Abstract
Introduction
Prior enterobiliary manipulation confers a high risk for liver abscess formation after hepatic ablation. We aimed to determine if prophylactic antibiotics could prevent post-ablation abscess in patients with a history of hepaticojejunostomy.
Materials and Methods
This single-institution retrospective study identified 262 patients who underwent 307 percutaneous liver ablation sessions between January 2010 and August 2014. Twelve (4.6%) patients with prior hepaticojejunostomy were included in this analysis. Ten (83>%) had received an aggressive prophylactic antibiotic regimen consisting of levofloxacin, metronidazole, neomycin, and erythromycin base. Two (16.6%) had received other antibiotic regimens. Clinical, laboratory, and imaging findings were used to identify abscess formation and antibiotic-related side effects.
Results
Twelve ablation sessions were performed during the period studied. During a mean follow-up period of 440 days (range, 77–1784 days), post-ablation abscesses had developed in 2 (16.6 %) patients, who both received the alternative antibiotic regimens. None of the 10 patients who received the aggressive prophylactic antibiotic regimen developed liver abscess. One of the 10 patients who received the aggressive prophylactic antibiotic regimen developed grade 2 antibiotic-related diarrhea and arthralgia.
Conclusion
An aggressive regimen of prophylactic antibiotics may be effective in preventing liver abscess formation after liver ablation in patients with prior hepaticojejunostomy.
Keywords: Abscess, Hepaticojejunostomy, Liver ablation, Antibiotics
Introduction
Liver ablation is an effective, minimally invasive procedure for treating small primary and metastatic tumors.1–3 Although ablation is generally considered safe, various complications have been reported, with rates of minor complications ranging from 5.0 to 8.9 % and major complications ranging from 2.2 to 5.7 %.4–6 Among the major complications, liver abscess formation is relatively rare, with reported incidences ranging from 0.3 to 2.0 %.5–8 However, in patients with sphincter of Oddi violation from previous bilioenteric anastomosis, sphincterectomy, biliary drainage or stent placement, the incidence of liver abscess following liver ablation is as high as 22 to 100 %.5,8,9 Therefore, a history of enterobiliary manipulation (EBM) is often considered a strong risk factor for development of liver abscess and, potentially, a contraindication for liver ablation.4,7,9,10
To date, no studies have reported that the use of a prophylactic antibiotic regimen in patients with a history of EBM undergoing liver ablation is of any benefit and investigations on that matter remain desired.5,7,8 Studies on the use of prophylactic antibiotics in patients with prior EBM undergoing transarterial hepatic embolotherapies have shown conflicting findings.11–13 Patel et al. 13 demonstrated a trend toward a lower rate of liver abscess formation in a study of 7 patients with a history of EBM who underwent transarterial chemoembolization (TACE) and received an aggressive prophylactic antibiotic regimen. An effective prophylactic antibiotic regimen for this high-risk patient populations could potentially reduce morbidity rates and ultimately expand the indication for hepatic ablation in patients with EBM.
The purpose of this single-institution retrospective study was to determine the utility of an aggressive prophylactic antibiotic regimen in preventing the development of liver abscesses following percutaneous liver ablation in patients with a history of hepaticojejunostomy.
Materials and Methods
Patient Population
This study complied with the requirements of the Health Insurance Portability and Accountability Act and was approved by our Institutional Review Board with a waiver of informed consent. We identified in our retrospective ablation databank 262 patients who underwent 307 percutaneous liver ablation sessions from January 2010 to May 2015. Among these, 12 (4.6 %) patients (6 female, 6 male; mean age 63.1 years [range, 37–78 years]) had a prior history of EBM, specifically hepaticojejunostomy as part of pancreaticoduodenectomy. We collected information on the following variables: age, sex, type of liver tumor(s), number of tumors, size of tumor(s), time interval between EBM and liver ablation procedures, ablation modality, and the prophylactic antibiotic regimen that had been administered.
Prophylactic Antibiotic Regimen
There is no consensus on the appropriate prophylactic measures to prevent liver abscess formation in patients with a history of EBM who are undergoing liver ablation. In the time period studied, our institution used three different regimens (Table 1). Ten patients utilized an aggressive regimen consisting of oral levofloxacin 500 mg daily and oral metronidazole 500 mg twice daily beginning 2 days prior to and continuing for 14 days after ablation, in addition to neomycin 1 g and erythromycin base 1 g orally at 1, 2, and 11 pm on the day before the ablation. This regimen was developed based on a previous report regarding its use on patients undergoing TACE.13 One patient received intravenous metronidazole 500 mg twice daily plus intravenous piperacillin/tazobactam 4.5 g four times a day on the day of the procedure followed by oral ciprofloxacin 500 mg twice daily and oral metronidazole 500 mg twice daily for 7 days following the procedure. Finally, one additional patient received metronidazole 500 mg orally twice daily for 10 days starting on the procedure's day.
Table 1. Clinical findings of 12 patients with a history of biliary manipulation prior to thermal ablation of liver lesion(s).
| Patient no. | Age (years) | Sex (M/F) | Primary tumor | Type of surgery causing biliary violation | Biliary manipulation to ablation (years) | Size of tumors (cm) | Thermal ablation modality | Previous TACE? | Antibiotic prophylaxis | Post-ablation abscess (yes/no) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | F | GIST | PD | 10.0 | 0.8 | MWA | No | APARa | No |
| 2 | 64 | F | PDAC | PD | 1.0 | 2 | RFA | No | APARa | No |
| 3 | 69 | M | Pancreatic NET | PD | 3.6 | 1.8 | MWA | No | APARa | No |
| 4 | 71 | F | LMS | PD | 1.7 | 2.5 | MWA | No | APARa | No |
| 5 | 42 | F | Ampullary carcinoma | PD | 1.5 | 1.8 | MWA | No | APARa | No |
| 6 | 54 | M | Pancreatic NET | PD | 0.8 | 1.2 | MWA | No | APARa | No |
| 7 | 72 | M | Pancreatic NET | PD | 16.5 | 1.7 | RFA | No | APARa | No |
| 8 | 65 | F | Pancreatic NET | PD | 2.0 | 1.0 | MWA | No | APARab | No |
| 9 | 65 | M | Ampullary carcinoma | PD | 2.9 | 1.4 | Cryo | No | APARa | No |
| 10 | 37 | M | Small bowel carcinoma | PD | 0.5 | 1.1 | MWA | No | APARa | No |
| 11 | 64 | F | Pancreatic NET | PD | 3.5 | (a) 1.8 (b)3.5 | (a) RFA (b)RFA | Yes | Other regimenc | Yes |
| 12 | 76 | M | PDAC | PD | 2.1 | 2.5 | MWA | No | Other regimend | Yes |
TACE transarterial chemoembolization, GIST gastrointestinal stromal tumor, PD pancreaticoduodenectomy, MWA microwave ablation, APAR aggressive prophylactic antibiotic regimen, PDAC pancreatic ductal adenocarcinoma, RFA radiofrequency ablation, NET neuroendocrine tumor, LMS leiomyosarcoma, Cryo cryoablation
APAR: Levofloxacin 500 mg orally daily and metronidazole 500 mg orally every 12 h beginning 2 days before the procedure and continuing for 14 days after the procedure; neomycin 1 g orally and erythromycin base 1 g orally at 1, 2, and 11 pm the day before the ablation
Patient was unable to complete antibiotic regimen because of antibiotic-related adverse events
Piperacillin/tazobactam 4.5 g intravenously four times a day plus metronidazole 500 mg intravenously twice daily within 1 h of procedure on the day of the procedure followed by ciprofloxacin and metronidazole 500 mg orally each twice a day for 7 days
Metronidazole 500 mg orally twice a day within 1 h of the procedure for 10 days
Percutaneous Ablation Procedure
All percutaneous liver ablations were performed with computed tomography (CT) guidance while the patients were under general anesthesia. Ultrasonography was also used at the operator's discretion. Radiofrequency ablation (Cool-tip ablation system, Covidien, Boulder, CO, USA), microwave ablation (CertusPR probe, Certus 140–2.4 GHz ablation system, Neuwave, Madison, WI, USA), or cryoablation (Galil Medical Inc., Arden Hills, MN, USA) were the ablation modalities utilized. At the end of the procedure, a triphasic contrast-enhanced CT study was performed to verify treatment completion and assess patients for potential complications. Patients were admitted for overnight observation and discharged home the next day.
Adverse Events Assessment
Patients were evaluated on the day after ablation, prior to discharge from the hospital. Per our institutional protocol, cross-sectional images of the liver were obtained using multi-detector CT or magnetic resonance imaging with a quadriphasic study protocol within 90 days after each ablation session, every 2 to 3 months after that for the first year after ablation, and every 6 months thereafter. All the 12 patients had post-ablation cross-sectional images available for review. All available images were retrospectively reviewed by two interventional radiologists (BO and SH) to identify findings suggestive of liver abscess. Liver abscess was defined as a discrete intrahepatic collection on cross-sectional imaging with aspiration yielding a microorganism, along with fever, chills, fatigue, and/or pain. All adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03.
Statistical Analysis
Continuous data were expressed as median (range) and compared using the Mann-Whitney U test. Categorical data were compared using the chi-square test. Statistical analyses were performed with GraphPad PRISM version 6.0 h (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Twelve patients with a history of hepaticojejunostomy underwent 12 percutaneous liver ablation procedures, with a total of 13 tumors treated. Table 1 contains complete patient details. The median time interval between the hepaticojejunostomy procedure (i.e., pancreaticoduodenectomy) and the ablation was 3.8 years (range, 0.5–16.5 years). The median size of the treated tumors was 1.75 cm (range, 0.8–3.5 cm). Radiofrequency ablation was performed for 4 tumors, microwave ablation for 8 tumors, and cryoablation for 1 tumor. One patient had one TACE session 24 h preceding the ablation procedure.
During a median follow-up period of 440 days (range, 77–1784 days), liver abscesses developed in 2 (16.6%) of 12 patients. Among the 10 patients who received the aggressive prophylactic antibiotic regimen of levofloxacin, metronidazole, neomycin, and erythromycin base, none developed abscesses. In contrast, the 2 patients who received other prophylactic antibiotic regimens developed low-grade fevers 14 and 21 days after the ablation procedures, respectively. CT showed a large intrahepatic abscess in each patient at 34 and 43 days post-ablation, respectively (Fig. 1). Both patients with abscesses were treated with catheter-directed aspiration and drainage. Intravenous antibiotics were administered based on bacterial cultures and antibiotic-sensitivity test results, which revealed the presence of Enterococcus faecalis sensitive to levofloxacin in 1 patient and multidrug-resistant Escherichia coli and Enterococcus faecium in the other patient. The abscesses subsequently resolved in both patients.
Fig. 1.
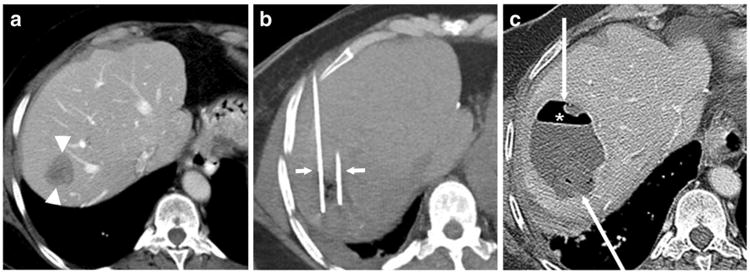
A 58-year-old woman with a history of pancreaticoduodenectomy for pancreatic neuroendocrine cancer presented with a liver metastasis. a Axial contrast-enhanced computed tomography (CT) scan demonstrates the metastasis in segment VII of the liver (arrowheads). b Ablation was performed with two microwave ablation antennas (short arrows) bracketing the lesion. c The patient presented 43 days after ablation with a low-grade temperature and malaise. Axial contrast-enhanced CT scan demonstrates a rim-enhancing mass (long arrows) containing an air-fluid level. Clinical and imaging findings were consistent with an abscess, which was treated successfully with catheter drainage and intravenous antibiotics
One patient who received the aggressive prophylactic antibiotic regimen developed arthralgia and diarrhea (CTCAE grade 2) 10 days after the hepatic ablation. The treating physicians attributed these symptoms to antibiotic-related side effects and instructed the patient to stop the antibiotics with 2 days remaining in the prescribed course. All other patients tolerated the antibiotic regimens.
Discussion
Liver abscesses are a common and significant complication following liver ablation in patients with prior EBM, including surgery, biliary drainage or stenting, and sphincterotomy. These interventions are strongly associated with retrograde chronic colonic-type-flora bacterial colonization in as many as 90 % of patients.14 Bacterial colonization of the hepaticojejunostomy has been observed as early as the first week after the surgical procedure.15–17 Although the vast majority of patients with bacterial colonization are asymptomatic, the communication of the colonized bile ducts with the ablation zone can promote bacterial colonization and growth at the thermally damaged tissue, a known favorable environment for bacterial dissemination.18
The existing literature shows conflicting results between abscess formation with the type of prior EBM, ablation modality, and type of prophylaxis. Table 2 reviews the incidence of abscess formation, prophylactic intervention(s), and time interval between ablation and abscess formation from previously published studies and our current case series. Elias et al.10 reviewed a cohort of 574 patients who were treated with thermal ablation of liver lesion(s) over an 8-year period. They found that among 9 patients who had a history of bilioenteric anastomosis prior to ablation, 4 (44 %) patients developed an abscess at the site of the ablation; the abscesses occurred despite the administration of various prophylactic antibiotic regimens. Shibata et al.7 evaluated the incidence and risk factors for the development of cholangitis and liver abscess in 358 patients who underwent 638 ablation procedures. Cholangitis and/or liver abscess occurred following 10 (1.6 %) ablation sessions. Bilioenteric anastomosis was the only significant risk factor strongly predisposing patients to cholangitis and/or liver abscess formation (P<0.001). While patients in that study had been administered prophylactic antibiotics, the study showed that antibiotic prophylaxis did not significantly protect patients against the development of liver abscesses (P = 0.31). In a series of 603 patients with hepatocellular carcinoma who underwent 751 thermal ablation sessions, Choi et al.8 described an abscess formation incidence of 1.7 % (13 sessions). Significant risk factors for abscess development included prior biliary manipulation (P = 0.0088), a tumor with retained iodized oil from prior transarterial chemoembolization (P = 0.040), and treatment with an internally cooled electrode system (P = 0.016).8 Of note, none of the patients with prior biliary manipulation received prophylactic antibiotics in that study. In our present series, 2 of 12 (16.7 %) patients who had a history of hepaticojejunostomy developed an abscess at the ablation site. Importantly, however, none of the 10 patients who had received the aggressive antibiotic regimen was among those who developed an abscess.
Table 2. Published retrospective studies evaluating liver abscess formation following liver ablation in the setting of prior biliary manipulation.
| First author and year of study | No. of patients with biliary manipulation and liver ablation/PEI | No. of patients who developed abscess (%) | Antibiotic prophylaxis | Time interval between ablation and abscess formation, range | Microorganisms in abscess |
|---|---|---|---|---|---|
| de Baere T 20035a | 3 | 3 (100) | Amoxicillin and clavulanate × 5 days | 13–60 days | Enterococcus faecalis, Escherichia coli, Clostridium species |
| Shibata T 20037 | 23 | 5 (21.7) | Cefazolin or cefmetazole 1 day before ablation and every 12 h until discharge | n/a | Enterococcus species, Enterobacter cloacae, Escherichia coli |
| Choi D 20058 | 9 | 2 (22.2) | None | 3–30 days | Clostridium perfringes, Staphylococcus aureus, Aeromonas hydrophila, Klebsiella pneumoniae, Enterococcus species (blood culture), gram-negative bacilli (blood culture) |
| Lida H 20149 | 8 | 6 (75) | Cefazolin × 1–2 days after ablation | 8–14 days | Enterococcus faecalis, Enterobacter cloacae, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter lwoffii, Streptococcus anginosus |
| Elias D 200610 | 9 | 4 (44.4) | Variable | 13–62 days | Enterococcus species, Enterococcus faecalis, Escherichia coli, Proteus vulgaris, Bacteroides fragilis |
| Current study | 10 | None (0) | APARb | n/a | n/a |
| Current study | 2 | 2 (100) | Other regimenscd | 34 and 43 days | Enterococcus faecalis, Enterococcus faecium, Escherichia coli |
PEI percutaneous ethanol injection, APAR aggressive prophylactic antibiotic regimen
Authors reported liver abscess formation after liver ablation in all patients (i.e., patients with prior enterobiliary manipulation were not reported separately)
APAR: Levofloxacin 500 mg orally every day and metronidazole 500 mg orally every 12 h beginning 2 days prior to the procedure and continuing for 14 days after the procedure, in addition to neomycin 1 g orally and erythromycin base 1 g orally at 1, 2, and 11 pm the day before the ablation
Piperacillin/tazobactam 4.5 g intravenously four times a day plus metronidazole 500 mg intravenously twice daily within 1 h of procedure on the day of the procedure followed by ciprofloxacin and metronidazole 500 mg orally each twice a day for 7 days
Metronidazole 500 mg orally twice a day within 1 h of the procedure for 10 days
The time between EBM and ablation may be associated with the likelihood of post-ablation bacterial infection. It is interesting to note that of the 13 liver ablation procedures in patients with biliary tract manipulation described by Elias et al.,10 no abscesses occurred when the EBM and radiofrequency ablation were performed synchronously (n = 4 procedures), whereas intrahepatic abscess occurred in 4 of 9 procedures when the ablation was performed after the EBM procedure. Given that the median time interval between biliary manipulation and ablation in our series was 3.8 years, our patients were likely at an increased risk for abscess formation given their potential higher chance to present with retrograde chronic colonic-type-flora bacterial colonization of the biliary system.
The organisms isolated from liver abscesses following ablation in patients who have undergone biliary manipulation are diverse, including gram-positive, gram-negative, and obligate anaerobic species of bacteria (Table 2). The most common organisms isolated were Enterococcus species and E. coli. The aggressive antibiotic regimen used in 10 of our patients, who had no incidence of abscess, consisted of levofloxacin, metronidazole, neomycin, and erythromycin base. Levofloxacin and metronidazole, which were started 2 days before the ablation and continued for 14 days following the ablation, are particularly effective against Enterococcus spp. and E. coli. While we included neomycin and erythromycin base in our aggressive antibiotic regimen based on evidence from a previous study,13 whether these antibiotics were actually useful in our series of patients remains uncertain. Neomycin and erythromycin base are known to prevent infectious complications following elective colonic surgery, with elevated drug levels found in the serum and colonic lumen.19–21 In our clinical setting, however, it remains unclear if these drugs were present within the proximal small bowel lumen and what effect, if any, they may have had on reducing intestinal flora.
The empiric use of antibiotics for prevention of infectious complications is not inconsequential. The increased associated costs of antibiotic therapy, its potential contribution to bacterial resistance, and its side effects are well-recognized limitations. The latter issue was illustrated in our study by the inability of one of our patients to complete the planned aggressive antibiotic regimen due to the development of CTCAE grade 2 antibiotic-related side effects. Because the patient was near the end of her prophylaxis period, no alternative antibiotics were administered. The early termination of antibiotic prophylaxis without subsequent development of an abscess suggests that systemic antibiotics may provide enduring effects, a possibility that needs further study.
Our study is limited by its retrospective design and small sample size. The small number of patients included in our study (n = 12 patients), the small number of patients who developed abscesses (n = 2 patients), and the lack of a control arm make it difficult to draw clinically significant conclusions regarding the role of pre-procedural antibiotics. Also, the history of TACE in one of our patients might have increased the risk of development of liver abscess since the compromised blood supply after TACE can contribute to abscess formation. Nonetheless, the lack of abscess formation observed in the subset of our study population who received the aggressive prophylactic antibiotic regimen is a favorable result as compared to other studies with similar numbers of patients.7,8,10 Finally, the absence of data on the presence and type of bacterial colonization of the patients' biliary systems limits our ability to conclusively determine the utility and efficacy of the specific types of antibiotics used.
In conclusion, the present study suggests that an aggressive prophylactic antibiotic regimen is well tolerated and may prevent the formation of liver abscesses following liver ablation in patients with prior hepaticojejunostomy. Our results will need to be validated with larger studies.
Acknowledgments
This research was supported, in part, by the National Institutes of Health through MD Anderson Cancer Center's Support Grant, CA016672. We would like to thank you Amy Ninetto by the scientific edition of the manuscript.
Footnotes
Compliance with Ethical Standards: Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Annals of surgery. 2006;243(3):321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234(3):961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 3.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–968. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 4.Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovascular and interventional radiology. 2010;33(1):11–17. doi: 10.1007/s00270-009-9736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Baere T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, Gamal El Din M, Letoublon C, Elias D. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR American journal of roentgenology. 2003;181(3):695–700. doi: 10.2214/ajr.181.3.1810695. [DOI] [PubMed] [Google Scholar]
- 6.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 7.Shibata T, Yamamoto Y, Yamamoto N, Maetani Y, Shibata T, Ikai I, Terajima H, Hatano E, Kubo T, Itoh K, Hiraoka M. Cholangitis and liver abscess after percutaneous ablation therapy for liver tumors: incidence and risk factors. Journal of vascular and interventional radiology : JVIR. 2003;14(12):1535–1542. doi: 10.1097/01.rvi.0000099532.29957.4f. [DOI] [PubMed] [Google Scholar]
- 8.Choi D, Lim HK, Kim MJ, Kim SJ, Kim SH, Lee WJ, Lim JH, Paik SW, Yoo BC, Choi MS, Kim S. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR American journal of roentgenology. 2005;184(6):1860–1867. doi: 10.2214/ajr.184.6.01841860. [DOI] [PubMed] [Google Scholar]
- 9.Iida H, Aihara T, Ikuta S, Yamanaka N. Risk of abscess formation after liver tumor radiofrequency ablation: a review of 8 cases wtih a history of enterobiliary anastomosis. Hepato-gastroenterology. 2014;61(135):1867–1870. [PubMed] [Google Scholar]
- 10.Elias D, Di Pietroantonio D, Gachot B, Menegon P, Hakime A, De Baere T. Liver abscess after radiofrequency ablation of tumors in patients with a biliary tract procedure. Gastroenterol Clin Biol. 2006;30(6–7):823–827. doi: 10.1016/s0399-8320(06)73327-9. [DOI] [PubMed] [Google Scholar]
- 11.Kim W, Clark TW, Baum RA, Soulen MC. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12(8):965–968. doi: 10.1016/s1051-0443(07)61577-2. [DOI] [PubMed] [Google Scholar]
- 12.Geschwind JF, Kaushik S, Ramsey DE, Choti MA, Fishman EK, Kobeiter H. Influence of a new prophylactic antibiotic therapy on the incidence of liver abscesses after chemoembolization treatment of liver tumors. Journal of vascular and interventional radiology : JVIR. 2002;13(11):1163–1166. doi: 10.1016/s1051-0443(07)61959-9. [DOI] [PubMed] [Google Scholar]
- 13.Patel S, Tuite CM, Mondschein JI, Soulen MC. Effectiveness of an aggressive antibiotic regimen for chemoembolization in patients with previous biliary intervention. Journal of vascular and interven-tional radiology : JVIR. 2006;17(12):1931–1934. doi: 10.1097/01.RVI.0000244854.79604.C1. [DOI] [PubMed] [Google Scholar]
- 14.Spies JB, Rosen RJ, Lebowitz AS. Antibiotic prophylaxis in vascular and interventional radiology: a rational approach. Radiology. 1988;166(2):381–387. doi: 10.1148/radiology.166.2.3275979. [DOI] [PubMed] [Google Scholar]
- 15.Chuang JH, Chen WJ, Lee SY, Chang NK. Prompt colonization of the hepaticojejunostomy and translocation of bacteria to liver after bile duct reconstruction. Journal of pediatric surgery. 1998;33(8):1215–1218. doi: 10.1016/s0022-3468(98)90153-1. [DOI] [PubMed] [Google Scholar]
- 16.Hitch DC, Lilly JR. Identification, quantification, and significance of bacterial growth within the biliary tract after Kasai's operation. Journal of pediatric surgery. 1978;13(6D):563–569. doi: 10.1016/s0022-3468(78)80094-3. [DOI] [PubMed] [Google Scholar]
- 17.Deguchi E, Yanagihara J, Shinjo H, Iwai N. Periodic bile cultures and irrigation of the external jejunostomy for cholangitis in biliary atresia. Pediatric surgery international. 1996;11(4):234–236. doi: 10.1007/BF00178425. [DOI] [PubMed] [Google Scholar]
- 18.Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, Lee WJ, Lim HK, Nam GJ, Han SS, Kim YH, Park CM, Kim PN, Byun JY. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics : a review publication of the Radiological Society of North America, Inc. 2003;23(1):123–34. doi: 10.1148/rg.231025054. discussion 34–36. [DOI] [PubMed] [Google Scholar]
- 19.DiPiro JT, Patrias JM, Townsend RJ, Bowden TA, Jr, Hooks VH, 3rd, Smith RB, Spiro TE. Oral neomycin sulfate and erythromycin base before colon surgery: a comparison of serum and tissue concentrations. Pharmacotherapy. 1985;5(2):91–94. doi: 10.1002/j.1875-9114.1985.tb03407.x. [DOI] [PubMed] [Google Scholar]
- 20.Nichols RL, Broido P, Condon RE, Gorbach SL, Nyhus LM. Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Annals of surgery. 1973;178(4):453–462. doi: 10.1097/00000658-197310000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannon JA, Altom LK, Deierhoi RJ, Morris M, Richman JS, Vick CC, Itani KM, Hawn MT. Preoperative oral antibiotics reduce surgical site infection following elective colorectal resections. Diseases of the colon and rectum. 2012;55(11):1160–1166. doi: 10.1097/DCR.0b013e3182684fac. [DOI] [PubMed] [Google Scholar]


