Abstract
Monodehydroascorbate reductase (MDHAR; EC 1.6.5.4) is an important enzyme for ascorbate recycling. To examine whether heterologous expression of MDHAR from Oryza sativa (OsMDHAR) can prevent the deleterious effects of unfavorable growth conditions, we constructed a transgenic yeast strain harboring a recombinant plasmid carrying OsMDHAR (p426GPD::OsMDHAR). OsMDHAR-expressing yeast cells displayed enhanced tolerance to hydrogen peroxide by maintaining redox homoeostasis, proteostasis, and the ascorbate (AsA)-like pool following the accumulation of antioxidant enzymes and molecules, metabolic enzymes, and molecular chaperones and their cofactors, compared to wild-type (WT) cells carrying vector alone. The addition of exogenous AsA or its analogue isoascorbic acid increased the viability of WT and ara2Δ cells under oxidative stress. Furthermore, the survival of OsMDHAR-expressing cells was greater than that of WT cells when cells at mid-log growth phase were exposed to high concentrations of ethanol. High OsMDHAR expression also improved the fermentative capacity of the yeast during glucose-based batch fermentation at a standard cultivation temperature (30°C). The alcohol yield of OsMDHAR-expressing transgenic yeast during fermentation was approximately 25% (0.18 g·g-1) higher than that of WT yeast. Accordingly, OsMDHAR-expressing transgenic yeast showed prolonged survival during the environmental stresses produced during fermentation. These results suggest that heterologous OsMDHAR expression increases tolerance to reactive oxygen species-induced oxidative stress by improving cellular redox homeostasis and improves survival during fermentation, which enhances fermentative capacity.
Introduction
In aerobic organisms, oxygen is a double-edged sword; it is absolutely required for normal metabolic growth, yet continuous exposure to oxygen can cause cellular damage. This is because molecular oxygen is continually reduced within cells to reactive oxygen species (ROS), such as superoxide anions (O2*−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH*). In addition, abiotic and biotic stimuli can lead to further ROS accumulation [1]. The produced ROS react readily with cellular components to generate acute or chronic damage that is sufficient to cause cell death, aging, and various disease [2]. Redox balance is maintained via the constitutive action of various antioxidant mechanisms that scavenge ROS, and both enzymatic and non-enzymatic processes can neutralize ROS [3].
Ascorbate (AsA)/D-erythroascorbate and glutathione are important antioxidants that are maintained in their reduced forms by enzymes in the AsA-glutathione cycle in higher eukaryotes, especially plants. The enzymes in this cycle, including AsA peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR), are considered critical for minimizing and/or protecting cells from ROS induced by abiotic stressors [4]. MDHAR belongs to the flavoprotein family of pyridine nucleotide-disulfide oxidoreductases, which includes thioredoxin reductase (Trr), lipoamide dehydrogenase, GR, and mercuric ion reductase, and it catalyzes the reduction of monodehydroascorbate (MDHA) and MDHA radicals to AsA using NAD(P)H as an electron donor [5]. Therefore, MDHAR plays an important role in maintaining the pool of AsA derived from MDHA. MDHAR is found in many eukaryotes, including cucumbers, potatoes, soybean root nodules, and rot fungus [6], and it is localized in chloroplasts, mitochondria, peroxisomes, and the cytosol in plants [7], and in microsomes, mitochondria, the Golgi apparatus, and erythrocytes in animals [8]. MDHAR cDNAs have been cloned from the following plants: Brassia rapa, cucumber, pea leaves, tomatoes, rice, Arabidopsis thaliana, and leaf mustard [9–12]. The MDHAR amino acid sequence is unique and it shares limited identity with other flavin oxidoreductases in eukaryotes [5]. Transcriptional activation of the MDHAR gene has been reported under some environmental stresses, including oxidative stress such as H2O2, paraquat (PQ), and salicylic acid in B. rapa [13]; strong illumination in wheat leaves [14]; SO2 and O3 in conifer needles [15]; drought in grasses [16]; and internode rubbing in tomato plants [17].
Saccharomyces cerevisiae is a eukaryotic unicellular microfungus that is widely distributed in natural environments and in association with various insects, animals, and plants. It plays a critical role in food chains and the carbon, nutrient cycles such as nitrogen and sulfur, and is used to produce food, beverages, chemicals, pharmaceuticals, biocontrol agents, and industrial enzymes as well as in agriculture [18]. S. cerevisiae is the main yeast responsible for biomass-based alcoholic fermentation using sugars derived from rice, wheat, barley, corn, and grape in a commercially important agriculture sector [19]. During fermentation, yeast cells are dynamically challenged by mixed and interrelated stressors, especially ethanol [20]. Increased ethanol concentration stresses the cells and has an impact on fermentation quality and yield. Furthermore, ethanol production is dependent on the ability of the yeast to adapt to sudden or continuous changes during fermentation [21]. Specific properties of yeast can be altered by genetic improvement through numerous methodologies, including sexual breeding, parasexual hybridization (known as genome shuffling), random mutagenesis, genetic engineering (such as single chromosomal transfer), and transformation using recombinant tools. Using transgenic biotechnology, yeast are engineered to express foreign genes for the generation of products of industrial interest, such as bioethanol and secondary metabolites [22,23]. Because of their academic importance, studies of stress tolerance in yeast are of fundamental scientific importance. Stress intolerance can be overcome through the development of stress- and ethanol-tolerant yeast strains via effective engineering of relevant genes.
Unlike in plants, little is known about the stress response involving MDHAR in yeast. In this study, a cDNA encoding the cytosolic MDHAR from the rice plant Oryza sativa (OsMDHAR) was cloned, and its function was analyzed in a genetically modified S. cerevisiae strain. Herein, we report that heterologous OsMDHAR expression improves tolerance to ROS-induced oxidative stress and fermentative capacity in S. cerevisiae. Furthermore, these findings improve our understanding of the development of ethanol-tolerant yeast strains and demonstrate the potential application of transgenic yeast in the fermentation field for industrial bioethanol production using glucose-based biomass.
Materials and Methods
Construction of OsMDHAR-expressing transgenic yeast
Total RNA was isolated from the leaves of 4-week-old O. sativa seedlings, and the cDNA was synthesized by reverse transcription-polymerase chain reaction (RT-PCR). The OsMDHAR coding region was amplified from the cDNA by PCR using Taq and Pfu polymerases (Roche, Basel, Switzerland). The reaction conditions were as follows: initial denaturation at 94°C for 3 min, followed by 30 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 2 min, and a final extension at 72°C for 7 min. The primer set used for PCR cloning of the OsMDHAR gene is shown in S1 Table. The PCR product was ligated into the pCR2.1® TOPO-TA cloning vector (Invitrogen, Carlsbad, CA, USA). After sequence confirmation and digestion with EcoRI, the DNA fragment containing the OsMDHAR gene was ligated into the yeast expression vector p426GPD (Euroscarf, Frankfurt, Germany). The construct was transformed into S. cerevisiae BY4741 cells (S2 Table) and derivatives using the PEG/LiCl method [24]. Transformants were selected by plating cells on minimal agar medium (0.67% yeast nitrogen base without amino acids and with ammonium sulfate and 0.192% yeast synthetic drop-out medium lacking uracil, with 2% glucose, and 2% bacto agar) at 28°C for 3 days. Single colonies were restreaked, cultured under the same conditions, and then used for subsequent experiments.
Semi-quantitative RT-PCR
Total RNA from mid-log phase yeast cells was obtained using the Total RNA Isolation Kit (Promega, Madison, WI, USA). RT-PCR was performed using Maxime RT-PCR Premix (iNtRON Biotechnology Inc., Seongnam, Korea) according to the manufacturer’s instructions. The oligonucleotide primers used were OsMDHAR-F and OsMDHAR-R (S1 Table). PCR amplicons were resolved in 1.5% agarose gels, stained with ethidium bromide, and photographed. The PDA1 amplicon was used as a reference [25].
Stress tolerance assay
To measure cell viability, cells (initial concentration adjusted to 1 × 106 cells per mL) grown overnight at 28°C were inoculated into YPD medium (1% yeast extract, 2% peptone, and 2% dextrose) containing various concentrations of H2O2. The cells were cultured for 16 h at 28°C with shaking (160 rpm). After culture, the optical density at 600 nm (OD600) was measured. To monitor thegrowth kinetics, cells (starting concentration adjusted to 1 × 106 cells per mL) were inoculated into YPD medium supplemented with 5 mM H2O2, and the OD600 was measured at 2-h intervals for the indicated times. For the spotting assay, mid-log phase cells (OD600 ≈ 2.0) were exposed to 20 mM H2O2 for 1 h at 28°C and serially diluted (100 to 10−4). Then, a 5-μL aliquot of each dilution was spotted onto YPD agar plates and incubated for 2–3 days at 28°C. Additionally, to determine the stress sensitivity of mutant cells (sod1Δ, tsa1Δ, por1Δ, por2Δ, and ara2Δ; S2 Table), the OD600 of each mutant was adjusted to about 1.5, and then cells were exposed to 10 mM H2O2 for 1 h at 28°C, serially diluted, and then spotted onto YPD agar plates.
Effect of exogenous antioxidants
Yeast cells in mid-log phase were treated with 10 mM AsA and 10 mM isoascorbate (IAA) for 1 h at 28°C, washed twice with cold phosphate-buffered saline (PBS) to remove residual antioxidants, and suspended in fresh YPD medium. Resuspended cells were exposed to 20 mM H2O2 for 1 h at 28°C with shaking, appropriately diluted, and spotted onto YPD agar plates. For growth kinetics, cells (1 × 106 cells/mL) grown at 28°C overnight were inoculated into YPD medium containing 10 mM AsA and 10 mM IAA in the absence or presence of 5 mM H2O2. The OD600 was measured at 2-h intervals for the indicated time.
Protein preparation
Mid-log phase cells were incubated in the absence or presence of 20 mM H2O2 for 1 h at 28°C and harvested by centrifugation at 4°C. Crude protein extracts were prepared using glass beads. Cells were washed three times with cold PBS and resuspended in lysis buffer (50 mM HEPES, pH 7.2, 5% glycerol, 1 mM PMSF, and protease inhibitor cocktail) with an equal volume of glass beads (425–600 μm; Sigma-Aldrich, St. Louis, MO, USA). After vigorously vortexing 4 times for 1 min each at 2-min intervals on ice, the lysates were centrifuged at 13,000 × g for 20 min at 4°C, and the supernatants were used as protein extracts. Protein concentration was determined by using Protein Dye Reagent (Bio-Rad, Hercules, CA, USA).
MDHAR activity assay
MDHAR activity was assayed spectrophotometrically. The assay was performed at 25°C with a reaction mixture containing 50 mM potassium phosphate, pH 7.2, 0.2 mM NADH, 2 mM AsA, 1 unit of AsA oxidase (Sigma-Aldrich), and crude extract. MDHAR activity was measured by monitoring the decrease in absorbance at 340 nm resulting from NADH oxidation. Activity was calculated using an absorbance coefficient of 6.2 mM−1cm−1. One unit is the amount of enzyme that oxidizes 1 nmol of NADH per min at 25°C [26].
Western blot analysis
To examine the expression changes in antioxidant proteins and metabolic enzymes, initial cell concentrations with 1 × 106 cells per mL were inoculated into YPD medium and cultured for 6 h at 28°C until the cells reached mid-log phase and were then exposed to 20 mM H2O2 for 1 h at 28°C with shaking. The cells were harvested and lysed with glass beads as described above. Total protein (20 μg) was resolved by SDS-PAGE and electrophoretically transferred to a PVDF membrane. The membrane was blocked with 5% non-fat skim milk in Tris/HCl-buffered saline containing 0.05% Tween 20 (TBST), and then incubated at 4°C overnight with each primary antibody appropriately diluted in TBST. The blot was washed four times for 40 min with TBST and then incubated for 90 min at room temperature with appropriately diluted secondary antibody. After washing four times for 40 min with TBST, the bands were visualized using ECL Western Blotting Detection Reagent (GE Healthcare, Little Chalfont, UK). The primary antibodies used were against the following proteins: hexokinase (Hxk), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), aldehyde dehydrogenase (Ald), alcohol dehydrogenase (Adh), Cu/Zn superoxide dismutase (Sod1), GR, glutathione peroxidase (Gpx), porin [27], thioredoxin isoform 2 (Trx2), Trx3, Trr, heat shock protein 104 (Hsp104), Hsp82, Hsp70 family (Ssa1 and Ssb2), Hsp60, Hsp42, Hsp30, and Hsp26. The primary and secondary antibodies were used as previously described [28]. Anti-tubulin antibody (Tub; Santa Cruz Biotechnology, Dallas, TX, USA) was used as a loading control. The anti-MDHAR antibody was produced in rabbits inoculated with purified MDHAR protein [29].
Measurement of AsA-like content
Yeast cells were treated with or without 20 mM H2O2 for 1 h at 28°C, harvested by centrifugation, and washed twice with cold PBS. AsA-like content was determined spectrophotometrically. Yeast cells were disrupted with glass beads and 5% m-phosphoric acid. The homogenate was centrifuged at 12,000 × g for 20 min. Total AsA content was determined in a reaction mixture containing 100 μL of supernatant, 500 μL of 150 mM KH2PO4 buffer (pH 7.4) containing 5 mM EDTA, and 100 μL of 10 mM dithiothreitol (DTT) to reduce dehydroascorbate [30] to AsA. The reaction mixtures were incubated for 10 min at room temperature. Then, 100 μL of 0.5% N-ethylmaleimide (NEM) was added to remove excess DTT. Ascorbic acid was assayed in a similar manner, except that 200 μL of deionized H2O was substituted for DTT and NEM. In both reactions, color was developed by adding 400 μL of 10% trichloroacetic acid, 400 μL of 44% o-phosphoric acid, 400 μL of α, α′-dipyridyl in 70% ethanol, and 200 μL of 30 g·L-1 FeCl3. The reaction mixtures were incubated at 40°C for 1 h and quantified spectrophotometrically at 525 nm. The concentration of DHA was estimated from the difference in the concentrations of total and reduced AsA. The concentrations of AsA and DHA were quantified by generating of a standard curve using AsA from 1 to 10 μM mL-1 [31].
Cellular redox state assay
Cells were cultured for 6 h at 28°C until they reached mid-log phase and then exposed to 20 mM H2O2 for 1 h at 28°C. The cells were harvested and lysed with glass beads. The intracellular hydroperoxide level was determined by ferrous ion oxidation in the presence of the ferric ion indicator xylenol orange [32]. Fifty microliters of crude cell extract were added to 950 μL of FOX reagent (100 μM xylenol orange [water-soluble form; Sigma-Aldrich], 250 μM ammonium ferrous sulfate, 100 mM sorbitol, and 25 mM sulfuric acid). The mixture was incubated at room temperature for 30 min and then centrifuged to remove any flocculated material before measuring the absorbance at 560 nm. Hydroperoxide concentration is reported as μg per mg of protein. For the fluorescence assay, yeast cells were treated with 0.1 mM dichlorodihydrofluorescein diacetate (DCFHDA) and 0.1 mM dihydrorhodamine 123 (DHR 123) for 20 min at 28°C with shaking before treatment with 10 mM or 20 mM H2O2 for 1 h at 28°C. Treated cells were washed twice with PBS and visualized by fluorescence microscopy (excitation, 488 nm; emission, 525 nm) [33]. Protein oxidation was determined using the Protein Carbonyl Content Assay Kit (Sigma-Aldrich) according to the manufacturer’s protocol.
Laboratory glucose-based batch fermentation
Cells (initial concentration adjusted to 1 × 106 cells per mL) grown at 30°C overnight were inoculated into YG medium containing 20% glucose and 1% yeast extract and then fermented under aerobic conditions on a shaker (160 rpm) for 72 h at 30°C. The alcohol concentration was determined based on the percentage (v/v) of alcohol in the distillate after fermentation, which was measured using an alcohol hydrometer (REF 503; Korins, Seoul, Korea). The residual glucose concentration was measured with a hand-held Refractometer N1 (Atago, Tokyo, Japan). Alcohol and residual glucose were measured after removing cell debris by centrifugation (2,000 × g, 3 min). Growth was monitored for 24 h under the same conditions. To investigate cell survival during fermentation, cells were harvested at three time points (24, 48, and 72 h) and serially diluted to 10−9 with deionized distilled water, and then 5 μL of each dilution was plated onto YPD agar, incubated for 3 days at 30°C, and then photographed. During fermentation, MDHAR expression was analyzed at various time points by western blotting as described above.
Statistical analysis
Significant differences in the measured parameters were identified using Origin Pro 8.0. Means were considered to be significantly different when P < 0.05. All experiments were independently performed at least three times, and the results are expressed as the mean ± standard deviation (SD). The results of the spotting, growth kinetics, and fermentation assays are representative of at least two independent experiments performed under identical conditions.
Results
Generation of OsMDHAR-expressing recombinant yeast and their stress response to an oxidant
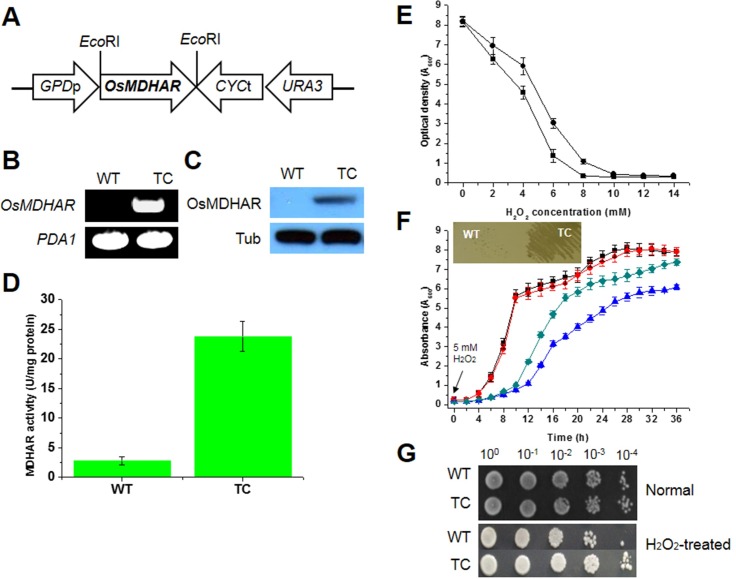
A cDNA containing the open reading frame (ORF) of MDHAR from O. sativa (OsMDHAR) was subcloned into the yeast expression vector p426GPD, which allows constitutive expression of a target gene under the control of the yeast GPD (glyceraldehyde-3-phosphate dehydrogenase) promoter (Fig 1A). To determine whether the OsMDHAR gene is expressed in yeast, semi-quantitative RT-PCR and immunoblotting were performed. A single DNA fragment of approximately 494 bp corresponding to a region within OsMDHAR was amplified from cells transformed with the p426GPD-OsMDHAR construct (TC), whereas no amplification signal was detected in wild-type cells transformed with vector alone (Fig 1B). To investigate whether the OsMDHAR protein is expressed, western blotting and enzymatic activity assays were performed. As shown in Fig 1C, immunoblotting analysis using an antibody raised against MDHAR showed a single band at 47 kDa derived from the MDHAR transgene in the crude extract prepared from the TC cells, but not in extract prepared from WT cells. The enzymatic activity of MDHAR in the TC cells was approximately 10-fold higher than that in the WT cells (Fig 1D). There was a clear correlation between the OsMDHAR mRNA level and the MDHAR protein level. To determine if OsMDHAR is related to stress tolerance in yeast, a cell survival assay was used. In this assay, the optical density of the TC cells was higher than that of the WT cells at a variety of H2O2 concentrations. The WT cells were completely inhibited in 8 mM H2O2, whereas the TC cells were inhibited in 10 mM H2O2 (Fig 1E). To confirm these results, growth kinetics, streaking, and spotting assays were performed. In the growth kinetics assay, the TC cells recovered more rapidly than the WT cells did in the presence of 5 mM H2O2 for the indicated times. However, under normal conditions, there was no difference between the cells (Fig 1F). The streaking assay results supported the growth rate findings (Fig 1F, boxed panel). The spotting assay also demonstrated that the TC cells had acquired increased stress tolerance when they were exposed to 20 mM H2O2 for 1 h at 28°C with shaking, compared to that of the WT cells (Fig 1G). These results suggest that OsMDHAR expressed in S. cerevisiae as a eukaryotic model system effectively enhances stress tolerance when the cells are challenged with oxidative stress.
Fig 1. Construction of an OsMDHAR-expressing yeast vector and the stress response of OsMDHAR-expressing yeast to hydrogen peroxide.
(A) Schematic diagram of the p426GPD::OsMDHAR construct. The OsMDHAR gene (approximately 1.5 kbp) was subcloned to generate the p426GPD::OsMDHAR construct with OsMDHAR under the control of the constitutive GPD promoter. Semi-quantitative RT-PCR (B), immunoblotting (C), and an enzymatic assay (D) were performed to examine whether OsMDHAR is expressed in this yeast strain. PDA1 and tubulin (Tub) were used as housekeeping controls for RT-PCR and western blotting, respectively. The molecular size of the PCR product and molecular weight of the detected band were approximately 494 bp and 47 kDa, respectively. Stress tolerance to hydrogen peroxide was evaluated by cell survival, growth kinetics, and spotting assays. (E) To monitor cell viability, yeast cells precultured in YPD medium were inoculated into fresh YPD medium and exposed to different concentrations of H2O2 for 16 h at 28°C. Then, the optical density at 600 nm (OD600) was measured. Circles, cells transformed with p426GPD-OsMDHAR (TC cells); squares, wild-type (WT) cells transformed with an empty vector. (F) For the growth kinetics assay, precultured yeast cells were inoculated into YPD medium containing 5 mM H2O2, and the OD600 was measured at 2-h intervals for 36 h. A streaking assay was also performed, in which mid-log phase yeast cells (OD600 ≈ 2.0) were streaked onto YPD agar plates supplemented with 5 mM H2O2. WT (squares) and TC (circles) cells in the absence of 5 mM H2O2; WT (upward triangles) and TC (diamonds) cells in the presence of 5 mM H2O2. (G) Mid-log phase yeast cells were exposed to 20 mM H2O2 for 1 h with shaking, and serially diluted with YPD medium. A 5-μL aliquot of each dilution was spotted onto YPD agar plates.
Expression of cell rescue proteins and the redox state under oxidative stress
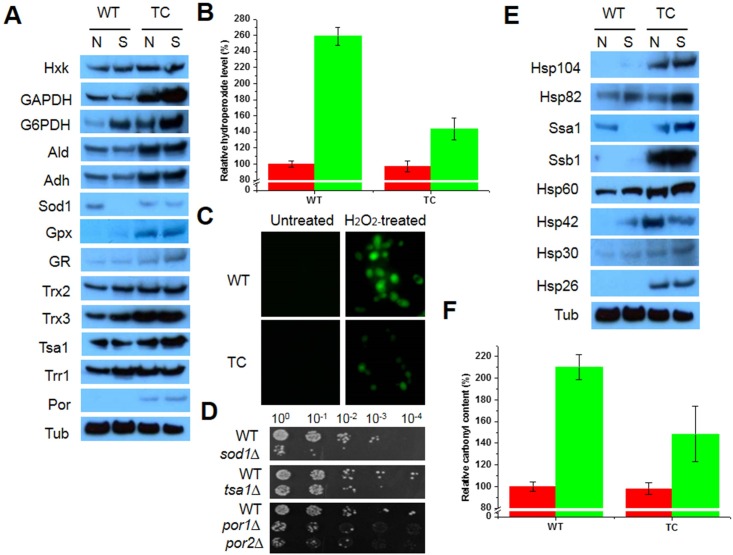
Compared to WT cells, TC cells showed increased survival following acquired tolerance under oxidative stress. To further understand the mechanism underlying the improved tolerance of TC cells, expression changes in rescue proteins were assessed by western blotting after exposure to oxidative stress. A wide range of proteins was upregulated in TC cells during oxidant challenge compared to the expression levels in WT cells (Fig 2). The expression of several metabolic enzymes, including Hxk, GAPDH, G6PDH, Ald, and Adh, was increased in the TC cells under oxidative stress, compared to that in WT cells. In addition, numerous antioxidant enzymes were upregulated, including Sod1, Gpx, GR, Trx2, Trx3, Tsa1, and Por, in TC cells. However, cytosolic Trr1 expression was similar in TC and WT cells grown under oxidative stress (Fig 2A). High expression of metabolic enzymes and antioxidant enzymes resulted in improved redox homeostasis in TC cells. The hydroperoxide level in TC cells was approximately 1.8-fold higher than that in WT cells cultured in the presence of H2O2; however, there was no difference between TC and WT cells cultured under normal conditions (Fig 2B). These results were supported by DCFHDA fluorescence, an indicator of cytosolic hydroperoxide, as DCFHDA fluorescence was lower in TC cells than in WT cells, although there was a change in signal intensity in both cells under oxidative stress (Fig 2C). Expression of Sod1, Tsa1, Por1, and Por2 was inhibited or unchanged in WT cells under oxidative stress, compared to the expression levels in TC cells. Mutants with deletions in antioxidant enzymes (sod1Δ, tsa1Δ, por1Δ, por2Δ, and ara2Δ) were hypersensitive to oxidative stress (Fig 2D). Unexpectedly, the recovery of por1Δ cells in growth kinetics and spotting assays was slower than that of WT cells without an empty vector (BY cells) in the presence of H2O2; however, there was no difference between these cells under normal condition (S1A and S1B Fig). Por1Δ cells displayed increased cytosolic and mitochondrial hydroperoxide levels under stress compared to the levels in BY cells (S1C Fig). In addition, por1Δ cells were sensitive to a wide range of stressors, including oxidants (menadione [MD] and tert-butylhydroperoxide [t-BOOH]), heat shock, metals (copper, iron, and zinc), heavy metals (aluminum and cobalt), acids (sulfuric acid and salicylic acid), and high salinity (NaCl) (S1D Fig). The expression of various molecular chaperones, HSPs, and cofactors was increased in TC cells under oxidative stress. The highly expressed proteins included Hsp104, Hsp82, Hsp60, Hsp30, Hsp26, and two members of the Hsp70 family (Ssa1 and Ssb1). Although the expression of Hsp42 in TC cells was lower under oxidative stress than under normal conditions, it was still higher in TC cells than in WT cells (Fig 2E). This high expression of chaperone proteins in TC cells resulted in decreased protein oxidation, and the protein carbonyl content of TC cells was lower than that of WT cells (Fig 2F). Thus, TC cells express a range of rescue proteins that enable them to respond to oxidative stress by improving redox homeostasis and proteostasis following the decreases in cellular hydroperoxide and protein oxidation levels.
Fig 2. Analyses of cell rescue proteins, redox state, and protein oxidation under oxidative conditions.
(A) Expression changes in antioxidant and metabolic enzymes in mid-log phase yeast cells exposed to 20 mM H2O2 for 1 h with shaking. Tubulin (Tub) was used as a loading control. (B) Hydroperoxide levels in TC cells in the absence (red bar) and presence (green bar) of 20 mM H2O2 were assessed using FOX reagent and were calculated relative to that in WT cells grown under normal conditions, which was set to 100%. (C) Mid-log phase yeast were exposed to 20 mM H2O2 for 1 h at 28°C with shaking. Redox state was analyzed by measuring DCFHDA oxidation as an indicator of cytosolic ROS. (D) Sensitivity of mutants (sod1Δ, tsa1Δ, por1Δ, and por2Δ) to oxidative stress. Yeast cells (OD600 ≈ 1.0) were exposed to 10 mM H2O2 for 1 h at 28°C with shaking, serially diluted with YPD medium, spotted onto YPD agar plates, and incubated for 2–3 days. (E) Expression changes in molecular chaperones in mid-log phase yeast cells exposed to 20 mM H2O2 for 1 h with shaking. Tubulin (Tub) was used as a loading control. (F) Protein carbonylation in yeast cells exposed to 20 mM H2O2 for 1 h was calculated relative to that in WT cells under normal conditions, which was set to 100%. Red bar, normal conditions; green bar, H2O2 treatment; WT, yeast cells with an empty vector; TC, OsMDHAR-expressing yeast cells; N, normal conditions; S, H2O2 treatment.
AsA-like content and its effect on H2O2
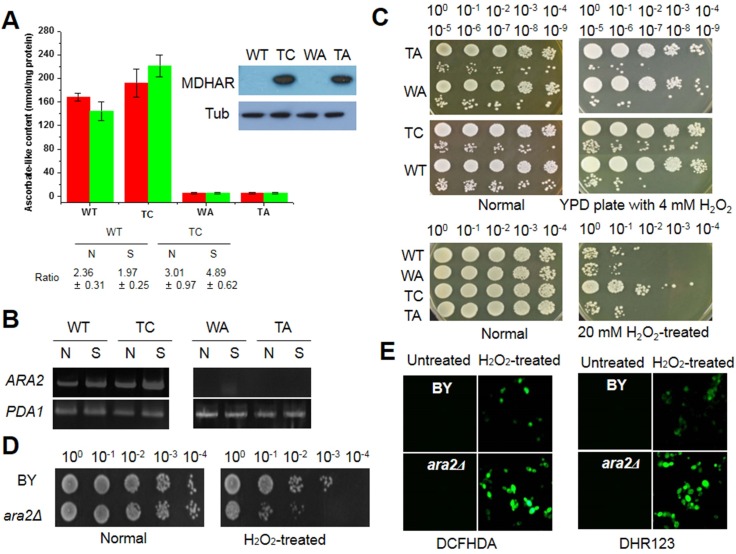
AsA and its analogue erythroascorbate (EAA), are antioxidant molecules that act as scavengers of free radicals and are essential for resistance and adaptation to abiotic stresses in yeast [34]. AsA is the most abundant antioxidant molecule in the cell, thus it is a good target for investigation of the intrinsic ability of OsMDHAR-expressing cells to eliminate free radicals. Since AsA-like content is an indication of the capacity of cells to manage exogenous stressors, we investigated a yeast strain in which the EAA biosynthesis-related gene ARA2 was deleted (ara2Δ). Deletion of ARA2 was confirmed by evaluating empty vector (p426GPD)- and OsMDHAR::p426GPD-transformed ara2Δ cells (WA and TA, respectively) by immunoblotting (Fig 3A, boxed panel). The EAA content of exponential phase WT, WA, TA, and TC cells was analyzed in YPD-grown cultures in the absence and presence of 20 mM H2O2. As shown in Fig 3A, significant differences in EAA content were observed between WT and TC cells. Although the EAA levels before and after stress were unchanged, the EAA pool in TC cells was approximately 1.3-fold higher than that in WT cells. In contrast, EAA was not detected in WA or TA cells. The accumulation of EAA in TC cells corresponded to the ratio of the reduced form to oxidized form in the presence of H2O2. To examine whether ARA2 is required for the increased EAA pool, ARA2 expression was measured by semi-quantitative RT-PCR. ARA2 expression was highest in TC cells exposed to oxidative stress, whereas it increased in WT cells under the same condition. In contrast, no expression was detected in WA or TA cells (Fig 3B). The importance of EAA in the response to oxidative stress was examined by a spotting assay and redox state analysis. Recovery capacity was higher in TC cells than in the other tested cells (WT, WA, and TA) in the presence of H2O2; however, no difference in growth kinetics was observed among the four cell types under normal conditions (Fig 3C). Ara2Δ cells were hypersensitive to oxidative stress compared to the sensitivity of BY cells (Fig 3D), and they displayed increased hydroperoxide levels in the cytosol and mitochondria, as measured using DCFHDA and DHR124 as indicator probes, respectively (Fig 3E). These results imply that EAA plays an important role in the oxidative stress response, and that in WT cells, the levels of EAA are insufficient to promote stress tolerance, compared to the levels in TC cells. Based on these results, in subsequent experiments, we tested the effects of exogenous AsA or its analogue IAA on the stress response of yeast cells. Although WT cells were more sensitive to oxidative stress than TC cells, spotting assays showed that WT cells reached the same growth levels as TC cells in the presence of exogenous AsA or IAA, which is indicative of adaptation capacity under oxidative stress (S2A Fig, upper panel). Supplementation with these molecules led to enhanced stress tolerance in ara2Δ cells under oxidative stress (S2A Fig, lower panel). Ara2Δ cells were also sensitive to various stressors, including heat shock, MD, t-BOOH, ethanol, NaCl, cadmium, zinc, and lactic acid (S2B Fig).
Fig 3. Stress response related to ascorbate (AsA)-like molecules.
(A) AsA-like content in yeast cells exposed to 20 mM H2O2 for 1 h was analyzed and is shown as nmol per mg protein. The ratio shown is that of the reduced form to oxidized form. (B) ARA2 expression was evaluated by semi-quantitative RT-PCR. PDA1 was used as a control. (C) Oxidative stress response of yeast cells in the absence and presence of ARA2. Mid-log phase cells were serially diluted, and 5 μL of the diluted solutions were spotted onto YPD agar plates containing 4 mM H2O2 (upper panels). Mid-log phase cells were treated with 20 mM H2O2 for 1 h with shaking, diluted with YPD medium, and spotted onto YPD agar plates. The plates were incubated for 2–3 days and photographed. (D) Stress sensitivity of ara2Δ yeast cells, in which the erythroascorbate (EAA) biosynthesis gene was deleted. Yeast cells (A600 ≈ 1.0) were exposed to 10 mM H2O2 for 1 h at 28°C with shaking, serially diluted with YPD medium, spotted onto YPD agar plates, and incubated for 2–3 days. (E) The redox state of ara2Δ yeast cells under oxidative conditions. Yeast cells (OD600 ≈ 1.0) were exposed to 10 mM H2O2 for 1 h after DCFHDA and DHAR 123 treatment for 30 min and washed twice with phosphate-buffered saline (PBS). Probe intensity was observed by fluorescence microscopy. BY, wild-type cells without an empty vector; ara2Δ, cells with a deletion of the EAA biosynthetic gene ARA2; WT, wild-type yeast cells with an empty vector; TC, yeast cells with p426GPD::OsMDHAR; WA, ara2Δ yeast cells with an empty vector; TA, ara2Δ yeast cells with p426GPD::OsMDHAR; N, normal conditions; S, in the presence of H2O2.
Effect of OsMDHAR expression under batch fermentation
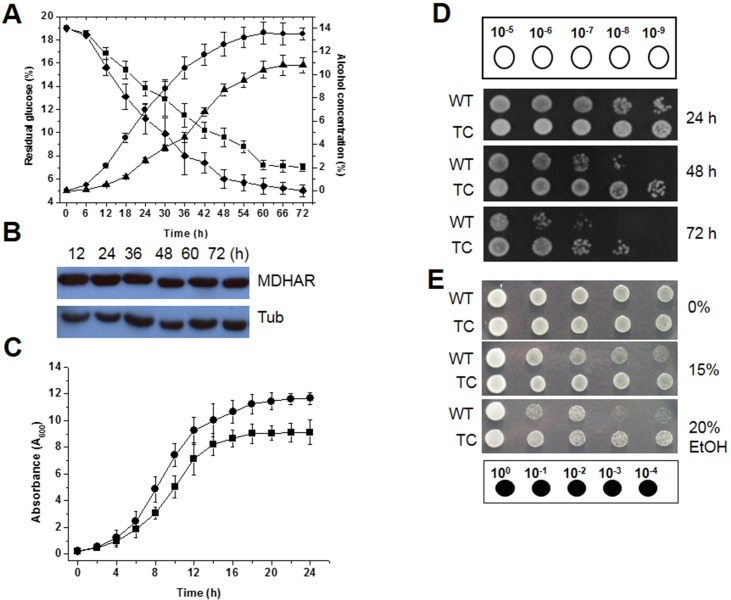
Since TC cells expressing OsMDHAR were more tolerant to oxidative stress than WT cells, these cells were tested in glucose-based batch fermentation in YG medium under aerobic conditions. Distinct differences in alcohol yield and residual glucose content were observed between TC and WT cells at 30°C, which is the temperature that is typically used for industrial fermentation. During fermentation for 72 h at 30°C, the alcohol yield of TC cells was approximately 25% higher than that of WT cells. The final alcohol concentration was approximately 13.5% and 10.2% with TC cells and WT cells, respectively, and the residual glucose concentration was inversely proportional to the alcohol concentration during fermentation (Fig 4A). OsMDHAR expression in the TC cells during fermentation was confirmed by western blotting, although protein expression decreased slightly over time (Fig 4B). In addition, distinct differences in growth kinetics and cell survival were observed between TC and WT cells during fermentation. The growth rate in early fermentation until stationary phase was higher in TC cells than in WT cells (Fig 4C). In particular, the survival of TC cells was time-dependently higher than that of WT cells during fermentation in YG medium for 72 h, although cell viability decreased gradually with time (Fig 4D). Next, to examine the response to various concentrations of ethanol, yeast cells were grown to log phase (OD600 = 2.0), and then exposed to 15% or 20% ethanol for 1 h with shaking. After exposure, the cultures were serially diluted and spotted onto YPD agar plates. TC cells were more tolerant to ethanol than WT cells (Fig 4E). In addition, TC cells expressing OsMDHAR were more resistant to ROS-induced oxidative stress (MD, t-BOOH, copper, iron, cadmium, and sodium dodecyl sulfate [SDS]) than WT cells (S3 Fig). Therefore, our findings indicate that OsMDHAR expression enhances fermentative capacity at moderate temperatures, which is an important factor for increased alcohol yield during fermentation.
Fig 4. Fermentative capacity and the survival of OsMDHAR-expressing yeast cells during batch fermentation.
(A) Fermentative capacity was analyzed by measuring the alcohol (AC) and residual glucose (RG) concentrations in YG medium after fermentation for 72 h at 30°C. Upward triangles, AC of WT cells; circles, AC of TC cells; squares, RG of WT cells; diamonds, RG of TC cells. (B) Time-dependent OsMDHAR expression during batch fermentation was evaluated by western blotting. Tubulin (Tub) was used as a loading control. (C) Growth kinetics during fermentation was assessed by measuring the OD600 at 2-h intervals for the indicated time. Squares, WT cells; circles, TC cells. (D) Cell viability during fermentation at 30°C was assessed by a spotting assay. Cells were harvested after 24 h (upper panel), 48 h (middle panel), and 72 h (lower panel) of fermentation and serially diluted to 10−9. A 5-μL aliquot of each diluted solution was spotted onto YPD agar plates. After incubation for 3 days, the plates were photographed. (E) Stress response to ethanol. Mid-log phase yeast cells (OD600 ≈ 2.0) were exposed to different concentrations of ethanol (0, 15%, and 20%) for 1 h, serially diluted, and spotted onto YPD agar plates. WT, yeast cells with an empty vector; TC, OsMDHAR-expressing yeast cells.
Discussion
We evaluated an OsMDHAR-expressing yeast strain and compared it a control WT strain to determine if OsMDHAR could improve the stress tolerance of S. cerevisiae as a eukaryotic model system. The viability of cells transformed with p426GPD::OsMDHAR (TC cells), which constitutively express OsMDHAR under the control of the GPD promoter, was inhibited by a higher concentration of H2O2, than the concentration that inhibited the viability of WT cells with an empty vector (Fig 1). In growth kinetics, streaking, and spotting assays, OsMDHAR-expressing TC cells were more resistant to H2O2 than WT cells. We measured the stress tolerance of TC cells containing recombinant OsMDHAR in the presence of 5 mM or 20 mM H2O2. OsMDHAR expression in yeast enhanced the acquired resistance to H2O2 (Fig 1) and to a wide range of ROS-induced oxidative stressors, including oxidants (MD and t-BOOH), redox-active metals (iron, copper, and cobalt), a non-redox-active metal (cadmium), SDS, and ethanol (S3 Fig). Although no previous studies have established a relationship between OsMDHAR expression and the stress response in yeast, the overexpression of eukaryotic antioxidant genes has been shown to improve stress resistance in both prokaryotes and eukaryotes [35–37].
To investigate whether the improved tolerance to oxidative stress in TC cells was due to an OsMDHAR-mediated response, the expression of various rescue proteins was analyzed. Under oxidative stress, TC cells upregulated several metabolic enzymes, including Hxk, GAPDH, Adh, Ald, and G6PDH, and antioxidant enzymes, including Sod1, Gpx, GR, Trx2, Trx3, Tsa1, and Por (Fig 2A). In contrast, Trr1 expression was the same in TC and WT cells under stress. The expression of most proteins was stress-dependently induced in TC cells. The major stress-protection mechanisms include synthesis of antioxidant proteins, proteins involved in metabolic and pentose phosphate pathways and molecular chaperones, and the accumulation of compatible solutes and hydrophylins as well as permeability adaptation of the plasma membrane [18]. Upregulation or overexpression of stress-related genes (SOD, CAT, GPX, TSA1, and TRX) enhances acquired resistance to ROS-induced oxidative stresses, including oxidants (O2*−, H2O2, and MD), ethanol, and lactic acid, by improving redox homeostasis following the reduction of oxidative damage through cross-protection between target proteins and other antioxidant enzymes in S. cerevisiae [38–42], whereas the direct interactions between H2O2 and other antioxidant proteins play a secondary role by protecting ribosomal proteins from stress-induced aggregation through the function of CAT and other peroxiredoxins [43,44]. In addition, the TRX system (TRX plus TRR) can act as an alternative system to reduce GSH disulfide (GSSG) in the presence of NADPH in glr1-deficient yeast cells and buffer oxidative stress [45]. Tomato MDHAR- and Arabidopsis thaliana MDHAR (AtMDHAR1)-overexpressing transgenic plants showed acquired tolerance against abiotic stressors (chilling and high temperature stresses, ozone, salt, polyethylene glycol, and radiation) by improving redox homeostasis through lowering the levels of hydrogen peroxide and lipid peroxidation and increasing the net photosynthetic rate, PSII effective quantum yield (Fv/Fm), fresh weight, and antioxidant’s enzyme activities (DHAR, GR, SOD and peroxidases) by elevating the AsA level compared to in WT plants [11,12,26]. In contrast, downregulation or repression of these antioxidant systems leads to a number of stress-dependent phenotypes, including genome instability [46], slow growth, and increased sensitivity to ROS-generating agents such as H2O2, which accelerates aging, auxotrophy, and cell death [47]. In addition, the suppression of proteins involved in the pentose phosphate pathway (i.e., G6PDH) and another pathway (isocitrate dehydrogenase) important for reducing power production increased the sensitivity to oxidative stress following an imbalance in NADPH homeostasis [47–49]. Furthermore, TC cells showed increased porin (Por) expression under oxidative stress (Fig 2A). Mitochondrial function affects redox homeostasis, which is mediated primarily by the voltage-dependent anion channel (VDAC; porin pore). VDAC releases superoxide anions from the mitochondria into the cytosol and increases cytosolic ROS [50]. However, por1Δ cells showed increased cytosolic and mitochondrial ROS levels (S1 Fig). Taken together, these findings highlight the importance of OsMDHAR-mediated mechanisms in maintaining ROS at non-toxic levels and cross-protection through the activation of various antioxidant enzymes.
In addition to metabolic and antioxidant enzymes, we observed increased expression of various HSPs, molecular chaperones, and cofactors, including Hsp104, Hsp90 (Hsp82), the Hsp70 family members (Ssa1 and Ssb1), Hsp60, Hsp42, Hsp30, and Hsp26, in TC cells under oxidative stress. (Fig 2E). Accumulation of chaperone proteins resulted in decreased stress-induced injury (Fig 2F). ROS-induced oxidative stress causes aggregation and inactivation of nascent proteins due to misfolding, which at high levels, can lead to neurodegenerative disease, cancer, viral infection, and cell death. To protect themselves from oxidative damage, yeast cells have evolved a wide range of molecular chaperones and cofactors, including Hsps that play a central role in protein homeostasis [51,52]. In eukaryotes, the cooperative functions of Hsp104, Ssa1, Hsp42, and Hsp26 rescue aggregated or misfolded proteins produced by oxidative stress through several folding process, such as general folding, nascent chain folding, and post-import folding following conformational changes associated with Fe/S proteins and substrate binding [51–54]. Hsp90 reactivates unfolded or misfolded proteins (referred to as client proteins) by interacting or assembling with co-chaperones and cofactors [51,55], which also participate in different important cellular functions, such as growth and differentiation, as well as stress responses [56]. As described above, sHsps such as Hsp42 and Hsp26 play a critical role in protein solubility when ROS-induced oxidative stress leads to general cytosolic protein unfolding; however, other sHsps, including Hsp30, have not been well characterized even though they also appear to play a role in stress tolerance [52]. Based on these observations, our results indicate that OsMDHAR expression leads to a marked accumulation of chaperone proteins, which facilitates protein refolding and minimizes oxidative damage by maintaining the balance of chaperone machinery systems, thus improving proteostasis under oxidative stress. However, the relationship between OsMDHAR and Hsp expression remains unclear.
OsMDHAR expression in TC cells increased the ratio of reduced and oxidized forms following the accumulation of the AsA-like molecule EAA under oxidative stress (Fig 3A). TC cells were more tolerant to ROS-induced oxidative stress than WT cells (Fig 3C). These results show that EAA in WT cells is insufficient to induce the necessary machinery for stress tolerance compared to TC cells. Treatment of WT cells with AsA or its analogue IAA improved the stress response, such that it was similar to that of TC cells. Supplementation with AsA or IAA also enhanced acquired tolerance in WA cells, although the stress resistance of WA cells was lower than that of TC and WT cells (S2 Fig). These results show that EAA as an AsA analogue plays an important role in tolerance to oxidative stress. From this perspective, the increase in the AsA-like pool is mainly the result of EAA recycling through OsMDHAR as well as EAA biosynthesis following upregulation of ARA2 under unfavorable conditions. MDHAR, which is widely found in eukaryotes, is an important antioxidant that functions as a key enzyme for maintaining AsA pools. AsA is synthesized by all higher plants and nearly all higher animals, with the exception of some species, including humans, primates, guinea pigs, some birds, and fish [57]. AsA and its analogue EAA are the most abundant water-soluble free radical scavenger antioxidants in living organisms. AsA serves as a cofactor for enzymes involved in hormone biosynthesis and is involved in the recycling of antioxidants such as AsA, α-tocopherol, and phenolics [49]. The precise mechanisms underlying these functions are not yet known. In Candida albicans, EAA peroxidase is capable of detoxifying H2O2 by converting it to water through oxidation of EAA, which increased tolerance to oxidative stress [34]. After participating in the reaction, EAA can be recycled by several different mechanisms. The EAA radical, produced following EAA oxidation, can be recycled following reduction by OsMDHAR or mitochondrial NADH-dependent cytochrome b5 reductase [58]. In bioassays using tobacco hornworm (Manduca sexta), EAA induced larval growth almost as well as AsA because AsA can be converted to EAA by a ubiquitin-mediated process [59]. Because of this OsMDHAR-mediated recycling, EAA content and its redox state are maintained, which is critical under ROS-induced oxidative stress. Additionally, EAA plays a role in cell growth and development, as well as in response to oxidative stress.
To further elucidate the functions of OsMDHAR, the glucose-based batch fermentative capacity of TC and WT cells was compared at 30°C (a typical industrial temperature) because Brazil, the United States, and Korea produced approximately 21.6, 50.3, and 0.15 billion gallons of bioethanol, respectively in 2012, which is more than double that produced in 2007 [60]. During batch fermentation at 30°C, OsMDHAR was constitutively expressed until the end of fermentation (Fig 2B). The alcohol concentrations produced by fermentation using TC and WT cells were 13.5% (0.71 ± 0.02 g·g−1) and 10.2% (0.53 ± 0.03 g·g−1), respectively (Fig 4A), and the alcohol concentration in TC cells was approximately 25% (0.18 g·g−1) higher than that in WT cells. In general, the theoretical ethanol yield from glucose fermentation by S. cerevisiae is 10–11% (0.50–0.55 g·g−1) [61]. Interestingly, the alcohol yield of TC cells in this study was 9.4% (0.24 g·g−1) higher than of WT cells reported previously [61]. Survival during fermentation at 30°C was also higher for TC cells than for WT cells (Fig 4D). The alcohol concentration of OsMDHAR-expressing TG yeast (13.5%; 0.71 ± 0.02 g·g−1) was higher than that of CaDHN- (13.3%; 0.63 ± 0.03 g·g−1) and OsTPX-expressing TG yeasts (12.9%; 0.61 ± 0.01 g·g−1); which were under the control of the same promoter) [62,63]. Current bioethanol production relies on agricultural products, including dextrose derived from corn in the US and sucrose derived from sugar in Brazil [60]. The ethanol produced during sugar-based fermentation affects cell viability, growth rate, and fermentation capacity and quality. To overcome these problems, genetically modified yeast strains have been introduced. Compared to S. cerevisiae, other yeasts have some limitations, such as low ethanol yield and poor ethanol tolerance in typical glucose-based fermentation [64,65]. Saccharomyces spp. is most attractive microorganism for fermenting sugars to ethanol. Besides its innate properties, overexpression of stress-related genes could be a useful method for developing ethanol-tolerant yeasts because ethanol toxicity is highly related to ROS generation. For example, overexpression of TRX2 increased fermentation capacity and wine yield and quality by preventing the oxidative damage to glycolytic and fermentation proteins (Ahp1) through cross-protection of transcription factor (Yap1) and antioxidant enzymes (Sod1, Sod2, and CAT) in industrial wine yeast during biomass-based batch- and fed-batch fermentation [42,66]. Upregulation of G6PDH (ZWF1) [36], MPR1 [67], and PAD [68] conferred tolerance to ethanol by enhancing NADPH and redox homeostasis or by detoxifying inhibitory compounds. To obtain a high ethanol yield in fermentation, improved S. cerevisiae strains that are tolerant of ethanol and different metabolic environments are required, such as the OsMDHAR-expressing transgenic yeast developed herein. As shown, OsMDHAR expression in TC cells increased tolerance to ethanol and sugar-induced osmotic and physiochemical stresses.
In conclusion, high expression of OsMDHAR in a transgenic yeast strain increased acquired tolerance to ROS-induced oxidative stress, such as that generated by H2O2, by maintaining balanced cellular redox homeostasis, proteostasis, and the pool of EAA as an AsA-like molecule. It is possible that the expression of OsMDHAR in yeast enhanced tolerance to oxidative stress and that all antioxidant defense mechanisms act synergistically, cross-protecting the cells at different levels and functioning to generate a fast and effective defense response under oxidative stress. In addition, heterologous OsMDHAR expression enhanced tolerance to high concentrations of ethanol, resulting in improved fermentative capacity. The genetically modified yeast used in this study show potential for use in the fermentation industry to improve ethanol yield. Further studies are needed to elucidate the mechanism underlying increased ethanol tolerance and yield in OsMDHAR-expressing S. cerevisiae during fermentation.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are available from Dryad (doi:10.5061/dryad.9bv73).
Funding Statement
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ011122022016), Rural Development Administration, Korea.
References
- 1. Jamieson DJ. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast. 1998; 14: 1511–1527. [DOI] [PubMed] [Google Scholar]
- 2.Noctor G, Mhamdi A, Foyer CH. Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ. 2016; 39: 1140–1160. 10.1111/pce.12726 [DOI] [PubMed] [Google Scholar]
- 3.Shigeoka S, Maruta T. Cellular redox regulation, signaling, and stress response in plants. Biosci Biotechnol Biochem. 20104; 78: 1457–1470. 10.1080/09168451.2014.942254 [DOI] [PubMed] [Google Scholar]
- 4.Gallie DR. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Exp Bot. 2013; 64: 433–443. 10.1093/jxb/ers330 [DOI] [PubMed] [Google Scholar]
- 5.Sano S, Asada K. cDNA cloning of monodehydroascorbate radical reductase from cucumber: a high degree of homology in terms of amino acid sequence between this enzyme and bacterial flavoenzymes. Plant Cell Physiol. 1994; 35: 425–437. [PubMed] [Google Scholar]
- 6.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010; 48: 909–930. 10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 7.Omoto E, Nagao H, Taniguchi M, Miyake H. Localization of reactive oxygen species and change of antioxidant capacities in mesophyll and bundle sheath chloroplasts of maize under salinity. Physiol Plant. 2013; 149: 1–12. 10.1111/ppl.12017 [DOI] [PubMed] [Google Scholar]
- 8.Sakihama Y, Mano J, Sano S, Asada K, Yamasaki H. Reduction of phenoxyl radicals mediated by monodehydroascorbate reductase. Biochem Biophys Res Commun. 2000; 279: 949–954. [DOI] [PubMed] [Google Scholar]
- 9.Eastmond PJ. MONODEHYROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. Plant Cell. 2007; 19: 1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drew DP, Lunde C, Lahnstein J, Fincher GB. Heterologous expression of cDNAs encoding monodehydroascorbate reductases from the moss, Physcomitrella patens and characterization of the expressed enzymes. Planta. 2007; 225: 945–954. [DOI] [PubMed] [Google Scholar]
- 11.Sultana S, Khew CY, Morshed MM, Namasivayam P, Napis S, Ho CL. Overexpression of monodehydroascorbate reductase from a mangrove plant (AeMDHAR) confers salt tolerance on rice. J Plant Physiol. 2012; 169: 311–318. 10.1016/j.jplph.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 12.Li F, Wu QY, Sun YL, Wang LY, Yang XH, Meng QW. Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiol Plant. 2010; 139: 421–434. 10.1111/j.1399-3054.2010.01369.x [DOI] [PubMed] [Google Scholar]
- 13.Yoon HS, Lee H, Lee IA, Kim KY, Jo J. Molecular cloning of the monodehydroascorbate reductase gene from Brassica campestris and analysis of its mRNA level in response to oxidative stress. Biochim Biophys Acta. 2004; 1658: 181–186. [DOI] [PubMed] [Google Scholar]
- 14.Mishra NP, Mishra RK, Singhal GS. Changes in the activities of anti-oxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol. 1993; 102: 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehlhorn H, Seufert G, Schmidt A, Kunert KJ. Effect of SO2 and O3 on production of antioxidants in conifers. Plant Physiol. 1986; 82: 336–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo N, Yu X, Liu J, Jiang Y. Nucleotide diversity and linkage disequilibrium in antioxidant genes of Brachypodium distachyon. Plant Sci. 2012; 197: 122–129. 10.1016/j.plantsci.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 17.Ben Rejeb I, Lenne C, Leblanc N, Julien JL, Ammar S, Bouzid S, et al. Iron-superoxide dismutase and monodehydroascorbate reductase transcripts accumulate in response to internode rubbing in tomato. C R Biol. 2004; 327: 679–686. [DOI] [PubMed] [Google Scholar]
- 18.Tange A, Prior B, Thevelein J. Yeast responses to stresses In: Rosa CA, Peter G, editors. Biodiversity and ecophysiology of yeasts: Springer-Verlag Berlin Heidelberg; 2006. pp.175–195. [Google Scholar]
- 19.Saini JK, Saini R, Tewari L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech. 2015; 5: 337–353. 10.1007/s13205-014-0246-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley D, Bandara A, Fraser S, Chambers PJ, Stanley GA. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J Appl Microbiol. 2010; 109: 13–24. 10.1111/j.1365-2672.2009.04657.x [DOI] [PubMed] [Google Scholar]
- 21.Ding J, Huang X, Zhang L, Zhao N, Yang D, Zhang K. Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2009; 85: 253–263. 10.1007/s00253-009-2223-1 [DOI] [PubMed] [Google Scholar]
- 22.Belloch C, Orlic S, Barrio E, Querol A. Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int J Food Microbiol. 2008; 122: 188–195. 10.1016/j.ijfoodmicro.2007.11.083 [DOI] [PubMed] [Google Scholar]
- 23.Husnik JI, Volschenk H, Bauer J, Colavizza D, Luo Z, van Vuuren HJ. Metabolic engineering of malolactic wine yeast. Metab Eng. 2006; 8: 315–323. [DOI] [PubMed] [Google Scholar]
- 24.Gietz RD, Woods RA. Genetic transformation of yeast. Biotechniques. 2001; 30: 816–820, 822–816, 828 passim. [DOI] [PubMed] [Google Scholar]
- 25.Wenzel TJ, Teunissen AW, de Steensma HY. PDA1 mRNA: a standard for quantitation of mRNA in Saccharomyces cerevisiae superior to ACT1 mRNA. Nucleic Acids Res. 1995; 23: 883–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, et al. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta. 2007; 225: 1255–1264. [DOI] [PubMed] [Google Scholar]
- 27.Compagno C, Brambilla L, Capitanio D, Boschi F, Ranzi BM, Porro D. Alterations of the glucose metabolism in a triose phosphate isomerase-negative Saccharomyces cerevisiae mutant. Yeast. 2001; 18: 663–670. [DOI] [PubMed] [Google Scholar]
- 28.Kim IS, Kim YS, Kim H, Jin I, Yoon HS. Saccharomyces cerevisiae KNU5377 stress response during high-temperature ethanol fermentation. Mol Cells. 2013; 35: 210–218. 10.1007/s10059-013-2258-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Do H, Kim IS, Kim YS, Shin SY, Kim JJ, Mok JE, et al. Purification, characterization and preliminary X-ray crystallographic studies of monodehydroascorbate reductase from Oryza sativa L. japonica. Acta Crystallogr F Struct Biol Commun. 2014; 70: 1244–1248. 10.1107/S2053230X14015908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sudhakar D, Fu X, Stoger E, Williams S, Spence J, Brown DP, et al. Expression and immunolocalisation of the snowdrop lectin, GNA in transgenic rice plants. Transgenic Res. 1998; 7: 371–378. [DOI] [PubMed] [Google Scholar]
- 31.Gillespie KM, Ainsworth EA. Measurement of reduced, oxidized and total ascorbate content in plants. Nat Protoc. 2007; 2: 871–874. [DOI] [PubMed] [Google Scholar]
- 32.Gay C, Collins J, Gebicki JM. Hydroperoxide assay with the ferric-xylenol orange complex. Anal Biochem. 1999; 273: 149–155. [DOI] [PubMed] [Google Scholar]
- 33.Kiani-Esfahani A, Bahrami S, Tavalaee M, Deemeh MR, Mahjour AA, Nasr-Esfahani MH. Cytosolic and mitochondrial ROS: which one is associated with poor chromatin remodeling? Syst Biol Reprod Med. 2013; 59: 352–359. 10.3109/19396368.2013.829536 [DOI] [PubMed] [Google Scholar]
- 34.Kwak MK, Song SH, Ku M, Kang SO. Candida albicans erythroascorbate peroxidase regulates intracellular methylglyoxal and reactive oxygen species independently of D-erythroascorbic acid. FEBS Lett. 2015; 589: 1863–1871. 10.1016/j.febslet.2015.04.050 [DOI] [PubMed] [Google Scholar]
- 35.Singh S, Brocker C, Koppaka V, Chen Y, Jackson BC, Matsumoto A, et al. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic Biol Med. 2013; 56: 89–101. 10.1016/j.freeradbiomed.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorsich SW, Dien BS, Nichols NN, Slininger PJ, Liu ZL, Skory CD. Tolerance to furfural-induced stress is associated with pentose phosphate pathway genes ZWF1, GND1, RPE1, and TKL1 in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2006; 71: 339–349. [DOI] [PubMed] [Google Scholar]
- 37.Grabowska D, Chelstowska A. The ALD6 gene product is indispensable for providing NADPH in yeast cells lacking glucose-6-phosphate dehydrogenase activity. J Biol Chem. 2003; 278: 13984–13988. [DOI] [PubMed] [Google Scholar]
- 38.Fierro-Risco J, Rincon AM, Benitez T, Codon AC. Overexpression of stress-related genes enhances cell viability and velum formation in Sherry wine yeasts. Appl Microbiol Biotechnol. 2013; 97: 6867–6881. 10.1007/s00253-013-4850-9 [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro TP, Fernandes C, Melo KV, Ferreira SS, Lessa JA, Franco RW, et al. Iron, copper, and manganese complexes with in vitro superoxide dismutase and/or catalase activities that keep Saccharomyces cerevisiae cells alive under severe oxidative stress. Free Radic Biol Med. 2015; 80: 67–76. 10.1016/j.freeradbiomed.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 40.Abbott DA, Suir E, Duong GH, de Hulster E, Pronk JT, van Maris AJ. Catalase overexpression reduces lactic acid-induced oxidative stress in Saccharomyces cerevisiae. Appl Environ Microbiol. 2009; 75: 2320–2325. 10.1128/AEM.00009-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandes PN, Mannarino SC, Silva CG, Pereira MD, Panek AD, Eleutherio EC. Oxidative stress response in eukaryotes: effect of glutathione, superoxide dismutase and catalase on adaptation to peroxide and menadione stresses in Saccharomyces cerevisiae. Redox Rep. 2007; 12: 236–244. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Pastor R, Perez-Torrado R, Cabiscol E, Ros J, Matallana E. Reduction of oxidative cellular damage by overexpression of the thioredoxin TRX2 gene improves yield and quality of wine yeast dry active biomass. Microb Cell Fact. 2010; 9: 9 10.1186/1475-2859-9-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rand JD, Grant CM. The thioredoxin system protects ribosomes against stress-induced aggregation. Mol Biol Cell. 200617: 387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munhoz DC, Netto LE. Cytosolic thioredoxin peroxidase I and II are important defenses of yeast against organic hydroperoxide insult: catalases and peroxiredoxins cooperate in the decomposition of H2O2 by yeast. J Biol Chem. 2004; 279: 35219–35227. [DOI] [PubMed] [Google Scholar]
- 45.Tan SX, Greetham D, Raeth S, Grant CM, Dawes IW, Perrone GG. The thioredoxin-thioredoxin reductase system can function in vivo as an alternative system to reduce oxidized glutathione in Saccharomyces cerevisiae. J Biol Chem. 2010; 285: 6118–6126. 10.1074/jbc.M109.062844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang HM, Siu KL, Wong CM, Jin DY. Loss of yeast peroxiredoxin Tsa1p induces genome instability through activation of the DNA damage checkpoint and elevation of dNTP levels. PLoS Genet. 2009; 5: e1000697 10.1371/journal.pgen.1000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan SX, Teo M, Lam YT, Dawes IW, Perrone GG. Cu, Zn superoxide dismutase and NADP(H) homeostasis are required for tolerance of endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol Biol Cell. 2009; 20: 1493–1508. 10.1091/mbc.E08-07-0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez-Mirabal HR, Winther JR. Redox characteristics of the eukaryotic cytosol. Biochim Biophys Acta. 2008; 1783: 629–640. [DOI] [PubMed] [Google Scholar]
- 49.Herrero E, Ros J, Belli G, Cabiscol E. Redox control and oxidative stress in yeast cells. Biochim Biophys Acta. 2008; 1780: 1217–1235. 10.1016/j.bbagen.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 50.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003; 278: 5557–5563. [DOI] [PubMed] [Google Scholar]
- 51.Morano KA, Grant CM, Moye-Rowley WS. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics. 2012; 190: 1157–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verghese J, Abrams J, Wang Y, Morano KA. Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol Mol Biol Rev. 2012; 76: 115–158. 10.1128/MMBR.05018-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Priya S, Sharma SK, Goloubinoff P. Molecular chaperones as enzymes that catalytically unfold misfolded polypeptides. FEBS Lett. 2013; 587: 1981–1987. 10.1016/j.febslet.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 54.Aguado A, Fernandez-Higuero JA, Moro F, Muga A. Chaperone-assisted protein aggregate reactivation: Different solutions for the same problem. Arch Biochem Biophys. 2015; 580: 121–134. 10.1016/j.abb.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 55.Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005; 120: 715–727. [DOI] [PubMed] [Google Scholar]
- 56.Breiman A. Plant Hsp90 and its co-chaperones. Curr Protein Pept Sci. 2014; 15: 232–244. [DOI] [PubMed] [Google Scholar]
- 57.Ostergaard J, Persiau G, Davey MW, Bauw G, Van Montagu M. Isolation of a cDNA coding for L-galactono-gamma-lactone dehydrogenase, an enzyme involved in the biosynthesis of ascorbic acid in plants. Purification, characterization, cDNA cloning, and expression in yeast. J Biol Chem. 1997; 272: 30009–30016. [DOI] [PubMed] [Google Scholar]
- 58.Lee JS, Huh WK, Lee BH, Baek YU, Hwang CS, Kim ST, et al. Mitochondrial NADH-cytochrome b5 reductase plays a crucial role in the reduction of D-erythroascorbyl free radical in Saccharomyces cerevisiae. Biochim Biophys Acta. 2001; 1527: 31–38. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Narasaki R, Kitano Y, Hasumi K. Ascorbic acid conversion to erythroascorbic acid, mediated by ubiquitin. Biochem Biophys Res Commun. 2009; 384: 210–214. 10.1016/j.bbrc.2009.04.101 [DOI] [PubMed] [Google Scholar]
- 60.Chum HL, Warner E, Seabra JEA, Macedo IC. A comparison of commersial ethanol production systems from Brazillian sugarcane and US corn. Biofuels Bioprod Bioref. 2013; 8: 205–223. 10.1002/bbb.1448 [DOI] [Google Scholar]
- 61.Zhao XQ, Bai FW. Mechanisms of yeast stress tolerance and its manipulation for efficient fuel ethanol production. J Biotechnol. 2009; 144: 23–30. 10.1016/j.jbiotec.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 62.Kim IS, Kim HY, Kim YS, Choi HG, Kang SH, Yoon HS. Expression of dehydrin gene from Arctic Cerastium arcticum increases abiotic stress tolerance and enhances the fermentation capacity of a genetically engineered Saccharomyces cerevisiae laboratory strain. Appl Microbiol Biotechnol. 2013; 97: 8997–9009. 10.1007/s00253-013-4729-9 [DOI] [PubMed] [Google Scholar]
- 63.Kim IS, Kim YS, Yoon HS. Expression of salt-induced 2-Cys peroxiredoxin from Oryza sativa increases stress tolerance and fermentation capacity in genetically engineered yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2013; 97: 3519–3533. 10.1007/s00253-012-4410-8 [DOI] [PubMed] [Google Scholar]
- 64.Steensels J, Snoek T, Meersman E, Picca Nicolino M, Voordeckers K, Verstrepen KJ. Improving industrial yeast strains: exploiting natural and artificial diversity. FEMS Microbiol Rev. 2014; 38: 947–995. 10.1111/1574-6976.12073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abreu-Cavalheiro A, Monteiro G. Solving ethanol production problems with genetically modified yeast strains. Braz J Microbiol. 2013; 44: 665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gomez-Pastor R, Perez-Torrado R, Cabiscol E, Ros J, Matallana E. Engineered Trx2p industrial yeast strain protects glycolysis and fermentation proteins from oxidative carbonylation during biomass propagation. Microb Cell Fact. 2012; 11: 4 10.1186/1475-2859-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du X, Takagi H. N-Acetyltransferase Mpr1 confers ethanol tolerance on Saccharomyces cerevisiae by reducing reactive oxygen species. Appl Microbiol Biotechnol. 2007; 75: 1343–1351. [DOI] [PubMed] [Google Scholar]
- 68.Larsson S, Nilvebrant NO, Jonsson LJ. Effect of overexpression of Saccharomyces cerevisiae Pad1p on the resistance to phenylacrylic acids and lignocellulose hydrolysates under aerobic and oxygen-limited conditions. Appl Microbiol Biotechnol. 2001; 57: 167–174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are available from Dryad (doi:10.5061/dryad.9bv73).