Abstract
Chemotherapy is an important strategy for the treatment of bladder cancer. However, the main problem limiting the success of chemotherapy is the development of multidrug resistance (MDR). To improve the management of bladder cancer, it is an urgent matter to search for strategies to reverse MDR. We chose three kinds of herbal medicines including ginsenoside Rh2, (-)-Epigallocatechin gallate (EGCG) and Tetramethylpyrazine (TMP) to detect their effects on bladder cancer. Reversal effects of these three herbal medicines for drug resistance in adriamycin (ADM)-resistant Pumc-91 cells (Pumc-91/ADM) were assessed by Cell Counting Kit-8 (CCK-8) cell proliferation assay system. The mechanisms of reversal effect for TMP were explored in Pumc-91/ADM and T24/DDP cells. After Pumc-91/ADM and T24/DDP cells were treated with TMP, cell cycle distribution analysis was performed by flow cytometry. The expression of MRP1, GST, BCL-2, LRP and TOPO-II was evaluated using quantitative real-time polymerase chain reaction (qRT-PCR), immunefluorescence assay and western blot. It was observed that TMP was capable of enhancing the cytotoxicity of anticancer agents on Pumc-91/ADM cells in response to ADM, however Rh2 and EGCG were unable to. The reversal effect of TMP was also demonstrated in T24/DDP cells. Moreover, the treatment with TMP in Pumc-91/ADM and T24/DDP cells led to an increased of G1 phase accompanied with a concomitant decrease of cell numbers in S phase. Compared to the control group, an obvious decrease of MRP1, GST, BCL-2 and an increase of TOPO-II were shown in TMP groups with a dose-dependency in mRNA and protein levels. However, there was no difference on LRP expression between TMP groups and the control group. TMP could effectively reverse MDR of Pumc-91/ADM and T24/DDP cells and its mechanisms might be correlated with the alteration of MRP1, GST, BCL-2 and TOPO-II. TMP might be a potential candidate for reversing drug resistance in bladder cancer chemotherapy.
Introduction
Globally, bladder cancer is the most common cancer of the genitourinary tract in men [1]. Approximately 70% of cancers are non-muscle invasive tumors with high recurrence, while the remaining 30% are muscle invasive with high risk of death from distant metastases [2]. The transurethral resection of bladder tumor (TURBT) is essential for non-muscle invasive bladder cancer treatment. With regard to low-grade Ta and T1 tumor, intravesical chemotherapy or immunotherapy is necessary. As for muscle-invasive bladder cancer, radical cystectomy and lymph nodes dissection is the standard operation [3]. Systemic chemotherapy is a reasonable alternative after surgery for patients with muscle invasive bladder cancers. Recent studies show that surgery combining with chemotherapy can improve the quality of life and improve survival [4]. However, cancer cells frequently develop an almost uncanny ability to resist the effects of cancer chemotherapeutic agents. Selection of cancer cells with one chemotherapeutic drug usually results in cross-resistance to other drugs with different cellular targets and structures. This phenomenon is known as multidrug resistance (MDR) [5]. The development of multidrug resistance in bladder cancer cells can severely impair the success of cancer systemic chemotherapy [6]. Adriamycin and cisplatin are important drugs used for chemotherapy against bladder cancer. However, due to the development of MDR, the treatment in bladder cancer with adriamycin, cisplatin or other agents often fails. The acquisition of MDR could be mediated via many mechanisms, including the increase in drug efflux, the decrease in drug influx, drug inactivation and alterations in the drug target, modification of cell cycle checkpoints and increased DNA damage repair and defective apoptotic pathways [7–9].
Some MDR proteins involve in the drug resistance of bladder cancer via reducing the intracellular drug concentrations. These proteins can predict poor outcomes after chemotherapy [10–12]. In bladder cancer, the expression of MDR1 mRNA in recurrent and residual tumors after doxorubicin chemotherapy was higher than that in untreated primary tumors [13]. Multidrug resistance protein 1 and lung resistance related protein were overexpressed in locally advanced bladder cancer. MRP1 expression correlated with a higher response and a higher probability of bladder preservation following neoadjuvant chemotherapy [14]. High LRP expression was significantly associated with a worse response to neoadjuvant chemotherapy and a decreased probability of bladder preservation [14].
Many multi-drug resistance modulators have been reported for their contribution to MDR. However because of the present several barriers, they limited therapeutic efficacy in the clinic. One common reason for clinical failure of MDR modulators is their non-specific toxicity to cancer patients. Another obstacle is the unexpected and undesired pharmacokinetic interactions between the modulators and the anti-cancer drugs used for the treatment of patients, which results in reducing doses of anticancer drugs and so inefficient benefit [15]. Therefore, it is an urgency to find novel MDR mediators which can effectively reverse the drug resistance to satisfy the requirement of chemotherapy.
Rh2 (Fig 1a), a ginsenoside with a dammarane skeleton, has been shown to suppress growth and induce apoptosis in vitro in many cancer cells [16–18]. Green tea, derived from the plant Camellia sinensis, is one of the most common beverages consumed worldwide, especially in China. The major catechins in green tea is (-)-epigallocatechin-3-gallate (EGCG) (Fig 1b) which accounts for 50–80% of catechin in green tea. It has been reported that EGCG has inhibitory activity against tumorigenesis [19,20]. Tetramethylpyrazine (Fig 1c) is one of the major bioactive components of Chuanxiong. It was confirmed that TMP was capable of reversing the MDR of BEL-7402/ADM cells in response to ADM [21]. But so far there were no studies on reversing drug resistance of Rh2, EGCG and TMP in bladder cancer chemotherapy.
Fig 1. The structure of three kind of herbal medicine.
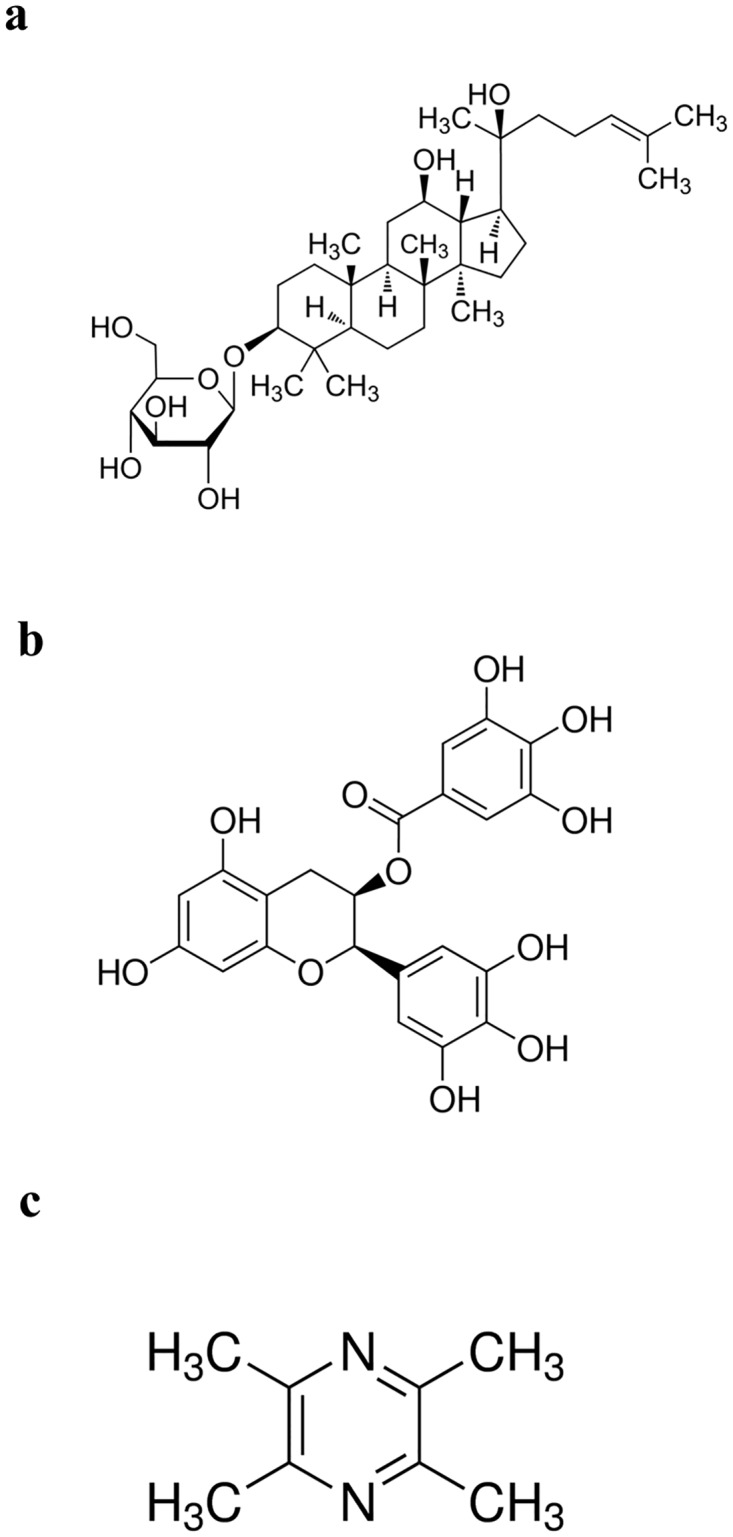
a. Rh2, b. EGCG, c.TMP.
In this study, the effects of Rh2, EGCG and TMP on cell viability were detected in multidrug resistant human bladder cancer cell line Pumc-91/ADM. The reversal effects of these three herbal medicines were also performed. Moreover, the reversal effect mechanisms of TMP on MDR were studied in Pumc-91/ADM and T24/DDP cells.
Materials and Methods
Cell culture
The human bladder cancer cell line Pumc-91 was provided by the Cell Laboratory of Peking Union Medical College Hospital. Pumc-91/ADM was a drug-resistant cell line that was established by stepwise exposure of Pumc-91 cells to escalating concentrations of adriamycin (aladdin, China), ranging from 0.02 μg/ml to 1.00 μg/ml for more than 6 months. The human bladder cancer cell line T24 was provided by Chinese Academy of Sciences Culture Collection. The human drug-resistant bladder cancer cell line T24/DDP was established by stepwise exposure of T24 cells to escalating concentrations of cisplatin (sigma), ranging from 0.01 μg/ml to 0.60 μg/ml for more than 6 months. The drug-resistant characteristics of Pumc-91/ADM and T24/DDP cell line were confirmed by MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide) assay, reverse transcription polymerase chain reaction (RT-PCR) and flow cytometry in previous studies [22,23]. The parental cell line was cultured in RPMI-1640 medium (Gibco, Invitrogen) which was supplemented with 10% heat-inactivated fetal bovine serum (Dingguo, Biotechnology), while drug-resistant cell lines were cultured in the above-mentioned medium with 18% fetal bovine serum. Cells were incubated at 37°C in a standard 5% CO2 cell incubator with a humidified atmosphere of 95% air. All experiments were carried out while cells were in exponential growth phase.
Cell viability assay
The effects of Rh2, EGCG and TMP on the viability of Pumc-91/ADM cells were measured using the Cell Counting Kit-8 (CCK-8) (dojindo) cell proliferation assay system. Pumc-91/ADM cells were seeded on 96 well plates with a density of 8×103 cells per well. After overnight incubation, cells were incubated with various concentrations of Rh2 (0–100 μM) (Sigma-Aldrich), EGCG (0–200 μM) (Sigma-Aldrich) and TMP (0–32 mM) (Sigma-Aldrich). Rh2 and EGCG were dissolved in dimeth-ylsulfoxide (DMSO) at a final concentration of less than 0.1% and TMP was dissolved in methyl alcohol at a final concentration of less than 0.1%. DMSO and methyl alcohol at a final concentration of less than 0.1% have no effect on cell viability. RPMI-1640 culture medium without cells served as a vehicle control and RPMI-1640 culture medium with cells served as a negative control. The cytotoxicity of these three traditional Chinese medicines was measured via CCK-8 assay. After cells were treated with Rh2, EGCG and TMP for 24h, 48h and 72h, 10μl of CCK-8 solution was added into each well. The absorbance was measured at a wavelength of 450 nm and 655 nm using a microplate reader (Model 680, BioRad Laboratories, Hercules, CA, USA) after cells incubated for 2h at 37°C. The proliferation ratio was calculated using the following equation: proliferation ratio (%) = (OD the drug treated group−OD the control group) / (ODthe control group—OD the vehicle group). The half maximal inhibitory concentration (IC50) was defined as the concentration of the drug that resulted in 50% inhibition of cell growth. IC50 was obtained via regression analysis between drug concentrations and the cell inhibition rate using SPSS 17.0 software. All of the experiments were repeated three times.
Multidrug resistance reversal assay
The reversal effect of Rh2, EGCG and TMP on Pumc-91/ADM cells was further detected with CCK-8. To minimize the effect of Rh2, EGCG and TMP themselves on cell growth, we chose lower concentrations of them in the reversal experiments. Cells were prior to seeding into 96-well plates and then treated with varying concentrations of ADM in the presence of Rh2 (0, 5, 10, 20 μM), EGCG (0, 10, 20, 40μM) and TMP (0, 1, 2, 4 mM) for 48h, respectively. Cells treated without herbal medicine were considered as the control group. Meanwhile, the reversal effect of TMP on T24/DDP cells was further detected with CCK-8. As described above, 10 μl of CCK-8 solution was added into each well. After cells were incubated at 37°C for 2h, the absorbance was recorded at 450 nm and 655 nm wavelength.
The reversal fold (RF) values, as potency parameter, were calculated from dividing IC50 of ADM alone by IC50 of ADM in combination with Rh2, EGCG or TMP in Pumc-91/ADM cells. RF of TMP in T24/DDP cells was calculated from dividing IC50 of DDP alone by IC50 of DDP in combination with TMP. The combinational index (CI) was calculated using the formula: CI = (Ea+b)/(Ea+Eb-Ea×Eb) [24]. In the formula, Ea+b represented the inhibition rate of ADM in combination with Rh2, EGCG, TMP or DDP in combination with TMP. Ea represented the individual inhibition rate of Rh2, EGCG or TMP and Eb represented the inhibition rate of ADM or DDP. The nature of the drug interaction was defined as: i) Additive (+) if CI ranged from 0.85 to 1.15; ii) synergism (++) if CI ranged from 1.15 to 2.0; iii) subtraction (-) if CI ranged from 0.85 to 0.55 and iv) antagonism (—) when the CI was <0.55.
Cell cycle distribution analysis
Pumc-91/ADM and T24/DDP cells were plated in culture dishes at concentrations determined to yield 50–60% confluence within 24h. Cells were then treated with different concentrations of TMP (0, 2 and 4 mM) for 48h. After that, cells were washed twice with cold dulbecco's phosphate-buffered saline (D-PBS) (Invitrogen) and then centrifuged at 140g for 5 min. The pellets were fixed with 70% ethanol in D-PBS and stored at 4°C for 1h. And then, cells were washed with D-PBS and resuspended with propidium iodide solution (0.01 mg/ml) (Sigma-Aldrich) containing RNase (100 μg/ml). After that, cells were incubated in the dark at room temperature for 30 min. The DNA content was then analyzed using the FACSCalibur flow cytometer with CellQuest software (BD Biosciences, San Jose, CA, USA). The data were analyzed using modfit software.
qRT-PCR
Total RNA was isolated with trizol reagent (Ambition, life technologies, USA) from Pumc-91/ADM and T24/DDP cells which were treated with TMP (0, 2 mM and 4 mM) according to manufacturer’s instructions. cDNA was generated using Oligo d (T) (Dingguo Biotech), dNTP (Genview) under the action of M-MLV reverse transcriptase (Promega). Quantitative real-time PCR was done by using TransStart Top Green qPCR SuperMix (Beijing TransGen Biotech Co., Ltd) with LightCycler 480 Real Time PCR System. The PCR primer sequences used were as follows: GAPDH forward: 5’-TTTGGTATCGTGGAAGGACT-3’ and reverse: 5’-AGTAGAGGCAGGGATGATGT-3’; MRP1 forward: 5’-TTGCCGTCTACGTGACCATT-3’ and reverse: 5’-AGGCGTTTGAGGGAGACACT-3’; LRP forward: 5’-TATGTGCCATCTGCCAAA GT-3’ and reverse: 5’-CATGTAGGTGCTTCCAATCA-3’; GST forward: 5’-TTCCTGTGGCATAATGTGAT-3’ and reverse: 5’-CTGATTCAA AGGCAAATCTC-3’; TOPO-II forward: 5’-AGGCATCGCATCTTGTTTAG-3’ and reverse: 5’ -CTGTCTCCGGTCTTCCATAA-3’; BCL-2 forward: 5’-GACAACATCGCCCTGTGGAT-3’ and reverse: 5’- AGGGCCAAACTGAGCAGAGT-3’. Quantitative real-time PCR were done by using TransStart Top Green qPCR SuperMix (Beijing TransGen Biotech Co., Ltd) with LightCycler 480 Real Time PCR System. GAPDH was used as endogenous reference. Relative gene expression was calculated using the 2-ΔΔCt method [25]. All of the experiments were repeated three times.
Immunofluorescence
When cultured to 90% confluence, cells were seeded on a glass slide at the density of 1×105 cells/ml and incubated with TMP (0, 2 mM, 4 mM) for 48h. After that, cells were washed three times with phosphate-buffered saline (PBS) (Hangzhou Sijiqing Company) and then fixed with 4% paraformaldehyde (Dingguo Biotechnology) for 15 min at room temperature. Cells were permeabilized in 0.1% Triton X-100/PBS (Sigma-Aldrich) for 10 min and incubated with 10% goat serum for 1h at 37°C. Cells were incubated with primary antibody of mouse monoclonal anti-MRP1 (1:100), rabbit monoclonal anti-LRP (1:100), rabbit polyclonal anti-GST (1:250), rabbit monoclonal anti-BCL-2 (1:100), rabbit polyclonal anti-TOPO-II (1:500), respectively (Abcam) at 37°C for 2h. After being washed with PBS three times, the cells were stained with AlexaFluor 488-conjugated secondary antibody (goat anti-mouse and goat anti-rabbit IgG, Abcam, 1:400) at 37°C in a moist cassette for 1h. The cells were washed with PBS three times and then incubated with Hoechst 33342 at the concentration of 5μg/ml for 10min at room temperature. Cell images were subsequently captured with fluorescence microscopy (Nikon 80i, Japan, ×400).
Western Blot
Pumc-91/ADM and T24/DDP cells were treated with TMP (0, 4 mM). After incubated for 48h, cells were collected and washed three times with PBS when in exponential growth phase. Protein extracts were prepared using a total protein extraction kit (KeyGen, Nanjing, China) according to the manufacturer's instructions. Protein (80 μg) per sample was separated in 8–12% SDS-PAGE and then was electrotransferred to a PVDF membrane (Bio-Rad, USA) for 1.5h. The membranes were blocked in solution with 5% nonfat milk in PBST for 2h at room temperature. Afterwards, the membranes were incubated with mouse or rabbit primary antibodies (Abcam, USA) against MRP1, LRP, GST, BCL-2 and TOPO-II at dilutions of 1:250, 1:1000, 1:250, 1:100, 1:500, respectively and β-actin (ZhongShan Co., China) at a dilution of 1:500 at 4°C overnight. Then the PVDF membrane was incubated in horseradish peroxidase-conjugated goat anti-mouse IgG antibody or anti-rabbit IgG antibody at a dilution of 1:1000 for 1.5h at room temperature. The bands were detected by enhanced HRP-DAB chromogenic kit. The integrated optical density (IOD) value of every band was analyzed with Image-Pro Plus v 6.0 software (MEDIA CYBERNETICS, USA). All of the experiments were repeated three times.
Statistical analysis
Statistical analysis was done by the SPSS 17.0 software (SPSS lnc., Chicago, USA). Student’s t test (two-tailed) and analysis of variance (one-way ANOVA) were used to analyze the control group and TMP groups. P value less than 0.01 was considered to indicate significant difference.
Results
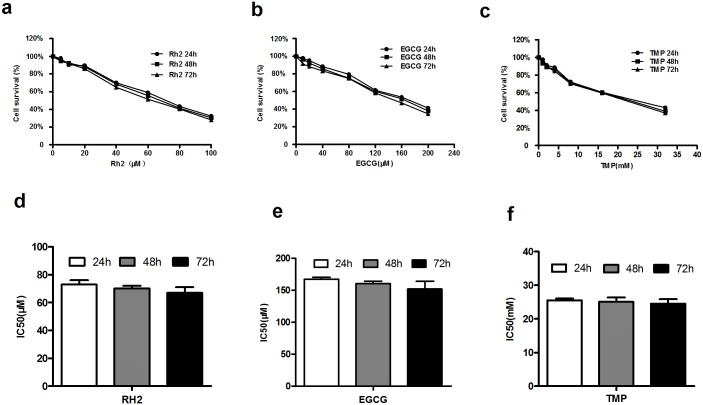
Growth inhibitory effect of three kinds of herbal medicines in Pumc-91/ADM cells
Rh2, EGCG and TMP could inhibit the proliferation of Pumc-91/ADM with dose-dependency but no time-dependency (Fig 2, S1 Table). With the increase of Rh2, EGCG and TMP concentration, the growth of cells gradually decreased. After cells were treated with Rh2 (5–100 μM), EGCG (10–200 μM) and TMP (0–32 mM) for 24h, the increase of cell inhibition rate ranged from 2.1% to 58.7%, 2.2% to 67.7%, 2.4% to 56.9%, for 48h ranged from 3.3% to 61.9%, 4.4% to 69.4%, 6.4% to 60.9% and for 72h ranged from 8.5% to 65.2%, 5.5% to 71.9%, 6.6% to 62.9% in Pumc-91/ADM cells, respectively (Fig 2a, 2b and 2c). The inhibitory concentration 50% values (IC50) at 24h, 48h and 72h were estimated to be 73, 70, 67 μM for Rh2 (P>0.01) (Fig 2d), 167, 160, 152 μM for EGCG (P>0.01) (Fig 2e) and 25.51, 25.08, 24.00 mM for TMP (P>0.01) (Fig 2f), respectively.
Fig 2. The inhibitory effect of three kind of herbal medicines in Pumc-91/ADM cell line.
Cell survival curves (a,b,c) and IC50 values (d, e, f) of Rh2, EGCG and TMP for 24h, 48h and 72h, respectively.
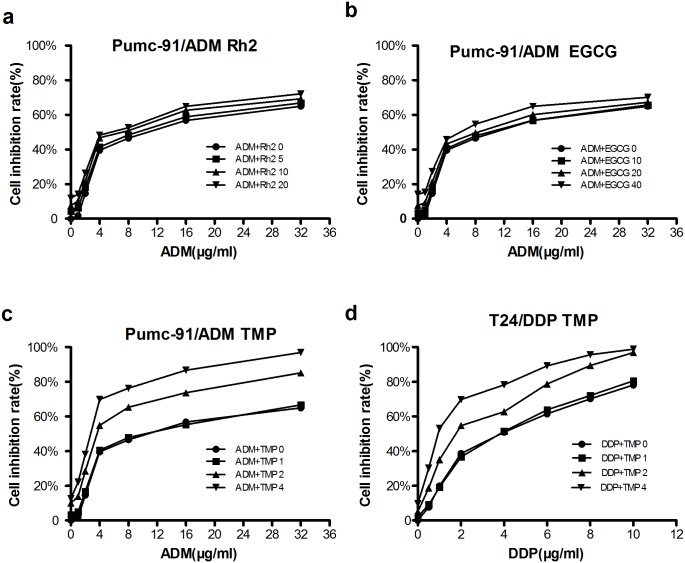
Reversal effects of Rh2, EGCG and TMP on multidrug resistance bladder cancer cells
We found that there was an obvious reversal effect of TMP among the three kinds of herbal medicines in Pumc-91/ADM cells (Fig 3c, S2 Table). But for Rh2 and EGCG (Fig 3a and 3b) (S2 Table), there were not. The IC50 values of ADM in combination with Rh2, EGCG or TMP for Pumc-91/ADM cells were shown in Table 1. Cells without herbal medicine were considered as the control group. Compared to the control group, the IC50 value of ADM in experimental groups treated with TMP was markedly decreased. The reversal fold was 1.05-fold (P>0.01), 2.48-fold (P<0.01) and 4.28-fold (P<0.01) in the groups of TMP at the concentration of 1mM, 2 mM and 4mM respectively. The combinational index (CI) was 0.99, 1.28 and 1.49 in TMP treated groups at the concentration of 1, 2 and 4 mM, respectively (Table 1). Meanwhile, the reversal effect of TMP on T24/DDP cells was also carried out (Fig 3d) (S3 Table). The reversal fold was 1.05-fold (P>0.01), 2.03-fold (P<0.01) and 3.25-fold (P<0.01) in the groups of TMP at the concentration of 1 mM, 2 mM and 4 mM, respectively. The combinational index (CI) was 0.98, 1.32 and 1.49 in TMP treated groups at the concentration of 1, 2 and 4 mM, respectively (Table 2). These results demonstrated that TMP had a reversal effect on multidrug resistance bladder cancer cells, with a dose-dependency.
Fig 3. The reversal effects of three kind of herbal medicines on multidrug resistance bladder cancer cells.
A range from 0 to 32 μg/ml of ADM was added into Pumc-91/ADM cells with (a) Rh2 (0, 5, 10, 20μM), (b) EGCG (0, 10, 20, 40μM) and (c) TMP (0, 1, 2, 4 mM) for 48 h. (d) A range from 0 to 10 μg/ml of DDP was added into T24/DDP cells with TMP (0, 1, 2, 4 mM).
Table 1. Effect of EGCG, Rh2 and TMP on ADM cytotoxicity in Pumc-91/ADM cells.
| Group | IC50(μg/ml) | RF | CI |
|---|---|---|---|
| ADM | 11.56 | 1.00 | |
| ADM+Rh2 5 | 10.21 | 1.13 | 1.00 |
| ADM+Rh2 10 | 8.40 | 1.38 | 1.05 |
| ADM+Rh2 20 | 7.10 | 1.63 | 1.04 |
| ADM+EGCG 10 | 10.94 | 1.06 | 0.98 |
| ADM+EGCG 20 | 9.44 | 1.22 | 0.99 |
| ADM+EGCG 40 | 7.55 | 1.55 | 1.00 |
| ADM+TMP 1 | 11.04 | 1.05 | 0.99 |
| ADM+TMP 2 | 4.67 | 2.48* | 1.28 |
| ADM+TMP 4 | 2.70 | 4.28* | 1.49 |
ADM, adriamycin;TMP, tetramethylpyrazine; Rh2, ginsenoside; EGCG, (−)-Epigallocatechin gallate; IC50, half maximal inhibitory concentration; RF, reversal fold; CI, combine index.
* p<0.01.
Table 2. Effect of TMP on DDP cytotoxicity in T24/DDP cells.
| Group | IC50(μg/ml) | RF | CI |
|---|---|---|---|
| DDP | 3.57 | 1.00 | |
| DDP+TMP 1 | 3.41 | 1.05 | 0.98 |
| DDP+TMP 2 | 1.76 | 2.03* | 1.32 |
| DDP+TMP 4 | 1.10 | 3.25* | 1.49 |
* p<0.01.
Nevertheless, RF was 1.13-fold, 1.38-fold, 1.63-fold (P>0.01) in the presence of Rh2 (5, 10 and 20 μM) and 1.06-fold, 1.22-fold, 1.55-fold (P>0.01) in the presence of EGCG (10, 20 and 40 μM), separately. The combinational index (CI) was 1.00, 1.05, 1.04 in Rh2 groups at the concentration of 5, 10, 20 μM and 0.98, 0.99, 1.00 in EGCG groups at the concentration of 10, 20, 40 μM, respectively (Table 1). These results indicated that Rh2 and EGCG both had no reversal effect on Pumc-91/ADM cells.
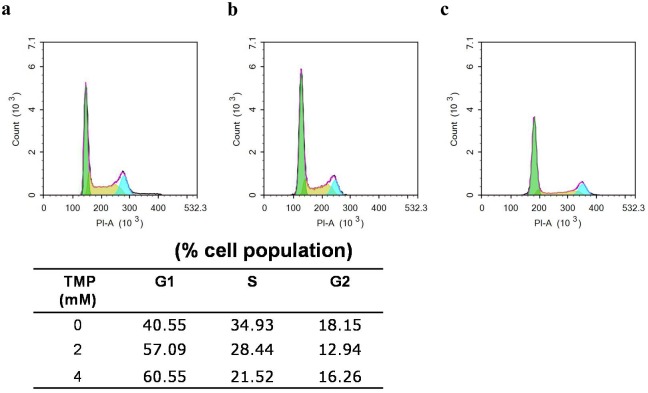
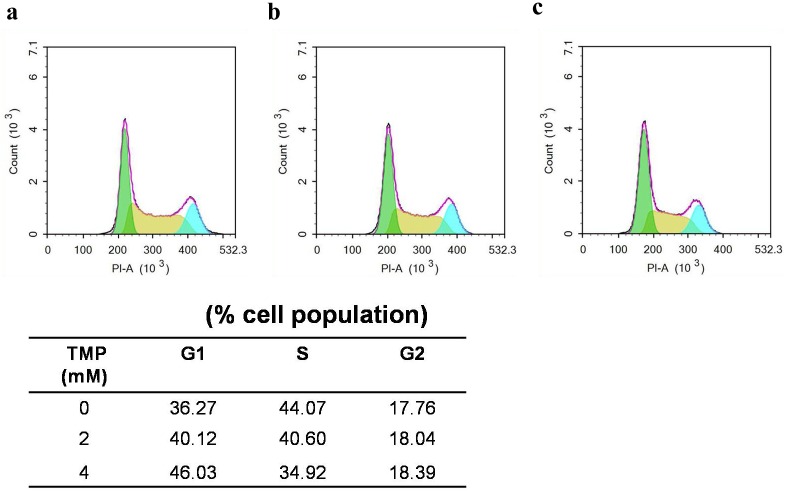
Cell cycle arrested at S phase after treatment with TMP
After Pumc-91/ADM and T24/DDP cells incubated with TMP for 48h, we examined the effect of TMP on cell cycle distributions. Compared to the control group, treatments with TMP (2, 4 mM) in experimental groups resulted in G1 phase arrest (57.09% and 60.55%, respectively, vs. 40.55%) in Pumc-91/ADM cells (Fig 4) and (40.12% and 46.03%, respectively, vs. 36.27%) in T24/DDP cells (Fig 5). These increases in the G1 cell population were accompanied with a decrease of S phase.
Fig 4. Cell cycle analysis of Pumc-91/ADM cells treated with TMP using flow cytometry.
Cells were cultured with different concentrations of TMP (a. 0 mM, b.2 mM, c.4 mM) for 48 h. G1, S and G2 indicated cell cycle phases.
Fig 5. Cell cycle analysis of T24/DDP cells treated with TMP using flow cytometry.
Cells were cultured with different concentrations of TMP (a. 0 mM, b.2 mM, c.4 mM) for 48 h. G1, S and G2 indicated cell cycle phases.
Effects of TMP on mRNA levels of MRP1, LRP, GST, BCL-2 and TOPO-II in multidrug resistance bladder cancer cells
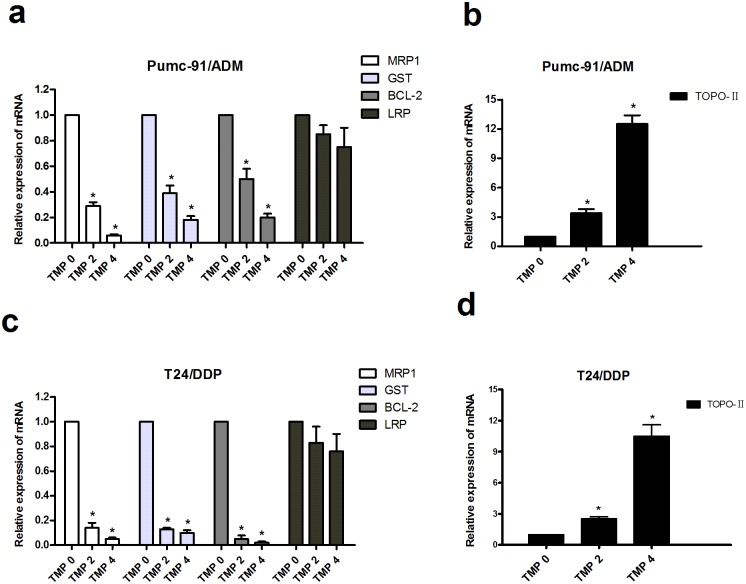
To determine whether MRP1, LRP, GST, BCL-2 and TOPO-II mRNA expression are involved in the reversal effect of TMP on MDR, the levels of MRP1, LRP, GST, BCL-2 and TOPO-II in Pumc-91/ADM and T24/DDP cells treated with different concentrations of TMP were detected by qRT-PCR. TMP treatment caused decreases of MRP1, GST, BCL-2 (Fig 6a and 6c, S4 and S5 Tables) and an increase of TOPO-II (Fig 6b and 6d, S4 and S5 Tables) at the mRNA level in Pumc-91/ADM and T24/DDP cells. However, there was no difference in the protein of LRP after cells treated with TMP. As compared to the control group, the levels of mRNA in TMP groups at concentrations of 2, 4 mM were 0.29-fold, 0.06-fold lower for MRP1 (P<0.01), 0.39-fold, 0.18-fold lower for GST (P<0.01), 0.50-fold, 0.20-fold lower for BCL-2 (P<0.01) and 0.85-fold, 0.75-fold lower for LRP (P>0.01) in Pumc-91/ADM cells (Fig 6a), respectively. There were prominent decreases accompanied by a dose dependency in TMP treated groups. In T24/DDP cells, the levels of mRNA in TMP groups at concentrations of 2, 4 mM were 0.14-fold, 0.01-fold lower for MRP1 (P<0.01), 0.13-fold, 0.10-fold lower for GST (P<0.01), 0.01-fold, 0.02-fold lower for BCL-2 (P<0.01) and 0.83-fold, 0.76-fold lower for LRP (P>0.01) (Fig 6c). However, the expression of TOPO-II in TMP groups was increased. Compared to the control group, the levels of TOPO-II mRNA in TMP groups at concentrations of 2, 4 mM were 3.4-fold, 12.5-fold higher in Pumc-91/ADM cells and 2.5-fold, 10.5-fold higher at concentrations of 2, 4 mM in T24/DDP cells, respectively (Fig 6b and 6d).
Fig 6. The relative expression of mRNA levels in Pumc-91/ADM and T24/DDP cells.
Cells were treated with different concentrations of TMP (0, 2, 4 mM) for 48 h. The group in the absence of TMP was considered as the control group. Results were expressed as a ratio of target gene to housekeeping gene (GAPDH). *p<0.01 compared with the control group. a, b. The mRNA levels of MRP1, GST, BCL-2, LRP and TOPO-IIin Pumc-91/ADM cells. c, d. The mRNA levels of MRP1, GST, BCL-2, LRP and TOPO-II in T24/DDP cells.
Effects of TMP on the protein levels of MRP1, LRP, GST, BCL-2 and TOPO-II in multidrug resistance bladder cancer cells
To investigate whether the mRNA levels of MRP1, LRP, GST, BCL-2 and TOPO-II in Pumc-91/ADM and T24/DDP cells were related to their protein expression of cells treated with TMP (2, 4 mM), immunefluorescence assay was carried out. Compared to the control group, the protein expressions of MRP1, GST, BCL-2 were significantly decreased in TMP groups (2, 4 mM) in accordance with their mRNA levels with a dose-dependency (Figs 7 and 8). But for TOPO-II, it was demonstrated that TMP could enhance its expression (Figs 7 and 8). With the concentrations of TMP increasing, the level of TOPO-II increased gradually in comparison to the untreated cells. But for LRP, there was no difference between the control group and TMP treated groups (2, 4 mM). These results indicated that TMP could down-regulate the expression of MRP1, GST, BCL-2 and up-regulate the expression of TOPO-II.
Fig 7. The expression of MRP1, LRP, GST, BCL-2 and TOPO-II at the protein levels examined by immunofluorescence assay in Pumc-91/ADM cells.
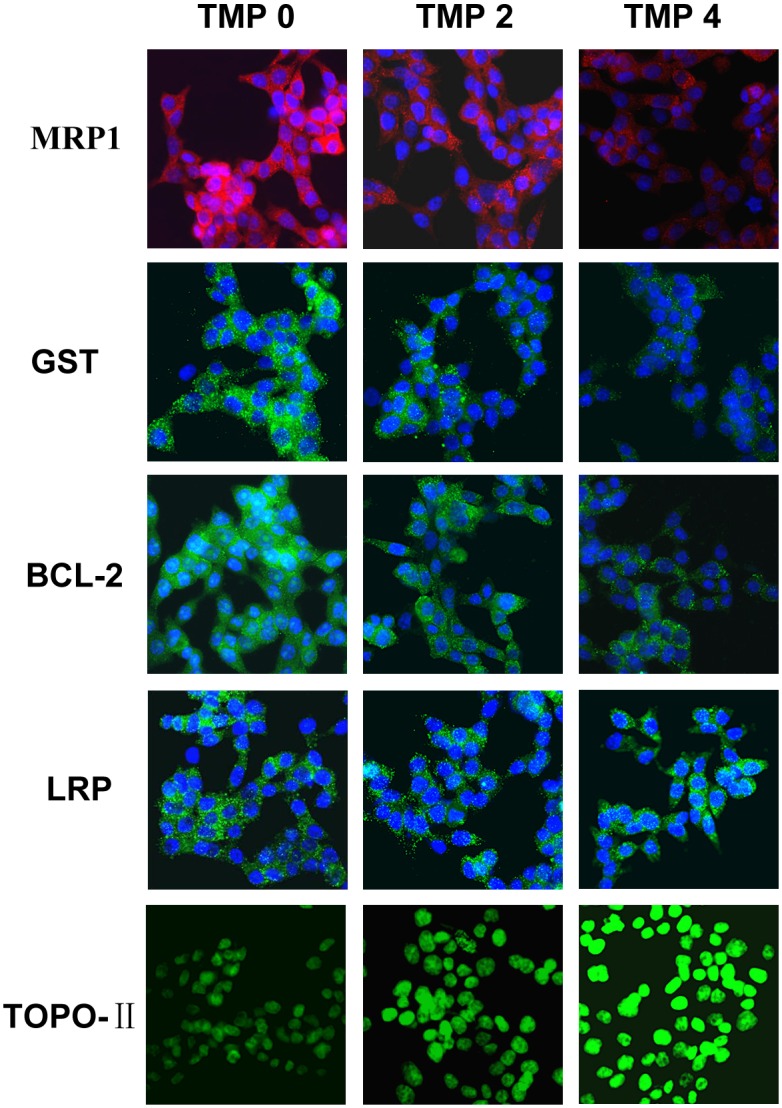
Cells were treated with different concentrations of TMP (0, 2, 4 mM) for 48 h. The group in the absence of TMP was considered as the control group (magnification, ×400).
Fig 8. The expression of MRP1, LRP, GST, BCL-2 and TOPO-II at the protein levels examined by immunofluorescence assay in T24/DDP cells.
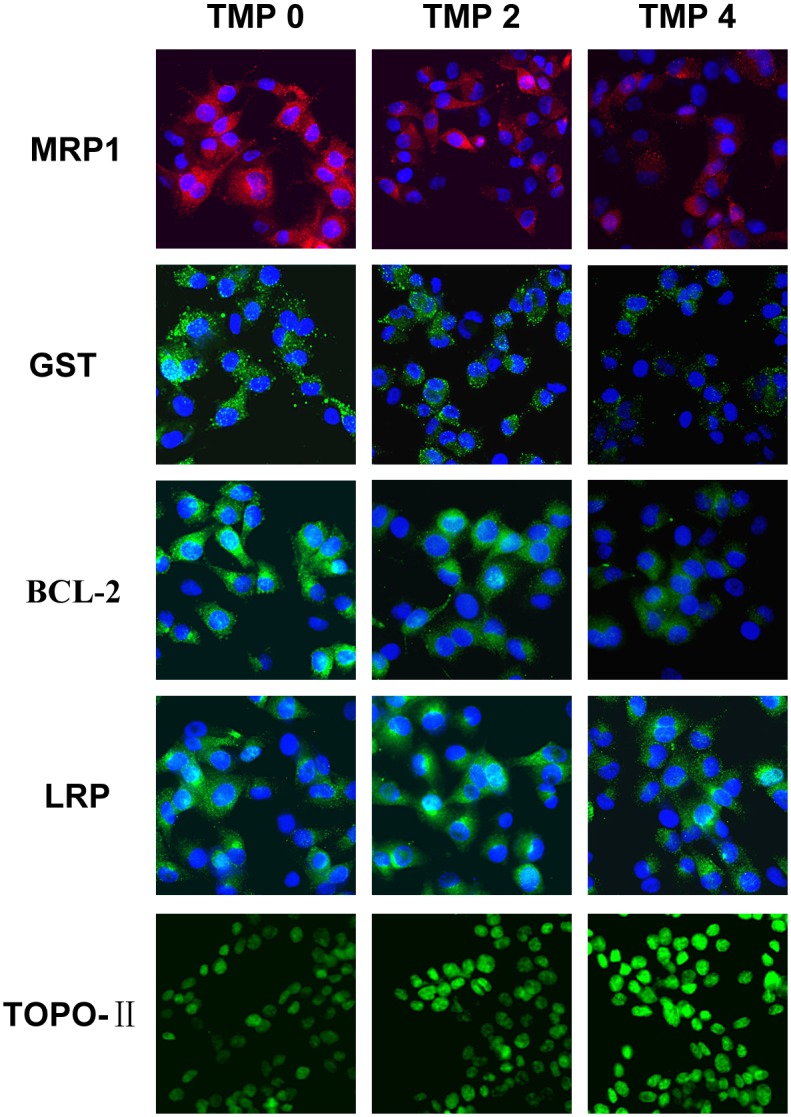
Cells were treated with different concentrations of TMP (0, 2, 4 mM) for 48 h. The group in the absence of TMP was considered as the control group (magnification, ×400).
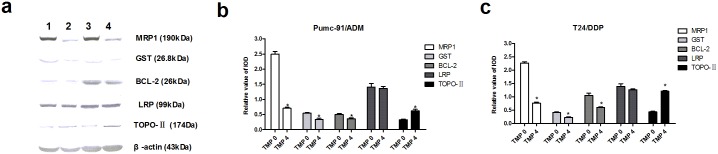
In order to quantify levels of these proteins, western blotting was performed on MRP1, GST, BCL-2, LRP and TOPO-II (Fig 9, S6 Table). The expression of MRP1, GST, BCL-2 was 0.28 fold, 0.62 fold and 0.72 fold lower in the TMP group than in the control group in Pumc-91/ADM cells (P<0.01), respectively. While the expression of MRP1, GST, BCL-2 was 0.34 fold, 0.56 fold and 0.57 fold lower in the TMP group than in the control group in T24/DDP cells (P<0.01). In contrast, TOPO-II expression was 1.9 fold and 2.75 fold higher in the TMP group compared to that in the control group in Pumc-91/ADM and T24/DDP cells, respectively (P<0.01). Whereas, there was no difference for LRP expression between the TMP groups and the control group in Pumc-91/ADM and T24/DDP cells.
Fig 9. The expression of MRP1, GST, BCL-2, LRP and TOPO-II at the protein levels examined by western blot in Pumc-91/ADM and T24/DDP cells.
a. electrophoregram. 1——Pumc-91/ADM TMP0, 2——Pumc-91/ADM TMP4, 3——T24/DDP TMP0, 4——T24/DDP TMP4. b, c. bar graph. *p<0.01 compared with the control group.
Discussion
Bladder cancer is one of the most important epithelial neoplasms worldwide. Patients with bladder cancer always show advanced stage disease in diagnosis, or relapse, invasion and dissemination after first-line therapy, causing a poor prognosis [26]. Adjuvant chemotherapy after surgery has been considered the major strategy for bladder cancer. Nevertheless, the main problem limiting the success of chemotherapy is the development of multidrug resistance [27,28]. Therefore, a combination therapy based on the synergistic effect between drugs has been used to overcome drug resistance. Herbal medicines are significant agents with pharmaceutical potential [29]. Modern pharmacological experiments have confirmed that the use of herbal medicines becomes increasingly popular, especially in cancer patients who exhibit chemotherapy resistance [30]. This approach may be a complementary and alternative therapy for chemotherapy. In consideration of the narrow therapeutic window of chemotherapy drugs, synergistic effect may improve therapeutic effects and reverse drug resistance because of the long-term chemotherapy.
Recently, many groups have reported synergistic anti-cancer effect in combination treatment with chemotherapeutic agents and a natural compound in vitro and in vivo cancer models [31,32]. It was reported that oral administration of Rh2 could inhibited the growth of ovarian xenografts in nude mice [33]. When Rh2 and cisplatin were intravenously administered to nude mice bearing ovarian xenografts, a synergistic effect was observed [34]. Synergy effect was also reported when murine hepatoma, sarcoma and melanoma models were treated with Rh2 and paclitaxel or mitoxantrone [35]. EGCG is synergistically cytotoxic to human cancer cells by modulating P-glycoprotein and the estrogen receptor [36,37]. It was also reported that EGCG could reverse cisplatin resistance through down regulating Axl and Tyro3 in human lung cancer cells [38]. TMP as one of the major bioactive components purified from the Chinese herb Ligusticum wallichii Franch., has been widely used in the treatment of cardiovascular and cerebral diseases in China [39]. It is reported that TMP acts as an anti-inflammatory agent in a rat asthma model [40]. Recently, many researchers have focused on the anti-tumorigenic activity in various cancer cells. Fu et al showed that TMP affected the growth and migration of the glioma cell line by the inhibition of calcium influx [41]. Similarly, it was confirmed that TMP could inhibit melanoma metastasis in vivo partly through suppressing the activity of vascular endothelial growth factor (VEGF) [42]. Therefore, TMP may constitute a potentially effective option for the treatment of tumors. One report demonstrated that TMP was capable of reversing the MDR of BEL-7402/ADM cells in response to ADM [21]. However, to the best of our knowledge, our research was the first time to detect the reversal effect of Rh2, EGCG and TMP in multidrug resistance bladder cancer cell line. In the present study, EGCG and Rh2 had no synergistic effect on bladder cancer. But for TMP, it was demonstrated that TMP was capable of enhancing the cytotoxicity of anticancer drugs by reversing MDR of Pumc-91/ADM cells in response to ADM. The reversal effect of TMP was also confirmed in T24/DDP cells. Compared to the control group, the IC50 of ADM and DDP was remarkably decreased with the concentration of TMP increasing, indicating that the reversal effect of TMP occurred with a dose-dependency. The exposure of Pumc-91/ADM and T24/DDP cells to TMP resulted in cell cycle arrest at G1 phase accompanied by a decrease of cell numbers in S phase. The important feature of cancer cells is that uncontrolled cell proliferation arises from defects throughout the cell cycle progression at G1, G2, S and mitotic phases. Cell cycle checkpoints, including a network of protein kinase signaling pathways, protect the cells from DNA damage induced by chemotherapeutics and provide the cells appropriate time to repair the damages [43]. Therefore, defects in cell cycle checkpoints could cause carcinogenesis and the development of drug resistance. Arrest of cell cycle progression at G2 phase generally gave the cells an opportunity to protect their viability after drug treatment [44,45]. In our study, cell cycle arrested at G1 phase inhibited uncontrolled cell proliferation and avoided the cell repairation for drug damage.
Drug resistance to chemotherapeutics occurs through several drug-metabolizing. Multi-drug resistance protein1 (MRP1) as an important ATP binding cassette transporter protein, affects the intracellular drug concentration through the alteration of drug influx or efflux. The overexpression of MRP1 can decrease intracellular concentration and cellular cytotoxicity of anticancer drug, such as adriamycin, vinblastine, mitoxantrone and etoposide [46–49]. GST has enzymatic activities with detoxification. It could decrease the concentrations of anticancer drugs through the GSH-conjugate export pump [50,51]. Cell death induced by apoptosis is one of the most common mechanisms of antineoplastic drugs. BCL-2 is considered as a main anti-apoptotic protein. In adriamycin-resistant H69AR human small cell lung cancer cells infected with BCL-2 interfering RNA, the level of BCL-2 decreased and also these cells were more sensitive to daunomycin than that of the parental cells [52]. Topoisomerase-II (TOPO-II) is the main target for many anticarcinogens. Drug resistance to TOPO-II occurs when the activity and sensitivity of the target enzyme TOPO-II are decreased by down-regulation or mutation [53]. Lung resistance protein (LRP) with a molecular weight of 99 kDa was identified as a major vault protein (MVP). It mediates drug resistance by pumping drugs away from intracellular drug targets through exocytotic vesicles or pump molecules [54]. Thus, we analyzed the expression of these drug resistance related proteins in this study. Our research demonstrated that there were obvious decreases in mRNA and protein levels of MRP1, GST, BCL-2 with TMP treatment in Pumc-91/ADM and T24/DDP cells. For TOPO-II, treatment with TMP resulted in an increase expression of TOPO-II in mRNA and protein levels. However, there was no difference in LRP expression between the control group and TMP treated groups. Thus, these dada demonstrated that TMP combined with adriamycin or cisplatin synergistically inhibited the proliferation of Pumc-91/ADM and T24/DDP cells. TMP could reverse the drug resistance of Pumc-91/ADM and T24/DDP cells.
Also, MDR expression and correlation with other receptors, such as Steroid and Xenobiotic Receptor (SXR) has been clearly demonstrated, as well as their correlation with clinical evolution and behavior. Jorge Rioja demonstrated that SXR expression had value as a predictor of survival independent of the standard pathological predictors for patients with invasive bladder cancer [55].
In conclusion, we demonstrated that TMP was able to enhance the cytotoxicity of anticarcinogen in Pumc-91/ADM and T24/DDP cells. Meanwhile, the mechanisms of this reversal effect were explored. TMP could induce accumulation of Pumc-91/ADM and T24/DDP cells at G1 phase in the cell cycle accompanied by a decrease of cell numbers in S phase. Several drug-metabolizing genes and proteins were detected after cells were treated with TMP. Compared to the control group, obvious decreases of MRP1, GST, BCL-2 and an increase of TOPO-II were shown in TMP treated groups at mRNA and protein levels. But for LRP, there was no difference between the control group and TMP groups. We concluded that MRP1, GST, BCL-2 and TOPO-II might be partly responsible for the reversal effects of TMP in Pumc-91/ADM and T24/DDP cells. These findings demonstrated that TMP might be a potential candidate for reversal effect of drug resistance in bladder cancer chemotherapy.
Supporting Information
Pumc-91/ADM cells were treated with Rh2, EGCG and TMP at 37°C for 24h, 48h and 72h, respectively.
(TIF)
A range from 0 to 32 μg/ml of ADM was added into Pumc-91/ADM cells with Rh2 (0, 5, 10, 20μM), EGCG (0, 10, 20, 40μM) and TMP (0, 1, 2, 4 mM) for 48 h.
(TIF)
A range from 0 to 10 μg/ml of DDP was added into T24/DDP cells with TMP (0, 1, 2, 4 mM).
(TIF)
Cells were treated with different concentrations of TMP (0, 2, 4 mM) for 48 h. The group in the absence of TMP was considered as the control group.
(TIF)
Cells were treated with different concentrations of TMP (0, 2, 4 mM) for 48 h. The group in the absence of TMP was considered as the control group.
(TIF)
Pumc-91/ADM and T24/DDP cells were treated with TMP at the concentration of 4 mM for 48h. Proteins levels were quantified by Image-Pro Plus v 6.0 software. A, Taget gene. B. β-actin.
(TIF)
Acknowledgments
This study was funded by Beijing Administration of traditional Chinese medical (2014-ZYJ04) and Beijing Key Laboratory of Urinary Cellular Molecular Diagnostics (Z151100001615060).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by Beijing Administration of traditional Chinese medical (2014-ZYJ04) and Beijing Key Laboratory of Urinary Cellular Molecular Diagnostics (Z151100001615060). Man Zhang received the funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65: 5–29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Arantes-Rodrigues R, Pinto-Leite R, Fidalgo-Goncalves L, Palmeira C, Santos L, Colaco A,et al. Synergistic effect between cisplatin and sunitinib malate on human urinary bladder-cancer cell lines. Biomed Res Int. 2013;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009; 374: 239–249. 10.1016/S0140-6736(09)60491-8 [DOI] [PubMed] [Google Scholar]
- 4.Atkins CD, Wrzesinski SH. Radiotherapy plus chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012; 367: 379–381 [DOI] [PubMed] [Google Scholar]
- 5.Krishna R, Mayer LD. Multidrug resistance (mdr) in cancer. Mechanisms, reversal using modulators of mdr and the role of mdr modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000; 11: 265–283. [DOI] [PubMed] [Google Scholar]
- 6.Xu N, Shen C, Luo Y, Xia L, Xue F, Xia Q, et al. Upregulated mir-130a increases drug resistance by regulating runx3 and wnt signaling in cisplatin-treated hcc cell. Biochem Biophys Res Commun. 2012; 425: 468–472. 10.1016/j.bbrc.2012.07.127 [DOI] [PubMed] [Google Scholar]
- 7.Hong L, Piao Y, Han Y, Wang J, Zhang X, Du Y, et al. Zinc ribbon domain-containing 1 (znrd1) mediates multidrug resistance of leukemia cells through regulation of p-glycoprotein and bcl-2. Mol Cancer Ther. 2005; 4: 1936–1942. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002; 53: 615–627. [DOI] [PubMed] [Google Scholar]
- 9.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005; 205: 275–292. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa S, Abe T, Naito S, Kotoh S, Kumazawa J, Hipfner DR, et al. Expression of multidrug resistance-associated protein (mrp), mdr1 and DNA topoisomerase ii in human multidrug-resistant bladder cancer cell lines. Br J Cancer. 1995; 71: 907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann AC, Wild P, Leicht C, Bertz S, Danenberg KD, Danenberg PV, et al. Mdr1 and ercc1 expression predict outcome of patients with locally advanced bladder cancer receiving adjuvant chemotherapy. Neoplasia. 2010; 12: 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serretta V, Pavone C, Allegro R, Vella M, Sanguedolce R, Porcasi R, et al. Correlation between gp-170 expression, prognosis, and chemoresistance of superficial bladder carcinoma. J Cancer Res Clin Oncol. 2003; 129: 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tada Y, Wada M, Migita T, Nagayama J, Hinoshita E, Mochida Y, et al. Increased expression of multidrug resistance-associated proteins in bladder cancer during clinical course and drug resistance to doxorubicin. Int J Cancer. 2002; 98: 630–635. [DOI] [PubMed] [Google Scholar]
- 14.Diestra JE, Condom E, Del Muro XG, Scheffer GL, Perez J, Zurita AJ, et al. Expression of multidrug resistance proteins p-glycoprotein, multidrug resistance protein 1, breast cancer resistance protein and lung resistance related protein in locally advanced bladder cancer treated with neoadjuvant chemotherapy: Biological and clinical implications. J Urol. 2003; 170: 1383–1387. [DOI] [PubMed] [Google Scholar]
- 15.Patel J, Mitra AK. Strategies to overcome simultaneous p-glycoprotein mediated efflux and cyp3a4 mediated metabolism of drugs. Pharmacogenomics. 2001; 2: 401–415. [DOI] [PubMed] [Google Scholar]
- 16.Kim HS, Lee EH, Ko SR, Choi KJ, Park JH, Im DS. Effects of ginsenosides rg3 and rh2 on the proliferation of prostate cancer cells. Arch Pharm Res. 2004; 27: 429–435. [DOI] [PubMed] [Google Scholar]
- 17.Fei XF, Wang BX, Tashiro S, Li TJ, Ma JS, Ikejima T. Apoptotic effects of ginsenoside rh2 on human malignant melanoma a375-s2 cells. Acta Pharmacol Sin. 2002; 23: 315–322. [PubMed] [Google Scholar]
- 18.Lee KY, Park JA, Chung E, Lee YH, Kim SI, Lee SK. Ginsenoside-rh2 blocks the cell cycle of sk-hep-1 cells at the g1/s boundary by selectively inducing the protein expression of p27kip1. Cancer Lett. 1996; 110: 193–200. [DOI] [PubMed] [Google Scholar]
- 19.Bode AM, Dong Z. Targeting signal transduction pathways by chemopreventive agents. Mutat Res. 2004; 555: 33–51. [DOI] [PubMed] [Google Scholar]
- 20.Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: Evidence from epidemiological and laboratory studies. Toxicol Lett. 2004; 150: 43–56. [DOI] [PubMed] [Google Scholar]
- 21.Wang XB, Wang SS, Zhang QF, Liu M, Li HL, Liu Y, et al. Inhibition of tetramethylpyrazine on p-gp, mrp2, mrp3 and mrp5 in multidrug resistant human hepatocellular carcinoma cells. Oncol Rep. 2009; 23: 211–215. [PubMed] [Google Scholar]
- 22.Zhang Min, Jin Sheng, Zhang Man. The establishment and biological characteristics evaluation of human multidrug-resistant bladder cancer Pumc-91/ADM cell line. Journal of Medical Research. 2009; 38(1):70–72. [Google Scholar]
- 23.Zhao Man, Zhang Man, Ma Ping. Establishment of cisplatin-multidrug resistance cell line of human bladder cancer T24 and its biological characteristics. Immunoassays and Clinical Medicine. 2015; 22(4):338–341. [Google Scholar]
- 24.Huang F, Wu XN, Chen J, Wang WX, Lu ZF. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp Ther Med. 2014; 7: 1611–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods. 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs BL, Lee CT, Montie JE. Bladder cancer in 2010: How far have we come? CA Cancer J Clin. 2010; 60: 244–272. 10.3322/caac.20077 [DOI] [PubMed] [Google Scholar]
- 27.Siddik ZH. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003; 22: 7265–7279. [DOI] [PubMed] [Google Scholar]
- 28.Koberle B, Tomicic MT, Usanova S, Kaina B. Cisplatin resistance: Preclinical findings and clinical implications. Biochim Biophys Acta. 2010; 1806: 172–182. 10.1016/j.bbcan.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 29.Xu HW, Xu L, Hao JH, Qin CY, Liu H: Expression of p-glycoprotein and multidrug resistance-associated protein is associated with multidrug resistance in gastric cancer. J Int Med Res. 2010; 38: 34–42. [DOI] [PubMed] [Google Scholar]
- 30.Cervello M, Montalto G. Cyclooxygenases in hepatocellular carcinoma. World J Gastroenterol. 2006; 12: 5113–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoshyomn S, Manske GC, Lew SM, Wald SL, Penar PL. Synergistic action of genistein and cisplatin on growth inhibition and cytotoxicity of human medulloblastoma cells. Pediatr Neurosurg. 2000; 33: 123–131. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H, Zhu W, Xie P, Li H, Zhang X, Sun X, et al. A phase i study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage iii non-small-cell lung cancer. Radiother Oncol. 2014; 110: 132–136. 10.1016/j.radonc.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 33.Nakata H, Kikuchi Y, Tode T, Hirata J, Kita T, Ishii K, et al. Inhibitory effects of ginsenoside rh2 on tumor growth in nude mice bearing human ovarian cancer cells. Jpn J Cancer Res. 1998; 89: 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kikuchi Y, Sasa H, Kita T, Hirata J, Tode T, Nagata I. Inhibition of human ovarian cancer cell proliferation in vitro by ginsenoside rh2 and adjuvant effects to cisplatin in vivo. Anticancer Drugs. 1991; 2: 63–67. [DOI] [PubMed] [Google Scholar]
- 35.Jia WW, Bu X, Philips D, Yan H, Liu G, Chen X, et al. Rh2, a compound extracted from ginseng, hypersensitizes multidrug-resistant tumor cells to chemotherapy. Can J Physiol Pharmacol. 2004; 82: 431–437. [DOI] [PubMed] [Google Scholar]
- 36.Chisholm K, Bray BJ, Rosengren RJ. Tamoxifen and epigallocatechin gallate are synergistically cytotoxic to mda-mb-231 human breast cancer cells. Anticancer Drugs. 2004; 15: 889–897. [DOI] [PubMed] [Google Scholar]
- 37.Qian F, Wei D, Zhang Q, Yang S. Modulation of p-glycoprotein function and reversal of multidrug resistance by (-)-epigallocatechin gallate in human cancer cells. Biomed Pharmacother. 2005; 59: 64–69. [DOI] [PubMed] [Google Scholar]
- 38.Kim KC, Lee C. Reversal of cisplatin resistance by epigallocatechin gallate is mediated by downregulation of axl and tyro 3 expression in human lung cancer cells. Korean J Physiol Pharmacol. 2014; 18: 61–66. 10.4196/kjpp.2014.18.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li WM, Liu HT, Li XY, Wu JY, Xu G, Teng YZ,et al. The effect of tetramethylpyrazine on hydrogen peroxide-induced oxidative damage in human umbilical vein endothelial cells. Basic Clin Pharmacol Toxicol. 2009; 106: 45–52. 10.1111/j.1742-7843.2009.00470.x [DOI] [PubMed] [Google Scholar]
- 40.Xiong L, Fang ZY, Tao XN, Bai M, Feng G. Effect and mechanism of ligustrazine on th1/th2 cytokines in a rat asthma model. Am J Chin Med. 2007; 35: 1011–1020. [DOI] [PubMed] [Google Scholar]
- 41.Fu YS, Lin YY, Chou SC, Tsai TH, Kao LS, Hsu SY, et al. Tetramethylpyrazine inhibits activities of glioma cells and glutamate neuro-excitotoxicity: Potential therapeutic application for treatment of gliomas. Neuro Oncol. 2008; 10: 139–152. 10.1215/15228517-2007-051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen L, Lu Y, Wu JM, Xu B, Zhang LJ, Gao M, et al. Ligustrazine inhibits b16f10 melanoma metastasis and suppresses angiogenesis induced by vascular endothelial growth factor. Biochem Biophys Res Commun. 2009; 386: 374–379. 10.1016/j.bbrc.2009.06.042 [DOI] [PubMed] [Google Scholar]
- 43.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S: Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004; 73: 39–85. [DOI] [PubMed] [Google Scholar]
- 44.Hapke G, Yin MB, Rustum YM. Targeting molecular signals in chk1 pathways as a new approach for overcoming drug resistance. Cancer Metastasis Rev. 2001; 20: 109–115. [DOI] [PubMed] [Google Scholar]
- 45.Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S, et al. Chk1 mediates s and g2 arrests through cdc25a degradation in response to DNA-damaging agents. J Biol Chem. 2003; 278: 21767–21773. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa Y, Nagai J, Okada Y, Sato K, Yumoto R, Takano M. Function and expression of atp-binding cassette transporters in cultured human y79 retinoblastoma cells. Biol Pharm Bull. 2010; 33: 504–511. [DOI] [PubMed] [Google Scholar]
- 47.Chen ZS, Furukawa T, Sumizawa T, Ono K, Ueda K, Seto K,et al. Atp-dependent efflux of cpt-11 and sn-38 by the multidrug resistance protein (mrp) and its inhibition by pak-104p. Mol Pharmacol. 1999; 55: 921–928. [PubMed] [Google Scholar]
- 48.Khodadadian M, Leroux ME, Auzenne E, Ghosh SC, Farquhar D, Evans R, et al. Mrp- and bcl-2-mediated drug resistance in human sclc: Effects of apoptotic sphingolipids in vitro. Lung Cancer. 2009; 66: 48–57. 10.1016/j.lungcan.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 49.Gruber BM, Bubko I, Krzyszton-Russjan J, Anuszewska EL. Synergistic action of doxorubicin and sulindac in human cervix carcinoma cells—studies on possible mechanisms. Med Sci Monit. 2009; 16: BR45–51. [PubMed] [Google Scholar]
- 50.Cullen KJ, Newkirk KA, Schumaker LM, Aldosari N, Rone JD, Haddad BR. Glutathione s-transferase pi amplification is associated with cisplatin resistance in head and neck squamous cell carcinoma cell lines and primary tumors. Cancer Res. 2003; 63: 8097–8102. [PubMed] [Google Scholar]
- 51.Sakamoto M, Kondo A, Kawasaki K, Goto T, Sakamoto H, Miyake K, et al. Analysis of gene expression profiles associated with cisplatin resistance in human ovarian cancer cell lines and tissues using cdna microarray. Hum Cell. 2001; 14: 305–315. [PubMed] [Google Scholar]
- 52.Palaniyandi K, Zhao Q, Chang XB. Infection of h69ar cells with retroviral particles harboring interfering rnai significantly reduced the multidrug resistance of these small cell lung cancer cells. Int J Biochem Mol Biol. 2011; 2: 155–167. [PMC free article] [PubMed] [Google Scholar]
- 53.Kellner U, Sehested M, Jensen PB, Gieseler F, Rudolph P. Culprit and victim—DNA topoisomerase ii. Lancet Oncol. 2002; 3: 235–243. [DOI] [PubMed] [Google Scholar]
- 54.Scheffer GL, Schroeijers AB, Izquierdo MA, Wiemer EA, Scheper RJ. Lung resistance-related protein/major vault protein and vaults in multidrug-resistant cancer. Curr Opin Oncol. 2000; 12: 550–556. [DOI] [PubMed] [Google Scholar]
- 55.Rioja J, Bandres E, Rosell Costa D, Rincon A, Lopez I, Zudaire Bergera JJ, et al. Association of steroid and xenobiotic receptor (sxr) and multidrug resistance 1 (mdr1) gene expression with survival among patients with invasive bladder carcinoma. BJU Int. 2010; 107: 1833–1838. 10.1111/j.1464-410X.2010.09653.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pumc-91/ADM cells were treated with Rh2, EGCG and TMP at 37°C for 24h, 48h and 72h, respectively.
(TIF)
A range from 0 to 32 μg/ml of ADM was added into Pumc-91/ADM cells with Rh2 (0, 5, 10, 20μM), EGCG (0, 10, 20, 40μM) and TMP (0, 1, 2, 4 mM) for 48 h.
(TIF)
A range from 0 to 10 μg/ml of DDP was added into T24/DDP cells with TMP (0, 1, 2, 4 mM).
(TIF)
Cells were treated with different concentrations of TMP (0, 2, 4 mM) for 48 h. The group in the absence of TMP was considered as the control group.
(TIF)
Cells were treated with different concentrations of TMP (0, 2, 4 mM) for 48 h. The group in the absence of TMP was considered as the control group.
(TIF)
Pumc-91/ADM and T24/DDP cells were treated with TMP at the concentration of 4 mM for 48h. Proteins levels were quantified by Image-Pro Plus v 6.0 software. A, Taget gene. B. β-actin.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.