ABSTRACT
Prior findings linking allergy and cancer have been inconsistent, which may be driven by diverse assessment methods. We used serum specific immunoglobulin E (IgE) against common inhalant allergens that was assessed prior to cancer diagnosis in studying this association. We selected 8,727 Swedish men and women who had measurements of serum allergen-specific IgE and total IgE between 1992 and 1996. Multivariable Cox regression using age as a timescale was performed to assess the associations of IgE sensitization, defined by any levels of serum specific IgE ≥35 kU/L, with risk of overall and specific cancers. A test for trend was performed by assigning scores derived from allergen-specific IgE levels at baseline as an ordinal scale. Kaplan–Meier curves and log-rank test were used to assess cancer survival by IgE sensitization status. During a mean follow-up of 16 year, 689 persons were diagnosed with cancer. We found an inverse association between IgE sensitization and cancer risk, with a hazard ratio (HR) of 0.83 and 95% confidence intervals (CI) of 0.70–0.99. A similar trend was seen with specific IgE scores overall (Ptrend = 0.007) and in women (Ptrend = 0.01). Although IgE sensitization was not associated with risk of common site-specific cancers, serum specific IgE scores were inversely associated with melanoma risk in men and women combined, and with risk of female breast and gynecological cancers combined. No association with survival was observed. The association between circulating IgE levels and incident cancer may point toward a role of T-helper 2 (TH2)-biased response in development of some cancers.
KEYWORDS: Allergy, atopy, cancer, immunoglobulin E, cohort
Introduction
Allergy is a hypersensitivity reaction initiated by specific immunologic mechanisms.1 Accruing evidence has indicated that allergy may be associated with the development of cancer, and is largely divided into two opposing views: (1) allergy may reduce cancer risk and (2) it may increase cancer risk.2 The first may be explained by the immunosurveillance hypothesis, which states that increased immune surveillance following hyper-reactive immune responses may hinder the development of cancer.2 Similarly, the prophylaxis hypothesis suggests that physical effects of allergy symptoms may prevent cancer by removal of potential carcinogens.3 The opposing hypotheses include a shift in T-helper balance, which determines the type of immune responses elicited. Predominance of TH2 over TH1 underlies the hypersensitivity reactions in allergy, and is thought to divert immune responses from the tumor-eradicating TH1 counterpart.4 Additionally, allergic inflammation may lead to initiation and promotion of cancer directly or through indirect mechanisms.5
Observational findings linking allergy and cancer are inconclusive.6 A crude way to determine presence of allergy would be using total IgE levels in the serum that may reflect the imbalance in the immune system, but without knowing its specificity makes the association between allergy and cancer challenging.7 Atopy, which refers to the genetic pre-disposition of developing IgE-mediated hypersensitivity or IgE sensitization against allergens,1 is often evaluated to unpick the role of allergy. Most previous studies crudely classified individuals into “atopic” and “non-atopic” based on self-reported history,8-10 which relies on an individual's recall and may incur a loss of time-specific information. Other studies defined atopy based on laboratory evidence, such as the presence of circulating IgE.1 The use of serum IgE against specific allergen provides more insight into how IgE sensitization may be associated to cancer, but may be hampered by the use of imprecise and/or different assessment methods. Findings based on pre-cancer diagnostic levels of allergen-specific IgE are limited and no studies have investigated the impact on cancer survival of pre-diagnostic allergen-specific IgE.11-13
In the Swedish Apolipoprotein Mortality Risk Study (AMORIS), we previously investigated the association between total IgE and the risk of cancer in 24,820 individuals. A weak inverse association was found albeit not statistically significant, and results were similar when data from our cohort was combined in a meta-analysis with previous findings.7 The recently updated AMORIS database now contains information on serum specific IgE utilizing comparable determinations, more participants, and a longer follow-up (up to 25 y). To gain further insight into the association between allergy and cancer, we now assessed serum specific IgE against common inhalant allergens in relation to risk of developing cancer and death after cancer diagnosis.
Results
Characteristics of study participants by IgE sensitization status are shown in Table 1. The average age at baseline was 40 y, and over half the study population were female (59%). During follow-up (median: 18.6 y), 689 incident cancer cases were identified. A total of 194 individuals died following cancer diagnosis, among which 146 died from cancer. The most common three types of cancer were prostate, female breast and colorectal cancers. The relative distribution of specific IgE scores to total IgE is shown in Table S1.
Table 1.
Characteristics of study participants by IgE sensitization status.
| IgE sensitization |
||
|---|---|---|
| No (n = 4,714) | Yes (n = 4,013) | |
| Age (years) | ||
| Mean (SD) | 42.26 (13.74) | 37.31 (12.66) |
| Sex, male, n (%) | 1661 (35.24) | 1945 (48.47) |
| Socioeconomic status, n (%) | ||
| High | 1912 (40.56) | 1423 (35.46) |
| Low | 2074 (44.00) | 1697 (42.49) |
| Unclassified/missing | 728 (15.44) | 893 (22.25) |
| History of chronic respiratory disease, n (%) | 67 (1.42) | 92 (2.29) |
| Year of measurement, n (%) | ||
| 1992–1994 | 1192 (25.29) | 986 (24.57) |
| 1994–1996 | 2682 (56.89) | 1997 (49.76) |
| 1996 | 840 (17.82) | 1030 (25.67) |
| Total IgE (kU/L) | ||
| <25 | 1765 (37.44) | 285 (7.10) |
| 25–100 | 1937 (41.09) | 1355 (33.77) |
| ≥100 | 1012 (21.47) | 2373 (59.13) |
| Mean follow-up in years, Mean (SD) | 15.86 (3.59) | 16.05 (3.23) |
| Any cancer during follow-up, n (%) | ||
| All cancer | 443 (9.40) | 246 (6.13) |
| Breast (female) | 115 (2.44) | 50 (1.25) |
| Prostate | 55 (1.17) | 42 (1.05) |
| Colorectal | 43 (0.91) | 21 (0.52) |
| Gynecological | 41 (0.87) | 12 (0.30) |
| hematological | 31 (0.66) | 22 (0.55) |
| Melanoma | 27 (0.57) | 10 (0.25) |
| Pulmonary | 21 (0.45) | 14 (0.35) |
| Bladder | 14 (0.30) | 11 (0.27) |
| NMSC | 16 (0.34) | 9 (0.22) |
| CNS | 11 (0.23) | 9 (0.22) |
| Kidney | 12 (0.25) | 9 (0.22) |
n = number of participants.
We assessed cancer risk based on IgE sensitization status and specific IgE scores as categories Table 2. When using 0.35 kU/L as the cut-off point for specific IgE, which indicates IgE sensitization, we did not observe any association with risk of cancer (Table 3). However, a statistically significant inverse trend was observed when using serum specific IgE scores in the overall study population (Ptrend = 0.03). In sex stratification, a similar association was observed in women. Adjustment for serum total IgE showed stronger associations and an inverse association between IgE sensitization and cancer risk in the overall population (HR: 0.83 (95% CI: 0.70–0.99). No association was noted in men and women separately. When stratifying the analyses by serum total IgE levels, the inverse trend was only seen between serum allergen-specific IgE scores and cancer risk in the overall population and in women with total IgE levels >100 kU/L (Table 3). Similar associations were observed when additionally adjusting our model for the number of allergen tested, the number of positive results, or the ratio between the two (results not shown).
Table 2.
Specific IgE scores in CALAB and corresponding serum concentrations.
| Specific IgE score | Serum concentrations (kU/L) | Serum IgE levels |
|---|---|---|
| 0 | <0.35 | Absent/undetectable |
| 1 | 0.35 – 0.70 | Low level |
| 2 | 0.70 – 3.50 | Moderate level |
| 3 | 3.50 – 17.5 | High level |
| 4 | 17.5 – 50 | Very high level |
| 5 | 50 – 100 | Very high level |
| 6 | ≥100 | Very high level |
Table 3.
Associations between IgE sensitization, serum specific IgE scores and risk of incident cancer overall and by sex with chronological age as timescale.
| HR (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|
| IgE sensitization |
Specific IgE scores§ |
|||||||
| n cancer/n total | No | Yes | 0 | 1–2 | 3–4 | 5–6 | Ptrend | |
| Both men and women | ||||||||
| n | 4714 | 4013 | 4714 | 6402 | 8331 | 8727 | ||
| Multivariable model | 689/8727 | 1.0 (Ref) | 0.88 (0.75–1.03) | 1.0 (Ref) | 1.00 (0.82–1.21) | 0.74 (0.59–0.93) | 0.91 (0.55–1.51) | 0.03 |
| Additional adjustment for total IgE | 689/8727 | 1.0 (Ref) | 0.83 (0.70–0.99) | 1.0 (Ref) | 0.95 (0.78–1.16) | 0.69 (0.54–0.88) | 0.79 (0.47–1.32) | 0.007 |
| Stratification by total IgE (kU/L) | ||||||||
| 191/2050 | 1.0 (Ref) | 0.79 (0.48–1.32) | 1.0 (Ref) | 0.86 (0.51–1.47) | 0.48 (0.12–1.99) | N/A | 0.29 | |
| 25–100 | 238/3292 | 1.0 (Ref) | 0.85 (0.64–1.13) | 1.0 (Ref) | 0.91 (0.65–1.28) | 0.77 (0.51–1.16) | N/A | 0.18 |
| ≥100 | 260/3385 | 1.0 (Ref) | 0.82 (0.63–1.06) | 1.0 (Ref) | 1.00 (0.74–1.34) | 0.66 (0.48–0.91) | 0.81 (0.47–1.39) | 0.02 |
| Men | ||||||||
| n | 1661 | 1945 | 1661 | 732 | 1003 | 210 | ||
| Multivariable model | 277/3606 | 1.0 (Ref) | 0.97 (0.76–1.23) | 1.0 (Ref) | 1.01 (0.75–1.36) | 0.89 (0.65–122) | 1.27 (0.64–2.52) | 0.80 |
| Additional adjustment for total IgE | 277/3606 | 1.0 (Ref) | 0.89 (0.69–1.17) | 1.0 (Ref) | 0.95 (0.69–1.29) | 0.83 (0.59–1.16) | 1.10 (0.54–2.23) | 0.42 |
| Stratification by total IgE (kU/L) | ||||||||
| <25 | 53/649 | 1.0 (Ref) | 0.71 (0.29–1.76) | 1.0 (Ref) | 0.59 (0.20–1.78) | 1.11 (0.27–4.67) | N/A | 0.64 |
| 25–100 | 97/1377 | 1.0 (Ref) | 0.83 (0.54–1.28) | 1.0 (Ref) | 0.99 (0.59–1.66) | 0.68 (0.38–1.23) | N/A | 0.24 |
| ≥100 | 127/1580 | 1.0 (Ref) | 0.97 (0.67–1.41) | 1.0 (Ref) | 1.00 (0.64–1.57) | 0.89 (0.57–1.40) | 1.23 (0.58–2.61) | 0.94 |
| Women | ||||||||
| n | 3053 | 2068 | 3053 | 956 | 926 | 186 | ||
| Multivariable model | 412/5121 | 1.0 (Ref) | 0.83 (0.67–1.03) | 1.0 (Ref) | 0.98 (0.76–1.27) | 0.64 (0.45–0.90) | 0.72 (0.34–1.53) | 0.01 |
| Additional adjustment for total IgE | 412/5121 | 1.0 (Ref) | 0.81 (0.64–1.02) | 1.0 (Ref) | 0.96 (0.74–1.24) | 0.61 (0.42–0.87) | 0.64 (0.30–1.39) | 0.01 |
| Stratification by total IgE (kU/L) | ||||||||
| <25 | 138/1401 | 1.0 (Ref) | 0.82 (0.44–1.53) | 1.0 (Ref) | 0.98 (0.53–1.83) | N/A | N/A | 0.30 |
| 25–100 | 141/1915 | 1.0 (Ref) | 0.87 (0.59–1.26) | 1.0 (Ref) | 0.85 (0.54–1.34) | 0.91 (0.52–1.58) | N/A | 0.51 |
| ≥100 | 133/1805 | 1.0 (Ref) | 0.73 (0.52–1.04) | 1.0 (Ref) | 0.98 (0.66–1.46) | 0.51 (0.31–0.83) | 0.63 (0.29–1.39) | 0.01 |
Highest specific IgE scores recorded at baseline.
N/A = not applicable; n = number of participants.
All models were adjusted for sex (except for sex-specific analysis), socioeconomic status, period of measurement and history of chronic pulmonary disease.
Similar associations to overall cancers were found when assessing all cancers excluding NMSC (Table 4). No statistically significant association was found between positive IgE sensitization and risk of specific cancer types. When observing trends across allergen-specific IgE scores, we found a lower risk of melanoma with higher specific IgE in both men and women combined (Ptrend = 0.04). No association was observed for other cancer sites. To further investigate the driver of the inverse association observed in women, we combined cancers of female genital organs (breast and gynecological cancers) as a single outcome and a protective effect of higher specific IgE scores was observed (Ptrend = 0.04).
Table 4.
Associations between IgE sensitization, serum specific IgE scores and risk of site-specific cancers by sex with chronological age as timescale.
| HR (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|
| IgE sensitization |
Specific IgE scores§ |
|||||||
| n cancer | No | Yes | 0 | 1–2 | 3–4 | 5–6 | Ptrend | |
| Both men and women | ||||||||
| All excluding NMSC | 664 | 1.0 (Ref) | 0.82 (0.69–0.99) | 1.0 (Ref) | 0.96 (0.78–1.18) | 0.67 (0.52–0.85) | 0.81 (0.48–1.36) | 0.005 |
| Colorectal | 64 | 1.0 (Ref) | 0.71 (0.39–1.26) | 1.0 (Ref) | 0.75 (0.38–1.51) | 0.60 (0.27–1.34) | 1.23 (0.28–5.42) | 0.32 |
| hematological | 53 | 1.0 (Ref) | 0.95 (0.51–1.76) | 1.0 (Ref) | 1.16 (0.58–2.30) | 0.66 (0.27–1.59) | 1.22 (0.27–5.46) | 0.62 |
| Melanoma | 37 | 1.0 (Ref) | 0.53 (0.24–1.17) | 1.0 (Ref) | 0.79 (0.33–1.88) | 0.32 (0.09–1.13) | N/A | 0.04 |
| Pulmonary | 35 | 1.0 (Ref) | 0.87 (0.41–1.84) | 1.0 (Ref) | 1.24 (0.56–2.76) | 0.41 (0.11–1.43) | 0.94 (0.12–7.44) | 0.33 |
| Bladder | 25 | 1.0 (Ref) | 1.20 (0.50–2.87) | 1.0 (Ref) | 1.45 (0.56–3.77) | 0.95 (0.29–3.14) | N/A | 0.89 |
| NMSC | 25 | 1.0 (Ref) | 0.97 (0.40–2.39) | 1.0 (Ref) | 0.62 (0.17–2.20) | 1.56 (0.55–4.44) | N/A | 0.74 |
| Kidney | 21 | 1.0 (Ref) | 1.09 (0.40–2.95) | 1.0 (Ref) | 1.50 (0.52–4.30) | 0.27 (0.03–2.23) | 3.42 (0.63–18.50) | 0.95 |
| CNS | 20 | 1.0 (Ref) | 1.33 (0.47–3.73) | 1.0 (Ref) | 1.82 (0.62–5.33) | 0.88 (0.21–3.61) | N/A | 0.74 |
| Men | ||||||||
| All excluding NMSC | 267 | 1.0 (Ref) | 0.89 (0.68–1.16) | 1.0 (Ref) | 0.95 (0.69–1.31) | 0.80 (0.57–1.13) | 1.12 (0.55–2.28) | 0.37 |
| Prostate | 97 | 1.0 (Ref) | 0.93 (0.38–1.04) | 1.0 (Ref) | 0.86 (0.50–1.48) | 0.93 (0.53–1.63) | 2.31 (0.78–6.82) | 0.79 |
| Colorectal | 35 | 1.0 (Ref) | 0.71 (0.34–1.49) | 1.0 (Ref) | 0.77 (0.31–1.88) | 0.54 (0.19–1.53) | 1.83 (0.38–8.77) | 0.52 |
| hematological | 23 | 1.0 (Ref) | 1.13 (0.44–2.89) | 1.0 (Ref) | 1.11 (0.36–3.35) | 1.12 (0.35–3.62) | 1.35 (0.15–12.15) | 0.78 |
| Pulmonary | 15 | 1.0 (Ref) | 1.07 (0.35–3.26) | 1.0 (Ref) | 1.41 (0.42–4.71) | 0.57 (0.11–3.02) | 2.38 (0.24–23.99) | 0.93 |
| Melanoma | 12 | 1.0 (Ref) | 0.56 (0.15–2.06) | 1.0 (Ref) | 0.65 (0.13–3.18) | 5.31 (0.10–2.77) | N/A | 0.31 |
| Women | ||||||||
| All excluding NMSC | 397 | 1.0 (Ref) | 0.81 (0.63–1.03) | 1.0 (Ref) | 0.98 (0.75–1.28) | 0.57 (0.39–0.83) | 0.65 (0.30–1.42) | 0.008 |
| Breast and gynecological | 218 | 1.0 (Ref) | 0.76 (0.55–1.06) | 1.0 (Ref) | 0.85 (0.58–1.24) | 0.68 (0.43–1.10) | 0.36 (0.09–1.47) | 0.04 |
| Breast | 165 | 1.0 (Ref) | 0.83 (0.57–1.20) | 1.0 (Ref) | 0.95 (0.63–1.45) | 0.69 (0.40–1.19) | 0.49 (0.12–2.05) | 0.14 |
| Gynecological | 53 | 1.0 (Ref) | 0.55 (0.27–1.13) | 1.0 (Ref) | 0.54 (0.22–1.32) | 0.65 (0.25–1.64) | N/A | 0.11 |
| Colorectal | 29 | 1.0 (Ref) | 0.75 (0.29–1.91) | 1.0 (Ref) | 0.75 (0.25–2.26) | 0.84 (0.23–3.10) | N/A | 0.53 |
| Hematological | 30 | 1.0 (Ref) | 0.83 (0.37–1.88) | 1.0 (Ref) | 1.13 (0.48–2.71) | 0.37 (0.08–1.65) | 1.10 (0.14–8.82) | 0.40 |
| Melanoma | 25 | 1.0 (Ref) | 5.13 (0.19–1.42) | 1.0 (Ref) | 0.86 (0.30–2.43) | 0.18 (0.02–1.49) | N/A | 0.07 |
Highest specific IgE scores recorded at baseline.
N/A = not applicable; n = number of participants; NMSC = nonmelanoma skin cancer; CNS = central nervous system.
All models were adjusted for sex (except for sex-specific analysis), socioeconomic status, period of measurement, history of chronic pulmonary disease and serum total IgE.
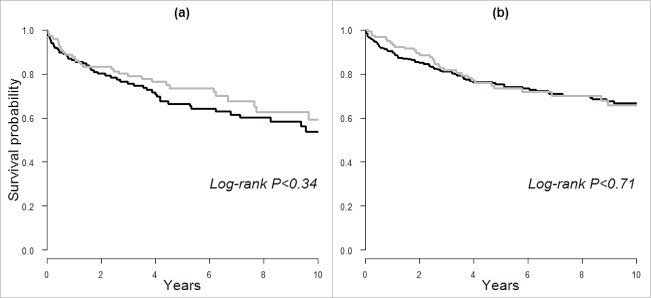
In our secondary analysis, we assessed risk of death following cancer diagnosis. As shown by the Kaplan–Meier curves in Fig. 1, the probability of survival was lower in men with IgE sensitization compared to those without in the long-term follow-up, but there were no statistically significant difference (Log-rank p > 0.05). Similarly, no differences were observed when categories of allergen-specific IgE scores were used (results not shown). We further evaluated this association by conducting Cox regression and found no clear associations between IgE sensitization or specific IgE scores and death from all-causes or cancer, e.g. HR for cancer death was 1.04 (95% CI: 0.59–1.84) and 1.54 (0.91–2.62) for men and women with compared to without IgE sensitization, respectively (results not shown in tables).
Figure 1.
Kaplan–Meier curves of 10-y survival following cancer diagnosis in (A) men and (B) women based on prediagnostic IgE sensitization. Black lines indicate IgE sensitization and the gray lines indicate a lack thereof.
Discussion
In the present study, IgE sensitization was associated with a lower risk of incident cancer. The inverse trend between allergen-specific IgE was more pronounced in women and among those with high total IgE levels. Among the most common cancers, no association was observed except for an inverse trend between serum specific IgE scores and risk of melanoma in the overall population, and with risk of breast and gynecological cancer in women. No associations between prediagnostic allergen-specific IgE and survival following cancer diagnosis were observed.
A shift toward immunosuppressive immune responses is characteristic of cancer.22 Nevertheless, little is known about the role of humoral immune responses, particularly IgE, in carcinogenesis. IgE production and class-switch recombination (CSR) to IgE from other immunoglobulin types such as IgG are regulated by TH2, and it has been suggested that a biased TH2 response underlies high IgE levels in allergic individuals.23 Since only limited responses following allergen exposure are observed for IgE,24 it is possible that any impact on carcinogenesis is secondary to the biased TH2 response rather than a result of high circulating IgE itself. In support of this, a temporal model of IgE and IgG has been proposed, in which the early-response IgE undergo sequential CSR to higher-affinity IgG3, then to IgG1, IgG2, and finally IgG4,25-27 the latter of which displays low immunoactivatory functions. Furthermore, inflammatory TH2-biased conditions such as IL-10, IL-4, VEGF and FoxP3+ Tregs that support class switching to IgG4 rather than IgE, and elevated IgG4 levels have been reported in different tumors including melanoma.28,29 Specifically in melanoma, elevated serum IgG4 levels and IgG4+ B cells in patient circulation are associated with worse clinical outcomes, implying a bias toward an alternative rather than an IgE-biased response associated with melanoma cancer growth.30,31 Taken together, these indications point toward a role of CSR dysregulation associated with TH2-biased response in driving the link between allergy, circulating IgE, and IgG4-related diseases including some cancers.32 Nevertheless, our findings in individuals with high total IgE (≥100 kU/L) may be consistent with a potential requirement for a critical threshold toward a classical IgE, rather than an alternative IgG4-biased TH2 immune response activation to confer for any potential protective benefits from cancer.
To date, only few prospective studies have examined possible associations between prediagnostic IgE sensitization and risk of incident cancer. In a recent study, Skaaby and colleagues evaluated serum specific evaluated IgE against inhalant allergen among 14,849 individuals.11 A lack of association between allergen-specific IgE levels and risk of overall cancer was reported for IgE sensitization.11 Similarly, we found a lack of a statistically significant association when assessing IgE sensitization against inhalant allergens in relation to overall incident cancer. However, when we took into account serum total IgE, an inverse association was found. Differences in follow-up periods and cohort composition may explain the discrepancy in the findings. Our study was based on a large cohort with a median follow-up of 18.6 y. In comparison, the study by Skaaby and colleagues comprised five cohorts spanning over different time periods, with a shorter overall median follow-up of 11.8 y.11 Adjustments for other risk factors such as smoking, alcohol consumption and physical activity did not alter findings in that study and are thus unlikely to explain the discrepancy with the results in our study in which risk factor information was unavailable.
For specific cancers, observational findings seem to vary by demographics and timing of specific IgE measurements. Two European nested case-control studies demonstrated an inverse association between IgE sensitization against inhalant allergens and risk of glioma in women but not men,13,33 whereas a lack of association was reported by another nested case-control study based on four US cohorts.34 Besides population attributes, a smaller number of cases in the latter study may explain this inconsistency. In case-control studies where allergen-specific IgE in cases was assessed after diagnosis, IgE sensitization was inversely associated with risk of lymphoid malignancies and positively with prostate cancer risk.35,36 However, no such association was observed in nested case-control studies where serum samples were prospectively collected before diagnosis.11,12,35
In our study population, we found no associations between IgE sensitization and risk of specific cancer sites, which is comparable with the study conducted by Skaaby and colleagues.11 However, an inverse association between serum specific IgE scores and risk of breast and gynecological cancers were observed in women. To date, evidence from observational studies on the role of allergy in these cancers remains unclear. In a meta-analysis, a lack of associations between history of any allergy, asthma or hay fever and breast cancer risk was suggested.9 On the other hand, a reduced incidence of ovarian, endometrial and cervical cancers have been reported in allergic patients.37-40 There is little evidence based on IgE sensitization status except for cancer of the uterus, where a lack of association was suggested.11 Considering the influence of estrogen in the development of these cancers, these results may indicate an interplay between immunologic and hormonal factors in carcinogenesis. Interestingly, endocrine treatment agents for estrogen-positive (ER+) breast cancers such as tamoxifen has been shown to reduce allergen-specific immunoglobulin levels including IgE in animal models of atopic dermatitis,41 which further suggests such complex associations.
There are several caveats in assessing serum allergen-specific IgE as a marker of allergy. Allergen-specific IgE levels represent the probability of having clinical allergic disease, therefore, use of a single allergen and/or cut-off to define IgE sensitization may not fully be representative of one's allergic symptoms.42 In line with this notion, we found stronger associations with categories of specific IgE compared to the conventional single cut-off point of specific IgE levels, which indicates that specific IgE scores or categories may be more useful than a single cut-off point in assessing cancer risk. Additionally, it was suggested that the number of positive inhalant allergens correlates better with allergic diseases compared to a single positive allergen-specific IgE test.43 In our study, we have addressed this possibility by an additional adjustment for the number of allergen tested, the number of positive results, or the ratio between the two, and they did not alter our findings. Finally, there is an indication that the sum of specific IgE levels against common inhalant allergens correlates better with clinical symptoms such as wheezing 44 and hospitalization with asthma,45 compared to individual levels of specific IgE. We were unable to assess cumulative levels of specific IgE in this study. Therefore, future studies assessing allergy-related cancer susceptibility may benefit from refined criteria of IgE sensitization.
To date, this is the first study documenting the association between IgE sensitization to common inhalant allergens and the risk of cancer using both serum allergen-specific and total IgE, and also the first study investigating the impact of pre-diagnostic IgE on cancer survival. Strengths of our study include the prospectively collected allergen-specific and total IgE levels prior to the diagnosis of cancer. Complete follow-up was obtained and all laboratory measurements were performed in one and the same laboratory.14 By using age as a timescale, we addressed the strong influence of age on absolute levels of specific IgE and its relative proportion to total IgE.19,46 A limitation of this study is the lack of information on clinical symptoms of allergy. Although information on specific types of allergens was available, we were unable to link individual allergens with risk of cancer due to the lack of number of cases. Our study population only included individuals who underwent IgE testing as part of a check-up or as outpatients and therefore may not be representative of the general population. However, this is not expected to influence the internal validity of this study. Allergy symptoms may have been confused with smoking-related respiratory disorders. To account for the lack of information on smoking, we adjusted our analysis for history of hospitalization with chronic obstructive pulmonary disease and asthma. Nevertheless, residual confounding may still have occurred. Lastly, spurious correlations may be of concern when performing multiple comparisons as shown in our study. However, we planned our analyses based on prior evidence and our results are explicable by suggested biological pathways and findings from other studies. Therefore, the observed association is unlikely to be spurious, although a discrepancy with the strength of the true association is possible due to the lack of cases.
In summary, our study suggests that IgE sensitization is weakly associated with a lower risk of malignancy in cancer-free individuals. These findings add to the evidence that immune responses involved in allergy contribute to the susceptibility of being diagnosed with cancer, particularly female breast and gynecological cancers and melanoma. In particular, our results may support a role of TH2-biased immune response in development of these cancers, indicated by a shift in the balance between circulating IgE and IgG subclasses including the low immunoactivatory IgG4, which urges further mechanistic investigations.
Methods
Study population
The AMORIS study has been described in detail elsewhere.14 Briefly, this study includes Swedish men and women with blood samples sequentially sent to the Central Automation Laboratory (CALAB) in Stockholm, Sweden14 Participants were either healthy and had a laboratory testing as a part of general health check-up, or were outpatients referred for laboratory testing. None of the participants were inpatients when samples were collected. In the AMORIS study, the CALAB database is linked to Swedish national registries, providing complete follow-up information on cancer diagnosis, death and emigration.15
Following a recent update, the AMORIS study now includes laboratory measurements of 812,073 individuals with follow-up information until 31 December 2011. From this population, we included 8,727 men and women aged 20 and older with no history of cancer who had baseline measurements of allergen-specific IgE against inhalant allergen and total IgE concentrations between 1992 and 1996. The study complied with the Declaration of Helsinki and was approved by the Ethics Review Board of the Karolinska Institutet.
Assessment of outcome
Cancer diagnosis was obtained from the population-based Swedish Cancer Register. International Classification of Diseases, seventh revision (ICD-7) codes were used to classify cancer sites. In addition to overall cancer (ICD-7: 140–207), we assessed all cancer excluding non-melanoma skin cancer (NMSC) (ICD-7: 140–207 excluding 191) and the 10 most frequently diagnosed cancers in our study population: prostate (ICD-7: 177), female breast (ICD-7: 170), colorectal (ICD-7: 153–154), gynecological including ovarian, uterus and cervix (ICD-7: 171–176), hematological (ICD-7: 200–207), melanoma skin (ICD-7: 190), pulmonary (primary; ICD-7: 162), bladder (ICD-7: 181), NMSC (ICD-7: 191), central nervous system (CNS; ICD-7: 193) and kidney cancer (ICD-7: 180). The secondary outcomes of this study were all-cause and cancer-specific deaths. Dates and causes of death were obtained from the Swedish Cause of Death Register, whereas information on emigration was retrieved from the Migration Register.
Assessment of exposures and covariates
Specific IgE concentrations against common inhalant allergens were measured using immunoassay. The test system, Pharmacia CAP® System (Thermo Fisher Scientific, formerly Pharmacia Diagnostics AB, Uppsala, Sweden), is based on solid phase coupled allergen, adsorbing the IgE antibodies in the sample and assessed by an anti-IgE antibody commercially developed. It has been well standardized, shows correct quantitative values and is reproducible over time.16 A list of inhalant allergens tested is available in Table S1. Results of allergen-specific IgE test were expressed as scores ranging from 0 to 6 which represent different levels of IgE from undetectable up to high concentrations of IgE (kU/L) as displayed in Table 2. Apart from these scores, no information on continuous levels of specific IgE was available. As with previous studies, any scores higher than 0 (which correspond to specific IgE levels of ≥0.35 kU/L) were defined as IgE sensitization and the presence of atopy.17 For consistency, the term IgE sensitization was used to describe specific IgE levels ≥0.35 kU/L throughout this study. When multiple allergens were tested at baseline, results for all allergen-specific IgE measurements were collected and positive IgE sensitization was defined as having at least one positive result among all the tested allergens. In addition to IgE sensitization status, highest specific IgE scores recorded at baseline examinations when multiple allergens were tested were used in the analysis. Serum total IgE (kU/L) was measured by enzyme-linked immunosorbent assay (ELISA) using Immunoassay System ES 700 (Boehringer-Mannheim, Germany). Coefficient of variation was less than 5%. Total IgE levels were categorized based on the clinical cut-off points into low (<25 kU/L), moderate (25–100 kU/L) or high levels (≥100 kU/L) as previously described (173).
Age at baseline measurement (years) was collected from the CALAB database. The period of measurement was categorized (1992–1993, 1994–1995, 1996) to account for a long recruitment period. Socioeconomic status (SES; white collar, blue collar, unemployed or unknown) was based on national Censuses.18 From the National Patient Register, we used data on history of hospitalization with chronic pulmonary disease including asthma (ever, never).
Statistical analysis
Multivariable Cox regression was used to estimate HR with corresponding 95% CI of overall risk of cancer by IgE sensitization status (yes, no) in all participants. Follow-up time was defined as the time from baseline measurement until cancer diagnosis, death from any cause, emigration or end of study, whichever occurred first. Additionally, the trend between allergen-specific IgE scores against inhalant allergens and risk of cancer was evaluated by assessing scores in groups (0, 1–2, 3–4, 5–6) as an ordinal scale. Levels of allergen-specific IgE are known to substantially decrease with age.19 Therefore, all analyses were performed using age as the time scale with delayed entry.
Models were adjusted for sex, SES, and period of measurement, history of chronic pulmonary disease to account for asthma and as a proxy for smoking given their association to IgE sensitivity and risk of lung cancer.20,21 Analyses were repeated in men and women separately. A further adjustment for categories of total IgE levels was performed in the second model. To evaluate any effect modification by total IgE levels, analyses were stratified according to total IgE levels. In an additional analysis, we adjusted our model for the number of allergen tested, the number of positive results or the ratio between the two. Analysis was subsequently performed for all cancer excluding NMSC and the 10 most common cancer sites with adjustment for total IgE levels. We repeated a similar analysis in men and women but only assessed the five sex-specific most common cancer sites due to the lack of number of cases.
For our secondary objective, we studied pre-diagnostic allergen-specific IgE in relation to survival after cancer diagnosis. Three cancer patients were excluded in the analysis because the diagnosis of cancer occurred at the time of death, leaving 686 individuals with cancer in the final analysis. Follow-up time was defined as the time from cancer diagnosis until death from any cause, emigration or end of study, whichever occurred first. Kaplan–Meier curves were used to assess overall survival by IgE sensitization status and scores of allergen-specific IgE, and statistical differences were assessed with the log-rank test. Cox regression was used to quantify the risks of all-cause and cancer-specific deaths by IgE sensitization status and allergen-specific IgE scores with age at diagnosis as time scale. The models were adjusted for the interval between baseline IgE measurements and cancer diagnosis, and total IgE levels.
All analyses were conducted with Statistical Analysis Software (SAS) release 9.4 (SAS Institute, Cary, NC) and R version 3.0.2 (R Foundation for Statistical Computing).
Supplementary Material
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests. Niklas Hammar is employed by the AstraZeneca, but the views expressed in the manuscript are his own and not those of AstraZeneca.
Funding
The research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The first author received a short-term fellowship grant from the European Federation of Immunological Societies/Immunology Letters to conduct part of the research. The authors acknowledge support by Cancer Research UK (C30122/A11527; C30122/A15774); the Academy of Medical Sciences; the Medical Research Council (MR/L023091/1); CRUK/NIHR in England/DoH for Scotland, Wales and Northern Ireland Experimental Cancer Medicine Center (C10355/A15587). The views expressed are those of the author(s) and not necessarily those of the funding organizations.
References
- 1.Johansson SGO, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TAE, Ring J et al.. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004; 113:832-6; PMID:15131563; http://dx.doi.org/ 10.1016/j.jaci.2003.12.591 [DOI] [PubMed] [Google Scholar]
- 2.Rittmeyer D, Lorentz A. Relationship between allergy and cancer: an overview. Int Arch Allergy Immunol 2012; 159:216-25; PMID:22722389; http://dx.doi.org/ 10.1159/000338994 [DOI] [PubMed] [Google Scholar]
- 3.Sherman PW, Holland E, Sherman JS. Allergies: their role in cancer prevention. Q Rev Biol 2008; 83:339-62; PMID:19143335; http://dx.doi.org/ 10.1086/592850 [DOI] [PubMed] [Google Scholar]
- 4.Simpson CR, Anderson WJA, Helms PJ, Taylor MW, Watson L, Prescott GJ, Godden DJ, Barker RN. Coincidence of immune-mediated diseases driven by Th1 and Th2 subsets suggests a common aetiology. A population-based study using computerized general practice data. Clin Exp Allergy [Internet] 2002; 32:37-42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12002734; PMID:12002734; http://dx.doi.org/ 10.1046/j.0022-0477.2001.01250.x [DOI] [PubMed] [Google Scholar]
- 5.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol [Internet] 2008. [cited 2015May15]; 9:628-38. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18628784; PMID:18628784; http://dx.doi.org/24074329 10.1038/nrm2455 [DOI] [PubMed] [Google Scholar]
- 6.Josephs DH, Spicer JF, Corrigan CJ, Gould HJ, Karagiannis SN. Epidemiological associations of allergy, IgE and cancer. Clin Exp Allergy [Internet] 2013. [cited 2014May15]; 43:1110-23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24074329; PMID:24074329; http://dx.doi.org/ 10.1111/cea.12178 [DOI] [PubMed] [Google Scholar]
- 7.Van Hemelrijck M, Garmo H, Binda E, Hayday A, Karagiannis SN, Hammar N, Walldius G, Lambe M, Jungner I, Holmberg L. Immunoglobulin E and cancer: a meta-analysis and a large Swedish cohort study. Cancer Causes Control [Internet] 2010. [cited 2014May10]; 21:1657-67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20533084; PMID:20533084; http://dx.doi.org/ 10.1007/s10552-010-9594-6 [DOI] [PubMed] [Google Scholar]
- 8.Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst [Internet] 2007. [cited 2014September26]; 99:1544-50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17925535; PMID:17925535; http://dx.doi.org/ 10.1093/jnci/djm170 [DOI] [PubMed] [Google Scholar]
- 9.Vojtechova P, Martin RM. The association of atopic diseases with breast, prostate, and colorectal cancers: a meta-analysis. Cancer Causes Control 2009; 20:1091-105; PMID:19340595; http://dx.doi.org/ 10.1007/s10552-009-9334-y [DOI] [PubMed] [Google Scholar]
- 10.Talbot-Smith A, Fritschi L, Divitini ML, Mallon DFJ, Knuiman MW. Allergy, atopy, and cancer: A prospective study of the 1981 Busselton cohort. Am J Epidemiol 2003; 157:606-12; PMID:12672680; http://dx.doi.org/ 10.1093/aje/kwg020 [DOI] [PubMed] [Google Scholar]
- 11.Skaaby T, Nystrup Husemoen LL, Roswall N, Thuesen BH, Linneberg A. Atopy and development of cancer: a population-based prospective study. J allergy Clin Immunol Pract [Internet] 2015; 2:779-85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25439371; PMID:25439371; http://dx.doi.org/25269801 10.1016/j.jaip.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 12.Nieters A, Luczynska A, Becker S, Becker N, Vermeulen R, Overvad K, Aleksandrova K, Boeing H, Lagiou P, Trichopoulos D et al.. Prediagnostic Immunoglobulin E levels and risk of chronic lymphocytic leukemia, other lymphomas and multiple myeloma- results of the European Prospective Investigation into Cancer and Nutrition. Carcinogenesis [Internet] 2014; 35:2716-22. Available from: http://www.carcin.oxfordjournals.org/cgi/doi/10.1093/carcin/bgu188; PMID:25269801; http://dx.doi.org/ 10.1093/carcin/bgu188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartzbaum J, Ding B, Johannesen TB, Osnes LTN, Karavodin L, Ahlbom A, Feychting M, Grimsrud TK. Association between prediagnostic IgE levels and risk of glioma. J Natl Cancer Inst [Internet] 2012. [cited 2014September21; 104:1251-9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3424222&tool=pmcentrez&rendertype=abstract; PMID:22855780; http://dx.doi.org/ 10.1093/jnci/djs315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Inflammatory markers, lipoprotein components and risk of major cardiovascular events in 65,005 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). Atherosclerosis [Internet] 2010. [cited 2014May2]; 213:299-305. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20843515; PMID:20843515; http://dx.doi.org/ 10.1016/j.atherosclerosis.2010.08.049 [DOI] [PubMed] [Google Scholar]
- 15.Wulaningsih W, Holmberg L, Garmo H, Malmstrom H, Lambe M, Hammar N, Walldius G, Jungner I, Van Hemelrijck M. Prediagnostic serum inflammatory markers in relation to breast cancer risk, severity at diagnosis and survival in breast cancer patients. Carcinogenesis [Internet] 2015; 36(10):1121–1128; Available from: http://www.ncbi.nlm.nih.gov/pubmed/26130675; PMID:26130675; http://dx.doi.org/ 10.1093/carcin/bgv096. [DOI] [PubMed] [Google Scholar]
- 16.Gleeson M, Cripps A W, Hensley MJ, Wlodarczyk JH, Henry RL, Clancy RL, Hensleyf MJ, Wlodarczykj JH, Henry RL, Clancy RL et al.. A clinical evaluation in children of the Pharmacia ImmunoCAP system for inhalant allergens. Clin Exp Allergy 1996; 26:697-702; PMID:8809427; http://dx.doi.org/ 10.1111/j.1365-2222.1996.tb00596.x [DOI] [PubMed] [Google Scholar]
- 17.Skaaby T, Husemoen LLN, Thuesen BH, Hammer-Helmich L, Linneberg A. Atopy and cause-specific mortality. Clin Exp Allergy [Internet] 2014; 44(11):1361-70:[cited 2014September26]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25220375; PMID:25220375; http://dx.doi.org/23706176 10.1111/cea.12408 [DOI] [PubMed] [Google Scholar]
- 18.Wulaningsih W, Michaelsson K, Garmo H, Hammar N, Jungner I, Walldius G, Holmberg L, Van Hemelrijck M. Inorganic phosphate and the risk of cancer in the Swedish AMORIS study. BMC Cancer 2013; 13:257; PMID:23706176; http://dx.doi.org/ 10.1186/1471-2407-13-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omenaas E, Bakke P, Elsayed S, Hanoa R, Gulsvik A. Total and specific serum IgE levels in adults: Relationship to sex, age and environmental factors. Clin Exp Allergy 1994; 24:530-9; PMID:7922774; http://dx.doi.org/ 10.1111/j.1365-2222.1994.tb00950.x [DOI] [PubMed] [Google Scholar]
- 20.Oettgen HC, Geha RS. IgE in asthma and atopy: Cellular and molecular connections. J Clin Invest 1999; 104:829-35; PMID:10510320; http://dx.doi.org/ 10.1172/JCI8205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DW, Young KE, Anda RF, Giles WH. Asthma and risk of death from lung cancer: NHANES II Mortality Study. J Asthma [Internet] 2005. [cited 2014January28]; 42:597-600. Available from: http://informahealthcare.com/doi/full/10.1080/02770900500216234; PMID:16169796; http://dx.doi.org/ 10.1080/02770900500216234 [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell [Internet] 2011. [cited 2014January9]; 144:646-74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21376230; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 23.Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol [Internet] 2003; 3:721-32. Available from: http://www.nature.com/doifinder/10.1038/nri1181; PMID:12949496; http://dx.doi.org/ 10.1038/nri1181 [DOI] [PubMed] [Google Scholar]
- 24.Wu LC, Zarrin Aa. The production and regulation of IgE by the immune system. Nat Rev Immunol [Internet] 2014; 14:247-59. Available from: http://www.nature.com/doifinder/10.1038/nri3632; PMID:24625841; http://dx.doi.org/ 10.1038/nri3632 [DOI] [PubMed] [Google Scholar]
- 25.Collins AM, Jackson KJL. A temporal model of human IgE and IgG antibody function. Front Immunol 2013; 4:1-6; PMID:23355837; http://dx.doi.org/ 10.3389/fimmu.2013.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson KJL, Wang Y, Collins AM. Human immunoglobulin classes and subclasses show variability in VDJ gene mutation levels. Immunol Cell Biol [Internet] 2014; 92:1-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24913324; PMID:24913324; http://dx.doi.org/25693563 10.1038/icb.2014.44 [DOI] [PubMed] [Google Scholar]
- 27.Consortium RE, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J et al.. Integrative analysis of 111 reference human epigenomes. Nature [Internet] 2015; 518:317-30. Available from: http://www.nature.com/doifinder/10.1038/nature14248; PMID:25693563; http://dx.doi.org/ 10.1038/nature14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, Correa I, Roberts L, Beddowes E, Koers A et al.. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest 2013; 123:1457-74; PMID:23454746; http://dx.doi.org/ 10.1172/JCI65579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harada K, Shimoda S, Kimura Y, Sato Y, Ikeda H, Igarashi S, Ren X-S, Sato H, Nakanuma Y. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology [Internet] 2012; 56:157-64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22290731; PMID:22290731; http://dx.doi.org/ 10.1002/hep.25627 [DOI] [PubMed] [Google Scholar]
- 30.Karagiannis P, Villanova F, Josephs DH, Correa I, Van Hemelrijck M, Hobbs C, Saul L, Egbuniwe IU, Tosi I, Ilieva KM et al.. Elevated IgG4 in patient circulation is associated with the risk of disease progression in melanoma. Oncoimmunology [Internet] 2015; 4:e1032492. Available from: http://www.tandfonline.com/doi/full/10.1080/2162402X.2015.1032492; PMID:26451312; http://dx.doi.org/ 10.1080/2162402X.2015.1032492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karagiannis P, Fittall M, Karagiannis SN. Evaluating biomarkers in melanoma. Front Oncol [Internet] 2014; 4:1-11. Available from: http://journal.frontiersin.org/article/10.3389/fonc.2014.00383/abstract; PMID:24478982; http://dx.doi.org/ 10.3389/fonc.2014.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karagiannis P, Gilbert AEA, Nestle FO, Karagiannis SN, Ri F. IgG4 antibodies and cancer-associated inflammation: Insights into a novel mechanism of immune escape. Oncoimmunology [Internet] 2013; 2(7):e24889:[cited 2014May15]; 2-4. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3782134/; PMID:24073371; http://dx.doi.org/ 10.4161/onci.24889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlehofer B, Siegmund B, Linseisen J, Schüz J, Rohrmann S, Becker S, Michaud D, Melin B, Bas Bueno-de-Mesquita H, Peeters PHM et al.. Primary brain tumours and specific serum immunoglobulin E: a case-control study nested in the European Prospective Investigation into Cancer and Nutrition cohort. Allergy [Internet] 2011. [cited 2014September21]; 66:1434-41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21726235; PMID:21726235; http://dx.doi.org/ 10.1111/j.1398-9995.2011.02670.x. [DOI] [PubMed] [Google Scholar]
- 34.Calboli FCF, Cox DG, Buring JE, Gaziano JM, Ma J, Stampfer M, Willett WC, Tworoger SS, Hunter DJ, Camargo CA et al.. Prediagnostic plasma IgE levels and risk of adult glioma in four prospective cohort studies. J Natl Cancer Inst [Internet] 2011. [cited 2014September21]; 103:1588-95. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3206038&tool=pmcentrez&rendertype=abstract; PMID:22010181; http://dx.doi.org/17227999 10.1093/jnci/djr361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melbye M, Smedby KE, Lehtinen T, Rostgaard K, Glimelius B, Munksgaard L, Schöllkopf C, Sundström C, Chang ET, Koskela P et al.. Atopy and risk of non-Hodgkin lymphoma. J Natl Cancer Inst [Internet] 2007. [cited 2014September26]; 99:158-66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17227999; PMID:17227999; http://dx.doi.org/ 10.1093/jnci/djk019 [DOI] [PubMed] [Google Scholar]
- 36.Ellison-Loschmann L, Benavente Y, Douwes J, Buendia E, Font R, Alvaro T, Kogevinas M, de Sanjosé S. Immunoglobulin E levels and risk of lymphoma in a case-control study in Spain. Cancer Epidemiol Biomarkers Prev [Internet] 2007. [cited 2015May15]; 16:1492-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17627016; PMID:17627016; http://dx.doi.org/ 10.1158/1055-9965.EPI-07-0176 [DOI] [PubMed] [Google Scholar]
- 37.Johnson LG, Schwartz SM, Malkki M, Du Q, Petersdorf EW, Galloway D a, Madeleine MM. Risk of cervical cancer associated with allergies and polymorphisms in genes in the chromosome 5 cytokine cluster. Cancer Epidemiol Biomarkers Prev 2011; 20:199-207; PMID:21071541; http://dx.doi.org/ 10.1158/1055-9965.EPI-10-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elmasri WM, Tran TH, Mulla ZD. A case-control study of asthma and ovarian cancer. Arch Environ Occup Health 2010; 65:101-5; PMID:20439229; http://dx.doi.org/ 10.1080/19338240903390297 [DOI] [PubMed] [Google Scholar]
- 39.Vesterinen E, Pukkala E, Timonen T, Aromaa A. Cancer incidence among 78,000 asthmatic patients. Int J Epidemiol 1993; 22:976-82; PMID:8144310; http://dx.doi.org/ 10.1093/ije/22.6.976 [DOI] [PubMed] [Google Scholar]
- 40.Kallen B, Gunnarskog J, Conradson TB. Cancer risk in asthmatic subjects selected from hospital discharge registry. Eur Respir J 1993; 6:694-7; PMID:8519380 [PubMed] [Google Scholar]
- 41.Babina M, Kirn F, Hoser D, Ernst D, Rohde W, Zuberbier T, Worm M. Tamoxifen counteracts the allergic immune response and improves allergen-induced dermatitis in mice. Clin Exp Allergy 2010; 40:1256-65; PMID:20337649; http://dx.doi.org/ 10.1111/j.1365-2222.2010.03472.x [DOI] [PubMed] [Google Scholar]
- 42.Ahlstedt S, Murray CS. In vitro diagnosis of allergy: how to interpret IgE antibody results in clinical practice. Prim Care Respir J [Internet] 2006; 15:228-36. Available from: http://www.nature.com/articles/pcrj2006054; PMID:16839813; http://dx.doi.org/ 10.1016/j.pcrj.2006.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wickman M, Lilja G, Söderström L, Van HageHamsten M, Ahlstedt S. Quantitative analysis of IgE antibodies to food and inhalant allergens in 4-year-old children reflects their likelihood of allergic disease. Allergy Eur J Allergy Clin Immunol 2005; 60:650-7; PMID:15813811; http://dx.doi.org/16378049 10.1111/j.1398-9995.2004.00764.x [DOI] [PubMed] [Google Scholar]
- 44.Heymann PW, Platts-Mills TAE, Johnston SL. Role of Viral Infections, Atopy and Antiviral Immunity in the Etiology of Wheezing Exacerbations Among Children and Young Adults. Pediatr Infect Dis J [Internet] 2005; 24:S217-22. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006454-200511001-00011; PMID:16378049 [DOI] [PubMed] [Google Scholar]
- 45.Simpson A, Soderstrom L, Ahlstedt S, Murray CS, Woodcock A, Custovic A. IgE antibody quantification and the probability of wheeze in preschool children. J Allergy Clin Immunol [Internet] 2005; 116:744-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16210045; PMID:16210045; http://dx.doi.org/20066506 10.1016/j.jaci.2005.06.032 [DOI] [PubMed] [Google Scholar]
- 46.Hamilton RG, MacGlashan DW, Saini SS. IgE antibody-specific activity in human allergic disease. Immunol Res 2010; 47:273-84; PMID:20066506; http://dx.doi.org/ 10.1007/s12026-009-8160-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.