Abstract
Sex differences in gene expression have been widely studied in Drosophila melanogaster. Sex differences vary across strains, but many molecular studies focus on only a single strain, or on genes that show sexually dimorphic expression in many strains. How extensive variability is and whether this variability occurs among genes regulated by sex determination hierarchy terminal transcription factors is unknown. To address these questions, we examine differences in sexually dimorphic gene expression between two strains in Drosophila adult head tissues. We also examine gene expression in doublesex (dsx) mutant strains to determine which sex-differentially expressed genes are regulated by DSX, and the mode by which DSX regulates expression. We find substantial variation in sex-differential expression. The sets of genes with sexually dimorphic expression in each strain show little overlap. The prevalence of different DSX regulatory modes also varies between the two strains. Neither the patterns of DSX DNA occupancy, nor mode of DSX regulation explain why some genes show consistent sex-differential expression across strains. We find that the genes identified as regulated by DSX in this study are enriched with known sites of DSX DNA occupancy. Finally, we find that sex-differentially expressed genes and genes regulated by DSX are highly enriched on the fourth chromosome. These results provide insights into a more complete pool of potential DSX targets, as well as revealing the molecular flexibility of DSX regulation.
Keywords: sex determination, Drosophila, sex hierarchy, doublesex, transcriptome, gene expression, sex bias, RNA-seq, Genetics of Sex
One major remaining question in biology is, ‘How does a shared genome give rise to two vastly different sexes?’ The Drosophila somatic sex determination hierarchy is responsible for directing sexual dimorphism in morphology, physiology, and adult behaviors (reviewed in Christiansen et al. 2002; Dauwalder 2011). Most sex-differential gene expression is expected to result from differences in how the terminal sex-specific transcription factors in this pathway regulate gene expression. How pervasive this regulation is, and how robust it is to genetic background and environmental differences, are open questions, especially given that both sex-differential expression and maleness/femaleness are quantitative traits.
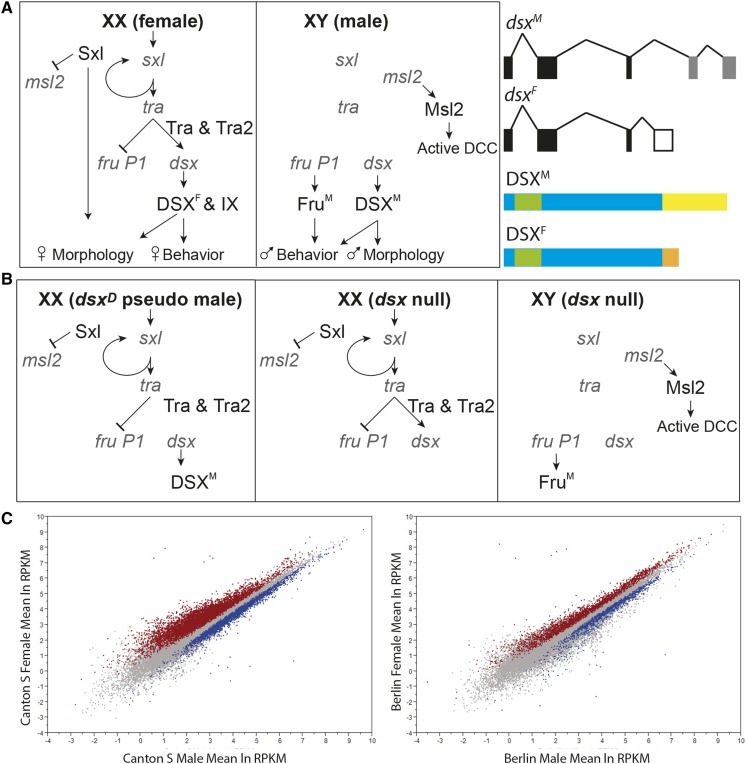
In Drosophila, sex differences in gene expression have been widely studied in different tissues, and at different developmental time points (reviewed in Samson and Rabinow 2014). The sex determination hierarchy specifies sex differences in somatic tissues. The hierarchy consists of an alternative pre-mRNA splicing cascade, responsive to the number of X chromosomes, which directs the production of sex-specific transcription factors encoded by doublesex (dsx) and fruitless (fru) (Figure 1) (reviewed in Salz 2011). dsx establishes nearly all known morphological sex differences, and also has a role in the nervous system, whereas fru has a primary role in directing reproductive potential in the nervous system (reviewed in Manoli et al. 2006; Yamamoto and Koganezawa 2013).
Figure 1.
Drosophila sex determination hierarchy, effects of mutant alleles, and sex differences in expression in wild-type animals. (A) The Drosophila somatic sex determination hierarchy. The primary determinant of sex is the number of X chromosomes. The sex hierarchy includes sex-differentially produced splicing factors encoded by sex-lethal (sxl), transformer (tra), and splicing factor transformer-2 (tra-2). Alternative splicing of dsx and fru P1 pre-mRNAs leads to sex-specific production of DSX and Fru transcription factors. In females, dosage compensation (DCC) is not active due to the production of Sxl, which inhibits translation of msl2. In females, DSXF together with IX regulate gene expression to direct female-specific behavior, morphology, and physiology. In males, DSXM and FruM regulate gene expression to direct male-specific behavior, morphology, and physiology. Splicing differences of dsx pre-mRNAs, and schematics of the DSX protein isoforms are shown. The common DNA binding domain is indicated with a green box. The sex differences at the carboxyl-termini are shown in yellow and orange. (B) dsxD pseudo males are chromosomally XX, but produce only the male specific DSX isoform. Above dsx, the sex hierarchy is genetically the same as in wild-type females. dsx null animals do not have DSX produced, but above dsx, the hierarchy is genetically the same as the female and male wild-type animals. (C) Scatter plots of ln(RPKM) values for CS and Ber, with red (female) and blue (male) indicating statistically significant expression differences between the sexes.
In this study, we focus on the mechanisms used by dsx to generate sexual dimorphism in gene expression, and address the relationship between dsx regulation and consistent sex-differential gene expression across strains. We examine the global transcriptional profile of adult head somatic tissues in two wild-type strains, and in two dsx mutant strains. The dsx/mab-3 (Dmrt) family of genes regulates sexual development across different taxa, and this role of the gene family appears to be an ancestral function (reviewed in Kopp 2012). Thus, this work could shed light on how quantitative differences between and among the sexes arise in diverse taxa.
In Drosophila, alternative sex-specific splicing of dsx pre-mRNAs generates male and female DSX isoforms (DSXM and DSXF), which share a common amino-terminal DNA binding domain and bind the same DNA sequence motif, but differ in their carboxyl-terminal region (Figure 1) (Burtis and Baker 1989; Erdman and Burtis 1993). This difference allows DSXF to interact with the product of intersex (ix), a homolog of a Mediator complex protein, which influences sex differences in DSX transcriptional activity (Garrett-Engele et al. 2002). Consistent with this, female ix mutants have the same intersexual phenotype as dsx null mutants, but male ix mutants do not (Baker and Ridge 1980).
Several genomic studies in Drosophila, as well as studies in other animals (reviewed in Ellegren and Parsch 2007), have suggested that sex differences in gene expression are pervasive. In fact, approximately 30–50% of the genome may show sexual dimorphism in transcript abundance (Jin et al. 2001; Arbeitman et al. 2002; Ranz et al. 2003; Gibson et al. 2004; Zhang et al. 2007; Graveley et al. 2011; Graze et al. 2014). However, in Drosophila, most of these differences are due to the presence of germline tissues, the ovary and testis (Arbeitman et al. 2002, 2004; Parisi et al. 2004; Goldman and Arbeitman 2007; Lebo et al. 2009; Catalan et al. 2012; Perry et al. 2014). Studies of sex differences in several somatic tissues have shown that far fewer genes show dimorphism in transcript abundance in somatic tissues, and that, for many of these genes, strain background has a large effect on sex-differential expression (Arbeitman et al. 2004; Tarone et al. 2005; Goldman and Arbeitman 2007; Lebo et al. 2009; Chatterjee et al. 2011; Catalan et al. 2012; Fear et al. 2015; Huylmans and Parsch 2015).
Given the importance of sex differences in the brain and other head tissues, the a priori expectation of many researchers has been that a sizeable proportion of genes expressed in the brain or head are regulated by dsx and fru, and that these genes should show large and consistent differences in expression between males and females. Several studies have identified sex-differentially expressed genes in these tissues, but these genes have varied across different studies (Goldman and Arbeitman 2007; Chang et al. 2011; Catalan et al. 2012). Disagreement across studies could result from differences in the strain used, tissues analyzed or experimental design. Assays of sex-differential expression in different strains, for the same tissue and using the same experimental design, have indicated that strain is an important factor. For example, in our previous genomic studies we identified genes with sex differences in expression that also showed sex-differential expression downstream of the sex hierarchy genes transformer and dsx (Figure 1 for hierarchy; and see Arbeitman et al. 2004; Goldman and Arbeitman 2007; Lebo et al. 2009; Chang et al. 2011). These latter studies required the sex differences to be present in different strain backgrounds. In all cases, this requirement substantially narrowed the starting list of genes identified with sex differences. Thus, these early studies of somatic gene expression differences identified genes with the most consistent sex hierarchy-dependent and strain-independent sex-differential expression. Additionally, previous direct examinations of variation across strains in sex-biased gene expression showed that strain background can have a significant effect (Tarone et al. 2005; Catalan et al. 2012; Huylmans and Parsch 2014).
Another contributing factor to differences observed across strains could be that DSX regulation appears to be flexible with respect to the regulatory mode by which sex-differential expression is produced. The regulatory mode could be associated with expression variation, if some modes produce more consistent sex-differential expression. Efforts to understand how DSX contributes to the generation of two different sexes have relied on early molecular-genetic studies of the Yolk Protein (Yp) genes, Yp1 and Yp2 (Baker and Ridge 1980; Bownes et al. 1983; Belote et al. 1985; Burtis et al. 1991). The Yps have highly female-biased expression in fat body tissue. It was shown that, in females, DSXF activates Yp expression, whereas, in males, DSXM represses Yp expression. These results suggest that sex differences are specified by sex-specific DSX isoforms having opposing roles. In one sex, sex-specific DSX activates expression of a set of genes, and, in the other sex, sex-specific DSX represses expression of these genes. Here, we call this mode of DSX regulation the opposing mode.
Subsequent genomic studies and gene-level validation studies showed that the opposing mode of DSX regulation is not the only mode (Arbeitman et al. 2004; Goldman and Arbeitman 2007; Lebo et al. 2009; Chatterjee et al. 2011; Luo and Baker 2015). Rather, additional modes were discovered that are more frequent (Arbeitman et al. 2004; Goldman and Arbeitman 2007; Lebo et al. 2009). The modes of DSX gene regulation, for a given gene, are: 1–4) DSX is an activator or repressor, but only in one sex; 5–6) DSX is either consistently an activator or a repressor in both sexes, but the extent of activation or repression is sex-specific; and 7) the opposing mode, for which Yps are the primary example. All modes were shown to be active in somatic tissues of the adult and in pupae (Arbeitman et al. 2004; Goldman and Arbeitman 2007; Lebo et al. 2009).
While the opposing mode of DSX gene regulation of Yps is highly consistent across strains, it is not clear if it is the DSX mode of regulation that leads to more consistent sex differences in expression across strain backgrounds. Furthermore, it is not clear if DSX DNA binding at a locus correlates with different DSX modes of gene regulation or influences consistent sex-differential gene expression across strains. The chromosomal locations of DSX targets are also underexplored; chromosomal bias has been observed for sex-differential expression and for regulatory targets of other sex regulatory hierarchy genes, such as fru (Parisi et al. 2003; Goldman and Arbeitman 2007; Chang et al. 2011; Catalan et al. 2012; Meisel et al. 2012; Dalton et al. 2013; Graze et al. 2014).
To address these questions we examined sex differences in gene expression in the head between males and females in two different wild-type laboratory strains: Canton-S (CS) and Berlin (Ber). To examine the modes of DSX regulation, we further examined sex differences in gene expression in chromosomally male and female dsx null mutants that have an intersexual phenotype, and in dsxD/dsxnull chromosomally XX pseudo males—an allele combination in which only the DSXM isoform is produced. We determined if the genes identified as regulated by DSX are enriched with those shown to be bound by DSX, using results from previous studies (Luo et al. 2011; Clough et al. 2014). Additionally, we determined if there is enrichment of DSX regulated genes on particular chromosome arms. Our results suggest that there is a large potential pool of DSX-regulated genes, with sex-differential expression dependent on strain, environment, and/or strain by environment interactions. Further, our results demonstrate that the fourth chromosome is enriched with DSX regulated genes.
Materials and Methods
Fly husbandry, tissue collection, and library preparation
Flies were raised on standard cornmeal food medium (33 l H2O, 237 g agar, 825 g dried deactivated yeast, 1560 g cornmeal, 3300 g dextrose, 52.5 g Tegosept in 270 ml 95% ethanol and 60 ml propionic acid). The incubator conditions are 25° on a 12-hr light:12-hr dark cycle.
Wild-type flies are the Canton-S and Berlin laboratory strains (obtained from U. Herberlein). Male (XY) and female (XX) dsx null flies are dsxd+r3/dsxm+r15 (Duncan and Kaufman 1975; Baker et al. 1991). The dsxD pseudo males (XX) are w,P{w+mc, Ubi-GFP}/+;dsxD, Sb1, e1/dsxm+r15 (for dsxD see Fung and Gowen 1957). The data for all experiments were generated at the same time, under the same environmental conditions. All flies were collected 0–16 hr post-eclosion under CO2 anesthetization, and allowed to recover for 8 hr before being snap-frozen in liquid nitrogen. All flies were stored at –80° until heads were collected. Adult heads were separated from the body by mechanical tapping in the cyrovial, and then separated while frozen on a piece of Plexiglass cooled on dry ice. Heads were immediately transferred to Trizol reagent. Libraries were prepared from three independent biological replicates for each condition. For each experimental condition, approximately 200 heads were used per library.
Data processing and analysis
Illumina library preparation and read mapping to the D. melanogaster Release 5 genome, using FB5.30 annotation, was previously described (Dalton et al. 2013; Fear et al. 2015). To account for sex differences in transcript isoform expression level, expression was measured and analyzed at the exonic level (Supplemental Material, Figure S1). For each gene model, exons were classified as single or overlapping across isoforms (mapping following Graze et al. 2012). Overlap occurs among annotated exons of a gene when isoforms differ in start and end positions of exons corresponding to the same genomic region. Expression was measured for each single exon, and for each region of exonic overlap, considering the entire region of overlap. For simplicity, both cases are referred to as exons throughout.
Different gene models can also overlap, resulting in ambiguity with respect to gene expression. These regions are not considered in our analysis. Genes, and corresponding exons, that were not detected in all samples were also excluded from analysis. For an exon to be considered detected, it needed to have average per nucleotide coverage greater than zero in all samples. Overall, of 14,092 annotated genes, and 57,962 corresponding exons, 9673 genes and 42,602 exons showed detectable gene expression in head tissues for all samples. To allow comparisons, we considered only those genes with DSX DNA occupancy data (Luo et al. 2011; Clough et al. 2014). We analyzed 9476 genes (41,720 exons) in the final analysis.
Expression was normalized as the natural log of the number of reads per kilobase per million mapped reads (RPKM) per exon, and a linear model was fit (for RPKM, see Mortazavi et al. 2008). Using this model, contrasts were performed to detect differential expression between wild-type females and males, between wild-type females and dsxD pseudo males, and between each sex and the corresponding dsx null genotype. The adjusted FDR P-value was calculated considering all tests together and significance was considered at levels FDR < 0.05, < 0.10 and < 0.20 (Benjamini and Hochberg 1995). The FDR < 0.05 level was used in all cases, with the exception of Table 2. Analytical results are provided in Table S1.
Table 2. DSX targets with consistent DSX regulation across strains.
| Gene | Chr. | Sex Bias | Regulatory Mode | DSX Occupancya | DSX Occupancyb |
|---|---|---|---|---|---|
| CG8539 | 3L | Female | Activator | 0 | 0 |
| Cpr72Ea | 3L | Female | Female specific activator | 0 | 0 |
| Mp20 | 2R | Female | Female specific activator | 0 | 1 |
| CG31522 | 3R | Female | Female specific activator | 1 | 0 |
| trpl | 2R | Female | Male specific repressor | 0 | 0 |
| CG3759 | 2L | Female | Male specific repressor | 1 | 0 |
| Yp2 | X | Female | Male specific repressor | 1 | 1 |
| Fmo-2 | 2R | Female | Male specific repressor | 1 | 1 |
| Yp3 | X | Female | Opposing effects | 0 | 1 |
| Yp1 | X | Female | Opposing effects | 1 | 1 |
| rost | 2L | Female | Opposing effects/ | 1 | 1 |
| Male specific repressor | |||||
| CG15012 | 3L | Male | Female specific repressor | 0 | 0 |
| mav | 4 | Male | Female specific repressor | 0 | 0 |
| CG12158 | 2R | Male | Female specific repressor | 0 | 0 |
| Cyp4d21 | 2L | Male | Female specific repressor | 0 | 0 |
| CG31145 | 3R | Male | Female specific repressor | 1 | 0 |
| CG18547 | 3R | Male | Female specific repressor | 1 | 0 |
| Cyp313a1 | 3R | Male | Opposing effects | 0 | 0 |
Genes with patterns of sex-differential expression in wild-type, dsxD pseudo male, and dsx null comparisons, and the same regulatory mode in both Canton S and Berlin strains (FDR < 0.10). Sex bias, the direction of sex-differential expression: Female (Female-biased) or Male (Male-biased); Regulatory mode, the seven types of DSX regulation; DSX occupancy.
DSXM and DSXF ChIP-seq in S2 cells and DSXM and DSXF Dam-ID in female ovaries, and male and female fat body (Clough et al. 2014; Table S1).
DSXF Dam-ID in adult females (Luo et al. 2011; Table S1). Bold indicates validated DSX targets.
Gene set enrichment analysis was conducted, using Fisher’s exact test, to determine if specific biological process, molecular function, or cellular component ontology terms are overrepresented among the set of genes corresponding to each DSX regulatory mode. For each of the biological process, molecular function, or cellular component ontologies, tests were performed with FDR correction for both strains and all modes considered together. Only genes with gene ontology (GO) annotation were considered in the enrichment analysis (Mootha et al. 2003; Rivals et al. 2007).
Data availability
These data have been deposited in GEO. The accession number for wild-type strains is GSE50515. The accession number for the dsx mutant strains is GSE67400 (Dalton et al. 2013; Fear et al. 2015).
Results
Here, we sought to understand the mechanism(s) that contribute to variability of sexually dimorphic gene expression across strains in the adult head (for example see Goldman and Arbeitman 2007). Sex differences in gene expression were analyzed at the exon level, to consider differences in transcript isoform abundance. When an exon for a gene is sex-differentially expressed, we also report differences at the gene level (Figure S1; and see Graze et al. 2012). Therefore, a gene with multiple transcript isoforms can be regulated by more than one DSX regulatory mode, depending on the exon/transcript isoform that is examined. Further, sex differences in particular transcript isoforms could be due to alternative pre-mRNA splicing, or differences in promoter deployment, which is not determined here (for example see Chang et al. 2011; Graveley et al. 2011). We examine sex-biased expression in two wild-type strains and dsx mutant strains, to identify the genes regulated by DSX, and to determine the mode of DSX regulation.
Sex-differential expression of DSX-regulated genes varies across strains
We examined two strains from genetically distinct populations: the laboratory strains Canton-S (CS; from Canton, OH) and Berlin (Ber; from Berlin, Germany). Here, we find that CS and Ber have substantial differences in the total number of genes with sex-differential expression (Figure 1, Table 1, Table S2, and Table S3). We also find that the majority of sex differences in expression are strain-specific, with few genes showing consistently biased expression in the two strains, in agreement with previous reports (Arbeitman et al. 2004; Tarone et al. 2005; Goldman and Arbeitman 2007; Chang et al. 2011; Huylmans and Parsch 2015).
Table 1. Sex-differential expression in Canton S and Berlin strains.
| Exons | Genes | |||
|---|---|---|---|---|
| WT Female to Male Comparison | WT Female to Male Comparison | |||
| Male-biased | Female-Biased | Male-Biased | Female-Biased | |
| Canton S | 2268 | 5686 | 1743 | 1672 |
| Berlin | 1070 | 2365 | 783 | 1611 |
| Both | 231 | 376 | 205 | 246 |
| WT Female to dsxD Comparisona | WT Female to dsxD Comparisona | |||
| Male-Biased | Female-Biased | Male-Biased | Female-Biased | |
| Canton S | 340 | 901 | 303 | 445 |
| Berlin | 78 | 207 | 69 | 168 |
| Both | 12 | 29 | 5 | 20 |
| WT to dsx Null Comparisonsb | WT to dsx Null Comparisonsb | |||
| Male-Biased | Female-Biased | Male-Biased | Female-Biased | |
| Canton S | 275 | 706 | 239 | 358 |
| Berlin | 52 | 120 | 44 | 105 |
| Both | 11 | 18 | 4 | 13 |
Each contrast compared the natural log of the RPKM normalized expression in wild-type females and males, wild-type females and dsxD pseudo males, and in wild-type females and dsx null females or wild-type males and dsx null males. A cut-off of FDR < 0.05 was used.
For each exonic region, the wild-type comparison was considered first, and if sex-biased in the wild-type comparison, the exon was considered sex-differentially expressed and regulated by dsx if there was also significant sex-differential expression that was biased in the same direction in the wild-type female to dsxD comparison.
If the exon was identified as a putative dsx target as in (a), then it was tested for a significant difference in either or both dsx null comparisons. Both exon level and corresponding gene level counts are reported.
To further examine the impact of dsx and strain background on sex-differential expression in each strain, we next identified exons that show sex-differential expression between males and females that are also regulated by dsx (Table 1), using data from dsxD pseudo males and dsx null genotypes. Here, the number of genes that meet the criteria for showing DSX regulation in all strains is substantially less than when we only considered sex-differential expression in each wild-type strain (Table 1), which could be due to strain background differences, or reflect lack of regulation by DSX.
The seven modes of DSX regulation and differences between strains
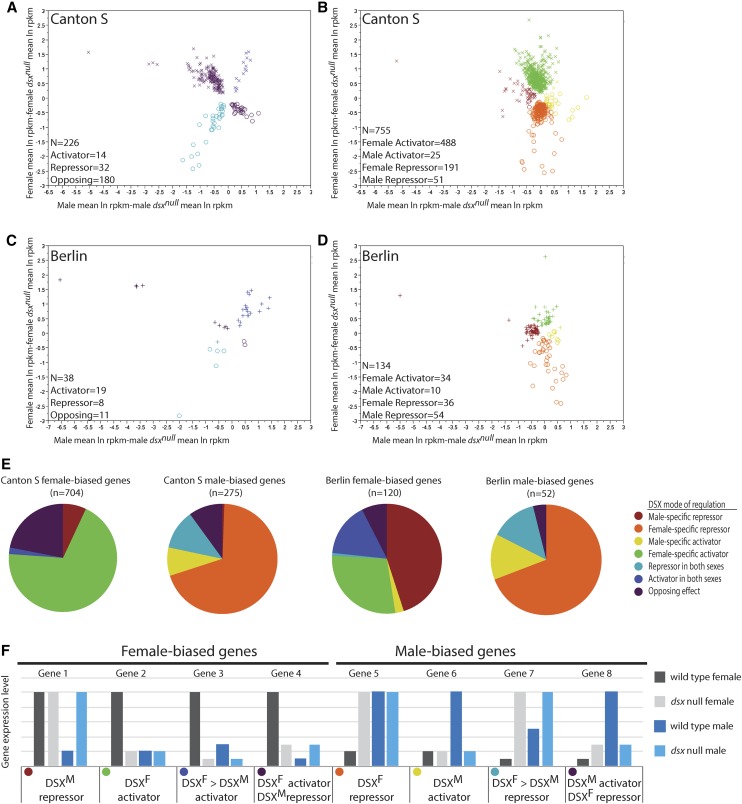
Using the data from the dsx null comparisons, we are able to determine which DSX regulatory mode is responsible for sex-differential expression (Figure 2F). The plots in Figure 2, A–D show that, for most genes, the magnitude of expression differences between the wild-type and dsx null genotypes is similar (clustered symbols), with a few genes having very large effects of the dsx null genotype (symbols on the periphery; these include the Yp genes).
Figure 2.
DSX modes of regulation and expression differences. The estimated expression differences between wild-type and dsx null animals are plotted for female comparisons by male comparisons [CS: (A) and (B); Ber: (C) and (D)]. In A–D male-biased genes (x) and female-biased genes (o) are indicated. (E) Pie charts showing the proportion of genes regulated by each DSX mode for female- and male-biased genes in CS and Ber. The legend on the right shows the colors used to indicate each DSX regulatory mode in this figure. (F) Hypothetical data demonstrating how DSX regulatory modes were determined (following Arbeitman et al. 2004; Goldman and Arbeitman 2007; Lebo et al. 2009). Expression in wild-type females or males of each strain was compared to expression in dsx null females or males, and both the significance of each test, and the direction of the mean difference in expression were considered. Thus, DSX can act as an activator or repressor in each sex, or both, and this defines the mode. If DSXF activates expression in females, the expectation is that gene expression will be significantly lower in the absence of activation in dsx null females. Similarly, if DSXF represses expression in females, gene expression is expected to be higher in the absence of DSXF repression in dsx null females. Activation and repression were similarly examined in males. We note that the mode classification is sensitive to our ability to statistically detect expression differences.
If we rank the DSX regulatory modes by number of genes that display regulation corresponding to each mode, we find that for male-biased genes the rank order between the two strains does not differ substantially. The most prevalent DSX regulatory mode for male-biased genes is female-specific repression (Figure 2E). An examination of the female-biased genes shows that the most prevalent regulatory mode in CS is female-specific activation by DSX, but in Ber it is male-specific repression by DSX. We previously found similar results on these DSX regulatory modes (Arbeitman et al. 2004; Goldman and Arbeitman 2007; Lebo et al. 2009). This suggests that there is regulatory flexibility in the generation of sex-differential gene expression, especially for genes with female-biased expression. While the opposing mode is the most well studied mode (darkest purple in Figure 2), it is not the most frequently observed mode for male- or female-biased genes.
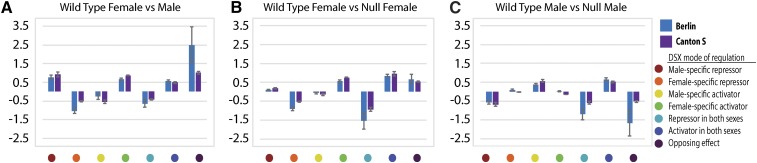
We next determined if a particular regulatory mode gives rise to consistently larger estimates for sex-differential expression when we compare gene expression in either CS or Ber wild-type animals (Figure 3A). A positive estimate indicates female-biased expression, and a negative estimate male-biased expression. We also examined the size of expression differences in the dsx null comparisons (Figure 3, B and C), with a positive estimate in the dsx null comparisons indicating that DSX is an inducer and negative estimate indicating that DSX is a repressor. We do not observe large changes in the magnitude of expression differences, with none of the regulatory modes consistently generating larger sex differences in the two strains examined (Figure 3 and Table S4). This further suggests that there is flexibility in the relationship between DSX mode and the extent of sex-differential expression.
Figure 3.
The effect of different DSX modes on regulation of expression. The estimate of differential expression for (A) wild-type females and males, (B) wild-type females and dsx null females, and (C) wild-type males and dsx null male comparisons, for each different DSX mode. The mean effect estimate, with standard error of the mean is shown for Ber (blue) and CS (purple) comparisons. The legend on the right shows the colors used to indicate each DSX regulatory mode.
DSX regulated genes and DSX DNA occupancy
We identified the set of genes that have the most consistent sex-differential expression, defined as those that are sex-differentially expressed in all strains used in this study and their DSX regulatory mode (Table 2, Table S5, and Table S6 for different FDR cut-off values). Of the most extensively validated DSX targets (Yp genes, bab1 and bab2, and Fmo-2), both the Yps and Fmo-2 are identified as showing sex-differential expression that is consistent across strains (Table 2).
To understand the relationship between expression patterns and DSX DNA binding, we determined if the genes that have sex-differential expression are enriched for genes that are also bound by DSX, as identified in previous studies (Luo et al. 2011; Clough et al. 2014). We find that genes with validated occupancy of DSX, from both previous studies, significantly overlap with the gene lists identified as DSX-regulated genes in either CS or Ber (Table 3). Further, genes regulated by DSX are equally likely to be bound by DSX, irrespective of the mode of regulation (data not shown).
Table 3. DSX regulated genes and binding site occupancy.
| Strain | Test (Fisher’s Exact) | P-Value | Obs (Exp) | Fold Enrichment |
|---|---|---|---|---|
| Canton S | Luo et al. 2011 Occupancy | <0.0001 | 62 (30) | 2.07 |
| Canton S | Clough et al. 2014 Occupancy | <0.0001 | 355 (198) | 1.79 |
| Berlin | Luo et al. 2011 Occupancy | <0.0001 | 21 (8) | 2.63 |
| Berlin | Clough et al. 2014 Occupancy | 0.0008 | 69 (49) | 1.41 |
Fisher’s exact test was performed to detect enrichment or depletion of genes with previously observed DSXM/DSXF occupancy in ovaries, fat body and in S2 cells (Clough et al. 2014), or in adult female flies (DSXF; Luo et al. 2011) among genes identified as regulated by dsx in 8- to 24-hr-old male or female heads (this study). While this study examines dsx regulation in males and females, the fold enrichment of genes with DSX occupancy in adult female flies (Luo et al. 2011) is greater than the fold enrichment of genes identified in the Clough et al. (2014) study.
We also determined if sex-differentially expressed genes are more likely to reside on different chromosome arms. Previous studies have shown that genes with male-biased expression in adult head tissues are enriched on the X chromosome (Goldman and Arbeitman 2007; Chang et al. 2011; Catalan et al. 2012), whereas other studies did not find a similar enrichment (discussed in Huylmans and Parsch 2015). Further analyses reconciled this conflicting observation, noting that the enrichment on the X of genes with male-biased expression in the head was likely due to expression in the central nervous system, and might be at the limit of detection in whole head studies (Huylmans and Parsch 2015). Here, we find a significant enrichment of sex-biased genes identified in CS on the X chromosome. We also find that the fourth chromosome is both significantly enriched for genes with sex-differential expression (Table 4 and Table S7) and significantly enriched for genes with validated occupancy by DSX (Table 5).
Table 4. Sex-differential expression and chromosome bias.
| Chr. | Strain | Sex-Differentially Expressed | DSX-Regulated | ||||
|---|---|---|---|---|---|---|---|
| P-Value | Obs (Exp) | Fold | P-Value | Obs (Exp) | Fold | ||
| X | Canton S | 0.02 | 587 (547) | 1.07 | N.S. | 144 (126) | 1.14 |
| 2L | Canton S | N.S. | 587 (569) | 1.03 | N.S. | 129 (131) | 0.98 |
| 2R | Canton S | 0.03 | 625 (665) | 0.94 | N.S. | 138 (153) | 0.90 |
| 3L | Canton S | N.S. | 599 (632) | 0.95 | N.S. | 143 (146) | 0.98 |
| 3R | Canton S | N.S. | 774 (790) | 0.97 | N.S. | 170 (182) | 0.93 |
| 4 | Canton S | < 0.0001 | 63 (27) | 2.33 | < 0.0001 | 21 (6) | 3.50 |
| X | Berlin | 0.01 | 436 (397) | 1.10 | N.S. | 39 (40) | 0.98 |
| 2L | Berlin | N.S. | 423 (413) | 1.02 | N.S. | 46 (42) | 1.09 |
| 2R | Berlin | N.S. | 511 (483) | 1.06 | 0.01 | 65 (49) | 1.33 |
| 3L | Berlin | 0.0003 | 398 (458) | 0.87 | N.S. | 38 (46) | 0.83 |
| 3R | Berlin | 0.02 | 531 (573) | 0.93 | N.S. | 46 (58) | 0.79 |
| 4 | Berlin | < 0.0001 | 46 (19) | 2.42 | N.S. | 2 (2) | 1.00 |
Fisher’s exact test was performed to detect enrichment or depletion of genes identified as sex-differentially expressed or genes identified as regulated by dsx, here, on each chromosome arm. The observed (Obs) and expected (Exp) number of genes are reported for each major chromosome arm, as well as the fold enrichment (Fold).
Table 5. Chromosome bias in DSX occupancy.
| Occupancy | Chr. | Obs (Exp) | Fold | P-Value |
|---|---|---|---|---|
| Clough et al. 2014 | X | 553 (530) | 1.04 | N.S. |
| 2L | 579 (551) | 1.05 | N.S. | |
| 2R | 619 (644) | 0.96 | N.S. | |
| 3L | 617 (612) | 1.01 | N.S. | |
| 3R | 727 (766) | 0.95 | N.S. | |
| 4 | 43 (26) | 1.65 | < 0.0001 | |
| Luo et al. 2011 | X | 76 (81) | 0.94 | N.S. |
| 2L | 84 (84) | 1.00 | N.S. | |
| 2R | 95 (98) | 0.97 | N.S. | |
| 3L | 113 (93) | 1.22 | 0.024 | |
| 3R | 107 (117) | 0.91 | N.S. | |
| 4 | 4 (4) | 1.00 | N.S. |
Fisher’s exact test was performed to detect enrichment or depletion of genes showing evidence of DSX occupancy in Clough et al. 2014, or in Luo et al. 2011, on each chromosome arm. The observed (Obs) and expected (Exp) number of genes are reported for each major chromosome arm, as well as the fold enrichment (Fold).
Discussion
The results of this study further confirm that sex differences in gene expression can show substantial differences across strains. While we found different sets of genes with sex-differential expression in each strain, the gene sets from both strains were highly significantly enriched for DSX DNA occupancy, suggesting that they are bona fide targets. There are many genes that have been implicated in being regulated by DSX using independent methodologies, including gene expression studies and direct observation of DSX DNA occupancy at specific loci. If one considers all of these studies and the observed differences across the studies, it suggests that there is a pool of DSX-regulated genes with the potential for sex-differential expression, but whether they will show sex-differential expression depends on strain, environment, and strain by environment interactions. This is the case even when the same tissue and developmental time points are examined. Perhaps having this pool of potential targets is one mechanism contributing to natural variation in gene expression, resulting in quantitative differences in maleness/femaleness among individuals in a population.
Given the seven different modes of DSX regulation, we wanted to determine if a particular DSX regulatory mode is responsible for the strain differences we observed. We hypothesized that some modes would show less consistency in generating sex-differential expression across the strains. The male-biased genes identified in CS and Ber had a similar rank order with respect to the number of genes regulated by a DSX mode, whereas female-biased genes did not. The results suggest that the regulatory modes to generate female-biased expression might be more flexible at a molecular level, but we found that all DSX modes of regulation are similarly sensitive to strain background. Further, we did not see clearly defined functional differences in the genes downstream of each DSX regulatory mode, on the basis of gene set enrichment analyses of ontologies (Table S8).
The difference in sex-differential expression between the two wild-type strains considered was striking. Why does CS display more significant sexual dimorphism of gene expression, as compared to Ber? It is possible that this is a consequence of their original genetic background or it could be due to differences in the conditions in which they have been reared, resulting in different selective pressures. It appears that both of these strains were initially collected in the wild and reared as laboratory strains beginning in the 1930s (Argelander and Strasburger 1939; Lindsley 1983; deBelle and Heisenberg 1996), though there is no clear documented lineage for the Ber wild-type strains in current use. It will be interesting to determine if variation in sex-differential expression is related to geographical region, the length of time strains have been reared in the laboratory, or differences in rearing conditions over time.
What are the molecular mechanisms driving strain differences? It is clear that sex-differential expression in the head is both dynamic across strains and genetically specified, with the molecular mechanisms that generate this type of gene regulation under active investigation. For example, studies of DSX cis-regulation of bab, Fad2 and fmo-1 show polymorphism in cis-regulatory elements can confer novel patterns of dsx-directed sexually dimorphic expression, while retaining ancestral monomorphic expression patterns that are essential in development (Kopp et al. 2000; Williams et al. 2008; Shirangi et al. 2009; Rogers et al. 2013; Luo and Baker 2015). It is unclear how similar cis polymorphism in DSX binding sites and associated DNA regions mediate natural variation in expression at the population level, but cis variation may contribute to the strain differences observed.
It is also possible that strain differences in sex-differential expression can be accounted for by strain differences in trans factors. This includes the abundance and/or splicing of sex hierarchy and cofactor mRNAs, such as those encoded by sxl, tra, tra-2, ix, hermaphrodite and/or dosage compensation genes, as was previously examined (Tarone et al. 2005; Fear et al. 2015; Li et al. 2015). An examination of expression levels for these genes in CS and Ber data did not reveal apparent differences in expression level that could account for the differences observed. Differences in biochemical activity, independent of expression levels, could also contribute to variation, including nonlinear responses due to differences in transcription factor binding affinity, or number of binding events, splicing efficiency, and co-factor protein interactions, as previously proposed (Tarone et al. 2005, 2012). In the case of dsx, differences in splicing efficiency could result in some cells expressing both the male- and female-specific DSX isoforms, which would influence DSX biochemical and regulatory activity, as DSX functions as a dimer (Cho and Wensink 1997). Previous loss-of-function and gain-of-function studies on DSX have suggested that the differences between the sexes for many phenotypes are a result of DSX functioning more like a dial than a switch (Jursnich and Burtis 1993). Our results are consistent with that idea, and go further to show that quantitative differences in regulation of DSX targets across strains are a likely mechanism contributing to natural variation in sexual dimorphism.
We note that DSX DNA binding does not appear to be sufficient to explain all DSX-regulated differences, as one of the studies examining DSX DNA occupancy, showed that DSX can be bound to a locus, independent of whether the gene is sex-differentially expressed (Clough et al. 2014). This suggests that more complex cis, trans, and cis by trans regulatory effects need to be considered for a more complete understanding of variation across strains. Future analyses will also benefit from a more thorough understanding of the nucleotide variation across strains, especially in DSX binding sites and their surrounding DNA, DSX transcriptional co-factors and deeper knowledge of the mechanisms of gene regulatory logic.
Our results further the idea that large-scale differences in interactions across branches of the sex hierarchy contribute to sex-differential expression. In previous work, we have suggested that sex-specific transcription factors may take advantage of the unique male and female nuclear environments, with dosage compensation or sex chromosome differences contributing to differences between males and females through interactions with sex hierarchy transcription factors (Goldman and Arbeitman 2007; Chang et al. 2011; Dalton et al. 2013). This idea can be extended in light of our observation that the fourth chromosome has a significant enrichment of genes identified as sex-differentially expressed in this study, and the fourth chromosome is also significantly enriched with genes with known DSX occupancy.
Interestingly, there is evidence that the Drosophila autosomal fourth chromosome evolved from an ancestral sex chromosome (Vicoso and Bachtrog 2013), and that regulation on the fourth and X chromosome share components of the dosage compensation machinery (Lundberg et al. 2013). Indeed, studies examining embryos and gonadal tissues, ovary and testis, showed an excess of female-biased expression and genes expressed in the ovary on the fourth chromosome in both D. melanogaster and in distantly related Brachyceran species, for which chromosome 4 (Muller’s F element) is a sex chromosome (Vicoso and Bachtrog 2013).
Regulation of sexually dimorphic expression by DSX and Fru, and their associated sex hierarchy splicing factors, predate the evolutionary turnover of sex chromosomes in Diptera (Davis et al. 2000; Kopp 2012). Furthermore, enrichment of sex-differential expression on sex chromosomes is thought to occur due to the unique molecular and evolutionary properties of sex chromosomes (reviewed in Vicoso and Charlesworth 2006; Ellegren and Parsch 2007). Thus, identifying targets of DSX and Fru and their chromosomal locations in a wider range of insect species may yield new insights into the molecular basis and evolution of sex-biased gene expression on sex chromosomes.
Sex differences in expression in the head were small in magnitude in many cases. Examining other tissues with larger effects will be important in the future. We also note that the dsx mutant alleles analyzed here are in different strain backgrounds than CS and Ber, and this could also influence our detection of the different DSX regulatory modes in CS and Ber. Furthermore, differences in fecundity are an important factor to consider, especially since the dsx mutants are sterile, and there are known tissue-interactions that influence gene expression in the adult head (Parisi et al. 2010). To fully understand variation in expression, future gene-level molecular analyses of tissue specific, cell-type specific or single-cell regulation, as well as developmental studies, will be important.
Wild animals experience different environmental conditions and are generally outbred. Thus, it is unclear a priori what will be a male- or female-biased gene in a wild population, because the sex-biased expression could vary both qualitatively and quantitatively. This type of regulatory variation may occur in other species and contribute to natural variation in sexual dimorphism. Indeed, our studies showed there is substantial variation in sexually dimorphic gene expression in the adult mouse hippocampus across strains (Vied et al. 2016). Our results also suggest that strain differences across studies may be contributing to the lack of reproducibility of some genomic studies, an important consideration as this issue is being widely evaluated in the scientific community (Editorial 2013). In the future, it will be interesting to perform similar studies at the population level, with an experimental design that allows one to ascertain cis, trans, and cis by trans regulatory effects, as well as population structure effects. In addition, understanding the molecular basis for variability of gene expression and flexibility in transcription factor regulatory mode, as we see for dsx regulated genes, is important in understanding how quantitative differences in femaleness/maleness arise.
Supplementary Material
Acknowledgments
We thank Lauren McIntyre and Sergey Nuzhdin for intellectual interactions that helped shape this study, and comments on the manuscript from our laboratories. We thank our reviewers for helpful suggestions that strengthened the manuscript. This work was supported by research start-up funds from Florida State university (FSU) College of Medicine (M.A. and J.D.), and National Institutes of Health (NIH) grants R01GM073039 (Principal Investigator is M.N.A.), and research start-up funds from the Department of Biological Sciences at Auburn University and from the Auburn University Office of Vice President for Research (R.M.G.). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors declare no competing interests.
Footnotes
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.027961/-/DC1
Communicating editor: H. K. Salz
Literature Cited
- Arbeitman M. N., Furlong E. E. M., Imam F., Johnson E., Null B. H., et al. , 2002. Gene expression during the life cycle of Drosophila melanogaster. Science 297: 2270–2275. [DOI] [PubMed] [Google Scholar]
- Arbeitman M. N., Fleming A. A., Siegal M. L., Null B. H., Baker B. S., 2004. A genomic analysis of Drosophila somatic sexual differentiation and its regulation. Development 131: 2007–2021. [DOI] [PubMed] [Google Scholar]
- Argelander, A., and E. H. Strasburger, 1939 A case of genetic disfunction of the ovaries. Drosophila Information Services 11: 43. [Google Scholar]
- Baker B. S., Ridge K. A., 1980. Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster. Genetics 94: 383–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. S., Hoff G., Kaufman T. C., Wolfner M. F., Hazelrigg T., 1991. The doublesex locus of Drosophila melanogaster and its flanking regions: a cytogenetic analysis. Genetics 127: 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belote J. M., Handler A. M., Wolfner M. F., Livak K. J., Baker B. S., 1985. Sex-specific regulation of yolk protein gene-expression in Drosophila. Cell 40: 339–348. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y., 1995. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57: 289–300. [Google Scholar]
- Bownes M., Dempster M., Blair M., 1983. Expression of the yolk-protein genes in the mutant doublesex dominant (Dsxd) of Drosophila melanogaster. J. Embryol. Exp. Morphol. 75: 241–257. [PubMed] [Google Scholar]
- Burtis K. C., Baker B. S., 1989. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell 56: 997–1010. [DOI] [PubMed] [Google Scholar]
- Burtis K. C., Coschigano K. T., Baker B. S., Wensink P. C., 1991. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 10: 2577–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan A., Hutter S., Parsch J., 2012. Population and sex differences in Drosophila melanogaster brain gene expression. BMC Genomics 13: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. L., Dunham J. P., Nuzhdin S. V., Arbeitman M. N., 2011. Somatic sex-specific transcriptome differences in Drosophila revealed by whole transcriptome sequencing. BMC Genomics 12: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S. S., Uppendahl L. D., Chowdhury M. A., Ip P. L., Siegal M. L., 2011. The female-specific doublesex isoform regulates pleiotropic transcription factors to pattern genital development in Drosophila. Development 138: 1099–1109. [DOI] [PubMed] [Google Scholar]
- Cho S., Wensink P. C., 1997. DNA binding by the male and female doublesex proteins of Drosophila melanogaster. J. Biol. Chem. 272: 3185–3189. [DOI] [PubMed] [Google Scholar]
- Christiansen A. E., Keisman E. L., Ahmad S. M., Baker B. S., 2002. Sex comes in from the cold: the integration of sex and pattern. Trends Genet. 18: 510–516. [DOI] [PubMed] [Google Scholar]
- Clough E., Jimenez E., Kim Y. A., Whitworth C., Neville M. C., et al. , 2014. Sex- and tissue-specific functions of Drosophila doublesex transcription factor target genes. Dev. Cell 31: 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton J. E., Fear J. M., Knott S., Baker B. S., McIntyre L. M., et al. , 2013. Male-specific Fruitless isoforms have different regulatory roles conferred by distinct zinc finger DNA binding domains. BMC Genomics 14: 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwalder B., 2011. The roles of fruitless and doublesex in the control of male courtship. Int. Rev. Neurobiol. 99: 87–105. [DOI] [PubMed] [Google Scholar]
- Davis T., Kurihara J., Yoshino E., Yamamoto D., 2000. Genomic organisation of the neural sex determination gene fruitless (fru) in the Hawaiian species Drosophila silvestris and the conservation of the fru BTB protein-protein-binding domain throughout evolution. Hereditas 132: 67–78. [DOI] [PubMed] [Google Scholar]
- deBelle J. S., Heisenberg M., 1996. Expression of Drosophila mushroom body mutations in alternative genetic backgrounds: a case study of the mushroom body miniature gene (mbm). Proc. Natl. Acad. Sci. USA 93: 9875–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan I., Kaufman T. C., 1975. Cytogenetic analysis of chromosome 3 in Drosophila melanogaster: mapping of the proximal portion of the right arm. Genetics 80: 733–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nature Editorial, 2013 Announcement: Reducing our irreproducibility. Nature. 496: 398. [Google Scholar]
- Ellegren H., Parsch J., 2007. The evolution of sex-biased genes and sex-biased gene expression. Nat. Rev. Genet. 8: 689–698. [DOI] [PubMed] [Google Scholar]
- Erdman S. E., Burtis K. C., 1993. The Drosophila doublesex proteins share a novel zinc finger related DNA-binding domain. EMBO J. 12: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fear J. M., Arbeitman M. N., Salomon M. P., Dalton J. E., Tower J., et al. , 2015. The wright stuff: reimagining path analysis reveals novel components of the sex determination hierarchy in Drosophila melanogaster. BMC Syst. Biol. 9: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung S. T. C., Gowen J. W., 1957. The developmental effect of a sex-limited gene in Drosophila melanogaster. J. Exp. Zool. 134: 515–532. [DOI] [PubMed] [Google Scholar]
- Garrett-Engele C. M., Siegal M. L., Manoli D. S., Williams B. C., Li H., et al. , 2002. intersex, a gene required for female sexual development in Drosophila, is expressed in both sexes and functions together with doublesex to regulate terminal differentiation. Development 129: 4661–4675. [DOI] [PubMed] [Google Scholar]
- Gibson G., Riley-Berger R., Harshman L., Kopp A., Vacha S., et al. , 2004. Extensive sex-specific nonadditivity of gene expression in Drosophila melanogaster. Genetics 167: 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman T. D., Arbeitman M. N., 2007. Genomic and functional studies of Drosophila sex hierarchy regulated gene expression in adult head and nervous system tissues. PLoS Genet. 3: 2278–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley B. R., Brooks A. N., Carlson J. W., Duff M. O., Landolin J. M., et al. , 2011. The developmental transcriptome of Drosophila melanogaster. Nature 471: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graze R. M., Novelo L. L., Amin V., Fear J. M., Casella G., et al. , 2012. Allelic imbalance in Drosophila hybrid heads: exons, isoforms, and evolution. Mol. Biol. Evol. 29: 1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graze R. M., McIntyre L. M., Morse A. M., Boyd B. M., Nuzhdin S. V., et al. , 2014. What the X has to do with it: differences in regulatory variability between the sexes in Drosophila simulans. Genome Biol. Evol. 6: 818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huylmans A. K., Parsch J., 2014. Population- and sex-biased gene expression in the excretion organs of Drosophila melanogaster. G3 (Bethesda) 4: 2307–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huylmans A. K., Parsch J., 2015. Variation in the X:autosome distribution of male-biased genes among Drosophila melanogaster tissues and its relationship with dosage compensation. Genome Biol. Evol. 7: 1960–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W., Riley R. M., Wolfinger R. D., White K. P., Passador-Gurgel G., et al. , 2001. The contributions of sex, genotype and age to transcriptional variance in Drosophila melanogaster. Nat. Genet. 29: 389–395. [DOI] [PubMed] [Google Scholar]
- Jursnich V. A., Burtis K. C., 1993. A positive role in differentiation for the male doublesex protein of Drosophila. Dev. Biol. 155: 235–249. [DOI] [PubMed] [Google Scholar]
- Kopp A., 2012. Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet. 28: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A., Duncan I., Godt D., Carroll S. B., 2000. Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature 408: 553–559. [DOI] [PubMed] [Google Scholar]
- Lebo M. S., Sanders L. E., Sun F. Z., Arbeitman M. N., 2009. Somatic, germline and sex hierarchy regulated gene expression during Drosophila metamorphosis. BMC Genomics 10: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. F., Rao X. Y., Mattox W. W., Amos C. I., Liu B., 2015. RNA-seq analysis of differential splice junction usage and intron retentions by DEXSeq. PLoS One 10: e0136653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D. L., 1983. Citation classic—genetic variations of Drosophila melanogaster. Current Contents Life Sci. 15: 15. [Google Scholar]
- Lundberg L. E., Kim M., Johansson A. M., Faucillion M. L., Josupeit R., et al. , 2013. Targeting of painting of fourth to roX1 and roX2 proximal sites suggests evolutionary links between dosage compensation and the regulation of the fourth chromosome in Drosophila melanogaster. G3 (Bethesda) 3: 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S. D., Baker B. S., 2015. Constraints on the evolution of a doublesex target gene arising from doublesex’s pleiotropic deployment. Proc. Natl. Acad. Sci. USA 112: E852–E861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S. D., Shi G. W., Baker B. S., 2011. Direct targets of the D. melanogaster DSXF protein and the evolution of sexual development. Development 138: 2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli D. S., Meissner G. W., Baker B. S., 2006. Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci. 29: 444–451. [DOI] [PubMed] [Google Scholar]
- Meisel R. P., Malone J. H., Clark A. G., 2012. Disentangling the relationship between sex-biased gene expression and X-linkage. Genome Res. 22: 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha V. K., Lindgren C. M., Eriksson K. F., Subramanian A., Sihag S., et al. , 2003. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34: 267–273. [DOI] [PubMed] [Google Scholar]
- Mortazavi A., Williams B. A., McCue K., Schaeffer L., Wold B., 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5: 621–628. [DOI] [PubMed] [Google Scholar]
- Parisi M., Nuttall R., Naiman D., Bouffard G., Malley J., et al. , 2003. Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science 299: 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi M., Nuttall R., Edwards P., Minor J., Naiman D., et al. , 2004. A survey of ovary-, testis-, and soma-biased gene expression in Drosophila melanogaster adults. Genome Biol. 5: R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi M. J., Gupta V., Sturgill D., Warren J. T., Jallon J. M., et al. , 2010. Germline-dependent gene expression in distant non-gonadal somatic tissues of Drosophila. BMC Genomics 11: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. C., Harrison P. W., Mank J. E., 2014. The ontogeny and evolution of sex-biased gene expression in Drosophila melanogaster. Mol. Biol. Evol. 31: 1206–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranz J. M., Castillo-Davis C. I., Meiklejohn C. D., Hartl D. L., 2003. Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science 300: 1742–1745. [DOI] [PubMed] [Google Scholar]
- Rivals I., Personnaz L., Taing L., Potier M. C., 2007. Enrichment or depletion of a GO category within a class of genes: which test? Bioinformatics 23: 401–407. [DOI] [PubMed] [Google Scholar]
- Rogers W. A., Salomone J. R., Tacy D. J., Camino E. M., Davis K. A., et al. , 2013. Recurrent modification of a conserved cis-regulatory element underlies fruit fly pigmentation diversity. PLoS Genet. 9: e1003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz H. K., 2011. Sex determination in insects: a binary decision based on alternative splicing. Curr. Opin. Genet. Dev. 21: 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M. L., Rabinow L., 2014. Transcriptomic analysis of sexual differentiation in somatic tissues of Drosophila melanogaster: successes and caveats. Sex Dev. 8: 113–126. [DOI] [PubMed] [Google Scholar]
- Shirangi T. R., Dufour H. D., Williams T. M., Carroll S. B., 2009. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol. 7: e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone A. M., Nasser Y. M., Nuzhdin S. V., 2005. Genetic variation for expression of the sex determination pathway genes in Drosophila melanogaster. Genet. Res. 86: 31–40. [DOI] [PubMed] [Google Scholar]
- Tarone A. M., McIntyre L. M., Harshman L. G., Nuzhdin S. V., 2012. Genetic variation in the Yolk protein expression network of Drosophila melanogaster: sex-biased negative correlations with longevity. Heredity 109: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicoso B., Bachtrog D., 2013. Reversal of an ancient sex chromosome to an autosome in Drosophila. Nature 499: 332–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicoso B., Charlesworth B., 2006. Evolution on the X chromosome: unusual patterns and processes. Nat. Rev. Genet. 7: 645–653. [DOI] [PubMed] [Google Scholar]
- Vied C., Ray S., Badger C. D., Bundy J. L., Arbeitman M. N., et al. , 2016. Transcriptomic analysis of the hippocampus from six inbred strains of mice suggests basis for sex-specific susceptibility and severity of neurological disorders. J. Comp. Neurol. (in press). [DOI] [PubMed] [Google Scholar]
- Williams T. M., Selegue J. E., Werner T., Gompel N., Kopp A., et al. , 2008. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 134: 610–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Koganezawa M., 2013. Genes and circuits of courtship behaviour in Drosophila males. Nat. Rev. Neurosci. 14: 681–692. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Sturgill D., Parisi M., Kumar S., Oliver B., 2007. Constraint and turnover in sex-biased gene expression in the genus Drosophila. Nature 450: 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
These data have been deposited in GEO. The accession number for wild-type strains is GSE50515. The accession number for the dsx mutant strains is GSE67400 (Dalton et al. 2013; Fear et al. 2015).