Abstract
Objective
To develop, in partnership with families of children with traumatic brain injury (TBI), a post-discharge intervention that is effective, simple and sustainable.
Design
Randomized Controlled Trial
Setting
Seven Level 1 Pediatric Trauma Centers in Argentina.
Patients
Persons less than 19 years of age admitted to one of the study hospitals with a diagnosis of severe, moderate, or complicated mild TBI, and were discharged alive.
Interventions
Patients were randomly assigned to either the Intervention or Standard Care group. A specially trained Community Resource Coordinator (CRC) was assigned to each family in the Intervention group. We hypothesized that children with severe, moderate, and complicated mild TBI who received the intervention would have significantly better functional outcomes at 6-months post-discharge than those who received standard care. We further hypothesized there would be a direct correlation between patient outcome and measures of family function.
Measurements and Main Results
The primary outcome measure was a composite measured at 6-months post-injury. There were 308 patients included in the study; 61% male. Forty-four percent sustained a complicated mild TBI, 18% moderate, and 38% severe. Sixty-five percent of the patients were 8 years old or younger, and over 70% were transported to the hospital without ambulance assistance. There was no significant difference between groups on the primary outcome measure. There was a statistically significant correlation between the primary outcome measure and scores on the Family Impact Module of the PedsQL (ρ= 0.57; p < 0.0001). Children with better outcomes lived with families reporting better function at 6-months post-injury.
Conclusions
While no significant effect of the intervention was demonstrated, this study represents the first conducted in Latin America that documents the complete course of treatment for pediatric patients with TBI spanning hospital transport through hospital care and into the post-discharge setting.
Keywords: Traumatic Brain Injury, Pediatrics, Outcomes, Latin America, Family Systems, Home Care
Introduction
The World Health Organization (WHO) estimates that by the year 2020, traumatic brain injury (TBI) will be the leading cause of death and disability worldwide (1). Pediatric TBI in Latin America is 2.97 times higher than the international average (2). Current epidemiological data, however, are scarce (3).
Previous research from Brazil suggests that for children with TBI, an intervention provided in the context of the family environment produced significantly better outcomes than an intervention in an institutional setting (4). However, the population in that study had access to high quality, multi-disciplinary rehabilitation resources not available in many low-and-middle-income countries (LMICs). Family support and family function have been shown to influence the recovery of survivors, as well as the well-being of the family as a whole (5, 6). Consequently, outcomes in response to an experimental treatment for a TBI patient must include, and be assessed in the context of, family function and well-being – that is, in the context of family as a system.
This project was conceived in response to the National Institutes of Health (NIH) Fogarty International Center (FIC) program, “Brain Disorders in the Developing World: Research Across the Lifespan.” The mandate of the program is two-fold – to conduct research in an LMIC about a brain disorder that is an important public health problem specific to that country; and while doing so, to build sustainable in-country capacity for ongoing research about the disorder. The purpose of this project was to develop, in partnership with families of children with TBI, a post-discharge intervention provided in the home that is simple and sustainable, and to introduce the intervention in a randomized controlled trial (RCT) in Argentina. Our preliminary research indicated a need for a post-discharge intervention for all levels of severity. We hypothesized that children with severe, moderate, and complicated mild TBI who received the intervention would have significantly better functional outcomes at 6-months post-discharge than those who received standard care. A secondary hypothesis was there would be a direct correlation between patient outcome and measures of family function.
Materials and Methods
Study overview
Background
Centro de Informática e Investigación Clínica (CIIC – Center for Informatics and Clinical Research) is a collaboration of physicians and researchers from Argentina and the United States who have been conducting research about TBI in Latin America since 2000. Academic and medical institutions within the collaboration include Hospital de Emergencias “Dr. Clemente Alvarez” (HECA – Rosario, Argentina), Universidad Nacional de Rosario – (UNR – Rosario, Argentina), and Oregon Health & Science University (OHSU – Portland, Oregon, USA). CIIC is the institution in Argentina responsible for this project. OHSU is the primary grantee.
Focus Groups
In April of 2010, we conducted 7 focus group meetings; three in Ecuador, and two each in Argentina, and Bolivia. A total of 70 people attended the meetings; 40 family members and 30 professionals. Their input was used to fully formulate this study. We chose this approach in order to fulfill on the FIC's priority that research in LMICs be generated by in-country personnel, and be relevant to their specific environments.
Center Selection
CIIC faculty surveyed pediatric trauma centers in Argentina, and selected sites based on the following criteria: previous research experience, annual TBI patient census, and presence of IRB at the study site. Seven centers were selected: Hospital de Niños Victor J. Vilela (Rosario), Hospital de Niños Sor Maria Ludovica (La Plata), Hospital El Cruce (Florencio Varela), Hospital de Niños de la Santísima Trinidad (Córdoba), Hospital de Niños Dr. Orlando Alassia (Santa Fe), Hospital Pediátrico Dr. Humberto Notti (Mendoza), Hospital Interzonal Materno Infantil Dr. Vitorio Tetamanti (Mar del Plata). Start dates for new patient entry ranged from August to October, 2011.
Funding
This project was funded by the Eunice Kennedy Schriber National Institute on Child Health and Human Development, and the Fogarty International Center of the National Institutes of Health (5R01-HD060570).
Structure, Responsibilities, and Oversight
Gustavo Petroni, MD, MCR, is the Principal Investigator from Latin America, and Nancy Carney, PhD, is the Principal Investigator from the United States. A Data and Safety Monitoring Committee (DSMC) was selected consisting of pediatric physicians, statisticians, and an ethicist – all from Argentina. One of the CIIC faculty, Gabriela Faguaga, is a pediatric physician who developed and manages an outpatient program for medically fragile children in one of the study hospitals. She was responsible for developing and managing the intervention for this study.
Setting
The setting for this study was 7 Level 1 pediatric trauma centers in Argentina (see Center Selection). All centers have intracranial pressure (ICP) monitoring as standard of care, and follow the Guidelines for the Acute Medical Management of Severe Traumatic Brain Injury in Infants, Children, and Adolescents – Second Edition (7). The World Bank status of Argentina is Upper Middle Income.
Study Site Team Structure
The study team for each site consisted of a Principal Investigator, two study coordinators who also collected acute care data, two Community Resource Coordinators (CRCs) who provided the intervention, and two outcome assessors.
Study Design
This was an RCT with blinded evaluation of outcome. It had a two-group parallel design.
Subjects
Inclusion/Exclusion Criteria
All consecutive patients admitted to the emergency departments (ED) of the 7 study hospitals were screened for eligibility (Table 1). Patients were enrolled from August 2011 through June 2013, with study duration varying across hospitals. The screening included information about the person's injury before and after admission to the study hospital. For eligible patients who did not consent, the screening provided information (de-identified data) about the pediatric TBI population against which we compare our consented study group, for the purposes of understanding external validity.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
| Admission to study hospital within 48 hours of injury |
| GCS < 13 on admission or within first 48 hours of injury, or GCS 14-15 with abnormal CT scan within first 48 hours of injury |
| GCS motor score < 5 for intubated patients |
| Age ≤ 18 years |
| Alive at hospital discharge |
| Discharge from acute care of primary study hospital (not from another one) |
| Clearly identifiable primary caregiver |
| Consented for inclusion into RCT |
| Exclusion Criteria |
| No contact information before discharge to allow post-discharge follow-up |
| No consent |
| Injury inflicted by parent or member of household |
| Patient hospitalized > 90 days |
GCS: Glasgow Coma Scale Score
Randomization
A patient was received in the participating hospital, evaluated by an ED physician, and identified as possibly meeting study criteria. After obtaining consent, just prior to the patient's hospital discharge, the study coordinator logged on to a secure database, verified his or her identity, and provided information about the subject to confirm eligibility and to determine the stratum. In addition to center, randomization was stratified by age group (0-2, >2-8, >8-11, >11-14, >14-18) and Pediatric Overall Performance Category (POPC) at discharge (1-2, 3-4, 5) (8). Randomization was blocked within each stratum to ensure balance. The program entered the subject information on the randomization log, retrieved the next assignment for that center and stratum, entered that on the randomization log, and sent the assignment to the study coordinator.
Treatment: Intervention and Standard Care
Standard Care Group
Post-discharge standard care varies according to the family's resources and abilities, and the available state health services. All patients randomized to either the Intervention Group or the Standard Care Group receive standard care.
Intervention Group
The main differences between Standard Care and the Intervention were (a) the post-discharge support to follow physician instructions, comply with medication prescriptions, and attend follow-up visits, (b) support for access to resources, and (c) access to a professional who could answer questions. A specially trained Community Resource Coordinator (CRC) was assigned to each family in the Intervention Group before discharge from the hospital. Approximately 2 – 3 days before hospital discharge, the CRC met with the primary caregiver of the patient and gave them a Family Resource Manual. The CRC and caregiver met again at the time of discharge. They completed a schedule of prescription drugs and follow-up visits with the primary care physician, and recorded relevant community resources in the manual. They scheduled a time for the CRC to contact the caregiver in one week. During the one-week call, the CRC answered questions, confirmed the next medical visit, and urged compliance with medical instructions. Similar calls were made each month for the 6-month duration of the intervention.
Baseline, Acute Care, and Hospital Ward Data Collection
After study enrollment, information was collected about demographics, injury characteristics, and pre-hospital transport and treatment. A comprehensive survey of social, cultural, economic, and psychological aspects of the family system was administered by the site PI or the Study Coordinator. The Abbreviated Injury Scale (AIS) (9) was recorded within 48 hours post-injury. ED and Intensive Care Unit (ICU) measures included Glasgow Coma Scale Score (GCS) (10), hypotension, hypoxia, pupillary reactivity, and CT scan results. Throughout the hospital stay vital signs, the Therapeutic Intensity Level (TIL), complications, neurosurgical interventions, and neuro-worsening data were recorded.
Outcome Measures
Outcome measures include the Cognitive Scale, Family Impact Module, and Quality of Life Module of the Pediatric Quality of Life Inventory (PedsQL) (11); the Pediatric Overall Performance Category (POPC); and Pediatric Cerebral Performance Category (PCPC) (8). The POPC and PCPC were administered at hospital discharge, and at 3- and 6-months post-injury. The PedsQL modules were administered at 6-months post-injury.
The PedsQL (Version 4.0) was used to examine outcomes at 6 months post-injury (11, 12). It is a generic, health related quality of life instrument developed to measure the core dimensions of physical, mental, social health, and role (school) function. The reliability and validity of the PedsQL have been examined in pediatric TBI studies in the United States (13, 14). The results demonstrated good internal consistency, test-retest reliability and construct validity. The inventory was also sensitive to TBI severity, and has been validated in Argentina (15).
The modules of the PedsQL are designed to measure behaviors and responses appropriate to each age that represent similar basic abilities and deficits (for example, crawling at ages 0-2, and walking for older children). Scoring of the modules is converted to a percentile score ranging from 0 to 100. The uniform metric allows meaningful composite scores across age groups. Higher scores indicate better function.
The POPC and PCPC are simple, 6-level scales (1 = normal; 6 = brain death) used to categorize pediatric level of overall function and cognitive function. They have demonstrated acceptable reliability and validity (16), and, in a study of 200 children treated in a pediatric ICU, they associated significantly with the Stanford-Binet Intelligence Scale 4th edition and the Bayley Scales of Infant Development (8). The POPC and PCPC were measured at hospital discharge, and again at 3 months and 6 months post-injury.
Blinding to Experimental Condition
All research personnel were blinded to the allocation of families into the Intervention and Standard Care Groups except for the Data Manager (who performed the random assignment, described above) and the CRCs who conducted the intervention. Outcome assessors were asked to report if blinding was violated.
Personnel Training and Set-Up
Prior to initiating this trial, key investigators from Argentina and the U.S. had participated in multiple studies of TBI using various research designs (17-20) as well as a formal trauma research training program funded by the Fogarty International Center (FIC) of the National Institutes of Health (NIH). In addition, a comprehensive training program specific to this study was conducted for all site personnel.
Data Quality and Monitoring
Data were collected on paper, then entered into a password-protected web-based data base using screens designed to look like the paper forms. The program automatically identified inconsistencies, as well as out-of-range errors. The data manager monitored data for inconsistencies and unusual values. The Data Manager and CRC Director conducted site visits at approximately 3-month intervals to audit data collection, correct errors, and train the site personnel.
Ethical Committee Approval
This study was approved by the local ethical committees responsible for each of the study sites, as well as by the institutional review board (IRB) at OHSU. All Latin American study sites possess current Federal Wide Assurance (FWA) approval from the U.S. Office of Health and Human Services. The study was not required to be registered with clinicaltrials.gov.
Data Analysis
The primary outcome was based on a composite measure and calculated as an average percentile over four elements – The PedsQL Quality of Life module, the PedsQL Cognitive Scale module, the POPC and the PCPC. For children from 0 to 4 years of age, parent responses were used for the PedsQL and PedsQL Cognitive Scale; for children from 5 to 18 years of age, child responses were used. A higher value on the composite outcome indicates a better overall functional outcome at 6-months post-discharge. Individual measures were summarized for each group and compared using Wilcoxon rank-sum test (for composite and PedsQL) and Fisher exact test (for POPC and PCPC).
The rationale for using a composite measure is that TBI can have a negative effect in many different, but important, domains. There is no single measure that captures them well. A good intervention will have a positive effect on most measures, and a detrimental effect on none. One could use multiple outcomes, but a very positive result on any outcome will lead to declaring the intervention effective and, if one corrects for multiple comparisons, the study loses power for consistent effects across measures. The composite average percentile is sensitive to interventions that have a positive effect on all the outcomes. We provided results on the individual measures as well as the composite.
The hypothesis was tested using linear regression controlling for site, age, POPC score at discharge from acute care, and severity. A 2-sided significance level of .048 was used in the final analysis to account for the interim analysis (see Results section). Analysis was according to the intention-to-treat principle, i.e., all randomized cases were followed and included with their assigned treatment group regardless of the home care that was actually received. To supplement the composite test of the overall hypothesis, individual measures were summarized for each group.
The correlation between patient outcome (primary composite score) and family function (Family Impact Module of the PedsQL) was assessed using Spearman's ρ coefficient.
Power and Sample Size
The null hypothesis was there would be no difference on the composite outcome between the Intervention and Standard Care Groups. A sample size of N = 274 would give the study 80% power to detect a difference of .34 standard deviations, in the range Cohen considers to be “small” (21), using a 2-sided significance level of .048 to account for one interim efficacy analysis (see Results section). Assuming a 90% follow up rate, the corrected sample size was 301 patients.
Results
One interim analysis was conducted in April of 2013, including all patients with 6-month follow-up assessments as of March 31st (N = 137). O'Brien-Fleming (22) boundaries were used to decide whether to terminate because of a positive effect of the experimental intervention. The decision was made to continue the study.
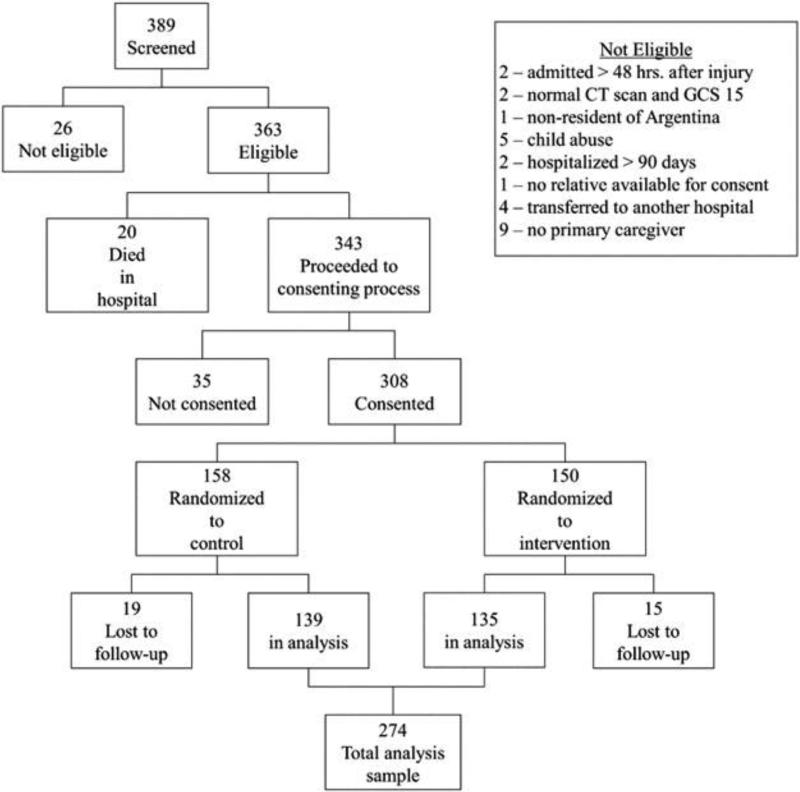
As of July 1st, 2013, 308 patients were randomized into the trial; 85% of all eligible patients who were screened. See Figure 1 for a summary of the screening and exclusions. Baseline characteristics of the sample of included patients are presented in Table 2. Forty-four percent sustained a complicated mild TBI, 18% moderate, and 38% severe. Sixty-five percent of the patients were 8 years old or younger, and over 70% were transported to the hospital without ambulance assistance. No patient in the study was discharged to a rehabilitation institution, and three patients received home care. There were no significant differences between the treatment groups at baseline. There were no significant differences in age and sex between the eligible patients who consented and those who did not (N = 35). There was a significant difference in the distribution of the GCS between these two groups, with a higher proportion of severe and mild but lower proportion of moderate patients in the group of consented patients.
Figure 1.
Patient screening and enrollment into study
Table 2.
Characteristics of the Participants by Treatment Group
| Treatment Group | ||||
|---|---|---|---|---|
| Intervention (N=150) | Control (N=158) | Overall (N=308) | P-value* | |
| Age | 0.87 | |||
| Early childhood: 0 to 2 years | 37 ( 24.7) | 40 ( 25.3) | 77 ( 25.0) | |
| Children: 3 to 8 years | 61 ( 40.7) | 61 ( 38.6) | 122 ( 39.6) | |
| Pre-puberty age 9 to 11 years | 19 ( 12.7) | 25 ( 15.8) | 44 ( 14.3) | |
| Early Adolescence:> 12 years to 14 years | 28 ( 18.7) | 29 ( 18.4) | 57 ( 18.5) | |
| Adolescence: 15 years to 18 years | 5 ( 3.3) | 3 ( 1.9) | 8 ( 2.6) | |
| Gender | 0.19 | |||
| Male | 86 ( 57.3) | 102 ( 64.6) | 188 ( 61.0) | |
| Female | 64 ( 42.7) | 56 ( 35.4) | 120 ( 39.0) | |
| Marshal Classification on initial CT | 0.33 | |||
| Diffuse Injury type I | 21 ( 14.0) | 14 ( 8.9) | 35 ( 11.4) | |
| Diffuse Injury type II | 84 ( 56.0) | 90 ( 57.0) | 174 ( 56.5) | |
| Diffuse Injury type III | 19 ( 12.7) | 18 ( 11.4) | 37 ( 12.0) | |
| Diffuse Injury type IV | 1 ( 0.7) | 1 ( 0.6) | 2 ( 0.6) | |
| Evacuated Mass Lesion | 12 ( 8.0) | 19 ( 12.0) | 31 ( 10.1) | |
| Non-evacuated Mass Lesion | 13 ( 8.7) | 16 ( 10.1) | 29 ( 9.4) | |
| Transport | 0.67 | |||
| Private Vehicle | 71 ( 47.3) | 70 ( 44.3) | 141 ( 45.8) | |
| Physician + Medical Ambulance | 41 ( 27.3) | 40 ( 25.3) | 81 ( 26.3) | |
| Unknown | 20 ( 13.3) | 29 ( 18.4) | 49 ( 15.9) | |
| Other | 8 ( 5.3) | 5 ( 3.2) | 13 ( 4.2) | |
| Police | 2( 1.3) | 5 ( 3.2) | 7 ( 2.3) | |
| Taxi | 3 ( 2.0) | 4 ( 2.5) | 7 ( 2.3) | |
| Ambulance (no doctor) | 2( 1.3) | 3 ( 1.9) | 5 ( 1.6) | |
| Helicopter | 2 ( 1.3) | 1 ( 0.6) | 3 ( 1.0) | |
| Firefighters | 1 ( 0.7) | 1 ( 0.6) | 2 ( 0.6) | |
| Initial Assistance | 0.81 | |||
| No assistance | 76 ( 50.7) | 83 ( 52.5) | 159 ( 51.6) | |
| Physician | 33 ( 22.0) | 30 ( 19.0) | 63 ( 20.5) | |
| Unknown | 20 ( 13.3) | 18 ( 11.4) | 38 ( 12.3) | |
| Untrained person (passerby) | 10 ( 6.7) | 9 ( 5.7) | 19 ( 6.2) | |
| Medical Rescue Team | 6 ( 4.0) | 9 ( 5.7) | 15 ( 4.9) | |
| Other | 4 ( 2.7) | 6 ( 3.8) | 10 ( 3.2) | |
| Nurse | 1 ( 0.7) | 2 ( 1.3) | 3( 1.0) | |
| Paramedic | 1 ( 0.6) | 1 ( 0.3) | ||
| Severity (GCS) | 0.90 | |||
| Mild | 65 ( 43.3) | 72 ( 45.6) | 137 ( 44.5) | |
| Moderate | 28 ( 18.7) | 27 ( 17.1) | 55 ( 17.9) | |
| Severe | 57 ( 38.0) | 59 ( 37.3) | 116 ( 37.7) | |
| Mechanism of Injury | 0.75 | |||
| Traffic accident | 63 ( 42.0) | 71 ( 44.9) | 134 ( 43.5) | |
| Fall from height | 50 ( 33.3) | 48 ( 30.4) | 98 ( 31.8) | |
| Strike | 20 ( 13.3) | 17 ( 10.8) | 37 ( 12.0) | |
| Other | 8 ( 5.3) | 10 ( 6.3) | 18 ( 5.8) | |
| Fall from own height | 4 ( 2.7) | 10 ( 6.3) | 14 ( 4.5) | |
| Gunshot wound | 4 ( 2.7) | 2 ( 1.3) | 6 ( 1.9) | |
| Unknown | 1 ( 0.7) | 1 ( 0.3) | ||
| POPC at discharge | 0.31 | |||
| 1 | 112 ( 74.7) | 121 ( 76.6) | 233 ( 75.6) | |
| 2 | 21 ( 14.0) | 12 ( 7.6) | 33 ( 10.7) | |
| 3 | 9 ( 6.0) | 10 ( 6.3) | 19 ( 6.2) | |
| 4 | 7 ( 4.7) | 13 ( 8.2) | 20 ( 6.5) | |
| 5 | 1 ( 0.7) | 2 ( 1.3) | 3 ( 1.0) | |
Note: Values expressed as N(%)
P-value comparisons across treatment groups for categorical variables are based on Chi-square test of homogeneity. Some categories were grouped when the Chi-square test was not valid due to cells with expected counts less than 5
The rate for follow-up at the 3-month time point was 92%, and at the 6-month time point was 89%. There are no significant differences between patients maintained and those lost to follow-up in age, gender, or GCS. There was no report of violation of outcome evaluators’ blinding status.
Effect of Intervention
Table 3 shows the results for the primary composite outcome and individual measures. No significant effect of the intervention was observed. The regression analysis indicates the intervention group had an average reduction of 0.85 points on the composite outcome (p-value: 0.61; 95%CI: −4.1, 2.4) when controlling for age, site, severity and POPC at hospital discharge. To supplement the composite test of the overall hypothesis, individual measures were summarized for each group and compared using Wilcoxon rank-sum test (for composite and PedsQL) and Fisher exact test (for POPC and PCPC). These unadjusted analyses are shown in Table 3. There was no mortality among the randomized sample between hospital discharge and 6 months post-injury.
Table 3.
Outcomes
| Intervention (N=135) | Control (N=139) | Overall (N=274) | P-value* | |
|---|---|---|---|---|
| Composite Outcome | 0.2598 | |||
| N | 135 | 139 | 274 | |
| Median | 51.4 | 54.4 | 54.2 | |
| Interquartile Range | 41.5-63.4 | 42.2-65.8 | 42.2-64.1 | |
| PedsQL Quality of Life Score | 0.3417 | |||
| N | 133 | 139 | 272 | |
| Median | 89.1 | 91.7 | 91.0 | |
| Interquartile Range | 79.3-97.2 | 80.6-97.8 | 80.4-97.8 | |
| PedsQL Cognitive Scale | 0.6119 | |||
| N | 117 | 112 | 229 | |
| Median | 93.8 | 95.8 | 93.8 | |
| Interquartile Range | 75.0-100. | 79.2-100. | 75.0-100. | |
| POPC at 6 months | 0.1609 | |||
| 1 | 116 ( 85.9) | 124 ( 89.2) | 240 ( 87.6) | |
| 2 | 15 ( 11.1) | 8 ( 5.8) | 23 ( 8.4) | |
| 3 | 3 ( 2.2) | 2 ( 1.4) | 5 ( 1.8) | |
| 4 | 1 ( 0.7) | 5 ( 3.6) | 6 ( 2.2) | |
| PCPC at 6 months | 0.4535 | |||
| 1 | 120 ( 88.9) | 119 ( 85.6) | 239 ( 87.2) | |
| 2 | 11 ( 8.1) | 11 ( 7.9) | 22 ( 8.0) | |
| 3 | 3 ( 2.2) | 4 ( 2.9) | 7 ( 2.6) | |
| 4 | 1 ( 0.7) | 5 ( 3.6) | 6 ( 2.2) |
P-values: Composite, PedsQL = Wilcoxon rank-sum, POPC and PCPC = Fisher exact test.
Correlation Analysis
There was a statistically significant correlation between the primary composite outcome and the scores on the Family Impact Module of the PedsQL (ρ= 0.57; p < 0.0001). Children with better outcomes lived with families reporting better function at 6-months post-injury.
Post-hoc Concordance Analysis. A post-hoc analysis indicates high concordance between child reports and parent reports for the PedsQL Quality of Life module (coefficient = 0.74 for both intervention and control groups; p = 0.26) and Cognitive Scale module (coefficient = 0.69 and 0.78 for intervention and controls groups, respectively; p = 0.75).
Discussion and Future Research
We conducted a randomized trial to test the effectiveness of a post-discharge intervention provided in the home for children who sustained severe, moderate, and complicated mild TBI. Our primary hypothesis, that patients who received the intervention would demonstrate better functional outcomes at 6-months post injury than those who did not, was not supported by the findings. Forty-four percent of this sample sustained complicated mild TBI, and 87% had good recovery at 6 months post-injury. An important next step in this line of research will be to test the intervention in a sample of patients with only severe TBI.
Our second hypothesis, that there would be a direct correlation between outcome and measures of family function, was supported by the findings. Children from families with better scores on the family function measure had better functional outcomes. This finding reinforces previous research, and provides information to encourage further development of home care interventions for children. However, this study was not designed to test causality between these variables. The recursive influence of the traumatized patient on family function and family function on outcome of the traumatized patient is likely not a linear relationship. An exploration of this relationship would require a study design employing a systems approach for analysis. Of interest, although the effect of the intervention was not statistically significant, the study hospitals elected to maintain the Community Resource Coordinator program.
While the pre-hospital transport information in this study is not directly relevant to the study hypotheses, it raises questions about methods of transport and their influence on mortality, as well as on the ability to accurately calculate mortality rates. Most patients were not transported by ambulance. Furthermore, the number of severely injured patients who die in ambulances and are diverted to the morgue is unknown. Finally, for patients who are dead-on-arrival, cause of death may not be clearly distinguished. These factors limit the generalizability of our findings.
Although WHO data have identified TBI as a serious pandemic (1), and it is reported that pediatric TBI in Latin America is almost 3 times that of the international average (2), in this study each hospital averaged less than 1 severe pediatric TBI patient per month. Furthermore, the majority of patients had returned to high function by 6 months post-injury. These data appear to contradict the epidemiological reports; they identify critical information gaps in the context of the pre-hospital setting, and constitute the primary weakness of this study. Future research needs to extend existing collaborations within the community, in order to access information about pre-hospital mortality and morbidity. This setting provides a unique opportunity to implement and test evidence-based guidelines for pre-hospital treatment of TBI.
Conclusion
The mandate of the NIH/FIC “Brain Disorders” program is to conduct research in LMICs about a brain disorder of critical importance in those communities, and concurrently to build sustainable in-country research capacity. Our research topic, traumatic brain injury, is a global epidemic. We collaborated with parents, patients, physicians, nurses, emergency medicine technicians, allied health professionals, and hospital administrators to develop a simple, inexpensive, sustainable intervention provided to families and their children with TBI. We implemented the intervention in 7 trauma centers in Argentina, and met the sample size target early, with exceptional enrollment and follow-up rates.
With this program, a research network was established. Sites were provided equipment and a web-based data collection system. Site personnel were trained in evidence-based research design and conduct, principles of data quality, and database management. They participated in a variety of different research methods, including focus groups, qualitative inquiry, field methods, observational research, and the randomized trial. They designed and developed their own intervention for this study.
Local leadership for this project was established by formal training in clinical research programs in the U.S. and Latin America, and by rigorous, on-site training specific to the project. That leadership established an independent non-profit entity – Centro de Informática e Investigación Clínica (CIIC) – to facilitate ongoing research. In addition to TBI research, CIIC currently serves communities in Latin America by conducting research on diverse topics such as chronic kidney disease, pesticide-associated birth defects, and breast cancer. In doing so, they have fulfilled the FIC mandate to establish sustainable research capacity.
Acknowledgments
Financial Support:
This project is funded by the Eunice Kennedy Schriber National Institute on Child Health and Human Development, and the Fogarty International Center of the National Institutes of Health (5R01-HD060570).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
Contributor Information
Nancy A. Carney, Oregon Health & Science University, Portland, OR..
Gustavo J. Petroni, Centro de Informática e Investigación Clínica, Rosario, Argentina..
Silvia B. Luján, Centro de Informática e Investigación Clínica, Rosario, Argentina..
Nicolás M. Ballarini, Centro de Informática e Investigación Clínica, Rosario, Argentina.; Universidad Nacional de Rosario, Rosario, Argentina.
Gabriela A. Faguaga, Centro de Informática e Investigación Clínica, Rosario, Argentina.; Hospital de Niños Víctor J Vilela, Rosario, Argentina.
Hugo E. M. du Coudray, Portland State University, Portland, OR.; Oregon Health & Science University, Portland, OR.
Amy E. Huddleston, Oregon Health & Science University, Portland, OR..
Gloria M. Baggio, Hospital de Niños Víctor J. Vilela, Rosario, Argentina..
Juan M. Becerra, Sistema Integrado de Emergencia Sanitaria, Rosario, Argentina..
Leonardo O. Busso, Hospital El Cruce, Florencio Varela, Argentina..
Sureyya S. Dikmen, University of Washington, Seattle, WA..
Roberto Falcone, Hospital Interzonal Especializado Materno Infantil Dr. Vitorio Tetamanti, Mar del Plata, Argentina..
Mirta E. García, Hospital de Niños Sor María Ludovica, La Plata, Argentina..
Osvaldo R. González Carrillo, Hospital de Niños “Dr. Orlando Alassia”, Santa Fe, Argentina..
Paula L. Medici, Hospital Interzonal Especializado Materno Infantil Dr. Vitorio Tetamanti, Mar del Plata, Argentina..
Marta B. Quaglino, Universidad Nacional de Rosario, Rosario, Argentina..
Carina A. Randisi, Sistema Integrado de Emergencia Sanitaria, Rosario, Argentina..
Silvia S. Sáenz, Hospital de Niños de la Santísima Trinidad, Córdoba, Argentina..
Nancy R. Temkin, University of Washington, Seattle, WA..
Elida E. Vanella, Hospital Pediátrico Dr. Humberto Notti, Mendoza, Argentina..
References
- 1.Hyder AA, Wunderlich CA, Puvanachandra P, et al. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22:341–353. [PubMed] [Google Scholar]
- 2.Murgio A, Fernandez Mila J, Manolio A, et al. Minor head injury at paediatric age in Argentina. J Neurosurg Sci. 1999;43:15–23. discussion 23-14. [PubMed] [Google Scholar]
- 3.Puvanachandra P, Hyder AA. Traumatic brain injury in Latin America and the Caribbean: a call for research. Salud Publica Mex. 2008;50(Suppl 1):S3–5. doi: 10.1590/s0036-36342008000700002. [DOI] [PubMed] [Google Scholar]
- 4.Braga L, Campos da Paz A Jr, editors. The Child with Traumatic Brain Injury or Cerebral Palsy: A context-sensitive, family-based approach to development. Taylor & Francis; Oxford: 2006. [Google Scholar]
- 5.Schmidt AT, Orsten KD, Hanten GR, et al. Family environment influences emotion recognition following paediatric traumatic brain injury. Brain Inj. 2010;24:1550–1560. doi: 10.3109/02699052.2010.523047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurowski BG, Taylor HG, Yeates KO, et al. Caregiver ratings of long-term executive dysfunction and attention problems after early childhood traumatic brain injury: family functioning is important. PM R. 2011;3:836–845. doi: 10.1016/j.pmrj.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 8.Fiser DH, Long N, Roberson PK, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 9.the AMA Committee on Medical Aspects of Automotive Safety: Rating the severity of tissue damage. I. The abbreviated scale. JAMA. 1971;215:277–280. doi: 10.1001/jama.1971.03180150059012. [DOI] [PubMed] [Google Scholar]
- 10.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 11.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy ML, MacKenzie EJ, Durbin DR, et al. The Pediatric Quality of Life Inventory: an evaluation of its reliability and validity for children with traumatic brain injury. Arch Phys Med Rehabil. 2005;86:1901–1909. doi: 10.1016/j.apmr.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Kruse S, Schneeberg A, Brussoni M. Construct validity and impact of mode of administration of the PedsQL among a pediatric injury population. Health and quality of Life outcomes. 2014;12:168. doi: 10.1186/s12955-014-0168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roizen M, Rodriguez S, Bauer G, et al. Initial validation of the Argentinean Spanish version of the PedsQL, 4.0 Generic Core Scales in children and adolescents with chronic diseases: acceptabiity in low-income settings. Health and Quality of Life Outcomes. 2008;6:59. doi: 10.1186/1477-7525-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 17.Rondina C, Videtta W, Petroni G, et al. Mortality and morbidity from moderate to severe traumatic brain injury in Argentina. J Head Trauma Rehabil. 2005;20:368–376. doi: 10.1097/00001199-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Petroni G, Quaglino M, Lujan S, et al. Early prognosis of severe traumatic brain injury in an urban argentinian trauma center. J Trauma. 2010;68:564–570. doi: 10.1097/TA.0b013e3181ce1eed. [DOI] [PubMed] [Google Scholar]
- 19.Carney N, Lujan S, Dikmen S, et al. Intracranial pressure monitoring in severe traumatic brain injury in latin america: process and methods for a multi-center randomized controlled trial. J Neurotrauma. 2012;29:2022–2029. doi: 10.1089/neu.2011.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesnut RM, Temkin N, Carney N, et al. Traumatic brain injury in Latin America: lifespan analysis randomized control trial protocol. Neurosurgery. 2012;71:1055–1063. doi: 10.1227/NEU.0b013e31827276b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical power analysis for the behavioral sciences. Second edn. Lawrence Erlbaum Associates; Hillsdale: 1988. [Google Scholar]
- 22.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]