Abstract
In addition to being a forage crop, Caliph medic (Medicago truncatula) is also a model legume plant and is used for research focusing on the molecular characterization of the interaction between rhizobia and plants. However, the endophytic microbiome in this plant is poorly defined. Endophytic bacteria play a role in supplying plants with the basic requirements necessary for growth and development. Moreover, these bacteria also play a role in the mechanism of salinity stress adaptation in plants. As a prelude to the isolation and utilization of these bacteria in Caliph medic farming, 41 bacterial OTUs were identified in this project from within the interior of the roots of this plant by pyrosequencing of the small ribosomal subunit gene (16S rDNA) using a cultivation-independent approach. In addition, the differential abundance of these bacteria was studied following exposure of the plants to salinity stress. About 29,064 high-quality reads were obtained from the sequencing of six libraries prepared from control and salinity-treated tissues. Statistical analysis revealed that the abundance of ~70% of the OTUs was significantly (p ≤ 0.05) altered in roots that were exposed to salinity stress. Sequence analysis showed a similarity between some of the identified species and other, known, growth-promoting bacteria, marine and salt-stressed soil-borne bacteria, and nitrogen-fixing bacterial isolates. Determination of the amendments to the bacterial community due to salinity stress in Caliph medic provides a crucial step toward developing an understanding of the association of these endophytes, under salt stress conditions, in this model plant. To provide direct evidence regarding their growth promoting activity, a group of endophytic bacteria were isolated from inside of plant roots using a cultivation-dependent approach. Several of these isolates were able to produce ACC-deaminase, ammonia and IAA; and to solubilize Zn+2 and PO4-3. This data is consistent with the predicted occurrence (based on cultivation-independent techniques) of these bacteria and provides some insight into the importance of the endophytic bacteria in Caliph medic when grown under normal and saline conditions.
Introduction
Endophytic bacteria refers to those species that are able to grow within plant tissues without showing disease symptoms, and survive by forming a symbiotic relationship with the host plant [1]. Endophytes can promote plant growth by increasing the availability of some nutrients, such as nitrogen, phosphorus, iron and zinc; by synthesizing growth hormones, such as indole-3 acetic acid, cytokinins and gibberellic acids [2]; and by producing 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase, an enzyme responsible for the cleavage of ACC, which is the immediate precursor of the hormone ethylene in all higher plants [3,4]. Endophytic bacteria are important for both the routine growth and the developmental processes of plants, as well as when plants experience biotic and abiotic stresses including salinity [5,6]. Under saline conditions, some endophytic microorganisms ameliorate the stress in plants by synthesizing osmoprotectant molecules, such as proline and/or trehalose [7], quaternary ammonium compounds in the cytoplasm [8,9], volatile organic molecules [10], and exopolysaccharides [9,11].
Salinity can severely affect plant health and yield by causing an imbalance in nutrient uptake, by increasing the negative osmotic water pressure on plant cells [12,13], and by reducing the availability of nutrients in the soil [14]. Recent research, however, has revealed that plant growth-promoting bacteria including both endophytic and rhizospheric bacteria can improve the survival chances and performance of plants under saline conditions [6,15,16]. For example, the inoculation of cucumber [17] and canola [3] with Pseudomonas putida UW4 has been found to enhance plant growth under saline conditions. Likewise, the treatment of pepper seedlings with Brevibacterium iodinum, Bacillus licheniformis and Zhihengliuela alba halotolerant bacteria reduced salt-induced ethylene synthesis and, as a result, promoted plant performance under the same conditions [18].
Alfalfa (Medicago sativa) and Caliph medic (Medicago truncatula) are important fodder crops worldwide, but their production has been severely reduced in recent decades due to soil salinity [19,20,21]. In addition, M. truncatula is considered to be a model legume plant for molecular research on rhizobium-legume symbiosis [22]. For this reason, M. truncatula was chosen for the current study into the impact of soil salinity on endophytic community richness in Caliph medic and other related species such as alfalfa.
Although numerous bacterial taxa were previously identified as endophytes in M. sativa, endophytes have not been studied in M. truncatula. For example, in addition to the well-known S. meliloti, a detailed study using Terminal-Restriction Fragment Length Polymorphism (T-RFLP) analysis, quantitative PCR and sequencing of the 16S rRNA gene showed that the root system of alfalfa was enriched with a wide range of species classified under the Sphingomonadaceae and Methylobacteriaceae bacteria families [23]. Other studies have shown that the bacteria community also includes Bacillus megaterium [24], Brevibacillus choshinensis and Microbacterium trichothecenolyticum [25], Endobacter medicaginis [26] and Micromonospora sp. [27].
Describing a microbial community structure, in which each bacterial member of the community is isolated, is not currently possible because we lack the knowledge to cultivate a large percentage of these microbes. In addition, determining the change to the community structure when the community is exposed to salinity stress requires a differential quantitative method in order to individually estimate the taxon abundance within the community. Currently, the use of the 16S rRNA gene sequencing in studying the microbial community structure is much more comprehensive than using culture-based approaches [28,29]. Therefore, in the project reported herein, a next-generation pyrosequencing method was used to characterize the structure of the endophytic community and to estimate the corresponding changes that occur in response to salinity stress in M. truncatula root tissues. These changes may provide an insight into the role of the endophytic microbial community in salinity tolerance in Caliph medic.
Material and Methods
Soil analysis
The physical and chemicals properties of the soil, including the electrical conductivity (E.C.) and the pH, were measured as described previously [30] by the Ministry of Agriculture and Fisheries’ soil analysis laboratories in Jomah, Oman.
Plant materials
Caliph medic (Medicago truncatula) seeds were surface-sterilized with 75% ethanol for 10 minutes and then with a 5% sodium hypochlorite solution for five minutes. Next, caliph medic seeds were rinsed three times with sterile distilled water. Seeds were planted in pots containing soil collected from fields used to grow different Medicago species such as alfalfa and caliph medic, which were located at the coordinates 23°39'42.5"N and 58°00'34.0"E, Jomah, Oman. Plants were grown in two–liter pots and placed in the field, where the day and night temperatures were respectively 28±2°C and 20±2°C, and in the natural daylight. Six pots were used, with each pot containing at least 10 seedlings. Three of the pots were used for the control treatment, while the other three pots were used for the NaCl treatment. The plants used in the control treatment were watered weekly with distilled water, while the salinity-treated plants were watered for the first two weeks with distilled water and then with increasing levels of saline solution on a weekly basis, starting with 50 mM, followed by 75 mM and then 100 mM NaCl for two successive weeks.
Plant roots were collected from the seedlings 50 days after planting. Roots of 10 different seedlings were pooled and considered as one replicate. Three replicates of the control and the NaCl treated pools were used in this experiment. The collected roots were surface-disinfected in line as described previously [31]. Briefly, a pool of roots from the control and the salinity-treated plants were separately washed in running water, then disinfected by treatment with 5.25% bleach for 3 minutes followed by 3% hydrogen peroxide solution for 3 minutes, and then washed twice with sterile distilled water containing a 10% solution of Tween 20. Finally, the roots were rinsed twice with sterile distilled water. To examine the surface disinfection efficiency, a sample of the roots from each pool was planted in solid TSA medium for one week at 28°C. Subsequently, the plates were examined for the presence of microbial-growing colonies. The surface-disinfected roots were flash-frozen in liquid nitrogen and kept at -80°C in a freezer until they were used for DNA extraction. The roots were grounded in liquid nitrogen using a sterile mortar and pestle. The DNeasy Plant Maxi Kit (Qiagen) was used to extract the total DNA, which contained both the plant cellular DNA and the microbial DNA of the endophytic community.
The bacteria communities were fingerprinted according to the ribosomal DNA (16S rRNA) sequences, using the pyrosequencing method (GS FLX+) and a 454 platform sequencer (Roche). The V3-V4 16S rRNA was amplified by PCR-fusion [32] using universal oligonucleotides (S1 Table). The PCR products were purified using AMPure9 beads and quantified using a Picogreen assay [33], while the CD-HIT-OTU (version 454–0.0.2) was used to assemble the raw data de novo. The amplicons were sequenced and assembled using the next-generation sequencing facilities at Macrogen, Inc. (Seoul, the Republic of Korea).
Isolation, identification and characterization of endophytic root bacteria
The endophytic bacteria were isolated from the surface sterilized roots of the Caliph medic plants grown under normal and saline conditions as previously described [6,31]. The bacterial strains were identified based on the 16S rDNA gene sequences. The 16S rDNA genes were amplified from the genomic DNA by PCR using the 27F and 1492R primers [34]. ACC deaminase activity in the newly isolated strains was determined using the procedure of Penrose and Glick [35]. The ability to produce IAA and similar compounds using the L-tryptophan was determined as previously described [6,31]. The capacity of the strains to produce ammonia was measured as previously described [36], while the ability to solubilize PO43- and Zn2+ using the Ca3(PO4)2 and the ZnO insoluble salts in the Pikovskaya’s agar media respectively, was determined using previously described methods [37].
16S rRNA sequence analysis
Raw data were demultiplexed using barcode sequences without allowing for any mismatch (Macrogen’s in-house software). Short reads were filtered, while tails and the reads that were too long were trimmed. Duplicates and chimeric reads were removed, with the resultant reads clustered with 100% identity using CD-HIT-DUP software [38]. Using a greedy algorithm [39], the remaining representative high-quality reads from the non-chimeric clusters were clustered into Operational Taxonomic Units (OTUs) with a similar cut-off identity at the species level as follows: for species 98%, for genus 94%, for family 90%, for order 85%, for class 80% and for phylum 75%.
Raw data were classified based on the barcode sequences of each sample. In order to find the best match, each sequence was compared (locally and globally) to the sequences available in the SILVA database. QIIME 1.8.0 software [40] was used to produce the OTU count. The similarity between the read sequences was examined in order to identify the OTUs as well as carry out statistical analysis on the diversity and evenness of the sample species. The Shannon, Simpson and Chao 1 indices were used to study the biodiversity based on the richness of the species and to estimate the abundance-based richness within the community [41].
The raw abundance value was used without rarefying for analyzing diversity statistics community richness and diversity using the biom files and the QIIME software. The goods coverage was also calculated using the calculator provided by software QIIME.
Each group (salinity and control treatments) was composed of three biological replicates and the validation of the data was based on p ≤ 0.05. With regard to the QIIME software, the Mann-Whitney U test, as a bootstrap version equal to 2,000 times, was used. The p-value was corrected by the Bonferroni procedure for multiple comparisons [42,43]. The complete linkage between the hierarchical cluster analysis and the visualization of the abundance values as a heat map were carried out using the PermutMatrix software [44]. The hierarchical cluster was set at a complete linkage and the dissimilarity was calculated based on the Euclidean distance. A neighbor-joining phylogenetic tree was built using the Mega software package 5.0 [45] and the default settings.
Principal Coordinate Analysis (PCoA) [46] analysis was used as an ordination-based approach to illustrate the variation between the bacterial community compositions in response to salinity treatment using the Past 3 software package [47] and the Gower’ distance matrix [48]. In addition, analysis of similarity test (ANOSIM) [49] and the Gower distance matrix index were used to assess the pairwise comparisons of significant differences between the microbial communities. The p-value was recalculated based on the Bonferroni significance.
The 16S rDNA sequences were deposited in GenBank/EMBL/DDBJ under the accession numbers KU587127-KU587167 and KX395941-KX396022.
Results and Discussion
Salinity stress impaired the growth of seedlings
Plant species vary in their ability to cope with salinity stresses. Although Caliph medic is considered to be a moderately salinity-tolerant plant [50], this tolerance depends on the growth stage and the genotype of the plant [20,51]. In this study, we have identified endophytic bacteria from Caliph medic at the seedling stage; therefore, the total endophytic microbial community of this plant may not be limited to those microbes described in this study. In comparison with the control experiment, the effect of the soil salinity stress was evident in the performance of the seedlings (Fig 1).
Fig 1. The influence of salinity treatment on Caliph medic seedlings.

This is quite normal since the soil salinity level measured as electrical conductivity (E.C.) dramatically increased due to the treatment from 1.23 (S.D. ±0.22) to 14.36 (S.D. ±0.4). That increase in the E.C. not only significantly increased the Na+ and Cl+ (p ≤ 0.05) (Table 1), but it also increased the soluble sulphate in the soil, presumably because sodium interacts with sulphate to form a soluble salt [52].
Table 1. Physicochemical properties of the soil used to grow Caliph medic seeds.
| Soil physicochemical properties | Average Content | p- value | |
|---|---|---|---|
| Control | NaCl-treatment | ||
| EC (dS/m) | 1.23 | 14.40 | 0.001 |
| pH | 7.30 | 7.30 | 1.000 |
| Na (meq/l) | 11.16 | 81.27 | 0.001 |
| K (meq/l) | 2.86 | 3.57 | 0.130 |
| Ca (meq/l) | 18.40 | 19.51 | 0.030 |
| Cl (meq/l) | 15.03 | 116.33 | 0.001 |
| HCO3 (meq/l) | 7.24 | 1.17 | 0.001 |
| SO4 (meq/l) | 8.15 | 11.78 | 0.001 |
| Gravel % | 18.17 | 18.14 | 0.970 |
| Sand % | 93.04 | 94.60 | 0.100 |
| Silt % | 3.20 | 3.20 | 1.000 |
| Clay % | 2.30 | 2.30 | 1.000 |
| CaCO3 | 22.10 | 21.89 | 0.001 |
| N % | 0.06 | 0.06 | 1.000 |
| P (ppm) | 89.70 | 110.80 | 1.000 |
| K (ppm) | 330.00 | 390.00 | 1.000 |
Soil analysis revealed a significant (p ≤ 0.05) reduction in the bicarbonate and a slight reduction in the calcium carbonate content of the soil in response to salinity treatment (Table 1). This is consistent with the observation that soil salinity reduces the global soil carbon stocks due to a reduction in microbial activity and, hence, in organic carbon decomposition rates [53].
16S rRNA sequencing uncovered the presence of a divergent endophytic bacterial community
Plants interact with the surrounding environment, including soil inhabitants such as bacteria; however, these microorganisms have only a minor effect on the structure of the endophytic bacterial community [54]. Since it was not possible to exclusively extract the microbial genomes from the root tissues, the total DNA, including the plant genomic DNA, was extracted from these tissues and used for bacterial DNA barcoding. Pyrosequencing of the resultant 16S rRNA libraries yielded a total of 119,381,266 pb from three control and three treatment libraries. The nucleotide sequences obtained from the control and the treated libraries were assembled into 167,698 reads, 94% were coded for high quality barcode sequences with an average length of ~712 bp. After the removal of the mitochondrion and chloroplast related sequences from the OTU list, a total of 29,064 high quality 16S rRNA sequences were obtained from the six libraries including 2,274 and 26,790 belonged to the control and the NaCl-treated libraries, respectively. The relatively high number of 16S rRNA reads obtained from the communities isolated from the roots grown in saline conditions is due to the presence of some overrepresented OTUs in these communities. For example, the reads associated with the three bacterial species Thalassospira povalilytica, Castellaniella hirudinis and Pseudomonas stutzeri account for 39.3% (10,528) of the total reads obtained from the sequencing of these libraries (Table 2).
Table 2. Bacterial OTUs identified from Caliph medic roots based on 16S rRNA DNA sequences and the mean of abundance in the libraries prepared from the control and salinity-treated plants.
Significant enrichment (p ≤ 0.05) of a certain OTU was calculated based on three biological replicates. The OTUs were arranged based on the descending p-value.
| OTU | Mean of abundance | p-value | OTU | Mean of abundance | p-value | ||
|---|---|---|---|---|---|---|---|
| Control | Treatment | Control | Treatment | ||||
| Brucella inopinata | 70.7 | 0 | 0.036 | Pseudoxanthomonas mexicana | 4.7 | 0.4 | 0.053 |
| Sphingobium xenophagum | 56.7 | 3.0 | 0.037 | Acidovorax soli | 4.0 | 0 | 0.055 |
| Tistrella mobilis | 12.0 | 0.0 | 0.041 | Enterobacter sp. | 0.4 | 744.4 | 0.055 |
| Streptomyces variabilis | 0.0 | 6.7 | 0.041 | Cellvibrio diazotrophicus | 3.4 | 85.7 | 0.057 |
| Enterobacter kobei | 0.0 | 79.4 | 0.043 | Pseudomonas stutzeri | 6.0 | 841.0 | 0.058 |
| Thalassospira xianhensis | 0.0 | 19.7 | 0.043 | Enterobacter cloacae | 5.0 | 704.0 | 0.058 |
| Pseudoxanthomonas broegbernensis | 6.4 | 0.0 | 0.044 | Achromobacter pulmonis | 0.7 | 10.7 | 0.058 |
| Pseudomonas resinovorans | 3.4 | 8.7 | 0.045 | Pseudomonas borbori | 3.4 | 474.0 | 0.058 |
| Marinobacter gudaonensis | 0.0 | 31.0 | 0.045 | Rhizobium rosettiformans | 40.4 | 244.4 | 0.063 |
| Shinella granuli | 85.0 | 4.7 | 0.045 | Pseudomonas indica | 3.0 | 610.4 | 0.067 |
| Fluviicola sp. | 0.0 | 13.0 | 0.045 | Salinicola salarius | 2.7 | 46.7 | 0.067 |
| Sphingopyxis macrogoltabida | 7.0 | 1.4 | 0.047 | Rheinheimera aquimaris | 62.7 | 765.0 | 0.068 |
| Inquilinus sp. | 0.0 | 67.0 | 0.048 | Pseudomonas aeruginosa | 185 | 330.7 | 0.068 |
| Halomonas lutea | 2.0 | 118.7 | 0.049 | Methylophaga sp. | 1.4 | 16.7 | 0.071 |
| Flavobacterium glycines | 0.0 | 64.4 | 0.049 | Halomonas sp. | 92.4 | 628.7 | 0.072 |
| Pseudomonas pseudoalcaligenes | 0.0 | 4.4 | 0.050 | Pseudomonas mendocina | 1.4 | 260.4 | 0.073 |
| Thalassospira povalilytica | 2.4 | 1351.7 | 0.051 | Sphingobacterium thalpophilum | 5.7 | 0.0 | 0.185 |
| Beijerinckia fluminensis | 46.7 | 0.0 | 0.052 | Rhizobium halotolerans | 0.0 | 5.7 | 0.190 |
| Marinobacter nanhaiticus | 17.0 | 62.0 | 0.053 | Sphingomonas koreensis | 3.7 | 2.4 | 0.583 |
| Pseudomonas sp. | 21.4 | 5.4 | 0.053 | Methylobacillus flagellatus | 2.4 | 2.0 | 0.893 |
| Castellaniella hirudinis | 0.0 | 1316.7 | 0.053 | ||||
The taxonomy abundance ratio and the sequence similarity analysis of the 16 rRNA using BLAST showed that about 99% of the 16S rRNAs were classified under the Proteobacteria phylum, while the rest were classified under the Actinobacteria and Bacteroidetes phyla. The Actinobacteria phylum included the Streptomyces family; the Bacteroidetes phylum included the Flavobacteria and Sphingobacteriia families; and the Proteobacteria included 14 different families including Chromatiaceae, Enterobacteriaceae, Pseudomonadaceae and Rhizobiaceae.
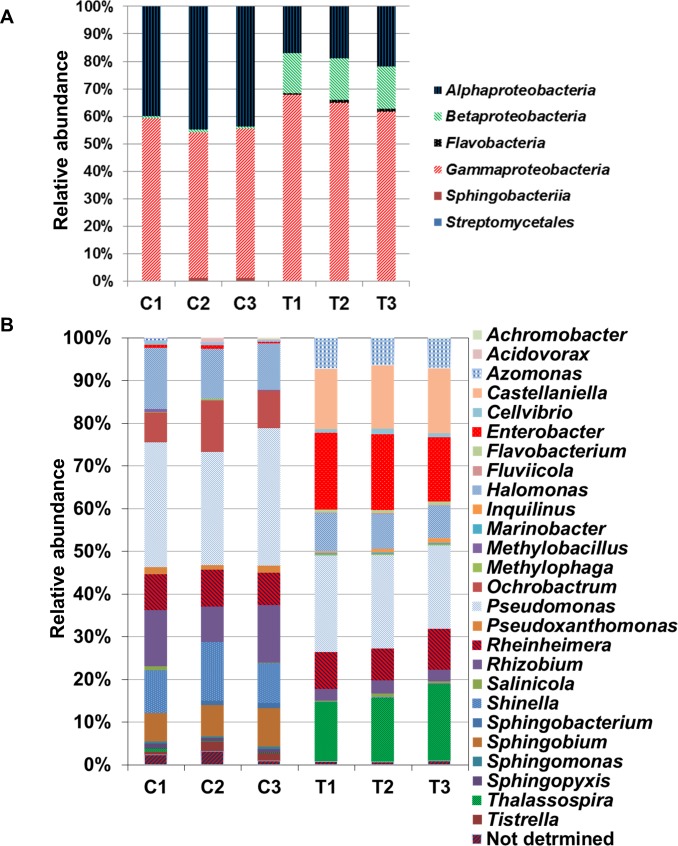
The proportional enrichment of Alphaproteobacteria class was high within the microbial community isolated from roots grown under normal conditions however, Gammaproteobacteria, Betaproteobacteria were dominant in response to salinity. In addition, salinity treatment leads to the appearance of Flavobacteria and Streptomycetales classes and disappearance of the Sphingobacteria class within the endophytic community (Fig 2A).
Fig 2.
Relative abundance of different class (A) and genus (B) in each replica of the control (C1-3) and NaCl treated (T1-3) samples. The abundance is expressed as the percentage in the total number of reads per each OTU.
The analysis also revealed the presence of a total of 41 OTUs, representing 27 unique genera, where Enterobacter, Halomonas, Marinobacter, Pseudomonas, Pseudoxanthomonas, Rhizobium and Thalassospira spp. were represented more than once in the communities (Table 2, and Fig 2B). It is quite normal to find that the majority of the identified bacteria were assigned to Proteobacteria because it is the second largest known bacterial phylum and one of the major ones in soil [55]. This phylum included eight different Pseudomonas species (Table 2), some of which previously showed plant growth-promoting activity, including P. aeruginosa [56] and P. stutzeri [57]. It is noteworthy that many bacteria have 5 to 10 copies of 16S rRNA therefore, there is the possibility of sequence divergence between different copies of the gene from the same organism [58].
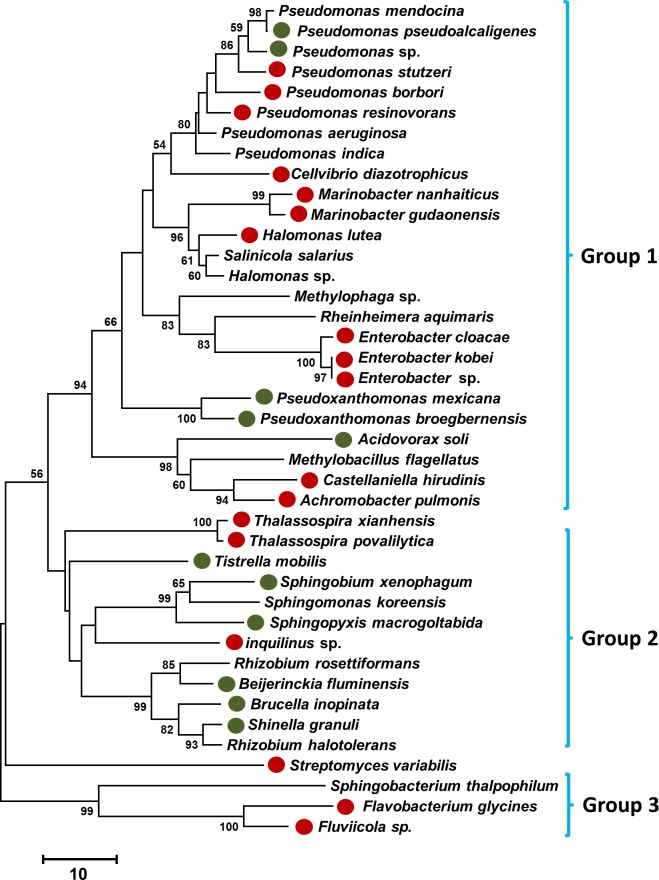
The relationship between the 16S rRNA gene sequences based on phylogenetic analysis showed that these sequences were clustered into three major groups, although the Streptomyces variabilis sequence was not among any of these clades because the unique 16S rRNA sequence belonged to the Actinobacteria phylum (Fig 3).
Fig 3. A neighbor-joining phylogenetic tree that was constructed based on the 16S rRNA DNA sequences, showing the relationships between the bacterial taxa identified in this study.
The bootstrap values >50% (based on 1,000 replications) are shown at branching points. Differential abundance OTUs (p ≤ 0.05) in the salinity-treated and control roots are indicated by closed red and green circles, respectively.
The influence of salinity stress on the microbial community structure
The sequencing of the 16S rRNA library revealed the presence of a relatively low-divergence endophytic community, indicated as the number of identified OTUs (Table 3). The biodiversity and the richness of the bacterial endophytic communities were assessed based on the OTUs, Shannon, Simpson, Chao 1 and Goods Coverage indices. Because it was not recommended to use high impact treatment of data, especially when the coverage of sample diversity is not high[59], the raw abundance value was used without rarefying.
Table 3. Changes in the community richness and biodiversity indices among the six 16S rRNA libraries in response to NaCl treatment in Caliph medic.
Significant changes based on p ≤ 0.05, n = 3, which were calculated using the one-way analysis of variance (ANOVA) test, are indicated by an asterisk.
| Index | Control | NaCl-Treated | p-value |
|---|---|---|---|
| OTUs | 29.33 | 34.33 | 0.03* |
| Chao1 | 31.25 | 34.83 | 0.21 |
| Shannon | 0.30 | 3.15 | 0.00* |
| Simpsoin | 0.06 | 0.79 | 0.00* |
| Goods Coverage | 99.99 | 99.99 | 0.56 |
The average number of OTUs and the evenness of species within the bacterial community were relatively low, but was significantly increased based on p ≤ 0.05, as indicated by the Shannon index, when plants were grown under saline conditions (Table 3). In addition, the probability that two randomly selected individuals in the habitat will belong to the same species was also increased, as indicated by the Simpson index under the same conditions; however, the estimated average richness for an OTU was unchanged in response to salinity stress, as indicated by the Chao 1 index (Table 3). The Goods Coverage index indicated that the samples were very well represented in the larger environment with an expectation value of more than 99% and this situation did not change in response to salinity (Table 3).
The low average number of the OTUs obtained from these communities is because of the fact that only a small number of the soil bacteria are facultative endophytes. Furthermore, the structure of a microbial community is altered based on the genetic basis of the microbial-host specificity, microbial-microbial interaction [60] and even microbial-tissue specificity (within the same host) [61], as well as based on the environmental conditions. For example, Dong and his colleagues [62] found that there are species-specific and inoculum-level variations in the ability of some bacteria to colonize M. sativa and M. truncatula seedlings.
The increase in the number of OTUs observed in the community, which were identified in salinity-stressed roots, is due to the alterations in the endophytic microbial ecosystem, which may also involve the presence of some opportunistic phytopathogens that may attack plants during periods when the plant is stressed. For example, P. aeruginosa is an opportunistic pathogen and was enriched in the communities isolated from the salinity treated roots [63]. The presence of some opportunistic phytopathogens may also associated with the appearance of some biocontrol microbes such as P. stutzeri (Table 2), a bacterium which has the ability to secrete hydrolytic enzymes against the Fusarium solani mycelia, the causative agent for the root rot disease in various plant species [64].
In fact, as a consequence of the salinity treatment, eight additional OTUs were observed within the community (p ≤ 0.05) while five other bacterial species disappeared (Table 2). For example, Thalassospira xianhensis and Castellaniella hirudinis were not represented in the bacterial communities isolated from the untreated roots while Brucella inopinata and Beijerinckia fluminensis were not represented in the communities of the salinity-treated roots (Table 2). Localization of the differentially enriched species of significant abundance (p ≤ 0.05) on the phylogenetic tree revealed that the three major clades of this tree equally embraced these bacteria, regardless of their species (Fig 3).
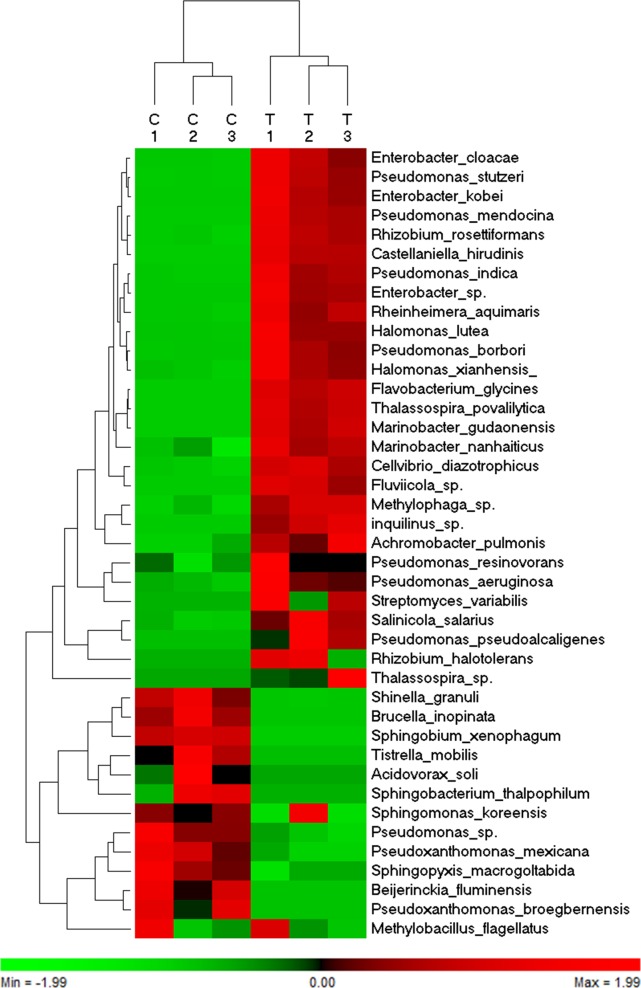
Hierarchical cluster analysis, based on the abundance of 41 bacterial species identified from six communities of roots grown in normal and saline conditions revealed that 39 species were clustered into two major groups (Fig 4). These groups shared a conserved abundance profile among the three libraries of the same treatment (control vs. salt). The first group included 28 OTUs with a high level of abundance in the bacterial communities identified in plants grown under saline conditions, whereas the second group included 11 OTUs with a high level of abundance in the communities identified in plants grown under normal conditions. Since they did not have a consistent richness among the three biological replicates, Methylobacillus flagellates was clustered out of the first group and the Sphingomonas koreensis was clustered out of the second group.
Fig 4. A heat map of the hierarchical cluster analysis and a dendrogram showing the normalized relative abundance of 41 identified species from three bacterial communities, which were prepared from roots grown under normal conditions (C1-3) and from three bacterial communities prepared from roots grown under NaCl stress (T1-3).
Differential enrichment analysis using the Mann-Whitney U test and based on the p ≤ 0.05 showed that, out of 41 OTUs identified in the bacterial community living in the roots, 29 were differentially enriched when the plants were exposed to salinity stress (Table 2), of which 10 were negatively affected and 19 were enriched due to salinity treatment. For example, Brucella inopinata, Sphingobium xenophagum and Shinella granuli were abundant in the root when grown under normal conditions. On the other hand, Enterobacter kobei, Halomonas lutea, Thalassospira povalilytica and Pseudomonas stutzeri were abundant in the root when grown under saline conditions.
The differentially enriched species in response to salinity included OTUs that were previously isolated from M. sativa, such as Endobacter medicaginis [26], and other OTUs were isolated from corn, such as Enterobacter kobei [65], and Enterobacter cloacae from date palm [6]. Previous studies showed that some strains of Enterobacter sp. are able to help plants growing under saline conditions by providing ACC deaminase, the phytohormone IAA and siderophores that facilitate iron acquisition under iron-limiting conditions.
It is noteworthy that other differentially enriched species identified in this study were previously isolated from marine and salt-polluted environments. For example, Marinobacter gudaonensis was isolated from oil-polluted saline soil [66] and a Marinobacter nanhaiticus strain was isolated from the sediment of the South China Sea [67]. The latter strain was able produce a suite of acylpeptidic marinobactin siderophores [68]. In addition, Streptomyces variabilis was isolated from the marine sponge Iotrochota sp. [69] and the Halomonas lutea sp. nov., which is a moderately halophilic bacterium, was isolated from a salt lake [70].
Some of the identified OTUs were assigned to nitrogen-fixing bacteria. For example, Cellvibrio diazotrophicus were isolated from the rhizosphere of salt meadow plants [71], Beijerinckia fluminensis from the giant reed and switchgrass rhizosphere [72] and acidic soil [73], and P. stutzeri [74] from chemically-stressed soil [75].
Identification of bacterial species from the M. truncatula roots, similar to those species isolated from saline and marine environments, is not surprising since the soil used in this experiment was mainly composed of a sandy texture (Table 1) and obtained from a field located near to the seashore; therefore, the microbial community within this soil would likely have been affected by the marine ecosystem.
The bacterial species identified in this study also included OTUs that potentially are useful in the environment. For example: Thalassospira xianhensis is a polycyclic aromatic hydrocarbon-degrading marine bacteria [76]; Achromobacter pulmonis was isolated from Phragmites australis (common reeds) and is able to remove carbamazepine [77]; Pseudomonas resinovorans is a carbazole- (CAR-) degrading bacterium [78]; Thalassospira povalilytica is a marine polyvinyl-alcohol degrading bacterium [79]; and Streptomyces variabilis is also a marine-derived bacteria, which produces the anti-cancer agent ammosamide [80].
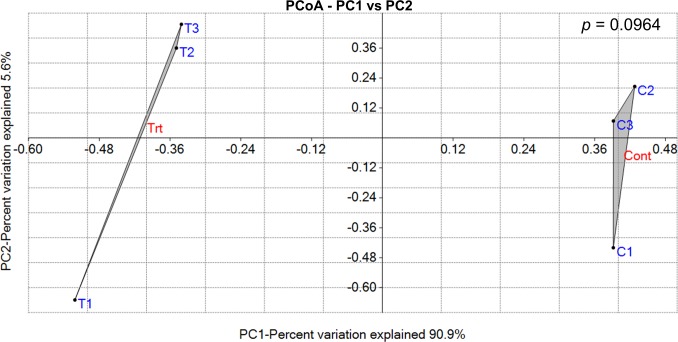
Despite the appearance of several new OTUs in response to salinity treatment, the pairwise overall variation analysis using the ordination-based and the ANOSIM similarity test approaches did not confirm the appearance of a significantly totally different endophytic community in Caliph medic roots in response to salinity stress (Fig 5).
Fig 5. Principal Coordinate Analysis (PCoA) illustrating distances between bacterial communities identified from control (C1-C3) and NaCl-treated roots (T1-T3) of Caliph medic.
The pairwise comparison using the ANOSIM test did not show significant variation (p = 0.0964) between the community groups identified from control plants (Cont) and NaCl plants (Trt). The first two coordinates explained about 96% while the third coordinate explained only 2.3% of the variation.
Unlike free living soil microbes, salinity may have a minor effect on the endophytic communities since plants may buffer, to some extent, the deleterious effects of salinity on the roots’ ecosystem therefore, the low bacterial variation between the communities is not unexpected.
In order to confirm the presence of endophytic plant growth promoting bacteria in the Caliph medic roots of plants grown under normal and salinity conditions, these bacteria were isolated, identified and their ability to produce ACC-deaminase, ammonia and IAA were measured. Moreover, the ability of these isolates to solubilize zinc and phosphorus were tested. The results showed that the newly isolated bacteria belonged to the Enterobacteriaceae and Pseudomonadaceae family. The isolated bacteria from plants grown under normal condition included strains similar to Enterobacter and Pseudomonas species (S2A Table), while those strains isolated from the plants grown under saline condition included strains similar to Enterobacter, Klebsiella and Pantoea species (S2B Table). Several of the isolated strains analyzed in this study showed the ability to catalyze the hydrolysis of the ACC to ɑ-ketobutyrate, to produce ammonia and IAA or similar compounds, and to solubilize zinc and phosphorus, regardless their original source (from salinity treated or untreated roots) (S3 Table). Therefore, the bacterial community present in the internal parts of the roots of Caliph medic have the potential role to promote plant growth under both normal and saline conditions. Several isolates identified in this experiment were also identified using the cultivation independent approach, however, both approaches (cultivation dependent and independent) are incomparable since other factors such as the use of different culture media will affect the enrichment. Furthermore, the cultivation dependent method is not quantitative.
Despite the identification of this set of bacterial species from M. truncatula roots, other bacteria species may remain unidentified. This is because a bacteria-host symbiotic relationship depends on the nature of the soil and other environmental factors, such as temperature. Additionally, by using a common DNA extraction method, it is difficult to ensure the extraction of genomic DNA and, in turn, the barcoding of every endophytic bacteria species from the root, since some species can form refractory-coated spore-like structures that prevent complete DNA extraction [81].
In conclusion, we were able in this report to identify a bacterial community containing a wide range of known and unknown endophytic species that are affected by the saline conditions in root tissues. The information obtained from this project is important for the isolation and further molecular characterization of endophytes from the model plant M. truncatula.
Supporting Information
(DOCX)
Endophytic bacterial strains isolated from Caliph medic roots when plant grew under normal (A) and saline conditions (B).
(XLSX)
Activity or product not detected in the assays is denoted by N.D.
(XLSX)
Acknowledgments
We would like to thank the Ministry of Agriculture and Fisheries and the College of Science, Sultan Qaboos University, Oman for funding this research project. Authors would like to thank Dr. Aliya Al-Ansari for the help in the bioinformatics analysis.
Data Availability
The 16S rDNA sequences were deposited in GenBank/EMBL/DDBJ under the accession numbers KU587127-KU587167 and KX395941-KX396022.
Funding Statement
This work was supported by the Ministry of Agriculture and Fisheries and the College of Science, Sultan Qaboos University, Oman. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 71: 4951–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 41: 109–117. [Google Scholar]
- 3.Cheng Z, Park E, Glick BR (2007) 1-Aminocyclopropane-1-carboxylate deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can. J. Microbiol. 53: 912–918. [DOI] [PubMed] [Google Scholar]
- 4.Glick BR (2004) Bacterial ACC deaminase and the alleviation of plant stress. Adv. Appl. Microbiol. 56: 291–312. [DOI] [PubMed] [Google Scholar]
- 5.Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaish MW, Antony I, Glick BR (2015) Isolation and characterization of endophytic plant growth-promoting bacteria from date palm tree (Phoenix dactylifera L.) and their potential role in salinity tolerance. Antonie Van Leeuwenhoek 107: 1519–1532. 10.1007/s10482-015-0445-z [DOI] [PubMed] [Google Scholar]
- 7.Suárez R, Wong A, Ramírez M, Barraza A, Orozco MdC, Cevallos MA, et al. (2008) Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol. Plant-Microbe Interact. 21: 958–966. 10.1094/MPMI-21-7-0958 [DOI] [PubMed] [Google Scholar]
- 8.Jha B, Gontia I, Hartmann A (2012) The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential. Plant Soil 356: 265–277. [Google Scholar]
- 9.Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 184: 13–24. 10.1016/j.micres.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 10.Timmusk S, El-Daim IAA, Copolovici L, Tanilas T, Kännaste A, Behers L, et al. (2014) Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PloS one 9: e96086 10.1371/journal.pone.0096086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szymańska S, Piernik A, Hrynkiewicz K (2013) Metabolic potential of microorganisms associated with the halophyte Aster tripolium L. in saline soils. Ecological Questions 18: 9–19. [Google Scholar]
- 12.Yaish MW (2015) Proline accumulation is a general response to abiotic stress in the date palm tree (Phoenix dactylifera L.). Genet Mol Res 14: 9943–9950. 10.4238/2015.August.19.30 [DOI] [PubMed] [Google Scholar]
- 13.Yaish MW, Kumar PP (2015) Salt tolerance research in date palm tree (Phoenix dactylifera L.), past, present, and future perspectives. Front. Plant Sci. 6: 348 10.3389/fpls.2015.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moradi A, Tahmourespour A, Hoodaji M, Khorsandi F (2011) Effect of salinity on free living-diazotroph and total bacterial populations of two saline soils. Afr. J. Microbiol. Res. 5: 144–148. [Google Scholar]
- 15.Ali S, Charles TC, Glick BR (2014) Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 80: 160–167. 10.1016/j.plaphy.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 16.Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol. Lett. 278: 1–9. [DOI] [PubMed] [Google Scholar]
- 17.Gamalero E, Berta G, Massa N, Glick B, Lingua G (2010) Interactions between Pseudomonas putida UW4 and Gigaspora rosea BEG9 and their consequences for the growth of cucumber under salt‐stress conditions. J. Appl. Microbiol 108: 236–245. 10.1111/j.1365-2672.2009.04414.x [DOI] [PubMed] [Google Scholar]
- 18.Siddikee MA, Glick BR, Chauhan PS, jong Yim W, Sa T (2011) Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol. Biochem. 49: 427–434. 10.1016/j.plaphy.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 19.Al-Lawati A, Al-Bahry S, Victor R, Al-Lawati A, Yaish M (2016) Salt stress alters DNA methylation levels in alfalfa (Medicago spp). Genet. Mol. Res. 15: 1. [DOI] [PubMed] [Google Scholar]
- 20.Peel MD, Waldron BL, Jensen KB, Chatterton NJ, Horton H, Dudley LM. (2004) Screening for salinity tolerance in alfalfa. Crop Sci. 44: 2049–2053. [Google Scholar]
- 21.Postnikova OA, Shao J, Nemchinov LG (2013) Analysis of the alfalfa root transcriptome in response to salinity stress. Plant Cell Physiol. 54: 1041–1055. 10.1093/pcp/pct056 [DOI] [PubMed] [Google Scholar]
- 22.Barker DG, Bianchi S, Blondon F, Dattée Y, Duc G, Essad S, et al. (1990) Medicago truncatula, a model plant for studying the molecular genetics of theRhizobium-legume symbiosis. Plant Mol. Biol. Report. 8: 40–49. [Google Scholar]
- 23.Pini F, Frascella A, Santopolo L, Bazzicalupo M, Biondi EG, Scotti C, et al. (2012) Exploring the plant-associated bacterial communities in Medicago sativa L. BMC Microbiol. 12: 78 10.1186/1471-2180-12-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalifa AY, Alsyeeh A-M, Almalki MA, Saleh FA (2016) Characterization of the plant growth promoting bacterium, Enterobacter cloacae MSR1, isolated from roots of non-nodulating Medicago sativa. Saudi J. Biol. Sci. 23: 79–86. 10.1016/j.sjbs.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stajković O, De Meyer S, Miličić B, Willems A, Delić D (2009) Isolation and characterization of endophytic non-rhizobial bacteria from root nodules of alfalfa (Medicago sativa L.). Bot. Serb. 33: 107–114. [Google Scholar]
- 26.Ramírez-Bahena MH, Tejedor C, Martín I, Velázquez E, Peix A (2013) Endobacter medicaginis gen. nov., sp. nov., isolated from alfalfa nodules in an acidic soil. Int. J. Syst. Evol. Microbiol. 63: 1760–1765. 10.1099/ijs.0.041368-0 [DOI] [PubMed] [Google Scholar]
- 27.Trujillo ME, Alonso-Vega P, Rodríguez R, Carro L, Cerda E, Alonso P, et al. (2010) The genus Micromonospora is widespread in legume root nodules: the example of Lupinus angustifolius. ISME J. 4: 1265–1281. 10.1038/ismej.2010.55 [DOI] [PubMed] [Google Scholar]
- 28.Lipson DA, Schmidt SK (2004) Seasonal changes in an alpine soil bacterial community in the Colorado Rocky Mountains. Appl. Environ. Microbiol. 70: 2867–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spain AM, Krumholz LR, Elshahed MS (2009) Abundance, composition, diversity and novelty of soil Proteobacteria. ISME J. 3: 992–1000. 10.1038/ismej.2009.43 [DOI] [PubMed] [Google Scholar]
- 30.Chapman HD, Pratt PF (1962) Methods of analysis for soils, plants and waters. Soil Sci. 93: 68. [Google Scholar]
- 31.Rashid S, Charles TC, Glick BR (2012) Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl. Soil Ecol. 61: 217–224. [Google Scholar]
- 32.Hattori M, Sakaki Y (1986) Dideoxy sequencing method using denatured plasmid templates. Anal. Biochem. 152: 232–238. [DOI] [PubMed] [Google Scholar]
- 33.Ahn SJ, Costa J, Emanuel JR (1996) PicoGreen quantitation of DNA: effective evaluation of samples pre-or psost-PCR. Nucleic Acids Res. 24: 2623–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane D (1991) 16S/23S rRNA sequencing. Nucleic acid techniques in bacterial systematics: 125–175. [Google Scholar]
- 35.Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase‐containing plant growth‐promoting rhizobacteria. Physiol. Plant. 118: 10–15. [DOI] [PubMed] [Google Scholar]
- 36.Marques AP, Pires C, Moreira H, Rangel AO, Castro PM (2010) Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol. Biochem. 42: 1229–1235. [Google Scholar]
- 37.Pikovskaya R (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17: e370. [Google Scholar]
- 38.Huang Y, Niu B, Gao Y, Fu L, Li W (2010) CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics 26: 680–682. 10.1093/bioinformatics/btq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J. Comp. Biol 7: 203–214. [DOI] [PubMed] [Google Scholar]
- 40.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7: 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magurran AE (2013) Measuring biological diversity: John Wiley & Sons. [Google Scholar]
- 42.Simes RJ (1986) An improved Bonferroni procedure for multiple tests of significance. Biometrika 73: 751–754. [Google Scholar]
- 43.Hochberg Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75: 800–802. [Google Scholar]
- 44.Caraux G, Pinloche S (2005) PermutMatrix: a graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics 21: 1280–1281. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9: 299–306. 10.1093/bib/bbn017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gower JC (1966) Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53: 325–338. [Google Scholar]
- 47.Hammer Ø, Harper D, Ryan P (2001) PAST: Paleontological Statistics Software Package for education and data analysis. Palaeontol. Electron 4. [Google Scholar]
- 48.Gower JC (1985) Measures of similarity, dissimilarity and distance. Ency. Stat. Sc. 5: 3. [Google Scholar]
- 49.Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18: 117–143. [Google Scholar]
- 50.Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59: 651–681. 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- 51.Howieson J, Ballard R (2004) Optimising the legume symbiosis in stressful and competitive environments within southern Australia—some contemporary thoughts. Soil Biol. Biochem. 36: 1261–1273. [Google Scholar]
- 52.Metternicht G, Zinck A (2008) Remote sensing of soil salinization: Impact on land management: CRC Press. [Google Scholar]
- 53.Setia R, Gottschalk P, Smith P, Marschner P, Baldock J, Setia D, et al. (2013) Soil salinity decreases global soil organic carbon stocks. Sci. Total Environ. 465: 267–272. 10.1016/j.scitotenv.2012.08.028 [DOI] [PubMed] [Google Scholar]
- 54.Johnston-Monje D, Mousa WK, Lazarovits G, Raizada MN (2014) Impact of swapping soils on the endophytic bacterial communities of pre-domesticated, ancient and modern maize. BMC Plant Biol. 14: 233 10.1186/s12870-014-0233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssen PH (2006) Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 72: 1719–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tewari S, Arora NK (2014) Multifunctional exopolysaccharides from Pseudomonas aeruginosa PF23 involved in plant growth stimulation, biocontrol and stress amelioration in sunflower under saline conditions. Curr. Microbiol. 69: 484–494. 10.1007/s00284-014-0612-x [DOI] [PubMed] [Google Scholar]
- 57.Han Y, Wang R, Yang Z, Zhan Y, Ma Y, Ping S, et al. (2015) 1-Aminocyclopropane-1-Carboxylate Deaminase from Pseudomonas stutzeri A1501 Facilitates the Growth of Rice in the Presence of Salt or Heavy Metals. J. Microbiol. Biotechnol. 25: 1119–1128. 10.4014/jmb.1412.12053 [DOI] [PubMed] [Google Scholar]
- 58.Pei AY, Oberdorf WE, Nossa CW, Agarwal A, Chokshi P, Gerz EA, et al. (2010) Diversity of 16S rRNA genes within individual prokaryotic genomes. Appl. Environ. Microbiol. 76: 3886–3897. 10.1128/AEM.02953-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McMurdie PJ, Holmes S (2014) Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol 10: e1003531 10.1371/journal.pcbi.1003531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kloepper JW (1996) Host specificity in microbe-microbe interactions. Bioscience 46: 406–409. [Google Scholar]
- 61.Zhang H, Kim M-S, Sun Y, Dowd SE, Shi H, Paré PW. (2008) Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant-Microbe Interact. 21: 737–744. 10.1094/MPMI-21-6-0737 [DOI] [PubMed] [Google Scholar]
- 62.Dong Y, Iniguez AL, Ahmer BM, Triplett EW (2003) Kinetics and strain specificity of rhizosphere and endophytic colonization by enteric bacteria on seedlings of Medicago sativa and Medicago truncatula. Appl. Environ. Microbiol. 69: 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stover CK, Pham XQ, Erwin A, Mizoguchi S, Warrener P, Hickey M, et al. (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406: 959–964. [DOI] [PubMed] [Google Scholar]
- 64.Lim H-S, Kim Y-S, Kim S-D (1991) Pseudomonas stutzeri YPL-1 genetic transformation and antifungal mechanism against Fusarium solani, an agent of plant root rot. Appl. Environ. Microbiol. 57: 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szilagyi-Zecchin VJ, Ikeda AC, Hungria M, Adamoski D, Kava-Cordeiro V, Glienke C, et al. (2014) Identification and characterization of endophytic bacteria from corn (Zea mays L.) roots with biotechnological potential in agriculture. AMB Express 4: 26 10.1186/s13568-014-0026-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu J, Cai H, Yu S-L, Qu R, Yin B, Guo Y-F, et al. (2007) Marinobacter gudaonensis sp. nov., isolated from an oil-polluted saline soil in a Chinese oilfield. Int. J. Syst. Evol. Microbiol. 57: 250–254. [DOI] [PubMed] [Google Scholar]
- 67.Gao W, Cui Z, Li Q, Xu G, Jia X, Zheng L. (2013) Marinobacter nanhaiticus sp. nov., polycyclic aromatic hydrocarbon-degrading bacterium isolated from the sediment of the South China Sea. Antonie van Leeuwenhoek 103: 485–491. 10.1007/s10482-012-9830-z [DOI] [PubMed] [Google Scholar]
- 68.Kem MP, Naka H, Iinishi A, Haygood MG, Butler A (2015) Fatty acid hydrolysis of acyl marinobactin siderophores by Marinobacter acylases. Biochemistry 54: 744–752. 10.1021/bi5013673 [DOI] [PubMed] [Google Scholar]
- 69.Jiang S, Li X, Zhang L, Sun W, Dai S, Xie L, et al. (2008) Culturable actinobacteria isolated from marine sponge Iotrochota sp. Mar. Biol. 153: 945–952. [Google Scholar]
- 70.Wang Y, Tang S-K, Lou K, Mao P-H, Jin X, Jiang C-L, et al. (2008) Halomonas lutea sp. nov., a moderately halophilic bacterium isolated from a salt lake. Int. J. Syst. Evol. Microbiol. 58: 2065–2069. 10.1099/ijs.0.65436-0 [DOI] [PubMed] [Google Scholar]
- 71.Suarez C, Ratering S, Kramer I, Schnell S (2014) Cellvibrio diazotrophicus sp. nov., a nitrogen-fixing bacteria isolated from the rhizosphere of salt meadow plants and emended description of the genus Cellvibrio. Int. J. Syst. Evol. Microbiol. 64: 481–486. 10.1099/ijs.0.054817-0 [DOI] [PubMed] [Google Scholar]
- 72.Xu J (2014) Isolation and Assessment of Nitrogen-Fixing and Phosphate-Solubilizing Bacteria for Use as Biofertilizers: Auburn University. [Google Scholar]
- 73.Oggerin M, Rubio V, Marín I, Arahal DR (2011) The status of the species Beijerinckia fluminensis Döbereiner and Ruschel 1958. Request for an Opinion. Int. J. Syst. Evol. Microbiol. 61: 1757–1759. 10.1099/ijs.0.032805-0 [DOI] [PubMed] [Google Scholar]
- 74.Yan Y, Yang J, Dou Y, Chen M, Ping S, Peng J, et al. (2008) Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. U.S.A. 105: 7564–7569. 10.1073/pnas.0801093105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Furukawa K, Hayase N, Taira K, Tomizuka N (1989) Molecular relationship of chromosomal genes encoding biphenyl/polychlorinated biphenyl catabolism: some soil bacteria possess a highly conserved bph operon. J. Bacteriol. 171: 5467–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao B, Wang H, Li R, Mao X (2010) Thalassospira xianhensis sp. nov., a polycyclic aromatic hydrocarbon-degrading marine bacterium. Int. J. Syst. Evol. Microbiol. 60: 1125–1129. 10.1099/ijs.0.013201-0 [DOI] [PubMed] [Google Scholar]
- 77.Sauvêtre A, Schröder P (2015) Uptake of carbamazepine by rhizomes and endophytic bacteria of Phragmites australis. Front. Plant Sci. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Widada J, Nojiri H, Yoshida T, Habe H, Omori T (2002) Enhanced degradation of carbazole and 2, 3-dichlorodibenzo-p-dioxin in soils by Pseudomonas resinovorans strain CA10. Chemosphere 49: 485–491. [DOI] [PubMed] [Google Scholar]
- 79.Nogi Y, Yoshizumi M, Miyazaki M (2014) Thalassospira povalilytica sp. nov., a polyvinyl-alcohol-degrading marine bacterium. Int. J. Syst. Evol. Microbiol.64: 1149–1153. 10.1099/ijs.0.058321-0 [DOI] [PubMed] [Google Scholar]
- 80.Pan E, Jamison M, Yousufuddin M, MacMillan JB (2012) Ammosamide D, an oxidatively ring opened ammosamide analog from a marine-derived Streptomyces variabilis. Org. Lett. 14: 2390–2393. 10.1021/ol300806e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sargent MG (1980) A procedure for isolating high quality DNA from spores of Bacillus subtilis 168. Microbiology 116: 511–514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Endophytic bacterial strains isolated from Caliph medic roots when plant grew under normal (A) and saline conditions (B).
(XLSX)
Activity or product not detected in the assays is denoted by N.D.
(XLSX)
Data Availability Statement
The 16S rDNA sequences were deposited in GenBank/EMBL/DDBJ under the accession numbers KU587127-KU587167 and KX395941-KX396022.