Abstract
Inflammatory cytokines, like tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), are elevated in ovarian cancer. Differences in cytokine expression by histologic subytpe or ovarian cancer risk factors can provide useful insight into ovarian cancer risk and etiology. We used ribonucleic acid (RNA) in-situ hybridization to assess TNF-α and IL-6 expression on tissue microarray slides from 78 epithelial ovarian carcinomas (51 serous, 12 endometrioid, 7 clear cell, 2 mucinous, 6 other) from a population-based case control study. Cytokine expression was scored semi-quantitatively and odds ratios (OR) and 95% confidence intervals (CI) were calculated using polytomous logistic regression. TNF-α was expressed in 46% of the tumors while sparse IL-6 expression was seen only 18% of the tumors. For both markers, expression was most common in high grade serous carcinomas followed by endometrioid carcinomas. Parity was associated with a reduced risk of TNF-α positive (OR=0.3, 95% CI: 0.1-0.7 for 3 or more children versus none) but not TNF-α negative tumors (p-heterogeneity=0.02). In contrast, current smoking was associated with a nearly three fold increase in risk of TNF-α negative (OR=2.8, 95% CI: 1.2, 6.6) but not TNF-α positive tumors (p-heterogeneity = 0.06). Our data suggests that TNF-α expression in ovarian carcinoma varies by histologic subtype and provides some support for the role of inflammation in ovarian carcinogenesis. The novel associations detected in our study need to be validated in a larger cohort of patients in future studies.
Keywords: Ovary, carcinoma, cytokines, TNF-α, parity, smoking
Introduction
Epidemiologic and biologic evidence supports the role of inflammation in the pathogenesis of ovarian cancer[1]. Ovulation is an inflammatory process involving localized elevations of prostaglandins and leukotrienes, wound healing and tissue remodeling[2]. Oral contraceptives and parity suppress ovulation and are associated with a reduced risk of ovarian cancer. Similarly, tubal ligation, which may block inflammatory mediators from reaching the ovary or fimbria, reduce risk of ovarian cancer by almost 50% and this protective association is remarkably consistent across studies[3]. In contrast, pro-inflammatory exposures such as genital powder use, endometriosis, and increased body mass index are known to increase ovarian cancer risk[4, 5],[6]. Results from several prospective studies suggest that elevated systemic markers of inflammation, specifically, serum levels of CRP, TNF-α and IL-6, are predictive of ovarian cancer development [7-12].
However, ovarian cancer is a heterogeneous disease with several distinct morphological phenotypes of surface epithelial carcinomas. Therefore, it is not surprising that multiple pathways of ovarian carcinogenesis have been proposed, the most notable ones being origin from inclusion cysts of ovarian surface epithelium[13, 14], high grade serous carcinomas with TP53 mutations originating in the fallopian tubes[15], endometrioid and clear cell carcinomas arising in association with endometriosis[6], and mucinous carcinomas arising in association with teratoma[16] or Brenner’s tumor[17] with a postulated role for metaplasias at the tuboperitoneal junction[18]. Whether pro-inflammatory exposures are relevant to risk of ovarian cancer development through all or only some of these pathways remains uncertain.
Prior studies have shown increased TNF-α mRNA levels in ovarian cancer as well as constitutive expression of other cytokines such as IL-1α, IL-6, CCL2, CXCL8, and M-CSF[19, 20]. However, most of these mRNA expression array studies used homogenized tumor tissue lysates and could not assess tissue localization of the various cytokines. Cytokine expression by the tumor cell epithelium could not be distinguished from cytokine expression by tumor infiltrating inflammatory cells. The lack of robust, validated anti-cytokine antibodies that can work well on paraffin sections has also hampered efforts to assess tissue localization of TNF-α and other cytokines. Recent novel developments in RNA in-situ hybridization (ISH) techniques offer a unique opportunity to study inflammatory mediators in formalin fixed paraffin embedded tissue[21]. In this study we evaluated the association between ovarian cancer risk factors and inflammatory exposures in relation to TNF-α and IL-6 expression in a well annotated set of ovarian cancers to understand the role of inflammatory mediators in the pathogenesis of ovarian carcinoma.
Materials and Methods
Study population
The New England Case Control Study (NECC) is a population-based case-control study conducted in three phases between 1992-2008[22]. Cases were recruited in Eastern Massachusetts and New Hampshire through statewide registries and tumor boards, while controls were identified through driver’s license lists (NH) and town resident lists (MA). In the current study, we selected participants from the last phase of the NECC conducted between 2003-2008. Participants were excluded if they were younger than 18 years, did not speak English, had moved elsewhere or were not accessible by phone, had a history of bilateral oophorectomy (controls) or if permission to contact them was denied by their physician (cases). Controls were frequency matched to the cases based on their age and their state of residence. Out of 1,610 potential cases 1,238 met the eligibility criteria, and 845 (68.3%) were enrolled between 2003-2008. We identified 2,523 potential controls, where 1,673 were eligible to participate, and 857 (51.2%) were enrolled. Information about reproductive and medical history and lifestyle factors was obtained through in-person interview. The study was approved by the institutional review boards at Brigham and Women’s Hospital and Dartmouth Medical School.
TMA preparation and RNA ISH assays
Pathology reports from the five year study period were reviewed by a gynecological pathologist (MG) to select cases of invasive surface epithelial carcinomas (low grade or high grade serous, mucinous, endometrioid, clear cell, and others) based on the current WHO classification of ovarian cancer[23]. Paraffin-embedded cancer tissue blocks were then requested for participants with invasive tumors, who had no history of neoadjuvant chemotherapy, and who had surgery at Brigham and Women’s Hospital, Boston, MA. Of the initially requested material (n=207) paraffin blocks were available for 78 tumors and this formed our final study group. Cases included in the TMA had similar characteristics to all the NECC confirmed invasive cases, and cases eligible for block collection with respect to ovarian cancer risk factors (age, parity, BMI, oral contraceptive use, tubal ligation, endometriosis, and family history of breast or ovarian cancer) and tumor characteristics (histology, grade, and stage) (Supplementary Table 1).
For each case, histopathology slides were reviewed by the study pathologist (MG) to select representative tumor blocks for inclusion in a tumor tissue microarray (TMA). Three separate areas of well-preserved tumor, away from foci of necrosis were then marked on the tumor tissue slides for TMA construction. TMA blocks were constructed using 1 mm diameter needles to extract the tissue cores that were then placed into predrilled holes of a recipient paraffin block to create a grid of tissue cores representing approximately 25 cases per TMA with up to three cores per case. Normal fallopian tubes and ovaries (n=18 cores) were also included as controls in the tumor TMA blocks.
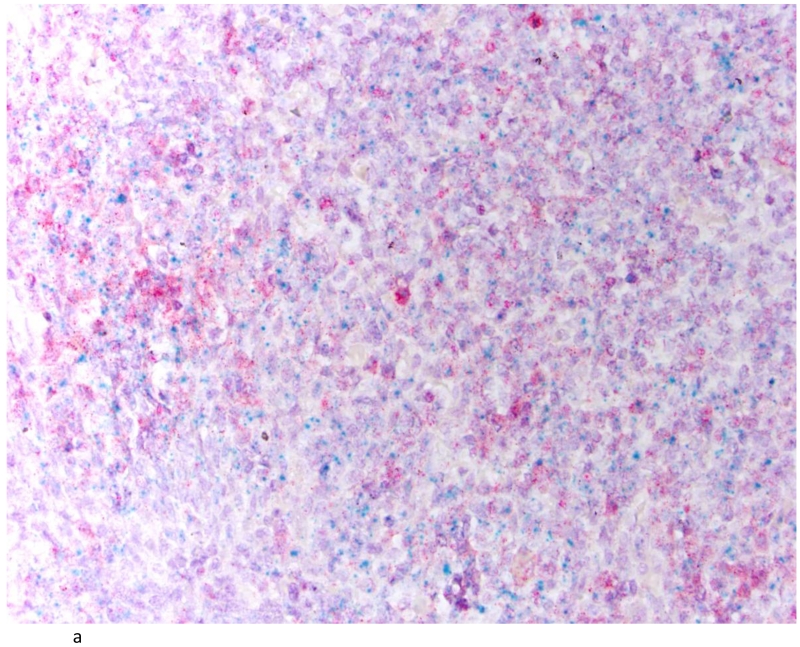
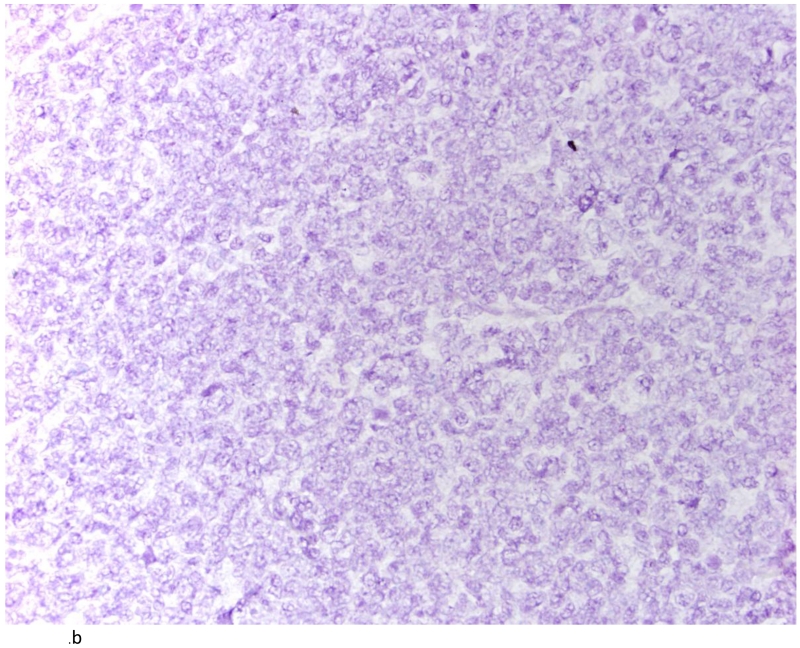
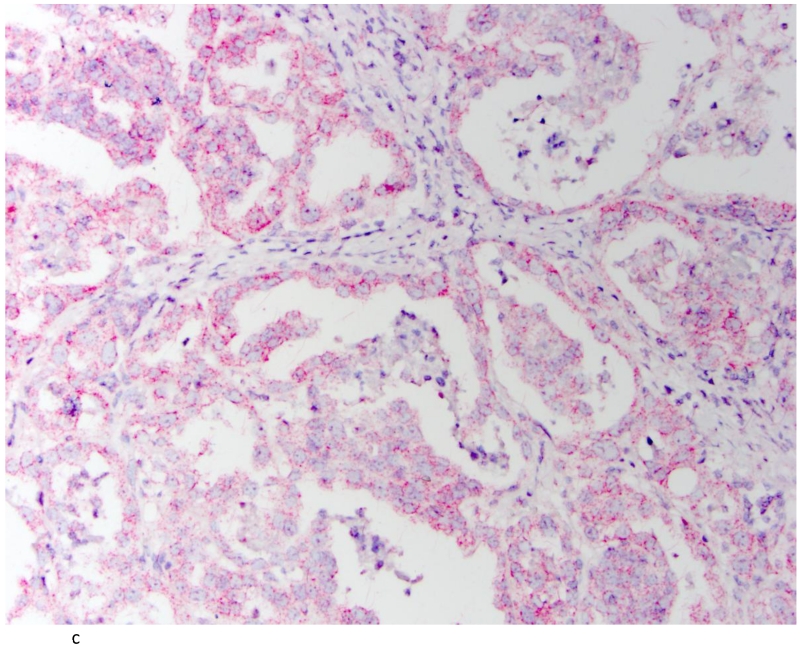
Tissue sections, 5μM in thickness, were cut from each TMA block and baked in a dry oven at 60° C for 1 hr before performing the RNAScope FFPE assay, as previously described[21]. Pre-assay optimization and target hybridization was performed according to ACD RNAscope 2-plex Chromogenic Assay on FFPE tissue. TNF-α positive signal was indicated by a red probe (Gene accession#: NM_000594.3: Homo sapiens TNF-α start position: 70/end position 1456) while IL-6 positive signal was indicated by a green probe (Gene accession #: NM_000600.3: Homo sapiens IL-6 start position: 27/end position 1112). Normal tonsil tissue was used as a positive control for TNF-α and IL-6 RNA transcripts (Figure 1A). DapB nuclear counterstain alone was used as a negative control (Figure 1B) and a ubiquitin probe (Gene accession # cd01803) was used to validate RNA integrity in the analyzed tissues (Figure 1C).
Fig. 1.
Tonsil as positive control for IL-6 and TNFα (A); tonsil with DapB as negative control (B) and Ubiquitin showing the integrity of RNA (C). (200×)
The RNA ISH stained TMA slides were then scored by the study pathologist (MG) to estimate the proportion of TNF-α and IL-6 positive tumor cells in each core using a semi-quantitative scoring scheme (0, <5%, 5-24%, 25-74%, ≥75%). For purpose of statistical analysis, we assigned the maximum value among the three tumor tissue cores to each case, based on prior analyses showing good correlation between maximum and mean scores[24]. Cores were not scored if <20 tumor cells were present in the RNA ISH stained tissue cores.
Assessment of Pro and Anti-inflammatory Exposures
Since tumor tissue samples were obtained only from cases diagnosed in Eastern Massachusetts, only controls recruited in Eastern Massachusetts (n=725) were included in this analysis. The history of known ovarian cancer risk factors, as well as pro-inflammatory and anti-inflammatory exposures was collected through in person interviews. Cases were asked to report exposures one year prior to diagnosis while controls were asked about exposures one year prior to the interview date. For this analysis, the following ovarian cancer risk factors were considered: age (continuous), parity (0, 1, or ≥2 children), oral contraceptive (OC) use (0, <2, 2-5, >5 years) and family history of breast or ovarian cancer (yes or no). In addition, the following inflammatory exposures were also included in the final analysis: endometriosis (yes or no), smoking status (never, current, former), body mass index (<25, ≥25-30, >30 kg/m2), genital powder use (yes or no), aspirin use (any aspirin use), ibuprofen use (any ibuprofen use), menstrual pain (none/mild or moderate/severe), and alcohol consumption (0-1.97, 1.97-5.97, 5.97-12.8, >12.8 g/day).
Statistical analysis
TNF-α status of each ovarian carcinoma case was categorized as positive if >5% of tumor cells were positive and negative if <5% of cells stained positive, based on the maximum score in any of the three tumor tissue cores, as described above. In contrast to TNF-α, the IL-6 positivity in ovarian cancer was always focal and less than 5% in all cases included in our study. Thus, the IL-6 status for each case was categorized as positive if greater than 0% of cells stained positive and negative if 0% of cells stained positive based on the maximum score in any of the three tumor tissue cores.
The association between known ovarian cancer risk factors and inflammatory exposures and ovarian cancer risk stratified by TNF-α staining status was assessed using polytomous logistic regression (PLR) with a three category outcome (TNF-α positive tumors, TNF-α negative tumors and controls). First, we evaluated ovarian cancer risk factors (age, parity, OC use, and family history of breast or ovarian cancer) in relation to tissue expression of TNF-α and IL-6, adjusted for age. Then, we built a multivariate model using significant variables from the univariate analysis. Finally, we assessed the risk associated with inflammatory exposures (endometriosis, smoking status, body mass index (BMI), genital powder use, aspirin use, ibuprofen use, menstrual pain, and alcohol consumption). To assess heterogeneity of risk associations by TNF-α status, we used the likelihood ratio test to compare a null model where exposure of interest was constrained to have the same association for TNF-α positive and TNF-α negative tumors, to an alternative model where the estimate of the association was allowed to vary. Statistical analyses were performed using SAS v9.3 (SAS Institute, Cary, NC), and Stata version 11.0 (StataCorp LP, College Station, TX, USA).
Results
We included 78 cases in three tissue microarrrays (Table 1). Of these, 50 (64%) were high grade serous, 1 (1%) low grade serous, 12 (15%) endometrioid, 7 (9%) clear cell, 2 (3%) mucinous, and 6 (8%) other histologic subtypes. On average, women were diagnosed at 58 years of age and had a mean BMI of 26. Approximately half of the cases reported ever using oral contraceptives, 72% were parous, and only 8% reported a tubal ligation. Ten percent of cases reported endometriosis and 23% had a family history of either breast or ovarian cancer.
Table 1.
Characteristics of cases according to TNF-α and IL-6 expression
| Characteristic | All cases | TNF-α positive cases |
IL-6 positive cases |
|---|---|---|---|
| n | 78 | 36 | 14 |
| Age at diagnosis (years), mean (sd) | 57.6 (11) | 54.2 (10) | 57.8 (12) |
| BMI (kg/m2), mean (sd) | 26.2 (6) | 26.7 (6) | 26.0 (5) |
| Oral contraceptive use, ever, n (%) | 40 (51) | 20 (56) | 7 (50) |
| Oral contraceptive use duration (months)a | 48.0 (50) | 52.0 (56) | 73.4 (61) |
| Parity, ever, n (%) | 56 (72) | 20 (56) | 10 (71) |
| Number of childrenb, mean (sd) | 2.9 (2) | 2.6 (1) | 2.5 (1) |
| Tubal ligation, ever, n (%) | 6 (8) | 0 (0) | 1 (7) |
| Endometriosis, n (%) | 8 (10) | 5 (14) | 0 (0) |
| Family history of breast or ovarian cancer, n (%) | 18 (23) | 7 (19) | 7 (50) |
| Genital powder use, n (%) | 23 (29) | 10(28) | 3 (21) |
| Aspirin use, n (%) | 17 (22) | 9 (25) | 4 (29) |
| Ibuprofen use, n (%) | 23 (30) | 12 (33) | 2 (14) |
| Menstrual pain, n (%) | 33 (42) | 16 (44) | 7 (50) |
| Smoking, n (%) | |||
|
| |||
| Never | 40 (51) | 20 (56) | 5 (36) |
|
| |||
| Former | 26 (33) | 13 (36) | 8 (57) |
|
| |||
| Current | 12 (15) | 3 (8) | 1 (7) |
| Smoking (pack-years), mean (sd)c | 18.1 (18) | 14.8 (16) | 14.1 (10) |
| Alcohol (g/day), mean (sd) | 6.9 (11) | 4.8 (7) | 7.9 (12) |
| Tumor characteristics | |||
| Histology, n (%) | |||
| High-grade serous | 50 (64) | 20 (56) | 12 (86) |
| Low-grade serous | 1 (1) | 1 (3) | 0 (0) |
| Endometrioid | 12 (15) | 10 (28) | 2 (14) |
| Clear cell | 7 (9) | 3 (8) | 0 (0) |
| Mucinous | 2 (3) | 1 (3) | 0(0) |
| Otherd | 6 (8) | 1 (3) | 0 (0) |
| Disease Stage, n (%) | |||
| I | 16 (21) | 9 (25) | 3 (21) |
| II | 8 (10) | 3 (8) | 0 (0) |
| III | 48 (62) | 23 (64) | 10 (71) |
| IV | 6 (8) | 1 (3) | 1 (7) |
| Grade, n (%) | |||
| 1 | 4 (5) | 2 (6) | 0 (0) |
| 2 | 15 (19) | 10 (28) | 5 (36) |
| 3 | 54 (69) | 20 (56) | 9 (64) |
| Ungraded/unknown | 5 (7) | 4 (11) | 0 (0) |
Abbreviations: TNF-α=tumor necrosis factor alpha, IL-6=interleukin 6, sd=standard deviation, BMI=body mass index
Among OC users
Total number of stillbirths and livebirths among parous
Among ever-smokers
Unspecified epithelial (n=2), mixed with clear cell or endometrioid components (n=4)
Overall, 36 (46%) cases expressed TNF-α and 14 (18%) expressed IL-6. Positive staining was localized to the nuclei in all cases. Women with TNF-α positive tumors generally had similar characteristics to all cases except that they were less likely to be parous, to have had a tubal ligation, or to be a current smoker (Table 1). For IL-6 positive cases, again characteristics were approximately the same as the whole case population, except these women were more likely to use OCs for a long duration, have a family history of breast or ovarian cancer, report severe menstrual pain, and have a history of smoking, but less likely to have endometriosis or used genital powder or ibuprofen.
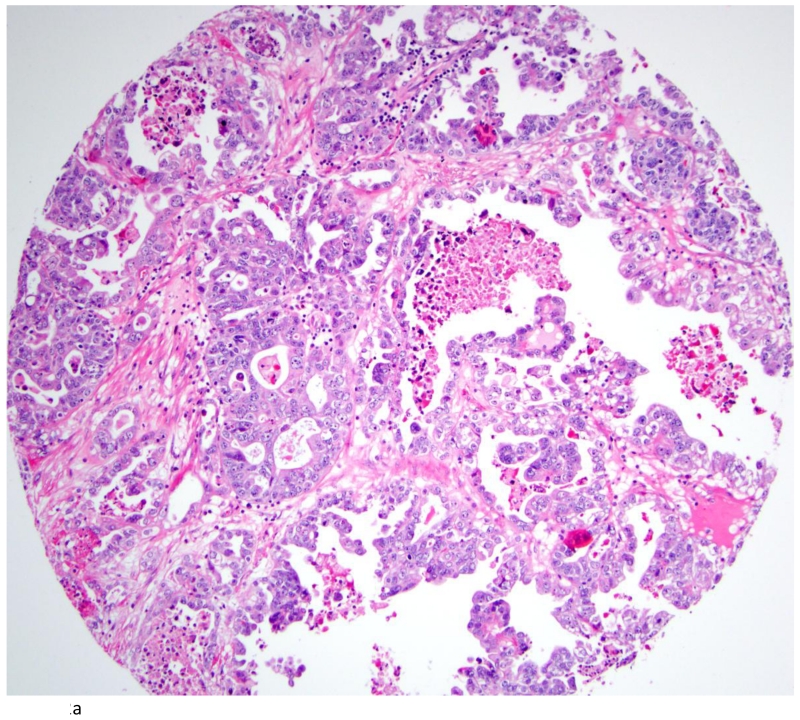
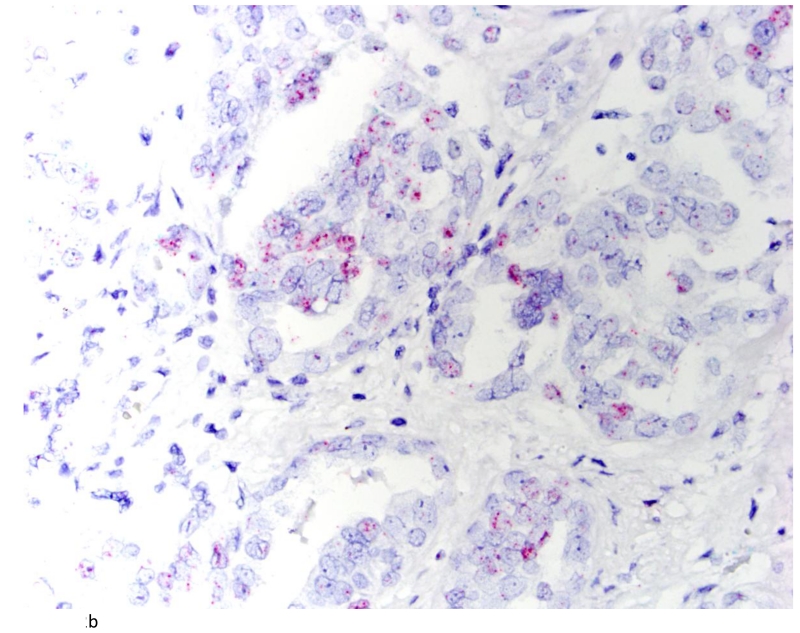
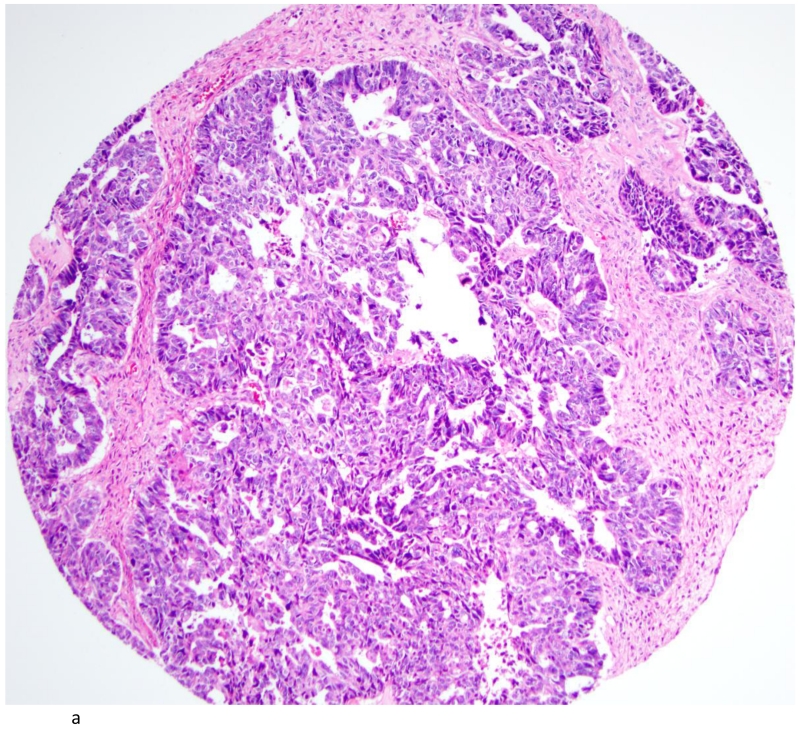
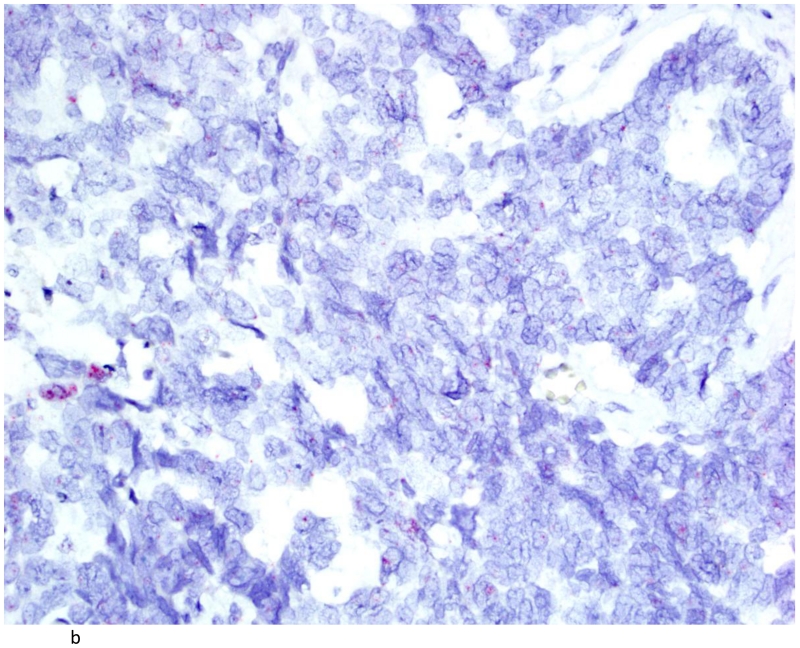
The distribution of TNF-α and IL-6 expression in ovarian tumors by histologic subtype is summarized in Table 2. A total of 42 (54%) ovarian carcinomas were categorized as TNF-α negative (<5% of tumor cells staining positive), and 36 (46%) as TNF-α positive. By histological subtype, 20(40%) of high-grade serous (Figures 2A and 2B), 1(100%) of low-grade serous, 1 (50%) mucinous, 10 (83%) endometrioid, and 3 (43%) clear cell tumors (Figures 3A & 3B) were TNF-α positive (Table 2). Few tumors expressed IL-6 and those that did expressed IL-6 in a small proportion of cells. Overall, only 14 tumors expressed IL-6 in our study population. Most of these were high grade serous accounting for 24% of the high grade serous tumors in our study. There was some heterogeneity in staining in the positive cases. For the 36 positive tumors, 11 were positive across all cores, 12 were positive in 2 of three cores, 3 were positive in 1 of 2 cores, and the remaining 10 were positive in only 1 of 3 cores. None of the normal fallopian tubes and ovaries included in the TMA were positive for either TNF-α or IL-6.
Table 2.
Distribution of TNF-α and IL-6 staining by histological subtype
| Cells stained, % |
High-grade serous n (%) |
Low-grade serous n (%) |
Mucinous n (%) |
Endometrioid n (%) |
Clear cell n (%) |
Other (n, %) |
Total |
|---|---|---|---|---|---|---|---|
| TNF-α | |||||||
| 0 | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 1 (14) | 1 (17) | 3 |
| <5 | 30 (60) | 0 (0) | 1 (50) | 1 (8) | 3 (63) | 4 (47) | 39 |
| 5-24 | 14 (28) | 0 (0) | 0 (0) | 6 (50) | 2 (29) | 1 (17) | 23 |
| 25-74 | 6 (12) | 1 (100) | 1 (50) | 3 (25) | 0 (0) | 0 (0) | 11 |
| ≥75 | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 1 (14) | 0 (0) | 2 |
| IL-6 | |||||||
| 0 | 38 (76) | 1 (100) | 2 (100) | 10 (83) | 7 (100) | 6 (0) | 64 |
| >0 | 12 (24) | 0 (0) | 0 (0) | 2 (17) | 0 (0) | 0 (0) | 14 |
| Total | 50 | 1 | 2 | 12 | 7 | 6 | 78 |
Abbreviations: TNF-α=tumor necrosis factor alpha, IL-6=interleukin 6
Fig. 2.
Clear cell carcinoma of the ovary (A; H&E stain; 200×) with positive TNFα signals (B). (400×)
Fig. 3.
High Grade Serous Carcinoma (A; H&E stain; 200×) with rare cells showing amplified signals for TNFα (B). (400×)
In the univariate model, the risk of ovarian cancer differed significantly by TNF-α status for parity but not oral contraceptive use, family history of breast or ovarian cancer, endometriosis, smoking, BMI, genital powder use, aspirin use, ibuprofen use, menstrual pain or alcohol intake (Table 3). Parity was associated with a decreased risk of TNF-α positive (OR=0.18, 95% CI: 0.08-0.43 for 1 or 2 children, OR=0.30, 95% CI: 0.13-0.71 for 3 or more children, compared to 0 children) but not TNF-α negative tumors (p-heterogeneity=0.02). Interestingly, we observed no TNF-α positive tumors among women with a history of tubal ligation. All six women with tubal ligation in our study population had TNF-α negative tumors.
Table 3.
Ovarian cancer risk factors and inflammatory exposures in relation to risk of TNF-α positive and negative tumors, univariate analysis.
| TNF-α negative (42 cases) |
TNF-α positive (36 cases) |
||||
|---|---|---|---|---|---|
| n cases (%) |
ORa (95% CI) |
n cases (%) |
ORa (95% CI) |
p-heterogeneityb | |
| Parity | 0.02 | ||||
| 0 children | 6 (14) | 1.00 (ref) | 16 (44) | 1.00 (ref) | |
| 1 or 2 children | 20 (48) | 0.99 (0.38, 2.54) | 9 (25) | 0.18 (0.08, 0.43) | |
| 3+ children | 16 (38) | 0.80 (0.29, 2.17) | 11 (41) | 0.30 (0.13, 0.71) | |
| Oral contraceptive use (years) | |||||
| 0 | 22 (52) | 1.00 (ref) | 16 (44) | 1.00 (ref) | 0.91 |
| <2 | 8 (19) | 1.01 (0.43, 2.39) | 7 (19) | 0.96 (0.38, 2.43) | |
| 2-5 | 6 (14) | 0.65 (0.25, 1.70) | 8 (22) | 0.78 (0.31, 1.92) | |
| >5 | 6 (14) | 0.36 (0.14, 0.96) | 5 (14) | 0.24 (0.08, 0.70) | |
| p-trend | 0.05 | 0.03 | |||
| Tubal ligation | 6 (14) | 1.61 (0.66, 3.90) | 0 (0) | n/a | n/a |
|
Family history of
breast or ovarian cancer |
11 (26) | 1.49 (0.72, 3.05) | 7 (19) | 1.12 (0.48, 2.63) | 0.61 |
|
Endometriosis
Smoking |
3 (7) | 0.85 (0.25, 2.86) | 5 (14) | 1.61 (0.60, 4.28) | 0.40 |
|
| |||||
| Never | 20 (48) | 1.00 (ref) | 20 (56) | 1.00 (ref) | 0.16 |
|
| |||||
| Former | 13 (31) | 0.61 (0.30, 1.26) | 13 (36) | 0.72 (0.35, 1.49) | |
|
| |||||
| Current | 9 (21) | 2.81 (1.20, 6.29) | 3 (8) | 0.84 (0.24, 2.93) | |
|
| |||||
| BMI (kg/m2) | |||||
|
| |||||
| <25 | 21 (50) | 1.00 (ref) | 19 (53) | 1.00 (ref) | 0.59 |
|
| |||||
| ≥25-30 | 12 (29) | 0.80 (0.39, 1.68) | 7 (19) | 0.58 (0.24, 1.41) | |
|
| |||||
| >30 | 9 (21) | 0.88 (0.39, 1.98) | 10 (28) | 1.25 (0.56, 2.78) | |
| Genital powder use | 13 (31) | 0.94 (0.48, 1.85) | 10 (28) | 0.89 (0.42, 1.88) | 0.91 |
| Aspirin use | 8 (19) | 0.56 (0.26, 1.34) | 9 (25) | 1.25 (0.55, 2.82) | 0.19 |
| Ibuprofen use | 11 (26) | 0.92 (0.44, 1.92) | 12 (33) | 0.95 (0.46, 1.98) | 0.95 |
|
Menstrual pain
Alcohol, g/day |
17 (40) | 1.59 (0.84, 3.02) | 16 (44) | 1.75 (0.89, 3.45) | 0.88 |
|
| |||||
| 0-1.97 | 16 (41) | 1.00 (ref) | 15 (44) | 1.00 (ref) | 0.46 |
|
| |||||
| 1.97-5.97 | 8 (21) | 1.02 (0.43, 2.41) | 11 (32) | 1.34 (0.61, 2.95) | |
|
| |||||
| 5.97-12.8 | 5 (13) | 0.66 (0.24, 1.83) | 4 (12) | 0.51 (0.17, 1.54) | |
|
| |||||
| >12.8 | 10 (26) | 1.20 (0.54, 2.67) | 4 (12) | 0.51 (0.17, 1.55) | |
Abbreviations: TNF-α=tumor necrosis factor alpha, IL-6=interleukin 6, BMI=body mass index
Adjusted for age (continuously)
p-value for heterogeneity was obtained using a likelihood ratio test
In the multivariate model (Table 4), adjusted for ovarian cancer risk factors, including age, parity, use of oral contraceptives and family history of breast or ovarian cancer, parity was associated with a decreased risk of TNF-α positive but not TNF-α negative cancers (p-heterogeneity=0.02). Compared to 0 children, having 1 or 2 children was associated with a 79% (OR=0.21, 95% CI: 0.09-0.49), and having 3 or more children was associated with a 69% (OR=0.31, 95% CI: 0.13-0.73) decreased risk of TNF-α positive ovarian carcinomas. In contrast, there was no association between parity and TNF-α negative ovarian cancer (OR=1.09, 95% CI: 0.42-2.83 for 1 or 2 children, and OR=0.81, 95% CI 0.29-2.21 for 3 or more children, compared to 0 children). The difference in association was unchanged after additional adjustment for endometriosis. For inflammatory-related exposures including endometriosis, BMI, genital powder use, aspirin use, ibuprofen use, menstrual pain or alcohol use, we observed no difference in risk by TNF-α staining status (p-heterogeneity >0.4). Interestingly, current smoking was associated with a 3 fold (OR=2.79, 95% CI: 1.18-6.62) increase in risk of TNF-α negative but not TNF-α positive ovarian cancer (OR=0.83, 95% CI: 0.23-2.97); however, the difference between the two associations was of borderline significance (p-heterogeneity=0.06).
Table 4.
Ovarian cancer risk factors and inflammatory exposures in relation to risk of TNF-α positive and TNF-α negative tumors.
| TNF-α negative cases | TNF-α positive cases | ||||
|---|---|---|---|---|---|
| n cases (%) |
ORa (95% CI) | n cases (%) |
ORa (95% CI) | p-heterogeneityb | |
| Parity | 0.02 | ||||
| 0 | 6 (14) | 1.00 (ref) | 16 (44) | 1.00 (ref) | |
| 1 or 2 | 20 (48) | 1.09 (0.42, 2.83) | 9 (25) | 0.21 (0.09, 0.49) | |
| 3 or more | 16 (38) | 0.81 (0.29, 2.21) | 11 (31) | 0.31 (0.13, 0.73) | |
| Oral contraceptive use (years) | |||||
| 0 | 22 (52) | 1.00 (ref) | 16 (44) | 1.00 (ref) | n/a |
| <2 | 8 (19) | 1.05 (0.54, 2.03) | 7 (19) | 1.05 (0.54, 2.03) | |
| 2-5 | 6 (14) | 0.78 (0.39, 1.56) | 8 (22) | 0.78 (0.39, 1.56) | |
| >5 | 6 (14) | 0.33 (0.16, 0.68) | 5 (14) | 0.33 (0.16, 0.68) | |
|
Family history of breast or
ovarian cancer |
11 (26) | 1.33 (0.75, 2.37) | 7 (19) | 1.33 (0.75, 2.37) | n/a |
| Endometriosis | 3 (7) | 0.76 (0.22, 2.57) | 5 (14) | 1.45 (0.53, 3.96) | 0.40 |
| Smoking | 0.16 | ||||
| Never | 20 (48) | 1.00 (ref) | 20 (56) | 1.00 (ref) | |
| Former | 13 (31) | 0.62 (0.30, 1.28) | 13 (36) | 0.75 (0.36, 1.58) | |
| Current | 9 (21) | 2.79 (1.18, 6.62) | 3 (8) | 0.83 (0.23, 2.97) | 0.06 |
| BMI (kg/m2) | 0.63 | ||||
| <25 | 21 (50) | 1.00 (ref) | 19 (53) | 1.00 (ref) | |
| ≥25-30 | 12 (29) | 0.80 (0.38, 1.68) | 7 (19) | 0.59 (0.24, 1.45) | |
| >30 | 9 (21) | 0.83 (0.37, 1.89) | 10 (28) | 1.17 (0.52, 2.63) | |
| Genital powder use | 13 (31) | 0.94 (0.48, 1.87) | 10 (28) | 0.91 (0.43, 1.96) | 0.95 |
| Aspirin use | 8 (19) | 0.60 (0.26, 1.35) | 9 (25) | 1.14 (0.50, 2.62) | 0.26 |
| Ibuprofen use | 11 (26) | 0.92 (0.44, 1.92) | 12 (33) | 0.87 (0.41, 1.83) | 0.92 |
| Menstrual pain | 17 (40) | 1.60 (0.83, 3.08) | 16 (44) | 1.63 (0.81, 3.27) | 0.97 |
| Alcohol (g/day) | 0.37 | ||||
| 0-1.97 | 16(41) | 1.00 (ref) | 15(44) | 1.00 (ref) | |
| 1.97-5.97 | 8(21) | 1.06 (0.45, 2.53) | 11(32) | 1.60 (0.71, 3.58) | |
| 5.97-12.8 | 5(13) | 0.69 (0.25, 1.92) | 4(12) | 0.51 (0.16, 1.57) | |
| >12.8 | 10(26) | 1.37 (0.61, 3.07) | 4(12) | 0.57 (0.18, 1.76) | |
Abbreviations: TNF-α=tumor necrosis factor alpha, IL-6=interleukin 6, BMI=body mass index
Adjusted for age, OC use, parity and family history or breast or ovarian cancer in the polytomous logistic regression model
p-value for heterogeneity was obtained using a likelihood ratio test
After restricting the analysis to high-grade serous carcinomas (n=50), the association between parity and TNF-α positive tumors persisted. Specifically, women with two or more children had a significant reduction in TNF-α positive (OR=0.35, 95% CI 0.13-0.93) but not TNF-α negative high-grade serous carcinomas (OR=2.4, 95% CI 0.55-10.38; p-heterogeneity = 0.06). An analysis of TNF-α staining status and other histologic subtypes of ovarian cancer could not be performed due to the small number of cases of other histological subtypes. Due to the limited extent of IL-6 staining across the entire study group, no further analysis was done to determine any associations between IL-6 and ovarian cancer risk factors and inflammatory exposures.
Discussion
Ovarian cancer is a heterogeneous disease in terms of pathogenesis and underlying molecular alterations. A fallopian tube origin has recently been postulated for high grade serous carcinomas that typically harbor TP53 mutations.[15] In contrast, the endometrioid and clear cell subtypes typically arise in association with endometriosis and are associated with PTEN or ARID1A mutations[25, 26] while mucinous adenocarcinomas are notable for KRAS mutations similar to their gastrointestinal counterparts[27]. There is also robust epidemiological evidence for the existence of an “inflammatory” pathway to ovarian carcinogenesis[1]. Multiparity, prolonged oral contraceptive use and tubal ligation are associated with a reduced risk of ovarian cancer while pro-inflammatory exposures such as endometriosis, increased body mass index, and genital powder use increase the risk of ovarian cancer. Moreover, systemic elevation of serum markers of inflammation such CRP[8-11], TNF-α and IL-6[28-30], have been shown to predict ovarian cancer development in prior studies. Our data suggests that the protective effect of multiparity is confined to reducing the risk of TNF-α positive ovarian cancer. These findings lend support to the hypothesis of an inflammatory pathway of ovarian carcinogenesis in which TNF-α may play a key pathogenic role. Compared to nulliparous women, parous women had a 69-79% reduced risk of developing TNF-α positive ovarian carcinoma but no reduction in risk of TNF-α negative tumors. Interestingly, current smokers in our study showed a nearly three-fold increased risk for developing TNF-α negative but not TNF-α positive ovarian cancer. Finally, the association between inflammatory exposures such as endometriosis, BMI, genital powder, aspirin or ibuprofen use and ovarian cancer did not differ by TNF-α expression status. IL-6 was focally present in a small proportion of high grade serous and endometrioid carcinomas but showed no significant epidemiological associations.
A strong reduction in risk of TNF-α positive tumors for parous women, but not for TNF-α negative tumors suggests that parity might protect against ovarian carcinomas that develop along an inflammatory pathway. The lack of a difference in risk by TNF-α staining status and oral contraceptive use suggests that the inverse association between parity and TNF-α positive tumors cannot be merely explained by suppression of ovulation suppression. If that were the case, we should have detected a similar inverse association between oral contraceptive use and TNF-α positive ovarian carcinomas. However, this finding must be interpreted with caution given the retrospective nature of our study design and small sample size. Similarly, our data suggest no association between inflammation-related exposures such as endometriosis, high BMI, genital powder use, alcohol use, menstrual pain, aspirin or ibuprofen use, and TNF-α status of ovarian cancer. However, the lack of any significant association may be due to small sample size and paucity of ovarian cancer subtypes such as clear cell and endometrioid carcinomas that are known to be associated with inflammatory exposures such as endometriosis.
Our findings suggest that ovarian cancer that arises in the setting of pro-inflammatory exposures is also a heterogeneous group and that TNF-α might play a pathogenic role in the development of some but not all such tumors. TNF-α is relevant to several aspects of ovarian function, including follicular growth, ovulation, and corpus luteum regression[31-33]. Whether TNF-α is relevant to initiation or maintainance of oncogenic transformation, or both, in ovarian surface epithelium remains to be determined in future studies.
Evaluation of TNF-α in ovarian tumors has been limited by methodological constraints. The lack of robust, validated antibodies that work well on paraffin sections has been a serious limitation. Prior studies that have used immunohistochemistry and/or in-situ hybridization techniques have shown discrepant results when used on the same set of tumors. In a small series of 25 cases, Takayama and colleagues showed positive TNF-α staining in 80% by immunohistochemistry but in only 35% by ISH suggesting non-specific immunoreactivity in the tumor cells[34]. Similarly, the distribution of TNF protein was noted to be different from that of TNF mRNA in a study by Naylor et al that showed majority of TNF protein localized to the stroma or the epithelial-stromal interface[35, 36] Others have used immunohistochemistry to evaluate expression of inflammatory markers, including TNF-α, IL-1, IL-6, IL-10, TGFβ and COX2, but have been limited either by high background on immunohistochemical staining[37] small sample size with a broad mix of benign and malignant ovarian tumors[38] or by lack of well annotated clinical data in order to detect significant epidemiological associations. TNF-α has also been evaluated in ovarian cancer by gene expression arrays but these are limited by their inability to distinguish between TNF-α expression by tumor cells versus tumor infiltrating inflammatory cells[19].
There is emerging data on the role of TNF-α in ovarian cancer pathogenesis. Cultured ovarian cancer cells show a 1000 fold increase in TNF-α mRNA levels compared to cultured normal ovarian surface epithelial cells[19]. Concurrent increase in TNF-α, CXCL12 and IL-6 mRNA levels was recently shown in ovarian cancers using gene expression arrays[39]. In this same study, upregulation of Notch signaling was seen with TNF-α, CXCL12 and IL-6 coexpressing tumors. Moreover, use of neutralizing antibodies or siRNA against these inflammatory mediators led to reduced angiogenesis and peritoneal tumor growth in in-vitro assays. It has also been postulated that the effects of TNF-α in ovarian cancer are mediated through its receptor TNFR2 since significantly higher levels of both TNF-α and TNFR2, but not TNFR1, were found in ovarian cancer compared to normal ovary[20]. The high levels of TNF-α mRNA in ovarian cancer cells can be partly suppressed by infliximab[19] which has been shown to be capable of binding to the surface of ovarian cancer cells[40]. However, none of the studies mentioned above attempted to evaluate the association between ovarian cancer risk factors or inflammatory exposures in relation to inflammatory cytokine expression in these tumors.
There are limitations to our study. Given the small sample size, the role of chance cannot be ruled out, particularly the ability to confidently determine no difference in TNF positive and TNF negative associations. However, we used polytomous logistic regression to evaluate how exposures influence the risk of TNF-α positive and negative tumors. This is a more robust statistical approach since it analyzes the whole dataset together instead of dividing it into two groups (TNF-α positive and TNF-α negative). A larger sample size will be needed to validate these results in an independent population. Furthermore, the small sample size precluded analysis by histologic subtype aside from restriction to high grade serous tumors. Thus, we were unable to analyze whether the protective role of parity is confined to a particular subset of ovarian cancer or is equally effective across all histological subtypes. We were also unable to effectively evaluate less common inflammatory exposures or associations of IL-6 expression since only focal expression was detected in a small number of tumors. Despite these limitations, using a novel RNA ISH method we were able to convincingly show presence of TNF-α transcripts in ovarian cancer epithelium and detect a significant association between parity and risk of developing TNF-α positive ovarian carcinoma.
In summary, TNF-α expression is detected by RNA ISH technique in nearly 50% of high grade serous and 83% of endometrioid ovarian carcinomas. Parity markedly reduces the risk of developing TNF-α positive ovarian cancer but does not affect the risk of developing TNF-α negative tumors, which lends additional support to the hypothesis of an inflammatory pathway of ovarian carcinogenesis. Whether TNF-α acts as an oncogenic stimulus by itself or promotes growth of tumor cells initiated by other oncogenic factors and whether targeting TNF-α can be an effective chemotherapeutic option remains to be determined in future studies.
Supplementary Material
Acknowledgments
This work was supported in part by the following grants: NIH R01 CA054419-10 and P50 CA105009, DOD W81XWH-10-1-0280, 5T32CA009001-38
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose
REFERENCES
- 1.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 2.Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50:233–238. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- 3.Rice MS, Murphy MA, Tworoger SS. Tubal ligation, hysterectomy and ovarian cancer: A meta-analysis. J Ovarian Res. 2012;5:13. doi: 10.1186/1757-2215-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terry KL, Karageorgi S, Shvetsov YB, Merritt MA, Lurie G, Thompson PJ, Carney ME, Weber RP, Akushevich L, Lo-Ciganic WH, Cushing-Haugen K, Sieh W, Moysich K, Doherty JA, Nagle CM, Berchuck A, Pearce CL, Pike M, Ness RB, Webb PM, Australian Cancer S. Australian Ovarian Cancer Study G. Rossing MA, Schildkraut J, Risch H, Goodman MT, Ovarian Cancer Association C Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res (Phila) 2013;6:811–821. doi: 10.1158/1940-6207.CAPR-13-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen CM, Nagle CM, Whiteman DC, Ness R, Pearce CL, Pike MC, Rossing MA, Terry KL, Wu AH, Australian Cancer S. Australian Ovarian Cancer Study G. Risch HA, Yu H, Doherty JA, Chang-Claude J, Hein R, Nickels S, Wang-Gohrke S, Goodman MT, Carney ME, Matsuno RK, Lurie G, Moysich K, Kjaer SK, Jensen A, Hogdall E, Goode EL, Fridley BL, Vierkant RA, Larson MC, Schildkraut J, Hoyo C, Moorman P, Weber RP, Cramer DW, Vitonis AF, Bandera EV, Olson SH, Rodriguez-Rodriguez L, King M, Brinton LA, Yang H, Garcia-Closas M, Lissowska J, Anton-Culver H, Ziogas A, Gayther SA, Ramus SJ, Menon U, Gentry-Maharaj A, Webb PM, Ovarian Cancer Association C Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;20:251–262. doi: 10.1530/ERC-12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, Chang-Claude J, Hein R, Lurie G, Wilkens LR, Carney ME, Goodman MT, Moysich K, Kjaer SK, Hogdall E, Jensen A, Goode EL, Fridley BL, Larson MC, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Vitonis AF, Titus LJ, Ziogas A, Brewster W, Anton-Culver H, Gentry-Maharaj A, Ramus SJ, Anderson AR, Brueggmann D, Fasching PA, Gayther SA, Huntsman DG, Menon U, Ness RB, Pike MC, Risch H, Wu AH, Berchuck A. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clendenen TV, Koenig KL, Arslan AA, Lukanova A, Berrino F, Gu Y, Hallmans G, Idahl A, Krogh V, Lokshin AE, Lundin E, Muti P, Marrangoni A, Nolen BM, Ohlson N, Shore RE, Sieri S, Zeleniuch-Jacquotte A. Factors associated with inflammation markers, a cross-sectional analysis. Cytokine. 2011;56:769–778. doi: 10.1016/j.cyto.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundin E, Dossus L, Clendenen T, Krogh V, Grankvist K, Wulff M, Sieri S, Arslan AA, Lenner P, Berrino F, Hallmans G, Zeleniuch-Jacquotte A, Toniolo P, Lukanova A. C-reactive protein and ovarian cancer: a prospective study nested in three cohorts (Sweden, USA, Italy) Cancer causes & control: CCC. 2009;20:1151–1159. doi: 10.1007/s10552-009-9330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McSorley MA, Alberg AJ, Allen DS, Allen NE, Brinton LA, Dorgan JF, Pollak M, Tao Y, Helzlsouer KJ. C-reactive protein concentrations and subsequent ovarian cancer risk. Obstet Gyncol. 2007;109:933–941. doi: 10.1097/01.AOG.0000257126.68803.03. [DOI] [PubMed] [Google Scholar]
- 10.Poole EM, Lee IM, Ridker PM, Buring JE, Hankinson SE, Tworoger SS. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor alpha receptor 2 levels and risk of ovarian cancer. Am J of Epid. 2013;178:1256–1264. doi: 10.1093/aje/kwt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toriola AT, Grankvist K, Agborsangaya CB, Lukanova A, Lehtinen M, Surcel HM. Changes in pre-diagnostic serum C-reactive protein concentrations and ovarian cancer risk: a longitudinal study. Annals of Onc: Official Journal of the European Society for Medical Oncology / ESMO. 2011;22:1916–1921. doi: 10.1093/annonc/mdq694. [DOI] [PubMed] [Google Scholar]
- 12.Trabert B, Pinto L, Hartge P, Kemp T, Black A, Sherman ME, Brinton LA, Pfeiffer RM, Shiels MS, Chaturvedi AK, Hildesheim A, Wentzensen N. Pre-diagnostic serum levels of inflammation markers and risk of ovarian cancer in the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial. Gynecol Oncol. 2014;135:297–304. doi: 10.1016/j.ygyno.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 14.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–1197. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 16.Ronnett BM, Seidman JD. Mucinous tumors arising in ovarian mature cystic teratomas: relationship to the clinical syndrome of pseudomyxoma peritonei. Am J Surg Pathol. 2003;27:650–657. doi: 10.1097/00000478-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Wu RC, Shwartz LE, Haley L, Lin MT, Shih Ie M, Kurman RJ. Clonality analysis of combined Brenner and mucinous tumours of the ovary reveals their monoclonal origin. J Pathol. 2015;237:146–151. doi: 10.1002/path.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szlosarek PW, Grimshaw MJ, Kulbe H, Wilson JL, Wilbanks GD, Burke F, Balkwill FR. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol Cancer Ther. 2006;5:382–390. doi: 10.1158/1535-7163.MCT-05-0303. [DOI] [PubMed] [Google Scholar]
- 20.Piura B, Medina L, Rabinovich A, Dyomin V, Levy RS, Huleihel M. Distinct expression and localization of TNF system in ovarian carcinoma tissues: possible involvement of TNF-alpha in morphological changes of ovarian cancerous cells. Anticancer Res. 2014;34:745–752. [PubMed] [Google Scholar]
- 21.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitonis AF, Titus-Ernstoff L, Cramer DW. Assessing ovarian cancer risk when considering elective oophorectomy at the time of hysterectomy. Obstet Gynecol. 2011;117:1042–1050. doi: 10.1097/AOG.0b013e318212fcb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serov SF, Scully RE. Collaboration with L.H. Sobin and pathologist in ten countries. World Health Organization; Geneva, Switzerland: Roto-Sadag, S.A., Geneva: 1973. International Histological Classification of Tumours. [Google Scholar]
- 24.Hecht JL, Kotsopoulos J, Gates MA, Hankinson SE, Tworoger SS. Validation of tissue microarray technology in ovarian cancer: results from the Nurses’ Health Study. Cancer Epidemiol Biomarkers Prev. 2008;17:3043–3050. doi: 10.1158/1055-9965.EPI-08-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho KR, Shih Ie M. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, Fereday S, Tam A, Galletta L, Tonin PN, Provencher D, Miller D, Jones SJ, Moore RA, Morin GB, Oloumi A, Boyd N, Aparicio SA, Shih Ie M, Mes-Masson AM, Bowtell DD, Hirst M, Gilks B, Marra MA, Huntsman DG. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gemignani ML, Schlaerth AC, Bogomolniy F, Barakat RR, Lin O, Soslow R, Venkatraman E, Boyd J. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol. 2003;90:378–381. doi: 10.1016/s0090-8258(03)00264-6. [DOI] [PubMed] [Google Scholar]
- 28.Maccio A, Lai P, Santona MC, Pagliara L, Melis GB, Mantovani G. High serum levels of soluble IL-2 receptor, cytokines, and C reactive protein correlate with impairment of T cell response in patients with advanced epithelial ovarian cancer. Gynecol Oncol. 1998;69:248–252. doi: 10.1006/gyno.1998.4974. [DOI] [PubMed] [Google Scholar]
- 29.Darai E, Detchev R, Hugol D, Quang NT. Serum and cyst fluid levels of interleukin (IL) -6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum Reprod. 2003;18:1681–1685. doi: 10.1093/humrep/deg321. [DOI] [PubMed] [Google Scholar]
- 30.Moradi MM, Carson LF, Weinberg B, Haney AF, Twiggs LB, Ramakrishnan S. Serum and ascitic fluid levels of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in patients with ovarian epithelial cancer. Cancer. 1993;72:2433–2440. doi: 10.1002/1097-0142(19931015)72:8<2433::aid-cncr2820720822>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 31.Brannstrom M, Norman RJ. Involvement of leukocytes and cytokines in the ovulatory process and corpus luteum function. Hum Reprod. 1993;8:1762–1775. doi: 10.1093/oxfordjournals.humrep.a137929. [DOI] [PubMed] [Google Scholar]
- 32.Norman RJ, Brannstrom M. Cytokines in the ovary: pathophysiology and potential for pharmacological intervention. Pharmacol Ther. 1996;69:219–236. doi: 10.1016/0163-7258(95)02064-0. [DOI] [PubMed] [Google Scholar]
- 33.Terranova PF, Rice VM. Review: cytokine involvement in ovarian processes. Am J Reprod Immunol. 1997;37:50–63. doi: 10.1111/j.1600-0897.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 34.Takeyama H, Wakamiya N, O’Hara C, Arthur K, Niloff J, Kufe D, Sakarai K, Spriggs D. Tumor necrosis factor expression by human ovarian carcinoma in vivo. Cancer Res. 1991;51:4476–4480. [PubMed] [Google Scholar]
- 35.Naylor MS, Malik ST, Stamp GW, Jobling T, Balkwill FR. In situ detection of tumour necrosis factor in human ovarian cancer specimens. Eur J Cancer. 1990;26:1027–1030. doi: 10.1016/0277-5379(90)90043-s. [DOI] [PubMed] [Google Scholar]
- 36.Naylor MS, Stamp GW, Foulkes WD, Eccles D, Balkwill FR. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J Clin Invest. 1993;91:2194–2206. doi: 10.1172/JCI116446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plewka D, Kowalczyk AE, Jakubiec-Bartnik B, Morek M, Bogunia E, Kmiec A, Wierzbicki PM, Plewka A. Immunohistochemical visualization of pro-inflammatory cytokines and enzymes in ovarian tumors. Folia Histochem Cytobiol. 2014;52:124–137. doi: 10.5603/FHC.2014.0015. [DOI] [PubMed] [Google Scholar]
- 38.Jammal MP, AA DAS, Filho AM, DECC E, Adad SJ, Murta EF, Nomelini RS. Immunohistochemical staining of tumor necrosis factor-alpha and interleukin-10 in benign and malignant ovarian neoplasms. Oncol Lett. 2015;9:979–983. doi: 10.3892/ol.2014.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulbe H, Chakravarty P, Leinster DA, Charles KA, Kwong J, Thompson RG, Coward JI, Schioppa T, Robinson SC, Gallagher WM, Galletta L, Australian Ovarian Cancer Study G. Salako MA, Smyth JF, Hagemann T, Brennan DJ, Bowtell DD, Balkwill FR. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72:66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naylor MS, Stamp GW, Balkwill FR. Investigation of cytokine gene expression in human colorectal cancer. Cancer Res. 1990;50:4436–4440. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.