Abstract
Rationale
Mitogen-activated protein kinase (MAPK) signaling regulates the growth response of the adult myocardium in response to increased cardiac workload or pathologic insults. The dual-specificity phosphatases (DUSPs) are critical effectors that dephosphorylate the MAPKs to control the basal tone, amplitude and duration of MAPK signaling.
Objective
To examine the dual-specificity phosphatase 8 (DUSP8) as a regulator of MAPK signaling in the heart and its impact on ventricular and cardiac myocyte growth dynamics.
Methods and Results
Dusp8 gene-deleted mice as well as transgenic mice with inducible expression of DUSP8 in the heart were used here to investigate how this MAPK-phosphatase might regulate intracellular signaling and cardiac growth dynamics in vivo. Dusp8 gene-deleted mice were mildly hypercontractile at baseline with a cardiac phenotype of concentric ventricular remodeling, which protected them from progressing towards heart failure in two surgery-induced disease models. Cardiac-specific overexpression of DUSP8 produced spontaneous eccentric remodeling and ventricular dilation with heart failure. At the cellular level, adult cardiac myocytes from Dusp8 gene-deleted mice were thicker and shorter, while DUSP8 overexpression promoted cardiac myocyte lengthening with a loss of thickness. Mechanistically, activation of extracellular signal-regulated kinases 1/2 (ERK1/2) were selectively increased in Dusp8 gene-deleted hearts at baseline as well as following acute pathologic stress stimulation, while p38 MAPK and c-Jun N-terminal kinases were unaffected.
Conclusions
These results indicate that DUSP8 controls basal and acute stress-induced ERK1/2 signaling in adult cardiac myocytes that then alters the length-width growth dynamics of individual cardiac myocytes, which further alters contractility, ventricular remodeling and disease susceptibility.
Keywords: Dual-specificity phosphatase, mitogen-activated protein kinase, concentric remodeling, dilated cardiomyopathy, cardiac hypertrophy, heart failure, signaling pathways, mice
INTRODUCTION
In its broadest sense, the mitogen-activated protein kinase (MAPK) signaling cascade consists of a sequence of successively acting kinases that result in dual phosphorylation and activation of three main branches identified by the terminal kinases, p38, c-Jun N-terminal kinases 1 and 2 (JNK1/2), and extracellular signal-regulated kinase 1 and 2 (ERK1/2).1, 2 Phosphorylation and activation of these three terminal MAPKs result from the upstream dual-specificity MAPK kinases (MAPKKs, also called MEKs or MKKs) that include MEK1/2 for ERK1/2, MKK3/MKK6 for p38, and MKK4/MKK7 for JNK1/2.2 Upstream of MAPKKs, multiple MAPKKKs form a complex network of kinases that either directly sense environmental stimulation, or are activated by effectors such as G proteins (Ras, Rac, Rho, and others) and G protein-coupled receptors (GPCRs).3–6 In the heart, MAPKs play a complex role in regulating cardiac hypertrophy and heart failure. For instance, cardiac-specific overexpression of dominant-negative mutants of p38α, MKK3 or MKK6 rendered the heart more susceptible to cardiac hypertrophy, and Mapk14 (p38) heart-specific null mice are more prone to heart failure.7, 8 Similarly, genetic inhibition of JNK1/2 also rendered the heart more susceptible to cardiac hypertrophy and failure, similar to the phenotype of mice with cardiac-specific deletion of the genes encoding MKK4 or MKK7 protein.9–11 With respect to ERK1/2 signaling, MEK1 heart-specific transgenic mice, which showed constitutive ERK1/2 signaling, were characterized by concentric hypertrophic remodeling with a relatively cardio-protective phenotype.12 By comparison, mice with heart-specific deletion of Mapk1/3 (ERK1/2) presented with extreme dilation and decompensation, a phenotype also observed in transgenic mice overexpressing the ERK1/2 inactivating dual-specificity phosphatase 6 (DUSP6) in cardiac myocytes of the heart. 13, 14
The magnitude and duration of MAPK phosphorylation are critical in determining the extent of the biologic signaling response, which while critically regulated by the upstream MAPKKs is also equally regulated by dephosphorylation and the subsequent recycling of the MAPKs. The MAPKs are phosphorylated at both threonine and tyrosine residues within the activation loop (TxY motif), which can also be directly dephosphorylated by either the Ser/Thr phosphatases protein phosphatase 1 (PP1), protein phosphatase 2 (PP2), protein tyrosine phosphatases (PTPs), or more specifically by dedicated dual-specificity phosphatases (DUSPs).15 There are 13 MAPK-specific DUSPs that have been characterized and classified into 3 subfamilies based on sequence homology, subcellular localization and substrate specificity.15, 16 A unique aspect of the DUSPs is that they are rapidly induced by stress stimulation where each gene is transcribed and translated within 15–40 minutes, providing a negative feedback mechanism to dephosphorylate and inactivate the MAPKs to allow their recycling.17–20 While the basic biology of the DUSPs is well understood, the field currently lacks an understanding of how these 13 different genes are integrated into stress responsiveness in vivo and the effects on organ physiology and disease.20–23
DUSP8, also known as M3/6, belongs to a group of DUSPs that also includes DUSP10 and DUSP16, all three of which have a more complicated domain structure compared with the other DUSPs, and all three are localized to both the cytoplasm and nucleus.24 While all Dusp genes appear to be transcriptionally inducible, DUSP8 also has basal levels of expression in the heart and brain.25 DUSP8 was reported to prefer p38 and JNK based on overexpression studies, although it can also affect ERK1/2.26–28 In this study we generated mice lacking the Dusp8 gene and transgenic mice with cardiac myocyte-specific overexpression of DUSP8 to further examine how it might regulate cardiac MAPK signaling and disease responsiveness. We observed that Dusp8 gene-deleted (KO, knock-out) mice presented with baseline concentric cardiac remodeling that was enhanced upon stress stimulation. This concentric ventricular remodeling was associated with increased cardiac contractility at baseline and protection from dilation and heart failure in 2 different surgical models of induced pathology. Mechanistically, loss of Dusp8 led to increased ERK1/2 activation at baseline as well as following acute pathologic stimulation, while p38 and JNK kinases were largely unaffected. Conversely, overexpression of DUSP8 resulted in decreased phosphorylation of all MAPKs investigated, ventricular dilation, and a greater propensity towards heart failure. Together, these data suggest that DUSP8 regulates the dynamics of cardiac MAPK signaling, which directly impacts ventricular remodeling and heart failure propensity.
METHODS
Generation of Dusp8 KO and transgenic mice
A vector targeting the catalytic domain of DUSP8 was generated to replace exons 5 and 6 of the Dusp8 gene with a neomycin cassette, which was electroporated into mouse SV129j-based embryonic stem (ES) cells. Correctly targeted ES cells were identified by Southern blotting and subsequently injected into C57Bl/6 blastocysts to generate chimeric mice, then germ line and homozygous Dusp8 null mice in a final C57Bl/6-SV129j background. The following PCR primers were used to genotype WT and KO mice. WT: forward, 5’-tgggcatgtcttctgacgac-3’, reverse, 5’-agtgaggtccatcagtctgc-3’; KO: forward, 5’-ctccaccatgccctcttc-3’, reverse, 5’-gcgcatcgccttctatcgc-3’. To generate DUSP8 inducible transgenic mice, a cDNA encoding this protein was cloned into SalI and HindIII sites of the modified murine α-myosin heavy chain (αMHC) promoter expression vector (Dr. Jeffrey Robbins, Cincinnati Children’s Hospital Medical Center) to allow for doxycycline-regulated expression of the DUSP8 transgene in the presence of a cardiac-specific tetracycline transactivator (tTA)-containing transgene. DUSP8 transgenic mice were genotyped using the following primers: forward, 5’-gggaagtggtggtgtaggaaag-3’, reverse, 5’-tttagggcaggagttgctgg-3’. Doxycycline (625 mg/kg) was administered in the food until weaning. Mice were then switched to regular chow diet for an additional 6 weeks to allow DUSP8 expression.14 All animal breeding and experimentation were approved by Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee.
Animal surgery, echocardiography, invasive hemodynamics, and histology
All surgeries were performed on 8–12 week-old mice. The surgical groups contained at least 4 animals of each genotype and the appropriate controls. Transverse aortic constriction (TAC) was previously described.29 A similar degree of aortic constriction between the groups of mice was confirmed by Doppler echocardiography. Alzet osmotic minipumps (Alzet, model 1002) containing angiotensin II (AngII, 1.5 mg/kg/day, Sigma, #A9525) and phenylephrine (PE, 50 mg/kg/day, Sigma, #P6126) diluted in phosphate buffered saline were implanted for 14 days as previously described.14 Ischemia/reperfusion (I/R) and myocardial infarction (MI) have been described previously.21 Cardiac function and dimensions were measured by echocardiography with a SONOS 5500 instrument (Hewlett-Packard) with a 15-MHz transducer. Left ventricular (LV) fractional shortening (FS) was calculated using left ventricle internal diameters at the end of systole and diastole (LVIDs and LVIDd, respectively) according to the formula: ([LVIDd-LVIDs]/LVIDd) ×100 (%).
For invasive hemodynamic measurements, mice were anesthetized by intraperitoneal injection of pentobarbital (6 mg/100 g body weight). Mice under anesthesia longer than 30 min were given a second dose as needed (3 mg/100 g body weight). For assessment of cardiac contractility, a high fidelity, solid state 1.2F pressure-volume catheter (Transonic Scisense Inc) was inserted into the left ventricle via a right carotid exposure and retrograde introduction of the catheter into the left ventricle. The signal was optimized by phase and magnitude channels. Data were collected with a PowerLab 8/36 (ADInstruments) work station and analyzed using LabChart 7 Pro (ADInstruments).
At the end of each surgical protocol, mice were sacrificed and hearts harvested for analysis of heart weight to body weight ratio calculations. For histological analysis, adult hearts were fixed overnight in 10% formalin-containing phosphate buffered saline and dehydrated for paraffin embedding. Serial 5-μm heart sections were stained with Masson trichrome to detect interstitial fibrosis as blue.
Cell analysis
Neonatal rat cardiac myocytes were generated as previously described from 1–2 day old newborns,30 and then infected with recombinant adenoviruses expressing either β-galactosidase (β-gal) or DUSP8 for 36 hours. Cells were serum starved for 1 hour, stimulated with 10 μmol/L of phenylephrine (PE, Sigma, # P6126)) for 5 or 15 minutes, and harvested for western blot analysis. Adult mouse ventricular myocytes were isolated from whole hearts on a hanging apparatus with a solution containing liberase blendzyme (Roche, #05401151001) as previously described.31 Following isolation, cardiac myocytes were plated on laminin-coated dishes and cultured in medium 199 (Corning, 10-060-CV). Cardiac myocyte length and width were measured with NIH ImageJ software on phase contrast images of fixed cells. Alternatively, adult ventricular myocytes were serum starved for 1 hour and then stimulated with 10 μM of PE or 0.1 μmol/L of angiotensin II (AngII, Sigma, #A9525), and harvested for RNA and protein analysis. Adult rat cardiac myocytes were isolated using a solution containing 0.5 mg/ml type II collagenase (Worthington, #LS004176) and 0.24 mg/ml hyaluronidase (Sigma, #H3506), cultured in DMEM (Hyclone, #SH30022.01) containing 1% fetal bovine serum (Sigma, #F2442). Rat cardiac myocytes were then infected with recombinant adenoviruses expressing either β-gal, MEK1, or DUSP8 for 48 hours. Alternatively, rat cardiac myocytes were transfected with 50 nM of control (Dharmacon, #D-001810-10-05) or Dusp8 siRNA (Dharmacon, #L-084829-02-0005) for 48 hours. Myocytes were either fixed for subsequent cell size analysis or stimulated with 10 μmol/L of PE for protein analysis.
Mouse embryonic fibroblasts (MEFs) were isolated from embryonic day 12.5 Dusp8 WT and KO embryos. Briefly, embryos were removed from the uterus and washed in phosphate buffered saline, the heads and other visceral organs were removed, then the remaining tissues were minced with a sterile razor blade and digested with 0.25% trypsin for 30–45 minutes at 37°C before the trypsin was inactivated with DMEM media containing 10% fetal bovine serum. The MEFs were collected as the cells growing out from the debris and cultured for 2 passages and harvested for western blot analysis in the following buffer: 10 mmol/L HEPES pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, 1 mmol/L DTT, 0.4% IGEPAL, and a protease/phosphatase inhibitor cocktail (Thermo Fisher Scientific, #78440). Protein samples were centrifuged at 15,000×g for 5 minutes to collect the supernatant as a cytoplasmic fraction, and the pellet as a nuclear fraction, which was then eluted into buffer containing 20 mmol/L HEPES pH 7.9, 0.4 mol/L NaCl, 1 mmol/L EDTA, 10% glycerol, 1 mmol/L DTT, and protease/phosphatase inhibitors. Equal amount of cytoplasmic and nuclear proteins were loaded for western blot analysis. For analysis of interaction between DUSP8 and MAPKs, HEK293 cells were transfected with Flag-DUSP8 for 36 hours, lysed into buffer containing 20 mmol/L HEPES, 0.5% triton X-100, 150 mmol/L NaCl, 1 mmol/L EDTA, and a protease/phosphatase inhibitor cocktail. Protein samples were incubated with anti-Flag M2 magnetic beads (Sigma, # M8823) for 1 h at room temperature, and eluted into 2x laemmli sample buffer.
RNA isolation and quantitative PCR
Total RNA was isolated from mouse hearts or cultured cells with the RNeasy Fibrous Tissue Kit (Qiagen, #74704) and quantified by the NANODROP 2000 Spectrophotometer (Thermo Scientific). cDNA was synthesized using the SuperScript III First-Strand Synthesis Kit (Invitrogen, #18080-051). Quantitative PCR was performed using SYBR green dye (Bio-Rad, #172-5274) on a CFX96 Real-Time PCR (RT-PCR) detection system (Bio-Rad). The primer sequences for real-time PCR were as follows: ribosomal protein 27, forward: 5’-ggacgctactccggacgcaaag-3’, reverse: 5’-cttcttgcccatg gcagctgtcac-3’; DUSP1, forward: 5’-aggacaaccacaaggcagac-3’, reverse: 5’-atactccgcctctgcttcac-3’; DUSP2, forward: 5’-tatgaccagggtggtcctgt-3’, reverse: 5’-ggcactgatctccaccatct-3’; DUSP4, forward: 5’-ggcttttgagttcgtcaagc-3’, reverse: 5’-cacagacacagggaagctga-3’; DUSP5, forward: 5’-tcgtgctggaccacggtag-3’, reverse: 5’-ctgagaacgggctttccaca-3’; DUSP6, forward: 5’-aggcaaaaactgtggtgtcc-3’, reverse: 5’-ccagggtcctttcaaagtca-3’; DUSP8, forward: 5’-tgacccaaaacggaataagc-3’; reverse: 5’-agagatgccagccagacagt-3’; DUSP10, forward: 5’-cagcaacaagcagaact tgc-3’, reverse: 5’-attggtcgtttgcctttgac-3’; DUSP16, forward: 5’-cagcgagatgtcctcaacaa-3’, reverse: 5’-ttggaggcttttgctttctc-3’; atrial natriuretic factor (ANF), forward: 5’-gccctgagtgagcagactg-3’, reverse: 5’-cggaagctgttgcagccta-3’; b-type natriuretic peptide (BNP), forward: 5’-ctgctggagctgataagaga-3’, reverse: 5’-agtcagaaactggagtctcc-3’; MHC, forward: 5’-acctaccagacagaggaaga-3’, αMHC reverse: 5’-attgtgtattggccacagcg-3’, βMHC reverse: 5’-ttgcaaagagtccaggtctgag-3’.
Western blotting
Cells or tissue samples were homogenized into lysis buffer containing 20 mM HEPES pH 7.4, 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, and a protease/phosphatase inhibitor cocktail. Protein samples were quantified and subjected to 10% SDS-PAGE. The following antibodies were used for Western blot detection: phospho-ERK1/2 (Thr202/Tyr204) (Cell Signaling Technology, #9101), phospho-p38 (Thr180/Tyr182) (Cell Signaling Technology, #9211), phospho-JNK1/2 (Thr183/Tyr 185) (Cell Signaling Technology, #4668), phospho-MEK1/2 (Ser217/221) (Cell Signaling Technology, #9154), phospho-MKK3/MKK6 (Ser189/207) (Cell Signaling Technology, #9231), phospho-MKK7 (Ser271/Thr275) (Cell Signaling Technology, #4171), ERK1/2 (Cell Signaling Technology, #9102), p38 (Cell Signaling Technology, #9212), JNK (Cell Signaling Technology, #9252), MEK1/2 (Cell Signaling Technology, #4694), MKK7 (Cell Signaling Technology, #4172), MKK6 (Cell Signaling Technology, #9264), Flag (Cell Signaling Technology, #2368), α tubulin (Santa Cruz biotechnology, sc-5286), lamin A/C (Cell Signaling Technology, #2032), DUSP8 (Abcam, #ab184134), sarcomeric α-actinin (Sigma, #A7811), and GAPDH (Fitzgerald, #10R-G109A). Western blots were quantified using NIH ImageJ and normalized to total protein levels.
Statistical analysis
All the results were presented as mean ± SEM. Student t test was performed to compare means between 2 groups. 1-way ANOVA with a Bonferroni post hoc test was used for comparison of differences across multiple groups. p<0.05 was considered statistically significant.
RESULTS
DUSP8 is induced by cardiac stress to regulate MAPK signaling
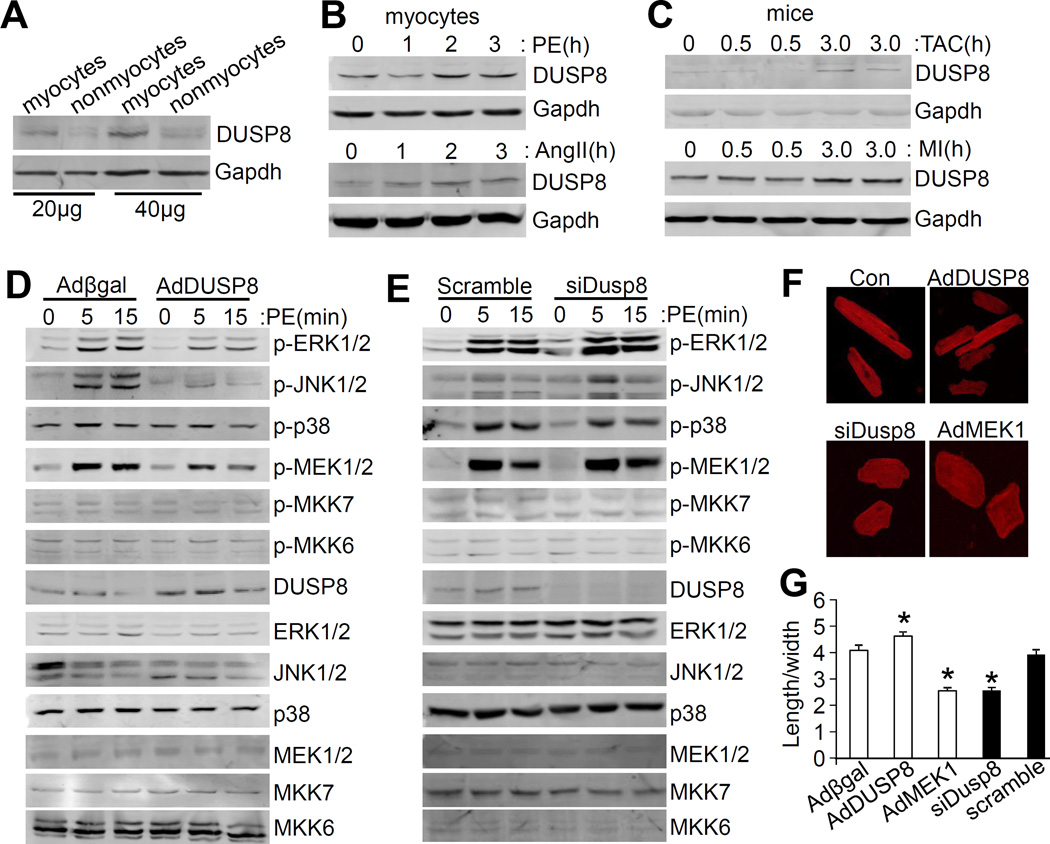
DUSP8 is predominantly expressed in the brain and heart where it is localized to both the cytoplasm and nucleus to inactivate MAPK signaling by direct dephosphorylation of the end effector kinases.25 To understand whether DUSP8 is functionally associated with cardiac diseases, we first analyzed DUSP8 expression from microarray data deposited in the public National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO) database. DUSP8 was upregulated in hearts from dilated cardiomyopathy (DCM) patients compared with non-disease control hearts (Online Figure I).32 We then investigated Dusp8 mRNA expression in myocytes and nonmyocytes isolated from neonatal or adult rodent hearts. Expression of Dusp8 mRNA was predominantly detected in myocytes (Online Figure IIA), which was further confirmed by protein analysis (Figure 1A; Online Figure IIB). We also assessed DUSP8 induction in cultured cardiac myocytes following 4 hours of AngII or PE stimulation, two agonists known to induce GPCR signaling and expression of other DUSP genes. 33 Both stimuli significantly enhanced levels of DUSP8 protein in cardiac myocytes, as well as increased Dusp8 mRNA in cardiac myocytes preferentially over nonmyocytes (Figure 1B; Online Figure IIC and IID). DUSP8 expression was also significantly increased in the hearts of mice subjected to TAC and MI surgical injury for 30 and 60 minutes (Figure 1C; Online Figure IIE and IIF). As previously reported, DUSP8 protein was localized to both the cytoplasm and nucleus in cells transfected with a Flag-DUSP8 fusion construct (Online Figure IIG and IIH).
Figure 1. DUSP8 is predominantly expressed in cardiac myocytes and regulates MAPK activity to influence myocyte growth.
A, Western blot analysis of DUSP8 levels in cardiac myocytes (myocytes) and nonmyocytes isolated from adult hearts. Exactly 20 and 40 μg of protein were loaded for the Western blotting. Gapdh was used as the loading control. B, Western blot analysis of DUSP8 induction in cultured adult cardiac myocytes in response to phenylephrine (PE, 10 μmol/L) or angiotensin II (AngII, 0.1 μmol/L) for the time shown in hours. C, Western blot analysis of DUSP8 induction in the hearts of mice subjected to TAC or MI, harvested at the times shown in hours after the procedure. D, Western blot analysis for total and phosphorylated MAPKs and MAPKKs from cultured neonatal rat cardiac myocytes infected with the 2 indicated adenoviruses. PE was used at 10 μmol/L for the indicated time in minutes. E, Western blot analysis for total and phosphorylated MAPKs and MAPKKs from cultured cardiac myocytes transfected with scramble siRNA or Dusp8 siRNA for 48 hours. PE was again given for the indicated times in minutes. F, Representative images of adult rat cardiac myocytes infected with Adβgal (Con), AdDUSP8, AdMEK1, or transfected with Dusp8 siRNA (siDusp8) for 48 hours. Myocytes were stained with antibody against sarcomeric α-actinin (red). Magnification is 400X total. G, Quantification of length/width ratio of adult myocytes shown in Figure 1F. *p<0.05 vs Adβgal or scramble. Approximately 60~80 cells were analyzed for the length/width ratio.
A recombinant adenovirus was also generated to overexpress DUSP8 in cultured neonatal rat cardiac myocytes to more carefully evaluate the effect on MAPK phosphorylation status at baseline and with 5 and 15 minutes of PE stimulation. Compared with Adβgal control infected cardiac myocytes, AdDUSP8 infection led to a significant reduction of PE-simulated phosphorylation of ERK1/2 and JNK1/2, but not p38 (Figure 1D; Online Figure II, panel I). Interestingly, among the MAPKKs, phosphorylation of MEK1/2 was also mildly diminished upon DUSP8 overexpression (Figure 1D). In contrast, knockdown of DUSP8 with small interfering RNA (siRNA) in cultured cardiac myocytes selectively increased the phosphorylation of ERK1/2 at baseline and with PE stimulation, without influencing JNK1/2 or p38 phosphorylation (Figure 1E; Online Figure IIJ).
These changes in ERK1/2 phosphorylation associated with manipulation in DUSP8 expression also resulted in a profound effect on myocyte morphology in culture. Adult rat cardiac myocytes infected with AdDUSP8 were thinner compared to control myocytes infected with Adβgal, while cardiac myocytes transfected with Dusp8 siRNA for 48 hours had increased width and reduced length, a phenomena similar to that of AdMEK1 overexpressing cells (Figure 1F). Indeed, analysis of length/width ratios revealed that loss of DUSP8 by siRNA led to a type of “concentric” growth of adult myocytes in culture, while overexpression of DUSP8 with AdDUSP8 promoted a type of dilated or eccentric growth (Figure 1G). Thus, DUSP8 primarily regulates ERK1/2 MAPK signaling in cardiac myocytes which influences the growth patterning characteristics of these cells.
Dusp8 KO mice have increased ERK1/2 phosphorylation and concentric remodeling of the heart
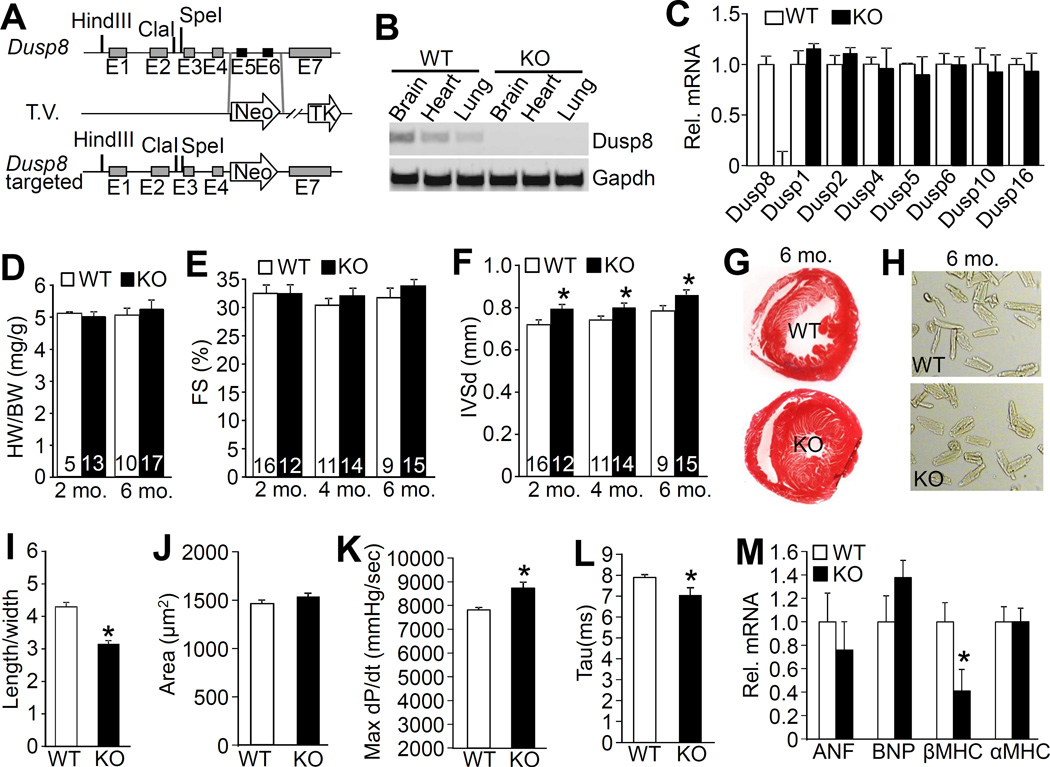
To assess the physiologic function of DUSP8 in the heart we generated Dusp8 KO mice by deleting exons 5 and 6 that encode the phosphatase domain (Figure 2A). We confirmed the absence of DUSP8 mRNA in the heart and other organs of the KO mice by RT-PCR analysis (Figure 2B). Importantly, loss of Dusp8 from the heart did not influence the mRNA expression of other Dusp genes that specifically regulate the MAPKs suggesting that DUSP8 might play a non-redundant role in the heart (Figure 2C). Dusp8 KO mice were viable and fertile and otherwise phenotypically normal (data not shown). With respect to the heart, loss of Dusp8 did not affect cardiac ventricular performance as assessed by echocardiography, nor were heart weights normalized to body weight at 2 and 6 months of age different (Figure 2D and 2E). However, echocardiography revealed a significant increase in septal thickness in the hearts of otherwise normal Dusp8 KO mice at 2, 4 and 6 months of age (Figure 2F), which was also visually apparent in histological sections of this organ (Figure 2G). The left ventricular free wall also appeared thicker by histological analysis although by echocardiography this did not achieve significance (Online Table I). In addition, left ventricular chamber dimension in Dusp8 KO mice during diastole was smaller than WT (Online Table I). This overall profile of a thicker heart with a reduced LV chamber was reminiscent of an increase in ERK1/2 signaling observed in heart-specific MEK1 transgenic mice, which resulted from a selective increase in cardiac myocyte width and a reduction in length.12 Indeed, the adult cardiac myocyte length/width ratio was significantly reduced in 6 month-old Dusp8 KO mice, yet total cardiac myocyte area was not different between WT and Dusp8 KO mice (Figure 2H-J), a phenotype consistent with concentric remodeling due to ERK1/2 signaling.13
Figure 2. Gene targeting of Dusp8 and resulting cardiac phenotype.
A, Schematic of the Dusp8 genetic locus and the targeting vector (T.V.) used to create Dusp8 gene-deleted embryonic stem cells, then mice. Restriction enzyme sites and exons are shown, and the neomycin (Neo) resistance cassette in the T.V. B, RT-PCR analysis of Dusp8 mRNA in the brain, heart and lung of 2 month-old WT versus Dusp8 KO mice. Gapdh was used as PCR control. C, Real-time PCR analysis of expression of multiple Dusp mRNAs in hearts of 2 month-old Dusp8 WT or KO mice. N=3 individual samples. D, Heart weight (HW) normalized to body weight (BW) in Dusp8 WT and KO mice at the indicated ages. Number of mice used is shown in the bars. E-F, Echocardiographic assessment of FS and interventricular septal thickness in diastole (IVSd) in Dusp8 WT and KO mice at the indicated ages. *p<0.05 vs WT. Number of mice used is shown in the bars. G, Representative Masson trichrome-stained histological sections from the hearts of Dusp8 WT and KO mice at 6 months of age. Magnification is 40x total. H, Representative microscopic phase-contrast images of adult cardiac myocytes isolated from 6 month-old Dusp8 WT and KO mice. Magnification is 200x total. I, Quantification of length/width ratio of adult cardiac myocytes isolated from Dusp8 WT and KO mice at 6 months of age as shown in panel “H”. A total of 248 myocytes were analyzed for each group. *p<0.05 vs WT. J, Analysis of the surface area of adult myocytes isolated from 6 month-old Dusp8 WT and KO mice as shown in panel “H”. A total of 248 myocytes were analyzed for each group. K and L, Invasive hemodynamic measurement of (K) cardiac contractility at baseline (load-dependent) as maximum rate of pressure change in the left ventricle over time (dP/dt max) or (L) time constant for isovolumetric relaxation (Tau) showing the exponential decay of ventricular pressure during isovolumic relaxation in Dusp8 WT and KO mice. N=3 for each group. *p<0.05 vs WT. M, Real-time PCR analysis for mRNA levels of atrial natriuretic factor (ANF), b-type natriuretic peptide (BNP), β-myosin heavy chain (βMHC), and α-myosin heavy chain (αMHC) in 2 month-old Dusp8 WT or KO mice. N=4 for each group. *p<0.05 vs WT.
Concentric remodeling of the heart can be an adaptive response to reduce wall stress, which could have some protection as shown previously in select mouse models.12, 13 Using invasive hemodynamic measurements in vivo, Dusp8 null mice showed a small, albeit significant increase in cardiac contractility and faster relaxation times compared to WT controls (Figure 2K and 2L). Interestingly, investigation of gene expression profiles in adult hearts from Dusp8 KO mice showed a significant and consistent reduction in βMHC mRNA levels compared with WT mice, but not other hypertrophic markers (Figure 2M). The βMHC gene encodes a slower ATPase isoform of myosin heavy chain compared with the αMHC isoform that is more highly expressed in the rodent heart.34
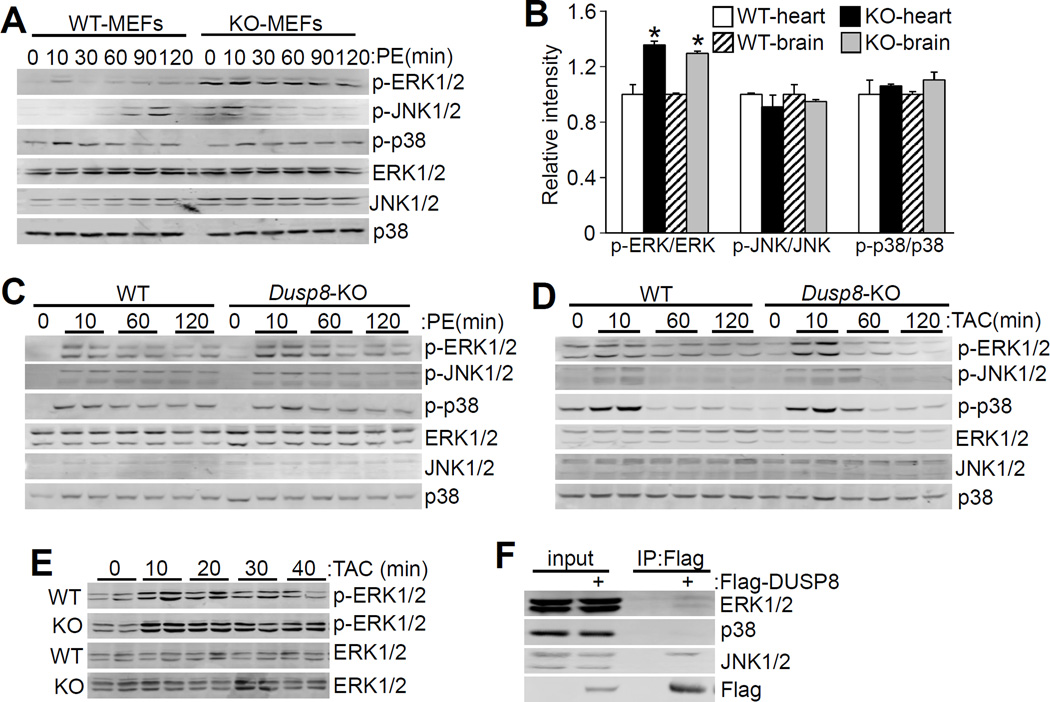
To investigate the underlying signaling mechanism for the concentric remodeling observed in Dusp8 KO hearts we analyzed total MAPK phosphorylation at 2 months of age. We have previously shown that Dusp1/4 double KO mice had a baseline augmentation in total phospho-p38 levels in the heart, while Dusp6 KO mice had a selective increase in total baseline ERK1/2 phosphorylation in the heart.20, 21 MEFs from WT and Dusp8 gene-deleted mice were first analyzed because these cells typically have less sporadic MAPK activation at baseline compared with cardiac myocytes (Liu and Molkentin, unpublished observations). Indeed, WT MEFs showed low levels of ERK1/2, JNK1/2 and p38 activation at baseline, yet Dusp8 null MEFs showed a dramatic increase in baseline and PE-stimulated ERK1/2 phosphorylation, with no changes in JNK1/2 or p38 (Figure 3A; Online Figure IIIA). These results suggest that DUSP8 has a specific function in dephosphorylating ERK1/2 in fibroblasts, but not other MAPKs.
Figure 3. Loss of Dusp8 leads to increased ERK1/2 phosphorylation.
A, Western blot assessment of MAPK phosphorylation and total MAPK levels in Dusp8 WT and KO MEFs in culture with or without PE stimulation at 10 μmol/L for the indicated times (0 time point is no PE stimulation). Of note, there is an open lane between the 6 WT and KO protein samples on the Western blot, given the variable bleed over from the adjacent lanes. B, Quantitative analysis of phosphorylated MAPKs relative to total MAPKs in 2 month-old Dusp8 WT and KO hearts and brains shown in Online Figure IIIB. N=4 for each group. *p<0.05 vs WT. C, D and E, Western blot analysis of cardiac MAPK phosphorylation and total MAPK levels in Dusp8 WT or KO mice after (C) PE or (D and E) TAC stimulation as indicated in the panel, for the indicated period of time in minutes. N=4 or greater for each. F, Western blot analysis of interaction between endogenous MAPKs (ERK1/2, p38, and JNK1/2) and exogenously expressed Flag-DUSP8 in HEK293 cells. This experiment was repeated three times with similar results.
Since DUSP8 is most highly expressed in heart and brain we also investigated baseline MAPK phosphorylation levels in these whole tissues in young adult mice. Consistent with the data in MEFs, ERK1/2, but not JNK1/2 or p38 phosphorylation levels were significantly elevated at baseline in both heart and brain tissue (Figure 3B; Online Figure IIIB). To analyze ERK1/2 phosphorylation in more depth, Dusp8 KO mice and WT controls were either subjected to acute PE injection or a TAC surgical procedure, and thereafter hearts were harvested 10, 60 and 120 minutes after stimulus onset for analysis of MAPK phosphorylation levels (Figure 3C and 3D; Online Figure IIIC-E). Here, the data show a significant increase only in ERK1/2 phosphorylation at 10 minutes, while by 60 or 120 minutes after stimulation ERK1/2 phosphorylation was equally downregulated between KO and WT mice (see discussion). There were no phosphorylation changes in p38 or JNK1/2 between WT and KO hearts (Figure 3C and 3D; Online Figure IIIC-E). The acute increase in ERK1/2 phosphorylation suggested that the effect of DUSP8 might be restricted to shorter durations, hence we repeated the analysis over a more refined acute time course of 10, 20, 30, and 40 minutes following TAC stimulation. The data showed significantly greater ERK1/2 phosphorylation at all 4 time points in Dusp8 KO hearts compared with WT controls (Figure 3E; Online Figure IIIF). Taken together these results suggest that DUSP8 regulates both baseline and short-term ERK1/2 signaling, but by 60 minutes and beyond other DUSPs are likely induced where they contribute enough to diminish this effect.
DUSP6 can also affect baseline ERK1/2 phosphorylation status in the heart and other tissues, as we have previously shown,21 which is likely due to a known direct interaction between this phosphatase and ERK2.35, 36 A Flag-DUSP8 encoding plasmid was transfected into HEK293 cells followed by pulldown of DUSP8 with a Flag resin, which identified both ERK1 and ERK2 bands, as well as the 54 kDa isoform of JNK1/2 (Figure 3F). Hence, DUSP8 is part of a complex with ERK1/2 and a specific isoform of JNK1/2, which could explain how it might regulate basal levels of phosphorylation of at least ERK1/2 in the heart and other tissues (MEFs).
Assessment of stress-induced hypertrophy in Dusp8 KO mice
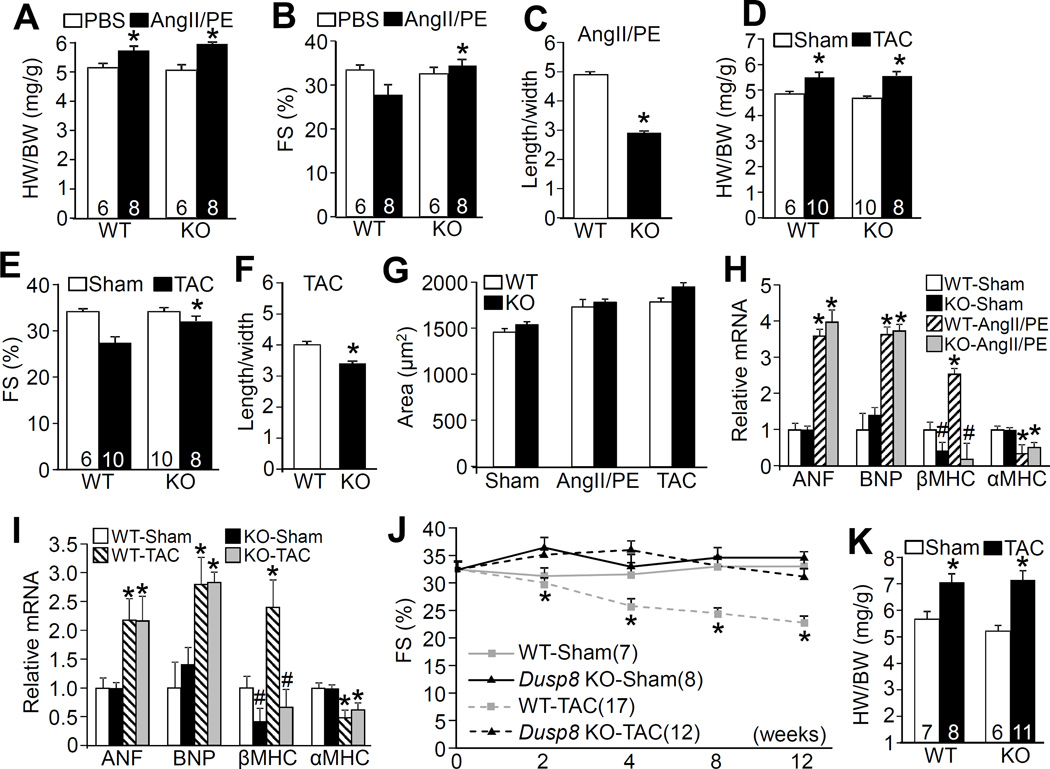
Heart-specific MEK1 transgenic mice, which have constitutively augmented ERK1/2 phosphorylation in the heart, have mild concentric hypertrophic remodeling, are hypercontractile, and are partially protected from heart failure-inducing stimuli.12, 37, 38 Thus, the mild concentric heart remodeling and increase in cardiac contractility we observed in Dusp8 KO mice was hypothesized to positively impact cardiac health after disease stimulation. First, we subjected WT and Dusp8 KO mice to infusion of AngII/PE or a TAC surgical procedure for 2 weeks, which induced an identical increase in heart weights in both groups (Figure 4A and 4D). However, Dusp8 KO mice maintained cardiac functional performance significantly better than WT controls subjected to either stimulus and they continued to show concentric ventricular remodeling (Figure 4B and 4E; Online Table II). Moreover, isolation of individual adult cardiac myocytes from these hearts after 2 weeks of stimulation showed the same propensity towards myocyte thickening without a matching increase in length, compared with WT controls, although total cellular areas were the same (Figure 4C, 4F, and 4G). There was still a significant reduction in βMHC mRNA levels in the heart after 2 weeks of AngII/PE stimulation or TAC in KO versus WT control mice. However the induction of ANF and BNP, and decrease in αMHC, following either stimulation were not statistically different between Dusp8 KO and WT mice (Figure 4H and 4I).
Figure 4. Analysis of cardiac hypertrophy and remodeling in Dusp8 KO mice after stress stimulation.
A, HW/BW ratio in Dusp8 WT and KO mice 2 weeks after AngII/PE infusion or PBS control. Number of mice used is shown within the bars. *p<0.05 vs PBS. B, Echocardiographic assessment of FS in Dusp8 WT and KO mice after 2 weeks of AngII/PE infusion. *p<0.05 vs WT AngII/PE. Number of mice analyzed is shown in the bars. C, Analysis of length/width ratio of adult cardiac myocytes isolated from Dusp8 WT and KO mice after AngII/PE infusion. Approximately 250 myocytes were analyzed for each group. *p<0.05 vs WT. D, HW/BW ratio in Dusp8 WT and KO mice 2 weeks after TAC or a sham procedure. Number of mice analyzed is shown in the bars. *p<0.05 vs Sham. E, Echocardiographic analysis of FS in Dusp8 WT or KO mice after 2 weeks of TAC or a sham procedure. *p<0.05 vs WT TAC. Number of mice analyzed is shown in the bars. F, Analysis of length/width ratio of adult cardiac myocytes isolated from Dusp8 WT and KO mice after 2 weeks of TAC. Approximately 250 myocytes were analyzed for each group.*p<0.05 vs WT. G, Analysis of adult cardiac myocyte area from dissociated hearts from Dusp8 WT and KO mice after AngII/PE, TAC or a sham procedure. Approximately 250 myocytes were analyzed for each group. H and I, Real-time PCR analysis of mRNA levels of ANF, BNP, βMHC, and αMHC from hearts of Dusp8 WT and KO mice after AngII/PE (H) or TAC (I) stimulation for 2 weeks compared to sham operated groups. At least 4 mice (hearts) were analyzed in each group. *p<0.05 vs WT or KO sham; #p<0.05 vs WT sham. J, Echocardiographic analysis of FS in Dusp8 WT and KO mice following TAC or a sham procedure for the indicated time in weeks. *p<0.05 vs KO TAC. Number of mice analyzed is shown. K, HW/BW ratio in Dusp8 WT and KO mice 12 weeks after a TAC or a sham procedure. Number of mice analyzed is shown in the bars. *p<0.05 vs Sham.
To investigate the effect of Dusp8 gene deletion on the propensity for heart failure progression in the setting of more severe disease, we subjected Dusp8 KO and WT control mice to 12 weeks of TAC stimulation. The data show that Dusp8 KO mice maintained cardiac function while the WT controls significantly decompensated in a progressive manner over the 12 weeks of TAC, although total increases in heart weight normalized to body weights were not different between the WT and KO groups (Figure 4J and 4K). These results indicate that the mild baseline concentric remodeling response and increased contractile performance characteristic of Dusp8 KO mice does not predispose to maladaptation, but instead provides prolonged protection.
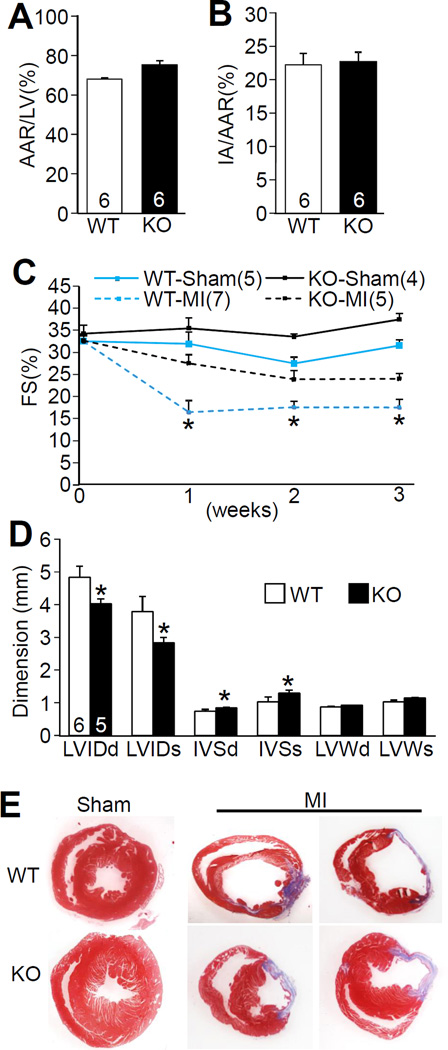
Compensatory remodeling and heart failure after MI injury in Dusp8 KO and WT mice was investigated as well. Importantly, loss of Dusp8 from the heart did not affect the degree of myocyte loss and total area of infarction after I/R injury to the heart, compared with WT hearts (Figure 5A and 5B). With respect to post-MI ventricular remodeling and heart failure, WT mice showed a significant reduction in cardiac ventricular performance as measured by echocardiography at 1, 2 and 3 weeks after injury, while Dusp8 KO mice were protected and maintained performance similar to sham surgical controls (Figure 5C). Further echocardiographic analysis also suggested protection as indicated by less ventricular dilation in Dusp8 null hearts 3 weeks after MI injury, as well as a maintained thickness in the septal wall and a trend toward greater thickness in the LV free wall in KO compared with WT mice (Figure 5D). Indeed, histological analysis of these hearts 3 weeks after MI showed greater ventricular dilation in WT mice while the KO mice had noticeably thicker septa and remaining LV free walls (Figure 5E). Thus, Dusp8 KO mice were partially protected from cardiac insults that would otherwise cause failure (see discussion).
Figure 5. Dusp8 KO mice are protected from heart failure following MI injury.
A, Area-at-risk (AAR) normalized to the perfused-area of the left ventricle (LV) in Dusp8 WT and KO mice subjected to 60 minutes of ischemia followed by 24 hours of reperfusion. Number of mice analyzed is shown in the bars. B, Assessment of infarct-area normalized to area-at-risk (IA/AAR) in the hearts of Dusp8 WT and KO mice after I/R injury. Number of mice analyzed is shown in the bars. C, Echocardiographic assessment of FS in Dusp8 WT and KO mice subjected to a sham or a MI procedure for the indicated times. *p<0.05 vs KO MI. D, Echocardiographic parameters in Dusp8 WT and KO mice 3 weeks after MI surgery. LVIDd and LVIDs, left ventricular end-diastolic and end-systolic chamber diameters; IVSd and IVSs, intraventricular end-diastolic and end-systolic septal thickness. LVWd and LVWs, left ventricular end-diastolic and end-systolic posterior wall thickness. *p<0.05 vs WT after MI. Number of mice analyzed is shown in the bars. E, Masson trichrome-stained histological heart sections from Dusp8 WT and KO mice 3 weeks after MI or sham.
Cardiac-specific overexpression of Dusp8 causes cardiomyopathy
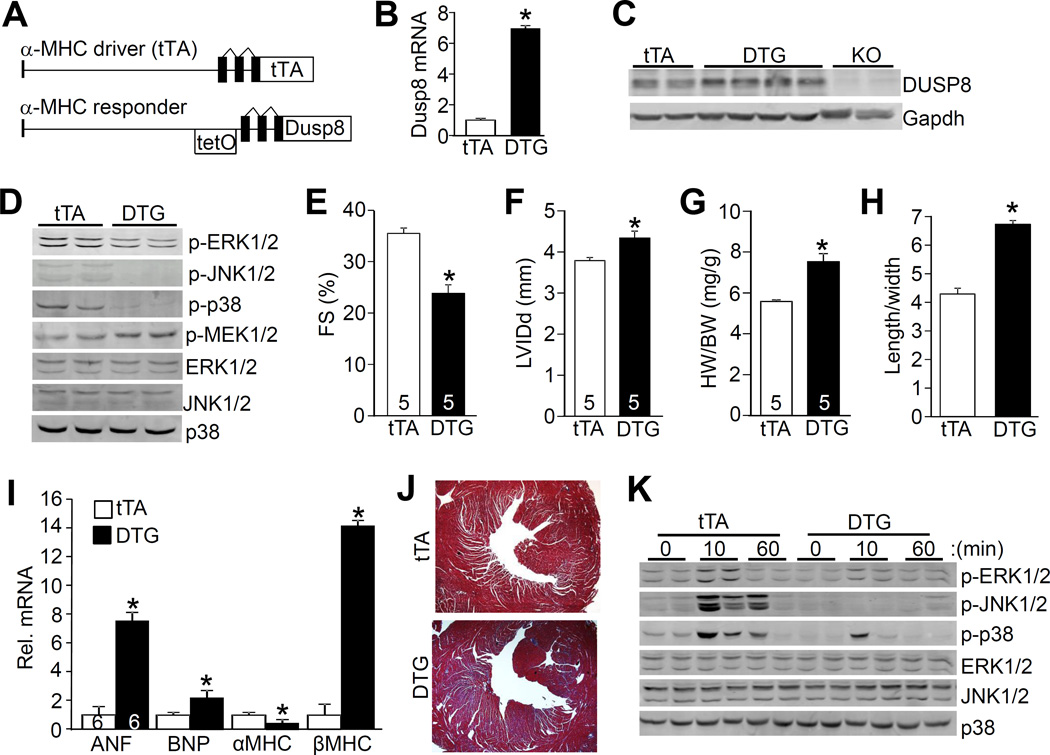
To further investigate the manner in which DUSP8 might alter MAPK signaling and ventricular remodeling, cardiac-specific inducible transgenic mice to overexpress this phosphatase in the heart were also generated (Figure 6A). The DUSP8 cDNA was cloned into the αMHC promoter-containing responder vector to generate transgenic mice. When bred with transgenic mice containing the αMHC-driven tetracycline transactivator (tTA) protein, and doxycycline (Dox) is absent, this line now produces expression in the heart (Figure 6A). Using this bi-transgenic strategy in the absence of Dox, virtually all double transgenic (DTG) mice died within 2 weeks of birth (data not shown), indicating that overexpression of DUSP8 is detrimental to the heart during development. However, treatment of pregnant mothers with Dox-containing chow to shut-down the inducible system during development and up to the first 3 weeks after birth resulted in viability of all DTG mice. Upon weaning, mice were removed from the Dox-dependent transgene repression, whereby DUSP8 mRNA and protein expression were strongly induced in the heart over the next 6 weeks (Figure 6B and 6C).
Figure 6. Generation of cardiac-specific DUSP8 transgenic mice and phenotypic characterization.
A, Schematic of the bi-transgenic inducible expression system used to regulate DUSP8 heart-specific overexpression. Abbreviations: tetO, tetracycline operator with response elements. B and C, Analysis of cardiac DUSP8 expression by real-time PCR in double transgenic mice (DTG) versus only single transgenic tTA mice (B) and Western blot (C) from hearts of mice 6 weeks after Dox removal to induce expression, 9 weeks-old in total. *p<0.05 vs tTA. Dusp8 KO heart samples are shown as a control. D, Western blot analysis for MAPK phosphorylation and total MAPKs in tTA controls and DTG mice at 9 weeks of age. At least six separate heart samples were analyzed for each group with similar results. E and F, Echocardiographic assessment of FS and LV internal chamber dimension at end diastole (LVIDd) in tTA controls and DTG mice at 9 weeks of age with 6 weeks of transgene induction. *p<0.05 vs tTA control. Number of mice analyzed is shown in the bars. G, HW/BW ratio in tTA controls and DTG mice at 9 weeks of age. *p<0.05 vs tTA. Number of mice analyzed is shown in the bars. H, Analysis of length/width ratio of adult cardiac myocytes isolated from hearts of tTA and DTG mice with 6 weeks of transgene induction. A total of 170 myocytes were analyzed for each group. *p<0.05 vs tTA. I, Real-time PCR analysis of mRNA for the indicated genes from the hearts of tTA control and DTG mice. N=4 hearts for each group. *p<0.05 vs tTA. J, Masson trichrome-stained histological sections of hearts from tTA and DTG mice at 9 weeks of age. Magnification is 40X total. K, Western blot analysis for MAPK phosphorylation and total MAPK levels from hearts of tTA controls and DTG mice after TAC stimulation for the indicated time in minutes. The 0 time point represents sham mice. At least 4 separate samples were analyzed.
Hearts from DUSP8 DTG mice were collected at 9 weeks of age for analysis of MAPK phosphorylation status. The data show down regulation in baseline ERK1/2, JNK1/2, and p38 phosphorylation in the heart due to DUSP8 overexpression, as well as a significant increase in MEK1 phosphorylation, similar to the effect observed in Mapk1/3 (ERK1/2) KO hearts through a feedback mechanism as previously observed (Figure 6D; Online Figure IVA).13 Associated with this global reduction in total MAPK phosphorylation in the heart was an increase in baseline dilation in LV chamber dimension and a loss in cardiac ventricular performance (Figure 6E and 6F; Online Figure IVB). Heart weight normalized to body weight was increased as was the length of individual adult myocytes isolated from DUSP8 DTG hearts, indicating dilated cardiac remodeling (Figure 6G and 6H). DUSP8 overexpression also induced cardiac hypertrophic marker gene expression with a dramatic increase in βMHC mRNA and a decrease in αMHC mRNA, as well as generalized cardiac fibrosis (Figure 6I and 6J). Overexpression of DUSP8 reduced the inducible phosphorylation of ERK1/2, p38, and JNK1/2 after TAC stimulation for 10 minutes, compared with control mice (Figure 6K; Online Figure IVC). TAC stimulation for 2 weeks also induced greater decompensation and loss of ventricular performance in DUSP8 DTG mice compared with tTA littermate controls (Online Figure IVD). While overexpression of DUSP8 models the known increase in this gene product in disease hearts, the levels achieved here in the transgenic approach were higher and might be responsible for some loss of specificity in also dephosphorylating JNK1/2 and p38. However, the transgenic approach still provides insight into how regulation of ERK1/2 by this DUSP family member can underlie cardiac remodeling and disease responsiveness (see discussion).
DISCUSSION
Loss of Dusp8 resulted in a profile of elevated ERK1/2 phosphorylation at baseline and with acute stimulation, resulting in a cardiac phenotype of concentric growth due to an increase in cardiac myocyte width, as well as a mild increase in contractility. This profile in Dusp8 null mice conferred protection to the heart in response to challenges that would otherwise lead to pronounced ventricular dilation and heart failure. Conversely, overexpression of DUSP8 decreased phosphorylation of ERK1/2, as well as p38 and JNK, resulting in the opposite phenotype of eccentric and dilatory ventricular remodeling that was associated with lengthening of individual cardiac myocytes. That ERK1/2 are the primary mechanistic explanation for eccentric versus concentric remodeling of the heart due to altered DUSP8 signaling is consistent with past data in genetically altered mice with specific manipulation of MEK1 or ERK1/2.12–14, 21
One of the interesting aspects of our study is the relationship between DUSP8 and MAPK signaling dynamics in the heart, which is also dependent on the backdrop of other available or co-induced Dusp genes in cardiac myocytes. We observed that loss of Dusp8 produced both an increase in baseline ERK1/2 phosphorylation as well as greater net phosphorylation levels in the first 40 minutes of stress to the heart. However, overexpression of DUSP8 in the heart or cultured cardiac myocytes caused dephosphorylation of all three major MAPK terminal effectors. Past literature has suggested that DUSP8 preferentially regulates p38 and JNK1/2 in select cell lines, although cardiac myocytes were not investigated, and these past approaches were also based on overexpression strategies. 39, 40 Thus, results from KO mice or KO cell lines are not in agreement with overexpression approaches in attributing the potential physiologic targets of DUSP8. This same general paradigm is reminiscent of data obtained with Dusp1 gene-targeted mice. Dusp1 KO mice showed no effect on ERK1/2 phosphorylation in the heart, yet cardiac overexpression of DUSP1 led to the equal inactivation of ERK1/2, JNK1/2, and p38.20, 41 Thus, loss-of-function approaches are likely of greater biologic relevance in ascertaining the true regulatory targets of various DUSP family members compared with overexpression approaches. In this case, our results suggest that DUSP8 is a more dedicated regulator of ERK1/2 in the heart, brain, and also fibroblasts.
Biochemical characterization studies revealed that MAPK family members have evolutionarily conserved interaction domain with two aspartic acids that are essential for binding to MAPKKs, phosphatases, and substrates.42 Similar to other DUSP proteins, DUSP8 possesses a docking site (56KRR58) in the N-terminus for possible ERK1/2 interaction. It will be interesting to understand the molecular basis for ERK1/2 recognition by DUSP8 by analyzing how amino acid residues within this domain might specify the types of MAPKs that can be bound. It is also likely that other regions outside of the docking domain could further specify ERK1/2 binding, as suggested by in depth biochemical analysis of the DUSP6-ERK2 interaction. 36
The profile of greater ERK1/2 phosphorylation in vivo in the absence of DUSP8 is intimately tied to the expression and induction of other DUSP family members that have activity towards ERK1/2. We previously showed that DUSP6 serves as a major dual-specificity phosphatase for regulating baseline ERK1/2 dephosphorylation status in cardiac myocytes, but not in response to stress induction.13, 14, 21 Thus, while both DUSP6 and DUSP8 appear to help establish a set-point in basal ERK1/2 phosphorylation status in the heart, DUSP8 can also regulate ERK1/2 dephosphorylation with acute stress stimulation over at least 40 minutes of time. After 60 minutes ERK1/2 now start to show dephosphorylation, likely due to the effects of other DUSP family members that are induced and that possibly play a greater role in long-term dampening of this signaling pathway, such as DUSP2, 5, and 7 that also have reported specificity for ERK1/2. Thus, DUSP8 appears to have a unique regulatory role in the heart by controlling both the baseline and acute amplitude of ERK1/2 phosphorylation during a stress or injury response.
Dusp8 KO mice also displayed an unexpected cardiac phenotype at baseline. Hearts from these mice were significantly remodeled with a profile of concentric ventricular hypertrophy and a mild but significant increase in baseline contractility, although absolute heart weights were not changed. Isolated adult cardiac myocytes from hearts of Dusp8 KO mice were significantly thicker and shorter, a phenotype that is reminiscent of transgenic mice expressing activated MEK1 in the heart (constitutive ERK1/2 activity). Indeed, both MEK1 transgenic and Dusp8 KO mice were significantly protected from pathologic insults that would otherwise cause heart failure.43 This profile of concentric remodeling with thicker cardiac myocytes appears to be protective, possibly by simply maintaining lower ventricular wall stress due to Laplace’s law. With respect to loss-of-function, mice with heart-specific deletion of Mapk1/3 (ERK1/2) showed extreme cardiac dilation characterized by longer cardiac myocytes that lacked thickness.13 This same general dilated profile was also observed in hearts of DUSP6 overexpressing transgenic mice that were completely inhibited for ERK1/2 phosphorylation.14 Thus, the dynamic equilibrium of ERK1/2 phosphorylation is intimately tied to an ill-defined molecular mechanism whereby cardiac myocyte length-width ratio is encoded, either developmentally or in response to new hypertrophic stimuli to the adult heart.
We believe that ERK1/2 are unique in their ability to promote cardiac myocyte thickening growth compared with the other MAPKs. Indeed, cardiac-specific MEK3 or MEK6 transgenic mice, which have greater p38 MAPK signaling, showed cardiomyopathy and ventricular dilation, similar to MKK7 TG mice with greater JNK1/2 activity.44, 45 By comparison, inhibition of p38 or JNK1/2 in the heart appeared to promote hypertrophic thickening of the heart.7, 9 Thus, p38 and JNK1/2 signaling have the opposite effect of ERK1/2 in affecting myocyte and ventricular growth dynamics, although we do not believe that p38 and JNK1/2 are as dedicated to regulating myocyte growth as are ERK1/2. Thus, while DUSP8 overexpression in the heart also promoted p38 and JNK1/2 inactivation in addition to ERK1/2, the hearts of these mice still dilated instead of becoming thicker, likely due to the more central role of ERK1/2 signaling. Indeed, we previously published that Dusp1/4 double KO mice, which have greater cardiac p38 signaling, but no effect on ERK1/2, develop cardiomyopathy and ventricular dilation during aging or with acute TAC stress stimulation.20 Thus, while there are 13 DUSP proteins that are dedicated to regulating and recycling the MAPKs, each appears to have a highly specialized regulatory role.
DUSP8 is one of only 3 family members that is present in both the cytoplasm and nucleus, and DUSP8 has a more complicated multidomain configuration with a unique C terminal PEST motif rich in proline [P], glutamic acid [E], serine [S], and threonine [T] that mediates its rapid degradation.46 Our loss-of-function study performed here now suggests for the first time that DUSP8 is a dedicated regulator of ERK1/2 in vivo. This specificity is likely tied to specific complexes that are formed amongst the MAPKs and the DUSP proteins, consistent with our observation that DUSP8 can form a complex with ERK1/2 in vivo. Indeed, our results suggest that DUSP8 is a dedicated regulator of ERK1/2 signaling in the heart, which impacts cardiac ventricular geometry, contractility and disease responsiveness.
Supplementary Material
Novelty and Significance.
What Is Known?
DUSP8 preferentially dephosphorylates JNK when overexpressed in culture.
DUSP8 is expressed in the heart.
MAPKs play a complex role in regulating cardiac hypertrophy and heart failure.
What New Information Does This Article Contain?
DUSP8 functions primarily through ERK1/2 in the heart.
DUSP8 regulates the magnitude and duration of ERK1/2 signaling following pathological stimulation to the adult heart.
DUSP8 regulates myocyte length-width growth dynamics through ERK1/2.
Loss of DUSP8 is cardioprotective and produces mild concentric remodelling.
This study was designed to examine the involvement of DUSP8 as a regulator of MAPK signaling in the heart and the impact on ventricular and cardiac myocyte growth dynamics. We demonstrate that DUSP8 functions primarily through ERK1/2 signaling in vivo. Deletion of Dusp8 in gene-targeted mice leads to concentric remodelling, greater contractility and cardioprotection, while cardiac specific expression of DUSP8 results in eccentric growth and cardiomyopathy. Our results suggest that DUSP8 regulates the dynamics of MAPK signaling to influence cardiac remodelling and disease propensity in vivo.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from the National Institutes of Health (to J.D. Molkentin). J.D. Molkentin was also supported by the Howard Hughes Medical Institute. R.L. was supported by a training grant from the National Heart Lung and Blood Institute of the NIH (T32HL125204).
Nonstandard Abbreviations and Acronyms
- α-MHC
α-myosin heavy chain
- AngII
angiotensin II
- β-gal
β-galactosidase
- DTG
double transgenic
- Dox
doxycycline
- DUSP
dual-specificity phosphatase
- ERK
extracellular signal-regulated kinase
- ES
embryonic stem
- FS
fractional shortening
- GPCR
G protein-coupled receptor
- I/R
ischemia/reperfusion
- JNK
c-Jun N-terminal kinase
- KO
knockout or gene-deleted
- LV
left ventricle
- MAPK
mitogen-activated protein kinase
- MAPKK
mitogen-activated protein kinase kinase
- MEF
mouse embryonic fibroblast
- MI
myocardial infarction
- PCR
polymerase chain reaction
- PE
phenylephrine
- siRNA
small interfering RNA
- TAC
transverse aortic constriction
- tTA
tetracycline transactivator
- WT
wild type
Footnotes
DISCLOSURES
No financial or other conflicts of interest exist with any of the authors.
REFERENCES
- 1.Kyriakis JM, Avruch J. Mammalian mapk signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 2.Cargnello M, Roux PP. Activation and function of the mapks and their substrates, the mapk-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldsmith ZG, Dhanasekaran DN. G protein regulation of mapk networks. Oncogene. 2007;26:3122–3142. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- 4.Hall A. Rho gtpases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 5.Goupil E, Wisehart V, Khoury E, Zimmerman B, Jaffal S, Hebert TE, Laporte SA. Biasing the prostaglandin f2alpha receptor responses toward egfr-dependent transactivation of mapk. Mol Endocrinol. 2012;26:1189–1202. doi: 10.1210/me.2011-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL. Angiotensin ii increases periostin expression via ras/p38 mapk/creb and erk1/2/tgf-beta1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91:80–89. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]
- 7.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 mapk promotes hypertrophic cardiomyopathy through upregulation of calcineurin-nfat signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, Takeda T, Osuka S, Morita T, Kondoh G, Uno Y, Kashiwase K, Taniike M, Nakai A, Matsumura Y, Miyazaki J, Sudo T, Hongo K, Kusakari Y, Kurihara S, Chien KR, Takeda J, Hori M, Otsu K. P38alpha mitogen-activated protein kinase plays a critical role in cardiac myocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. C-jun n-terminal kinases (jnk) antagonize cardiac growth through cross-talk with calcineurin-nfat signaling. EMBO J. 2003;22:5079–5089. doi: 10.1093/emboj/cdg474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W, Zi M, Jin J, Prehar S, Oceandy D, Kimura TE, Lei M, Neyses L, Weston AH, Cartwright EJ, Wang X. Cardiac-specific deletion of mkk4 reveals its role in pathological hypertrophic remodeling but not in physiological cardiac growth. Circ Res. 2009;104:905–914. doi: 10.1161/CIRCRESAHA.108.188292. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Zi M, Chi H, Jin J, Prehar S, Neyses L, Cartwright EJ, Flavell RA, Davis RJ, Wang X. Deprivation of mkk7 in cardiac myocytes provokes heart failure in mice when exposed to pressure overload. J Mol Cell Cardiol. 2011;50:702–711. doi: 10.1016/j.yjmcc.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The mek1-erk1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, York AJ, Lorenz JN, Zimmermann WH, Meloche S, Molkentin JD. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac erk1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caunt CJ, Keyse SM. Dual-specificity map kinase phosphatases (mkps): Shaping the outcome of map kinase signalling. FEBS J. 2013;280:489–504. doi: 10.1111/j.1742-4658.2012.08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salojin K, Oravecz T. Regulation of innate immunity by mapk dual-specificity phosphatases: Knockout models reveal new tricks of old genes. J Leukoc Biol. 2007;81:860–869. doi: 10.1189/jlb.1006639. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Gorospe M, Hutter D, Barnes J, Keyse SM, Liu Y. Transcriptional induction of mkp-1 in response to stress is associated with histone h3 phosphorylation-acetylation. Mol Cell Biol. 2001;21:8213–8224. doi: 10.1128/MCB.21.23.8213-8224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori Sequeiros Garcia M, Gomez NV, Gorostizaga A, Acquier A, Gonzalez-Calvar SI, Mendez CF, Paz C. Map kinase phosphatase-3 (mkp-3) is transcriptionally and post-translationally up-regulated by hcg and modulates camp-induced p21 expression in ma-10 leydig cells. Mol Cell Endocrinol. 2013;371:174–181. doi: 10.1016/j.mce.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Yang D, Xie P, Liu Z. Ischemia/reperfusion-induced mkp-3 impairs endothelial no formation via inactivation of erk1/2 pathway. PLoS One. 2012;7:e42076. doi: 10.1371/journal.pone.0042076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auger-Messier M, Accornero F, Goonasekera SA, Bueno OF, Lorenz JN, van Berlo JH, Willette RN, Molkentin JD. Unrestrained p38 mapk activation in dusp1/4 double-null mice induces cardiomyopathy. Circ Res. 2013;112:48–56. doi: 10.1161/CIRCRESAHA.112.272963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maillet M, Purcell NH, Sargent MA, York AJ, Bueno OF, Molkentin JD. Dusp6 (mkp3) null mice show enhanced erk1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem. 2008;283:31246–31255. doi: 10.1074/jbc.M806085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson BS, Harrison BC, Jeong MY, Reid BG, Wempe MF, Wagner FF, Holson EB, McKinsey TA. Signal-dependent repression of dusp5 by class i hdacs controls nuclear erk activity and cardiac myocyte hypertrophy. Proc Natl Acad Sci U S A. 2013;110:9806–9811. doi: 10.1073/pnas.1301509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molina G, Vogt A, Bakan A, Dai W, Queiroz de Oliveira P, Znosko W, Smithgall TE, Bahar I, Lazo JS, Day BW, Tsang M. Zebrafish chemical screening reveals an inhibitor of dusp6 that expands cardiac cell lineages. Nat Chem Biol. 2009;5:680–687. doi: 10.1038/nchembio.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang CY, Tan TH. Dusps, to map kinases and beyond. Cell Biosci. 2012;2:24. doi: 10.1186/2045-3701-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martell KJ, Seasholtz AF, Kwak SP, Clemens KK, Dixon JE. Hvh-5: A protein tyrosine phosphatase abundant in brain that inactivates mitogen-activated protein kinase. J Neurochem. 1995;65:1823–1833. doi: 10.1046/j.1471-4159.1995.65041823.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen YR, Shrivastava A, Tan TH. Down-regulation of the c-jun n-terminal kinase (jnk) phosphatase m3/6 and activation of jnk by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene. 2001;20:367–374. doi: 10.1038/sj.onc.1204105. [DOI] [PubMed] [Google Scholar]
- 27.Theodosiou A, Ashworth A. Differential effects of stress stimuli on a jnk-inactivating phosphatase. Oncogene. 2002;21:2387–2397. doi: 10.1038/sj.onc.1205309. [DOI] [PubMed] [Google Scholar]
- 28.Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. The dual specificity phosphatases m3/6 and mkp-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem. 1996;271:27205–27208. doi: 10.1074/jbc.271.44.27205. [DOI] [PubMed] [Google Scholar]
- 29.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/nfat coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 30.De Windt LJ, Lim HW, Haq S, Force T, Molkentin JD. Calcineurin promotes protein kinase c and c-jun nh2-terminal kinase activation in the heart. Cross-talk between cardiac hypertrophic signaling pathways. J Biol Chem. 2000;275:13571–13579. doi: 10.1074/jbc.275.18.13571. [DOI] [PubMed] [Google Scholar]
- 31.Liu R, Correll RN, Davis J, Vagnozzi RJ, York AJ, Sargent MA, Nairn AC, Molkentin JD. Cardiac-specific deletion of protein phosphatase 1beta promotes increased myofilament protein phosphorylation and contractile alterations. J Mol Cell Cardiol. 2015;87:204–213. doi: 10.1016/j.yjmcc.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barth AS, Kuner R, Buness A, Ruschhaupt M, Merk S, Zwermann L, Kaab S, Kreuzer E, Steinbeck G, Mansmann U, Poustka A, Nabauer M, Sultmann H. Identification of a common gene expression signature in dilated cardiomyopathy across independent microarray studies. J Am Coll Cardiol. 2006;48:1610–1617. doi: 10.1016/j.jacc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 33.Lim HW, New L, Han J, Molkentin JD. Calcineurin enhances mapk phosphatase-1 expression and p38 mapk inactivation in cardiac myocytes. J Biol Chem. 2001;276:15913–15919. doi: 10.1074/jbc.M100452200. [DOI] [PubMed] [Google Scholar]
- 34.Tardiff JC, Hewett TE, Factor SM, Vikstrom KL, Robbins J, Leinwand LA. Expression of the beta (slow)-isoform of mhc in the adult mouse heart causes dominant-negative functional effects. Am J Physiol Heart Circ Physiol. 2000;278:H412–H419. doi: 10.1152/ajpheart.2000.278.2.H412. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Sun JP, Zhou B, Zhang ZY. Structural basis of docking interactions between erk2 and map kinase phosphatase 3. Proc Natl Acad Sci U S A. 2006;103:5326–5331. doi: 10.1073/pnas.0510506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou B, Zhang J, Liu S, Reddy S, Wang F, Zhang ZY. Mapping erk2-mkp3 binding interfaces by hydrogen/deuterium exchange mass spectrometry. J Biol Chem. 2006;281:38834–38844. doi: 10.1074/jbc.M608916200. [DOI] [PubMed] [Google Scholar]
- 37.Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, Voisin L, Saba-El-Leil MK, Meloche S, Pouyssegur J, Pages G, De Windt LJ, Doevendans PA, Molkentin JD. Mek1-erk2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 38.Maejima Y, Galeotti J, Molkentin JD, Sadoshima J, Zhai P. Constitutively active mek1 rescues cardiac dysfunction caused by overexpressed gsk-3alpha during aging and hemodynamic pressure overload. Am J Physiol Heart Circ Physiol. 2012;303:H979–H988. doi: 10.1152/ajpheart.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palacios C, Collins MK, Perkins GR. The jnk phosphatase m3/6 is inhibited by protein-damaging stress. Curr Biol. 2001;11:1439–1443. doi: 10.1016/s0960-9822(01)00426-2. [DOI] [PubMed] [Google Scholar]
- 40.Cotsiki M, Oehrl W, Samiotaki M, Theodosiou A, Panayotou G. Phosphorylation of the m3/6 dual-specificity phosphatase enhances the activation of jnk by arsenite. Cell Signal. 2012;24:664–676. doi: 10.1016/j.cellsig.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Bueno OF, De Windt LJ, Lim HW, Tymitz KM, Witt SA, Kimball TR, Molkentin JD. The dual-specificity phosphatase mkp-1 limits the cardiac hypertrophic response in vitro and in vivo. Circ Res. 2001;88:88–96. doi: 10.1161/01.res.88.1.88. [DOI] [PubMed] [Google Scholar]
- 42.Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in map kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 43.Kehat I, Molkentin JD. Extracellular signal-regulated kinase 1/2 (erk1/2) signaling in cardiac hypertrophy. Ann N Y Acad Sci. 2010;1188:96–102. doi: 10.1111/j.1749-6632.2009.05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiser RA, Liang Q, Bueno O, Huang Y, Lackey T, Klevitsky R, Hewett TE, Molkentin JD. Genetic inhibition or activation of jnk1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo. J Biol Chem. 2005;280:32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- 45.Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien K, Xiao RP, Kass DA, Wang Y. The in vivo role of p38 map kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci U S A. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rechsteiner M, Rogers SW. Pest sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.