Abstract
Previous studies from our lab have demonstrated that mild cognitive impairments identified early in life are predictive of cognitive deficits that develop with age, suggesting that enhancements in cognition at an early age can provide a buffer against age-related cognitive decline. Environmental enrichment has been shown to improve learning and memory in the rodent, but the impact of enrichment on synaptic plasticity and the molecular mechanisms behind enrichment are not completely understood. To address these unresolved issues, we have housed 2-month old rats in environmentally enriched (EE), socially enriched (SE), or standard housing (SC) and conducted tests of learning and memory formation at various time intervals. Here we demonstrate that animals that have been exposed to one month of social or environmental enrichment demonstrate enhanced learning and memory relative to standard housed controls. However, we have found that after 4 months EE animals perform better than both SE and SC groups and demonstrate an enhanced hippocampal LTP. Our results demonstrate that this LTP is dependent on mGluR5 signaling, activation of ERK and mTOR signaling cascades, and sustained phosphorylation of p70s6 kinase, thus providing a potential target mechanism for future studies of cognitive enhancement in the rodent.
Keywords: Young rats, Environmental enrichment, Metabotropic glutamate receptor, Morris water maze, Object recognition, p70s6 kinase
1. Introduction
Behavioral and pharmacological treatments for cognitive impairments associated with the aging process are becoming a growing focus of concern as the elderly population continues to expand worldwide. Previous studies from our lab demonstrate that cognitive impairment can be identified in young rats using cognitively demanding versions of the Morris water maze and novel object recognition tasks, and that these impairments are predictive of cognitive impairments at 12 months of age (Hullinger & Burger, 2015). This suggests that interventions focused on improving cognition from an early age may provide a buffer against age related cognitive decline.
One potential noninvasive technique to ensure cognitive training from a young age is the use of environmental enrichment. Environmental enrichment has been used to improve behavioral and cellular consequences of various neurological and psychiatric disorders in animal models, including Parkinson’s disease, stroke, traumatic brain injury, epilepsy, multiple sclerosis, depression, schizophrenia, and autism spectrum disorders (Burrows, McOmish, Buret, Van den Buuse, & Hannan, 2015; Hannan, 2014; Mazarakis et al., 2014; Pang & Hannan, 2013). In addition, environmental enrichment has been shown to improve learning and memory retention in behavioral tasks such as fear avoidance conditioning, the radial maze, and the Morris water maze (Harati et al., 2011; Harburger, Lambert, & Frick, 2007; Mora-Gallegos et al., 2015; Novkovic, Mittmann, & Manahan-Vaughan, 2015; Sampedro-Piquero, Arias, & Begega, 2014). However, the mechanisms behind environmental enrichment remain unclear. Furthermore, studies suggest that environmental enrichment may have differential effects on behavior and molecular mechanisms in young and aged rodents (Harburger et al., 2007; Mora-Gallegos et al., 2015; Sampedro-Piquero, Begega, Zancada-Menendez, Cuesta, & Arias, 2013). In addition, the impact of environmental enrichment on synaptic plasticity remains disputed; previous studies have shown that enrichment enhances hippocampal LTP ((Kumar, Rani, Tchigranova, Lee, & Foster, 2012; Malik & Chattarji, 2012; Novkovic et al., 2015) whereas other studies have found no effect (Eckert, Bilkey, & Abraham, 2010; Foster & Dumas, 2001). These contradictory results may arise because there is no set definition of “enrichment” and many variables have been changed in the current literature, including the actual enrichment with toys, ropes, tunnels, and houses but also the number of rats per cage, the size of the cage, enrichment time, and the amount of handling the rats receive.
Previous studies have demonstrated that genes related to memory formation, synaptic plasticity, and protein synthesis may be upregulated by a period of environmental enrichment (Lichti et al., 2014; Novkovic et al., 2015; Sampedro-Piquero et al., 2014) including group 1 metabotropic glutamate receptors (mGluR1/5) (Melendez, Gregory, Bardo, & Kalivas, 2004). Indeed, the role of metabotropic glutamate receptor subtype 5 (mGluR5) in learning and memory is well established; mice lacking mGluR5 show impaired learning on the Morris water maze and contextual fear conditioning tasks (Lu et al., 1997), and the daily application of the mGluR5 antagonist 2-methyl-6-(phenylethyl)pyridine (MPEP) causes impairments in working and reference memory in rats (Manahan-Vaughan & Braunewell, 2005). Furthermore, mice lacking mGluR5 do not experience the same increases in dendritic branch formation or hippocampal BDNF levels as WT controls following a period of environmental enrichment (Burrows et al., 2015). Therefore, one potential mechanism by which enrichment may improve learning and memory and synaptic plasticity is through an enhancement of mGluR5 activity and subsequent activation downstream signaling pathways such as the ERK and mTOR signaling pathways.
To avoid the confounding effects changing multiple variables in the enriched condition, three housing conditions were used for this study: environmentally enriched (EE), socially enriched (SE), and standard controls (SC). We found that one month of EE or SE improves learning and memory in 2-month old rats, whereas after 4 months environmentally enriched rats demonstrate improved performance relative to both SE and SC groups. Furthermore, we demonstrate that at this time point, EE animals demonstrate enhanced hippocampal LTP, and that this LTP is dependent on mGluR5 signaling, activation of the ERK and mTOR signaling cascades, and sustained phosphorylation of p70s6 kinase (p70s6k). Taken together, these results provide both a behavioral and pharmaceutical target for enhancing cognition from a young age in order to provide a buffer against age-related cognitive decline.
2. Methods
2.1. Animal subjects
32 2-month old F344 rats from the National Institute of Aging (NIA) rodent colony were used in this study. These 2-month old young adult rats were selected in order to investigate whether enrichment could improve cognition from a young age. Specifically, F344 from the NIA were selected to conduct experiments in parallel with studies of enrichment in aged rats (unpublished data). All animals had free access to water and food. 12 h dark and light cycles were maintained. Behavioral tests were given during the dark cycle. All procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee and were conducted in accordance with the U.S. National Institutes of Health ‘Guide for the Care and Use of Laboratory Animals’.
2.2. Environmental enrichment paradigm
Three housing conditions were used for this study: enriched, social, and standard (Fig. 1). Enriched cages housed 6 rats in 2 × 2 ft plastic cages equipped with PVC pipes, a plastic hut, soft nesting material, and various objects to stimulate exploration. The location of these objects was changed once a week and novel objects introduced weekly to maintain novelty. Social cages housed 6 rats in 2 × 2 ft plastic cages with no objects other than normal bedding, and served as a control to determine whether social enrichment alone was beneficial for the animals. Standard cages housed 2 rats in standard shoebox cages provided by university laboratory animal resources. Upon arrival, animals were divided randomly such that each age group consisted of 6 enriched rats, 6 social rats, and 4 standard housed rats. Animals were housed in their respective environments and tested at various time points to determine the length of enrichment necessary to improve learning and memory. Time points of 1 month and 4 month were selected in order to determine the minimum time necessary for environmental enrichment to improve cognition based upon the varying lengths tested in previous studies (Eckert et al., 2010; Harburger et al., 2007; Malik & Chattarji, 2012; Mora-Gallegos et al., 2015).
Fig. 1.
Environmental enrichment consisted of 6 animals housed in a 2 × 2′ cage with various objects and enriching toys changed weekly. Social enrichment cages were identical, except they lacked toys.
2.3. Behavior experiments
2.3.1. Novel object recognition
We used a two-day version of the Novel Object Recognition paradigm to assess learning and memory in rats following a period of enrichment. On the first day of the paradigm (training day), rats were trained on the locations of two identical objects. Miniature flamingo figurines were placed in corner locations of a square arena (40 × 40 cm) with corncob bedding spread 5 cm thick from the floor. Rats were individually placed in the arena and given six minutes to explore. In between each animal, the bedding was stirred and the toys cleaned with 70% ethanol to minimize olfactory cues. Testing of object recognition memory occurred 24 h after training. During testing, one flamingo was replaced with a finch figurine, and rats were again placed in the cage and given six minutes to explore freely. Two cohorts of rats were tested at one month, and then at 4 months using different objects (Cohort 1; EE, n = 6; SE, n = 6; SC, n = 4. Cohort 2: EE, n = 6; SE, n = 6; SC, n = 4).
2.3.1.1. Data analysis
All trials on both the Training and Testing days were videotaped and analyzed by an experimenter blind to the identity of the rat, using Videotrack software by ViewPoint Life Sciences (Montreal, CANADA). Total amount of time spent directly sniffing, rubbing, licking, or biting the objects (exploration) was recorded for each animal. The relative exploration time were recorded for each object and expressed as a novelty score (Time Spent (s) Investigating Novel Object/Time Spent (s) Investigating Both Objects in Total). One-way ANOVA with Tukey’s multiple comparison tests was conducted to determine significance of differences in novelty score between enriched, social, and standard rats.
2.3.2. Morris water maze
Rats were placed in a large blue tank (173 cm diameter) filled with room temperature water and trained to use external cues to locate a clear Lucite platform submerged approximately 5 cm beneath the surface of the water. To ensure that all animals had sufficient visual acuity and swimming ability, a single day of visible platform training consisting of 4 trials was conducted at the beginning of the task. In this session, a visible platform was placed in the center of the pool and the animals were dropped from all 4 quadrants and given 90 s to find the platform.
The day following visible platform training, hidden platform training was conducted. On hidden platform trials, the platform was always located in the Southeast quadrant of the pool. The training consisted of 4 trials a day over 7 consecutive days in the 1 month of enrichment condition (cohort 1; only tested at 1 month in MWM: EE, n = 6; SE, n = 6; SC, n = 4), and over 3 consecutive days in the 4 months of enrichment condition (cohort 2; untested at one month in MWM: EE, n = 6; SE, n = 6; SC, n = 4). Animals were tested only once in the MWM (either at 1 month or 4 months) to avoid confounds of training due to long lasting memory for this task. In each trial the animal was dropped from the North, East, South, or West quadrant in a randomized order and given 90 s to find the platform. Upon finding the platform the animal was allowed to sit for 10 s before being removed, towel tried, and placed back in the pool for the next training session. If the animal did not find the platform it was guided to the platform by the experimenter.
Following hidden platform training a probe trial was conducted to test for retention of the platform location. For the probe trial, the platform was removed from the pool and the animal was given 60 s to swim before being removed from the pool, thoroughly dried in the heated cage, and placed back into his home cage.
2.3.2.1. Data analysis
For hidden platform training, the distance traveled before reaching the platform was analyzed and measured using Videotrack software by ViewPoint Life Sciences (Montreal, Canada). Platform crossings in the probe trial were calculated by tallying the number of times each subject entered the platform zone during the 60 s trial. Two-way ANOVA with Bonferroni post hoc tests was conducted on hidden platform training to determine significance of differences between enriched, social, and standard rats on all days of training. One-way ANOVA with Tukey’s multiple comparison tests was conducted on probe trial crossings to determine differences between the three groups.
2.4. Electrophysiology
For extracellular recordings of field excitatory postsynaptic potentials (fEPSP), acute hippocampal slices (400 μm) were prepared from rats as previously described (Gerstein, O’Riordan, Osting, Schwarz, & Burger, 2012) Enameled bipolar platinum–tungsten stimulating electrodes were placed along Schaffer collaterals, and fEPSPs were recorded with ACSF filled recording electrodes (2–4 MOhm) from area CA1 stratum radiatum. Baseline responses were set to an intensity that evoked an fEPSP with a slope of 66% that of the maximum evoked response (O’Riordan et al., 2014). Half-Train theta burst (0.5 TBS) consisted of a total of five bursts (each burst consisting of four stimulations at a frequency of 100 Hz) with an interburst interval of 200 ms.
2.5. Drug treatment
DHPG, MPEP, LY367385, Okadaic Acid (OA), and PF4708671 (PF) were purchased from Tocris Bioscience (Missouri, USA). Rapamycin and U0126 were purchased from Sigma. DHPG (20 mM) and MPEP (4 mM) stock solutions were prepared in H2O. LY367385 (5 mM), Rapamycin (2 mM), OA (30 uM), PF (50 μM), and U0126 (3 mM) stock solutions were prepared in >99.9% dimethyl sulfoxide (DMSO). All solutions were stored in aliquots at −20 °C and used within 2 weeks after preparation.
LTP was induced in standard housed and socially enriched rats by priming with DHPG followed by 0.5 TBS. After collecting a stable baseline, DHPG was applied to the chamber for 10 min (10 μM final concentration), followed by a 20 min washout to ensure return to baseline before the 0.5TBS. MPEP (40 μM), LY367385 (100 μM), and U0126 (30 μM) were applied after collecting a stable baseline 25 min prior to stimulation for 15 min, followed by a 10 min washout. After the drugs were washed out, slices received the 0.5TBS. Rapamycin (200 nM) was applied for 20 min prior to stimulation, and kept on the slices for 20 min after 0.5TBS. Following this 20 min period chambers were returned to ACSF circulation. The p70s6k specific inhibitor PF4708671 (PF: 500 nM) was applied 5 min after 0.5TBS and was kept on the slices for the duration of recording. Okadaic acid (OA: 3 nM) was applied to slices 2 h prior to stimulation and kept on the slices for the entire duration of recording.
After electrophysiological recordings, slices were frozen and saved for Western blot analysis. All numerical data were expressed as mean ± SEM. Data were analyzed by two-way ANOVA (treatment and time) with repeated measures (mixed model) and Bonferroni post hoc tests.
2.6. Western blot analysis
400 μM hippocampal slices were collected, snap frozen in liquid nitrogen, and stored at −80 °C until ready to process. Slices were triturated with a 28.5 gauge insulin needle in RIPA buffer (50 mmol/L Tris, pH 7.8, 150 mmol/L HCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) in the presence of mammalian protease inhibitors (1:100, Sigma, St. Louis, MO) and phosphatase inhibitors (in mmol/L: 10 NaF, 2 Na Vanadate, 4 Na pyrophosphate, 10 b-glycerophosphate). Lysates were spun down at 12,000 rpm for 30 min, and the supernatant was transferred to a clean tube. Protein extract was separated using 4–15% gradient SDS–polyacrylamide gel electrophoresis (PAGE) precast gels from Bio-Rad (Hercules, CA) and then transferred to nitrocellulose membranes using Trans-Blot Turbo Transfer System (Bio- Rad). Primary antibodies used were total mTOR (Cell Signaling #2972, 1:1000), phospho-mTOR (Cell Signaling #2971, 1:1000), total ERK (Cell Signaling #4695, 1:1000), phospho-ERK (Cell Signaling #9101, 1:1000), phospho-p70s6k (Cell Signaling #9205, 1:1000), and GAPDH (Millipore #2302, 1:2000). All primary anti-bodies were diluted in 5% BSA and incubated at 4 °C overnight with shaking. Secondary antibodies used were anti-mouse and anti-rabbit in 5% BSA, all from Santa Cruz Biotechnology. Secondary antibodies were applied for 1 h at room temperature with shaking.
Western blots were developed with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce Protein Research, Rockford, IL) and imaged with the GelDoc-It Imaging Systems by UVP LLC (Upland, CA). Bands were normalized against GAPDH expression and densitometric quantitation of immuno-positive bands was performed using ImageJ software (National Institutes of Health). Two-tailed Student’s t-test was used for statistical analysis using Prism 5 (GraphPad Software, La Jolla CA).
3. Results
3.1. EE improves performance on MWM and NOR
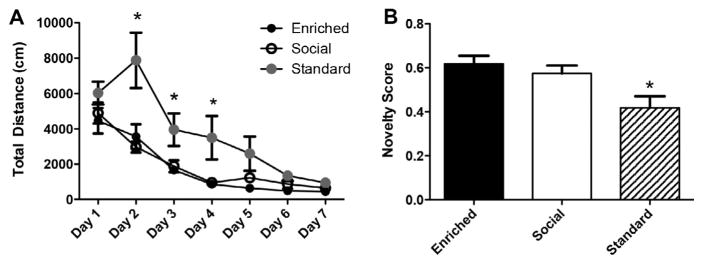
We found that after one month, both EE and SE animals demonstrated improved spatial and object recognition memory relative to standard housed controls. We found that EE and SE groups swam significantly shorter distances in hidden platform training of the MWM (Fig. 2A, repeated measures ANOVA, F(2,13) = 9.874, p < .01). EE and SE rats also demonstrated superior performance in NOR, whereas SC animals performed only at chance levels (50%; Fig. 2B, one-way ANOVA, F(2,12) = 5.925, p = .016). However, at this time point there was no distinction between performance of social and enriched animals, suggesting that both forms of enrichment have beneficial effects on learning and memory for this age group of rats.
Fig. 2.
1 month of social and environmental enrichment enhances learning and memory. (A) SE and EE groups perform equally well in the hidden platform training phase of the MWM, and both groups perform significantly better than SC rats on days 2 and 4 of training. (B) EE and SE groups perform significantly better than SC animals in NOR. (EE, n = 6; SE, n = 6; SC, n = 4).
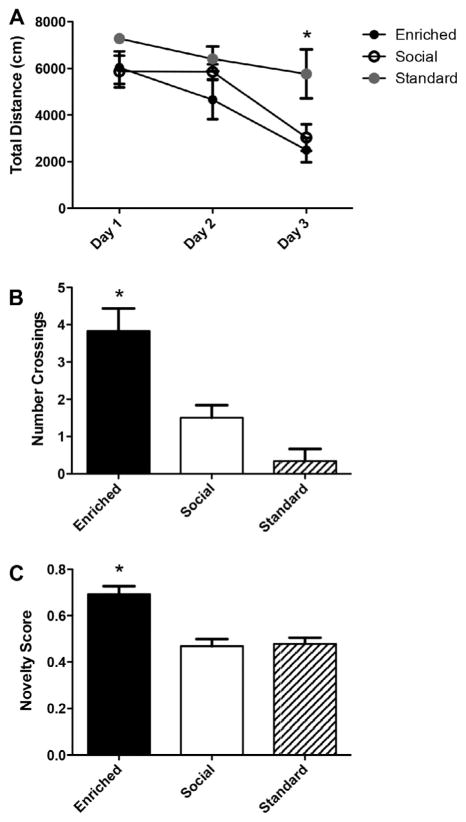
To determine whether social enrichment would be as effective as environmental enrichment over longer periods of time, animals were housed in EE, SE, or SC housing for a period of 4 months. After 4 months of environmental enrichment, animals were tested in MWM and NOR. Since we found that all groups reached asymptote performance after day 4 of MWM training in the one month time point (Fig. 2A), we decided to test a naive cohort of animals just for 3 days at the 4 month time point (Fig. 3A). Again, we found that EE and SE animals performed better in hidden training than SC animals, as demonstrated by decreased distance swam in hidden platform training (Fig. 3A, repeated-measures ANOVA, F(2,12) = 5.013, p < .05). However, during the probe trial following the last hidden trial on day 3, EE animals demonstrated significantly more platform crossings than both SE and SC animals (Fig. 3B, one-way ANOVA, F(2,12) = 11.76, p = .0015), suggesting a more precise memory of the platform location is formed in the environmentally enriched animals. Furthermore, we found that after 4 months the EE animals also scored significantly higher in NOR than both SE and SC groups (Fig. 3C, one-way ANOVA, F(2,29) = 16.47, p < .0001).
Fig. 3.
After 4 months of enrichment, EE animals display superior learning and memory compared to SE and SC groups. (A) EE and SE animals swim significantly shorter distances than SC groups during the hidden platform phase of MWM. (B) EE rats display significantly more platform crossings during the probe trial than SE and SC groups. (EE, n = 6; SE, n = 6; SC, n = 4) (C) EE animals outperform both SE and SC groups in NOR. (EE, n = 12; SE, n = 12; SC, n = 8).
3.2. EE enhances mGluR5-dependent hippocampal LTP
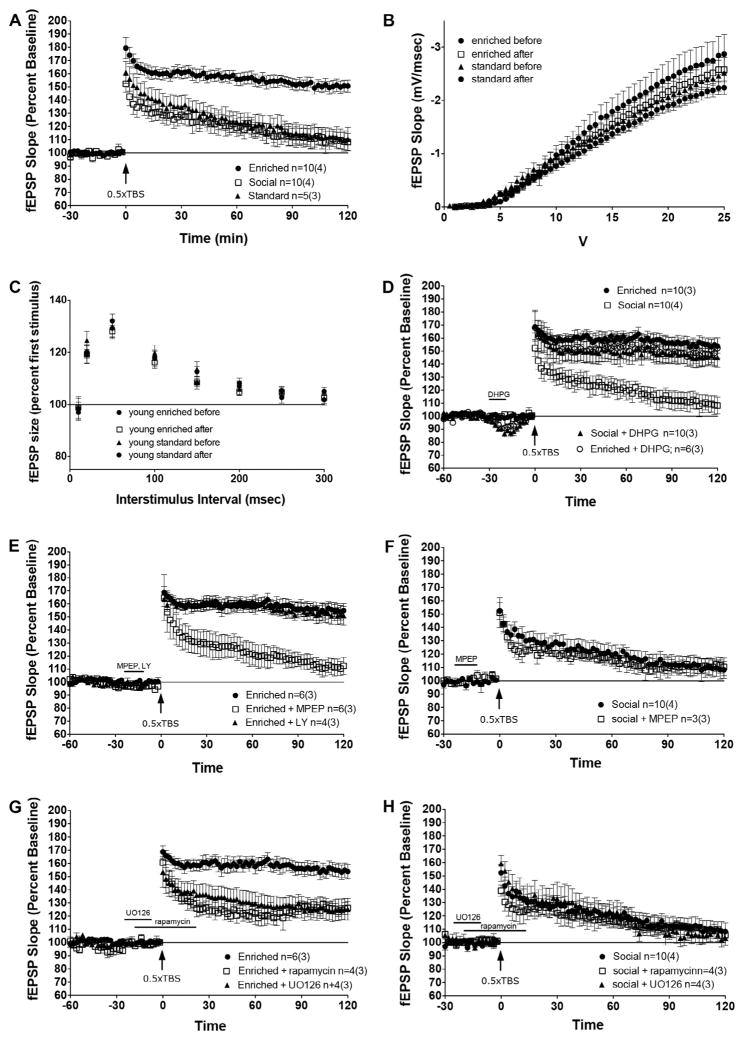
We first sought to investigate whether hippocampal LTP could be enhanced by a period of environmental enrichment. To measure this, we delivered a one half train theta burst stimulation (0.5 TBS; see methods for details) to acute hippocampal slices and measured the evoked excitatory postsynaptic potential in area CA3 along the Schaffer-collateral pathway. It has been previously demonstrated that the 0.5 TBS in the presence of the mGluR1/5 selective agonist 3,5-dihydroxyphenylglycine (DHPG, 10 μM) can convert an acute short-term potentiation into robust, long lasting LTP (Bashir et al., 1993; Bortolotto, Bashir, Davies, & Collingridge, 1994; Cohen & Abraham, 1996; O’Riordan et al., 2014). It is thought that this conversion depends specifically on activity of mGluR5 (Bortolotto et al., 2005; O’Riordan et al., 2014). Interestingly, we found that for enriched animals, 0.5 TBS in the absence of DHPG induced a robust and long lasting LTP that was not seen in SE or SC animals (Fig. 4A, repeated measures ANOVA, F(2,22) = 13.41, p < .001). We did not observe changes in basal synaptic transmission properties following environmental enrichment as measured by input/output curves (Fig. 4B) and paired-pulse facilitation (Fig. 4C). As anticipated, we found that DHPG induced LTP in standard and socially housed rats to levels seen in the enriched condition in the absence of agonist priming (Fig. 4D, only SE shown). However, we did not see any further increase in LTP in slices from enriched animals exposed to DHPG, indicating that the LTP seen in these animals has achieved maximal levels (Fig. 4D).
Fig. 4.
Environmental enrichment results in LTP expression through a mechanism dependent on mGluR5 signaling and subsequent activation of the ERK and mTOR signaling pathways. (A) Rats exposed to 4 months of EE express LTP using a subthreshold 0.5 TBS, whereas SE and SC groups do not. (B) No difference in input/output curves was observed between groups. (C) No differences in paired pulse facilitation were observed between groups. (D) The application of DHPG results in LTP expression in SE animals to levels similar to those observed in EE. (E) Application of the mGluR5 antagonist MPEP, but not the mGluR1 antagonist LY367385, inhibits LTP in EE animals. (F) MPEP does not diminish the synaptic response seen in SE controls. (G) Application of rapamycin or U0126 significantly reduces LTP in EE rats. (H) Rapamycin and U0126 have no significant effect on potentiation in SE controls.
3.3. ERK and mTOR signaling are not specifically enhanced by EE
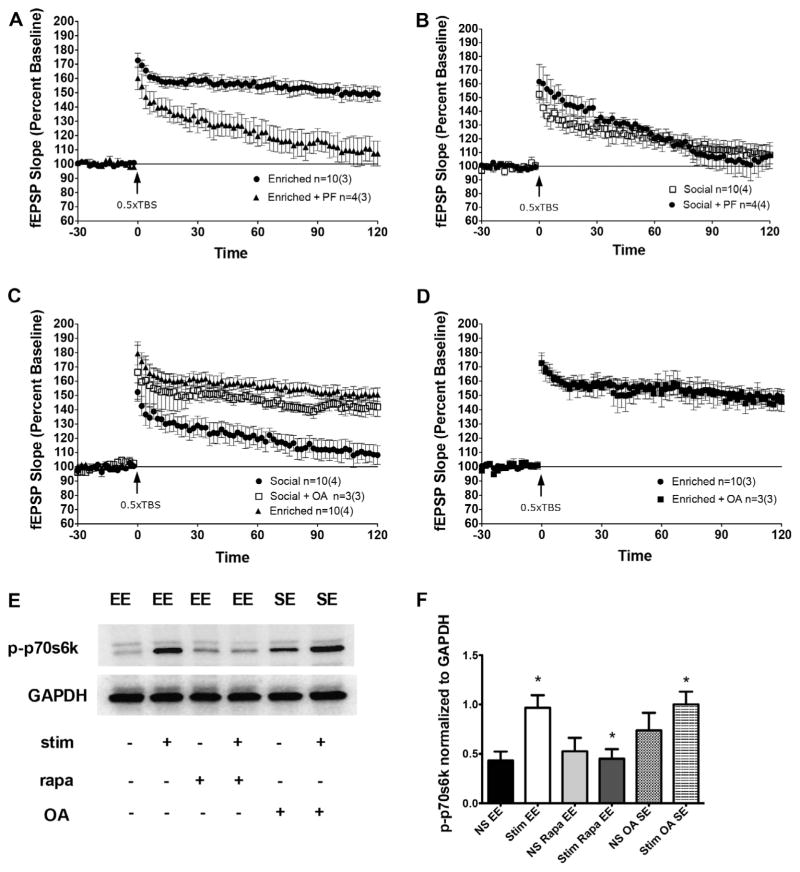
Next, we wanted to determine which signaling pathways are activated that lead to enhanced LTP in enriched animals. We have previously shown that this form of mGluR-dependent LTP requires mGluR5 and not mGluR1α activation in normal mice and Homer1 knockout mice overexpressing Homer1c (O’Riordan et al., 2014); therefore, we sought to determine whether this mechanism was also participating in EE. To test this hypothesis, the mGluR5-selective noncompetitive antagonist, 2-methyl-6-(phenylethy nyl)-pyridine (MPEP, 40 μM), and mGluR1α-selective competitive antagonist LY367385 (LY, 100 μM) were applied to slices before 0.5 TBS. We found that MPEP, but not LY, selectively reduced LTP in enriched animals (Fig. 4E, repeated measures ANOVA, F(2,13) = 13.3, p < .001), suggesting that the LTP in these animals specifically requires intact mGluR5 activation. As a control, we also applied MPEP to slices from SE animals. We chose to focus on SE animals because we wanted to investigate molecular differences between the two forms of enrichment in order to better understand why EE had a stronger effect on learning and memory than SE. We found that MPEP had no impact on the response of SE slices to 0.5 TBS, indicating that suppression of mGluR5 specifically reduces the LTP seen in enriched animals and is not simply indicative of a general suppression of synaptic activity (Fig. 4F).
We next sought to clarify whether specific downstream signaling cascades that are recruited following 0.5 TBS are differentially activated by EE to cause robust LTP in the enriched animals in the absence of pharmacological activation of mGluR5. We chose to investigate the role of the PI3K-mTOR and MEK-ERK pathways, given that these cascades have been implicated in mGluR1/5 function and are essential for hippocampal dependent memory formation (Gafford, Parsons, & Helmstetter, 2011, 2013; Giovannini, Lana, & Pepeu, 2015; Pollard, Willett, & Morley, 2014). The allosteric mTOR antagonist rapamycin (200 nM) and competitive MEK inhibitor U0126 (30 μM) were applied to acute slices prior to 0.5 TBS. We found that selective inhibition of either mTOR or ERK signaling disrupted LTP in enriched animals, causing a significant decrease in fEPSPs compared to untreated slices (Fig. 4G, repeated-measures ANOVA, F(3,20) = 7.487 p < .01). However, this inhibition did not completely suppress LTP, the slices still maintained activity above baseline. This suggests that these pathways operate synergistically, and the activity of one is able to partially compensate for the suppression of another. As a control, we also applied rapamycin and U0126 to slices from SE animals. We found that neither drug affected the synaptic response in SE animals (Fig. 4H), demonstrating that the inhibition of mTOR and ERK specifically impairs the LTP seen in EE animals and is not indicative of a general reduction in synaptic activity.
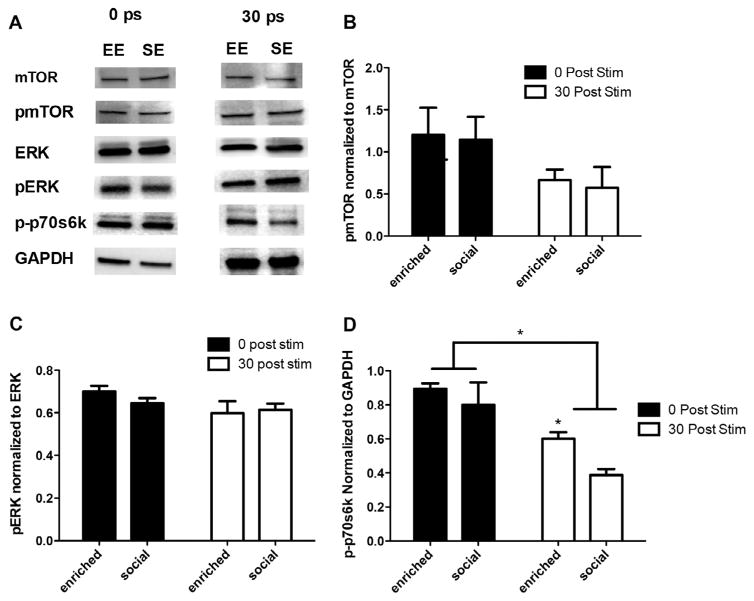
To confirm our electrophysiology results, we conducted western blot analysis to determine whether the ERK and mTOR signaling cascades are differentially activated in enriched animals relative SE controls. Socially enriched animals performed equally well as environmentally enriched during hidden platform training of the MWM (but poorer performance in probe trial, and in NOR; Fig. 3) and yet did not demonstrate enhanced LTP, so we chose to focus on SE rather than SC animals to investigate molecular differences between EE and SE animals that might explain this discrepancy. To accomplish this, slices were given a 0.5TBS and collected immediately after or 30 min after stimulation. Surprisingly, we found no differences in expression of pERK or pmTOR between EE and SE animals immediately following or 30 min after stimulation (Fig. 5A–C). This demonstrates that although activation of ERK and mTOR is necessary for the enhancement in LTP seen in enriched animals, enhanced activation of these pathways does not explain why enriched animals demonstrate LTP in the absence of pharmacological activation of mGluR5 whereas socially enriched animals do not.
Fig. 5.
(A) Slices harvested immediately after 0.5 TBS revealed no differences in the expression of mTOR, pMTOR, ERK, pERK, or p-p70s6k in EE and SE animals. At 30 min, p-p70s6k was the only protein with increased expression in EE compared to SE groups. All other proteins displayed similar levels of expression. (B) Analysis of pmTOR expression indicates no significant changes in the expression of pmTOR between EE and SE groups. (C) Analysis of pERK expression indicates no significant changes in the expression of pERK between EE and SE groups. (D) Analysis of p-p70s6k indicates a sustained phosphorylation in enriched animals relative to SE. Stim = 0.5 TBS.
3.4. EE promotes sustained phosphorylation of p70s6k
Given the lack of differences seen in pERK and pmTOR expression between EE and SE animals, we decided to examine p70s6k, a downstream kinase of both ERK and mTOR that regulates transcription. We found no differences in phospho-p70s6k expression between EE and SE groups immediately following 0.5 TBS; however, 30 min after stimulation EE animals demonstrate sustained phosphorylation of p70s6k relative to SE controls (Fig. 5A and D, unpaired t-test, t = 4.172, df = 4, p = .014).
To further examine the possibility that a sustained phosphorylation of p70s6k may account for enhanced LTP in the enriched animals, we applied the membrane permeable p70s6k specific inhibitor PF4708671 to slices 5 min following 0.5 TBS. We selected 5 min post-stimulation in order to disrupt the maintenance of phosphorylation rather than the initial activation of p70s6k. We found that PF significantly inhibited LTP in enriched animals (Fig. 6A, repeated-measures ANOVA, F(2,13) = 9.28, p < .001). As a control, we also incubated slices from SE animals with PF 5 min post 0.5 TBS, and found that PF did not affect the synaptic response in these animals (Fig. 6B). Taken together, these data suggest that environmental enrichment elicits robust LTP through specific targeting of p70s6k, inducing a sustained phosphorylated state following 0.5 TBS. To further validate our hypothesis, we pharmacologically induced sustained phosphorylation of this kinase in slices collected from socially housed controls. To accomplish this, we incubated slices for 2 h prior to stimulation with the phosphatase inhibitor okadaic acid (OA). PP2A is the phosphatase known to dephosphorylate p70s6k and was therefore specifically targeted using a low concentration of OA, 3 nM (Cohen, Klumpp, & Schelling, 1989). We found that incubation in OA resulted in LTP expression following 0.5 TBS in the SE animals to the levels seen in the environmentally enriched group (Fig. 6C, repeated-measures ANOVA, F(2,21) = 14.3, p = .0001), and that OA had no further enhancing effect on slices from EE animals (Fig. 6D). To confirm that this elevation is due to sustained phosphorylation of p70s6k, we collected SE slices that had been incubated in OA 30 min following 0.5 TBS and compared levels of phospho-p70s6k to those seen in slices collected from EE animals in the absence of OA. We found that the addition of OA to in SE animals enhanced phosphorylation of p70s6k to the levels seen in environmentally enriched slices (Fig. 6E and F). Interestingly, we also saw an increase in phospho-p70s6k expression in the absence of any theta burst stimulation. Although this effect is not significant, it suggests that OA incubation enhances basal levels of phosphorylated p70s6k. To confirm the importance of this sustained phosphorylation for hippocampal LTP in enriched rats, we examined levels of phospho-p70s6k 30 min after stimulation in slices that had received 0.5 TBS in the presence of rapamycin, which had previously been shown to disrupt LTP in enriched animals (Fig. 4D). We confirmed that EE animals demonstrate enhanced phosphorylation of p70s6k following 0.5 TBS relative to levels seen in unstimulated slices (Fig. 6E and F, unpaired t-test, t = 3.407, df = 6, p = .014), and demonstrated that rapamycin effectively prevents this stimulation induced rise in expression (Fig. 6E and F unpaired t-test, t = 3.218, df = 6, p = .018).
Fig. 6.
Sustained phosphorylation of p70s6k is necessary for LTP in enriched animals. (A) The p70s6k inhibitor PF impairs LTP in enriched animals. (B) PF does not impact the synaptic response seen in SE animals. (C) OA enhances LTP in SE animals to levels similar to those seen in EE following 0.5 TBS. (D) OA does not enhance LTP in EE animals. (E) Western blot analysis reveals that p-p70s6k expression is significantly sustained 30 min following 0.5 TBS and that this expression is inhibited by rapamycin in EE rats. OA enhances p-p70s6k expression in SE rats. Results are quantified in panel F. Stim = 0.5 TBS. NS = non-stimulated slices.
4. Discussion
The present study investigated the mechanisms by which environmental enrichment improves cognition and synaptic plasticity in young adult rats. We have discovered that one month of social or environmental enrichment enhances performance in MWM and NOR, but that after 4 months environmentally enriched animals perform better than both socially enriched and standard housed controls. Furthermore, we have demonstrated that EE results in LTP expression following a subthreshold 0.5 TBS that is dependent on mGluR5 signaling, downstream activation of the ERK and mTOR signaling pathways, and sustained phosphorylation of p70s6k. These data suggest that although social enrichment is equally effective at enhancing cognition in the short term, the long term effect of environmental enrichment with inanimate objects in the home cage is stronger than that of social enrichment. This may be because the objects were changed regularly, and the novelty of new objects provided more continuous stimulation than social enrichment with the same group of rats. Importantly, our studies were conducted in male F344 rats and behavioral testing occurred during the dark cycle, which may eliminate confounding factors such as grogginess, alertness, and anxiety during behavior testing. These differences between our experimental design and that described in previous research are important to consider when determining the efficacy of environmental enrichment on various rat strains, ages, and testing cycles.
Interestingly, we found that although the selective inhibition of ERK and mTOR signaling impaired LTP in enriched animals, western blot revealed no differences in the phosphorylation state of ERK or mTOR between EE and SC animals at the time points selected for this study. This data suggests that although activation of ERK and mTOR are necessary for LTP to occur in enriched animals, the enhanced LTP seen in enriched animals is not necessarily a result of enhanced activation of ERK or mTOR. To further probe this apparent discrepancy, we investigated the activity of p70s6k, a downstream kinase in the ERK and mTOR signaling pathways. Western blot analysis revealed that although the initial activation of p70s6k was the same in EE and SE animals, EE animals displayed sustained phosphorylation of the kinase 30 min after 0.5 TBS whereas SE animals did not. Furthermore, we found that the inhibition of p70s6k activity 5 min after 0.5 TBS eliminated LTP seen in EE animals. These data indicate that ERK and mTOR are essential for LTP to occur due to their mutual activation of p70s6k, and that this activation and subsequent sustained phosphorylation is essential for LTP expression seen in enriched animals.
The phosphorylation status of p70s6k is regulated by multiple signaling cascades, including ERK, mTOR, PDK1, PKC, and PP2A. It is possible that enriched animals exhibit elevated levels of phosphorylated p70s6k due to mildly enhanced activity of multiple p70s6k activators, and that the combined effect of this collective enhancement produces significant differences in p70s6k phosphorylation despite individual differences not being detectable by western blot analysis. This conclusion is supported by the observation that although inhibition of ERK or mTOR diminished LTP seen in the enriched animals, it did not completely abolish it. This suggests that collective activity of multiple pathways might be responsible for the LTP seen in enriched animals, potentially due to their mutual activation of p70s6k. It is also possible that enriched animals maintain the phosphorylated state of p70s6k due to decreased activity of PP2A, the phosphatase known to dephosphorylate p70s6k. To assess this hypothesis, slices from SE animals were incubated in okadaic acid at concentrations that specifically inhibit PP2A (Cohen et al., 1989). We found that this inhibition increased the synaptic response in SE slices, resulting in LTP expression similar to that seen in EE groups following 0.5 TBS. Overall, these experiments suggest that enriched animals display LTP where controls do not, specifically due sustained phosphorylation of p70s6k, perhaps through a general enhancement in the activity of multiple upstream kinases, a downregulation of PP2A activity, or both.
Our results parallel previous studies implicating p70s6k and PP2A in normal learning and memory formation. Previous studies have demonstrated that PP2A expression is increased following lead exposure in the rodent, and that this elevation in PP2A causes deficits in the acquisition phase and short term (48 h after training) probe trials that parallel those seen in standard housed animals relative to enriched (Rahman, Khan, Al-Khaledi, Khan, & Al-Shemary, 2012). Furthermore, animals that systemically lack p70s6k show impairments in hippocampal dependent memory tasks, including the Morris water maze, contextual fear conditioning, and conditioned taste aversion (Antion et al., 2008). Studies have shown that p70s6k activation specifically in the hippocampus is required for the acquisition of conditioned place preference, inhibitory avoidance and both the formation and retrieval of contextual fear memories (Bekinschtein et al., 2007; Cui et al., 2010) In addition, it has been demonstrated that aged animals with unimpaired cognition express higher basal levels of p70s6k than their aged counterparts with cognitive deficits (Ménard & Quirion, 2012). These data indicate that activation of p70s6k and the maintenance of phospho-p70s6k through proper expression of PP2A are vital for normal learning and memory formation. In the context of our current experiments, it also suggests that cognitive training could provide a buffer against age-related cognitive decline specifically by enhancing expression of phospho-p70s6k in the hippocampus.
Previous studies from our lab indicate that mild cognitive impairments can be found in rats as early as 3 months of age, and that these deficits early in life are predictive of cognitive deficits that develop with age (Hullinger & Burger, 2015). This suggests that behavioral intervention focused on improving cognition from an early time point could provide a buffer against age-related cognitive decline. This study demonstrates that environmental enrichment improves cognition and hippocampal LTP in 2 month old rats by a mechanism involving mGluR5 activation and sustained phosphorylation of p70s6k, providing both a behavioral means of improving cognition and a potential therapeutic target for behavioral enhancement. The results of this study will have important implications for the future studies of memory enhancement as well as successful cognitive aging, as it provides a means to enhance cognition from a young age and potentially target prevention of age related cognitive decline. In addition, this and other studies of enrichment in rodents suggest that environmental enrichment could be a potentially useful technique in young and aged humans, and open the door for future studies investigating effectiveness of environmental enrichment in the treatment of disorders of cognition in human populations.
Acknowledgments
This research was supported by funds from the University of Wisconsin Graduate School, School of Medicine and Public Health and Department of Neurology to C.B. R.H. was supported by the University of Wisconsin Neuroscience Training Program Grant NIH/NIGMS T32GM007507 and a NSF National Science Foundation Graduate Research Fellowship Program (GRFP) Fellowship. We would like to acknowledge the rat subjects who contributed to this study.
References
- Antion MD, Merhav M, Hoeffer CA, Reis G, Kozma SC, Thomas G, … Klann E. Removal of S6K1 and S6K2 leads to divergent alterations in learning, memory, and synaptic plasticity. Learning and Memory. 2008;3(15):29–38. doi: 10.1101/lm.661908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, … Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363(6427):347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH. MTOR signaling in the hippocampus is necessary for memory formation. Neurobiology of Learning and Memory. 2007;87(2):303–307. doi: 10.1016/j.nlm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. A molecular switch activated by metabotropic glutamate receptors regulates induction of long term potentiation. Nature. 1994;368(6473):740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Collett VJ, Conquet F, Jia Z, van der Putten H, Collingridge GL. The regulation of hippocampal LTP by the molecular switch, a form of metaplasticity, requires mGlu5 receptors. Neuropharmacology. 2005;49(1):13–25. doi: 10.1016/j.neuropharm.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Burrows EL, McOmish CE, Buret LS, Van den Buuse M, Hannan AJ. Environmental enrichment ameliorates behavioral impairments modeling schizophrenia in mice lacking metabotropic glutamate receptor. Neuropsychopharmacology. 2015;5 doi: 10.1038/npp.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Abraham WC. Facilitation of long-term potentiation by prior activation of metabotropic glutamate receptors. Journal of Neurophysiology. 1996;76(2):953–962. doi: 10.1152/jn.1996.76.2.953. [DOI] [PubMed] [Google Scholar]
- Cohen S, Klumpp S, Schelling DL. An improved procedure for identifying and quantitating phosphatases in mammalian tissues. FEBS Letters. 1989;250(2):596–600. doi: 10.1016/0014-5793(89)80803-8. [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhang XQ, Cui Y, Xin WJ, Jing J, Liu XG. Activation of phosphatidylinositol 3-kinase/Akt-mammalian target of Rapamycin signaling pathway in the hippocampus is essential for the acquisition of morphine-induced place preference in rats. Neuroscience. 2010;24(171):134–143. doi: 10.1016/j.neuroscience.2010.08.064. [DOI] [PubMed] [Google Scholar]
- Eckert MJ, Bilkey DK, Abraham WC. Altered plasticity in hippocampal CA1, but not dentate gyrus, following long-term environmental enrichment. Journal of Neurophysiology. 2010;103(6):3320–3329. doi: 10.1152/jn.01037.2009. [DOI] [PubMed] [Google Scholar]
- Foster TC, Dumas TC. Mechanism for increased hippocampal synaptic strength following differential experience. Journal of Neurophysiology. 2001;85(4):1377–1383. doi: 10.1152/jn.2001.85.4.1377. [DOI] [PubMed] [Google Scholar]
- Gafford GM, Parsons RG, Helmstetter FJ. Consolidation and reconsolidation of contextual fear memory requires mammalian target of rapamycin-dependent translation in the dorsal hippocampus. Neuroscience. 2011;19(182):98–104. doi: 10.1016/j.neuroscience.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford GM, Parsons RG, Helmstetter FJ. Memory accuracy predicts hippocampal mTOR pathway activation following retrieval of contextual fear memory. Hippocampus. 2013;23(9):842–847. doi: 10.1002/hipo.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein H, O’Riordan K, Osting S, Schwarz M, Burger C. Rescue of synaptic plasticity and spatial learning deficits in the hippocampus of Homer1 knockout mice by recombinant Adeno-associated viral gene delivery of Homer1c. Neurobiology of Learning and Memory. 2012;97(1):17–29. doi: 10.1016/j.nlm.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini MG, Lana D, Pepeu G. The integrated role of Ach, ERK, and mTOR in the mechanisms of hippocampal inhibitory avoidance memory. Neurobiology of Learning and Memory. 2015;119:18–33. doi: 10.1016/j.nlm.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Hannan AJ. Environmental enrichment and brain repair: Harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathology and Applied Neurobiology. 2014;40(1):13–25. doi: 10.1111/nan.12102. [DOI] [PubMed] [Google Scholar]
- Harati H, Majchrzak M, Cosquer B, Galani R, Kelche C, Cassel JC, Barbelivien A. Attention and memory in aged rats: Impact of lifelong environmental enrichment. Neurobiology of Aging. 2011;32(4):718–736. doi: 10.1016/j.neurobiolaging.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Lambert TJ, Frick KM. Age-dependent effects of environmental enrichment on spatial reference memory in male mice. Behavioral Brain Research. 2007;185(1):43–48. doi: 10.1016/j.bbr.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullinger R, Burger C. Learning impairments identified early in life are predictive of future impairments associated with aging. Behavioural Brain Research. 2015;294:224–233. doi: 10.1016/j.bbr.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiology of Aging. 2012;33(4):1–17. doi: 10.1016/j.neurobiolaging.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti CF, Fan X, English RD, Zhang Y, Li D, Kong F, … Green TA. Environmental enrichment alters protein expression as well as the proteomic response to cocaine in the rat nucleus accumbens. 2014;21(8):246. doi: 10.3389/fnbeh.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long term potentiation (LTP) but normal CA3 LTP. Journal of Neuroscience. 1997;17(13):5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Chattarji S. Enhanced intrinsic excitability and EPSP-spike coupling accompany enriched environment-induced facilitation of LTP in hippocampal CA1 pyramidal neurons. Journal of Neurophysiology. 2012;107(5):1366–1378. doi: 10.1152/jn.01009.2011. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cerebral Cortex. 2005;15(11):1703–1713. doi: 10.1093/cercor/bhi047. [DOI] [PubMed] [Google Scholar]
- Mazarakis NK, Mo C, Renoir T, van Dellen A, Deacon R, Blakemore C, Hannan AJ. ‘Super-enrichment’ reveals dose-dependent therapeutic effects of environmental stimulation in a transgenic mouse model of Huntington’s disease. Journal of Huntingtins Disease. 2014;3(3):299–309. doi: 10.3233/JHD-140118. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology. 2004;29(11):1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- Ménard C, Quirion R. Successful cognitive aging in rats: a role for mGluR5 glutamate receptors homer 1 proteins and downstream signaling pathways. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0028666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Gallegos A, Rojas-Carvajal M, Salas S, Saborío-Arce A, Fornaguera-Trías J, Brenes JC. Age-dependent effects of environmental enrichment on spatial memory and neurochemistry. Neurobiology of Learning and Memory. 2015:96–104. doi: 10.1016/j.nlm.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Novkovic T, Mittmann T, Manahan-Vaughan D. BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus. 2015;25(1):1–15. doi: 10.1002/hipo.22342. [DOI] [PubMed] [Google Scholar]
- O’Riordan K, Gerstein H, Hullinger R, Burger C. The role of Homer1c in metabotropic glutamate receptor-dependent long-term potentiation. Hippocampus. 2014;24(1):1–6. doi: 10.1002/hipo.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang TY, Hannan AJ. Enhancement of cognitive function in models of brain disease through environmental enrichment and physical activity. Neuropharmacology. 2013:515–528. doi: 10.1016/j.neuropharm.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Pollard HJ, Willett M, Morley SJ. MTOR kinase-dependent, but raptor independent regulation of downstream signaling is important for cell cycle exit and myogenic differentiation. Cell Cycle. 2014;13(16):2517–2525. doi: 10.4161/15384101.2014.941747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Khan KM, Al-Khaledi G, Khan I, Al-Shemary T. Over activation of hippocampal serine/threonine protein phosphatases PP1 and PP2A is involved in lead-induced deficits in learning and memory in young rats. Neurotoxicology. 2012;33(3):370–383. doi: 10.1016/j.neuro.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Sampedro-Piquero P, Arias JL, Begega A. Behavioral testing-related changes in the expression of Synapsin 1 and glucocorticoid receptors in standard and enriched aged Wistar rats. Experimental Gerontology. 2014:292–302. doi: 10.1016/j.exger.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Sampedro-Piquero P, Begega A, Zancada-Menendez C, Cuesta M, Arias JL. Age-dependent effects of environmental enrichment on brain networks and spatial memory in Wistar rats. Neuroscience. 2013;17(248):43–53. doi: 10.1016/j.neuroscience.2013.06.003. [DOI] [PubMed] [Google Scholar]