follows:
iTIL: Areas within the invasive tumor cells, mainly in closely packed pattern without relevant intervening stroma between the invasive tumor cells. This compartment represents a direct contact of TIL with the tumor cells.
sTIL: Interstitial areas within the invasive tumor area containing sufficient amount of connective tissue without the presence of invasive tumor cells.
imTIL: Peripheral areas of the invasive tumor cells representing the invasion front of the tumor.
The assessment of the immunohistochemical stains was conducted in all three anato-pathological compartments in an identical manner: three different areas of interest in the 40x objective magnification were evaluated in a semi-quantitative way following a recently recommended assessment in the literature for breast cancer.24 The frequency of TIL was given in a semi-quantitative scale (results in 5% steps beginning with 0%) as percentage of stained lymphocytes per 40x objective field in the specific investigated compartment area. One field of pre-defined interest may correspond to max. 1mm2. Importantly, TIL scoring was performed as the percentage only of the given predefined tumor compartment alone, areas not belonging to the given compartment were not included in the assessed surface area. In order to minimize biasing due to tumor heterogeneity, we evaluated three different areas per respective tumor compartment of each section. The average percentages of these three fields per TIL subset and per tumor compartment were used for further evaluation such as calculation of mean and median. Illustrative areas of all three compartments in primary and metastatic lesions are shown in a representative primary breast cancer with its corresponding brain metastasis (Fig. 1).
Figure 1.
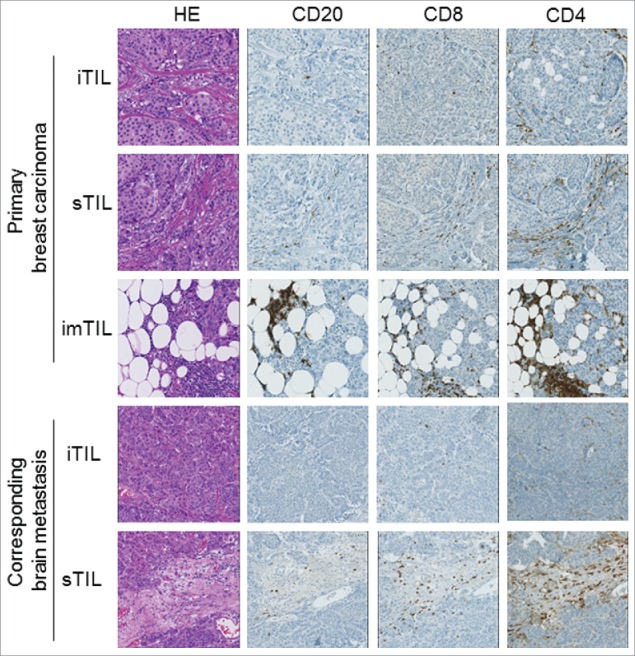
Distribution pattern of TIL subsets in the three tumor compartment of a representative primary breast cancer and its corresponding brain metastasis. Illustration of a primary breast carcinoma and its corresponding brain metastasis with HE as well as CD4+, CD8+ and CD20 immunostainings in all three examined tumor compartments are depicted (iTIL: intratumoral infiltrating lymphocytes, sTIL: stromal infiltrating lymphocytes, imTIL: infiltrative margin infiltrating lymphocytes).
Scores were assessed in the first run by two pathologists (ZV, BS) and were randomly re-assessed by one investigator (BS) after a period of time of at least a few weeks.
Statistical analysis
Evaluation of the TIL frequencies and their correlation to clinicopathological parameters was performed using one-way ANOVA analysis with Bonferroni post testing. Disease free survival defined as the time between diagnosis of the PT and the occurrence of the respective distant metastasis was computed using Kaplan–Meier estimator. All data are shown as mean with standard deviation (SD). Significances are displayed as follows: ns = p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001. Analyses were performed using SPSS (version 22) and GraphPad Prism (version 5.04) software.
Results
We semi-quantitatively analyzed CD4+, CD8+ and CD20+ infiltrating lymphocyte (TIL) subsets within three tumor compartments. According to previous recommendations in literature(24also see Material and Methods), we evaluated intratumoral (iTIL) and stromal (sTIL) percentage of TIL for the PT and the site of metastasis (Met) in each case as well as TIL at the invasive margin (imTIL) for each PT and also for some metastatic lesions with clear infiltrative margins. Due to rather diagnostic biopsy sampling than total resection of distant metastasis, the invasive margin was available in only 48% of all corresponding meta-stases.
Distribution of TIL in primary tumor
Within the PT, we found significantly more imTIL than sTIL and iTIL than in the metastases (p < 0.0001) (Tables 2 and 3). This finding was irrespective of the anatomical site of their metastasis (Fig. 2A–D). Lymphocyte subsets consistently displayed a distribution pattern with CD4+ TIL being most frequent followed by CD8+ TIL and finally CD20+ TIL within the stromal and invasive margin compartment again irrespectively of the site of metastasis (Fig. 2A–D). This TIL distribution pattern was not as evident for the intratumoral compartment (Table 2) generally displaying very low frequencies of TIL. As such, for all PT, the average CD4+/CD8+ ratio for sTIL and imTIL was >1. Additionally, in all three tumor compartments CD20+ TIL were generally less frequent than infiltrating T cells.
Table 2.
Distribution of tumor infiltrating lymphocytes (TIL) subsets within the primary tumor. Mean and median are displayed for CD4+, CD8+ and CD20+ lymphocytes within the three investigated primary tumor compartments consisting of intratumoral (iTIL), stromal (sTIL) and invasive margin (imTIL) TIL divided into the four anatomical sites (brain, bone, liver and soft tissue) of the corresponding metastasis.
| Site of Metastasis |
||||||||
|---|---|---|---|---|---|---|---|---|
| Brain (n = 21) |
Bone (n = 24) |
Liver (n = 11) |
Soft tissue (n = 31) |
|||||
| TIL within the primary tumor | Mean | Median | Mean | Median | Mean | Median | Mean | Median |
| iCD4+ T cells | 4.8 | 3.7 | 5.1 | 2.3 | 1.8 | .7 | 7.3 | 5.3 |
| iCD8+ T cells | 5.7 | 3.7 | 12.7 | 8.3 | 4.8 | 2.0 | 9.3 | 6.7 |
| iCD20+ B cells | 0.7 | 0.3 | 0.3 | 0.3 | 0.4 | 0.3 | 0.5 | 0.3 |
| sCD4+ T cells | 18.0 | 13.3 | 19.4 | 16.7 | 11.6 | 13.3 | 21.3 | 20.0 |
| sCD8+ T cells | 11.4 | 8.3 | 14.8 | 12.5 | 7.1 | 6.7 | 16.2 | 13.3 |
| sCD20+ B cells | 1.2 | 0.3 | 2.0 | 0.7 | 2.0 | 0.7 | 4.5 | 2.0 |
| imCD4+ T cells | 42.9 | 36.7 | 38.8 | 33.3 | 38.8 | 26.7 | 39.2 | 36.7 |
| imCD8+ T cells | 26.6 | 20.0 | 28.0 | 23.3 | 22.3 | 13.3 | 31.1 | 26.7 |
| imCD20+ B cells | 5.6 | 5.0 | 11.1 | 7.5 | 10.1 | 8.3 | 20.6 | 16.7 |
Table 3.
Distribution of metastasis infiltrating lymphocytes (mTIL) subsets within the site of metastasis. Mean and median are displayed for CD4+, CD8+ and CD20+ lymphocytes within the two investigated metastasis compartments consisting of intratumoral (iTIL) and stromal (sTIL) divided into the four anatomical sites (brain, bone, liver and soft tissue) of the corresponding metastasis.
| Site of Metastasis |
||||||||
|---|---|---|---|---|---|---|---|---|
| Brain (n = 21) |
Bone (n = 24) |
Liver (n = 11) |
Soft tissue (n = 31) |
|||||
| TIL within the site of metastasis | Mean | Median | Mean | Median | Mean | Median | Mean | Median |
| iCD4+ T cells | 2.3 | 2.0 | 1.2 | 0.3 | 0.8 | 0.3 | 7.3 | 5.0 |
| iCD8+ T cells | 3.1 | 2.0 | 2.8 | 0.7 | 1.1 | 0.7 | 4.9 | 3.7 |
| iCD20+ B cells | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 1.2 | 0.3 |
| sCD4+ T cells | 7.9 | 7.0 | 4.9 | 2.2 | 2.6 | 0.7 | 15.1 | 8.3 |
| sCD8+ T cells | 6.2 | 6.7 | 3.2 | 2.2 | 4.4 | 5.0 | 10.5 | 8.3 |
| sCD20+ B cells | 1.8 | 0.3 | 0.6 | 0.3 | 0.5 | 0.3 | 5.2 | 2.0 |
Figure 2.
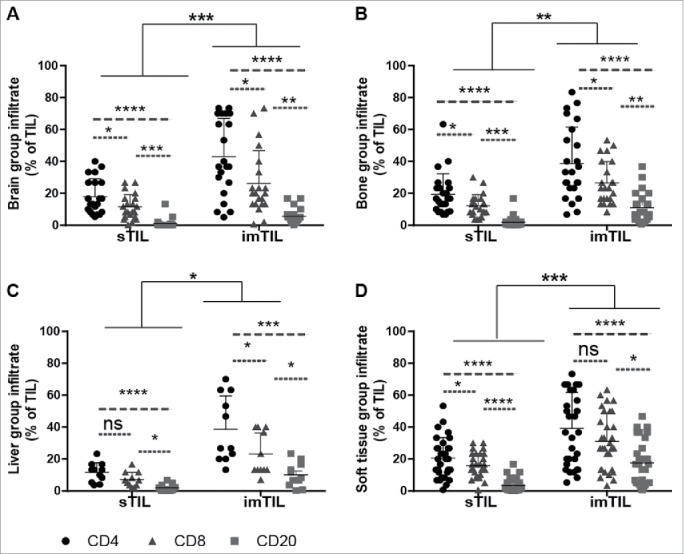
Similar distribution of TIL with regard to the investigated primary tumor compartments and the lymphocyte subtypes but irrespectively of the site of distant metastasis. Scatter graphs show that within the primary tumor compartments (iTIL (not shown), sTIL and imTIL) invasive margin TIL (imTIL) are significantly increased when compared to stromal (sTIL) TIL irrespectively of the particular site (A–D) to which metastasis had occurred. For all three compartments (iTIL not shown) CD4+, CD8+ and CD20+ lymphocyte subsets always followed the same gradient with CD4+ lymphocytes being the most prominent, followed by CD8+ lymphocytes and at last CD20+ lymphocytes again irrespectively of the anatomical site of distant metastasis (A–D). Significances are displayed as follows: ns = p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001.
Distribution of TIL in the metastastic lesions
With respect to the corresponding sites of metastasis, in general mTIL were less frequent in the metastatic lesions than TIL of the PT (Tables 2 and 3). The distribution pattern of mTIL within the compartments displayed a similar tendency both for the frequency (sTIL > iTIL) and for the composition of the TIL subsets (frequency of CD4+TIL>CD8+TIL>CD20+TIL) irrespectively of the anatomical site at which metastasis had occurred (Table 3). When comparing the frequency of TIL subsets within the two investigated compartments, we found significantly more CD4+ and CD8+ TIL in the PT than in the metastases (Fig. 3A–D, showing stromal compartiments).
Figure 3.
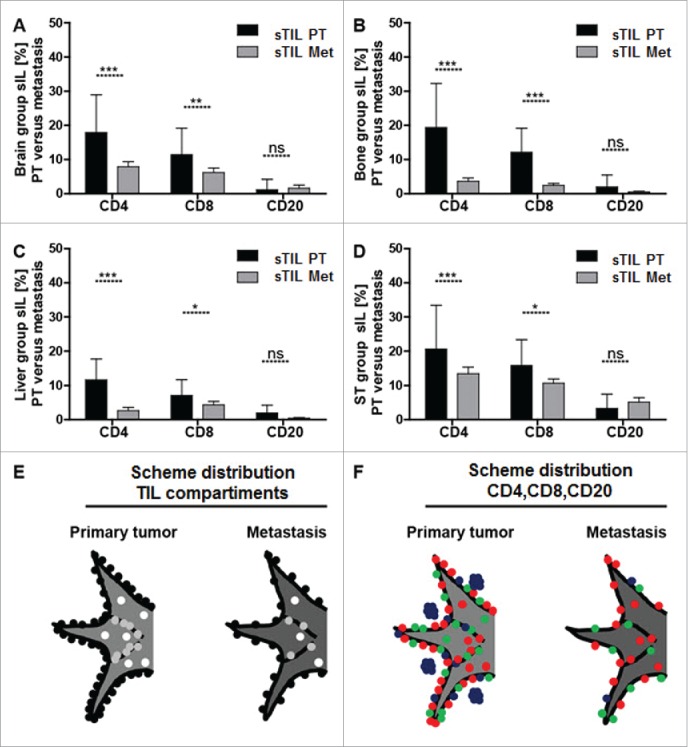
Significantly more sTIL within the primary tumor than mTIL within the site of the corresponding metastasis. Bar graphs for CD4+, CD8+ and CD20+ stromal infiltrating lymphocyte subsets show that there are significantly more sTIL within the primary tumor than mTIL within the site of the corresponding metastasis irrespectively of the anatomical site of distant metastasis (A–D). The distribution of infiltrating lymphocytes is schematically visualized with respect to the three primary tumor/metastasis compartments (E) consisting of intratumoral lymphocytes (iTIL_PT/iTIL_Met = white dots), stromal lymphocytes (sTIL_PT/sTIL_Met = gray dots) and invasive margin lymphocytes (imTIL_PT/imTIL_Met = black dots) and with respect to the lymphocyte subsets within these compartments (F) comprising CD4+ infiltrating lymphocytes (red dots), CD8+ infiltrating lymphocytes (green dots) and CD20+ infiltrating lymphocytes (blue dots). Significances are displayed as follows: ns = p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001.
Different distribution pattern of TIL in primary and metastastic lesions
We observed a distinct TIL distribution pattern schematically summarized as imTIL > sTIL > iTIL (Fig. 3E) and CD4+TIL>CD8+TIL>CD20+TIL subsets (Fig. 3F). We found this distribution pattern within the PT and the site of the corresponding metastasis, irrespectively of the anatomical site at which metastasis had occurred.
Correlation of TIL in primary and metastastic lesions with disease free survival
High numbers of CD8+ TIL at the invasive margin were associated with a longer DFS (p < 0.05) whereas low numbers of CD8+ imTIL correlated with a shorter DFS regarding the whole cohort. Interestingly, if the results were stratified according to the site of the metastatic lesion, we could not determine any correlation to DFS and imCD8+ T cells among cases with soft tissue metastases, however, patients with metastasis to brain, bone or liver exhibited the same survival benefit of increased imCD8+ T cells (Fig. 4, liver not shown). In contrast, there was no significant correlation to DFS with regard to the frequency of CD4+ positive TIL in any locations analyzed (data not shown).
Figure 4.
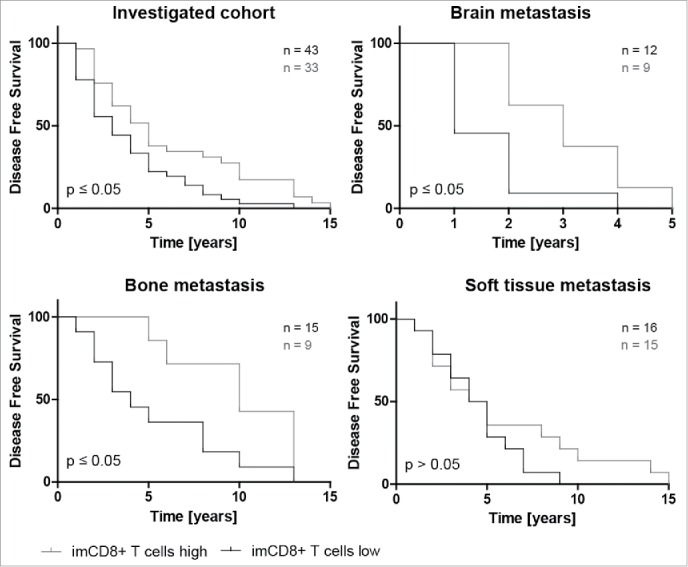
Higher amounts of CD8+ lymphocytes within the invasive margin is associated with significantly longer disease free survival (DFS). Kaplan–Meier plots of disease free survival dichotomized at the respective CD8+ imTIL median of the primary tumor (also see Table 2) into CD8+ T cells high and CD8+ T cells low show that higher amounts of CD8+ T cells within the invasive margin of the primary tumor are generally (A) but also in the subgroups with distant metastasis into brain and bone except for soft tissue metastasis associated with a with significantly longer DFS again (B–D).
Correlation of intrinsic subtypes and metastatic sites to overall survival and ITL in patients with brain metastases
Patients with brain metastasis differed from the other breast cancer patients suffering from bone, liver or soft tissue metastasis in terms of clinic-pathological data and CD20+ imTIL. Patients developing brain metastasis were significantly younger at the age of the onset of the PT and the metastasis (Fig. 5A). They had a significantly shorter DFS (Fig. 5B) and displayed most often HER2+ or basal like breast cancer subtypes. (Fig. 5C). Interestingly, we found significantly less CD20+ imTIL in those PT metastasizing into the brain when compared to the PT groups showing bone, liver or soft tissue metastasis (Fig. 5E). Additionally, Luminal A and Luminal B tumors displayed a similar long-term better overall survival than HER2 positive and TN cases, which showed also a similar follow-up (Fig. 5F). There was no correlation between TIL with any of the analyzed markers and the anatomical compartments and the intrinsic subtypes when analyzed separately.
Figure 5.
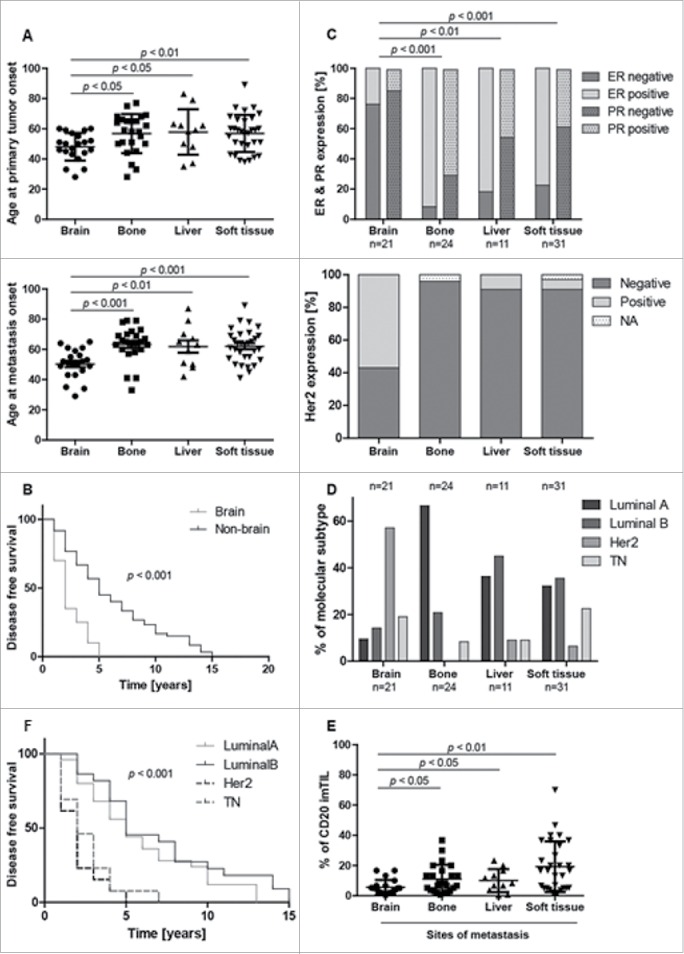
Clinico-pathological correlation of intrinsic subtypes and different metastastic sites of breast cancer to overall survival and to CD20 distribution at the invasion front. Brain metastasis group shows different clinicopathological and CD20+ imTIL behavior. Patients with distant metastasis to the brain show a significantly younger age at onset of both primary tumor and metastasis when compared to the other groups (A). Time to occurrence of distant brain metastasis (= disease free survival) is significantly reduced in the brain group when compared to all other groups (B). Patients with brain metastasis show significantly less estrogen receptor (ER) and progesterone (PR) but significantly more Her2 receptor expression in the primary tumor (C,D). Curiously, CD20 tumor infiltrating lymphocytes at the infiltrative margin (imTIL) of the primary tumor are significantly reduced in the brain group when compared to the other groups where distant metastasis had occurred to bone, liver or soft tissue (E). When correlating intrinsic subtypes to overall survival, Luminal A and Luminal B tumors are separated from HER2 positive and TN breast cancer cases by a similarly improved overall survival (F).
Discussion
We investigated TIL at four different anatomical sites at which metastasis had occurred and compared amount and frequency of TIL with their matched TIL in the primary breast cancer. In our study, we could demonstrate that TIL were significantly reduced in the corresponding metastastic site if compared with the PT. However, the composition of the TIL subsets and their distribution in the predefined anato-pathological locations remained basically unaltered in the metastatic process irrespectively from the anatomical site of the metastases. As to the prognostic value of TIL, we could show that patients with brain metastases exhibited a different clinical course including younger age at the time of metastases and a distinct immunophenotype regarding CD20 positive B-cells at the infiltration margin.
We subdivided infiltrating lymphocytes into intratumoral, stromal and infiltrative margin TIL, corresponding to the three established tumor compartments debated to fulfill functionally different roles.24 For this purpose, we considered large sections superior to punched material applied for the widely used TMA investigations. Important features such as the invasive margin compartment may potentially be underrepresented both due to the method itself and due to tumor and lymphocyte inhomogeneity.
By applying the recently suggested method by Salgado et al.,24 in evaluating TIL overall we found fewer mTIL than their corresponding TIL irrespective of the site of metastasis. This was in line with results described in a small cohort pilot study using TMA material from primary and matched metastatic breast cancer.31 With regard to the three tumor compartments, we observed an TIL gradient with TIL being most present at the invasive margin followed by stromal TIL and least frequent within the tumor center. As recently reviewed in detail,24 iTIL were detected in lower numbers than sTIL or imTIL. Interestingly, we observed this trend also in the metastases independently of the anatomical site at which metastasis had occurred. Regarding the TIL subtypes, we found higher infiltrates of CD4+ TIL than CD8+ TIL corresponding to a CD4+/CD8+ ratio > 1. We observed higher infiltrates of CD8+ TIL to correlate with a better DFS in line with previous studies describing CD8+ TIL to be associated with a better clinical outcome and prognosis in breast cancer.13,14,32 However, patients with soft tissue metastases, could not be further stratified based on their CD8+ positive T cells. Interestingly, TIL in the metastastic lesions displayed a similar arrangement of lymphocyte subsets with CD4+ T cells being most abundant followed by CD8+ T cells and CD20+ B cells. It is therefore to assume, that the composition of mTIL in the metastastic site mirrors TIL in their matched PT regardless of the anatomical metastatic location suggesting an intrinsic role of the PT influencing the immune composition at least for the metastatic sites investi-gated.
Apart from these findings, patients suffering from brain metastasis differed from the other patients both with respect to their clinicopathological parameters and their TIL distribution. In line with previous findings, their PT displayed significantly more HER2+ or basal like subtypes (p < 0.01), patients were younger at the time of diagnosis (p < 0.05) and suffered from a shorter distant metastasis-free survival (p < 0.01).38 Curiously, we found less (p < 0.05) CD20+ imTIL in PT metastasizing into the brain. While some groups describe a metastasis-promoting behavior of CD20+ TIL others suggested B cells to contribute to antitumor immunity by antibody production.39-41 According to the latter, in our case the significantly reduced number of CD20+ imTIL may have added to the aggressive behavior of these PT preferably metastasizing to the brain.
In conclusion, our data propose an intrinsic role of the PT influencing the immune composition within the corresponding metastasis at least for the metastatic sites investigated. Further investigation is required to find out whether an individual “tumor immune signature” could already be established by characterizing the TIL composite within the PT potentially allowing a targeted treatment of the corresponding metastatic disease.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Martina Storz and André Fitsche for outstanding technical assistance.
References
- 1.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C et al.. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 2010; 7:e1000279; PMID:20520800; http://dx.doi.org/ 10.1371/journal.pmed.1000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eyles J, Puaux AL, Wang X, Toh B, Prakash C, Hong M, Tan TG, Zheng L, Ong LC, Jin Y et al.. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest 2010; 120:2030-9; PMID:20501944; http://dx.doi.org/ 10.1172/JCI42002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G et al.. Systemic spread is an early step in breast cancer. Cancer Cell 2008; 13:58-68; PMID:18167340; http://dx.doi.org/ 10.1016/j.ccr.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 4.Zhang XH, Giuliano M, Trivedi MV, Schiff R, Osborne CK. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res 2013; 19:6389-97; PMID:24298069; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-0838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.org/ 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 6.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C et al.. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 2014; 232:199-209; PMID:24122236; http://dx.doi.org/ 10.1002/path.4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F et al.. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011; 29:610-8; PMID:21245428; http://dx.doi.org/ 10.1200/JCO.2010.30.5425 [DOI] [PubMed] [Google Scholar]
- 8.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:22419253; http://dx.doi.org/ 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P et al.. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 10.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013; 39:11-26; PMID:23890060; http://dx.doi.org/ 10.1016/j.immuni.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 11.Kristensen VN, Vaske CJ, Ursini-Siegel J, Van Loo P, Nordgard SH, Sachidanandam R, Sørlie T, Wärnberg F, Haakensen VD, Helland Å et al.. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci U S A 2012; 109:2802-7; PMID:21908711; http://dx.doi.org/ 10.1073/pnas.1108781108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, Yin D, Gu F, Yao Z, Fu L. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat 2011; 130:645-55; PMID:21717105; http://dx.doi.org/ 10.1007/s10549-011-1647-3 [DOI] [PubMed] [Google Scholar]
- 13.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29:1949-55; PMID:21483002; http://dx.doi.org/ 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 14.Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, Earl HM, Poole CJ, Hiller L, Dunn JA et al.. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 2014; 25:1536-43; PMID:24915873; http://dx.doi.org/ 10.1093/annonc/mdu191 [DOI] [PubMed] [Google Scholar]
- 15.Issa-Nummer Y, Loibl S, von Minckwitz G, Denkert C. Tumor-infiltrating lymphocytes in breast cancer: A new predictor for responses to therapy. Oncoimmunology 2014; 3:e27926; PMID:25340002; http://dx.doi.org/ 10.4161/onci.27926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C et al.. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28:105-13; PMID:19917869; http://dx.doi.org/ 10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 17.West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res 2011; 13:R126; PMID:22151962; http://dx.doi.org/ 10.1186/bcr3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K et al.. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat 2012; 132:793-805; PMID:21562709; http://dx.doi.org/ 10.1007/s10549-011-1554-7 [DOI] [PubMed] [Google Scholar]
- 19.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20:5064-74; PMID:24714771; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, Dorazio P, Trosko JA, Rüter J, Mariani GL et al.. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res 2010; 16:3485-94; PMID:20479064; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-0505 [DOI] [PubMed] [Google Scholar]
- 23.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G et al.. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123:2873-92; PMID:23778140; http://dx.doi.org/ 10.1172/JCI67428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F et al.. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2014; 26:259-71; PMID:25214542; http://dx.doi.org/10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goc J, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology 2013; 2:e26836; PMID:24498556; http://dx.doi.org/ 10.4161/onci.26836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weigelt B, Peterse JL, van 't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer 2005; 5:591-602; PMID:16056258; http://dx.doi.org/ 10.1038/nrc1670 [DOI] [PubMed] [Google Scholar]
- 27.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res 2007; 67:5064-6; PMID:17545580; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0912 [DOI] [PubMed] [Google Scholar]
- 28.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4:71-8; PMID:14708027; http://dx.doi.org/ 10.1038/nrc1256 [DOI] [PubMed] [Google Scholar]
- 29.Pukrop T, Dehghani F, Chuang HN, Lohaus R, Bayanga K, Heermann S, Regen T, Van Rossum D, Klemm F, Schulz M et al.. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 2010; 58:1477-89; PMID:20549749; http://dx.doi.org/10.1002/glia.21022 [DOI] [PubMed] [Google Scholar]
- 30.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009; 16:91-102; PMID:19647220; http://dx.doi.org/ 10.1016/j.ccr.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cimino-Mathews A, Ye X, Meeker A, Argani P, Emens LA. Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol 2013; 44:2055-63; PMID:23701942; http://dx.doi.org/ 10.1016/j.humpath.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res 2012; 14:R48; PMID:22420471; http://dx.doi.org/ 10.1186/bcr3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer 2015; 112:1782-90; PMID:25942397; http://dx.doi.org/ 10.1038/bjc.2015.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrama D, thor Straten P, Fischer WH, McLellan AD, Bröcker EB, Reisfeld RA, Becker JC. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity 2001; 14:111-21; PMID:11239444; http://dx.doi.org/ 10.1016/S1074-7613(01)00094-2 [DOI] [PubMed] [Google Scholar]
- 35.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L et al.. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26:4410-7; PMID:18802153; http://dx.doi.org/ 10.1200/JCO.2007.15.0284 [DOI] [PubMed] [Google Scholar]
- 36.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 2011; 71:6391-9; PMID:21900403; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-0952 [DOI] [PubMed] [Google Scholar]
- 37.Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, Mulé JJ. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol 2011; 179:37-45; PMID:21703392; http://dx.doi.org/ 10.1016/j.ajpath.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010; 28:3271-7; PMID:20498394; http://dx.doi.org/ 10.1200/JCO.2009.25.9820 [DOI] [PubMed] [Google Scholar]
- 39.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 2005; 7:411-23; PMID:15894262; http://dx.doi.org/ 10.1016/j.ccr.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 40.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res 2011; 71:3505-15; PMID:21444674; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother 2003; 52:715-38; PMID:12920480; http://dx.doi.org/ 10.1007/s00262-003-0409-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


