Abstract
Objective
Patients with single ventricle can develop aortic-to-pulmonary collaterals (APCs). Along with systemic-to-pulmonary artery shunts, these structures represent a direct pathway from systemic to pulmonary circulations, and may limit cerebral blood flow (CBF). This study investigated the relationship between CBF and APC flow on room air and in hypercarbia, which increases CBF in patients with single ventricle.
Methods
106 consecutive patients with single ventricle underwent 118 cardiac magnetic resonance (CMR) scans in this cross-sectional study; 34 prior to bidirectional Glenn (BDG) (0.50±0.30 years old), 50 prior to Fontan (3.19±1.03 years old) and 34 3–9 months after Fontan (3.98±1.39 years old). Velocity mapping measured flows in the aorta, cavae and jugular veins. Analysis of variance (ANOVA) and multiple linear regression were used. Significance was p<0.05.
Results
A strong inverse correlation was noted between CBF and APC/shunt both on room air and with hypercarbia whether CBF was indexed to aortic flow or body surface area, independent of age, cardiopulmonary bypass time, Po2 and Pco2 (R=−0.67–−0.70 for all patients on room air, p<0.01 and R=−0.49–−0.90 in hypercarbia, p<0.01). Correlations were not different between surgical stages. CBF was lower, and APCs/shunt flow was higher prior to BDG than in other stages.
Conclusions
There is a strong inverse relationship between CBF and APC/shunt flow in patients with single ventricle throughout surgical reconstruction on room air and in hypercarbia independent of other factors. We speculate that APC/shunt flow may have a negative impact on cerebral development and neurodevelopmental outcome. Interventions on APC may modify CBF, holding out the prospect for improving neurodevelopmental trajectory.
Trial Registration Number
INTRODUCTION
Among the many known complications that patients with single ventricle must face1,2 are the development of aortic-to-pulmonary collaterals (APCs),3–6 which have been identified on angiography for many years. APC flow places a volume load on an already compromised ventricle and is linked to short-term adverse outcomes.4,7 Only recently has cardiac magnetic resonance (CMR) developed the capability to quantify flow in these vessels.3–6 In a control group from our institution, APC flow has been demonstrated to be 0.18 +0.18 L/min/m2, which is 5±5% of aortic flow.3
We speculated that APC flow may also complicate brain development by affecting cerebral blood flow (CBF), potentially raising issues regarding neurological outcome. Presumably due to both delayed maturation and brain injury,8–10 adverse neurodevelopment in patients with single ventricle has been documented in numerous studies.11,12 APCs represent a low resistance runoff of systemic flow into the pulmonary bed, which may divert blood from the developing brain to the lungs. This would decrease CBF, possibly contributing to both delayed or abnormal brain maturation and injury, resulting in lower than normal intelligence. Numerous studies have linked CBF to lower IQ testing.13,14 Therefore, we hypothesise that APCs may directly contribute to decreasing CBF, and indirectly complicate brain development as one of many contributors to ultimate outcome.
The purpose of this investigation was to examine the relationship of APC flow with CBF under room air and hypercarbia, which dilates the cerebral vascular bed. We hypothesise that APC flow will negatively impact CBF, and speculate that it may represent a risk factor for poor neurodevelopmental outcome. Because APCs may be embolised in the catheterisation laboratory5 or manipulated pharmacologically,15 patients at risk can potentially undergo early intervention making the relationship of APCs to CBF important to identify and characterise for clinical outcome.
METHODS
Patients
This was a cross-sectional subanalysis of a single-centre prospective National Institutes of Health study of CBF in patients with single ventricle throughout staged surgical reconstruction. Patients were enrolled from April 2009 to March 2012. The inclusion criteria included any patient <10 years of age with single-ventricle physiology undergoing surgery at our institution. Patients with a Sano shunt or patent main pulmonary artery prior to bidirectional Glenn (pre-BDG)/hemi-Fontan were excluded because they were few in numbers, and no definitive statistical statement could be made; they could not be combined with those who underwent a Blalock–Taussig shunt for reasons mentioned below. Subjects needed to have phase-encoded velocity mapping in the jugular veins to measure CBF. As a measure of APC, patients had phase-encoded velocity mapping across the systemic semilunar valve(s), superior vena cava (SVC) and either the inferior vena cava (IVC) or descending aorta (DAo) at the diaphragm. Exclusion criteria included any contraindication to CMR. Informed consent for the larger study was obtained from all participants’ families. The hospital’s institutional review board approved this study.
Study procedure
Patients underwent a CMR immediately prior to heart surgery (either pre-BDG or prior to Fontan) or 3–9 months after Fontan. In those prior to surgery, patients were prepared in the operating room with intravenous and arterial line placement; all participants were administered general anaesthesia of nitrous oxide and sevoflurane of ≤1 minimum alveolar concentration (MAC) and mechanically ventilated to achieve a Paco2 of 40±2 mm Hg on arterial blood gas measurement. A Siemens Avanto 1.5 Twhole-body MRI system (Siemens Medical Solutions, Malvern, Pennsylvania, USA) was used for CMR. The patient was placed in the supine position, head first into the scanner using the six-channel head coil and eight-channel body array coil; all imaging were performed at iso-center. Flows of patients in the pre-BDG and BDG groups were initially measured on room air; however, after ~20–25 min, 3%–7% CO2 was administered to create hypercarbic conditions, increasing CBF; this lasted 15–20 min, during which time, measurement of flows were repeated. Hypercarbic conditions aimed for a Paco2 in the 60 s mm Hg on arterial blood gas. In those who were 3–9 months after Fontan, only room-air conditions were studied since an arterial line was not present to measure arterial blood gases. These studies lasted ~1 h.
CMR protocol
After localisers, a stack of static steady-state free-precession images of the thorax was obtained to assess cardiovascular anatomy and to obtain the exact slice positions and orientations for retrospective, ECG-gated phase contrast magnetic resonance, which was performed across: (a) right and left jugular veins, (b) SVC and IVC, (c) the aorta or neoaorta, (d) DAo at the diaphragm and (e) for those after Fontan, across the right and left pulmonary arteries. Multiple excitations were used to offset respiratory motion. Table 1 lists the parameters.
Table 1.
Parameters used in phase contrast magnetic resonance
| TR (ms) | 2.8–3.9 |
| TE (ms) | 1.3–2.8 |
| Field of view (cm) | 180–260 |
| Slice thickness (mm) | 4–5 |
| Flip angle (degrees) | 25 |
| VENC (cm/s) | 60–90 for venous flow, 150–200 for aorta |
| Number of phases across cardiac cycle | 20–30, depending upon heart rate |
| Bandwidth (Hz/pixel) | 375 |
TE, echo time; TR, repetition time; VENC, velocity encoding.
Analysis of flow images was performed on a Siemens Leonardo workstation using Argus. The sum jugular flow represented CBF, which has been validated in numerous studies.16,17 APC flow was measured two ways: (a) aorta-(SVC+IVC) flow3 and (b) aorta-(SVC+DAo) flow.6 These measures are not interchangeable, and each has its drawbacks (using IVC or DAo alone may yield erroneous results because of veno–veno collaterals or APC from the abdominal DAo, respectively). If two ventricular outflows were present, each was interrogated separately, and the flows summed as aortic output. In pre-BDG patients, the difference between aortic and the caval flow sum (or SVC +DAo flow) represented the sum of APC flow and the Blalock–Taussig shunt. Because of time considerations, a second measure of APC flow (the difference between pulmonary artery and pulmonary venous flow) could not be obtained. Including pre-BDG in the study despite the result being the sum of APC flow and the Blalock–Taussig shunt was important because whether the source is APC or a Blalock–Taussig shunt, the concept of access to the pulmonary bed from the systemic circulation may have a bearing on a ‘steal’ phenomenon, decreasing CBF. Patients with a Sano shunt or patent main pulmonary artery (small number in this cohort) could not be combined with patients who had a Blalock–Taussig shunt for this reason. Those with a Sano shunt or patent main pulmonary artery would only have APC calculated in the pre-BDG group while those with a Blalock–Taussig shunt would have both APC and shunt flow measured using the formulas above. Both CBF and APC were indexed to body surface area (BSA) as well as to aortic flow (expressed as a per cent to account for redistribution of cardiac output; this made sense since it is aortic flow being redistributed between the cerebral and pulmonary beds). Fenestration flow in patients after Fontan was calculated as SVC+IVC – sum of branch pulmonary arteries).
Statistics
Descriptive statistics were presented as frequency counts for categorical variables and mean±SD for normally distributed or median (range) for non-normally distributed continuous variables. Mann–Whitney U test was used to compare non-normally distributed variables between two groups. The Kruskal–Wallis test (non-parametric one-way analysis of variance (ANOVA)) and Dunn’s multiple comparison test or Shapiro–Wilk test and Šidák multiple comparison test were used to compare three groups. Simple and multiple linear regression models were applied to assess the relationships of aorta-(SVC+IVC) flow and aorta-(SVC+DAo) with CBF. Models were adjusted for age, cardiopulmonary bypass time, Po2 and Pco2. The Fisher r-to-z transformation was used to compare correlation coefficients. Sensitivity analyses were applied to examine data points that were outliers. The assessment of models via residual analysis showed that the residuals from each model were approximately normally distributed based on visual examination of histograms and normal probability plots. Statistical significance level was set at p value ≤0.05. Ranksum (Mann–Whitney U test), dunntest (Dunn’s multiple comparison test), swilk (Shapiro–Wilk normality test), oneway (ANOVA), regress (regression analyses), corr (correlation analyses) and tabulate (Fisher’s exact test) procedures in Stata V.13.1 (Stata, College Station, Texas, USA) were used to conduct the statistical analyses.
RESULTS
Study population
Table 2 delineates the demographics of the 106 patients enrolled divided by surgical stage, which were all statistically different from each other with few exceptions. Table 2 also provides a breakdown diagnosis as well; over half had hypoplastic left heart syndrome and owing to that, the vast majority were functional single RVs. No patient had coarctation of the aorta or stenosis of aortic branches. Two patients in the BDG group underwent APC embolisation prior to the study.
Table 2.
Baseline characteristics of patients undergoing CMR for CBF and APC flow
| All | Pre-BDG | BDG | Fontan | p Value | |
|---|---|---|---|---|---|
| Number of patients (N) | 106 pts, 118 scans | 34 | 50 | 34 | |
| Male/female | 72/46 (scans) | 20/14 | 27/23 | 25/9 | 0.193 |
| Age at CMR (years) | 2.8 (0.27, 8.4) | 0.41 (0.27, 2.01) | 3.06 (1.8, 7.6) | 3.6 (2.0, 8.4) | <0.001 |
| Weight (kg) | 12.7 (4.9, 28.0) | 6.3 (4.9, 9.2) | 12.8 (10.6, 28) | 15.5 (11.4, 27.7) | <0.001 |
| Height (cm) | 90.0 (36.0, 121.9) | 63.5 (57.0, 69.1) | 90.5 (36, 120) | 100.4 (83.8, 121.9) | <0.001 |
| BSA (m2) | 0.55 (0.26, 0.96) | 0.32 (0.26, 0.42) | 0.55 (0.49, 0.92) | 0.63 (0.53, 0.96) | <0.001 |
| Age at stage of surgery (years) | 0.01 (0.01, 0.07) | 0.42 (0.27, 0.70) | 3.1 (1.3, 7.6) | <0.001 | |
| Time: surgery to CMR (years) | 0.38 (0.27, 0.71) | 2.7 (1.4, 7.0) | 0.63 (0.33, 2.7) | <0.001 | |
| CPB time (min) | 82 (29, 147) | 62 (14, 102) | 62.5 (52, 124) | <0.001 | |
| Circ arrest time (min) | 42 (0, 76) | 18 (0, 45) | 23.5 (0, 49) | <0.001 | |
| Po2 RA (mm Hg) | 46 (36, 69) | 50.5 (34, 67) | <0.001 | ||
| Pco2 RA (mm Hg) | 40.0+4.8 | 38.6+4.9 | 0.11* | ||
| Po2 hypercarbia (mm Hg) | 51 (34, 93) | 61 (24, 79) | <0.001 | ||
| Pco2 hypercarbia (mm Hg) | 68 (35, 96) | 67 (33, 110) | 0.43 | ||
| Morphology | |||||
| DILV | 6 | 0 | 3 | 3 | 0.24 |
| DORV | 14 | 5 | 6 | 3 | 0.72 |
| HLHS | 64 | 19 | 27 | 18 | 0.97 |
| Heterotaxy | 6 | 0 | 3 | 3 | 0.24 |
| PA | 2 | 0 | 1 | 1 | 0.99 |
| PA/Intact Ventricular Septum | 2 | 1 | 1 | 0.99 | |
| SV with TGA | 4 | 1 | 1 | 2 | 0.82 |
| Tricuspid Atresia | 9 | 3 | 4 | 2 | 0.99 |
| Other SV | 11 | 5 | 4 | 2 | 0.52 |
All pre-BDG patients had a Blalock–Taussig shunt. All comparisons failed either normality or equal variance test and were compared with non-parametric testing with the exception of Pco2 in RA. Values expressed as mean±SD or median (minimum, maximum) depending upon whether the data were normally distributed or not.
APC, aortic-to-pulmonary collateral; BDG, bidirectional Glenn; BSA, body surface area; CBF, cerebral blood flow; Circ, circulatory; CMR, cardiac magnetic resonance; CPB, cardiopulmonary bypass; DILV, double inlet LV; DORV, double outlet RV; HLHS, hypoplastic left heart syndrome; N, number; PA, pulmonary atresia; Pco2, partial pressure of carbondioxide; Po2, partial pressure of oxygen; pts, patients; RA, room air; SV, single ventricle; TGA, transposition of the great arteries.
Absolute flows in aorta and systemic veins
Table 3 lists the raw flow data used in CBF and APC calculations by surgical stage as well as room air and hypercarbia. A significant steady decline on room air aortic flow was noted as patients progressed through surgical reconstruction, although no difference was noted between pre-BDG and BDG stages during hypercarbia. Significantly higher total jugular, IVC and DAo flows on room air as well as hypercarbic conditions were present in the BDG group compared with the pre-BDG and Fontan groups. SVC flow on room air was significantly lower in the Fontan group when compared with the other two groups. In the Fontan group, caval return was 2.6±0.73 L/min/m2, pulmonary artery flow was 2.0±0.7 L/min/m2 with a calculated fenestration flow of 0.7±0.5L/min/m2 (17% of aortic output).
Table 3.
Flows measured in the aorta and systemic veins on room air and in hypercarbic conditions
| RA |
CO2 |
||||
|---|---|---|---|---|---|
| Flows (in L/min/m2) | Pre-BDG | BDG | Fontan | Pre-BDG | BDG |
| Aortic valve(s)* | 6.2 (3.0, 9.9) | 5.5 ( 3.6, 7.9) | 4.21±0.80 | 7.02±1.55 | 7.18±1.20 |
| Total jugular† | 1.42±0.44 | 1.78±0.49 | 1.28±0.54 | 1.98±0.54 | 2.39±0.54 |
| SVC‡ | 1.94±0.50 | 2.09±0.57 | 1.50±0.55 | 2.53±0.67 | 2.93±0.72 |
| IVC§ | 1.13 (0.48, 2.20) | 1.79 (0.87, 3.35) | 1.01 (0.62, 2.23) | 1.19 (0.51, 2.53) | 2.44 (1.19, 5.24) |
| DAo¶ | 1.28 (0.75, 2.77) | 1.58 (0.70, 2.80) | 1.2 (0.47, 1.98) | 1.48 (0.63, 2.26) | 2.36 (1.17, 4.72) |
| CBF (% Ao flow)** | 24.6±11.3 | 32.7±9.6 | 31.0±12.0 | 31.2±14.3 | 34.3±9.3 |
| APC flow using IVC (% Ao flow)†† | 50.1 (15.4, 74.0) | 29.74 (5.0, 50.0) | 34.9 (3.6, 75.9) | 46.6 (8.3, 68.19) | 22.2 (5.2, 42.5) |
| APC flow using DAo (% Ao flow)‡‡ | 44.1 (11.4, 72.5) | 31.2 (9.5, 56.1) | 34.4 (7.0, 62.1) | 38.3±17.2 | 28.5±13.3 |
Values expressed as mean±SD or median (minimum, maximum) depending upon whether the data was normally distributed or not.
RA flow significantly different between all three stages (p<0.001, non-parametric test).
RA flow of BDG significantly higher than Fontan and pre-BDG (p<0.01, parametric test) and hypercarbic flow of BDG significantly higher than pre-BDG (p=0.002, parametric test).
RA flow of Fontan significantly lower than pre-BDG and BDG (p<0.001, parametric test) and hypercarbic flow of BDG significantly higher than pre-BDG (p=0.01, parametric test).
RA flow of BDG significantly higher from Fontan and pre-BDG (p<0.001, non-parametric test) and hypercarbic flow of BDG significantly higher than pre-BDG (p<0.001, non-parametric test).
RA flow of BDG significantly higher from Fontan and pre-BDG (p<0.001, non-parametric test) and hypercarbic flow of BDG significantly higher than pre-BDG (p<0.001, non-parametric test).
RA flow significantly lower in pre-BDG than other two stages (parametric test).
RA and hypercarbic flow significantly higher in pre-BDG than other two stages (p<0.001, non-parametric test).
RA (p=0.03, non-parametric test) and hypercarbic flow (p=0.01, parametric test) significantly higher in pre-BDG than other two stages.
Ao, aorta; APC, aortic-to-pulmonary collateral; BDG, bidirectional Glenn; CBF, cerebral blood flow; CO2, carbon dioxide; DAo, descending aorta; IVC, inferior vena cava; RA, room air; SVC, superior vena cava.
CBF and APC flows, parsed by surgical stage, are also listed in table 3 on room air and in hypercarbia. CBF as a per cent of aortic flow on room air was significantly lower in the pre-BDG group than in either the BDG or Fontan groups. In hypercarbia, pre-BDG flow was similar to BDG flow. APC/shunt flow as a per cent of the aortic flow on room air was significantly higher in the pre-BDG group than in the BDG or Fontan groups whether using IVC or DAo flow. In hypercarbia, pre-BDG APC/shunt flow was also significantly higher than BDG flow again whether using IVC or DAo flow.
Correlation between CBF and APC
Figures 1 and 2 graphically depict the relationship between CBF and APC/shunt as a group and divided by surgical stage, respectively. A significant inverse correlation existed between CBF and APC in the entire cohort as well as each surgical stage whether under room air or hypercarbia. On room air, correlations between stages did not differ (p=0.06–0.29); however, under hypercarbia, the correlation was significantly higher for pre-BDG than for the BDG (p≤0.001). This inverse relationship was stronger under hypercarbia than room air for the pre-BDG group. The correlation was independent of age, cardiopulmonary bypass time, Po2 and Pco2 with the exception of the Fontan stage on room air (significant with linear regression and not significant after adjusting for age and cardiopulmonary bypass time) and the BDG in hypercarbia (significant with linear regression and significant with multiple linear regression after adjusting for age, Po2 and Pco2).
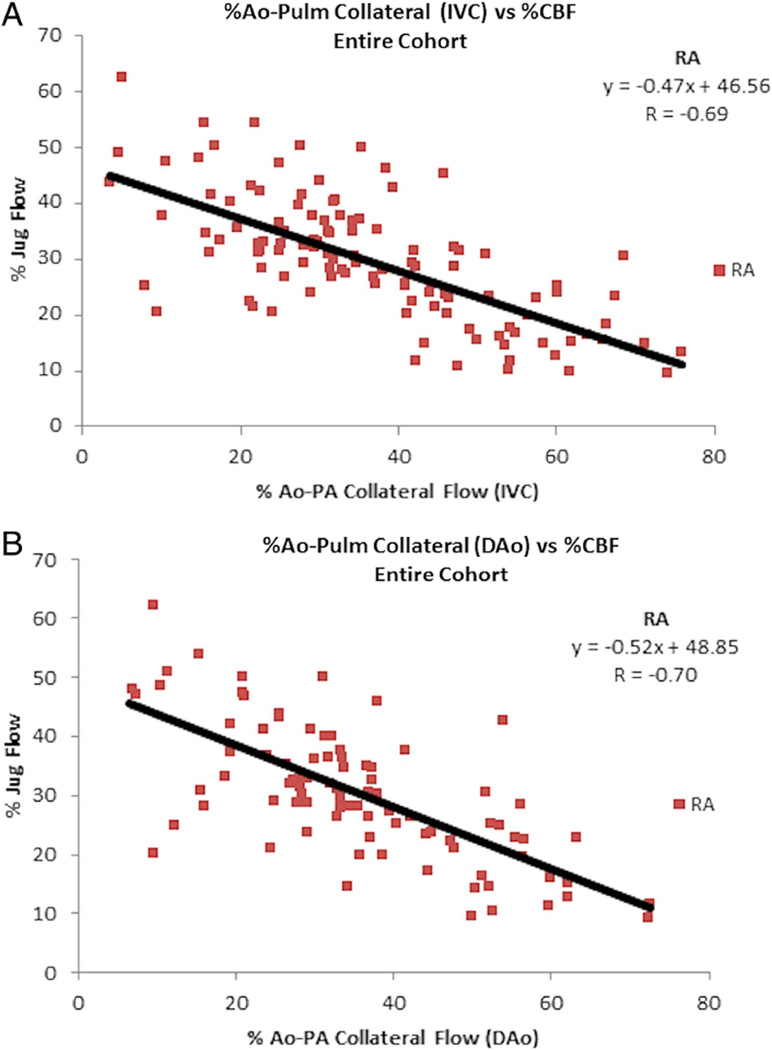
Figure 1.
Relationship between CBF and APC flow indexed to aortic output in the entire cohort. Note the inverse correlation between CBF and APC flow on room air whether APC flow calculated by either IVC (A) or DAo flow (B). Ao=aorta; APC, aortic-to-pulmonary collateral; CBF, cerebral blood flow; DAo, descending aorta; IVC, inferior vena cava; Jug, jugular; Pulm, pulmonary; RA, room air.
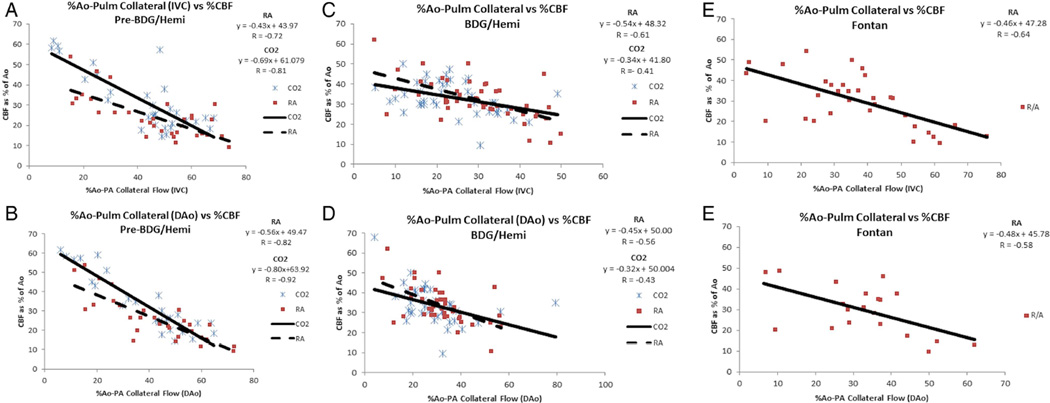
Figure 2.
Relationship between CBF and APC flow indexed to aortic output in the entire cohort. Pre-BDG (A), after BDG (B), after Fontan (C). IVC is used for top figure and DAo is used in bottom figure. Note the inverse correlation between CBF and APC flow at all stages. This remains true whether on room air (solid line, boxes) or in hypercarbia (dashed line, cross-hatch). Ao, aorta; APC, aortic-to-pulmonary collateral; BDG, bidirectional Glenn; CBF, cerebral blood flow; CO2, carbon dioxide; DAo, descending aorta; IVC, inferior vena cava; RA, room air.
Table 4 lists the correlation coefficients when determining the relationship of the absolute amount of CBF compared with APC flow (as a per cent of aortic flow) for each stage as well as the entire cohort. R values, which were all significant, varied between −0.37 and −0.68.
Table 4.
Correlation coefficients of APC flow as a per cent of aortic flow versus CBF indexed to BSA and significance
| APC flow as % of Ao using IVC vs Jug flow indexed to BSA |
APC flow as % of Ao using DAo vs Jug flow indexed to BSA |
||||
|---|---|---|---|---|---|
| Room air | Hypercarbia | Room air | Hypercarbia | ||
| Pre-BDG | R=−0.37 (p=0.04) | R=−0.68 (p<0.01) | Pre-BDG | R=−0.41 ( p=0.03) | R=−0.68 (p<0.01) |
| BDG | R=−0.56 (p<0.01) | R=−0.50 (p<0.01) | BDG | R=−0.49 ( p<0.01) | R=−0.52 (p<0.01) |
| Fontan | R=−0.51 (p<0.01) | N/A | Fontan | R=−0.47 ( p=0.04) | N/A |
| All | R=−0.51 (p<0.01) | N/A | All | R=−0.53 ( p<0.01) | N/A |
Ao, aorta; APC, aortic-to-pulmonary collateral; BDG, bidirectional Glenn; BSA, body surface area; CBF, cerebral blood flow; DAo, descending aorta; IVC, inferior vena cava.
DISCUSSION
The major finding of this subanalysis was a significant inverse correlation between CBF and APC/shunt flow, whether CBF was indexed to aortic flow or BSA under conditions of room air or hypercarbia, at each stage of surgical reconstruction. This suggests a redistribution of flow and ‘steal’ phenomenon whereby runoff into the pulmonary bed diverts blood away from the cerebral circulation. This inverse relationship was stronger in pre-BDG partly due to the surgically created aortic-to-pulmonary shunt; this assertion is supported by the lower indexed CBF and higher APC/shunt flow in pre-BDG patients than in those after BDG or 3–9 months after Fontan. To put normal APC flow into perspective, in a control group from our institution consisting of two-ventricle patients with either arch anomalies and no prior surgery or postoperative patients with no known shunts, APC flow was 0.18±0.18 L/min/m2, which is 5±5% of aortic flow.3
This study also noted a steady decline in aortic output as patients progressed through surgical reconstruction; nevertheless, aortic output equalised between pre-BDG and BDG patients during hypercarbia (both rising), consistent with relaxation of the cerebral and systemic vascular bed. CBF and APC/shunt flow were indexed to aortic flow since aortic output was the blood pool being redistributed. CBF indexed to BSA, however, is also important in cerebral development, and its relationship with APC/shunt flow was also investigated.
Absolute jugular flows were higher in BDG patients than pre-BDG patients as the BDG connection places the cerebral and lower resistance pulmonary vascular beds exclusively in series with each other, increasing CBF. IVC and DAo flows were also higher in BDG patients than pre-BDG patients as there was less APC/shunt flow in the BDG to divert blood from CBF.
Hypercarbia relaxes the cerebral and systemic vascular bed, increasing CBF in normal individuals. The increase in aortic output and Po2 in pre-BDG is also partially related to that. Increased Pco2 also increases pulmonary vascular resistance, decreasing APC flow and indirectly affecting CBF, especially in the BDG stage. In pre-BDG patients, the CBF–APC curve in hypercarbia is shifted upwards when compared with the room air consistent with increased CBF at all levels of APC/shunt. The hypercarbia curve, however, also has an increased negative slope compared with room air, indicating that hypercarbia increases pulmonary vascular resistance less than the decrease in cerebral vascular resistance in the delicate balancing act between the two. In BDG patients, however, hypercarbia does not shift the curve upwards, but instead is nearly identical to room air, demonstrating the decreased cerebral vascular resistance is offset by the increased pulmonary vascular resistance with increased Pco2. The effects of hypercarbia on CBF relative to APC/shunt between stages may be either age related or the difference in physiology; pre-BDG, CBF and pulmonary blood flow are in parallel whereas in the BDG stage, CBF is in series with pulmonary blood flow and in parallel with pulmonary blood flow via APC vessels.
As SVC flow is similar to total jugular flow (CBF) and is used in APC/shunt flow calculations, correlation of CBF and APC/shunt flow may be expected. Two major considerations, however, make the association unlikely. The SVC accepts variable amounts of flow from the scalp, cranium and upper extremities in children, making it not comparable with CBF. Indeed, table 3 demonstrates 0.2–0.6 L/min/m2 higher SVC flow than total jugular flow. In addition, the APC flow equation has IVC and DAo flow as independent variables; APC flow, therefore, can affect IVC and DAo flow and leave CBF unchanged.
It has been known for many years that APCs are present in single ventricles);18 however, there is debate regarding their clinical impact.19 Recently, CMR has evolved to accurately quantify APC flow,3,6 demonstrating associations with age, ventricular function20 and clinical outcomes (eg, hospital stay and pleural effusions).4,21 Presumably, APC flow may also divert variable amounts of blood from other organs, including the coronaries. Interventions on APCs pharmacologically15 and via catheter5 can decrease APC flow, which may potentially be useful in affecting the patient’s neurodevelopmental outcome.
The inverse relationship between APC and CBF suggests an adverse relationship, possibly leading to poor neurodevelopment. It also suggests, however, that manipulating APC flow via catheter5 or pharmacology15 may mitigate these effects. CBF is related to intelligence in adults13,22 and cognitive function in children.14,23 For example, a study by Ley et al14 measured fetal aortic blood flow velocities and intellectual ability at 6.5 years of age (presuming aortic flow an indirect measure of CBF). Verbal and global IQ scores were lower in patients with reduced fetal aortic flow. They concluded that abnormal fetal aortic velocity both independently and in combination with other factors was a significant predictor of impaired intellectual outcome. Lou et al23 demonstrated that neonatal CBF measurements correlated with neuropsychological testing at ages 9–10. In a larger study of preterm infants, Hunt et al24 demonstrated that low SVC flow was associated with an abnormal developmental quotient (p=0.006). Even in a mixture of patients with congenital heart disease such as tetralogy of Fallot, d-transposition of the great arteries and isolated ventricular septal defects, Cheng et al25 noted that decreased CBF velocities were associated with poorer developmental testing. Therefore, altering APC flow holds the possibility of altering CBF, which in turn may ultimately lead to better neurocognitive outcomes.
LIMITATIONS
Because of time limitations, APC flow could not be teased out from Blalock–Taussig shunt flow; that would have required measuring branch pulmonary artery and the pulmonary venous flow adding more time onto scanning. This, however, does not negate the findings of this investigation; whether the source is APC or a Blalock–Taussig shunt, systemic-to-pulmonary flow has an inverse relationship with CBF and the concept of a ‘steal’ when the pulmonary bed has direct access to the systemic circulation. This study also did not investigate the relationship of APCs with other organ systems, but it at least suggests that those organ systems may also be at risk for hypoperfusion.
In addition, because of time limitations, a second measure of APC flow (ie, difference in pulmonary artery and pulmonary venous flow) was not able to be obtained; nevertheless, it has been noted in other studies that both measures of APC flow were nearly identical.3,6
Multiple studies have used CBF velocities25,26 or SVC flow indexed to weight24 when correlating CBF with neurological outcome. As one measure, our study used indexed CBF-to-aortic flow since aortic flow is the blood pool which is being distributed between brain and APC/shunt. It would be interesting to examine cerebral oxygen delivery relative to APC/shunt, and this will be the subject of a future investigation.
Jugular flow does not purely measure CBF as it contains a small amount of non-brain head and neck flow. However, jugular flow has been validated as a true measure of CBF.16,17 In addition, our own laboratory has correlated this technique with spectroscopy.27
This study did not relate manipulating APC with CBF or the relationship of this manipulation with neurodevelopment. A larger prospective trial would be necessary to prove this speculation.
CONCLUSION
There is a strong inverse relationship between CBF and APC/shunt flow in patients with single ventricle throughout surgical reconstruction, which is present on room air and in hypercarbia. This relationship is independent of age, cardiopulmonary bypass time, Po2 and Pco2. Because pre-BDG patients have higher APC/shunt flow, their CBF is lower at the other two stages. We speculate that this may have a negative impact on cerebral development and neurodevelopmental outcome. In addition, we speculate that interventions on APC may be able to modify CBF, holding out the prospect for improving the trajectory of neurodevelopmental outcome.
Key messages.
What is already known on this subject?
Aortic-to-pulmonary collaterals (APCs) are known to develop in patients with single ventricles who also have less than optimal neurodevelopmental outcomes. Cerebral blood flow (CBF) has been linked to poor cognitive development.
What might this study add?
It is unclear how APC flow affects CBF, which in turn may affect neurodevelopment; this study provides that link.
How might this impact on clinical practice?
Because APCs may be abolished by embolisation in the cardiac catheterisation laboratory, a link between that flow and CBF may contribute to the decision to embolise these collaterals.
Acknowledgments
Funding This study was funded by National Heart Lung and Blood Institute grant 1R01HL090615-01 (PI Fogel), NINDS NS- 072338 (PI Licht) and support from the June and Steve Wolfson Family Foundation. Dr Fogel receives grants from Edwards Life Sciences as a CMR Core Lab (COMPASSION trial), a grant from AMAG for the FACT trial and is the medical monitor for Kereos for an angiogenesis imaging agent.
Footnotes
Contributors All authors have participated in conception and design or analysis and interpretation of data, or both; drafting of the manuscript or revising it critically for important intellectual content; and final approval of the manuscript submitted.
Competing interests None declared.
Ethics approval IRB at The Children’s Hospital of Philadelphia.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Sundareswaran KS, de Zélicourt D, Sharma S, et al. Correction of pulmonary arteriovenous malformation using image based surgical planning. JACC Cardiovasc Imaging. 2009;2:1024–1030. doi: 10.1016/j.jcmg.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MI, Bush DM, Ferry RJ, Jr, et al. Somatic growth failure after the Fontan operation. Cardiol Young. 2000;10:447–457. doi: 10.1017/s1047951100008118. [DOI] [PubMed] [Google Scholar]
- 3.Whitehead KK, Gillespie MJ, Harris MA, et al. Noninvasive quantification of systemic to pulmonary collateral flow: a major source of inefficiency in patients with superior cavopulmonary connections. Circ Cardiovasc Imaging. 2009;2:405–411. doi: 10.1161/CIRCIMAGING.108.832113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glatz AC, Rome JJ, Small AJ, et al. Systemic-to-pulmonary collateral flow as measured by cardiac magnetic resonance imaging is associated with acute post-Fontan clinical outcomes. Circ Cardiovasc Imaging. 2012;5:218–225. doi: 10.1161/CIRCIMAGING.111.966986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dori Y, Glatz AC, Hanna BD, et al. Acute effects of embolizing systemic-to-pulmonary arterial collaterals on blood flow in patients with superior cavopulmonary connections: a pilot study. Circ Cardiovasc Interv. 2013;6:101–106. doi: 10.1161/CIRCINTERVENTIONS.112.972265. [DOI] [PubMed] [Google Scholar]
- 6.Grosse-Wortmann L, Al-Otay A, Yoo SJ. Aortopulmonary collaterals after bidirectional cavopulmonary connection or Fontan completion: quantification with MRI. Circ Cardiovasc Imaging. 2009;2:219–225. doi: 10.1161/CIRCIMAGING.108.834192. [DOI] [PubMed] [Google Scholar]
- 7.Prakash A, Satiroglu E, Porras D, et al. Risk factors for profuse systemic-to-pulmonary artery collateral burden in hypoplastic left heart syndrome. Am J Cardiol. 2013;112:400–404. doi: 10.1016/j.amjcard.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(Suppl I):I109–I114. [PubMed] [Google Scholar]
- 9.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavani F, Zimmerman RA, Clancy RR, et al. Incidental intracranial hemorrhage after uncomplicated birth: MRI before and after neonatal heart surgery. Neuroradiology. 2003;45:253–258. doi: 10.1007/s00234-003-0946-8. [DOI] [PubMed] [Google Scholar]
- 11.Ravishankar C, Zak V, Williams IA, et al. for the Pediatric Heart Network Investigators. Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. 2013;162:250.e2–256.e2. doi: 10.1016/j.jpeds.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wernovsky G, Stiles KM, Gauvreau K, et al. Cognitive development after the Fontan operation. Circulation. 2000;102:883–889. doi: 10.1161/01.cir.102.8.883. [DOI] [PubMed] [Google Scholar]
- 13.Koide H, Kobayashi S, Kitani M, et al. Improvement of cerebral blood flow and cognitive function following pacemaker implantation in patients with bradycardia. Gerontology. 1994;40:279–285. doi: 10.1159/000213597. [DOI] [PubMed] [Google Scholar]
- 14.Ley D, Tideman E, Laurin J, et al. Abnormal fetal aortic velocity waveform and intellectual function at 7 years of age. Ultrasound Obstet Gynecol. 1996;8:160–165. doi: 10.1046/j.1469-0705.1996.08030160.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee KJ, Yoo SJ, Holtby H, et al. Acute effects of the ACE inhibitor enalaprilat on the pulmonary, cerebral and systemic blood flow and resistance after the bidirectional cavopulmonary connection. Heart. 2011;97:1343–1348. doi: 10.1136/hrt.2011.225656. [DOI] [PubMed] [Google Scholar]
- 16.Wilson EM, Halsey JH, Vitek JJ. Validation of jugular venous flow as an index to total cerebral blood flow. Stroke. 1972;3:300–321. doi: 10.1161/01.str.3.3.300. [DOI] [PubMed] [Google Scholar]
- 17.Meyer JS, Ishikawa S, Lee TK, et al. Quantitative measurement of cerebral blood flow with electromagnetic flow meters, recording internal jugular venous flow in monkey and man. Trans Amer Neurol Assoc. 1963;88:78–83. [PubMed] [Google Scholar]
- 18.Triedman JK, Bridges ND, Mayer JE, et al. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol. 1993;22:207–215. doi: 10.1016/0735-1097(93)90836-p. [DOI] [PubMed] [Google Scholar]
- 19.Bradley SM, McCall MM, Sistino JJ, et al. Aortopulmonary collateral flow in the Fontan patient: does it matter? Ann Thorac Surg. 2001;72:408–415. doi: 10.1016/s0003-4975(01)02813-2. [DOI] [PubMed] [Google Scholar]
- 20.Prakash A, Rathod RH, Powell AJ, et al. Relation of systemic-to-pulmonary artery collateral flow in single ventricle physiology to palliative stage and clinical status. Am J Cardiol. 2012;109:1038–1045. doi: 10.1016/j.amjcard.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosse-Wortmann L, Drolet C, Dragulescu A, et al. Aortopulmonary collateral flow volume affects early postoperative outcome after Fontan completion: a multimodality study. J Thorac Cardiovasc Surg. 2012;144:1329–1336. doi: 10.1016/j.jtcvs.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Butler RW, Dickinson WA, Katholi C, et al. The comparative effects of organic brain disease on cerebral blood flow and measured intelligence. Ann Neurol. 1983;13:155–159. doi: 10.1002/ana.410130208. [DOI] [PubMed] [Google Scholar]
- 23.Lou HC, Skov H, Henriksen L. Intellectual impairment with regional cerebral dysfunction after low neonatal cerebral blood flow. Acta Paediatr Scand. 1989;360:72–82. doi: 10.1111/j.1651-2227.1989.tb11285.x. [DOI] [PubMed] [Google Scholar]
- 24.Hunt RW, Evans N, Reger I, et al. Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. J Pediatr. 2004;145:588–592. doi: 10.1016/j.jpeds.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 25.Cheng HH, Wypij D, Laussen PC, et al. Cerebral blood flow velocity and neurodevelopmental outcome in infants undergoing surgery for congenital heart disease. Ann Thorac Surg. 2014;98:125–132. doi: 10.1016/j.athoracsur.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eixarch E, Meler E, Iraola A. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow distribution. Ultrasound Obstet Gynecol. 2008;32:894–899. doi: 10.1002/uog.6249. [DOI] [PubMed] [Google Scholar]
- 27.Buckley EM, Hance D, Pawlowski T, et al. Validation of diffuse correlation spectroscopic measurements of cerebral blood flow using phase-encoded velocity mapping MRI. J Biomed Opt. 2012;17:037007. doi: 10.1117/1.JBO.17.3.037007. [DOI] [PMC free article] [PubMed] [Google Scholar]