Abstract
Despite the fact that squab is consumed throughout the world because of its high nutritional value and appreciated sensory attributes, aspects related to its characterization, and in particular genetic issues, have rarely been studied. In this study, meat traits in terms of pH, water-holding capacity, intramuscular fat content, and fatty acid profile of the breast muscle of squabs from two meat pigeon breeds were determined. Breed-specific differences were detected in fat-related traits of intramuscular fat content and fatty acid composition. RNA-Sequencing was applied to compare the transcriptomes of muscle and liver tissues between squabs of two breeds to identify candidate genes associated with the differences in the capacity of fat deposition. A total of 27 differentially expressed genes assigned to pathways of lipid metabolism were identified, of which, six genes belonged to the peroxisome proliferator-activated receptor signaling pathway along with four other genes. Our results confirmed in part previous reports in livestock and provided also a number of genes which had not been related to fat deposition so far. These genes can serve as a basis for further investigations to screen markers closely associated with intramuscular fat content and fatty acid composition in squabs. The data from this study were deposited in the National Center for Biotechnology Information (NCBI)’s Sequence Read Archive under the accession numbers SRX1680021 and SRX1680022. This is the first transcriptome analysis of the muscle and liver tissue in Columba using next generation sequencing technology. Data provided here are of potential value to dissect functional genes influencing fat deposition in squabs.
Keywords: transcriptome, squab, intramuscular fat, fatty acid composition
Squab (young domestic pigeon under 4 wk old) has been consumed as a food by many nations for centuries. In China, squabs have been commercially raised on a large scale since the early 1970s (Tong et al. 2014). As the high nutritious value of squabs has become apparent to more and more people, the demand for them has rapidly expanded. In 2015, there were 30 million pairs of breeding pigeons in China (Cao and Chen 2015). With the development of the pigeon industry, squab production has progressively shifted from providing large amounts of protein to nourish populations, into promoting meats of consistent eating quality. The pigeon industry is beginning to consider breed differences in regard to nutritional values and meat quality, such as fatty acid (FA) composition, total lipids content, and tenderness, in addition to growth rate and meat yield (Bu et al. 2010; Zhang et al. 2012; Tong et al. 2014).
The total lipid content of muscle, intramuscular fat (IMF), and its FA composition play major roles in the quality attributes of meats, including sensory properties (flavor, juiciness, and tenderness) and nutritional values (Hocquette et al. 2010). It is generally assumed that IMF is important to induce a flavorful, juicy, and tender meat. The positive influences of IMF on the sensory quality traits of meat have been documented in pork (Gao and Zhao 2009), lamb (Watkins et al. 2013), and beef (Mateescu et al. 2015). As the unfavorable effects of dietary saturated FA exerted on health have been realized by an ever increasing number of consumers, strategies intending to modify the FA composition in meat have been applied in livestock to produce healthier products (Tous et al. 2013; Scollan et al. 2014). Up till now, breed-related differences in the capacity of lipid accumulation have been documented in pig (Wood et al. 2004a; Tyra et al. 2013), cattle (Gotoh et al. 2009), sheep (Qiao et al. 2008), and chicken (Wang et al. 2016). A number of single nucleotide polymorphisms (SNPs) significantly associated with the IMF trait of meat have been detected in functional genes involved in the lipid metabolism, which include the FABP (fatty acid-binding protein) (pig, Cho et al. 2011; beef cattle, Avilés et al. 2013), LPL (lipoprotein lipase) (chicken, Zhang et al. 2015), and DAGT1 (diacylglycerol acyltransferase) (cattle, Wu et al. 2012). Meanwhile, effects of genetic variants on FA composition were also demonstrated (Taniguchi et al. 2004; Wood et al. 2008). SNPs that have an influence on the FA profile of meat have been identified in the SCD (stearoyl-CoA desaturase) (beef cattle, Li et al. 2012; goat, Avilés et al. 2016), FASN (fatty acid synthase) (cattle, Bartoň et al. 2016), and LEPR (leptin receptor) (pig, Li et al. 2010) genes. These intensively documented reports indicated the possibility of improving meat quality and its prohealth properties by increasing the IMF content and improving FA composition through genetic selection in livestock. However, comparatively little work has been conducted in squabs. Consequently, the biological mechanisms responsible for the deposition of fat in meat-producing animals (pig and cattle, especially) have been intensively explored, while limited information is available in squabs.
The development of the RNA-Sequencing (RNA-Seq) technique has opened new opportunities for researchers to obtain huge amounts of information about the transcriptome profile of tissues of interest, which help in the acquisition of knowledge concerning the mechanism of lipid deposition in livestock (Jeong et al. 2013; Chen et al. 2015; Wang Z. et al. 2015; Miao et al. 2015). To our best knowledge, there are no data available in the literature regarding the gene expression profiles in the muscle and liver tissues of squabs. In order to identify candidate genes that have effects on the IMF content and FA composition in the muscle of squabs, this present study was undertaken to: (1) demonstrate the differences in the genetic capacity of IMF deposition between squabs of different breeds; (2) analyze the FA profile of IMF in the breast muscle of squabs; and (3) identify candidate genes involved in the lipid metabolism that might be closely related to the IMF trait through comparison of transcriptome profiles of muscle and liver tissues between squabs with divergent IMF content. Results from our study will be helpful in understanding the background genetic mechanisms involved in IMF accumulation in squabs.
Materials and Methods
Ethics statement
All experiments with animals were performed following the “Guide for the Care and Use of Laboratory Animals” of the Comparative Medical Centre of Yangzhou University (a registered animal facility for supervising experiments on laboratory animals), and were in accordance with a protocol approved by the animal use committee of the Chinese Ministry of Agriculture.
Animals and sample collection
All birds used in this study were reared under the same management system and processed in facilities operated by the same commercial supplier (Jiangnan Pigeon Industry Co. Ltd., Changzhou, China). Pigeons were kept in a stacked-cage raising system and squabs were housed within the same cage with both parents. A total of 68 female squabs, 34 from White Carneau (breed A) and 34 from Europigeon (breed B), were killed at 28 d of age by cervical dislocation. Live body weight of each bird was recorded. After euthanasia, roughly 1–2 g of pectoral muscle samples (on the left side) and liver samples were collected at necropsy and immediately flash frozen in liquid nitrogen. Breast muscles of both sides, including the pectoralis major and minor muscles, were removed from the carcasses (skinned and deboned), trimmed of visible adipose and connective tissues, placed into plastic bags individually, vacuum-sealed, and weighed. Left breast muscles were quickly frozen at −20° for later determination of intramuscular fat content and FA composition, which were undertaken not more than 1 wk later. Right breast muscles were submerged in ice for transport to the laboratory where they were subjected to 24 hr aging in a refrigerator (4°) prior to further analysis of pH, water-holding capacity, and tenderness.
Determination of physical and chemical traits of breast muscles
Meats traits recorded in this study were determined in the Institute of Poultry Science, Chinese Academy of Agricultural Science, Yangzhou, Jiangsu, China.
The pH value of each breast muscle sample (pectoralis major) was measured 24 hr postmortem (pH24) at a depth of 1–2 cm using a portable pH meter (Testo 205, Testo AG, Lenzkirch, Germany) equipped with a penetrating electrode. Water-holding capacity (WHC) was calculated by difference in weight of a meat sample, ∼ 0.5 g, before and after being subjected to a pressure of 35 kg for 5 min. Warner−Bratzler shear force (WBSF) was measured on uncooked meat aged at 4° for 24 hr. Briefly, three rectangular samples (1 cm × 1 cm × 3 cm) were cut from each breast muscle parallel to the direction of muscle fibers. Tenderness was determined by WBSF measurement (C-LM3B, Tenovo, Beijing, China) by shearing meat samples perpendicular to the fiber direction. The shear force value was presented as the mean of the forces required to shear each set of samples and data were reported in Newtons. For each sample, pH24, WHC, and WBSF were determined from triplicate samples.
For the determination of IMF content, breast muscles were homogenized individually in a meat grinder after being thawed. About 1.5 g of each muscle homogenate was dried with sand to a constant weight at 103 ± 2° followed by cooling in a desiccator for at least 30 min. The IMF contents in breast muscles were measured by placing dried samples into a fat-free extraction sleeve and extracted in a Soxhlet device (VELP Scientifica, SER 148, Usmate, Italy) using anhydrous ether as the solvent, and results were expressed as percentages, on the basis of wet tissue weight.
The FA composition of IMF of the breast muscle was further investigated. FAs were transmethylated according to Morrison and Smith (1964). Analysis of fatty acid methyl esters (FAME) was performed with the gas chromatography (GC) method, using Agilent 7890A GC system equipped with a flame ionization detector and an Agilent J&W advanced capillary GC column (Agilent 122-2361, 60 m, 0.25 mm internal diameter, 0.15 μm film thickness). Samples were injected by an auto-sampler (Agilent Technologies 7683 Series, Santa Clara, CA). Fatty acids were identified by comparing the retention times with those of a standard FAME mixture, CDDE-GLC-NESTLE 37 FAME Mix (Nu-Check-Prep Inc., Elysian, MN). For each sample, GC analysis was carried out in duplicate and the results were expressed as percent of total FA methyl esters present in the sample. The sum of saturated fatty acid (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) was also determined.
Statistical analysis
All statistical procedures were performed by the SPSS 17.0 software package (SPSS Inc., Chicago, IL). An independent-samples t-test of the variance between two different pigeon breeds was carried out. Two-sided P-values of less than 0.05 were considered statistically significant. Results were given as mean ± SD in the text. The Pearson correlation coefficient between IMF content and WBSF value was estimated and significance was detected at the 5% level.
RNA-Seq library construction for Illumina sequencing
The following protocols were performed by staff at the Oebiotech Co., Ltd., Shanghai, China. Three animals from each pigeon breed were used for the RNA-Seq analysis. Briefly, total RNA was isolated from the muscle and liver tissues using the TRIzol total RNA extraction kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The amount and purity of RNA were determined by NANODROP 2000c Spectrophotometer (Thermo Scientific) and the integrity was assessed with the Bioanalyzer 2100 (Agilent Technologies). Samples with RNA integrity number larger than 8.8 were considered acceptable for sequencing. mRNA libraries were constructed using the TruSeq RNA Sample Preparation Kit v2 (Illumina, Inc., San Diego, CA) according to the TrueSeq protocol and then sequenced in one lane using an Illumina Hiseq 2500 (Illumina Inc.) instrument.
Data filtering and mapping of reads
The quality of the raw data was checked with FastQC v0.10.1 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Raw sequence reads with a Q-score < 20 and with a length shorter than 90 bp were removed for quality control and the remaining (clean) reads were mapped to the reference genome of pigeon (ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF_000337935.1_Cliv_1.0) using bowtie 2 and TopHat v1.0.10 software (Trapnell et al. 2009). For functional annotation, the transcripts were compared against the reference genes (ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF_000337935.1_Cliv_1.0/GCF_000337935.1_Cliv_1.0_rna.fna.gz).
Differential gene expression analysis and functional annotation
The fragments per kilobase per million reads (FPKM) method was used to eliminate the influence of different gene length and sequencing level on the calculation of gene expression. The calculated gene expression was directly used for comparing the difference of gene expression between samples by the R package DESeq (http://bioconductor.org/packages/release/bioc/html/DESeq.html) (Anders and Huber 2010). If the FPKM value of either sample was zero, 0.01 was used instead of 0 to calculate the fold change. Genes with adjusted P-value of less than 0.05 and fold change > 1.5 were considered to be differentially expressed genes (DEGs). These DEGs were then subjected to Gene ontology (GO) annotation analysis (ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF_000337935.1_Cliv_1.0/GCF_000337935.1_Cliv_1.0_genomic.gff.gz) and functional enrichment analysis using the classic algorithm and Fisher’s exact test. To further characterize the metabolic pathways of DEGs, the Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used to analyze pathways (Kanehisa et al. 2008).
Validation of RNA-Seq results
After total RNA was reverse-transcribed, quantitative real-time PCR (qRT-PCR) was performed on an ABI 7500 thermocycler (Applied Biosystems). The GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as the internal reference gene for normalization of expression data (Wang Y. et al. 2015). Primers used in the present study are listed in Supplemental Material, Table S1. qRT-PCR in a 10-μl reaction volume with 0.1 μM of each primer (forward and reverse) and a quantity of cDNA corresponding to 10 ng of total RNA was performed using SYBR Premix Ex Taq reagents (Takara, Dalian, China). The validation performed on the same three samples per breed per tissue, which were used for RNA-Seq analysis, were run with three technical replicates. The PCR conditions were as follows: 2 min at 95° for the initial denaturation, followed by 40 cycles of denaturation at 95° for 5 sec, and 60° for 34 sec. The melt analysis curve was observed to confirm the amplification of single specific PCR fragments. Gene expression data were normalized to the GAPDH. Data from the relative quantification were transformed using the 2−ΔΔCt method described by Livak and Schmittgen (2001). Significance was determined at P < 0.05.
The data from this study were deposited in NCBI’s Sequence Read Archive under the accession numbers SRX1680021 and SRX1680022. Supplemental materials included: Table S1, which contains the information on primer sequences used in qRT-PCR; File S1 and File S2, which contain the significantly enriched GO terms in the liver and muscle tissues, respectively; and File S3, which contains the detected significantly expressed genes involved in the lipid metabolism and the peroxisome proliferator-activated receptor (PPAR) pathway.
Data availability
The data from this study were deposited in NCBI’s Sequence Read Archive under the accession numbers SRX1680021 and SRX1680022.
Results
Physical traits of the breast muscle
At the age of 28 d, no significant differences in live body weight (BW), breast muscle weight (BMW), and pH24 were observed between squabs from two breeds studied. However, significant differences were detected in other traits with respect to the ratio of breast muscle weight to live body weight (BMW/BW), WHC, and WBSF (shown in Table 1). Compared to birds from breed B, squabs from breed A had favorable meat traits in terms of a significantly higher proportion of breast muscle, better WHC, and lower value of WBSF (tender meat).
Table 1. Physical traits of the breast muscle recorded in the present study.
| Breed A (n = 34) | Breed B (n = 34) | P-Value | |
|---|---|---|---|
| BW (g) | 484.15 ± 24.94 | 488.11 ± 24.62 | 0.512 |
| BMW (g) | 372.55 ± 52.66 | 350.59 ± 42.58 | 0.063 |
| BMW/BW (%)a | 76.86 ± 9.43 | 71.88 ± 8.37 | 0.024 |
| pH24 | 6.05 ± 0.14 | 6.01 ± 0.18 | 0.202 |
| WHC (%)a | 79.59 ± 4.43 | 76.72 ± 6.25 | 0.033 |
| WBSFa (Newtons) | 11.54 ± 2.97 | 15.04 ± 2.87 | 0.000 |
Data are expressed as mean ± SD. Breed A, White Carneau; breed B, Europigeon. BW, live body weight; BMW, breast muscle weight; BMW/BW, the ratio of breast muscle weight to live body weight; pH24, pH value of the breast muscle 24 hr postmortem; WHC, water-holding capacity; WBSF, Warner−Bratzler shear force.
Means differed significantly between squabs from two breeds (P < 0.05).
Chemical traits of the breast muscle
A significant difference in the average IMF content was detected in the breast muscle samples from two pigeon breeds, 4.22 ± 0.77% and 3.61 ± 0.64% for breed A and breed B, respectively (P = 0.004). In this study, across all birds, there was a medium but significant (P < 0.001) correlation between WBSF value and IMF content (r = −0.517). As IMF content increased, less force was needed to cut through the meat.
Further analysis of the FA profile revealed that the main FAs identified in the breast muscle samples were palmitic (C16:0, 13.34–18.70%) and stearic (C18:0, 8.76–12.42%) acids for SFAs, palmitoleic (C16:1c9, 4.33–8.58%) and oleic (C18:1c9, 28.19–37.13%) acids for MUFAs, and linoleic (C18:2c9,c12, 13.65–18.64%) and eicosatrienoic (C20:3c11,c14,c17, 3.59–5.41%) acids for PUFAs. Differences between squabs from two pigeon breeds were significant when comparing concentrations (expressed as a percentage) of individual FAs in the IMF. In the breast muscle samples from breed A, the sum of SFAs was significantly lower, while the sum of MUFAs was significantly higher than that in breed B. The observed significant difference in the total content of MUFAs was mainly due to the significantly higher level of the predominant MUFA, oleic acid, in breed A. No significant differences were detected in the sum of omega 3 and omega 6 FAs or their ratio (shown in Table 2).
Table 2. Fatty acid composition of IMF from breast muscle samples of squabs.
| FAME | Breed A (n = 14) | Breed B (n = 14) | P-Value |
|---|---|---|---|
| C16:0 | 15.4895 ± 0.3850 | 15.3696 ± 0.2786 | 0.803 |
| C18:0 | 10.0805 ± 0.2365 | 9.7712 ± 0.2076 | 0.335 |
| C10:0 | 0.1157 ± 0.0178 | 0.1507 ± 0.0125 | 0.119 |
| C12:0 | 0.1527 ± 0.0263 | 0.1727 ± 0.0244 | 0.583 |
| C13:0 | 0.1092 ± 0.0138 | 0.1239 ± 0.0048 | 0.323 |
| C14:0a | 0.2913 ± 0.0315 | 0.3779 ± 0.0251 | 0.041 |
| C15:0a | 0.1157 ± 0.0141 | 0.2350 ± 0.0504 | 0.038 |
| C17:0a | 0.7085 ± 0.0694 | 1.0627 ± 0.0992 | 0.007 |
| C20:0a | 0.6875 ± 0.1316 | 1.1255 ± 0.0710 | 0.008 |
| C21:0 | 1.0505 ± 0.0967 | 1.1094 ± 0.0335 | 0.570 |
| C22:0 | 0.6779 ± 0.0373 | 0.7694 ± 0.0445 | 0.127 |
| C23:0 | 0.4894 ± 0.0442 | 0.6581 ± 0.0985 | 0.130 |
| C18:1c9a | 32.2607 ± 0.6585 | 30.6222 ± 0.4520 | 0.050 |
| C16:1c9 | 6.4010 ± 0.2891 | 6.6256 ± 0.1040 | 0.471 |
| C14:1c9a | 0.0979 ± 0.0099 | 0.1926 ± 0.0236 | 0.001 |
| C15:1c10 | 0.9772 ± 0.0484 | 0.8852 ± 0.0683 | 0.282 |
| C17:1c10 | 1.1355 ± 0.2063 | 1.0084 ± 0.1314 | 0.608 |
| C18:1t9 | 0.7260 ± 0.1073 | 0.5854 ± 0.1330 | 0.418 |
| C20:1c11 | 1.3144 ± 0.1551 | 1.2893 ± 0.1505 | 0.908 |
| C24:1c15 | 0.8442 ± 0.0350 | 0.9206 ± 0.0308 | 0.113 |
| C18:2c9,c12 | 15.4767 ± 0.4062 | 15.5508 ± 0.3440 | 0.890 |
| C18:2t9,t12 | 0.4280 ± 0.2789 | 0.1940 ± 0.0459 | 0.415 |
| C18:3c6,c9,c12 | 1.6038 ± 0.2583 | 1.3369 ± 0.0764 | 0.331 |
| C20:2c11,c14 | 0.4693 ± 0.0518 | 0.5959 ± 0.0375 | 0.058 |
| C20:4c5,c8,c11,c14a | 0.3997 ± 0.0492 | 0.7141 ± 0.0731 | 0.001 |
| C22:2c13,c16 | 0.6902 ± 0.1129 | 0.8465 ± 0.0677 | 0.246 |
| C18:3c9,c12,c15 | 1.1975 ± 0.0850 | 1.3377 ± 0.0537 | 0.175 |
| C20:3c11,c14,c17 | 4.5368 ± 0.1443 | 4.6063 ± 0.0715 | 0.671 |
| C20:5c5,c8,c11,c14,c17 | 0.4261 ± 0.0271 | 0.4433 ± 0.0239 | 0.639 |
| C22:6c4,c7,c10,c13,c16,c19 | 0.3612 ± 0.0336 | 0.4525 ± 0.0328 | 0.063 |
| ∑SFAa | 29.9684 ± 0.2561 | 30.9258 ± 0.3636 | 0.041 |
| ∑MUFAa | 43.7568 ± 0.5990 | 42.1293 ± 0.4501 | 0.039 |
| ∑PUFA | 25.5894 ± 0.5442 | 26.0779 ± 0.3057 | 0.441 |
| P/S | 0.8548 ± 0.0203 | 0.8450 ± 0.0148 | 0.698 |
| ∑n-6 | 19.0677 ± 1.6118 | 19.2381 ± 0.9752 | 0.738 |
| ∑n-3 | 6.5217 ± 0.8445 | 6.8398 ± 0.4088 | 0.216 |
| n-6/n-3 | 2.9695 ± 0.4736 | 2.8200 ± 0.1902 | 0.283 |
Values are presented as mean ± SD. Breed A, White Carneau; breed B, Europigeon. ∑SFA, total saturated fatty acids; ∑MUFA, total monounsaturated fatty acids; ∑PUFA, total polyunsaturated fatty acids; P/S, the ratio of PUFAs to SFAs; ∑n-6, total omega 6 fatty acids; ∑n-3, total omega 3 fatty acids.
Means differed significantly between two breeds (P < 0.05).
Transcriptome analysis and DEGs screening
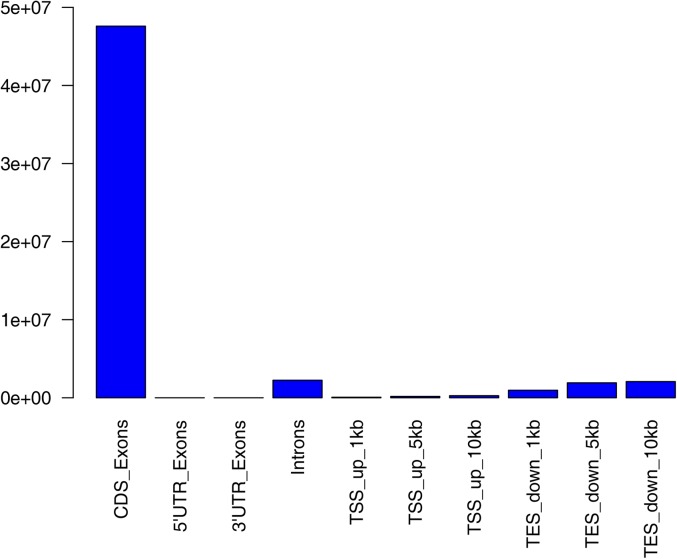
To identify DEGs related to the differences in IMF content in the breast muscle, RNA-Seq was used to obtain the transcriptomes of liver and muscle tissues from squabs of two pigeon breeds. Detailed results of sequencing and assembly are shown in Table 3. A total amount of 603.58 million reads with an average length of 125 nucleotides was acquired from the RNA-Seq experiment. On average, 51.71 ± 6.59 and 48.88 ± 3.20 million reads were obtained for libraries from liver and muscle samples, respectively. About 73.06% (70.50–75.93%) of reads were mapped to the reference genome, of which 96.91% mapped to unique genomic locations. An average of 60.44% of the mapped reads corresponded to annotated genes, 90.03% (88.55–91.26%) of them were located in exons, and 4.43% (4.10–4.95%) were in introns. The detailed distribution of reads is shown in Figure 1.
Table 3. Summary of Illumina sequencing.
| Tissue | Sample Name | Raw Reads | Clean Reads | Clean Bases (bp) | Valid Ratio (%) | Q30 (%) | GC Content (%) | Total Mapped Reads |
|---|---|---|---|---|---|---|---|---|
| Liver | A14G | 54272912 | 53984128 | 6743324339 | 99.39 | 92.50 | 49.0 | 37007492 |
| Liver | A17G | 54897634 | 54612062 | 6821811425 | 99.41 | 92.62 | 49.0 | 37514969 |
| Liver | A24G | 58981376 | 58670858 | 7328755933 | 99.40 | 92.40 | 49.0 | 41828564 |
| Liver | B6G | 43045126 | 42823256 | 5349250748 | 99.41 | 92.62 | 49.0 | 29871220 |
| Liver | B14G | 56363624 | 56062464 | 7003011885 | 99.39 | 92.55 | 48.5 | 38557847 |
| Liver | B15G | 44367286 | 44136162 | 5513068527 | 99.40 | 92.27 | 48.0 | 30338161 |
| Muscle | A14M | 51679634 | 51416892 | 6421765893 | 99.40 | 91.69 | 49.0 | 25474058 |
| Muscle | A17M | 46154200 | 45912278 | 5735008428 | 99.40 | 92.32 | 49.0 | 23944779 |
| Muscle | A24M | 54084880 | 53808252 | 6721243425 | 99.41 | 92.30 | 50.0 | 28855995 |
| Muscle | B6M | 46911708 | 46665656 | 5829074439 | 99.40 | 92.34 | 49.0 | 23568399 |
| Muscle | B14M | 49465288 | 49209082 | 6146719244 | 99.41 | 92.28 | 49.0 | 25481567 |
| Muscle | B15M | 46522126 | 46280970 | 5781030700 | 99.41 | 92.29 | 49.0 | 24009210 |
A, White Carneau; B, Europigeon.
Figure 1.
Detailed distribution of reads mapped to the reference genome. CDS, coding sequence; TES_down, downstream of the transcription ending site; TSS_up, upstream of the transcription starting site; UTR, untranslated regions.
The mean gene expression in the muscle and liver tissues between squabs from two breeds was highly correlated (P < 0.0001). The Pearson correlation coefficient was 0.99 and 0.97 for the muscle and liver tissue, respectively, showing that most of the genes had a similar behavior. Using the DESeq program, 1027 transcripts were identified as significantly differentially expressed in the liver tissue between two breeds, of which, 372 transcripts (mapped to 224 unique genes) were up-regulated and 655 transcripts (mapped to 412 unique genes) were down-regulated in breed A. In the muscle tissue, a total of 767 DEGs were identified, of which, 268 transcripts (mapped to 151 unique genes) were up-regulated and the remaining 499 transcripts (mapped to 298 unique genes) were down-regulated in breed A. These unigenes were successfully categorized into the three main GO categories of biological process, cellular component, and molecular function. The proportion of these DEGs categorized into GO biological process of lipid metabolic and regulatory process was 9.4% (60/636) and 5.6% (25/449) in liver and muscle tissues, respectively. They were involved in GO terms such as medium- and very long-chain fatty-acyl-CoA metabolic process, triglyceride homeostasis, FA oxidation, and phospholipid biosynthetic process. Significantly enriched GO terms in DEGs were shown in File S2 (liver) and File S3 (muscle).
In the present study, the KEGG database was used to analyze the DEGs in the metabolic pathway. Since the IMF content of muscle results from the balance between uptake, synthesis, and degradation of lipids, we focused mainly on the pathways directly involved in the lipid metabolism (Table 4). After the zero-expression genes were removed from the analysis, a total of 27 unique DEGs involved in 12 different lipid metabolic pathways were identified in liver. The fold changes for those DEGs ranged from 1.47 to 11.27. We noticed that two DEGs also belonged to the PPAR signaling pathway, which was considered to be one of the most important pathways involved in the regulation of lipid metabolism. In the muscle tissue, 16 DEGs involved in 12 different lipid metabolic pathways were identified. The fold changes for those DEGs ranged from 1.62 to 8.12. Four of these DEGs were also involved in the PPAR pathway. Including these six DEGs, a total of 10 significant DEGs were identified from the PPAR pathway with fold changes ranging from 1.64 to 11.27. Most of them (7/10) were significantly up-regulated in liver and/or muscle tissues from breed A. Significant DEGs involved in the lipid metabolism were listed in File S3.
Table 4. Significant DEGs involved in the pathways of lipid metabolism.
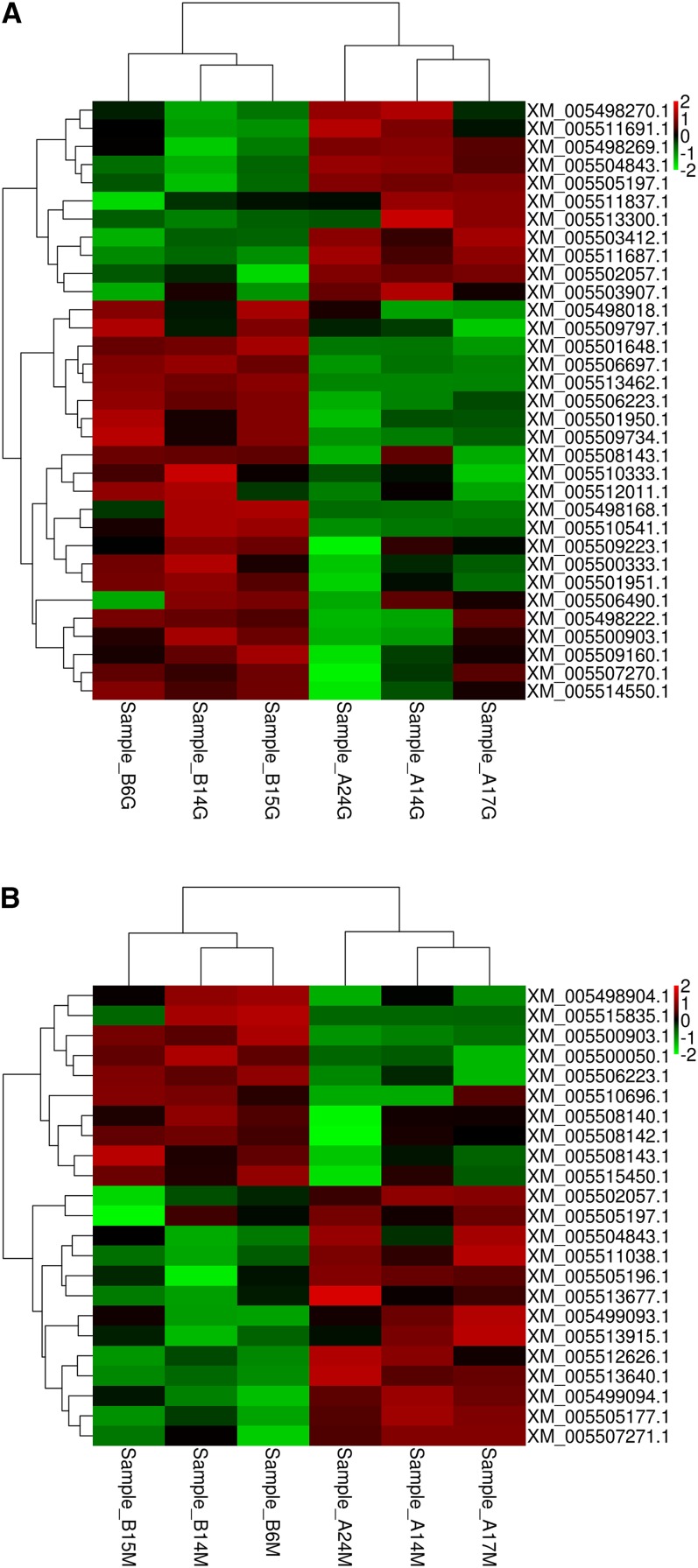
Hierarchical clustering analysis was applied to compare the expression patterns of these DEGs in the liver (Figure 2A) and muscle (Figure 2B) tissues. The expression patterns of these genes can be divided into two clusters and the birds could be differentiated on the basis of the breed.
Figure 2.
(A) Heatmap showing expression data for 33 differentially expressed transcripts in the liver tissue. Rows indicate genes with significant expression differences between the two breeds; columns represent individual samples from two pigeon breeds (Sample_A17G, Sample_A14G, Sample_A24G, and Sample_B15G, Sample_B14G, Sample_B6G were from breed A and breed B, respectively). Breed A, White Carneau; breed B, Europigeon. (B) Heatmap showing expression data for 23 differentially expressed transcripts in the muscle tissue. Rows indicate genes with significant expression differences between the two breeds; columns represent individual samples from two pigeon breeds (Sample_A17M, Sample_A14M, Sample_A24M, and Sample_B6M, Sample_B14M, Sample_B15M, were from breed A and breed B, respectively). Breed A, White Carneau; breed B, Europigeon.
Gene expression validation of DEGs by qRT-PCR
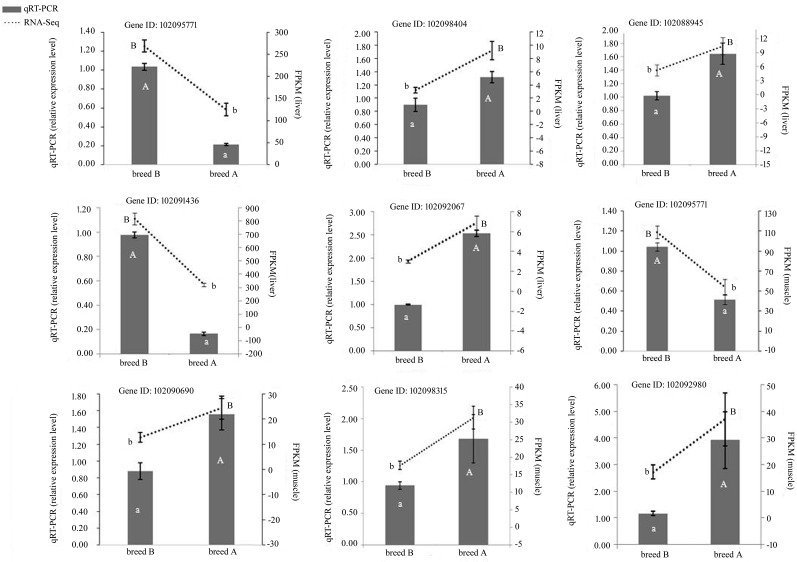
In the five pathways involved in the synthesis and degradation of ketone bodies, FA biosynthesis and degradation, biosynthesis of unsaturated FAs, and arachidonic acid metabolism, we randomly selected eight DEGs to validate the expression data identified by RNA-Seq, of which, five DEGs were expressed in the liver, four DEGs were expressed in the muscle, and one was expressed in both tissues. qRT-PCR analysis confirmed the direction of changes in the expression level of DEGs detected by RNA-Seq (Figure 3), which demonstrated that data from RNA-Seq and qRT-PCR were consistent in quantitatively estimating the transcription levels of the tested transcripts.
Figure 3.
Expression of eight significant DEGs detected by RNA-Seq and validated by qRT-PCR. Results from RNA-Seq are shown by line graphs on the top and values are shown on the right Y-axis as FPKM. Results from qRT-PCR are shown by bar graphs on the bottom and values are shown on the left Y-axis as relative expression level. Breed A, White Carneau; breed B, Europigeon. “A” and “a” indicate significant difference in the relative expression of genes detected via qRT-PCR at P < 0.05. “B” and “b” indicate significant difference in the expression of mRNA detected from RNA-Seq at P < 0.05. Data are presented as mean ± SE.
Discussion
In this study, squabs from two breeds had significant differences in traits regarding meat production performance and meat quality. Birds from breed A had a significantly higher proportion of breast muscle, which was favored in regard to increasing meat yields in the pigeon industry. Muscle samples from breed A also had significantly higher WHC than those from breed B. Since higher WHC is associated with reduced drip loss, better appearance of fresh meat, and better sensory properties of cooked meat (Pedersen et al. 2003), improvement in WHC of meat is desired both by industry and consumers. Besides these two favorable traits, the breast muscle from breed A also had significantly higher IMF content and lower WBSF value, which characterized a tender meat. A significantly negative correlation between IMF and average shear force was detected in this study, which was consistent with previous findings that demonstrated the favorable effects of increased IMF level on meat tenderness although the strength of the correlation varied between studies (Van Laack et al. 2001; Fortin et al. 2005; Teye et al. 2006; Jeleníková et al. 2008). Our results revealed that under the same rearing conditions, squabs from breed A were more efficient in increasing breast muscle yield and had better meat quality in terms of WHC, IMF content, and tenderness.
It has been widely accepted that IMF content is one of the key determinants of meat tenderness, flavor, and juiciness (Hodgson et al. 1991; Fernandez et al. 1999). Since the IMF content of meat can be influenced by both genetic and environmental factors (Dikeman et al. 2005), care was taken in the present study to ensure that the IMF level was assessed under conditions where other factors known to affect lipid deposition, such as slaughter age, muscle type, gender, and feeding (Dodson et al., 2010; Geldenhuys et al. 2013; Anderson et al. 2015), were kept to a minimum level of variation. Therefore, breed was retained as the main source of the significant difference observed in IMF level.
It is know that IMF contributes importantly not only to various aspects of meat quality but also to the nutritional value of meat. The P/S and n-6/n-3 ratios are normally used to assess the nutritional value of fat. The recommended dietary ratios for P/S and n-6/n-3 were > 0.4 and < 4.0, respectively (Wood et al. 2004b). Analysis of the FA composition of IMF in the breast muscle of squabs indicated that the mean P/S ratio, 0.88 for the breast muscle, was higher than that in the breast and thigh meat of broilers, 0.54 and 0.62, respectively (Ahmed et al. 2015). It was also higher than the P/S ratios of other red meats, such as lamb (0.32, longissimus lumborum) (Majdoub-Mathlouthi et al. 2015), beef (0.44, longissimus thoracis) (Andreo et al. 2016), and pork (0.55−0.62, longissimus thoracis) (Alonso et al. 2015). As to the n-6/n-3 ratio in the breast muscle of squabs, it was also within the recommended range. It was favorably lower (2.86) in squabs compared with the n-6/n-3 ratio in beef (greater than 17.4, longissimus muscle) (Indurain et al. 2010), pork (greater than 9.0, longissimus thoracis) (Ayuso et al. 2015), broiler breast meat, and thigh meat (6.89 and 4.86, respectively) (Ahmed et al. 2015). Given the relationship found between incidence of coronary diseases and high ratios of n-6/n-3 FAs in meat (Santos-Silva et al. 2002), results from our study indicated that the breast muscle of squabs had a high ratio of P/S and a more favorable balance between n-6 and n-3 PUFA, which was beneficial and healthy for consumers.
Our results also demonstrated the better meat quality of squabs from breed A due to the higher IMF content (tender meat) and favorable FA composition (higher MUFA and lower SFA content), which met the recommendation of lowering the dietary intake of SFA and increasing that of unsaturated FAs to decrease the risk of cardiovascular diseases (Simopoulos 2002; Gidding et al. 2005). This breed-specific difference in meat quality might be used for selection programs in the pigeon industry to produce a product that has enough IMF to ensure a pleasant eating experience and at the same time alleviate the health concerns associated with high-SFA meat. Further research needs to be carried out to determine whether the inclusion of breed A in the parental line will result in an increase in IMF and improvement in the FA profile in the offspring to make the squab meat more attractive for health reasons.
In order to identify genes closely associated with the genetic variation in IMF deposition and FA composition in squabs, transcriptomes of the breast muscle and liver tissue were compared between squabs from two pigeon breeds which differed significantly in these traits. Of all the significant DEGs involved in lipid metabolism, several functional genes have been previously reported to be prosperous candidate genes related to IMF trait or FA composition in livestock, including ADH (alcohol dehydrogenase 1) (Ward et al. 2012), ANGPTL4 (angiopoietin-like 4) (Ren et al. 2014), FADS1 (fatty acid desaturase 1) (Ibeagha-Awemu et al. 2014), ACSL4 (acyl-CoA synthetase long-chain family member 4) (Ruść et al., 2011; Chen et al. 2014), and LPL (lipoprotein lipase) (Wang et al. 2013; Zhang et al. 2015). However, the majority of significant DEGs involved in lipid metabolism have not been documented to be associated with the differences in the capacity of lipid accumulation. Interestingly, the widely reported FABP genes, including the muscle-and-heart type (FABP3), adipocyte type (FABP4), and liver type (FABP1), which have been revealed to be significantly related to the variations of IMF level in pig (Zhao et al. 2009; Lee et al. 2010; Han et al. 2012), chicken (Ye et al. 2010; Wang et al. 2016), duck (He et al. 2012), and milk FA composition in dairy cattle (Nafikov et al. 2013), were not identified as significant DEGs in this study although the mRNA expression levels of FABP3, FABP4, and FABP1 in the liver tissues of breed A were higher than those of breed B (with an up-regulated fold change of 1.10, 1.97, and 4.73, respectively). These results led us to suggest that either FABP might not regulate the FA metabolism at the transcriptomic level or there might be digestive, nutritional, or metabolic particularities between squabs and other meat-producing animals. Further studies need to screen potential SNPs in these DEGs in different pigeon populations to assess possible associations between genetic variability and IMF content, as well as the FA profiles in meat before markers identified might be used in improvement programs aiming to produce a more competitive product with tender squab meat and favorable FA composition.
We also focused on significant DEGs identified in the PPAR signaling pathway because there is increasing evidence suggesting the involvement of this pathway in lipid metabolism (Doran et al. 2014; Hausman et al. 2014; Zheng et al. 2015). Association studies between polymorphisms in genes in the PPAR signaling pathway and porcine meat quality traits have been carried out (He et al. 2013) and the positive correlation between IMF deposition and PPAR signaling genes has been reported in chicken (Cui et al. 2012). In this study, we found that seven of the ten unique genes identified in the PPAR signaling pathway were significantly up-regulated in the high-IMF breed A, including three genes involved in acyl-CoA synthesis, ACSL4, ACSL5 (acyl-CoA synthetase long-chain family member 5), and ACSBG2 (acyl-CoA synthetase bubblegum family member 2), indicative of an enhanced lipogenesis. Our results were in agreement with previous studies that documented these genes as potential candidate genes associated with the difference in lipid metabolism (Pan et al. 2010; Corominas et al. 2012; Claire D’Andre et al. 2013). While ACSBG1 (acyl-CoA synthetase bubblegum family member 1), the fourth gene encoding an acyl-CoA synthetase, was up-regulated in low-IMF breed B, its significant up-regulation in skeletal muscle was observed in mice administered metformin, which suppressed lipid accumulation by promoting FA oxidation (Wang et al. 2014). We suggested that these genes may form the foundation for further investigation to identify causative mutations involved in fat deposition and FA composition in the muscle of squabs. Also, future studies on the translational level of proteins encoded by these DEGs as well as information about the expression changes due to different variants would be needed to elucidate their effects on lipid metabolism in squabs.
Conclusions
In this study, we demonstrated that squabs from two meat pigeon breeds, White Carneau and Europigeon, had significant differences in meat traits with regard to WHC, tenderness, IMF content, and FA composition. Datasets from transcriptome profiling of the breast muscle and liver tissue were generated and functional genes differentially expressed between the high- and low-IMF squabs were identified, among which, genes involved in lipid metabolism and the PPAR signaling pathway might be potential candidate genes associated with the variation of IMF content and FA composition in the muscle of squabs. These genes will help to identify DNA markers to predict the ability of squabs to deposit IMF and use them in selection programs in order to provide squab with enhanced meat quality and ensure the competitiveness of the pigeon industry.
Supplementary Material
Acknowledgments
This work was financially supported by the National Science Foundation of China (31402062), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.029793/-/DC1
Communicating editor: D. J. de Koning
Literature Cited
- Ahmed S. T., Islam M. M., Bostami A. B., Mun H. S., Kim Y. J., et al. , 2015. Meat composition, fatty acid profile and oxidative stability of meat from broilers supplemented with pomegranate (Punica granatum L.) by-products. Food Chem. 188: 481–488. [DOI] [PubMed] [Google Scholar]
- Alonso V., Muela E., Gutiérrez B., Calanche J. B., Roncalés P., et al. , 2015. The inclusion of Duroc breed in maternal line affects pork quality and fatty acid profile. Meat Sci. 107: 49–56. [DOI] [PubMed] [Google Scholar]
- Anderson F., Pannier L., Pethick D. W., Gardner G. E., 2015. Intramuscular fat in lamb muscle and the impact of selection for improved carcass lean meat yield. Animal 9: 1081–1090. [DOI] [PubMed] [Google Scholar]
- Anders S., Huber W., 2010. Differential expression analysis for sequence count data. Genome Biol. 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreo N., Bridi A. M., Soares A. L., Prohmann P. E. F., Peres L. M., et al. , 2016. Fatty acid profile of beef from immunocastrated (BOPRIVA) Nellore bulls. Meat Sci. 117: 12–17. [DOI] [PubMed] [Google Scholar]
- Avilés C., Polvillo O., Peña F., Juárez M., Martínez A. L., et al. , 2013. Associations between DGAT1, FABP4, LEP, RORC, and SCD1 gene polymorphisms and fat deposition in Spanish commercial beef. J. Anim. Sci. 91: 4571–4577. [DOI] [PubMed] [Google Scholar]
- Avilés C., Horcada A., Polvillo O., Membrillo A., Anaya G., et al. , 2016. Association study between variability in the SCD gene and the fatty acid profile in perirenal and intramuscular fat deposits from Spanish goat populations. Small Rumin. Res. 136: 127–131. [Google Scholar]
- Ayuso M., Óvilo C., Rodríguez-Bertos A., Rey A. I., Daza A., et al. , 2015. Dietary vitamin A restriction affects adipocyte differentiation and fatty acid composition of intramuscular fat in Iberian pigs. Meat Sci. 108: 9–16. [DOI] [PubMed] [Google Scholar]
- Bartoň L., Bureš D., Kott T., Řehák D., 2016. Associations of polymorphisms in bovine DGAT1, FABP4, FASN, and PPARGC1A genes with intramuscular fat content and the fatty acid composition of muscle and subcutaneous fat in Fleckvieh bulls. Meat Sci. 114: 18–23. [DOI] [PubMed] [Google Scholar]
- Bu Z., Li B. L., Zhao Z. H., Wang Q., Chen H. S., et al. , 2010. Resources of pigeon and current breeding situation in China. China Animal Husbandry & Veterinary Medicine 37: 116–119. [Google Scholar]
- Cao Y. C., Chen J. S., 2015. The current status of intensive farming of meat pigeon and strategies for its development. Guangdong Journal of Animal and Veterinary Science 40: 18–20. [Google Scholar]
- Chen D., Li W., Du M., Wu M., Cao B., 2015. Sequencing and characterization of divergent marbling levels in the beef cattle (Longissimus dorsi muscle). Transcriptome. Asian Australas J. Anim. Sci. 28: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. N., Jiang Y. Z., Cen W. M., Xing S. H., Zhu L., et al. , 2014. Distribution of H-FABP and ACSL4 gene polymorphisms and their associations with intramuscular fat content and backfat thickness in different pig populations. Genet. Mol. Res. 13(3): 6759–6772. [DOI] [PubMed] [Google Scholar]
- Cho K. H., Kim M. J., Jeon G. J., Chung H. Y., 2011. Association of genetic variants for FABP3 gene with back fat thickness and intramuscular fat content in pig. Mol. Biol. Rep. 38: 2161–2166. [DOI] [PubMed] [Google Scholar]
- Claire D’Andre H., Paul W., Shen X., Jia X., Zhang R., et al. , 2013. Identification and characterization of genes that control fat deposition in chickens. J. Anim. Sci. Biotechnol. 4: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corominas J., Ramayo-Caldas Y., Castelló A., Muñoz M., Ibáñez-Escriche N., et al. , 2012. Evaluation of the porcine ACSL4 gene as a candidate gene for meat quality traits in pigs. Anim. Genet. 43: 714–720. [DOI] [PubMed] [Google Scholar]
- Cui H. X., Liu R. R., Zhao G. P., Zheng M. Q., Chen J. L., et al. , 2012. Identification of differentially expressed genes and pathways for intramuscular fat deposition in pectoralis major tissues of fast-and slow-growing chickens. BMC Genomics 13: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikeman M. E., Pollak E. J., Zhang Z., Moser D. W., Gill C. A., et al. , 2005. Phenotypic ranges and relationships among carcass and meat palatability traits for fourteen cattle breeds, and heritabilities and expected progeny differences for Warner-Bratzler shear force in three beef cattle breeds. J. Anim. Sci. 83: 2461–2467. [DOI] [PubMed] [Google Scholar]
- Dodson M. V., Jiang Z., Chen J., Hausman G. J., Guan L. L., et al. , 2010. Allied industry approaches to alter intramuscular fat content and composition in beef animals. J. Food Sci. 75: R1–R8. [DOI] [PubMed] [Google Scholar]
- Doran A. G., Berry D. P., Creevey C. J., 2014. Whole genome association study identifies regions of the bovine genome and biological pathways involved in carcass trait performance in Holstein-Friesian cattle. BMC Genomics 15: 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez X., Monin G., Talmant A., Mourot J., Lebret B., 1999. Influences of intramuscular fat content on the quality of pig meat - 2. Consumer acceptability of m. longissimus lumborum. Meat Sci. 53: 67–72. [DOI] [PubMed] [Google Scholar]
- Fortin A., Robertson W. M., Tong A. K., 2005. The eating quality of Canadian pork and its relationship with intramuscular fat. Meat Sci. 69: 297–305. [DOI] [PubMed] [Google Scholar]
- Gao S. Z., Zhao S. M., 2009. Physiology, affecting factors and strategies for control of pig meat intramuscular fat. Recent Pat. Food Nutr. Agric. 1: 59–74. [PubMed] [Google Scholar]
- Geldenhuys G., Hoffman L. C., Muller N., 2013. The effect of season, sex, and portion on the carcass characteristics, pH, color, and proximate composition of Egyptian Goose (Alopochen aegyptiacus) meat. Poult. Sci. 92: 3283–3291. [DOI] [PubMed] [Google Scholar]
- Gidding S. S., Dennison B. A., Birch L. L., Daniels S. R., Gillman M. W., et al. , 2005. Dietary recommendations for children and adolescents: a guide for practitioners: consensus statement from the American Heart Association. Circulation 112: 2061–2075. [DOI] [PubMed] [Google Scholar]
- Gotoh T., Albrecht E., Teuscher F., Kawabata K., Sakashita K., et al. , 2009. Differences in muscle and fat accretion in Japanese Black and European cattle. Meat Sci. 82: 300–308. [DOI] [PubMed] [Google Scholar]
- Han X., Jiang T., Yang H., Zhang Q., Wang W., et al. , 2012. Investigation of four porcine candidate genes (H-FABP, MYOD1, UCP3 and MASTR) for meat quality traits in Large White pigs. Mol. Biol. Rep. 39: 6599–6605. [DOI] [PubMed] [Google Scholar]
- Hausman G. J., Basu U., Du M., Fernyhough-Culver M., Dodson M. V., 2014. Intermuscular and intramuscular adipose tissues: Bad vs. good adipose tissues. Adipocyte 3: 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Chen J., Lu L., Tian Y., Tao Z., et al. , 2012. A novel SNP of liver-type fatty acid-binding protein gene in duck and its associations with the intramuscular fat. Mol. Biol. Rep. 39: 1073–1077. [DOI] [PubMed] [Google Scholar]
- He K., Wang Q., Wang Z., Pan Y., 2013. Association study between gene polymorphisms in PPAR signaling pathway and porcine meat quality traits. Mamm. Genome 24: 322–331. [DOI] [PubMed] [Google Scholar]
- Hocquette J. F., Gondret F., Baeza E., Medale F., Jurie C., et al. , 2010. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 4: 303–319. [DOI] [PubMed] [Google Scholar]
- Hodgson R. R., Davis G. W., Smith G. C., Savell J. W., Cross H. R., 1991. Relationships between pork loin palatability traits and physical characteristics of cooked chops. J. Anim. Sci. 69: 4858–4865. [DOI] [PubMed] [Google Scholar]
- Ibeagha-Awemu E. M., Akwanji K. A., Beaudoin F., Zhao X., 2014. Associations between variants of FADS genes and omega-3 and omega-6 milk fatty acids of Canadian Holstein cows. BMC Genet. 15: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indurain G., Beriain M. J., Sarries M. V., Insausti K., 2010. Effect of weight at slaughter and breed on beef intramuscular lipid classes and fatty acid profile. Animal 4: 1771–1780. [DOI] [PubMed] [Google Scholar]
- Jeleníková J., Pipek P., Miyahara M., 2008. The effects of breed, sex, intramuscular fat and ultimate pH on pork tenderness. Eur. Food Res. Technol. 227: 989–994. [Google Scholar]
- Jeong J., Bong J., Kim G. D., Joo S. T., Lee H. J., et al. , 2013. Transcriptome changes favoring intramuscular fat deposition in the longissimus muscle following castration of bulls. J. Anim. Sci. 91: 4692–4704. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., et al. , 2008. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36: D480–D484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Choi Y. M., Choe J. H., Kim J. M., Hong K. C., et al. , 2010. Association between polymorphisms of the heart fatty acid binding protein gene and intramuscular fat content, fatty acid composition, and meat quality in Berkshire breed. Meat Sci. 86: 794–800. [DOI] [PubMed] [Google Scholar]
- Li C., Aldai N., Vinsky M., Dugan M. E., McAllister T. A., 2012. Association analyses of single nucleotide polymorphisms in bovine stearoyl-CoA desaturase and fatty acid synthase genes with fatty acid composition in commercial cross-bred beef steers. Anim. Genet. 43: 93–97. [DOI] [PubMed] [Google Scholar]
- Li X., Kim S. W., Choi J. S., Lee Y. M., Lee C. K., et al. , 2010. Investigation of porcine FABP3 and LEPR gene polymorphisms and mRNA expression for variation in intramuscular fat content. Mol. Biol. Rep. 37: 3931–3939. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)). Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Majdoub-Mathlouthi L., Saïd B., Kraiem K., 2015. Carcass traits and meat fatty acid composition of Barbarine lambs reared on rangelands or indoors on hay and concentrate. Animal 9: 2065–2071. [DOI] [PubMed] [Google Scholar]
- Mateescu R. G., Garrick D. J., Garmyn A. J., VanOverbeke D. L., Mafi G. G., et al. , 2015. Genetic parameters for sensory traits in longissimus muscle and their associations with tenderness, marbling score, and intramuscular fat in Angus cattle. J. Anim. Sci. 93: 21–27. [DOI] [PubMed] [Google Scholar]
- Miao X., Luo Q., Qin X., Guo Y., Zhao H., 2015. Genome-wide mRNA-seq profiling reveals predominant down-regulation of lipid metabolic processes in adipose tissues of Small Tail Han than Dorset sheep. Biochem. Biophys. Res. Commun. 467: 413–420. [DOI] [PubMed] [Google Scholar]
- Morrison W. R., Smith L. M., 1964. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 5: 600–608. [PubMed] [Google Scholar]
- Nafikov R. A., Schoonmaker J. P., Korn K. T., Noack K., Garrick D. J., et al. , 2013. Association of polymorphisms in solute carrier family 27, isoform A6 (SLC27A6) and fatty acid-binding protein-3 and fatty acid-binding protein-4 (FABP3 and FABP4) with fatty acid composition of bovine milk. J. Dairy Sci. 96: 6007–6021. [DOI] [PubMed] [Google Scholar]
- Pan Z., Wang J., Han C., Zhai N., Lv J., et al. , 2010. Identification of differentially expressed genes between hepatocytes of Landes geese (Anser anser) and Sichuan White geese (Anser cygnoides). Mol. Biol. Rep. 37: 4059–4066. [DOI] [PubMed] [Google Scholar]
- Pedersen D. K., More S., Andersen H. J., Balling Engelsen S., 2003. Early prediction of water-holding capacity in meat by multivariate vibrational spectroscopy. Meat Sci. 65: 581–592. [DOI] [PubMed] [Google Scholar]
- Qiao Y., Huang Z. G., Li Q. F., Liu Z. S., Dai R., et al. , 2008. Developmental changes of the LPL mRNA expression and its effect on IMF content in sheep muscle. Agric. Sci. China 7: 104–111. [Google Scholar]
- Ren Z. Q., Wu W. J., Liu W. H., Zheng R., Li J. L., et al. , 2014. Differential expression and effect of the porcine ANGPTL4 gene on intramuscular fat. Genet. Mol. Res. 13: 2949–2958. [DOI] [PubMed] [Google Scholar]
- Ruść A., Sieczkowska H., Krzęcio E., Antosik K., Zybert A., et al. , 2011. The association between acyl-CoA synthetase (ACSL4) polymorphism and intramuscular fat content in (Landrace × Yorkshire) × Duroc pigs. Meat Sci. 89: 440–443. [DOI] [PubMed] [Google Scholar]
- Santos-Silva J., Bessa R. J. B., Santos-Silva F., 2002. Effect of genotype, feeding system and slaughter weight on the quality of light lambs. II. Fatty acid composition of meat. Livest. Prod. Sci. 77: 187–194. [Google Scholar]
- Scollan N. D., Dannenberger D., Nuernberg K., Richardson I., MacKintosh S., et al. , 2014. Enhancing the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 97: 384–394. [DOI] [PubMed] [Google Scholar]
- Simopoulos A. P., 2002. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 21: 495–505. [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Utsugi T., Oyama K., Mannen H., Kobayashi M., et al. , 2004. Genotype of stearoyl-CoA desaturase is associated with fatty acid composition in Japanese Black cattle. Mamm. Genome 15: 142–148. [DOI] [PubMed] [Google Scholar]
- Teye G. A., Sheard P. R., Whittington F. M., Nute G. R., Stewart A., et al. , 2006. Influence of dietary oils and protein level on pork quality. 1. Effects on muscle fatty acid composition, carcass, meat and eating quality. Meat Sci. 73: 157–165. [DOI] [PubMed] [Google Scholar]
- Tong H. B., Xie P., Bu Z., Xu M., 2014. The application of breeding techniques used in meat pigeon and strategies for the development of pigeon industry. China Poultry 36: 2–5. [Google Scholar]
- Tous N., Lizardo R., Vilà B., Gispert M., Font-i-Furnols M., et al. , 2013. Effect of a high dose of CLA in finishing pig diets on fat deposition and fatty acid composition in intramuscular fat and other fat depots. Meat Sci. 93: 517–524. [DOI] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S. L., 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyra M., Ropka-Molik K., Terman A., Piórkowska K., Oczkowicz M., et al. , 2013. Association between subcutaneous and intramuscular fat content in porcine ham and loin depending on age, breed and FABP3 and LEPR genes transcript abundance. Mol. Biol. Rep. 40: 2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laack R. L., Stevens S. G., Stalder K. J., 2001. The influence of ultimate pH and intramuscular fat content on pork tenderness and tenderization. J. Anim. Sci. 79: 392–397. [DOI] [PubMed] [Google Scholar]
- Wang C., Liu F., Yuan Y., Wu J., Wang H., et al. , 2014. Metformin suppresses lipid accumulation in skeletal muscle by promoting fatty acid oxidation. Clin. Lab. 60: 887–896. [DOI] [PubMed] [Google Scholar]
- Wang W., Xue W., Jin B., Zhang X., Ma F., et al. , 2013. Candidate gene expression affects intramuscular fat content and fatty acid composition in pigs. J. Appl. Genet. 54: 113–118. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ding J. T., Yang H. M., Yan Z. J., Cao W., et al. , 2015. Analysis of pigeon (Columba) ovary transcriptomes to identify genes involved in blue light regulation. PLoS One 10: e0143568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hui X., Wang H., Kurban T., Hang C., et al. , 2016. Association of H-FABP gene polymorphisms with intramuscular fat content in Three-yellow chickens and Hetian-black chickens. J. Anim. Sci. Biotechnol. 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Li Q., Chamba Y., Zhang B., Shang P., et al. , 2015. Identification of genes related to growth and lipid deposition from transcriptome profiles of pig muscle tissue. PLoS One 10: e0141138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. K., McKinnon J. J., Hendrick S., Buchanan F. C., 2012. The impact of vitamin A restriction and ADH1C genotype on marbling in feedlot steers. J. Anim. Sci. 90: 2476–2483. [DOI] [PubMed] [Google Scholar]
- Watkins P. J., Frank D., Singh T. K., Young O. A., Warner R. D., 2013. Sheepmeat flavor and the effect of different feeding systems: a review. J. Agric. Food Chem. 61: 3561–3579. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Nute G. R., Richardson R. I., Whittington F. M., Southwood O., et al. , 2004a Effects of breed, diet and muscle on fat deposition and eating quality in pigs. Meat Sci. 67: 651–667. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Richardson R. I., Nute G. R., Fisher A. V., Campo M. M., et al. , 2004b Effects of fatty acids on meat quality: a review. Meat Sci. 66: 21–32. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Enser M., Fisher A. V., Nute G. R., Sheard P. R., et al. , 2008. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 78: 343–358. [DOI] [PubMed] [Google Scholar]
- Wu X. X., Yang Z. P., Shi X. K., Li J. Y., Ji D. J., et al. , 2012. Association of SCD1 and DGAT1 SNPs with the intramuscular fat traits in Chinese Simmental cattle and their distribution in eight Chinese cattle breeds. Mol. Biol. Rep. 39: 1065–1071. [DOI] [PubMed] [Google Scholar]
- Ye M. H., Chen J. L., Zhao G. P., Zheng M. Q., Wen J., 2010. Associations of A-FABP and H-FABP markers with the content of intramuscular fat in Beijing-You chicken. Anim. Biotechnol. 21: 14–24. [DOI] [PubMed] [Google Scholar]
- Zhang H. K., Xin W., Tong H. B., Bu Z., Xu S. J., et al. , 2012. The application of comprehensive selection index in the breeding of meat pigeon. Poult. Sci. 6: 12–14. [Google Scholar]
- Zhang X. D., Li Q. H., Lou L. F., Liu J., Chen X. H., et al. , 2015. High-resolution melting curve analysis of the ADSL and LPL genes and their correlation with meat quality and blood parameters in chickens. Genet. Mol. Res. 14: 2031–2040. [DOI] [PubMed] [Google Scholar]
- Zhao S. M., Ren L. J., Chen L., Zhang X., Cheng M. L., et al. , 2009. Differential expression of lipid metabolism related genes in porcine muscle tissue leading to different intramuscular fat deposition. Lipids 44: 1029–1037. [DOI] [PubMed] [Google Scholar]
- Zheng J., Xiao X., Zhang Q., Yu M., Xu J., et al. , 2015. Maternal protein restriction induces early-onset glucose intolerance and alters hepatic genes expression in the peroxisome proliferator-activated receptor pathway in offspring. J. Diabetes Investig. 6: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from this study were deposited in NCBI’s Sequence Read Archive under the accession numbers SRX1680021 and SRX1680022.