Abstract
Purpose
Oral supplementation with omega 3 (ω-3) and/or 6 (ω-6) fatty acids (FAs) has been reported to alleviate the signs and symptoms of dry eye disease (DED), and to improve the expressibility and quality of meibum, in patients with meibomian gland dysfunction (MGD). We tested our hypothesis that these FA effects may reflect a direct FA action on human meibomian gland epithelial cells (HMGECs).
Methods
Immortalized (I) HMGECs were cultured with ω-3, ω-6 or both FAs together for up to 7 days in the presence or absence of serum. Following FA exposure, cells were analyzed for lipid expression, lysosome content and proliferative ability.
Results
Our research shows that ω-3 and ω-6 stimulate the accumulation of small neutral lipid-containing vesicles, but not lysosomes, in IHMGECs. This vesicular effect was associated with a 2.4- to 3.7-fold increase in the cellular content of triglycerides following ω-3 and ω-6 treatment, respectively. The combination of both FAs together also enhanced triglyceride levels. Of interest, culture of IHMGECs with ω-3 and azithromycin (AZM), a known inducer of IHMGEC differentiation, led to a significantly greater amount of total neutral lipids, relative to that found with AZM alone. Cellular exposure to the FAs did not alter the expression of free or esterified cholesterol, or phospholipids. Further, these FAs, alone or together, prevented the proliferation of IHMGECs in serum-free, but not serum-containing, media.
Conclusions
Our findings support our hypothesis and demonstrate that ω-3 and ω-6 can act directly on IHMGECs to influence the quality and quantity of intracellular lipids.
Keywords: Human, meibomian gland, epithelial cells, omega 3 and 6 fatty acids
Introduction
Throughout the world countless individuals suffer from tear film dysfunctions, which are collectively diagnosed as dry eye disease (DED).1,2 Indeed, DED afflicts over 40 million in the USA alone, and is one of the most frequent reasons for patient visits to eye care practitioners.1 DED is characterized by a vicious cycle of tear film hyperosmolarity and instability and corneal stress, leading to increased friction, inflammation, ocular surface damage and decreased visual acuity.1,2 The major cause of DED is obstructive meibomian gland dysfunction (MGD).3,4 MGD, in turn, is due to hyperkeratinization of the ductal epithelium and an increased viscosity (i.e. reduced quality) of meibum, resulting in lipid insufficiency, and a heightened evaporation and instability of the tear film.3,5
There is no global cure for either DED or MGD. However, investigators have recently reported that oral supplementation with omega 3 (ω-3) and/or 6 (ω-6) fatty acids (FAs) may alleviate the signs and symptoms of DED, and improve the expressibility and quality of meibum, in patients with MGD.6–11 These findings have led to the use of ω-FAs as a DED and/or MGD treatment.12–14 Yet the mechanism(s) underlying these ω-FA effects are not completely understood.15
We hypothesize that these ω-FA actions reflect, at least in part, a direct influence on the quality and quantity of lipids produced by human meibomian gland epithelial cells (HMGECs). The purpose of this study was to test our hypothesis.
Material and Methods
Cell cultures
Immortalized human meibomian gland epithelial cells (IHMGECs) were cultured in the presence or absence of 10% fetal bovine serum, according to published protocols.16–18 After reaching 80 to 90% confluence (~ 5 × 10/6 well), cells were exposed to ethanol vehicle, linolenic acid (ω-3, 10−5 M; Santa Cruz Biotechnology, Dallas, TX), linoleic acid (ω-6, 10−5 M; Sigma-Aldrich, St. Louis, MO) or linolenic and linoleic acids together (0.5×10−5+ 0.5×10−5M), for 5 to 7 days. Azithromycin (AZM, 10 μg/ml; Santa Cruz Biotechnology) was used as a positive control in all experiments, because this antibiotic has well-defined effects on both the proliferation and differentiation of IHMGECs.19–22 Following treatment, cells were processed for enumerative, histological and biochemical procedures.
Cell analyses
Cells were counted with a hemocytometer. Total cellular neutral lipid and lysosome accumulation were evaluated by staining cells with LipidTOX green neutral lipid stain (Invitrogen, Grand Island, NY) and LysoTracker® Red DND-99 (Invitrogen), a fluorescent probe designed for labeling acidic organelles (e.g. lysosomes), as previously reported.19–22 The cellular content of free and esterified cholesterol, triglycerides, phosphatidylethanolamine, phosphatidylcholine and phosphatidylinositol were determined by high-performance thin-layer chromatography (HPTLC; Silica Gel 60, Merck, Darmstadt, Germany), as described.20,22 The cellular lysate levels of Lamp-1 and LC3 proteins, which are biomarkers for lysosomes and autophagosomes23,24, were assessed by Western blots. Methods involved use of primary antibodies specific for Lamp-1 (H4A3, 1:350; Developmental Studies Hybridoma Bank, Iowa City, IA), LC3 (1:1,000, Cell Signaling Technology, Danvers, MA) or β-actin (1:10,000; Cell Signaling Technology), followed by development with HRP-conjugated secondary antibodies (1:5,000; Sigma-Aldrich).
Each experiment was performed in duplicate or triplicate under the same conditions and repeated at least 3 times. Staining intensities were quantified with ImageJ (http://rsbweb.nih.gov/ij/index.html). Data were analyzed by ANOVA and Newman-Keuls multiple comparisons tests by using Prism 5 software (GraphPad Software, Inc., La Jolla, CA).
Results
Influence of ω-3 and/or ω-6 on lipid expression in IHMGECs
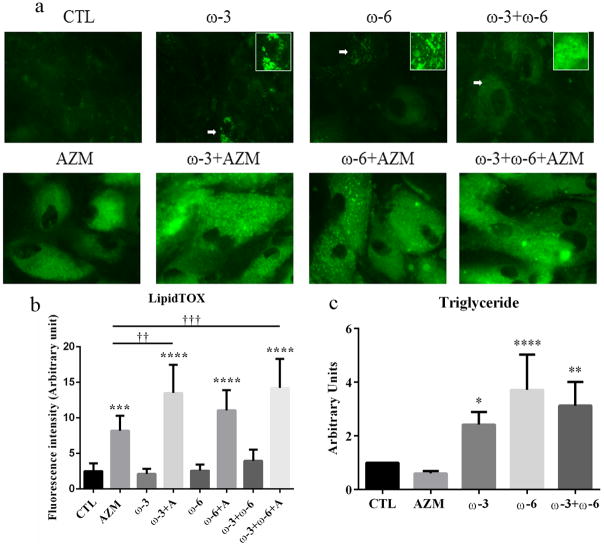
To determine whether ω-3 and/or ω-6 influence the quantity and quality of lipids in IHMGECs, we treated cells with these FAs or vehicle for 7 days and then processed samples for histological and biochemical procedures. Our findings demonstrate that ω-3, ω-6, as well as their combination, stimulate the accumulation of small neutral lipid-containing vesicles in IHMGECs (Figure 1). This vesicular effect was associated with a significant 2.4- to 3.7-fold increase in the cellular content of triglycerides following ω-3 and ω-6 treatment, respectively. The combination of both FAs together also enhanced triglyceride levels. Of particular interest, culture of IHMGECs with ω-3 and AZM led to a significantly greater amount of total neutral lipids, relative to that found with AZM alone (Figure 1). IHMGEC exposure to the FAs did not alter the expression of free or esterified cholesterol, or phospholipids (data not shown).
Figure 1.
Effects of ω-3 and/or ω-6 on the quantity and quality of lipids in IHMGECs. Cells were treated with vehicle, 10−5M ω-3, 10−5M ω-6, ω-3+ω-6 (each 0.5 × 10−5), or FAs combined with 10 μg/ml AZM for 7 days. a. Cells were stained with LipidTOX, and the green color indicates neutral lipids. The images were obtained with a fluorescent microscope. b. The fluorescence intensity of Lipid TOX staining was measured by using ImageJ. ***p<0.001 and ****p < 0.0001 versus control. **†<0.01 and ***†<0.001 versus AZM. The experiments were repeated 3 times, and the results from a single experiment are shown. c. The lipid extractions were analyzed by HPTLC, and the band intensities were measured by using ImageJ; control band instensity was set to 1, and data (mean ± SE) are reported as fold-change compared to control values. The results displayed are from 5 separate experiments.
Effect of ω-3 and/or ω-6 on the accumulation of acidic organelles in IHMGECs
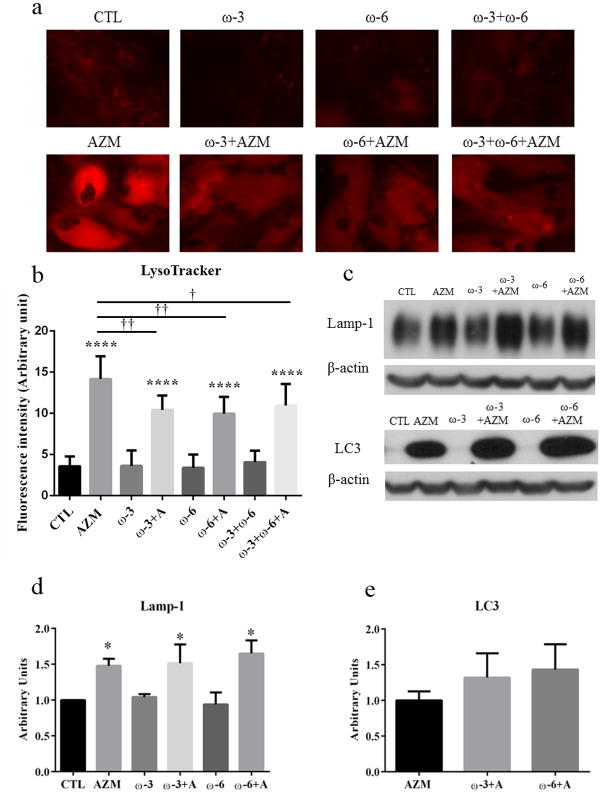
To evaluate whether ω-3 and/or ω-6 promote the accumulation of acidic organelles in IHMGECs, we cultured cells with FAs or vehicle, with or without AZM, for up to 7 days before analyzing samples with microscopy and Western blots. As shown in Figure 2, our results demonstrate that these FAs, whether alone or together, had no effect on the relative number of these organelles. However, these FAs did reduce the stimulatory effect of AZM on the organelle accumulation. This antagonistic influence of ω-3 and/or ω-6 did not extend to the levels of Lamp-1 or LC3. The FAs had no impact on these protein amounts, regardless of whether IHMGECs were cultured with ω-3 and/or ω-6 in the presence or absence of AZM.
Figure 2.
Influence of ω-3 and/or ω-6 on the accumulation of acidic organelles in IHMGECs. Cells were cultured with vehicle, 10−5M ω-3, 10−5M ω-6, ω-3+ω-6 (each 0.5 × 10−5), or FAs combined with 10 μg/ml AZM for 7 days in a and b, and 5 days for c, d and e. a. Cell samples in duplicate were exposed to LysoTracker, which stains acidic organelles a red color. Images were obtained with a fluorescent microscope. b. The fluorescence intensities were measured by using ImageJ. ****p < 0.0001 versus control. *†<0.05, **†<0.01 versus AZM. The staining was repeated 3 times, and the results shown are from a single experiment. c. Cell lysates were evaluated on Western blots for Lamp-1 and LC3 in triplicate. d. The samples containing AZM showed a significant increase of Lamp-1 level comparing to control. *p<0.05. e. There was no significant difference between the effects of ω-3 or ω-6 combined with AZM or AZM alone. The remaining samples displayed weak signals for LC3 expression.
Influence of ω-3 and/or ω-6 on the proliferation of IHMGECs
To assess whether ω-3 and/or ω-6 influence the proliferation of IHMGECs, we cultured cells with these FAs or vehicle in serum-free or serum-containing media for 5 days. We also compared their effect, if any, to that of AZM, which is known to reduce the proliferation of IHMGECs in serum-free conditions.21 Our findings demonstrate ω-3, ω-6, alone or together, prevented, whereas AZM decreased, the proliferation of IHMGECs in serum-free media (Figure 3). In contrast, these treatments had no impact on IHMGEC proliferation in serum-containing media.
Figure 3.
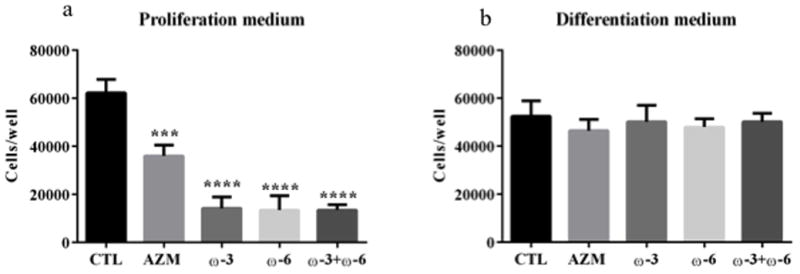
Impact of ω-3 and/or ω-6 on the proliferation of IHMGECs. Cells were seeded at a 10,000 cells/well in serum-free media (a), and 50,000 cells/well in serum-containing media (b), in12-well plates (n=3 wells/treatment). IHMGECs were treated with vehicle, 10−5M ω-3, 10−5M ω-6, ω-3+ω-6 (each 0.5 × 10−5), or AZM (10 μg/ml) for 5 days before cell counting. Results were reported as mean ± SE. Data from one experiment are shown as a representative of three studies performed under the same conditions. ***p<0.001 and ****p<0.0001.
Discussion
Our findings support our hypothesis and demonstrate that ω-3 and ω-6 can act directly on IHMGECs to influence the quality and quantity of intracellular lipids. Our research shows that ω-3 and ω-6 stimulate the accumulation of small neutral lipid-containing vesicles, but not lysosomes, in IHMGECs. This vesicular effect was associated with an increase in the cellular content of triglycerides. Cellular exposure to ω-3 also enhanced AZM’s impact on neutral lipid accumulation. In contrast, the ω-FAs prevented IHMGEC proliferation in serum-free media.
Our discovery that ω-FAs act directly on IHMGECs to influence lipid expression is not surprising. The meibomian gland is a large sebaceous gland, and researchers have reported that ω-6 stimulates the differentiation of, and lipogenesis in, sebaceous gland epithelial cells (sebocytes).25–27 Further, we have previously found that ω-3 intake is associated with a significant change in the lipid profile of human meibum.28 It is possible that these types of responses may contribute to the qualitative improvement of human meibomian gland secretions, and the reduction in meibomian gland obstruction, in MGD patients following oral ω-FA intake.8
Of particular interest was our finding that ω-FAs promote the IHMGEC accumulation of small neutral lipid-containing vesicles, which appear to be enriched in triglycerides. These vesicles may well be lipid droplets, which are the main storage organelles for triglycerides in eukaryotic cells and play roles in membrane biosynthesis, lipid homeostasis and defense against lipotoxicity.29 In support of this possibility, lipid droplets are known to be present in sebocytes, and their number is increased by ω-FA exposure.30 This sebaceous response, in turn, is paralleled by a heightened mRNA expression of perilipins (PLIN; also called lipid storage protein 5 [LSDP5]) in general, and of PLIN2 protein (also called adipose differentiation-related protein [ADRP]) in particular.30 Perilipins are proteins that coat lipid droplets and regulate their storage and utilization of lipids.31 We have found LSDP5 mRNA in human meibomian glands (DA Sullivan et al, unpublished data), as well as ADRP mRNA in mouse meibomian glands.32 Moreover, investigators have identified ADRP in lipid droplets of rat meibomian gland epithelial cells.33,34 In effect, ω-FAs may modulate lipid droplet dynamics in HMGECs, although additional research is required to demonstrate this action.
The accumulation of lipid droplets, and the presence of ADRP, have been linked to differentiation, and not proliferation, of rat meibomian gland epithelial cells.33,34 Similarly, we have discovered that the upregulation of genes promoting lipid biosynthesis and ADRP appearance is associated with increased differentiative, and decreased proliferative, cellular processes in the human and mouse meibomian gland.32,35 These findings suggest that ω-FA treatment enhances the differentiation and not the proliferation of IHMGECs. Consistent with this suggestion is our observation that the ω-FAs prevented the proliferation of IHMGECs in serum-free (i.e. proliferating) media. Such prevention was not found in serum-containing (i.e. differentiating) media, given that this culture condition does not promote IHMGEC proliferation.17 The ability of ω-FAs to suppress cell growth has also been found in pancreatic and colorectal cells.36,37
We found that ω-3 and ω-6, alone or together, significantly increased the triglyceride content in IHMGECs. This effect might explain how the ω-FAs enhanced AZM’s impact on neutral lipid accumulation, given that AZM typically elevates the IHMGEC levels of cholesterol, cholesterol ester and phospholipids, but reduces those of triglycerides.19,21,22 The ω-FAs may have countered the AZM-induced loss of triglyceriedes, leading to increased LipidTox staining of the IHMGECs. In contrast, the ω-FAs appeared to decrease the AZM-induced generation of lysosomes, as identified by LysoTracker. LysoTracker is an acidotropic probe that selectively accumulates in acidic compartments. This attenuated lysosome finding is likely a technical artifact. Both ω-3 and ω-6 cause pH changes in cellular organelles, which may lead to diminished LysoTracker staining.38 Given that the protein levels of Lamp-1 and LC3, which are biomarkers for lysosomes and autophagosomes23,24, were not reduced by ω-FA exposure, we conclude that no alterations occurred in lysosome numbers. This inconsistency between the staining and immunoblot results suggests that LysoTracker staining may not be the best way to monitor lysosomes in experiments involving ω-FAs.
Overall, our study demonstrates that ω-3 and ω-6 directly influence the quality and quantity of lipids and lipid-containing vesicles in IHMGECs, and appear to promote the differentiation, but not the proliferation, of these cells. Our results are analogous to some of those recently found with the ω-3 metabolites, docosahexaenoic and eicosapentaenoic acids, in IHMGECs.39 These cellular effects may contribute to the reported benefit of oral ω-FAs in the management of DED and MGD.6–11 However, as in other studies40,41, the full impact of ω-3 and ω-6 on the meibomian gland and ocular surface health remains to be determined.
Acknowledgments
Source of Funding: This research was supported by NIH grant EY05612, the Margaret S. Sinon Scholar in Ocular Surface Research Fund, and the Guoxing Yao Research Fund.
Footnotes
Conflicts of Interest
A patent application has been filed related to azithromycin. The intellectual property for this application is owned by the Schepens Eye Research Institute/Massachusetts Eye and Ear. Otherwise, the authors have no conflict of interest.
References
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Chao W, Belmonte C, Benitez Del Castillo J, et al. Report of the Inaugural Meeting of the TFOS i2 = initiating innovation Series: Targeting the Unmet Need for Dry Eye Treatment (London, United Kingdom, March 21, 2015) Ocul Surf. 2016 doi: 10.1016/j.jtos.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Knop E, Knop N, Millar T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472–478. doi: 10.1097/ICO.0b013e318225415a. [DOI] [PubMed] [Google Scholar]
- 5.Green-Church KB, Butovich I, Willcox M, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52:1979–1993. doi: 10.1167/iovs.10-6997d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korb DR, Blackie CA, Finnemore VM, et al. Effect of using a combination of lid wipes, eye drops, and omega-3 supplements on meibomian gland functionality in patients with lipid deficient/evaporative dry eye. Cornea. 2015;34:407–412. doi: 10.1097/ICO.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 7.Sheppard JD, Jr, Singh R, McClellan AJ, et al. Long-term Supplementation With n-6 and n-3 PUFAs Improves Moderate-to-Severe Keratoconjunctivitis Sicca: A Randomized Double-Blind Clinical Trial. Cornea. 2013;32:1297–1304. doi: 10.1097/ICO.0b013e318299549c. [DOI] [PubMed] [Google Scholar]
- 8.Pinna A, Piccinini P, Carta F. Effect of oral linoleic and gamma-linolenic acid on meibomian gland dysfunction. Cornea. 2007;26:260–264. doi: 10.1097/ICO.0b013e318033d79b. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra C, Singh S, Chakma P, et al. Effect of oral omega-3 Fatty Acid supplementation on contrast sensitivity in patients with moderate meibomian gland dysfunction: a prospective placebo-controlled study. Cornea. 2015;34:637–643. doi: 10.1097/ICO.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 10.Olenik A, Jimenez-Alfaro I, Alejandre-Alba N, et al. A randomized, double-masked study to evaluate the effect of omega-3 fatty acids supplementation in meibomian gland dysfunction. Clin Interv Aging. 2013;8:1133–1138. doi: 10.2147/CIA.S48955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojtowicz JC, Butovich I, Uchiyama E, et al. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30:308–314. doi: 10.1097/ICO.0b013e3181f22e03. [DOI] [PubMed] [Google Scholar]
- 12.Rand AL, Asbell PA. Nutritional supplements for dry eye syndrome. Curr Opin Ophthalmol. 2011;22:279–282. doi: 10.1097/ICU.0b013e3283477d23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown NA, Bron AJ, Harding JJ, et al. Nutrition supplements and the eye. Eye (Lond) 1998;12(Pt 1):127–133. doi: 10.1038/eye.1998.21. [DOI] [PubMed] [Google Scholar]
- 14.Downie LE, Keller PR, Vingrys AJ. An evidence-based analysis of Australian optometrists’ dry eye practices. Optom Vis Sci. 2013;90:1385–1395. doi: 10.1097/OPX.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 15.Hom MM, Asbell P, Barry B. Omegas and Dry Eye: More Knowledge, More Questions. Optom Vis Sci. 2015;92:948–956. doi: 10.1097/OPX.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Hatton MP, Khandelwal P, et al. Culture, immortalization, and characterization of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:3993–4005. doi: 10.1167/iovs.09-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, Kam WR, Ding J, et al. Effect of growth factors on the proliferation and gene expression of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2013;54:2541–2550. doi: 10.1167/iovs.12-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan DA, Liu Y, Kam WR, et al. Serum-induced differentiation of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2014;55:3866–3877. doi: 10.1167/iovs.13-13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Ding J. The combined effect of azithromycin and insulin-like growth factor-1 on cultured human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2014;55:5596–5601. doi: 10.1167/iovs.14-14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Kam WR, Ding J, et al. One man’s poison is another man’s meat: using azithromycin-induced phospholipidosis to promote ocular surface health. Toxicology. 2014;320:1–5. doi: 10.1016/j.tox.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Kam WR, Ding J, et al. Effect of azithromycin on lipid accumulation in immortalized human meibomian gland epithelial cells. JAMA Ophthalmol. 2014;132:226–228. doi: 10.1001/jamaophthalmol.2013.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Kam WR, Ding J, et al. Can tetracycline antibiotics duplicate the ability of azithromycin to stimulate human meibomian gland epithelial cell differentiation? Cornea. 2015;34:342–346. doi: 10.1097/ICO.0000000000000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eskelinen EL. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med. 2006;27:495–502. doi: 10.1016/j.mam.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 25.Kim SY, Hyun MY, Go KC, et al. Resveratrol exerts growth inhibitory effects on human SZ95 sebocytes through the inactivation of the PI3-K/Akt pathway. Int J Mol Med. 2015;35:1042–1050. doi: 10.3892/ijmm.2015.2098. [DOI] [PubMed] [Google Scholar]
- 26.Zouboulis CC, Angres S, Seltmann H. Regulation of stearoyl-coenzyme A desaturase and fatty acid delta-6 desaturase-2 expression by linoleic acid and arachidonic acid in human sebocytes leads to enhancement of proinflammatory activity but does not affect lipogenesis. Br J Dermatol. 2011;165:269–276. doi: 10.1111/j.1365-2133.2011.10340.x. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfield RL, Kentsis A, Deplewski D, et al. Rat preputial sebocyte differentiation involves peroxisome proliferator-activated receptors. J Invest Dermatol. 1999;112:226–232. doi: 10.1046/j.1523-1747.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan BD, Cermak JM, Sullivan RM, et al. Correlations between nutrient intake and the polar lipid profiles of meibomian gland secretions in women with Sjogren’s syndrome. Adv Exp Med Biol. 2002;506:441–447. doi: 10.1007/978-1-4615-0717-8_62. [DOI] [PubMed] [Google Scholar]
- 29.Brown DA. Lipid droplets: proteins floating on a pool of fat. Curr Biol. 2001;11:R446–449. doi: 10.1016/s0960-9822(01)00257-3. [DOI] [PubMed] [Google Scholar]
- 30.Dahlhoff M, Camera E, Picardo M, et al. PLIN2, the major perilipin regulated during sebocyte differentiation, controls sebaceous lipid accumulation in vitro and sebaceous gland size in vivo. Biochim Biophys Acta. 2013;1830:4642–4649. doi: 10.1016/j.bbagen.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujimoto T, Parton RG. Not just fat: the structure and function of the lipid droplet. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schirra F, Suzuki T, Richards SM, et al. Androgen control of gene expression in the mouse meibomian gland. Invest Ophthalmol Vis Sci. 2005;46:3666–3675. doi: 10.1167/iovs.05-0426. [DOI] [PubMed] [Google Scholar]
- 33.Sumida M, Kutsuna M, Kodama T, et al. Gene expression and fat deposit in primary cultures of rat meibomian gland cells. Adv Exp Med Biol. 2002;506:489–493. doi: 10.1007/978-1-4615-0717-8_68. [DOI] [PubMed] [Google Scholar]
- 34.Kutsuna M, Kodama T, Sumida M, et al. Presence of adipose differentiation-related protein in rat meibomian gland cells. Exp Eye Res. 2007;84:687–693. doi: 10.1016/j.exer.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Khandelwal P, Liu S, Sullivan DA. Androgen regulation of gene expression in human meibomian gland and conjunctival epithelial cells. Mol Vis. 2012;18:1055–1067. [PMC free article] [PubMed] [Google Scholar]
- 36.Dommels YE, Haring MM, Keestra NG, et al. The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE(2) synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis. 2003;24:385–392. doi: 10.1093/carcin/24.3.385. [DOI] [PubMed] [Google Scholar]
- 37.Song KS, Jing K, Kim JS, et al. Omega-3-polyunsaturated fatty acids suppress pancreatic cancer cell growth in vitro and in vivo via downregulation of Wnt/Beta-catenin signaling. Pancreatology. 2011;11:574–584. doi: 10.1159/000334468. [DOI] [PubMed] [Google Scholar]
- 38.Las G, Serada SB, Wikstrom JD, et al. Fatty acids suppress autophagic turnover in beta-cells. J Biol Chem. 2011;286:42534–42544. doi: 10.1074/jbc.M111.242412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hampel U, Kruger M, Kunnen C, et al. In vitro effects of docosahexaenoic and eicosapentaenoic acid on human meibomian gland epithelial cells. Exp Eye Res. 2015;140:139–148. doi: 10.1016/j.exer.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 40.Weylandt KH, Serini S, Chen YQ, et al. Omega-3 Polyunsaturated Fatty Acids: The Way Forward in Times of Mixed Evidence. Biomed Res Int. 2015;2015:143109. doi: 10.1155/2015/143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calder PC. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J Parenter Enteral Nutr. 2015;39:18S–32S. doi: 10.1177/0148607115595980. [DOI] [PubMed] [Google Scholar]