Abstract
Pericytes are modified smooth muscle cells that closely enwrap small blood vessels, regulating and supporting the microvasculature through direct endothelial contact. Pericytes demonstrate a distinct immunohistochemical profile, including expression of αSMA (Smooth Muscle Actin), CD146, PDGFRβ (Platelet Derived Growth Factor Receptor β), and RGS5 (regulator of G-protein signaling 5). Previously, pericyte related antigens have been observed to be present among a group of soft tissue tumors with a perivascular growth pattern, including glomus tumor, myopericytoma and angioleiomyoma. Similarly, malignant tumor cells have been shown to pericyte-like immunoprofile when present in a perivascular location, seen in malignant melanoma, glioblastoma, and adenocarcinoma. Here, we examine well-differentiated liposarcoma specimens, which showed some element of perivascular areas with the appearance of smooth muscle (N=7 tumors). Immunohistochemical staining was performed for pericyte antigens, including αSMA, CD146, PDGFRβ, and RGS5. Results showed consistent pericytic marker expression among liposarcoma tumor cells within a perivascular distribution. MDM2 immunohistochemistry and fluorescence in situ hybridization for MDM2 revealed that these perivascular cells were of tumor origin (7/7 tumors), while double immunohistochemical detection for CD31/CD146 ruled out an endothelial cell contribution. These findings further support the concept of pericytic mimicry, already established in diverse malignancies, and its presence in well-differentiated liposarcoma. The extent to which pericytic mimicry has prognostic significance in liposarcoma is as yet unknown.
Keywords: liposarcoma, pericyte, SMA, CD146, PDGFRB
1. Introduction
Pericytes are modified smooth muscle cells that closely enwrap small blood vessels, regulating and supporting the microvasculature through direct endothelial contact. Pericytes demonstrate a distinct immunohistochemical profile, including expression of αSMA (Smooth Muscle Actin), CD146, PDGFRβ (Platelet Derived Growth Factor Receptor β), and RGS5 (regulator of G-protein signaling 5), without evidence of endothelial differentiation 1, 2. Current interests in pericytes stem in large part from the growing understanding that this cell type represents a native mesenchymal stem cell (MSC) progenitor cell 1, 3–9. Indeed, purified pericytes give rise to multiple mesodermal tissues after in vitro differentiation or in vivo transplantation, including bone, adipose, cartilage, and muscle – features identical to traditionally derived bone marrow MSC 9, 10.
Growing data suggests that when tumor cells adopt a perivascular location, they also adopt cell surface markers characteristic of pericytes. This phenomenon is best understood in malignant melanoma, where tumor cells adopt a perivascular migration pattern, or ‘angiotropism’ 11–13. This perivascular invasion, also called extravascular migratory metastasis (EVMM), is an under recognized route of tumor spread, and associated with a poor prognosis in melanoma 13. Similarly, in the malignant brain tumor glioblastoma, tumor cells adopt a pericyte-like location associated with perivascular invasion 14, 15. In fact, using cell-tracking techniques, investigators have found that the majority of vessel-lining pericyte-like cells in glioblastoma are actually of tumor cell origin. Likewise, recent research suggests that pancreatic and prostatic adenocarcinoma exhibit perivascular invasion for regional spread, although this has been less well studied 16, 17. In summary, pericyte marker expression, also termed ‘pericyte mimicry’ is a characteristic finding across all studied tumors with a perivascular tumor growth.
A subset of liposarcomas display heterologous differentiation, including smooth muscle, bone, and cartilage. Non-neoplastic adipose tissue is a rich source of pericytes 18–22, and it is currently assumed that tissue resident MSC are the precursors for these heterologous components within liposarcoma. Of particular interest are areas with the appearance of smooth muscle within well-differentiated liposarcoma 23–26. Areas resembling smooth muscle often appear to arise from and radiate outwards from intratumoral blood vessels, usually in a multifocal fashion 23. These perivascular proliferations house atypical stromal cells characteristic of liposarcoma, often within the vessel wall. Of note, these perivascular cells demonstrate immunoreactivity Smooth Muscle Actin (SMA)23, but a more thorough examination of pericyte markers has not yet been performed.
Here, we examine well-differentiated liposarcoma specimens. We hypothesized that these perivascular areas with the appearance of smooth muscle would be associated with a pericyte immunophenotype, or ‘pericytic mimicry,’ within liposarcoma tumor cells.
2. Materials and Methods
2.1 Tissue Identification and Histology
Tumors were identified using a retrospective review of the pathology slide archives of the Department of Pathology and Laboratory Medicine at the University of California, Los Angeles (UCLA), examining 55 cases with a diagnosis of ‘well-differentiated liposarcoma’ or ‘atypical lipomatous tumor.’ Slides were reviewed by two independent pathologists to ensure accuracy of diagnosis, and to identify tumors with some element of perivascular areas with the appearance of smooth muscle (S.M.D and A.W.J). Cases with dedifferentiation or greater than 5 mitotic figures per 10 HPF were not included. Patient information was obtained, including age, sex, tumor location, tumor size, and previous cytogenetic studies performed during the initial diagnostic evaluation. Formalin fixed paraffin embedded (FFPE) tumor tissue from patients were acquired from the tissue archives, under UCLA IRB approval # 13-000918.
2.2 Immunohistochemistry
Immunohistochemistry for pericyte markers was performed using the ABC method (Vectastain Elite ABC, Vector Laboratories, Burlingame, CA, USA) using DAB as the chromogen (ImmPACT DAB, Vector Laboratories). Multiple antigens were detected by multiplexing the ABC method and DAB chromogen with an alkaline phosphatase polymer detection method (ImmPress-AP Polymer Detection, Anti-mouse IG, Vector Laboratories) and Vector Red® chromogen (Vector Red® Alkaline Phosphatase Substrate, Vector Laboratories).
The following primary antibodies were used: monoclonal mouse anti-MDM2 (1/100, EMD Millipore), monoclonal mouse anti-αSMA (1/75, [1A4], ABCAM), monoclonal rabbit anti-CD146 (1/500, EPR3208, ABCAM, Cambridge, MA, USA), monoclonal rabbit anti-PDGFRβ (1/100, [2E8E1], monoclonal mouse anti-RGS5 (1/100, [89C2], Cell Signaling Technologies), and monoclonal mouse anti-CD31 (1/100, [89C2], Cell Signaling Technologies). The following secondary antibodies were used: polyclonal goat biotinylated anti-rabbit IgG (1/500, Sigma, St. Louis, MO, USA), polyclonal horse anti-mouse IgG (1/500,[H + L], Vector Laboratories), polyclonal goat anti-rat Ig (1/500, Becton Dickinson and Company).
Heat mediated antigen retrieval was performed for all immunohistochemical stains in 1 mM tris-EDTA, 0.01% Tween-20 (Sigma), pH 8. Non-specific antibody binding was blocked (IHC-TEK Antibody Diluent, pH 7.4, IHC World, LLC, Woodstock, MD, USA). Endogenous peroxidase and alkaline phosphatase blocking solution was used (BLOXALL Endogenous Peroxidase and Alkaline Phosphatase Blocking Solution, Vector Laboratories). Mayer’s hematoxylin was used as a nuclear counterstain (1/5, ABCAM) and slides were mounted using an aqueous media (VectaMount AQ, Vector Laboratories). In all cases, immunohistochemical staining without primary antibody was used as a negative control.
2.3 Fluorescence in situ hybridization (FISH)
Fluorescence in situ hybridization (FISH) was performed using commercially available fluorescently labeled dual-color MDM2 (red)/CEP 12 (green) probe sets from Abbott-Molecular, IL, USA. The FISH hybridization and analyses were performed on 2 µm thick formalin fixed paraffin tissue sections, following the manufacturer’s suggested protocols. The cells were counterstained with DAPI (4',6-diamidino-2-phenylindole) and the red and green fluorescent probe signals were simultaneously observed and imaged under a Zeiss (Axiophot) Fluorescent Microscope equipped with dual- and triple-color filters. A stained slide with the perivascular areas of interest was marked and used for assessing FISH signal patterns. The MDM2 amplified cells exhibited a very high number of red signals relative to the number of centromere control signals (CEP 12). As well as being in greater number, the amplified MDM2 signals were usually smaller and tightly clustered. Representative images of the perivascular areas were captured using a cooled charged coupled device camera.
3. Results
3.1 Perivascular smooth muscle proliferation in well-differentiated liposarcoma / atypical lipomatous tumor (WDLPS / ALT)
Seven cases of WDLPS / ALT with perivascular areas with the appearance of smooth muscle were identified in our retrospective examination of case files (Table 1). Most cases arose in either the retroperitoneum (n=3) or the lower extremity (n=3), and affected individuals were in their 5th to 7th decades of life. Tumors ranged in size from 8.5 – 41 cm in greatest dimension. Clinical follow-up was 13.7 years (range 2–19 years). Two patients suffered local recurrences (28.5% of cases). Karyotype studies obtained in one case, showed supernumerary ring chromosomes, characteristic of WDLPS / ALT. All cases (100%, 7 of 7) demonstrated amplification of MDM2 by FISH, which was confirmed by MDM2 immunohistochemistry (100%, 7 of 7).
Table 1.
Patient demographics
| Sample # |
Diagnosis | Gender (M/F) |
Age (yrs) |
Location | Size (cm) |
Recurrence | Time to recurrence (mo) |
|---|---|---|---|---|---|---|---|
| 1 | ALT | M | 65 | Thigh | 25 | No | - |
| 2 | LPS, WD | M | 64 | Retroperitoneum | 24 | No | - |
| 3 | LPS, WD | F | 54 | Retroperitoneum | 25 | Yes | 93 |
| 4 | ALT | F | 43 | Back | 8.5 | No | - |
| 5 | LPS, WD | M | 58 | Retroperitoneum | 11 | Yes | 20 |
| 6 | ALT | F | 53 | Calf | 20 | No | - |
| 7 | ALT | M | 63 | Thigh | 41 | No | - |
ALT: Atypical lipomatous tumor; LPS: Liposarcoma; WD: Well-differentiated
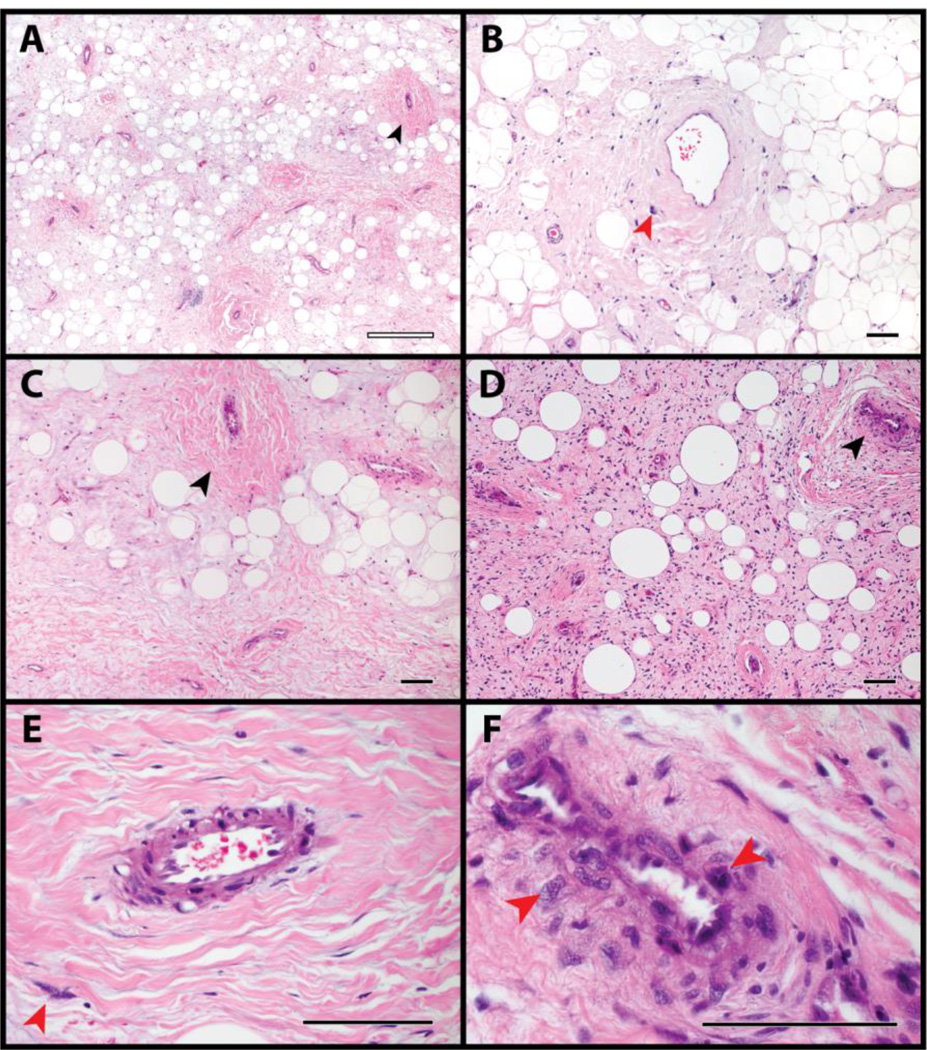
The histologic appearance of the seven cases of WDLPS / ALT was first examined (Fig. 1). Tumors showed a variable lipomatous to sclerotic background. Perivascular areas with the appearance of smooth muscle were most commonly in a multifocal distribution, often clustered around groups of adjacent blood vessels (black arrowheads, Fig. 1). Enlarged, hyperchromatic and atypical stromal cells characteristic of liposarcoma were found within these perivascular areas (red arrowheads, Fig. 1). These were seen both intimately associated with the endothelium and also at a distance from the vessel wall (compare Fig. 1F to 1E). One case showed patchy heterologous bone formation (not shown).
Fig. 1. Appearance of liposarcoma / atypical lipomatous tumor.
(A-F) Histological appearance of well-differentiated liposarcoma / atypical lipomatous tumor, by H&E staining. Areas of variably prominent perivascular smooth muscle differentiation were imaged, including atypical stromal cells in proximity to these areas. Red arrowheads indicate atypical enlarged stromal cells, while black arrowheads indicate areas of perivascular smooth muscle differentiation. Black scale bar: 100 µm. White scale bar: 500 µm.
3.2 Pericyte marker expression in liposarcoma / atypical lipomatous tumor (WDLPS / ALT)
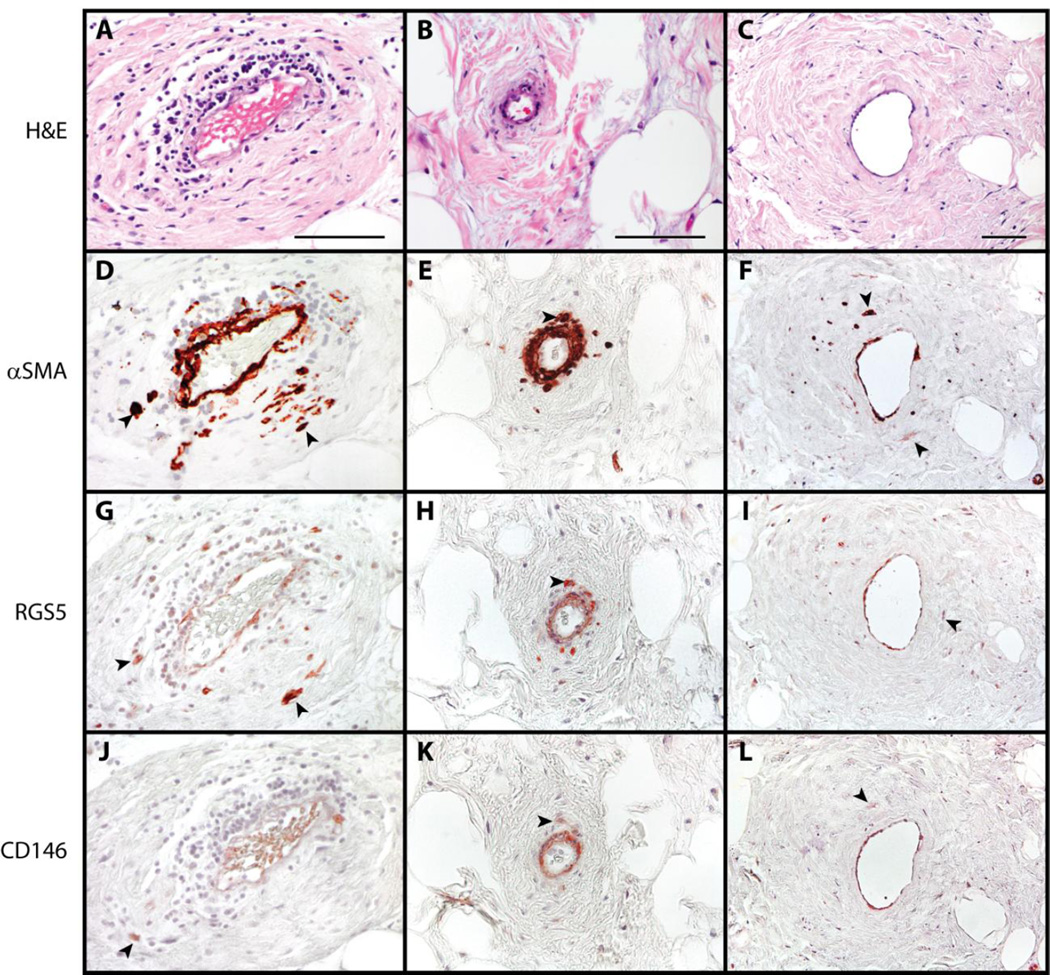
Next, pericyte marker expression was evaluated by immunohistochemical stainings. First, perivascular areas were examined (Fig. 2, three representative areas in different tumors shown). In each instance, atypical stromal cells were seen within a perivascular location. Interestingly, these atypical stromal cells were found to be frequently positive for the majority of pericyte antigens (Fig. 2D-L, black arrowheads). This included consistent immunoreactivity for αSMA, CD146, and RGS5. Of note, PDGFRβ showed minimal staining in most cases (not shown).
Fig. 2. Pericytic markers in liposarcoma / atypical lipomatous tumor.
(A-C) H&E appearance. (D-F) α Smooth Muscle Actin (αSMA) immunohistochemical staining. (G-I) RGS5 immunohistochemical staining. (J-L) CD146 immunohistochemical staining. Black arrowheads indicate atypical enlarged stromal cells with positive staining. Black scale bar: 100 µm.
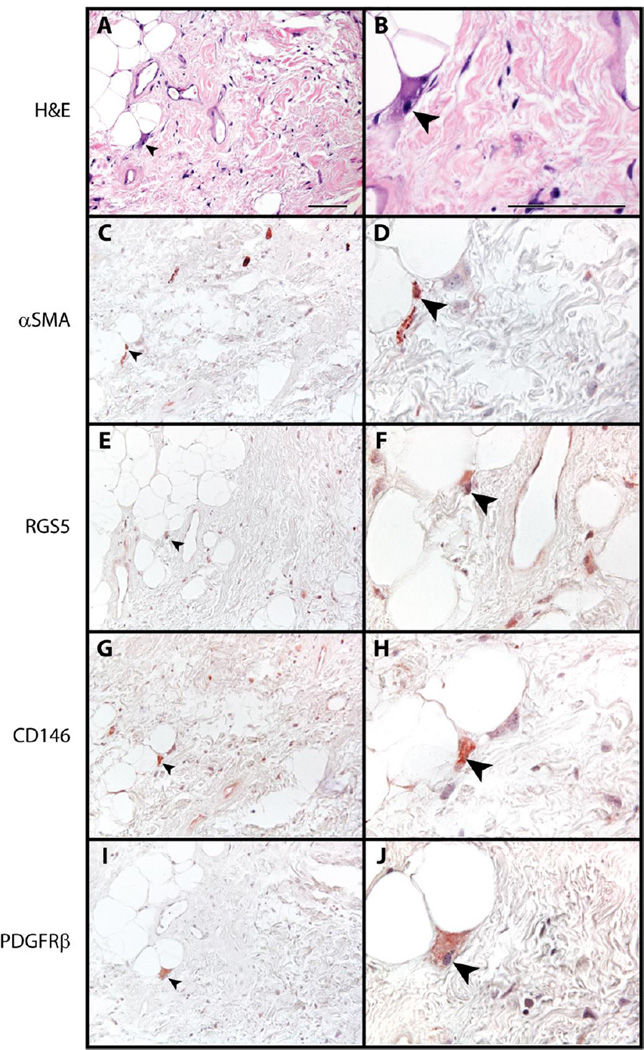
Next, areas within the tumor substance and apart from prominent vasculature were examined (Fig. 3). For the most part, pericyte antigens were not commonly seen among lipoblasts or enlarged stromal cells within WDLPS / ALT apart from the perivascular areas. However, on detailed examination, rare to scattered atypical stromal cells were seen with pan-positivity for pericyte markers (αSMA, CD146, RGS5, and PDGFRβ shown, black arrowheads).
Fig. 3. Pericytic markers in liposarcoma / atypical lipomatous tumor.
(A,B) H&E appearance. (C,D) α Smooth Muscle Actin (αSMA) immunohistochemical staining. (E,F) RGS5 immunohistochemical staining. (G,H) CD146 immunohistochemical staining. (I,J) PDGFRβ immunohistochemical staining. Black arrowheads indicate atypical enlarged stromal cells with positive staining. Black scale bar: 100 µm.
3.3 Confirmation of tumor cell origin among perivascular cells
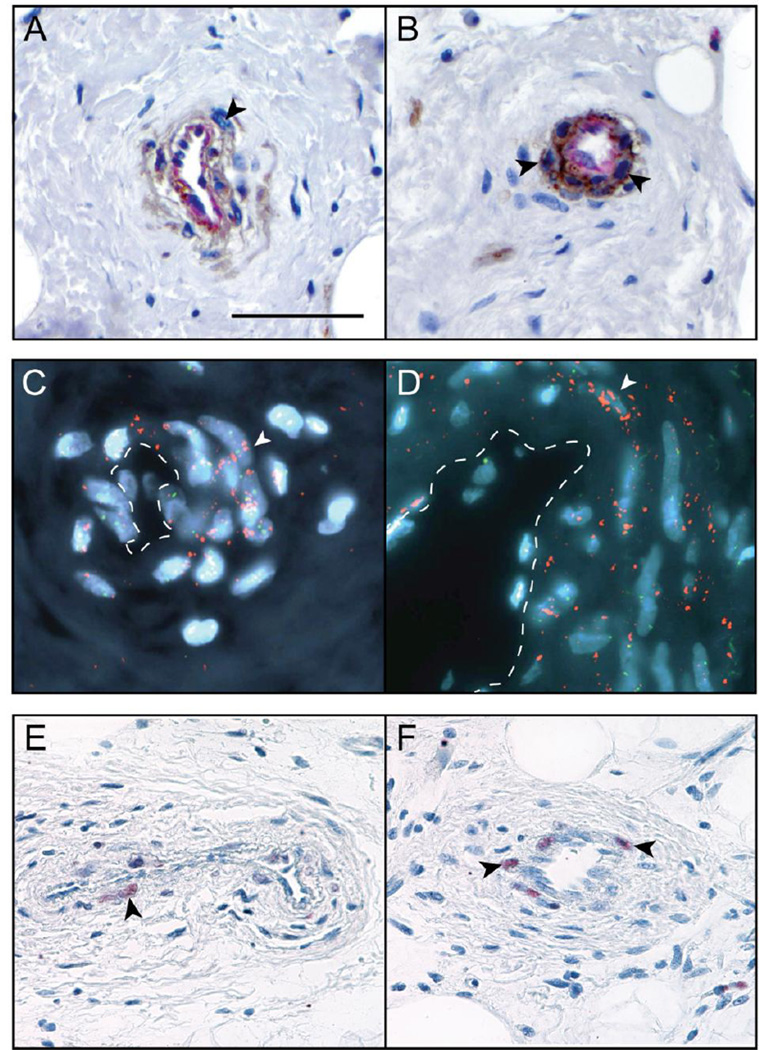
Nuclear enlargement and hyperchromasia among perivascular cells has previously led to the assumption that these were of tumor cell origin 23. In order to confirm this, markers of endothelial differentiation (CD31) and liposarcomatous origin (MDM2 gene amplification and MDM2 expression by IHC) were assayed (Fig. 4). First, dual immunohistochemistry was performed for CD31 (highlighting endothelium red) and CD146 (highlighting perivascular cells in brown) (Fig. 4A,B). As expected CD31 immunostaining was restricted to the endothelium, while CD146 highlighted both perivascular and endothelial cells. Next, MDM2 amplification within perivascular cells was assayed by fluorescence in situ hybridization (Fig. 4C,D). A marked increase in MDM2 signals (red) was observed among perivascular tumor cells (vascular lumen highlighted with dashed white lines). These findings were confirmed with MDM2 immunohistochemical staining, demonstrating atypical perivascular cells with nuclear MDM2 immunoreactivity (Fig. 4E,F). In aggregate, these studies confirm the tumor cell origin of perivascular cells with pericyte antigen expression in WDLPS / ALT.
Fig. 4. Tumor cell origin of perivascular cells in liposarcoma / atypical lipomatous tumor.
(A,B) Dual immunohistochemical staining for CD31 (endothelial marker, appearing red) and CD146 (pericyte marker, appearing brown). Please note that CD146 is known to also highlight endothelial cells. (C,D) MDM2 amplification by Fluorescence In Situ Hybridization. MDM2 appears red, CEP 12 appears green, and DAPI nuclear counter stain appears blue. White dashed lines highlight vascular lumen. (E,F) Nuclear MDM2 immunohistochemical staining. Black and white arrowheads indicate atypical enlarged stromal cells with positive staining. Black scale bar: 100 µm.
4. Discussion
In summary, pericyte antigen expression is present among WDLPS / ALT tumor cells and is found predominantly in a perivascular distribution. Scattered single atypical stromal cells unassociated with vascular smooth muscle proliferation also showed pericyte antigen expression, albeit less frequently. The presence of multiple pericyte antigens among these vasculature-associated atypical stromal cells has strong parallels to pericytic mimicry, a phenomenon best described in malignant melanoma 11–13, 27. Pericytic mimicry has also been termed extravascular migratory metastasis (EVMM) or angiotropism. In general, angiotropic tumor cells are defined histologically as tumor cells closely associated with the endothelium of vascular channels in a pericytic location, and do not show intravasation into the blood vessel. While in melanoma, pericytic mimicry is generally described at the advancing front of the tumor, in WDLPS / ALT this phenomenon was observed in a multicentric fashion in association with the vasculature.
Pericytic mimicry has been shown to be an independent poor prognostic marker in melanoma 13. Specifically, analysis of 52 patients with malignant melanoma showed a significant association between angiotropism and development of metastasis, with other prognostic features such as tumor thickness being identical. Although somewhat limited in number, our cases followed a typical disease course for WDLPS / ALT, characterized by local recurrence in 2/7 cases (28.5%). Of note, Gronchi et al. recently found that myogenic differentiation was associated with a poor prognosis in retroperitoneal liposarcoma 28. However this study examined both well-differentiated and dedifferentiated tumors, and included both markers of smooth and skeletal muscle differentiation. Thus, we consider this ’myogenic differentiation’ wholly distinct from pericytic mimicry.
In our previous reports, we examined pericyte antigen expression among soft tissue tumors with a perivascular growth pattern 29, 30. These included glomus tumor, myopericytoma, and angioleiomyoma. Results showed that among all tumors a consistent expression pattern of pericyte markers was observed in relation to their perivascular pattern of growth, including diffuse immunoreactivity for αSMA, CD146, and PDGFRβ. Later studies found that angiomyolipoma and related PEComa family tumors showed relatively consistent, although patchy, pericytic marker expression 30. These expression patterns are distinct from the predominantly perivascular distribution of pericyte antigens in WDLPS / ALT.
Unfortunately, no known pericytic markers are absolutely specific. For example, RGS5 expression has been observed in a number of malignancies, including lung 31, gastric 32, and hepatocellular carcinomas 33, as well as various lymphomas 33. Likewise, CD146 expression can be found in mucoepidermoid carcinoma, gestational trophoblastic tumors, angiosarcoma, and Kaposi’s sarcoma, among other tumor types 34. However, based on the available literature and the present study, co-expression of multiple pericyte markers (including αSMA, CD146, PDGFRβ, and RGS5) is quite specific for pericytic differentiation. Other possible pericyte markers have yet to be investigated in soft tissue tumors, including Ang-1, Ang-2 35, and Nestin 36, 37.
In summary, pericyte antigen expression is present among WDLPS / ALT tumor cells and is found predominantly in a perivascular distribution. This phenomenon parallels pericyte mimicry, found in melanoma and other malignant neoplasms. The extent to which pericytic mimicry has prognostic significance in liposarcoma is as yet unknown.
Highlights.
Pericyte mimicry has been studied in melanocytic but not mesenchymal tumors
Liposarcoma shows aberrant pericyte markers in a perivascular fashion
Pericyte mimicry is present in cells of liposarcoma rather than endothelial origin
Acknowledgments
The present work was supported by the UCLA Daljit S. and Elaine Sarkaria Fellowship award, the Orthopaedic Research and Education Foundation with funding provided by the Musculoskeletal Transplant Foundation, and NIH/NIAMS K08 AR068316. The authors thank the staff of UCLA Translational Pathology Core Laboratory, Y. Yen, and A.S. James for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest
The authors have no conflicts of interest.
References
- 1.Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21(8):1299–1308. doi: 10.1089/scd.2011.0200. PubMed PMID: 21861688; PMCID: PMC3353742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C, Peault B. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cellular and molecular life sciences : CMLS. 2014;71(8):1353–1374. doi: 10.1007/s00018-013-1462-6. Epub 2013/10/26. PubMed PMID: 24158496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CW, Montelatici E, Crisan M, Corselli M, Huard J, Lazzari L, Peault B. Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev. 2009;20(5–6):429–434. doi: 10.1016/j.cytogfr.2009.10.014. PubMed PMID: 19926515. [DOI] [PubMed] [Google Scholar]
- 4.Chen CW, Okada M, Proto JD, Gao X, Sekiya N, Beckman SA, Corselli M, Crisan M, Saparov A, Tobita K, Peault B, Huard J. Human pericytes for ischemic heart repair. Stem cells (Dayton, Ohio) 2013;31(2):305–316. doi: 10.1002/stem.1285. Epub 2012/11/21. PubMed PMID: 23165704; PMCID: 3572307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corselli M, Chen CW, Crisan M, Lazzari L, Peault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30(6):1104–1109. doi: 10.1161/ATVBAHA.109.191643. Epub 2010/05/11. PubMed PMID: 20453168. [DOI] [PubMed] [Google Scholar]
- 6.Crisan M, Chen CW, Corselli M, Andriolo G, Lazzari L, Peault B. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176:118–123. doi: 10.1111/j.1749-6632.2009.04967.x. PubMed PMID: 19796239. [DOI] [PubMed] [Google Scholar]
- 7.Crisan M, Deasy B, Gavina M, Zheng B, Huard J, Lazzari L, Peault B. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods Cell Biol. 2008;86:295–309. doi: 10.1016/S0091-679X(08)00013-7. PubMed PMID: 18442653. [DOI] [PubMed] [Google Scholar]
- 8.Crisan M, Huard J, Zheng B, Sun B, Yap S, Logar A, Giacobino JP, Casteilla L, Peault B. Purification and culture of human blood vessel-associated progenitor cells. Chapter 2:Unit 2B 1-2B 13. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc02b02s4. PubMed PMID: 18770640. http://onlinelibrary.wiley.com/doi/10.1002/9780470151808.sc02b02s4/abstract?systemMessage=Wiley+Online+Library+will+be+unavailable+on+Saturday+27th+February+from+09%3A00-14%3A00+GMT+%2F+04%3A00-09%3A00+EST+%2F+17%3A00-22%3A00+SGT+for+essential+maintenance.++Apologies+for+the+inconvenience. [DOI] [PubMed] [Google Scholar]
- 9.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. PubMed PMID: 18786417. [DOI] [PubMed] [Google Scholar]
- 10.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science (New York, NY. 2008;322(5901):583–586. doi: 10.1126/science.1156232. Epub 2008/09/20. PubMed PMID: 18801968; PMCID: 2597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lugassy C, Peault B, Wadehra M, Kleinman HK, Barnhill RL. Could pericytic mimicry represent another type of melanoma cell plasticity with embryonic properties? Pigment Cell Melanoma Res. 2013;26(5):746–754. doi: 10.1111/pcmr.12120. Epub 2013/06/25. PubMed PMID: 23789776. [DOI] [PubMed] [Google Scholar]
- 12.Lugassy C, Wadehra M, Li X, Corselli M, Akhavan D, Binder SW, Peault B, Cochran AJ, Mischel PS, Kleinman HK, Barnhill RL. Pilot study on "pericytic mimicry" and potential embryonic/stem cell properties of angiotropic melanoma cells interacting with the abluminal vascular surface. Cancer Microenviron. 2013;6(1):19–29. doi: 10.1007/s12307-012-0128-5. Epub 2013/01/01. PubMed PMID: 23275074; PMCID: 3601217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnhill RL, Lugassy C. Angiotropic malignant melanoma and extravascular migratory metastasis: description of 36 cases with emphasis on a new mechanism of tumour spread. Pathology. 2004;36(5):485–490. doi: 10.1080/00313020412331282708. Epub 2004/09/17. PubMed PMID: 15370120. [DOI] [PubMed] [Google Scholar]
- 14.Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, Min W, McLendon RE, Rich JN, Bao S. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153(1):139–152. doi: 10.1016/j.cell.2013.02.021. Epub 2013/04/02. PubMed PMID: 23540695; PMCID: 3638263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu AY, Ouyang G. Tumor angiogenesis: a new source of pericytes. Curr Biol. 2013;23(13):R565–R568. doi: 10.1016/j.cub.2013.05.023. Epub 2013/07/13. PubMed PMID: 23845244. [DOI] [PubMed] [Google Scholar]
- 16.Levy MJ, Gleeson FC, Zhang L. Endoscopic ultrasound fine-needle aspiration detection of extravascular migratory metastasis from a remotely located pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7(2):246–248. doi: 10.1016/j.cgh.2008.09.010. Epub 2009/01/13. PubMed PMID: 19135552. [DOI] [PubMed] [Google Scholar]
- 17.Lugassy C, Vernon SE, Warner JW, Le CQ, Manyak M, Patierno SR, Barnhill RL. Angiotropism of human prostate cancer cells: implications for extravascular migratory metastasis. BJU Int. 2005;95(7):1099–1103. doi: 10.1111/j.1464-410X.2005.05474.x. Epub 2005/04/21. PubMed PMID: 15839940. [DOI] [PubMed] [Google Scholar]
- 18.James AW, Zara JN, Corselli M, Askarinam A, Zhou AM, Hourfar A, Nguyen A, Megerdichian S, Asatrian G, Pang S, Stoker D, Zhang X, Wu B, Ting K, Peault B, Soo C. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med. 2012;1(9):673–684. doi: 10.5966/sctm.2012-0053. Epub 2012/12/01. PubMed PMID: 23197874; PMCID: 3659737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James AW, Zara JN, Corselli M, Chiang M, Yuan W, Nguyen V, Askarinam A, Goyal R, Siu RK, Scott V, Lee M, Ting K, Peault B, Soo C. Use of human perivascular stem cells for bone regeneration. J Vis Exp. 2012;(63):e2952. doi: 10.3791/2952. Epub 2012/06/06. PubMed PMID: 22664543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James AW, Zara J, Zhang X, Askarinam A, Goyal R, Chiang M, Yuan W, Chang L, Corselli M, Shen J, Pang S, Stoker D, Ting K, Peault B, Soo C. Perivascular Stem Cells: A Prospectively Purified Mesenchymal Stem Cell Population for Bone Tissue Engineering. Stem Cell Translational Medicine. 2012;1(6):510–519. doi: 10.5966/sctm.2012-0002. PubMed PMID: 23197855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Askarinam A, James AW, Zara JN, Goyal R, Corselli M, Pan A, Liang P, Chang L, Rackohn T, Stoker D, Zhang X, Ting K, Peault B, Soo C. Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein. Tissue Eng Part A. 2013;19(11–12):1386–1397. doi: 10.1089/ten.tea.2012.0367. Epub 2013/02/15. PubMed PMID: 23406369; PMCID: 3638559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Peault B, Chen W, Li W, Corselli M, James AW, Lee M, Siu RK, Shen P, Zheng Z, Shen J, Kwak J, Zara JN, Chen F, Zhang H, Yin Z, Wu B, Ting K, Soo C. The Nell-1 growth factor stimulates bone formation by purified human perivascular cells. Tissue Eng Part A. 2011;17(19–20):2497–2509. doi: 10.1089/ten.tea.2010.0705. Epub 2011/05/28. PubMed PMID: 21615216; PMCID: 3179623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folpe AL, Weiss SW. Lipoleiomyosarcoma (well-differentiated liposarcoma with leiomyosarcomatous differentiation): a clinicopathologic study of nine cases including one with dedifferentiation. Am J Surg Pathol. 2002;26(6):742–749. doi: 10.1097/00000478-200206000-00007. PubMed PMID: 12023578. [DOI] [PubMed] [Google Scholar]
- 24.Nahal A, Meterissian S. Lipoleiomyosarcoma of the rectosigmoid colon: a unique site for a rare variant of liposarcoma. Am J Clin Oncol. 2009;32(4):353–355. doi: 10.1097/COC.0b013e31818c0926. PubMed PMID: 19363435. [DOI] [PubMed] [Google Scholar]
- 25.Boland JM, Colby TV, Folpe AL. Liposarcomas of the mediastinum and thorax: a clinicopathologic and molecular cytogenetic study of 24 cases, emphasizing unusual and diverse histologic features. Am J Surg Pathol. 2012;36(9):1395–1403. doi: 10.1097/PAS.0b013e3182562bc1. PubMed PMID: 22895273. [DOI] [PubMed] [Google Scholar]
- 26.Ortega P, Suster D, Falconieri G, Zambrano E, Moran CA, Morrison C, Suster S. Liposarcomas of the posterior mediastinum: clinicopathologic study of 18 cases. Mod Pathol. 2015;28(5):721–731. doi: 10.1038/modpathol.2014.152. PubMed PMID: 25475695. [DOI] [PubMed] [Google Scholar]
- 27.Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Hömig-Hölzel C, Reuten R, Schadow B, Weighardt H, Wenzel D, Helfrich I, Schadendorf D, Bloch W, Bianchi ME, Lugassy C, Barnhill RL, Koch M, Fleischmann BK, Förster I, Kastenmüller W, Kolanus W, Hölzel M, Gaffal E, Tüting T. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507(7490):109–113. doi: 10.1038/nature13111. PubMed PMID: 24572365. [DOI] [PubMed] [Google Scholar]
- 28.Gronchi A, Collini P, Miceli R, Valeri B, Renne SL, Dagrada G, Fiore M, Sanfilippo R, Barisella M, Colombo C, Morosi C, Stacchiotti S, Casali PG, Dei Tos AP, Pilotti S. Myogenic differentiation and histologic grading are major prognostic determinants in retroperitoneal liposarcoma. Am J Surg Pathol. 2015;39(3):383–393. doi: 10.1097/PAS.0000000000000366. PubMed PMID: 25581729. [DOI] [PubMed] [Google Scholar]
- 29.Shen J, Shrestha S, Yen Y, Asatrian G, Mravic M, Soo C, Ting K, Dry S, Peault B, James A. Pericyte antigens in perivascular soft tissue tumors. Int J Surg Path. 2015;23(8):638–648. doi: 10.1177/1066896915591272. PubMed PMID: 26085647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen J, Shrestha S, Yen YH, Scott MA, Asatrian G, Barnhill R, Lugassy C, Soo C, Ting K, Peault B, Dry SM, James AW. Pericyte antigens in angiomyolipoma and PEComa family tumors. Med Oncol. 2015;32(8):659. doi: 10.1007/s12032-015-0659-y. PubMed PMID: 26123600. [DOI] [PubMed] [Google Scholar]
- 31.Huang G, Song H, Wang R, Han X, Chen L. The relationship between RGS5 expression and cancer differentiation and metastasis in non-small cell lung cancer. J Surg Oncol. 2012;105(4):420–424. doi: 10.1002/jso.22033. PubMed PMID: 21780128. [DOI] [PubMed] [Google Scholar]
- 32.Wang JH, Huang WS, Hu CR, Guan XX, Zhou HB, Chen LB. Relationship between RGS5 expression and differentiation and angiogenesis of gastric carcinoma. World J Gastroenterol. 2010;16(44):5642–5646. doi: 10.3748/wjg.v16.i44.5642. PubMed PMID: 21105200; PMCID: PMC2992685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu M, Chen X, Zhang J, Wang D, Fang X, Wang X, Wang G, Chen G, Jiang X, Xia H, Wang Y. Over-expression of regulator of G protein signaling 5 promotes tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma cells. J Surg Oncol. 2013;108(3):192–196. doi: 10.1002/jso.23367. PubMed PMID: 23868206. [DOI] [PubMed] [Google Scholar]
- 34.Shih IM, Nesbit M, Herlyn M, Kurman RJ. A new Mel-CAM (CD146)-specific monoclonal antibody, MN-4, on paraffin-embedded tissue. Mod Pathol. 1998;11(11):1098–1106. Epub 1998/11/27. PubMed PMID: 9831208. [PubMed] [Google Scholar]
- 35.Wakui S, Yokoo K, Muto T, Suzuki Y, Takahashi H, Furusato M, Hano H, Endou H, Kanai Y. Localization of Ang-1-2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Laboratory investigation; a journal of technical methods and pathology. 2006;86(11):1172–1184. doi: 10.1038/labinvest.3700476. Epub 2006/09/14. PubMed PMID: 16969369. [DOI] [PubMed] [Google Scholar]
- 36.Alliot F, Rutin J, Leenen PJ, Pessac B. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. Journal of neuroscience research. 1999;58(3):367–378. Epub 1999/10/13. PubMed PMID: 10518110. [PubMed] [Google Scholar]
- 37.Klein D, Meissner N, Kleff V, Jastrow H, Yamaguchi M, Ergun S, Jendrossek V. Nestin(+) tissue-resident multipotent stem cells contribute to tumor progression by differentiating into pericytes and smooth muscle cells resulting in blood vessel remodeling. Front Oncol. 2014;4:169. doi: 10.3389/fonc.2014.00169. Epub 2014/07/16. PubMed PMID: 25019063; PMCID: 4072089. [DOI] [PMC free article] [PubMed] [Google Scholar]