Abstract
Free will is a perception that people have that they choose to make their movements. This perception includes a sense of willing the movement and self-agency that they are responsible for the movement. If there is a “free will force” that plays a role in movement selection, it should precede movement. There is no evidence for a driving force, and the perception of willing is not fully processed until after the movement. The perceptions of free will likely arise from an interaction between frontal and parietal areas. Free will might be considered to exist if a person’s brain is functioning normally without coercion.
Keywords: Free will, volition, willing, agency, self-agency, consciousness, qualia, Bereitschaftspotential, neuroimaging, transcranial magnetic stimulation
Everyone has the sense that their movements are ordinarily freely chosen and their thoughts are freely made. I decide that I want to pick up this chocolate bar and eat it, and then I do it. This sense of free will is important to a person’s sense of themselves. I think, therefore I am. I make my movements, therefore I am, and, moreover, others can see who I am. Despite this crucial, universal sense, the nature of free will itself is somewhat obscure and controversial. What does free will mean? Does it really exist? If so, what is its physiology? Our science at the moment does have answers, or at least partial answers, to these questions.
Definitions must come first. Free will is a person’s perception that thoughts and movements made are a person’s choice. In this discussion, I will focus mostly on movements since they are more easily measurable. Free will for movement also includes a sense that the decision itself is a driving force for that movement. It is because of the decision that the movement occurs. That there is a perception of free will is clear; that there is a driving force is murky.
The topic of free will has been the subject of philosophical discussion for centuries.1, 2 One school of philosophy says that there cannot be free will since the world is deterministic, and free will is incompatible with determinism. Everything is predictable on the basis of fundamental physics. There cannot be free will since all movement is fully predicable from what has happened before. An opposite school says that free will can exist because the world is not fully deterministic. Yet another school says that free will and determinism are actually compatible, since if a course of action is chosen, then that choice drives the movement. There are also theological schools of thought. One postulates that only God makes all the choices, while another grants free will to persons and sets God up in judgment as to whether you did the right thing. Another philosophical issue, which is also a legal issue, is when persons can be deemed responsible for their behavior. This topic is beyond the scope of this discussion.
Heisenberg physics rather than Newtonian physics allows that physical laws are not fully deterministic. Some events are stochastic and that the future can only be probabilistically predicted. It is also possible that some laws of nature are top down as opposed to bottom up, and that may confound determinism.3 For the purposes here, I will assume that free will is possible; that physics or God have not determined the course of the universe. Given that it is possible, the rest of this essay will summarize what we know about its physiology.
There are two aspects of free will, willing and self-agency. The sense of willing is the freely made decision to initiate a movement. The general view would be that this decision and its perception occur at the same time, and that this time is before the movement. Indeed, it would have to be before the movement if it was to be causal. Cause must precede effect. Self-agency is the sense that the person himself is responsible for the movement that just occurred. Willing and agency are separable. Willing can occur without agency; consider patients with a stroke, spinal cord injury, or peripheral injury; they can will, but nothing will happen. Self-agency generally requires both willing and the movement to occur, but, in certain types of anosognosia, patients can have the sense that they have voluntarily moved, when in fact they have not.
The timing of the sense of willing
Movements are easily measured and timed. Perceptions not so. Perceptions are subjectively reportable data. They are elements of what we are consciously aware. These elements are called qualia, and willing is a quale. Qualia are quite real to each of us, yet they are “creations of the mind”. Illusions show us that the mind is easily fooled, and neurologists regularly see that confabulation can be a personal reality. Yet it is possible to use perceptions and their timing as data. We just need to be careful.
The timing of the quale of willing can be determined. Libet and colleagues,4 in a now classic paper, first determined the timing of willing compared with the timing of movement and its associated brain activity. The time of willing was called W, and the time of the sense that the movement occurred was called M. Subjects sat in front of a clock face with a ball that moved around the clock in about 3 seconds. Subjects made movements at freely chosen times and subsequently reported either the time of W or the time of M depending on where the ball was at the subjective moment (Fig. 1). These times were compared with the actual time of movement and the EEG activity that preceded the movement. The EEG activity preceding movement is a slowly rising negative potential called the Bereitschaftspotential (BP), as originally named,5 or Readiness Potential (RP), as Libet et al. used in English translation. This potential typically begins about 1 to 1.5 sec prior to movement. Without going into detail, M was close to movement onset, W was about 300 ms prior to movement onset, and RP onset was about 1 sec prior to movement. Hence RP onset was about 700 ms prior to W. Superficially, these data suggest that the brain has initiated the process of movement initiation long before the subjective sense of willing the movement. This would violate the ordinary sense that the decision to move is primary, followed by movement initiation. These results have been duplicated many times. The data are clear, the interpretation difficult. Some have said that these data disprove that free will exists. Libet et al. said that, yes, movement initiates unconsciously, but free will is actually still possible because a movement once initiated by the brain can be consciously vetoed. It might be said then that they did not believe in free will, only “free won’t”. Others commenting on these data have raised a variety of issues, even raising problems with the experiment itself, saying that the experiment cannot answer the question.
Fig. 1.
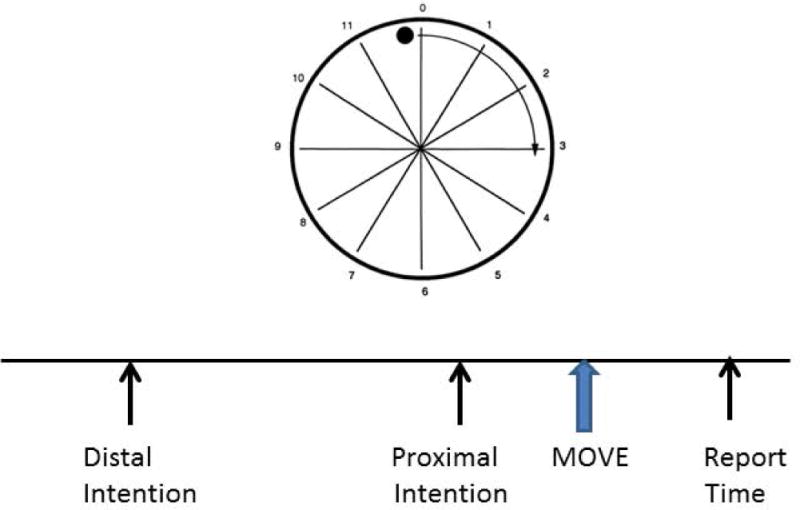
Diagrammatic set-up for the experiments of Libet et al.4 and Vinding et al.8 Subjects sit in front of a “clock” with a ball that moves around the circumference every 3 seconds. They make movements whenever they want, and then afterwards report the “time” of distal intention (thinking about making the movement sometime in future), proximal intention (willing of the movement, W), or the movement itself (M). In the Libet et al. experiment, the EEG is backaveraged from the movement; in the Vinding et al. experiment, the EEG is backaveraged from the time of distal intention.
A Libet-like experiment was also done with fMRI.6 In this experiment, subjects in the scanner made movements of right or left hand at freely chosen times while watching a series of letters. After the movement, subjects were asked when they decided to move either hand by indicating what letter they saw at the time of the decision. In this experiment, it was possible to identify with 60% probability (slightly more than chance) which hand would be moved as long as 8 sec before the movement, while the subjective perception was within the second prior to movement.
One argument against the Libet experiment is that making small movements in the laboratory has nothing to do with free will.7 The subject is just following orders. Free will is the decision to agree to do the experiment in the first place; it is a thought and not a movement. This argument distinguishes between distal and proximal intentions. The distal intention, the thought, is the agreement to participate; the proximal intention is the stereotyped movements. In order to get around this argument, in part, it is possible to do a Libet-like experiment where there are both distal and proximal intentions. In this experiment, the subject makes a decision (distal intension) to move at a time in the future, and the time of this decision is noted on the clock (Fig. 1). The EEG can then be averaged on the decision times. In such experiments, a BP like potential can be seen prior to the distal intention.8 Thus, the brain can be seen to be unconsciously active prior to a “thought”, not only a movement. In another recent experiment, a BP like potential was seen prior to subjective time of selection of a letter whether or not there was an associated movement.9 The investigators concluded that the BP could be associated just with decision making.
Another way of getting at the timing of the subjective perception of willing was reported by Matsuhashi and myself.10 Again, subjects made movements at freely chosen times, but now while listening to tones (Fig. 2). If a tone came after the intention to move, but before the movement occurred, then the subject was to veto the movement. This is an experiment dealing with free won’t, but the beginning of the time period of vetoing should be the time of willing, here called T for “thinking” about moving. The end of the period of vetoing is the point of no return, when the movement will occur despite attempts to veto it. The period of vetoing is identified by a histogram of the times of tones prior to movements. While we originally thought T would be similar to W, it was earlier with respect to movement onset, about 1400 ms on average, but still shorter than the time of the beginning of the BP, which was 2200 ms in our experiments. To explain this discrepancy, we suggested that the period between T and W would be a period of “probe awareness”. Probe awareness is a quale just below the “awareness” threshold and can be brought into awareness by probing for it. This experiment would then suggest that movement initiation begins fully unconsciously, followed by probe awareness, followed by full awareness.
Fig. 2.
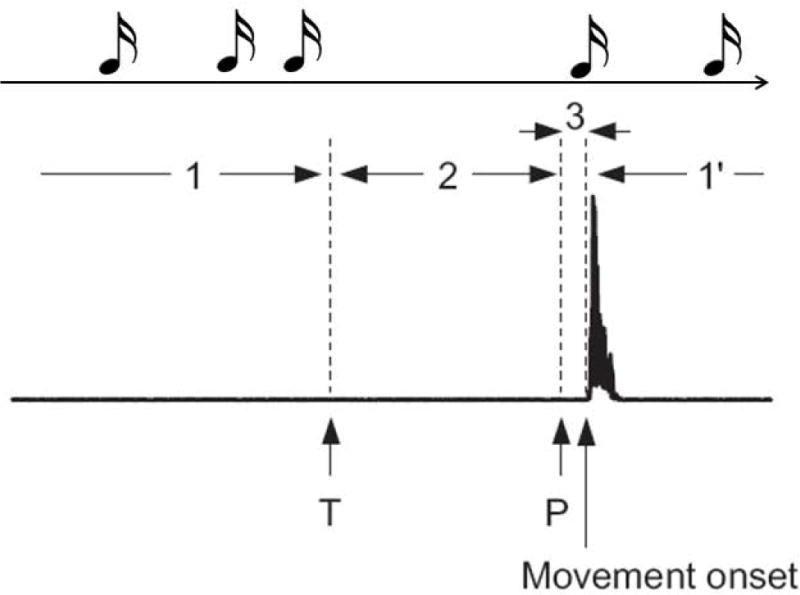
Diagrammatic set-up for the experiment of Matsuhashi and Hallett.10 Subjects made movements at freely chosen times as soon as they thought about doing so (time T). They also listened to tones delivered randomly. If they heard a tone after deciding to move, then they should veto the movement. Hence, looking at the timing of tones with respect to movements, in period 1, tones will occur since T had no happened yet, in period 2, there should not be any tones since the movement will be vetoed, in period 3, tones will occur again since the point of no return (P) will have been reached and the movement cannot be vetoed. Diagram is modified from the original paper.
If movement truly begins unconsciously, then it should be possible to show that persons are thinking about something else when the EEG shows that a movement is being initiated. While the BP indicates movement preparation, it is ordinarily obtained by off-line averaging of about 50 trials. To use the BP for this purpose, it would have to be recognized in real time in single trials. This engineering feat was accomplished by Bai et al.11 using an array of EEG electrodes and all the EEG frequency content. Then we could do the following experiment.12 Subjects were asked to make movements at freely chosen times, and when movement happened a light went on (Fig. 3). First, the EEG before a movement was identified individually, and this was typically mainly activity in the beta band over the central region contralateral to the movement. The prediction algorithm was calculated to have low false positive rate, not worrying about false negatives. Second, subjects were again asked to move freely and when the algorithm predicted a movement, the computer turned the light on. A number of events were produced by this. Movements by the subject followed by the light (false negatives), lights followed quickly by movement (subjects thought that “we beat them to it”), and lights without movement. The latter could have been due to several reasons: (1) there had been movement intention, but there was “no need” to move since the light was already turned on, (2) there was no movement intention and a false positive prediction, and (3) there was no movement intention but a true prediction. The last two reasons could not be separated, but taken together there were twice as many of them as would be predicted from our false positive rate. Hence, some must have been true predictions. (As a third step of the experiment, we tested the model again to prove that it had not changed and the false positive rate had not increased.)
Fig. 3.
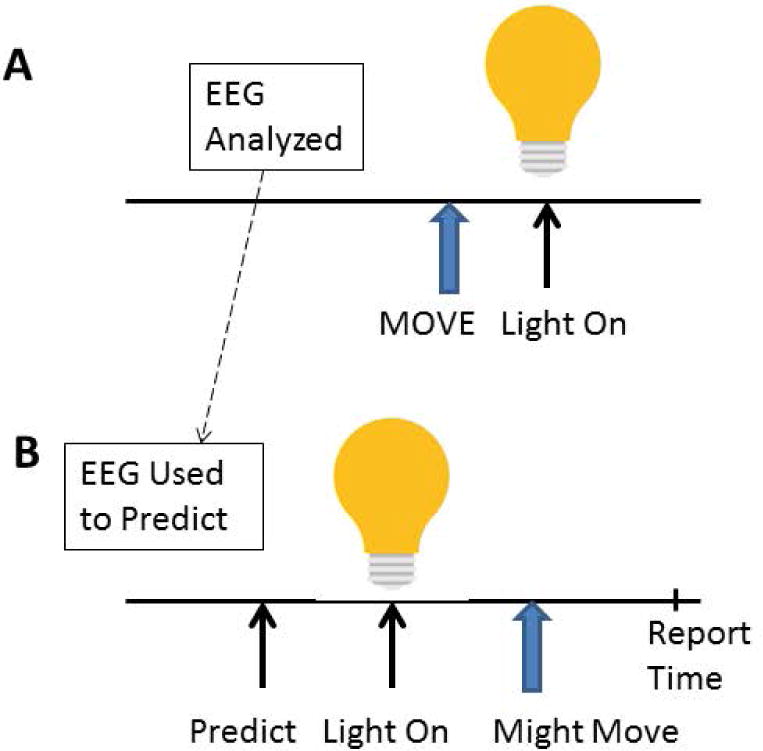
Diagrammatic set-up for the experiment of Schneider et al.12 In the first part of the experiment, A, subjects made movements freely and when they moved a light went on. EEG was analyzed during these movements and a prediction algorithm was calculated. In the second part of the experiment, B, the situation for the subject was the same, but if the algorithm detected that the subject was going to make a movement, then the computer turned on the light. After that the subject sometimes did and sometimes did not move. Following the light and possible move, we asked the subject to report what they were thinking when the light went on.
The interesting part of the experiment was asking persons what they were thinking when the light went on and they had not moved. Sometimes, as noted above, it was movement intention. Other times, it was various random thoughts such as what was going to happen for dinner. Thus, it was possible to demonstrate directly that persons could be thinking about something else when the brain was preparing to move.
Using single cell recordings from human premotor cortex (during neurosurgical procedures), activity could be seen prior to freely chosen movement.13 Similar to what Bai et al did, it was possible to create a model with this activity to predict when movement would occur. The model with these data was much more accurate than what could be done with EEG; however, the investigators did not duplicate the second part of the experiment, making the predictions before the subjects actually moved.
Manipulating the timing of the sense of willing
Curiously, it is possible to manipulate the timing of W in Libet-like experiments. Single pulse transcranial magnetic stimulation (TMS) was given to either the motor cortex or the supplementary motor area (SMA) either at the time of the freely chosen movement or 200 ms later.14 There was no effect of stimulation over the motor cortex, but stimulation over SMA could influence the reported time of W at either time point of stimulation. W is presumably occurring before movement, yet its reported time could be influenced by TMS done later. Transcranial direct current stimulation over either angular gyrus or primary motor cortex during a Libet-like experiment caused W to be earlier.15 Moreover, the effect on W could be accounted for by changes in slow wave activity both before and after the movement.
Another way of influencing W is perhaps even more remarkable. The Libet-like experiment is done with a button press that leads to production of a tone.16 The tone is delayed in intervals from 5 to 60 ms after the press. In this circumstance, W is delayed linearly with the delay of the tone. In only the 5 ms interval was W prior to the press itself; in the other delays W was said to be after the button press. Thus W is influenced in a systematic fashion after the movement is done. Similar experiments by the same authors show the same effect on M.17
Anatomy of the sense of willing
In fMRI experiments, paying attention to a phenomenon will increase the activation associated with it. Attention to intention increases activity in the temporoparietal junction region, the SMA and the dorsolateral prefrontal cortex.18
At the time of neurosurgical procedures parts of the brain can be stimulated to determine their function. This is useful, of course, for mapping prior to any intervention. Stimulating the motor cortex produces a movement which is well known. Stimulation of SMA provokes an “urge” to move, while stimulation of the parietal cortex provokes an “intention” to move.19 It is claimed that “urge” might only be a “wanting to move”, while “intention” is the feeling that actually precedes a specific movement. Whether these sensations are actually different, both are proximate to movement. Patients with bilateral parietal lesions, in the Libet experiment, have an abnormal W, very close to movement onset, but the behavioral correlate of that was not described in the paper where this was reported.20 However, the authors had described loss of agency in patients with apraxia from parietal lesions earlier,21 and this has been confirmed by others.22
Interpretation of the data concerning willing
It is important to recognize that conscious perceptions are delayed compared to real world events. In essence, we live in the past. It takes time for peripheral stimuli to reach the central nervous system and be processed into perceptions.23 This would be the order of one or several hundreds of milliseconds. Libet and his colleagues also did experiments with direct continuous stimulation of the sensory cortex, seeing how long it would be until subjects recognized a sensation. This process took several hundred msec, but then the time of the stimulus was referred to when the stimulus began.24
W, M and T are examples of subjective perceptions. Movement and the BP are real world events. If this is the case, then the brain processes underlying W, M and T are actually occurring over a period of time and the end of that period is after the time they are reported. Hence, it is therefore possible to influence W and M after the movement since the processing of these perceptions is likely not finished until after the movement.25 See Fig. 4 for timing of the phenomena.
Fig. 4.
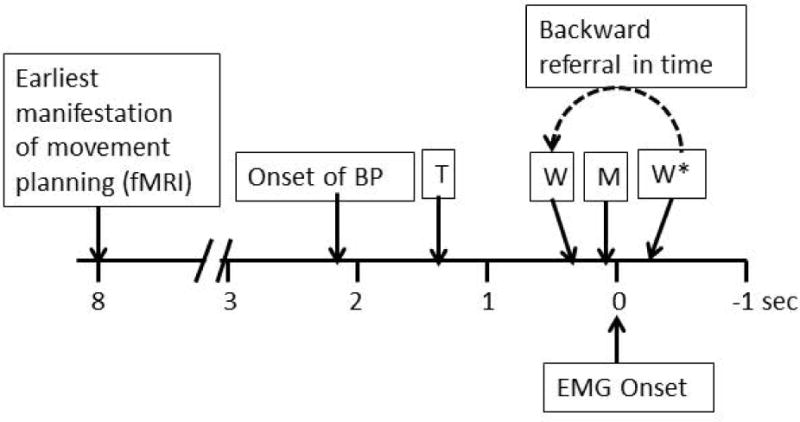
Time line for events related to movement initiation and execution with associated subjective perceptions. BP= Bereitschaftspotential. T=time of thinking about making a movement. W=time of willing a movement. M=time that a movement is thought to be initiated. W*=end of the time when the perception of W can be influenced. Note that the time of W is referred backward in time even though its processing is not completed until after the movement in already initiated.
The physiology of the sense of willing gives some answers and one result is clear: the sense of willing is not contemporaneous with movement initiation. It certainly seems that movement is generated subconsciously and conscious awareness of this intention occurs late in the process, even possibly after the movement itself. Moreover, the sense of willing is not even time-locked to any feature of movement initiation and can be manipulated.
Agency
As noted earlier, agency (specifically self-agency) is the sense that you have made the movement that has just happened. Generally, there must be willing of a movement and then the observation of a compatible movement that follows. As emphasized by Wegner,26 for W to be the cause of M, W must precede M. The process must also involve a match (or mismatch) detector. The movement must be pretty close to the movement that has been willed. The motor system generates feedforward signals to the rest of the brain when it issues a motor command. The sensory system detects the feedback of what movements have occurred. The match of feedforward and feedback are routine for the brain and are consistent with agency, if one chooses to think about it (most of the time, we don’t bother to think about it). Mismatch is somewhat jarring to the nervous system and might well set up an alert—a willed movement did not occur or occurred abnormally. To a certain extent, it is difficult to separate mismatch and agency as they are part of the same process. Per force, the sense of agency must occur (when it occurs) after movement. Studies have focused on the anatomy.
One way of investigating agency is to have persons make movements and then modulate the correctness of the feedback. One of the first experiments of this type was done by Farrer et al.27 They had persons make joystick movements and modulated the feedback which indicated a compatible or incompatible result. The main findings with blood flow PET were that the right TPJ region was negatively modulated with the amount of agency and anterior insula was positively modulated. We did a similar experiment in more detail with fMRI.28 Subjects had their right hand in a data glove and made successive movements of the fingers. The data glove captured the movements at each joint, and this information was used to drive an image of the glove on a computer screen. In the 100% condition, the movement of the image mirrored the hand movement exactly, and, after looking at this for even a short time, subjects would get the sense of agency for moving the image, and, of course, this was true. It was also possible for the experimenter to drive the image with arbitrary movements, and thus with various mixtures of the true and arbitrary signals, get what would be 80%, 60%, 40%, 20% and 0% control. Subjects were pretty good at specifying how much control/agency they had, and the fMRI signal was related to the subjective amount of agency they had in each 20 sec block of performance. All areas of modulation were negative with respect to agency. There were two prominent temporal patterns of these areas. The first, called leading, had an early peak with moderate decline over most of the rest of the block of performance. The second, called lagging, had a somewhat more sluggish onset and then only a very slow decline through the block. The most prominently activated leading regions, all on the right side, were the TPJ, superior frontal gyrus, anterior insula, and precuneus. The most prominently activated lagging regions, again on the right side, included the middle frontal gyrus and inferior parietal lobule. The results were interpreted that the early areas were principally concerned with feedback analysis, and the later areas were more related to the sense of agency. It certainly would take some time experiencing a certain level of control to make an assessment of the amount of agency. This study was repeated using EEG,29 and the most prominent correlate to agency, again inverse, was alpha band coherence in the frontal areas, perhaps correlating with the lagging areas in the fMRI study.
A meta-analysis of 15 studies of agency identified an inverse relationship in the TPJ, pre-SMA, precuneus, and the dorsomedial prefrontal cortex.30 A direct relationship was found in the insula. As with willing, the anatomy of agency has both a frontal and parietal locus, and these neuroimaging studies are confirmed with brain stimulation studies.
Non-invasive brain stimulation in the parietal area can influence the experience of agency. Subjects made reaching movements and observed a virtual hand on a computer screen.31 The virtual hand was either perturbed or not (corresponding to no agency and agency, respectively), and single pulse TMS was delivered on some trials to the right parietal cortex during the movement. With stimuli, subjects were more likely to say that they did not have agency for both types of movement. A similar experiment with 10Hz online rTMS to the right parietal cortex had similar results.32 A third study used a different type of task where button presses produced pictures of colored disks and subjects had to judge whether they had agency for producing the specific color.33 Movements were triggered by visual stimuli after receiving subliminal primes, and single pulse TMS was delivered to the right parietal area at different times during the trials. In this case, TMS interfered with the sense of agency only during motor preparation, not during motor execution, suggesting an effect on feedforward signaling rather than feedback.
A frequently used experimental paradigm for studying agency is called intentional binding.34 In such experiments, a Libet clock is used to time when persons identify the time of a button press and the time of a tone, produced with variable delay after the button press (Fig. 5). Times of button press alone and tone alone are also measured. When the button press produces the tone, the button press is appreciated later and the tone is appreciated earlier. Thus, intention binds the action and consequence together. Cathodal theta-burst TMS (inhibitory) over the pre-SMA disrupted intentional binding.35 This result has been repeated with anodal and cathodal (facilitatory and inhibitory, respectively) transcranial direct current stimulation (tDCS) over the pre-SMA.36 In another experiment, intentional binding was disrupted by anodal tDCS over the left parietal area.37
Fig. 5.
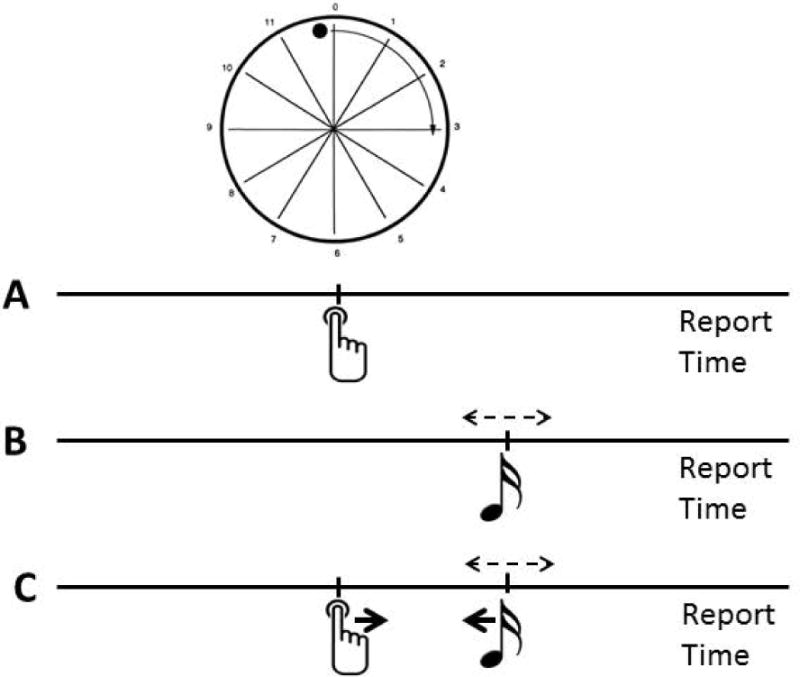
Diagrammatic set-up for intentional binding experiments. Subjects use a “Libet clock” for subjective timing. First, A, the timing of button pressing is noted (similar to Libet’s M) and, B, the timing of a randomly occurring tone. Then, C, the tone is yoked to the button press at varying intervals, and again subjects are asked to specify when the button press and tones occurred. In the setting of C, the button press subjectively occurred later and the tone occurred earlier. Hence, the two events are bound together by the intentional act.
Factors in the brain influencing choice
The perception of willing cannot drive movement; it is a perception and perceptions are passive. If there is a “free will force”, its action does not occur contemporaneously with the perception. How is it decided by the brain to make certain movements at specific times? Is there a free will force contributing to this decision making process? The processes of choice, movement planning and initiation are becoming well known, and no free will force has been recognized – nor is it necessary to describe what is happening.
Movements occur when the motor cortex issues a command. The motor cortex gets its input, directly or indirectly, from the entire brain. The posterior part of the brain provides sensory input and helps guide movement related to external stimuli; for example, getting the key in the lock. The anterior part of the brain provides drives such as hunger, thirst, sex, other pleasures, and homeostasis, coming from limbic and hypothalamic influences. The anterior part of the brain is also modulated strongly by reward, both negative and positive, and reward is mediated in part by dopamine signaling. We tend to do what has been rewarding in the past. Cortical areas are all modulated by basal ganglia and cerebellar influences. The basal ganglia in particular appear to play a role in what movement to do and what not to do. There is also some neural noise that introduces some apparent randomness. All this input comes to premotor and eventually to motor cortex; the various factors are weighed and the output determined by the strongest factors at the time. Movement can be predicted probabilistically. The more we know about a person and the factors at any time, the better our prediction can be. If asked why we have made the choice that we have made, we can always come up with a reason. The reason may indeed to relate to some of these factors, but likely some important ones are unconscious and hence our explanation might be partly confabulatory. See Fig. 6 for a diagram of how the brain might be organized to produce movement and the associated perceptions.
Fig. 6.
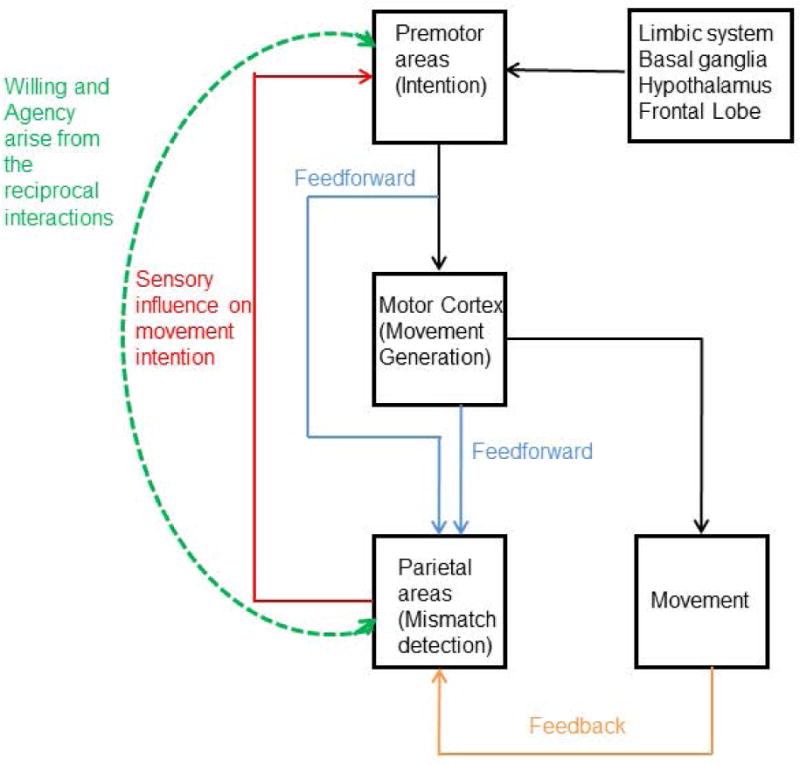
Block diagram of different parts of the brain and how they connect to produce movement and the perceptions of willing and agency. Movement intention forms in the premotor areas under internal influences of limbic system, basal ganglia, hypothalamus and frontal lobe and external influences from parietal lobe. Premotor cortex sends a command to motor cortex that produces a movement. Feedforward signals come from premotor and motor cortices to parietal area and such processing can give rise to the sense of willing. Comparing feedforward signals with feedback from the movement generated looks for mismatch. If no mismatch, then a perception of agency can arise. Note that it appears that the perceptions of willing and agency both employ a network between parietal and frontal areas.
I have previously described the salted peanut problem.25 We sit in front of a bowl of peanuts, eat some, and then say we have had enough. In less than a minute, our hand is going out for more peanuts. The peanuts are just too tasty, too rewarding, and motor system goes for the peanuts despite the content of our consciousness.
The mind-body problem
These days most of us believe that the mind-body problem is settled. The mind is a product of the brain. On the other hand, we often talk as if we do not understand what that means. With respect to the Libet et al. experiment, it is often said that “the brain knows before you do that you will be making a movement”. On the face of it, this does not make sense. You are your brain. Hence, that statement brings up a difference without a distinction. It appears that there are many things going on in the brain at all times; only one or two are in consciousness at a particular time. Much of what the brain does is unconscious, and for much of what we generally do, we do not concern ourselves with whether a movement is freely chosen or not. We just go about our business. A difficult choice might bring a particular brain process into consciousness, but that does not mean that consciousness acts upon the choice. We do not know what consciousness is or how awareness comes about, but it seems passive, the most prominent brain network operative at a particular time.38, 39 Hence, you are your brain and therefore you are making your movements (or, synonymously, your brain is making your movements).
Neurological disorders of volition
Neurologists should care about this topic or, at least, understand about it since we run into issues of volition with patients regularly. I have called these situations the neurological disorders of volition.25 They include functional movement disorders, tic, alien hand, anosognosia, and the passivity phenomena of schizophrenia; we have expanded on them elsewhere.40
Contemporary view of free will
Free will issues have been a matter of philosophy, but now are matters of science. Given that the brain makes all our movements and that the sense of free will is a passive event late in the process, what are we to make of free will itself? Is everything we do, just determined by the laws of physics? As noted in the beginning, contemporary physics would seem to allow some stochastic events. Behavior can be explained probabilistically based on past history and current factors. That is the way the brain works. If the brain is allowed to function this way, then it is free, and it is likely that this is the sense of freedom that we can appreciate. The brain is not free if in the midst of a seizure or if a gun is held to the head. In the first instance, the brain is malfunctioning, and, in the second instance, there is a strong external factor influencing what the brain will choose to do. In relation to the latter, it has been recently demonstrated that if persons are coerced to make movements in the intentional binding experiment, the amount of binding is less.41 Hence, we can have free will if our brains are free, and this is true most of the time for most people. Remaining to be explained is conscious awareness itself, what is its physiology, why do we have it, what is its function. We might need a deeper level of scientific explanation to tackle these interesting questions.
Acknowledgments
This work was supported by the NINDS Intramural Program.
Footnotes
Potential conflicts of interest: None
References
- 1.Griffith M. Free Will; The Basics. London: Routledge; 2013. [Google Scholar]
- 2.Kane R, editor. The Oxford Handbook of Free Will. 2nd. Oxford: Oxford University Press; 2011. [Google Scholar]
- 3.Murphy N, Ellis GFR, O’Connor T, editors. Downward Causation of the Neurobiology of Free Will. Berlin: Springer; 2009. [Google Scholar]
- 4.Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106(Pt 3):623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- 5.Kornhuber HH, Deecke L. Changes in the Brain Potential in Voluntary Movements and Passive Movements in Man: Readiness Potential and Reafferent Potentials. Pflugers Archiv fur die gesamte Physiologie des Menschen und der Tiere. 1965;284:1–17. [PubMed] [Google Scholar]
- 6.Soon CS, Brass M, Heinze HJ, Haynes JD. Unconscious determinants of free decisions in the human brain. Nat Neurosci. 2008;11(5):543–545. doi: 10.1038/nn.2112. [DOI] [PubMed] [Google Scholar]
- 7.Mele AR. Decision, intentions, urges and free will: Why Libet has not shown what he says he has. In: Campbell J, O’Rourke M, Shier D, editors. Explanation and Causation: Topics in Comtemporary Philosophy. Boston: MIT Press; 2007. [Google Scholar]
- 8.Vinding MC, Jensen M, Overgaard M. Distinct electrophysiological potentials for intention in action and prior intention for action. Cortex. 2014;50:86–99. doi: 10.1016/j.cortex.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Alexander P, Schlegel A, Sinnott-Armstrong W, Roskies AL, Wheatley T, Tse PU. Readiness potentials driven by non-motoric processes. Consciousness and cognition. 2016;39:38–47. doi: 10.1016/j.concog.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Matsuhashi M, Hallett M. The timing of the conscious intention to move. Eur J Neurosci. 2008;28(11):2344–2351. doi: 10.1111/j.1460-9568.2008.06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai O, Rathi V, Lin P, et al. Prediction of human voluntary movement before it occurs. Clin Neurophysiol. 2011;122(2):364–372. doi: 10.1016/j.clinph.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider L, Houdayer E, Bai O, Hallett M. What we think before a voluntary movement. Journal of cognitive neuroscience. 2013;25(6):822–829. doi: 10.1162/jocn_a_00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried I, Mukamel R, Kreiman G. Internally generated preactivation of single neurons in human medial frontal cortex predicts volition. Neuron. 2011;69(3):548–562. doi: 10.1016/j.neuron.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau HC, Rogers RD, Passingham RE. Manipulating the experienced onset of intention after action execution. Journal of cognitive neuroscience. 2007;19(1):81–90. doi: 10.1162/jocn.2007.19.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Douglas ZH, Maniscalco B, Hallett M, Wassermann EM, He BJ. Modulating conscious movement intention by noninvasive brain stimulation and the underlying neural mechanisms. J Neurosci. 2015;35(18):7239–7255. doi: 10.1523/JNEUROSCI.4894-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banks WP, Isham EA. We infer rather than perceive the moment we decided to act. Psychological science. 2009;20(1):17–21. doi: 10.1111/j.1467-9280.2008.02254.x. [DOI] [PubMed] [Google Scholar]
- 17.Banks WP, Isham EA. Do we really know what we are doing? Implications of reported time of decision for theories of volition. In: Sinncott-Armstrong W, Nadel L, editors. Conscious Will and Responsibility. Oxford: Oxford Uniiversity Press; 2011. pp. 47–60. [Google Scholar]
- 18.Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science. 2004;303(5661):1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- 19.Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. Movement intention after parietal cortex stimulation in humans. Science. 2009;324(5928):811–813. doi: 10.1126/science.1169896. [DOI] [PubMed] [Google Scholar]
- 20.Sirigu A, Daprati E, Ciancia S, et al. Altered awareness of voluntary action after damage to the parietal cortex. Nat Neurosci. 2004;7(1):80–84. doi: 10.1038/nn1160. [DOI] [PubMed] [Google Scholar]
- 21.Sirigu A, Daprati E, Pradat-Diehl P, Franck N, Jeannerod M. Perception of self-generated movement following left parietal lesion. Brain. 1999;122(Pt 10):1867–1874. doi: 10.1093/brain/122.10.1867. [DOI] [PubMed] [Google Scholar]
- 22.Pazzaglia M, Galli G. Loss of agency in apraxia. Frontiers in human neuroscience. 2014;8:751. doi: 10.3389/fnhum.2014.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eagleman DM, Tse PU, Buonomano D, Janssen P, Nobre AC, Holcombe AO. Time and the brain: how subjective time relates to neural time. J Neurosci. 2005;25(45):10369–10371. doi: 10.1523/JNEUROSCI.3487-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libet B, Wright EW, Jr, Feinstein B, Pearl DK. Subjective referral of the timing for a conscious sensory experience: a functional role for the somatosensory specific projection system in man. Brain. 1979;102(1):193–224. doi: 10.1093/brain/102.1.193. [DOI] [PubMed] [Google Scholar]
- 25.Hallett M. Volitional control of movement: The physiology of free will. Clin Neurophysiol. 2007;118(6):1179–1192. doi: 10.1016/j.clinph.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wegner DM. Precis of the illusion of conscious will. Behav Brain Sci. 2004;27(5):649–659. doi: 10.1017/s0140525x04000159. discussion 659–692. [DOI] [PubMed] [Google Scholar]
- 27.Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. Neuroimage. 2003;18(2):324–333. doi: 10.1016/s1053-8119(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 28.Nahab FB, Kundu P, Gallea C, et al. The neural processes underlying self-agency. Cereb Cortex. 2011;21(1):48–55. doi: 10.1093/cercor/bhq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang SY, Im CH, Shim M, et al. Brain Networks Responsible for Sense of Agency: An EEG Study. PLoS One. 2015;10(8):e0135261. doi: 10.1371/journal.pone.0135261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sperduti M, Delaveau P, Fossati P, Nadel J. Different brain structures related to self- and external-agency attribution: a brief review and meta-analysis. Brain structure & function. 2011;216(2):151–157. doi: 10.1007/s00429-010-0298-1. [DOI] [PubMed] [Google Scholar]
- 31.Preston C, Newport R. Misattribution of movement agency following right parietal TMS. Social Cognitive and Affective Neuroscience. 2008;3(1):26–32. doi: 10.1093/scan/nsm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritterband-Rosenbaum A, Karabanov AN, Christensen MS, Nielsen JB. 10 Hz rTMS over right parietal cortex alters sense of agency during self-controlled movements. Frontiers in human neuroscience. 2014;8:471. doi: 10.3389/fnhum.2014.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambon V, Moore JW, Haggard P. TMS stimulation over the inferior parietal cortex disrupts prospective sense of agency. Brain structure & function. 2015;220(6):3627–3639. doi: 10.1007/s00429-014-0878-6. [DOI] [PubMed] [Google Scholar]
- 34.Haggard P, Clark S, Kalogeras J. Voluntary action and conscious awareness. Nat Neurosci. 2002;5(4):382–385. doi: 10.1038/nn827. [DOI] [PubMed] [Google Scholar]
- 35.Moore JW, Ruge D, Wenke D, Rothwell J, Haggard P. Disrupting the experience of control in the human brain: pre-supplementary motor area contributes to the sense of agency. Proc Biol Sci. 2010;277(1693):2503–2509. doi: 10.1098/rspb.2010.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavazzana A, Penolazzi B, Begliomini C, Bisiacchi PS. Neural underpinnings of the ‘agent brain’: new evidence from transcranial direct current stimulation. Eur J Neurosci. 2015;42(3):1889–1894. doi: 10.1111/ejn.12937. [DOI] [PubMed] [Google Scholar]
- 37.Khalighinejad N, Haggard P. Modulating human sense of agency with non-invasive brain stimulation. Cortex. 2015;69:93–103. doi: 10.1016/j.cortex.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Baars BJ, Franklin S, Ramsoy TZ. Global workspace dynamics: cortical “binding and propagation” enables conscious contents. Front Psychol. 2013;4:200. doi: 10.3389/fpsyg.2013.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci U S A. 2015;112(3):887–892. doi: 10.1073/pnas.1418031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kranick SM, Hallett M. Neurology of Volition. Exp Brain Res. 2013;229(3):313–327. doi: 10.1007/s00221-013-3399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caspar EA, Christensen JF, Cleeremans A, Haggard P. Coercion Changes the Sense of Agency in the Human Brain. Curr Biol. 2016;26(5):585–592. doi: 10.1016/j.cub.2015.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]


