Abstract
Background
Testicular torsion is an acute urologic emergency occurring in male newborns, children or adolescents. Prolonged ischemia for more than six hours can lead to irreversible testicular damage. Surgical detorsion allows reperfusion and is the only treatment currently available. The aim of this study was to evaluate the antioxidant effect of apigenin (APG) on the testicular ischemia-reperfusion (I/R) injury.
Methods
Forty-two Wistar rats were randomly divided into five groups. Sham group underwent operation of the left testis. In the torsion-detorsion groups C15 and C120, the left testis was rotated 1080o for three hours. The treatment groups Ap15 and Ap120 received the same surgical procedure as groups C15 and C120, but APG was administered intravenously at the same time of detorsion via the right femoral vein. Left orchiectomy was performed 15 min after detorsion at groups C15 and Ap15, and at 120 min at groups C120 and Ap120 for histopathologic and immunohistochemical evaluation.
Results
In I/R-untreated groups C15 and C120, there was a moderate to severe distortion of the tubules with lesions that varied between grades III and IV according to histopathological finding. In APG-treated groups Ap15 and Ap120, most of the lesions showed injuries of grades II and III with mild and moderate histopathological features. In Terminal deoxynucleotide transferase dUTP Nick End Labeling (Tunel) assay, APG-treated animals showed a statistically significantly decreased number of apoptotic cells compared to groups C15 and C120.
Conclusion
Intravenous administration of APG seems to have a protective effect on testicular ischemia-reperfusion injury after testicular torsion and detorsion. Hippokratia 2015; 19 (3): 225-230.
Keywords: Apigenin, testicular torsion, ischemia/reperfusion injury, rat
Introduction
Testicular torsion is an acute and relatively rare condition that occurs soon after birth or in childhood and adolescence. The incidence of this clinical entity represents even nowadays a very interesting experimental field aiming at the prevention of male reproductive organ injuries.
Since prolonged ischemia of the testis of more than six hours can cause irreversible damage, urgent surgical treatment is mandatory1,2. The severity of testicular damage is related to the degree and duration of torsion, with testicular salvage rates of surgical detorsion ranging between 42%-88%3,4. Whether or not spermatogenic testicular function is preserved remains unknown.
It seems that the main pathophysiology of testicular torsion-detorsion is ischemia-reperfusion (I/R) injury of the testes. In ischemia-reperfusion injury, overgeneration of reactive oxygen species (ROS) is thought to play a critical role in the loss of ipsilateral testicular spermatogenesis5. Reactive oxygen species are produced after reperfusion, and the release of cytokines, such as interleukin-1b and tumor necrosis factor-a (TNF-a), results in recruitment of neutrophils and macrophages, causing testicular atrophy, germ cell apoptosis and disruption of spermatogenesis6-9. ROS can cause tissue damage or death through cell membrane lipid peroxidation, protein denaturation, and DNA impairment10-12.
Apigenin (APG) is a natural dietary plant-derived compound that belongs to the group of flavones, the aglycone of several naturally occurring glycosides. Shown to prevent lipid peroxidation and protect the antioxidant system13, APG also appears to inhibit proliferation and induce apoptosis of NCI-H460 cells (human non-small cell lung cancer cells line), possibly by the upregulation of Bax expression and caspase-3, and the down-regulation of Bcl-2 expression14.
The aim of this study was to evaluate the antioxidant effect of APG on testicular ischemia-reperfusion injury.
Materials and methods
Animals and surgical procedure
Forty-two male Wistar rats were used in this study. The rats aged between 14 and 16 weeks and weighed 250-300g. The animals were kept in groups of four in special plastic cages under controlled lighting conditions (12h light-dark cycle) and at a temperature of 23°C and relative humidity. They were fed following a standard laboratory diet, except for the night before surgery when food was withheld. The experiment was conducted in accordance with the animal research protocol approved by the Ethical Committee of Democritus University of Thrace (Agricultural Economy and Veterinary Authorities of Thrace, Decision Number 8268 28/05/2012) and the Veterinary Authorities of Greece in agreement with Law 160/1991. The rats were anesthetized by using diethyl ether and intraperitoneal ketamine injection (50 mg/kg), and surgery was performed under sterile conditions. In the sham group, the left testis was brought out through a left-sided inguino-scrotal incision and was returned to the scrotum; the incision was then closed. Testicular I/R injury was induced by testicular torsion-detorsion. In the torsion-detorsion groups C15 and C120, the left testis was exposed through a similar incision. The left testis was rotated 1080 degrees in a counterclockwise direction and was maintained in this torsion position by fixing the testis to the scrotum with 6/0 prolene sutures; the trauma was then closed. Even thought the bibliography suggests a 720-degree rotation, this was not found adequate in our case; thus, we decided to increase the degrees of the rotation to 1080 degrees, getting a satisfactory outcome. After three hours of torsion, under general anesthesia, the scrotum was reopened, and the testis was counter-rotated to the natural position. The left testis was still viable for restoration of blood flow and was reinserted into the scrotum. The treatment groups Ap15 and Ap120 underwent the same surgical procedure as the torsion-detorsion groups, but at the time of detorsion APG was injected intravenously (10 mg/kg) via the right femoral vein. APG (5 mg), obtained from Merck Millipore S.A., Hellas, was diluted in normal saline solution of 0.3 mL NaCl 0.9% and 0.2 mL dimethyl sulfoxide solvent (DMSO). The rats were randomly divided into five groups (Table 1). The animals were sacrificed under general anesthesia by isoflurane at the end of the reperfusion time. Blood sampling of 5-7 mL was taken from the vena cava, which corresponded to almost the entire blood volume.
Table 1. Experimental groups in which 42 male Wistar rats were randomly divided, to evaluate the antioxidant effect of apigenin on testicular ischemia-reperfusion injury.
Group Sham: left-sided inguino-scrotal incision; Groups C15 and C120: left testis was rotated for three hours, reperfusion followed at 15 and 120 min, respectively; Groups Ap15 and Ap120: same surgical procedure as groups C15 and C120, but apigenin was administered intravenously at the time of detorsion.
Histopathology and histopathologic evaluation
Specimens chosen for microscopic examination were fixed immediately in 10% formaldehyde and then embedded in paraffin wax, sectioned serially at 4μm, and stained with hematoxylin and eosin (H&E). The sections were examined by two blinded investigators using a light microscope (Nikon eclipse 50i, Nikon Corporation, Japan) and assessed according to the Cosentino et al15 classification.
Immunohistochemistry
Tissue specimens were fixed in formalin and embedded in paraffin blocks according to standard procedures. Four-micron sections (4µm) of representative blocks from each case were deparaffinized, rehydrated, and incubated for 30 min at 37oC with trypsin. Endogenous peroxidase activity was quenched by 15-minute incubation of slides with 0.3% H2O2. The biotin-streptavidin method was applied for immunohistochemistry (IHC), using the Kit Chemicon IHC Select-Immunoperoxidase secondary detection system (Chemicon, USA). Phosphate buffer saline (PBS) was used to wash slides for five minutes; the blocking reagent was subsequently added for 10 minutes. Slides were then incubated for 60 minutes in a humidified atmosphere with one of the polyclonal antibodies, as follows: Anti-Interleukin-10 (IL-10; Abcam, USA) at 1:400 dilution, anti-Tumor Necrosis Factor (TNF-α; Acris, Germany) at 1:200 dilution. Negative control slides were, in parallel, incubated with 10% Normal Rabbit Serum/Phosphate Buffer Saline (NRS/PBS). After washing with PBS, the biotinylated secondary antibody was added for 10 minutes, followed by streptavidin horseradish peroxidase (HRP) incubation for 10 minutes. Bound antibody complexes were stained with 0.05% diaminobenzidine chromogen (DAKO, USA) for 10 minutes. Finally, sections were briefly counterstained with Mayer’s haematoxylin, mounted and examined under a Nikon microscope at x200 original magnification. A homogenous, light brown staining of the cytoplasm revealed positive cells.
Each slide was individually evaluated and scored in a blinded fashion by two independent observers (M.L. and N.P.). Sections with >10% stained cells were considered as being positive16. The average labeling index of TNF-a and IL-10 was assessed according to the proportion of positive cells, after scanning the entire section of the specimen. The results of expression were graded as: negative (0) for ≤10% of cells stained; low (I) >10% and ≤30% of cells stained; moderate (II) >30% and ≤70% of cells stained; and high (III) for >70% of cells stained (0: negative; I: low; II: moderate; and III: high expression).
Terminal deoxynucleotide transferase dUTP Nick End Labeling (Tunel) assay
We performed the Tunel assay using the in situ Death Detection kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Control slides were incubated for the same period with 50 μL Label solution (negative control). Finally, slides were stained for 10 min with 0.05% diaminobenzidine. Then, sections were briefly counterstained with Mayer’s haematoxylin, mounted, and examined under a Nikon Eclipse 50i microscope at 400x magnification. Positive cells were indicated by light brown staining of the nucleus and cytoplasm. In order to avoid an overestimation of apoptosis, positive cells were considered only those that exhibited both morphologic features of apoptosis on light microscope (cytoplasmic fragmentation and nuclear condensation)17. Apoptotic cells were counted in at least 30 circular seminiferous tubular cross sections per testis, and their total number was divided by the number of tubules to determine the apoptotic index. All slides were photographed with Nikon Digital Sight SD-SI (Nikon Corporation, Japan).
Statistical analysis
We performed statistical analysis of the data using the Statistical Package for the Social Sciences, version 19.0 (SPSS, IBM, NY, USA). The normality of quantitative variables was determined by the Kolmogorov-Smirnov test. Tunel, TNF-a, IL-10 and the total histological score were expressed as mean ± standard deviation (SD). Within groups, the differences of these indices were examined by Mann-Whitney test. Between groups, differences were assessed by Kruskal-Wallis test; post hoc analysis was performed using the Mann-Whitney test, with adjusted level of significance at a=0.017, according to Bonferroni’s correction. All tests were two-tailed, and statistical significance was considered as p values <0.05.
Results
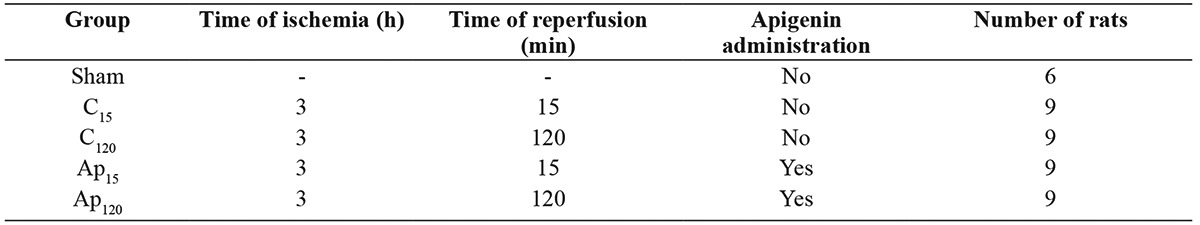
The rats in the sham group showed normal testicular architecture with normal seminiferous tubule morphology (Figure 1a). In I/R-untreated groups C15 and C120, there was a moderate to severe distortion of the tubules with lesions that varied between disordered sloughed germinal cells with shrunken pyknotic nuclei and less distinct seminiferous tubule borders (grade III) and closely packed seminiferous tubules with coagulative necrosis of the germinal cells (grade IV) (Figure 1b). In APG-treated groups Ap15 and Ap120, most of the lesions showed injuries characterized by less orderly, non-cohesive germinal cells and closely packed seminiferous tubules (grade II) and grade III, with mild and moderate histopathological features (Figure 1c). No significant differences were found between groups C15 and C12015.
Figure 1. Testis tissue section from control group (a: normal testicular architecture); ischemia-reperfusion group (b: moderate to severe distortion of the tubules); apigenin group (c: mild and moderate histopathological features). Hematoxylin & Eosin, original magnification x100.
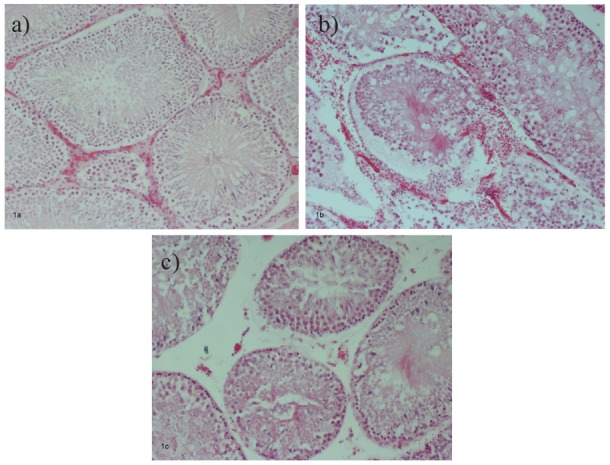
Tissue sections from the testis of sham-operated animals stained by the Tunel technique showed very few stained nuclei (Figure 2a). However, sections taken from I/R-untreated groups C15 and C120 revealed an increased number of apoptotic germ cells (Figure 2b). APG-treated animals showed a decreased number of apoptotic cells compared to groups C15 and C120 that was statistically significant (p=0.006) (Figure 2c). Animals with delayed reperfusion time (Group Ap120) showed even less apoptotic cells compared against those of group Ap15, but the difference was statistically insignificant (p=0.545).
Figure 2. Expression of apoptotic cells’ marker Tunel in control group (a: absence of apoptotic cells); ischemiareperfusion group (b: increased apoptotic germ cells, arrows); apigenin group (c: less apoptotic cells than I-R group, arrows). Immunostaining, original magnification x200.
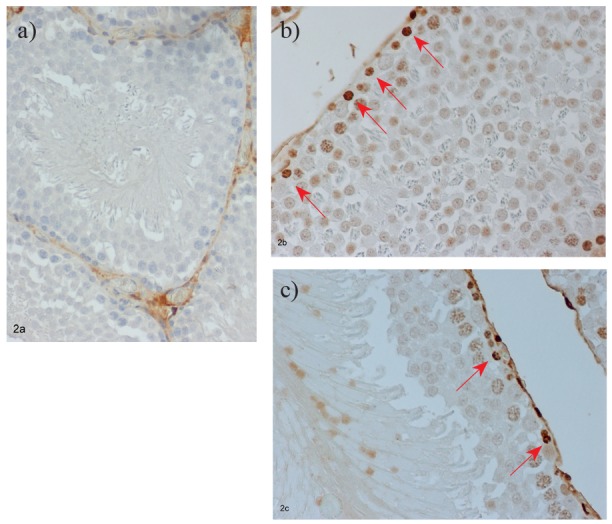
Tissue sections from the testis of sham-operated animals stained for TNF-a, and IL-10 were negative (Figure 3a, b). I/R untreated animals showed increased immunopositivity of grade III for TNF-a compared to grade II for IL-10 (Figure 3c, d). On the contrary, tissue sections from I/R APG-treated animals from groups Ap15 and Ap120 revealed increased positivity of grade II for IL-10 and decreased for TNF-a, with most rats showing grade I positivity (Figure 3e, f).
Figure 3. Expression of TNF-a, and IL-10 in (a, d: low expression) control group; (b, e: high expression) ischemiareperfusion group; (c, f: moderate expression) apigenin group. Immunostaining, original magnification x200.
The results of the two observers indicated very good inter-observer reliability, with an interclass correlation coefficient of 0.978 (p <0.001) for Tunel, and Cohen’s kappa of 0.829 (p <0.001) for TNF-a, and 0.811 (p <0.001) for IL-10.
The mean values of Tunel, TNF-a, IL-10, and the total histological score in the three studied groups are shown in Table 2. The values of the above indices were compared within each group according to the different time of reperfusion. Within the control group, it was observed that 120 min reperfusion was followed by statistically significant higher values of Tunel (by 20.83%, p =0.006), TNF-a (by 43.82%, p =0.020) and total histological score (by 7.52%, p =0.042) compared to 15 min reperfusion. On the contrary, within the APG group, the total histological score was significantly lower (by -23.03%, p <0.001) after 120 min reperfusion compared to 15 min reperfusion; mean values of Tunel, TNF-a, and IL-10 were similar after 15 min and 120 min reperfusion (p =0.545, p =0.317, and p =0.072, respectively).
Table 2. Expression of antibodies and effect of apigenin on testicular ischemia-reperfusion injury.
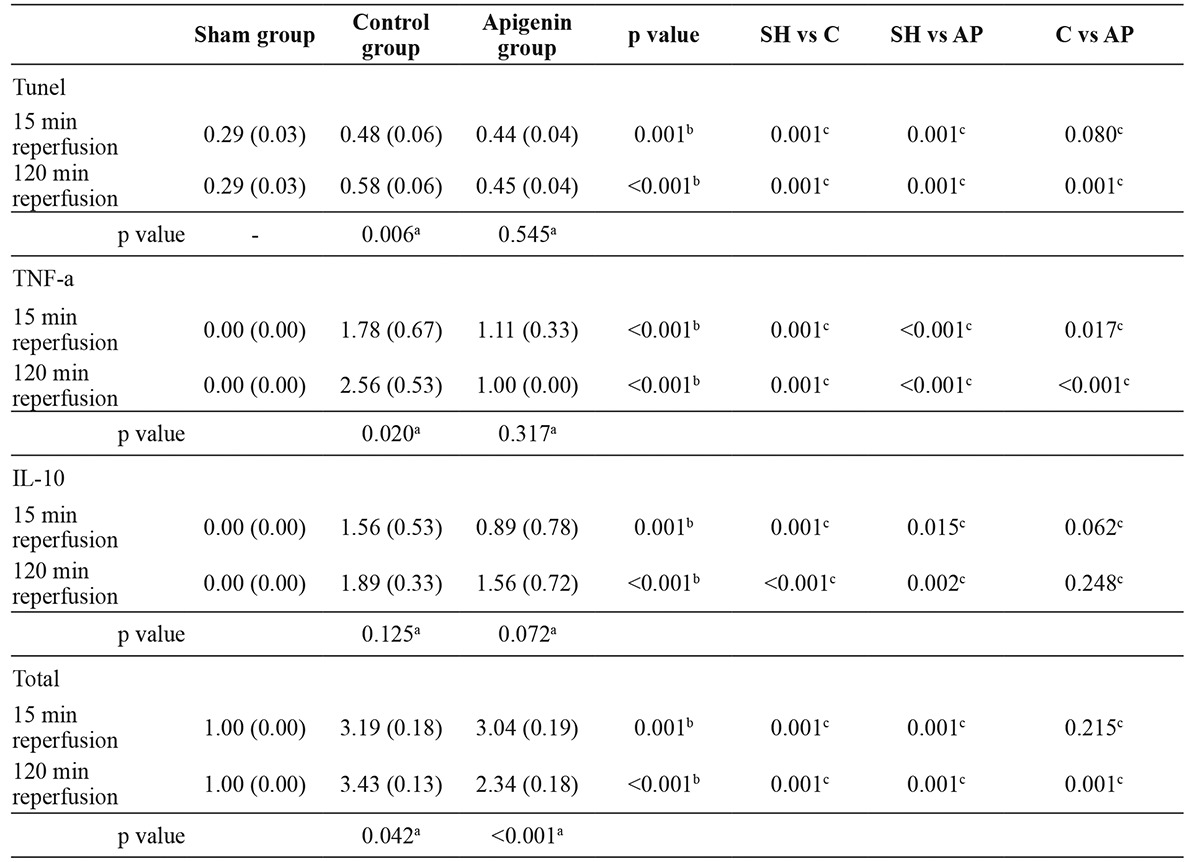
SH: sham group, C: Control group (Torsion-detorsion without Apigenin), AP: Apigenin group. All indices were expressed as Mean (Standard Deviation); a indicates statistical significance within each group (Mann-Whitney test); b indicates statistical significance between the three different groups (Kruskal-Wallis test); c indicates statistical significance for pairwise comparisons (Mann-Whitney test with Bonferroni’s correction).
In the sequence, the Kruskal-Wallis test showed statistically significant differences among all the four indices studied herein, after 15 min or 120 min reperfusion, between the three groups (all p ≤0.001; Table 2). Post hoc analysis, with adjusted level of significance at a =0.017 for pairwise comparisons according to Bonferroni’s correction, showed: i) statistically significantly higher values of all indices in the control group and APG group compared to the sham group (all p ≤0.015; Table 2); ii) the APG group displayed lower values of TNF-a (by -37.64%, p =0.017) after 15 min reperfusion compared to the control group; iii) the APG group exhibited lower values of Tunel (by -22.41%, p =0.001), TNF-a (by -60.94%, p <0.001) and total histological score (by -31.78%, p =0.001) after 120 min reperfusion compared to the control group; iv) the APG group was associated with a tendency towards lower values of Tunel (by -8.33%, p =0.080) and IL-10 (by -42.95%, p =0.062) after 15 min reperfusion compared to the control group.
Discussion
Testicular torsion-detorsion is an ischemia-reperfusion process of the testis. In the course of testicular ischemia-reperfusion, ROS are overproduced5. Increased production of ROS inflicts significant injury on ischemic tissue through oxidation of cell membrane lipids, proteins, and DNA10,11. Testicular torsion-detorsion is thought to play a critical role in the loss of ipsilateral testicular spermatogenesis5. As a response to the acute inflammation due to I/R, release of pro-inflammatory cytokines, such as interleukin-1b and TNF-a, accelerate the inflammatory processes by inducing inflammatory molecules, thereby triggering apoptosis12.
Some published studies18-22 demonstrated that injuries were attenuated in shorter ischemic time with the use of various agents. However, bearing in mind that in clinical practice the delivery time can differ among agents with some taking longer than calculated in our experiment, we prolonged the period of testicular ischemia to enable the examination of deeper injuries not previously studied in the literature. It became apparent from the published data and observations made in our research that it was important to have active substances that could minimize testicular damage in longer ischemic times. One such substance is APG which we chose for our experiment with the aim of investigating its efficacy. Our results showed reduced apoptosis.
Endogenous IL-10 is an important aspect of the natural anti-inflammatory response that limits the deleterious effects of the pro-inflammatory cascade of ischemia-reperfusion injury. IL-10 has been shown in vitro23,24 and in vivo25,26 to be also capable of regulating and inhibiting TNF-a production by neutrophils, macrophages, and Th1-cells.
In our experiment, immunohistochemical studies demonstrated increased positivity for TNF-a of grade III and less intense positivity of grade II for IL-10 in the I/R-untreated groups, whereas APG-treated groups showed decreased immunoreactivity for TNF-a and more intense for IL-10. In association with the significantly improved histopathological picture in the respective groups, these findings suggest that the synergistic action of APG and endogenous IL-10 offers protection against inflammatory and apoptotic cascades that progressively lead to testicular cell injury.
APG is a natural product belonging to the group of flavones family of the flavonoid compounds, which is widely distributed in many vegetables and fruits, and has the capacity for diverse pharmacological effects including antioxidant and anti-carcinogenic potential27. The antioxidant effect lies in the H+donating potential of its aromatic OH-group28.
Results of its use in our experimental laboratory and other institutions have been quite encouraging. In our experiment, it was used for its antioxidant potential since the results reported by Tsalkidou et al29 and Lampropoulos et al30 were also favourable. We found APG to have a positive effect on reperfusion, indicating promise for its use in clinical practice. In our experiment, although APG clearance time for the dose of 10 mg/kg was about 12 hours, the duration of reperfusion time after the administration of APG at the time of detorsion in treated groups Ap15 and Ap120 was that of Tmax and T½, respectively31. Despite the short reperfusion time, we found that animals treated with APG had a significant decrease in the stained apoptotic germ cell number and showed less severe histological findings compared to the I/R-untreated animals. These results show that APG may prevent testicular injury by its antioxidant potential.
In the present study, we did not investigate administering APG at different doses and different times or at prolonged reperfusion time. Given that these factors may influence the effect of APG, additional studies are required to determine the optimal dose and administration time of APG. We also believe that an increase in the reperfusion time could promote even better results for APG efficiency.
Conclusion
This is the first study to demonstrate the protective effect of APG on testicular ischemia-reperfusion injury, due to its antioxidant properties. We propose that APG could be clinically used in patients with testicular torsion.
Conflict of Interest
Authors declare no conflict of interests regarding this publication.
References
- 1.Anderson JB, Williamson RC. The fate of the human testes following unilateral torsion of the spermatic cord. Br J Urol. 1986;58:698–704. doi: 10.1111/j.1464-410x.1986.tb05916.x. [DOI] [PubMed] [Google Scholar]
- 2.Williamson RC. The continuing conundrum of testicular torsion. Br J Surg. 1985;72:509–510. doi: 10.1002/bjs.1800720702. [DOI] [PubMed] [Google Scholar]
- 3.Parker RM, Robison JR. Anatomy and diagnosis of torsion of the testicle. J Urol. 1971;106:243–247. doi: 10.1016/s0022-5347(17)61267-9. [DOI] [PubMed] [Google Scholar]
- 4.Cattolica EV, Karol JB, Rankin KN, Klein RS. High testicular salvage rate in torsion of the spermatic cord. J Urol. 1982;128:66–68. doi: 10.1016/s0022-5347(17)52758-5. [DOI] [PubMed] [Google Scholar]
- 5.Turner TT, Tung KS, Tomomasa H, Wilson LW. Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod. 1997;57:1267–1274. doi: 10.1095/biolreprod57.6.1267. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor S. Testicular torsion: a race against time. Int J Clin Pract. 2008;62:821–827. doi: 10.1111/j.1742-1241.2008.01727.x. [DOI] [PubMed] [Google Scholar]
- 7.Turner TT, Bang HJ, Lysiak JL. The molecular pathology of experimental testicular torsion suggests adjunct therapy to surgical repair. J Urol. 2004;172:2574–2578. doi: 10.1097/01.ju.0000144203.30718.19. [DOI] [PubMed] [Google Scholar]
- 8.Altavilla D, Romeo C, Squadrito F, Marini H, Morgia G, Antonuccio P, et al. Molecular pathways involved in the early and late damage induced by testis ischemia: evidence for a rational pharmacological modulation. Curr Med Chem. 2012;19:1219–1224. doi: 10.2174/092986712799320538. [DOI] [PubMed] [Google Scholar]
- 9.Lysiak JJ. The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod Biol Endocrinol. 2004;2:9. doi: 10.1186/1477-7827-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filho DW, Torres MA, Bordin AL, Crezcynski-Pasa TB, Boveris A. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol Aspects Med. 2004;25:199–210. doi: 10.1016/j.mam.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Prillaman HM, Turner TT. Rescue of testicular function after acute experimental torsion. J Urol. 1997;157:340–345. [PubMed] [Google Scholar]
- 12.Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- 13.Singh JP, Selvendiran K, Banu SM, Padmavathi R, Sakthisekaran D. Protective role of Apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine. 2004;11:309–314. doi: 10.1078/0944711041495254. [DOI] [PubMed] [Google Scholar]
- 14.Pan X, Yang Z, Yang Z, Zhou S, Zhang H, Zang L. [Effect of apigenin on proliferation and apoptosis of human lung cancer NCI-H460 cells] Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:1137–1140. [PubMed] [Google Scholar]
- 15.Cosentino MJ, Nishida M, Rabinowitz R, Cockett AT. Histological changes occurring in the contralateral testes of prepubertal rats subjected to various durations of unilateral spermatic cord torsion. J Urol. 1985;133:906–911. doi: 10.1016/s0022-5347(17)49278-0. [DOI] [PubMed] [Google Scholar]
- 16.Lambropoulou M, Papadopoulos N, Tripsianis G, Alexiadis G, Pagonopoulou O, Kiziridou A, et al. Co-expression of survivin, c-erbB2, and cyclooxygenase-2 (COX-2): prognostic value and survival of endometrial cancer patients. J Cancer Res Clin Oncol. 2010;136:427–435. doi: 10.1007/s00432-009-0673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ypsilantis P, Lambropoulou M, Grapsa A, Tentes I, Tsigalou C, Panopoulou M, et al. Pringle maneuver deteriorates gut barrier dysfunction induced by extended-liver radiofrequency ablation. Dig Dis Sci. 2011;56:1548–1556. doi: 10.1007/s10620-010-1462-4. [DOI] [PubMed] [Google Scholar]
- 18.Zavras N, Kostakis ID, Sakellariou S, Damaskos C, Roupakas E, Tsagkari E, et al. Comparison of erythropoietin and sildenafil protective role against ischemia/reperfusion injury of the testis in adult rats. Int Urol Nephrol. 2014;46:731–736. doi: 10.1007/s11255-013-0569-x. [DOI] [PubMed] [Google Scholar]
- 19.Dokmeci D, Kanter M, Inan M, Aydogdu N, Basaran UN, Yalcin O, et al. Protective effects of ibuprofen on testicular torsion/detorsion-induced ischemia/reperfusion injury in rats. Arch Toxicol. 2007;81:655–663. doi: 10.1007/s00204-007-0189-2. [DOI] [PubMed] [Google Scholar]
- 20.Dokmeci D, Inan Μ, Basaran UΝ, Yalcin O, Aydogdu N, Turan FN, et al. Protective effect of L-Carnitine on testicular iscaemia-reperfusion injury in rats. Cell Biochem Funct. 2007;25:611–618. doi: 10.1002/cbf.1355. [DOI] [PubMed] [Google Scholar]
- 21.Wei SM, Yan ZZ, Zhou J. Beneficial effect of taurine on testicular ischemia-reperfusion injury in rats. Urology. 2007;70:1237–1242. doi: 10.1016/j.urology.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 22.Wei SM, Yan ZZ, Zhou J. Protective effect of rutin on testicular ischemia-reperfusion injury. J Pediatr Surg. 2011;46:1419–1424. doi: 10.1016/j.jpedsurg.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 23.Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G. Interleucin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leucocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178:2207–2211. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Moine O, Stordeur P, Schandene L, Marchant A, de Groote D, Goldman M, et al. Adenosine enhances IL-10 secretion by human monocytes. J Immunol. 1996;156:4408–4414. [PubMed] [Google Scholar]
- 25.Marchant A, Bruyns C, Vandenabeele P, Ducarme M, Gérard C, Delvaux A, et al. Interleucin-10 controls interferon-gamma and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol. 1994;24:1167–1171. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- 26.Gerard C, Bruyns C, Merchant A, Abramowicz D, Vandenabeele P, Delvaux A, et al. Interleucin-10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 28.Nagaraja HS, Jassal RS, Chakravarthi S, Thanikachalam P, Lee N, Anupama BK. Apigenin reduces cyclosporine-A induced changes in lipid hydroperoxides and total antioxidants in Sprague-Dawley rats. J Chin Clin Med. 2009;4:26–31. [Google Scholar]
- 29.Tsalkidou EG, Tsaroucha AK, Chatzaki E, Lambropoulou M, Papachristou F, Trypsianis G, et al. The effects of apigenin on the expression of Fas/FasL apoptotic pathway in warm liver ischemia-reperfusion injury in rats. Biomed Res Int. 2014;2014:157216. doi: 10.1155/2014/157216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lampropoulos P, Lambropoulou M, Papalois A, Basios N, Manousi M, Simopoulos C, et al. The role of apigenin in an experimental model of acute pancreatitis. J Surg Res. 2013;183:129–137. doi: 10.1016/j.jss.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 31.Chen ZX, Ying XX, Meng S, Zhu X, Jiang H, Cao Q, et al. High-performance Liquid Chromatographic determination and pharmacokinetic study of apigenin in rat plasma after intravenous administration. Arch Pharm Res. 2011;34:741–746. doi: 10.1007/s12272-011-0507-3. [DOI] [PubMed] [Google Scholar]


