Abstract
Background:
The National Health Service Health Check program in England is the largest cardiovascular risk assessment and management program in the world. We assessed the effect of this program on modelled risk of cardiovascular disease, individual risk factors for cardiovascular disease, prescribing of relevant medications and diagnosis of vascular disease.
Methods:
We obtained retrospective electronic medical records for a randomly selected sample of 138 788 patients aged 40–74 years registered with 462 English general practices participating in the Clinical Practice Research Datalink between 2009 and 2013. We used a quasi-experimental design of difference-indifferences matching analysis to compare changes in outcomes between Health Check attendees and nonattendees, with a median follow-up time of 2 years.
Results:
Overall, 21.4% of the eligible population attended a Health Check. After matching (n = 29 672 in each group), attendees had a significant absolute reduction in modelled risk for cardiovascular disease (−0.21%, 95% confidence interval [CI] −0.24% to −0.19%) and individual risk factors: systolic blood pressure (−2.51 mm Hg, 95% CI −2.77 to −2.25 mm Hg), diastolic blood pressure (−1.46 mm Hg, 95% CI −1.62 to −1.29 mm Hg), body mass index (−0.27, 95% CI −0.34 to −0.20) and total cholesterol (−0.15 mmol/L, 95% CI −0.18 to −0.13 mmol/L). Statins were prescribed for 39.9% of attendees who were at high risk for cardiovascular disease. The program resulted in significantly more diagnoses of selected vascular diseases among attendees, with the largest increases for hypertension (2.99%) and type 2 diabetes mellitus (1.31%).
Interpretation:
The National Health Service Health Check program had statistically significant but clinically modest impacts on modelled risk for cardiovascular disease and individual risk factors, although diagnosis of vascular disease increased. Overall program performance was substantially below national and international targets, which highlights the need for careful planning, monitoring and evaluation of similar initiatives internationally.
The World Health Organization has set a target to reduce premature death from cardiovascular disease by 25% by 2025,1 a goal that was affirmed in the recently published Sustainable Development Goals.2 International protocols recommend the implementation of risk assessment for cardiovascular disease and management programs as integral components of strategies to achieve this target.3–6 Although different in scale and settings, many countries have launched risk assessment programs, including the Million Hearts initiative in the United States and a program of “more heart and diabetes checks” in New Zealand.4–6 In Canada, the recommendation to deliver cardiovascular screening, education and follow-up programs in a variety of community settings was published in the Canadian Heart Health Strategy and Action Plan in 2009.7 The action plan aimed to improve heart health in the Canadian population, although its funding was never secured.7,8 However, the recently launched Choosing Wisely Canada campaign, which aims to promote the cost-effective use of medicines, has challenged the usefulness of annual health examinations for asymptomatic adults who have no apparent risk factors.9
The Health Check program of the National Health Service in England is the largest and most ambitious risk assessment and management program for cardiovascular disease worldwide. Since its inception in 2009, the program has offered, to all adults aged 40–74 years with no known vascular disease, a risk assessment every 5 years, with tailored management strategies, including lifestyle advice. Introduction of the Health Check program has been controversial, and its appropriateness and benefits have been continually challenged.10–13 Previous assessments have been limited to evaluations of local programs, have had only short follow-up and have not taken underlying trends in cardiovascular risk into account, although some of these analyses have identified significant but modest reductions in modelled risk for cardiovascular disease and individual risk factors.14–16
Evaluation of cardiovascular risk assessment programs delivered in routine care settings internationally are sparse. The impact of the Health Check program, which is being delivered in the context of a universal health system with well-developed primary care and high penetration of electronic medical records, has international significance. The objectives of this study were to assess the impact of the Health Check program on changes in modelled risk for cardiovascular disease, individual risk factors for cardiovascular disease, prescribing of relevant medications and identification of new diagnoses of vascular disease.
Methods
Data source
We used data from the Clinical Practice Research Datalink, one of the largest electronic medical records databases in the world (www.cprd.com/).17 This database routinely collects longitudinal and anonymized primary care data from general practices in the United Kingdom, providing a nationally representative sample consisting of about 7% of the population.17–20 The data are subject to regular quality checks and are widely used for research studies.21 Data stored in the database include each patient’s demographic information and registration status, medical history and diagnosis, laboratory test results (e.g., cholesterol level), drug prescriptions and referrals to secondary care. Ethics approval for the study protocol was obtained from the Independent Scientific Advisory Committee of the Clinical Practice Research Datalink (protocol number: 12_039).
Study population
We extracted data for a computer-selected random sample of 194 248 English residents aged 40–74 years who were registered with a practice that was participating in the Clinical Practice Research Datalink during the first 4 years of the Health Check program (Apr. 1, 2009, to Mar. 31, 2013), which we defined as the intervention period. According to the Health Check eligibility criteria, we excluded patients with a previous diagnosis of vascular disease (atrial fibrillation, chronic kidney disease, coronary artery disease, hypercholesterolemia, heart failure, hypertension, peripheral vascular disease, stroke, transient ischemic attack and diabetes mellitus).
Patients were categorized into 2 groups: Health Check attendees and nonattendees. Because of a delay in publicizing a universal code for recording Health Check attendance in general practice, we identified attendees following the best practice guidance of the Health Check program.22 We defined attendance as the recording of 4 risk factors (blood pressure, body mass index [BMI], cholesterol and smoking status) within a 6-month period when a patient was continuously eligible for the program and a Health Check attendance date as the day when the last of the 4 risk factors was recorded.23,24 Our method of identifying Health Check attendance has achieved good validity, as documented previously.24
Outcome measures
Our study outcomes were the modelled risk score for cardiovascular disease as computed by the QRISK2 algorithm, which is recommended by the National Institute for Health and Care Excellence (because the Framingham algorithm overestimates cardiovascular risk in the UK population), systolic blood pressure, diastolic blood pressure, BMI, total cholesterol, prevalence of smoking, prescribing of statin and antihypertensive medications, and diagnosis of the following vascular diseases: atrial fibrillation, chronic kidney disease, coronary artery disease (including myocardial infarction), familial hypercholesterolemia, heart failure, hypertension, peripheral vascular disease, stroke, transient ischemic attack and type 2 diabetes mellitus.25,26
We generated cardiovascular risk scores using a licensed QRISK2 batch processor (.NET version 2014.0, ClinRisk Ltd.), which computes risk scores from the following data: systolic blood pressure, BMI, ratio of total to high-density-lipoprotein (HDL) cholesterol, smoking status and pre-existing vascular diseases (atrial fibrillation, chronic kidney disease, hypertension and diabetes), none of which apply to patients who are eligible for the Health Check program; and other risk factors, including the patient’s age, sex, ethnicity, family history of premature coronary artery disease, and the Townsend index value (explained below) or postcode.25 Patient age was held constant (from the year 2009) throughout the intervention period, to isolate the effect of aging on risk scores.16 We assigned ethnicity as “missing” if it was not recorded (which occurred for 32.8% of the study population), we assigned a smoking status of “nonsmoker” if there was no indication of the patient being a smoker in the past,27 and we assumed no family history of premature coronary artery disease if such a history had never been recorded.
The Townsend index is a composite small-area measure of deprivation for England, which is based on the 2001 census data reported for unemployment, household overcrowding, and non-ownership of a house or a car.28 The Clinical Practice Research Datalink mapped an individual’s postcode to the area’s Townsend score but supplied only quintiles of the Townsend deprivation index values, where the quintile with the lowest index values represented the least-deprived neighbourhoods and the quintile with the highest index values represented the most-deprived neighbourhoods. Therefore, we assigned the median Townsend index value for each quintile using data obtained nationally for QRISK2 calculations.29 For patients with missing Townsend data, the QRISK2 batch processor assigned a score of 0.
Statistical analysis
We used a difference-in-differences matching model, as first proposed by Heckman and associates, 30 which is commonly employed in health services research and policy evaluations.31–36 The model begins with propensity score matching, which ensures that the observed characteristics of the intervention and matched control group are comparable, consequently eliminating as much observed heterogeneity as possible.37 Because the matched control group provides a counterfactual for the intervention group had there been no intervention, the difference-in-differences part of the model removes unobserved heterogeneity that was fixed over time or that followed parallel time trends between groups, thus providing a robust estimator.30,34,35,38
Our statistical model required a comparison of individual-level data between baseline and follow-up periods in relation to an intervention date. We defined the intervention date as the Health Check attendance date, as described above, for attendees and as the midpoint of the eligibility period (defined by the age and registration status of individual patients) for nonattendees. Exploiting the longitudinal nature of the Clinical Practice Research Datalink, we obtained each individual’s baseline data from the latest measurements taken on or within 5 years before the intervention date and follow-up data from the latest measurements taken after the intervention date but before the end of the study (on Mar. 31, 2013). For prescribing of medications, we considered a patient to be taking a medication if there were prescriptions recorded within 12 months before or after the intervention date for baseline and follow-up data, respectively. Participants in our study had no diagnosis at baseline because of the Health Check eligibility criteria, and we considered any diagnosis within 3 months after the intervention date to be associated with the intervention.23
Missing data are a common problem in routine health care data.39 Failure to consider missing risk factor data in our dataset could have resulted in analysis of a highly selected subset of the study population, which would have wasted valuable observed risk factor data for most patients.40,41 However, we have included findings from complete case analyses for comparison.
We therefore used multiple imputation by chained equations to estimate missing data for blood pressure, BMI and log-transformed (for skewed distribution) total and HDL cholesterol at both baseline and follow-up. We included in the imputation model all input variables for the QRISK2 algorithm, data on English geographic region and an indicator for Health Check attendance. We generated 10 imputed datasets and ran the QRISK2 algorithm on each one to compute cardiovascular risk scores.
We calculated the means (or proportions) of the outcomes before and after the intervention for both Health Check attendees and nonattendees. We compared the changes in outcome from baseline to follow-up using paired t tests, and we assessed the difference in changes between attendees and nonattendees using t tests (unadjusted difference-indifferences). We then ran the difference-indifferences matching model for every outcome on each imputed dataset and combined the point estimates and standard errors using the Rubin rule, to produce the adjusted difference-in-differences estimator.42
All data management and statistical analyses were conducted in STATA SE software, version 12.1. We used psmatch2 in the STATA software, with specification of kernel matching with appropriate bandwidth (i.e., between 0.05 and 0.1), and allowed matching to build on the following variables: patient’s age, sex, ethnicity (white, nonwhite or missing), quintile of the Index of Multiple Deprivation (2010) mapped to practice postcode and English region. We also ran models that included clinical risk factor levels at baseline (systolic blood pressure, BMI, total cholesterol and smoking status) in the matching process, to assess the robustness of the results. The Index of Multiple Deprivation is a composite score of socioeconomic status similar to the Townsend deprivation index, but it was assigned to each “Lower Layer Super Output Area” (geographic area containing about 650 households) in England, on the basis of 7 principal domains of deprivation (income, employment, health deprivation and disability, education skills and training, barriers to housing and services, crime, living environment).43 We did not use individual-level Townsend data, as these were missing for 21.1% of the sample.
To evaluate the levels of impact by population subgroups, we stratified the analyses by pre-intervention modelled cardiovascular risk categories (< 10%, 10%–20% or ≥ 20%) or by whether the following risk factors were above recommended levels: systolic blood pressure (≥ 140 mm Hg), diastolic blood pressure (≥ 90 mm Hg), BMI (≥ 30) and total cholesterol (≥ 5 mmol/L).
Results
We identified a cohort of 138 788 patients from 462 practices who were eligible for a Health Check between Apr. 1, 2009, and Mar. 31, 2013, for inclusion in our analyses, after excluding 55 460 patients with a previous diagnosis of vascular disease (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151201/-/DC1). Of the eligible patients, 21.4% (29 672) attended a Health Check during the intervention period (median follow-up 2 yr).
Table 1 compares the demographic characteristics of attendees and nonattendees before and after propensity score matching. Compared with nonattendees before matching, Health Check attendees were older (mean age 53.5 v. 50.1), more likely to be women (52.6% v. 50.0%) and more likely to be from a white ethnic group (71.9% v. 54.8%) (all p < 0.001). Differences between attendees and nonattendees were nonsignificant (p > 0.05) after matching, which indicates that the propensity score method substantially eliminated between-group differences in observed characteristics.
Table 1:
Demographic characteristics of National Health Service Health Check attendees and nonattendees before and after matching (n = 138 788)
| Characteristic | Group; % of group before matching* | Group; % of group after matching* | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Attendees n = 29 672 |
Nonattendees n = 109 116 |
p value | Attendees n = 29 672 |
Nonattendees n = 29 672† |
p value | |
| Age, yr, mean | 53.5 | 50.1 | < 0.001 | 53.5 | 53.4 | 0.2 |
|
| ||||||
| Sex, female | 52.6 | 50.0 | < 0.001 | 52.6 | 52.6 | 0.9 |
|
| ||||||
| Ethnicity | ||||||
|
| ||||||
| White | 71.9 | 54.8 | < 0.001 | 71.9 | 71.4 | 0.2 |
|
| ||||||
| Nonwhite | 7.7 | 5.0 | < 0.001 | 7.7 | 7.6 | 0.6 |
|
| ||||||
| Missing | 20.2 | 40.1 | < 0.001 | 20.2 | 20.9 | 0.054 |
|
| ||||||
| IMD | ||||||
|
| ||||||
| Quintile 1 (least deprived) | 18.0 | 19.4 | < 0.001 | 18.0 | 18.1 | 0.7 |
|
| ||||||
| Quintile 2 | 21.7 | 21.9 | 0.5 | 21.7 | 21.9 | 0.5 |
|
| ||||||
| Quintile 3 | 19.7 | 22.4 | < 0.001 | 19.7 | 20.0 | 0.3 |
|
| ||||||
| Quintile 4 | 21.1 | 20.4 | 0.004 | 21.1 | 20.9 | 0.5 |
|
| ||||||
| Quintile 5 (most deprived) | 19.3 | 15.7 | < 0.001 | 19.3 | 18.7 | 0.1 |
|
| ||||||
| Region | ||||||
|
| ||||||
| North East | 2.8 | 2.0 | < 0.001 | 2.8 | 2.5 | 0.04 |
|
| ||||||
| North West | 18.4 | 13.8 | < 0.001 | 18.4 | 17.9 | 0.08 |
|
| ||||||
| Yorkshire and Humber | 2.7 | 3.4 | < 0.001 | 2.7 | 2.7 | 0.9 |
|
| ||||||
| East Midlands | 2.8 | 4.7 | < 0.001 | 2.8 | 2.8 | 0.9 |
|
| ||||||
| West Midlands | 11.4 | 10.1 | < 0.001 | 11.4 | 11.4 | 0.7 |
|
| ||||||
| East of England | 10.0 | 10.9 | < 0.001 | 10.0 | 10.2 | 0.3 |
|
| ||||||
| South West | 9.0 | 11.6 | < 0.001 | 9.0 | 9.4 | 0.1 |
|
| ||||||
| South Central | 14.1 | 15.4 | < 0.001 | 14.1 | 14.3 | 0.5 |
|
| ||||||
| London | 16.9 | 13.6 | < 0.001 | 16.9 | 16.5 | 0.2 |
|
| ||||||
| South East Coast | 11.3 | 14.1 | < 0.001 | 11.3 | 11.8 | 0.054 |
Note: IMD = index of multiple deprivation (2010).
Unless indicated otherwise.
Resultant sample size once kernel weight from propensity score matching was applied.
Modelled risk for cardiovascular disease
Before the intervention, Health Check attendees had a higher mean QRISK2 score than nonattendees (6.7% v. 5.1%) (Table 2). Although both groups had absolute reductions in cardiovascular risk after the intervention (reduced to 6.2% and 4.9%, respectively), there was a small but significantly greater reduction among attendees after matching (−0.21%, 95% confidence interval [CI] −0.24% to −0.19%). This reduction is equivalent to one additional cardiovascular event being prevented every year for every 4762 (95% CI 4167 to 5263) people who attend a Health Check. Our robustness tests indicated that the results remained broadly similar when patients were additionally matched on the basis of 4 clinical risk factor levels at baseline (systolic blood pressure, BMI, total cholesterol and smoking status) (see Appendices 2 and 3, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151201/-/DC1).
Table 2:
Overall effect of the National Health Service Health Check program on modelled risk for cardiovascular disease, risk factors for cardiovascular disease and prescribing (n = 138 788)
| Risk factor and group | n | Timeframe; mean ± SD* | Difference, by paired t test (95% CI) | Crude DID (95% CI) | DID matching estimator (95% CI) | |
|---|---|---|---|---|---|---|
| Before intervention | After intervention | |||||
| QRISK2, % 10-yr risk | ||||||
| Attendees | 29 672 | 6.7 ± 5.9 | 6.2 ± 5.3 | −0.48 (−0.50 to −0.46) | −0.29 (−0.31 to −0.27) | −0.21 (−0.24 to −0.19) |
| Nonattendees | 109 116 | 5.1 ± 5.3 | 4.9 ± 5.0 | −0.19 (−0.19 to −0.18) | ||
| Systolic BP, mm Hg | ||||||
| Attendees | 29 672 | 131.9 ± 17.4 | 130.0 ± 12.7 | −1.92 (−2.09 to −1.75) | −2.72 (−2.88 to −2.56) | −2.51 (−2.77 to −2.25) |
| Nonattendees | 109 116 | 128.5 ± 13.6 | 129.3 ± 11.3 | 0.79 (0.73 to 0.86) | ||
| Diastolic BP, mm Hg | ||||||
| Attendees | 29 672 | 80.2 ± 10.5 | 78.5 ± 7.7 | −1.71 (−1.82 to −1.60) | −1.74 (−1.84 to −1.64) | −1.46 (−1.62 to −1.29) |
| Nonattendees | 109 116 | 78.7 ± 8.2 | 78.7 ± 6.7 | 0.02 (−0.01 to 0.07) | ||
| Body mass index | ||||||
| Attendees | 29 672 | 27.7 ± 5.1 | 27.7 ± 5.0 | 0.01 (−0.003 to 0.02) | −0.28 (−0.30 to −0.27) | −0.27 (−0.34 to −0.20) |
| Nonattendees | 109 116 | 26.9 ± 4.1 | 27.2 ± 4.0 | 0.30 (0.29 to 0.30) | ||
| Total cholesterol, mmol/L | ||||||
| Attendees | 29 672 | 5.5 ± 1.0 | 5.3 ± 0.8 | −0.21 (−0.22 to −0.20) | −0.20 (−0.20 to −0.19) | −0.15 (−0.18 to −0.13) |
| Nonattendees | 109 116 | 5.3 ± 0.6 | 5.3 ± 0.6 | −0.01 (−0.01 to −0.01) | ||
| Smoking prevalence, % of group | ||||||
| Attendees | 29 672 | 17.9 | 16.3 | −1.60 (−1.80 to −1.39) | −0.22 (−0.46 to 0.01) | −0.11 (−0.35 to 0.13) |
| Nonattendees | 109 116 | 22.2 | 20.8 | −1.37 (−1.48 to −1.26) | ||
| Statin prescribed, % of group | ||||||
| Attendees | 29 672 | 9.7 | 15.3 | 5.60 (5.29 to 5.90) | 4.40 (4.17 to 4.62) | 3.83 (3.52 to 4.14) |
| Nonattendees | 109 116 | 3.1 | 4.3 | 1.20 (1.11 to 1.28) | ||
| Antihypertensive prescribed, % of group | ||||||
| Attendees | 29 672 | 4.8 | 9.9 | 5.05 (4.76 to 5.33) | 2.45 (2.20 to 2.71) | 1.37 (1.08 to 1.66) |
| Nonattendees | 109 116 | 1.8 | 4.4 | 2.59 (2.48 to 2.70) | ||
Note: BP = blood pressure, CI = confidence interval, crude DID = difference-in-differences without matching, DID = difference-in-differences, QRISK2 = algorithm to calculate cardiovascular risk level, SD = standard deviation.
Unless indicated otherwise.
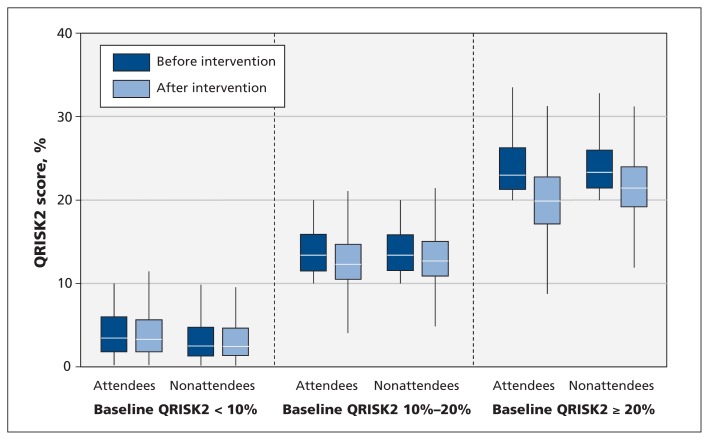
After stratification by pre-intervention cardiovascular risk categories, the absolute risk reduction for attendees with a baseline risk of 20% or higher (−0.54%, 95% CI −0.93% to −0.15%) was not significantly greater than for those with lower risk (10%−20% risk: −0.34%, 95% CI −0.44% to −0.24%; < 10% risk: −0.14%, 95% CI −0.16% to −0.12%) (Figure 1; Appendix 4, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151201/-/DC1).
Figure 1:
Baseline and post-intervention QRISK2 scores for Health Check attendees and nonattendees, stratified by pre-intervention cardiovascular risk.
Individual risk factors for cardiovascular disease
Relative to nonattendees, Health Check attendees had higher pre-intervention mean systolic blood pressure (131.9 v. 128.5 mm Hg), higher mean diastolic blood pressure (80.2 v. 78.7 mm Hg), higher mean BMI (27.7 v. 26.9) and slightly higher mean total cholesterol (5.5 v. 5.3 mmol/L), but a lower prevalence of current smoking (17.9% v. 22.2%) (Table 2). After the intervention, Health Check attendees had absolute reductions in systolic and diastolic blood pressure, total cholesterol and smoking prevalence, but not in BMI. After matching, there were significant reductions in the following individual risk factors among Health Check attendees: systolic blood pressure (−2.51 mm Hg, 95% CI −2.77 to −2.25 mm Hg), diastolic blood pressure (−1.46 mm Hg, 95% CI −1.62 to −1.29 mm Hg), BMI (−0.27, 95% CI −0.34 to −0.20) and total cholesterol (−0.15 mmol/L, 95% CI −0.18 to −0.13 mmol/L).
Reductions in diastolic blood pressure, BMI and total cholesterol were similar among Health Check attendees, irrespective of modelled cardiovascular risk levels at baseline (Appendix 4). Reductions in systolic blood pressure were significantly greater among Health Check attendees with higher modelled cardiovascular risk at baseline: the reductions were −4.54 mm Hg (95% CI −6.04 to −3.03 mm Hg) for individuals with 20% risk or higher at baseline, −3.16 mm Hg (95% CI −3.84 to −2.47 mm Hg) for those with 10%−20% risk at baseline, and −2.16 mm Hg (95% CI −2.46 to −1.86 mm Hg) for those with less than 10% risk at baseline.
Table 3 presents the impact of Health Check attendance on blood pressure, BMI and total cholesterol stratified by individual risk factor levels at baseline. Health Check attendance was associated with significant decreases in all 3 risk factors after matching, with those who had elevated risk levels at baseline experiencing the greatest reduction, except for BMI. For example, Health Check attendees with elevated blood pressure at baseline experienced a greater reduction in systolic blood pressure than those with normal blood pressure at baseline (−3.22 mm Hg [95% CI −3.63 to −2.81 mm Hg] v. −1.20 mm Hg [95% CI −1.51 to −0.89 mm Hg]).
Table 3:
Impact of the National Health Service Health Check program, stratified by individual risk levels before the intervention (n = 138 788)
| Outcome and pre-intervention risk category | n | Timeframe; mean ± SD | Difference, by paired t test (95% CI) | Crude DID (95% CI) | DID matching estimator (95% CI) | |
|---|---|---|---|---|---|---|
| Before intervention | After intervention | |||||
| Systolic BP, mm Hg | ||||||
| BP < 140/90 mm Hg | ||||||
| Attendees | 19 028 | 122.2 ± 10.6 | 125.7 ± 10.9 | 3.58 (3.43 to 3.74) | 0.35 (0.19 to 0.51) | −1.20 (−1.51 to −0.89) |
| Nonattendees | 84 455 | 123.5 ± 10.0 | 126.7 ± 9.8 | 3.23 (3.16 to 3.30) | ||
| BP ≥ 140/90 mm Hg | ||||||
| Attendees | 10 644 | 149.3 ± 13.3 | 137.5 ± 12.2 | −11.7 (−12.0 to −11.4) | −4.23 (−4.54 to −3.92) | −3.22 (−3.63 to −2.81) |
| Nonattendees | 24 661 | 145.5 ± 10.3 | 137.9 ± 11.5 | −7.54 (−7.70 to −7.38) | ||
| Diastolic BP, mm Hg | ||||||
| BP < 140/90 mm Hg | ||||||
| Attendees | 19 028 | 75.5 ± 7.6 | 76.6 ± 6.8 | 1.12 (1.01 to 1.23) | −0.13 (−0.24 to −0.02) | −0.65 (−0.85 to −0.44) |
| Nonattendees | 84 455 | 76.5 ± 6.8 | 77.8 ± 6.1 | 1.26 (1.21 to 1.30) | ||
| BP ≥ 140/90 mm Hg | ||||||
| Attendees | 10 644 | 88.6 ± 9.5 | 81.8 ± 8.0 | −6.79 (−6.98 to −6.60) | −2.59 (−2.80 to −2.39) | −2.05 (−2.32 to −1.78) |
| Nonattendees | 24 661 | 86.2 ± 8.2 | 82.0 ± 7.3 | −4.20 (−4.30 to −4.09) | ||
| Body mass index (BMI) | ||||||
| BMI < 30 | ||||||
| Attendees | 21 238 | 25.1 ± 2.8 | 25.3 ± 2.9 | 0.18 (0.17 to 0.20) | −0.17 (−0.18 to −0.15) | −0.23 (−0.30 to −0.16) |
| Nonattendees | 91 282 | 25.6 ± 2.6 | 26.0 ± 2.7 | 0.36 (0.35 to 0.36) | ||
| BMI ≥ 30 | ||||||
| Attendees | 8 434 | 34.2 ± 3.9 | 33.8 ± 4.0 | −0.42 (−0.46 to −0.38) | −0.42 (−0.47 to −0.37) | −0.30 (−0.39 to −0.21) |
| Nonattendees | 17 834 | 33.5 ± 3.7 | 33.5 ± 3.9 | −0.003 (−0.02 to 0.02) | ||
| Total cholesterol, mmol/L | ||||||
| Total cholesterol < 5 mmol/L | ||||||
| Attendees | 8 979 | 4.3 ± 0.4 | 4.6 ± 0.5 | 0.29 (0.28 to 0.30) | 0.05 (0.04 to 0.06) | −0.08 (−0.10 to −0.05) |
| Nonattendees | 24 928 | 4.6 ± 0.4 | 4.8 ± 0.5 | 0.24 (0.23 to 0.24) | ||
| Total cholesterol ≥ 5 mmol/L | ||||||
| Attendees | 20 693 | 6.0 ± 0.7 | 5.6 ± 0.7 | −0.44 (−0.45 to −0.43) | −0.34 (−0.35 to −0.33) | −0.13 (−0.15 to −0.11) |
| Nonattendees | 84 188 | 5.6 ± 0.5 | 5.5 ± 0.5 | −0.09 (−0.09 to −0.08) | ||
Note: BP = blood pressure, CI = confidence interval, Crude DID = difference-in-differences without matching, DID = difference-in-differences, SD = standard deviation.
Prescribing of statins and antihypertensive medications
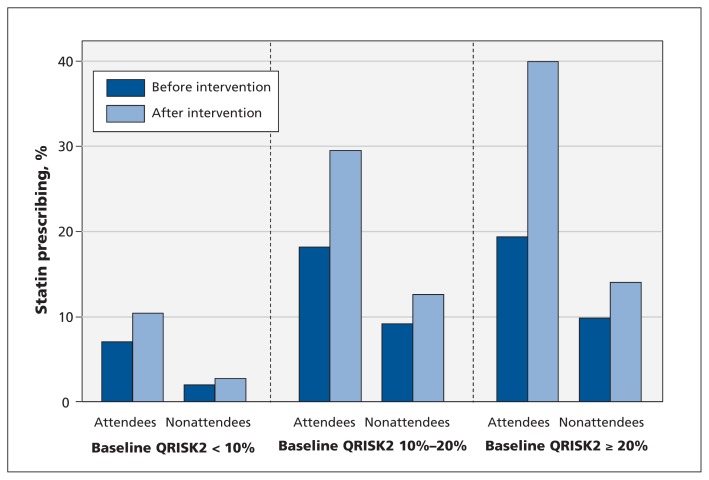
Before the intervention, Health Check attendees were more likely than nonattendees to receive a prescription for a statin (9.7% v. 3.1%) or an antihypertensive medication (4.8% v. 1.8%) (Table 2). After matching, Health Check attendance was associated with significantly greater absolute increases in prescribing of statins (+3.83%, 95% CI +3.52% to +4.14%) and antihypertensive medications (+1.37%, 95% CI +1.08% to +1.66%) (Table 2). Statin prescribing increased significantly among Health Check attendees, irrespective of modelled cardiovascular risk at baseline (Figure 2, Appendix 4). However, the increases were greatest among Health Check attendees with cardiovascular risk 20% or higher at baseline (+15.2%, 95% CI +12.2% to +18.1%), followed by those with 10%–20% risk (+7.22%, 95% CI +6.20% to +8.24%) and those at less than 10% risk (+2.23%, 95% CI +1.94% to +2.52%). The level of prescribing for Health Check attendees remained low after the intervention, with prescribing of statins and antihypertensive medications for only 39.9% and 23.4%, respectively, of attendees with cardiovascular risk of 20% or higher at baseline.
Figure 2:
Baseline and post-intervention prescribing of statins for Health Check attendees and nonattendees, stratified by pre-intervention cardiovascular risk.
Diagnosis of vascular disease
During the study period, a vascular disease was diagnosed in 6.4% (1 894/29 672) of the Health Check attendees and 1.3% (1 465/109 116) of the nonattendees. Table 4 shows that, after matching, the following diseases were diagnosed significantly more frequently among Health Check attendees: 0.17% (95% CI 0.11% to 0.23%) for chronic kidney disease, 0.09% (95% CI 0.07% to 0.11%) for familial hypercholesterolemia, 2.99% (95% CI 2.77% to 3.21%) for hypertension, 0.03% (95% CI 0.01% to 0.05%) for peripheral vascular disease and 1.31% (95% CI 1.17% to 1.45%) for type 2 diabetes mellitus (where the values shown are matching estimators of the differences between attendees and nonattendees). There was no significant increase in diagnosis of atrial fibrillation, coronary artery disease, heart failure or transient ischemic attack.
Table 4:
Overall impact of the National Health Service Health Check program on diagnosis of vascular disease (n = 138 788)
| Diagnosis | Group; % with diagnosis after intervention | Crude difference, % (95% CI) | Matching estimator, % (95% CI) | |
|---|---|---|---|---|
| Attendees n = 29 672 |
Nonattendees n = 109 116 |
|||
| Atrial fibrillation | 0.10 | 0.04 | 0.05 (0.02 to 0.08) | 0.02 (−0.02 to 0.06) |
| Chronic kidney disease | 0.34 | 0.11 | 0.23 (0.18 to 0.28) | 0.17 (0.11 to 0.23) |
| Coronary artery disease | 0.24 | 0.13 | 0.10 (0.05 to 0.15) | 0.02 (−0.04 to 0.08) |
| Familial hypercholesterolemia | 0.10 | 0.006 | 0.10 (0.07 to 0.12) | 0.09 (0.07 to 0.11) |
| Heart failure | 0.03 | 0.01 | 0.02 (0.005 to 0.04) | 0.01 (−0.01 to 0.03) |
| Hypertension | 4.08 | 0.76 | 3.31 (3.15 to 3.46) | 2.99 (2.77 to 3.21) |
| Peripheral vascular disease | 0.07 | 0.02 | 0.04 (0.02 to 0.06) | 0.03 (0.01 to 0.05) |
| Stroke | 0.04 | 0.04 | −0.002 (−0.03 to 0.02) | −0.03 (−0.05 to −0.01) |
| Transient ischemic attack | 0.05 | 0.03 | 0.02 (−0.0004 to 0.04) | 0.008 (−0.01 to 0.03) |
| Type 2 diabetes mellitus | 1.62 | 0.22 | 1.40 (1.30 to 1.49) | 1.31 (1.17 to 1.45) |
Note: CI = confidence interval.
Comparison with complete case analysis
In the complete case analysis, Health Check attendees did not experience a significant reduction in modelled cardiovascular risk after matching (Appendices 5 and 6, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151201/-/DC1). Reductions in individual risk factor levels among Health Check attendees were broadly comparable in the complete case and main (imputed) analyses (Appendices 5, 7 and 8, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.151201/-/DC1).
Interpretation
In this national evaluation based on routine primary care data, we found that attendance of the Health Check program was associated with statistically significant but clinically modest overall reductions in modelled cardiovascular risk and individual risk factors (except for smoking prevalence). Reductions in modelled cardiovascular risk, diastolic blood pressure, BMI and total cholesterol were similar for all Health Check attendees, irrespective of modelled cardiovascular risk levels at baseline. Levels of medication prescribing remained suboptimal for Health Check attendees at high cardiovascular risk. The program resulted in significantly more diagnoses of selected vascular diseases among attendees, with the largest increases for hypertension and type 2 diabetes.
Evaluation of cardiovascular risk assessment and management programs in routine care settings is limited. Si and colleagues44 performed a systematic review and meta-analysis of the effectiveness of general health checks on surrogate outcomes (systolic and diastolic blood pressure, BMI and total cholesterol) using several randomized controlled trials, including the Oxford and Collaborators Health CHECK Trial (OXCHECK) and EUROACTION studies, the British Family Heart Study and a trial from Denmark. The meta-analysis showed that practice-based health checks were associated with significant and beneficial effects favouring the intervention group, and the result was consistent across all studies, but the magnitude of changes in surrogate outcomes remained uncertain because of the limited number of studies available.44 Although the design and content (e.g., invitation methods, age of the population, duration and method of follow-up) of those randomized controlled trials are not directly comparable to characteristics of the Health Check program, our findings are consistent with those reported in the meta-analysis,44 with significantly greater reductions in blood pressure, BMI and total cholesterol being observed among Health Check attendees. Our findings are also consistent with 2 previous evaluations of local Health Check programs in England, which showed significant reductions in modelled cardiovascular risk and individual risk factors (except for BMI) at 1 year after the intervention among those who attended a Health Check.15,16
Our findings raise a question about the potential contribution of risk assessment and management programs to achieving international targets for cardiovascular mortality reduction, especially in low-resource settings and in countries where similar programs have not been deployed. The performance of the Health Check program has fallen well short of national and international performance targets for cardiovascular risk assessment programs. This outcome may be due to several factors, including poor initial planning of the program and inadequate engagement of health care professionals and the public about potential program benefits.45,46 A recent study reported that one-third of nonattendees did not receive invitations, while other nonattendees suggested that they lacked information about and understanding of the program.46 Inflexible appointment times and venues were also identified as barriers to accessing the program.45,46
In a previous study, we found important variations in program performance, including variations in coverage between geographic areas and lower coverage among younger persons and those from a black African or Chinese ethnic background.24 Early modelling of the Health Check program undertaken by the English Department of Health indicated that the program would need to achieve 75% coverage, with 85% of high-risk attendees receiving statins, to be cost-effective.47 At the international level, the World Health Organization has set a target for risk assessment programs of 50% of individuals with high risk for cardiovascular disease receiving drug therapy.1 The Health Check program failed to meet all of these targets, with only 21.4% coverage, and only 39.9% of high-risk patients receiving statins. These findings are concerning, given that the program is being delivered in the context of a universal health care system with well-developed primary care and high penetration of electronic medical records.
Strengths and limitations
The Health Check program has been criticized because it has not been subjected to a randomized controlled trial.48 However, public health agencies in England opted to roll out the program nationally and have emphasized the value of observational studies for policy evaluation.49 We employed a robust quasi-experimental study design to evaluate the Health Check program, an approach that permits causal inference of the program’s impacts.38 Although we cannot rule out completely the possibility of bias, we used robust matching, which ensured that the baseline outcomes were similar between groups. This method should reduce bias, including regression to the mean, adequately. Use of alternative experimental designs, such as interrupted time series, was not feasible because of incompleteness of the risk factor data (such data would be required to generate time trends in our outcome measures).
The study’s limitations included missing risk factor data, which we addressed by means of multiple imputation. This approach is robust when data are missing at random, and we justified using this method by including all variables that might be predictive of the missing risk factors in our dataset.41,50 Nonetheless, the results for changes in individual risk factors were broadly similar to those from the complete case analysis. This study was also limited by poor initial coding of Health Check attendance in general practice information systems, because of the delay in publicizing a universal code. Although this problem may have resulted in some misclassification of Health Check attendance in our sample, our definition of attendance has been previously validated.24
Conclusion
Our results highlight the need for careful monitoring and evaluation of risk assessment programs for cardiovascular disease internationally. They also emphasize the need for high-quality research to identify effective strategies to improve program performance.
Acknowledgements
Imperial College London is grateful for support from the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre and the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) Northwest London and the NIHR CLAHRC East Midlands.
Footnotes
Competing interests: Michael Soljak is a member of the Health Check National Expert Scientific and Clinical Advisory Panel. Kamlesh Khunti has served as a consultant and speaker for AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Janssen and Boehringer Ingelheim. He has received grants in support of investigator and investigator-initiated trials from AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim, Merck Sharp & Dohme, and Roche. He has served on advisory boards for AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Janssen and Boehringer Ingelheim. He chaired the NICE (National Institute for Health and Care Excellence) guidance on identification and prevention for people at high risk of diabetes and is an advisor to the National Health Service Health Check program. Azeem Majeed is a principal in a general practice that participates in the Health Check program. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Michael Soljak, Azeem Majeed and Christopher Millett conceived the study. Kiara Chang, John Lee and Christopher Millett designed the study. Kiara Chang analyzed the data, and John Lee provided advice and reviewed the statistical analysis. All of the authors discussed the data analyses and interpreted the results. Kiara Chang, John Lee, Azeem Majeed and Christopher Millett wrote the first draft of the manuscript. All of the authors critically revised the manuscript, approved the final manuscript for publication and agreed to act as guarantors of the work. Kiara Chang has full access to all the data used in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This study was an independent research study commissioned and funded by the UK Department of Health Policy Research Programme (National Evaluation of the National Health Service Health Check program 009/0051). The views expressed in this article are those of the authors and not necessarily those of the UK Department of Health.
References
- 1.Global status report on noncommunicable diseases 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.Transforming our world: the 2030 Agenda for Sustainable Development. New York: United Nations, Division for Sustainable Development; 2015. Available: https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed 2015 Sept. 7). [Google Scholar]
- 3.Package of Essential Noncommunicable (PEN) disease interventions for primary health care in low-resource settings. Geneva: World Health Organization; 2010. [Google Scholar]
- 4.Health targets: more heart and diabetes checks. Wellington (NZ): Government of New Zealand, Ministry of Health; 2014. Available: www.health.govt.nz/new-zealand-health-system/health-targets/about-health-targets/health-targets-more-heart-and-diabetes-checks (accessed 2015 Mar. 24). [Google Scholar]
- 5.Frieden TR, Berwick DM. The “Million Hearts” initiative— preventing heart attacks and strokes. N Engl J Med 2011; 365:e27. [DOI] [PubMed] [Google Scholar]
- 6.Cardiovascular diseases [fact sheet]. New Delhi (India): Office of the WHO Representative to India; Available: www.searo.who.int/india/topics/cardiovascular_diseases/CVD_fact_sheet.pdf?ua=1 (accessed 2015 July 16). [Google Scholar]
- 7.Smith ECHHS-AP Steering Committee. Canadian Heart Health Strategy and Action Plan: building a heart healthy Canada. Ottawa: Canadian Heart Health Strategy and Action Plan; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith ER. The Canadian Heart Health Strategy and Action Plan. Can J Cardiol 2009;25:451–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponka D. The periodic health examination in adults. CMAJ 2014;186:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gøtzsche PC, Jørgensen KJ, Krogsbøll LT. Authors’ reply to Lauritzen and colleagues, Newton and colleagues, and Mangin. BMJ 2014;349:g4790. [DOI] [PubMed] [Google Scholar]
- 11.Newton JN, Davis A, Waterall J, et al. NHS Health Check programme: too early to conclude. BMJ 2014;349:g4785. [DOI] [PubMed] [Google Scholar]
- 12.Gøtzsche PC, Jørgensen KJ, Krogsbøll LT. General health checks don’t work. BMJ 2014;348:g3680. [DOI] [PubMed] [Google Scholar]
- 13.Lauritzen T, Sandbaek A, Borch-Johnsen K. General health checks may work. BMJ 2014;349:g4697. [DOI] [PubMed] [Google Scholar]
- 14.Forster AS, Dodhia H, Booth H, et al. Estimating the yield of NHS Health Checks in England: a population-based cohort study. J Public Health (Oxf) 2015;37:234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochrane T, Davey R, Iqbal Z, et al. NHS health checks through general practice: randomised trial of population cardiovascular risk reduction. BMC Public Health 2012;12:944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artac M, Dalton AR, Majeed A, et al. Effectiveness of a national cardiovascular disease risk assessment program (NHS Health Check): results after one year. Prev Med 2013;57:129–34. [DOI] [PubMed] [Google Scholar]
- 17.Clinical Practice Research Datalink. London (UK): The Clinical Practice Research Datalink Group; 2015. Available: www.cprd.com/intro.asp (accessed 2015 Aug. 20). [Google Scholar]
- 18.Lawrenson R, Williams T, Farmer R. Clinical information for research; the use of general practice databases. J Public Health Med 1999;21:299–304. [DOI] [PubMed] [Google Scholar]
- 19.van Staa TP, Smeeth L, Ng ES, et al. The efficiency of cardiovascular risk assessment: Do the right patients get statin treatment? Heart 2013;99:1597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrett E, Thomas SL, Schoonen WM, et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Putting prevention first. NHS Health Check: vascular risk assessment and management. Best practice guidance. London: National Health Service, Department of Health; 2009. [Google Scholar]
- 23.Daykin A, White H. Vascular health check: business rules paper. Version 1.0. In: NHS Health Check secondary use data set, NHS Health Check data set – documentation. West Yorkshire (UK): Health and Social Care Information Centre; 2015. Available: www.hscic.gov.uk/nhshealthcheck (accessed 2015 Aug. 20). [Google Scholar]
- 24.Chang KCM, Soljak M, Lee JT, et al. Coverage of a national cardiovascular risk assessment and management programme (NHS Health Check): retrospective database study. Prev Med 2015;78:1–8. [DOI] [PubMed] [Google Scholar]
- 25.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipid modification: cardiovascular risk assessment and modification of blood lipids for the primary and secondary prevention of cardiovascular disease. London (UK): National Institute for Health and Care Excellence; 2014. [PubMed] [Google Scholar]
- 27.Lewis JD, Brensinger C. Agreement between GPRD smoking data: a survey of general practitioners and a population-based survey. Pharmacoepidemiol Drug Saf 2004;13:437–41. [DOI] [PubMed] [Google Scholar]
- 28.Townsend P, Phillimore P, Beattie A, eds. Health and deprivation. Inequality and the North. London (UK): Croom Helm; 1987. [Google Scholar]
- 29.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Performance of the QRISK cardiovascular risk prediction algorithm in an independent UK sample of patients from general practice: a validation study. Heart 2008;94:34–9. [DOI] [PubMed] [Google Scholar]
- 30.Heckman JJ, Ichimura H, Todd PE. Matching as an econometric evaluation estimator: evidence from evaluating a job training programme. Rev Econ Stud 1997;64:605–54. [Google Scholar]
- 31.Smith JA, Todd PE. Does matching overcome LaLonde’s critique of nonexperimental estimators? J Econom 2005;125:305–53. [Google Scholar]
- 32.Buscha F, Maurel A, Page L, et al. The effect of employment while in high school on educational attainment: a conditional difference-in-differences approach. Oxf Bull Econ Stat 2012;74:380–96. [Google Scholar]
- 33.Rawat R, Kadiyala S, McNamara PE. The impact of food assistance on weight gain and disease progression among HIV-infected individuals accessing AIDS care and treatment services in Uganda. BMC Public Health 2010;10:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng L, Liu H, Zhang Y, et al. The impact of health insurance on health outcomes and spending of the elderly: evidence from China’s new cooperative medical scheme. Health Econ 2015;24: 672–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imbens GM, Wooldridge JM. Recent developments in the econometrics of program evaluation. NBER Work Pap 14251. Cambridge (MA): National Bureau of Economic Research; 2008. [Google Scholar]
- 36.Kantarevic J, Kralj B. Link between pay for performance incentives and physician payment mechanisms: evidence from the diabetes management incentive in Ontario. Health Econ 2013;22:1417–39. [DOI] [PubMed] [Google Scholar]
- 37.Blundell R, Costa Dias M. Evaluation methods for non-experimental data. Fisc Stud 2000;21:427–68. [Google Scholar]
- 38.Jones AM, Rice N. Econometric evaluation of health policies. HEDG Work Pap 09/09. York (UK): University of York, Health Economics and Data Group; 2009. [Google Scholar]
- 39.Altman DG, Bland JM. Missing data. BMJ 2007;334:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen KJ, Donders ART, Harrell FE, et al. Missing covariate data in medical research: to impute is better than to ignore. J Clin Epidemiol 2010;63:721–7. [DOI] [PubMed] [Google Scholar]
- 41.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons; 2004. [Google Scholar]
- 43.Lad M. The English indices of deprivation 2010. London (UK): Department for Communities and Local Government (UK); 2011. Available: www.gov.uk/government/uploads/system/uploads/attachment_data/file/6871/1871208.pdf (accessed 2015 Aug. 20). [Google Scholar]
- 44.Si S, Moss JR, Sullivan TR, et al. Effectiveness of general practice-based health checks: a systematic review and metaanalysis. Br J Gen Pract 2014;64:e47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry C, Thurston M, Alford S, et al. The NHS health check programme in England: a qualitative study. Health Promot Int 2016;31:106–15. [DOI] [PubMed] [Google Scholar]
- 46.Ellis N, Gidlow C, Cowap L, et al. A qualitative investigation of non-response in NHS health checks. Arch Public Health 2015;73:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vascular Policy Team. Economic modelling for vascular checks. London (UK): Department of Health; 2008. [Google Scholar]
- 48.Capewell S, McCartney M, Holland W. NHS Health Checks— a naked emperor? [invited debate]. J Public Health (Oxf) 2015;37:187–92. [DOI] [PubMed] [Google Scholar]
- 49.Waterall J, Greaves F, Gresser C, et al. Response to Capewell et al. [invited debate]. J Public Health (Oxf) 2015;37:193–4. [DOI] [PubMed] [Google Scholar]
- 50.Coventry P, Lovell K, Dickens C, et al. Integrated primary care for patients with mental and physical multimorbidity: cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ 2015;350:h638. [DOI] [PMC free article] [PubMed] [Google Scholar]