Abstract
The suprachiasmatic nucleus (SCN) of the anterior hypothalamus is the master circadian clock that coordinates daily rhythms in behavior and physiology in mammals. Like other hypothalamic nuclei, the SCN displays an impressive array of distinct cell types characterized by differences in neurotransmitter and neuropeptide expression. Individual SCN neurons and glia are able to display self-sustained circadian rhythms in cellular function that are regulated at the molecular level by a 24 h transcriptional-translational feedback loop. Remarkably, SCN cells are able to harmonize with one another to sustain coherent rhythms at the tissue level. Mechanisms of cellular communication in the SCN network are not completely understood, but recent progress has provided insight into the functional roles of several SCN signaling factors. This review discusses SCN organization, how intercellular communication is critical for maintaining network function, and the signaling mechanisms that play a role in this process. Despite recent progress, our understanding of SCN circuitry and coupling is far from complete. Further work is needed to map the circuitry of the SCN fully and define the signaling mechanisms that allow for collective timekeeping in the SCN network.
Introduction
Daily rhythms in metabolic and endocrine function serve to anticipate predictable changes in the environment. These rhythms are not driven externally, but instead arise from an intrinsic cellular process that tracks the hours of the day. In other words, cells are daily clocks. Because cellular clocks tick with a period close to but not exactly 24 h, they are referred to as “circadian” (i.e., about a day). Biological timekeeping at the circadian timescale ensures that physiological and behavioral processes occur at the appropriate time of day. In this respect, it is an essential and ubiquitous feature of life on this planet. For example, glucocorticoid release surges just before wakening to proactively marshal important energy resources (Figure 1, Takahashi, et al. 1968). Likewise, the secretion of growth hormone is highest during slow-wave sleep when its ability to repair and strengthen bones is most effective (Figure 1, Takahashi et al. 1968). In both cases, the timing of these rhythms is programmed by a biological clock and influenced by sleep (Czeisler and Klerman 1999). Over the last few decades, we have gained a deep understanding of the mechanisms that produce circadian timekeeping at the molecular and cellular levels. A key remaining question is how the numerous clock cells of our bodies form a coordinated system.
Figure 1.
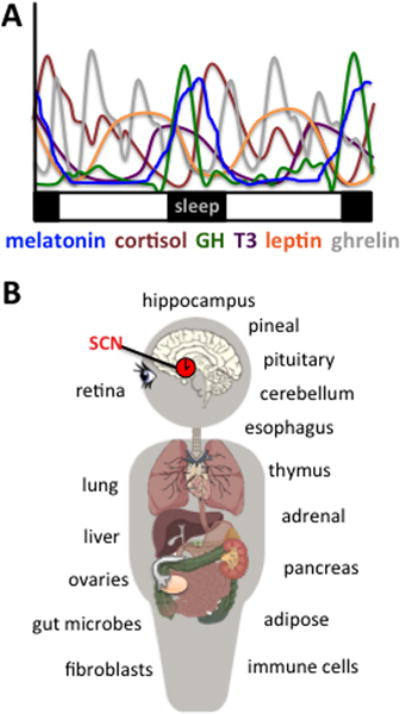
Daily hormone rhythms are regulated by the circadian timekeeping system. A. Hormone release fluctuates over the circadian cycle, with peak times occurring at specific times of day. Figure based on data from (Gavrila, et al. 2003; Koutkia, et al. 2005; Natalucci, et al. 2005; Russell, et al. 2008; Takahashi et al. 1968). B. The circadian timekeeping system is a hierarchical collection of tissue clocks located throughout the brain and body, many of which are endocrine tissues that regulate hormone synthesis and release across the day.
In mammals, the circadian system is a hierarchical collection of tissue clocks located throughout the brain and body (Mohawk, et al. 2012). Many of these clocks are endocrine tissues that regulate hormone synthesis and release across the day (Figure 1). Examples include the pituitary gland, the pineal gland, the adrenal gland, adipose tissue, and immune cells. These myriad body clocks are coordinated by the suprachiasmatic nucleus (SCN) of the anterior hypothalamus (Mohawk and Takahashi 2011). The SCN serves as a master clock that receives cues from the environment (e.g., light) and relays them to downstream tissues through a variety of outputs (e.g, synaptic connections, humoral cues, behavioral and physiological control of sleep and body temperature). In this manner, the SCN harmonizes the various body clocks with one another and with the local time zone. Classic work demonstrates that the SCN is necessary for maintaining circadian rhythms in numerous processes, including sleep, feeding, drinking, melatonin production, and reproductive function (Klein, et al. 1991; Weaver 1998). Given its extensive influence on endocrine function, understanding SCN circuitry is of prime importance.
The SCN is a neuronal network of cellular clocks that coordinate with one another to form a functional and cohesive population (Welsh, et al. 2010). Like other hypothalamic nuclei, the SCN produces a diverse range of neurotransmitters and neuropeptides (Lee, et al. 2013; van den Pol and Tsujimoto 1985), which have been used to define distinct neuronal subclasses (Antle and Silver 2005). In addition to relaying information to downstream clocks, SCN signaling molecules serve as local coupling factors that are important for maintaining network function. In particular, SCN neurons in the network produce rhythms that are more precise, higher amplitude, and more robust than those displayed by isolated SCN neurons. Thus, intercellular communication within the SCN network is critically important for ensuring the fidelity of outputs to downstream tissues. Moreover, SCN neurons coordinate as a population to encode environmental conditions that are critical to survival (e.g., seasonal changes in day length). Despite its importance, the process by which SCN neurons influence one another is not completely understood. For instance, it is not known precisely how different SCN factors contribute to network function, how these signals are transmitted, and how they act to influence cellular rhythms. This review will describe recent progress that has advanced understanding of SCN circuitry and highlight several issues that remain outstanding.
Circadian timekeeping at the cellular level
As a tissue, the SCN displays daily rhythms in numerous cellular processes, including metabolism, electrical activity, gene/protein expression, peptide release, and response to photic stimulation (Klein et al. 1991; Weaver 1998). Most of these rhythms persist when the SCN is studied in isolation from the environment and the rest of the brain. Thus, autonomous rhythmicity is an intrinsic property of the SCN itself. A key question posed almost immediately after this discovery was whether SCN rhythms are a network- or cellular-driven phenomenon. The simple answer – SCN neurons are intrinsic clock cells. Pioneering work demonstrated that individual SCN neurons are capable of sustaining cellular rhythms even when dispersed at low density (Herzog, et al. 1998; Honma, et al. 1998; Welsh, et al. 1995). Based on these results, it is commonly stated that SCN neurons are autonomous, self-sustained clocks. But the reality of the situation is slightly more complex because SCN neurons communicate with one another in ways that strengthen cellular rhythms (see more in next section).
But how does a cellular clock keep circadian time? Research conducted over the last few decades has revealed an elegant molecular mechanism that operates in nearly every cell of the body (Buhr and Takahashi 2013). Briefly, cellular rhythms are generated by interlocking feedback loops controlling the daily transcription of “clock genes” and “clock-controlled genes” (Figure 2). At its core, the molecular circadian clock is a delayed negative feedback loop, with positive elements that drive transcription and negative elements that repress transcription on a daily basis. The positive elements are the transcription factors CLOCK and BMAL1, whereas the negative elements are PERIOD and CRYTOCHROME proteins. Daily transcription is initiated when CLOCK and BMAL1 form a dimer that activates expression of a family of Period (Per1, Per2, Per3) and Cryptochrome genes (Cry1, Cry2). This increases levels of PER and CRY proteins, which dimerize, translocate into the nucleus, and inhibit their own transcription by repressing CLOCK-BMAL1 function (Figure 2). This transcriptional repression, along with ubiquitination and degradation, cause PER and CRY levels to decline. Falling levels of PER and CRY then allow for de-repression of CLOCK-BMAL1, which re-activates transcription of Per and Cry. And so the cycle renews the following day. This type of negative feedback mechanism forms the basis of circadian timekeeping in a wide range of organisms – bacteria, fungi, plants, insects, and mammals – although the genes involved differ (Mackey 2007).
Figure 2.
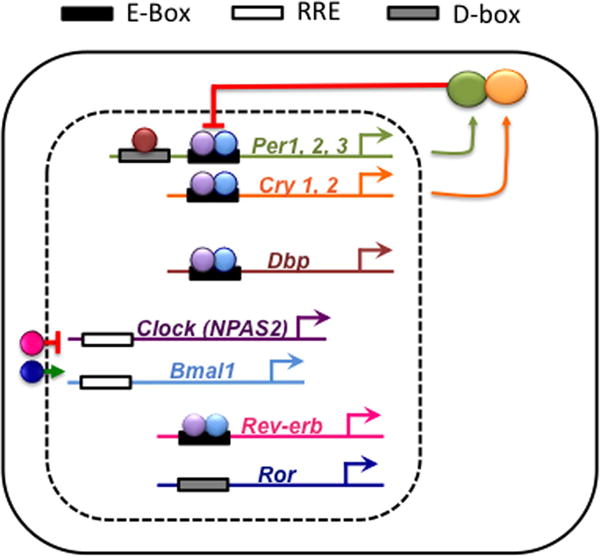
The molecular circadian clock is comprised of interlocking transcriptional-translational feedback loops. CLOCK and BMAL1 are transcription factors that bind to E-box elements within the promoter sequences of a variety of clock genes. The protein products of Period (Per) and Cryptochrome (Cry) genes form repressors that inhibit their own transcription. Additional feedback loops involve other clock genes that interact with the elements of the core loop to amplify and stabilize molecular clock function. For example, the protein products of Rev-erb and Ror genes compete for binding at ROR elements to influence Bmal1 and Clock transcription. Further, the protein products of clock-controlled genes, such as Dbp, can influence both downstream molecular targets and core clock genes by binding at D-box elements.
It remains a key area of research to understand fully the molecular control of cellular rhythms (Zhang and Kay 2010). With each passing day, our understanding of the circadian network at this level grows. We now appreciate that there are additional gene networks that regulate the core loop described above. For example, one interconnected loop regulates Bmal1 and Clock transcription via opposing actions of ROR and REV-ERBα (Figure 2). This ancillary loop regulates the precision, robustness, and amplitude of core clock gene expression. Also, there are numerous “clock-controlled genes” that are influenced by the molecular clock either directly or indirectly. For example, the transcription factor D-binding protein (DBP) is directly controlled by CLOCK-BMAL1 (Figure 2). By regulating the timing of DBP expression, the core molecular clock can influence myriad downstream targets. To further demonstrate the interlocked nature of these feedback loops, DBP can influence Per transcription by binding to an upstream promoter sequence. Moreover, clock genes may contain additional regulatory elements (e.g., CRE) through which their expression is regulated by cellular and environmental signals. Collectively, the circadian molecular clock operates in nearly every cell of the body to regulate at least half the genome (Zhang, et al. 2014).
In SCN neurons, the molecular oscillator controls myriad cellular processes (Kuhlman 2007). Notably, SCN neurons typically display daily rhythms in membrane potential with high firing during the day and low firing during the night (Brown and Piggins 2007). The temporal patterning of SCN electrical activity has important implications for network signaling and outputs to downstream tissues, so this is a critical area of research. Yet this approach is often limited in the number of SCN neurons that can be recorded at the same time. Recent technological advances enable a more comprehensive view of SCN network function using real-time imaging of molecular activity. Over the years, researchers have engineered an impressive array of mouse models with genetically encoded optical reporters (e.g., Per1-GFP, Per1-luciferase, PER2::LUCIFERASE, Bmal1-luciferase, Cry1-luciferase) that allow one to track molecular clock function in real-time (Kuhlman, et al. 2000; Maywood, et al. 2013; Noguchi, et al. 2010; Yamaguchi, et al. 2003; Yoo, et al. 2004). In addition, genetic mouse models and viral tools have been developed to monitor daily rhythms in Ca+2 and other intracellular signals (Brancaccio, et al. 2013; Enoki, et al. 2012; Irwin and Allen 2013). Provided there is good penetrance and faithful recapitulation of native molecular function, real-time imaging approaches provide an unprecedented view of the dynamic clock because they can be used to monitor hundreds of SCN neurons simultaneously. Relative to standard electrophysiological approaches, they also allow for cellular recordings to be performed in a relatively non-invasive manner for many days in culture. Together with more traditional approaches, real-time imaging techniques provide excellent tools to investigate cellular clock function and network properties.
The SCN network: Greater than the sum of its parts
It is now well established that the SCN contains multiple cellular clocks that interact with one another to form a functional network. As described above, individual SCN cells can express self-sustained circadian rhythms (Herzog et al. 1998; Honma et al. 1998; Welsh et al. 1995). Nevertheless, it is clear that SCN neurons are weaker clocks when isolated from one another. For instance, ~60% of SCN neurons are competent oscillators when dispersed in low-density culture, but only ~30% of neurons are rhythmic under conditions of complete isolation (Webb, et al. 2009). Strikingly, this is much lower than that observed when SCN neurons are connected in the network, with >90% of cells sustaining rhythms in a slice preparation. Further, coupled SCN neurons display rhythms that are more precise, higher amplitude, and more robust to perturbation and stochastic noise (Abraham, et al. 2010; Buhr, et al. 2010; Herzog, et al. 2004). Remarkably, network interactions can even preserve cellular clock function when the molecular oscillator is crippled by genetic defect (Evans, et al. 2012; Ko, et al. 2010; Liu, et al. 2007; Nakamura, et al. 2002). This series of observations indicates that SCN neurons can keep time by themselves, but are more capable clocks when they receive signals from other cells in the network. Of note, this is not true for all types of clock cells. Fibroblasts are strong circadian oscillators that appear to depend very little on one another for sustaining cellular rhythms (Leise, et al. 2012; Nagoshi, et al. 2004). While this may be viewed as evidence that SCN neurons are “weaker” clocks than fibroblasts, this is not necessarily the case. Fibroblast clocks may oscillate well as individual cells, but they lack network level properties that can buffer them from clock gene mutations (Liu et al. 2007). In contrast, SCN neurons have strength in numbers because they influence and bolster one another.
Another key property of SCN neurons is that they are able to synchronize with one another. SCN neurons embedded within the network display well-coordinated rhythms both in vivo and in vitro. In contrast, SCN neurons that are dissociated “run at different speeds” due to the expression of different period lengths (Herzog et al. 1998; Honma et al. 1998; Welsh et al. 1995). As a consequence, dissociated SCN neurons gradually lose synchrony with one another over time in culture. But SCN neurons remain synchronized when they are able to interact fully with one another. These results indicate that SCN neurons adopt a common period due to intercellular communication, which prevents loss of synchrony among SCN neurons. Studies of mutant SCN neurons likewise suggest they interact with one another to determine overall period (Herzog et al. 1998; Liu, et al. 1997; Low-Zeddies and Takahashi 2001). Due to their ability to synchronize, SCN neurons remain coordinated as a population, maintain tissue-level rhythms, and send strong outputs to downstream tissues. Importantly, the coherence of SCN rhythms is a key determinant of behavioral and physiological rhythmicity (Ciarleglio, et al. 2009). This is a property that appears to be unique to the SCN because most other types of cells appear unable to synchronize with one another (Nagoshi et al. 2004; Welsh, et al. 2004; Yamazaki, et al. 2000). Cellular clocks that lack communication would be expected to rely on SCN-controlled signals to maintain tissue coherence (Farnell, et al. 2011). For example, fibroblasts are strong cellular oscillators, but they desynchronize and lose population-level rhythms (Welsh et al. 2004). Interestingly, it may be their strongly autonomous cellular clock that prevents fibroblasts from synchronizing with one another (Locke, et al. 2008).
Lastly, network interactions influence the relative timing of neuronal activity to regulate the waveform of their collective rhythm. When embedded in the network, SCN neurons “prefer” to adopt specific phase relationships, with electrical and molecular rhythms that are slightly dispersed in time rather than occurring all at the same time (Brancaccio et al. 2013; Evans, et al. 2011; Hamada, et al. 2004; Myung, et al. 2012; Quintero, et al. 2003; Rohling, et al. 2006; Saeb-Parsy and Dyball 2003; Yamaguchi et al. 2003; Yan, et al. 2007). This is not a random event; rather there are clear regional phase differences that are reproducible and stereotyped across animals. Phase mapping analyses of PERIOD2::LUCIFERASE (PER2::LUC) expression in the murine SCN reveal several consistent patterns (Evans et al. 2011). First, the caudal SCN typically assumes an earlier phase than the rostral SCN. Also, there are fairly complex gradients of expression, with the dorsal SCN phase-leading more central and ventral regions. These spatiotemporal arrangements are intrinsically regulated by the network itself (Evans et al. 2011; Quintero et al. 2003; Yamaguchi et al. 2003), and yet can be modulated markedly by environmental lighting conditions (reviewed in Meijer, et al. 2012; Evans & Gorman, in press).
In terms of environmental modulation of SCN temporal organization, one of the most studied contexts is seasonal changes in day length. Day length regulates the phase coherence of SCN neurons, with more clustered phases under short winter-like days than long summer-like days (Evans, et al. 2013; Hazlerigg, et al. 2005; Inagaki, et al. 2007; Jagota, et al. 2000; Myung, et al. 2015; Naito, et al. 2008). Temporal coherence of phase among SCN neurons influences the overall waveform of rhythms produced by the network, with longer duration of clock gene expression and electrical activity under long days. This photoperiodic encoding is determined largely by changes in SCN phase relationships rather than changes in cellular rhythms (Brown and Piggins 2009; Rohling et al. 2006). Effectively, photoperiodic encoding by the SCN network alters the patterning of outputs transmitted under summer versus winter conditions. Seasonal changes in SCN outputs provide time of year information to downstream tissues to produce seasonal changes in behavior and physiology (Inagaki et al. 2007; Schaap, et al. 2003). Thus, the SCN network is both a daily clock and an annual calendar.
In addition to its ecological significance, plasticity in SCN phase relationships can be exploited to test mechanisms of intercellular communication (Evans et al. 2013; Evans, et al. 2015). In this approach, SCN neurons are desynchronized by light in vivo and then allowed to re-synchronize in vitro so that the process of network coupling can be tracked in real time. Specifically, very long day lengths reorganize the SCN network so that it adopts a highly polarized state with two groups of SCN neurons cycling in anti-phase. After release from these lighting conditions, SCN neurons within these two groups interact with one another and gradually resynchronize over the course of a week. Leveraging this form of network plasticity, the process of SCN coupling can be tracked in real-time using an ex vivo slice preparation. By capturing the dynamic process of SCN communication, this analytical assay may provide a novel discovery tool for further defining the circuitry of the SCN network (Evans et al. 2013).
SCN network organization: Functional differences among neuronal subclasses
The SCN contains local projection neurons that communicate within one another and other hypothalamic structures (Abrahamson and Moore 2001; Moore, et al. 2002). The axons of many SCN neurons terminate within the nucleus itself, thus forming local circuit connections and/or collaterals from longer-range projections. Nearly all SCN neurons produce γ-aminobutyric acid (GABA), yet they can be categorized into distinct subgroups based on co-expression of different neuropeptides (Abrahamson and Moore 2001; Moore and Speh 1993). The SCN is typically subdivided into two spatially segregated compartments: the shell and the core (Moore and Silver 1998). These two compartments contain distinct subclasses of neurons that differ neurochemically (Figure 3). The SCN shell contains a dense population of neurons that express arginine vasopressin (AVP), as well as other types of neurons. The SCN core contains a variety of subclasses, including neurons that express vasoactive intestinal polypeptide (VIP) or gastrin releasing peptide (GRP). These subgroups have distinct developmental patterns (Antle, et al. 2005b) and are thought to represent distinct subclasses with minimal overlap in neuropeptide expression. However, as the index of SCN peptides grows, it is becoming apparent that SCN neurons can co-express different peptides that may contribute to their functional activity (e.g., Atkins, et al. 2010; Drouyer, et al. 2010; Geoghegan and Carter 2008; Hundahl, et al. 2012; Lee, et al. 2015). Although the shell-core scheme of the SCN network continues to be a convenient construct, it will likely morph to become more sophisticated as understanding of SCN circuitry increases (Morin 2007, 2012).
Figure 3.
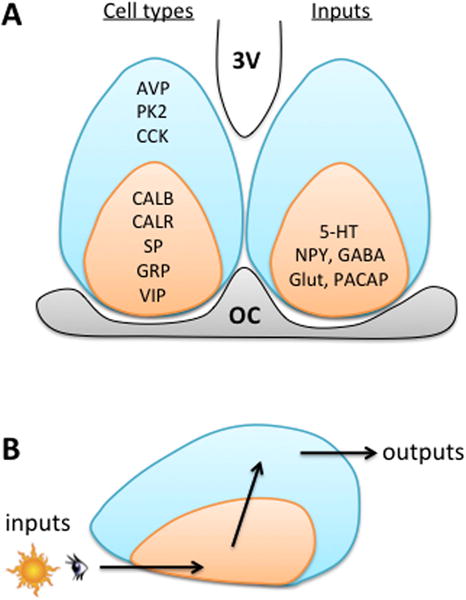
The SCN network is a heterogeneous population of cellular clocks. A. Organization of the mouse SCN, illustrating the compartmentalization of cell types and inputs in the coronal plane. AVP: Arginine Vasopressin, PK2: Prokineticin 2, CCK: Cholecystokinin, CALB: Calbindin, CALR: Calretinin, SP: Substance P, GRP: Gastrin-Releasing Peptide, VIP: Vasoactive Intestinal Polypeptide, 5-HT: Serotonin (from dorsal raphe), NPY: Neuropeptide Y (from intergeniculate leaflet of the thalamus, which also releases GABA, neurotensin, and enkephalin), Glut: Glutamate (from retina), PACAP: Pituitary Adenylate Cyclase-Activating Polypeptide (from retina), 3V: Third ventricle, OC: Optic Chiasm. B. The shell-core model of the SCN network, representing how each compartment is thought to contribute to pacemaker function.
The expression and location of AVP and VIP neurons is consistent across mammalian species, but regional anatomy and chemoarchitecture can vary (Cassone, et al. 1988; Morin 2007). For example, the SCN of rodents contains neurons that express calcium-binding proteins, but the location and specific proteins expressed differ among rodent species. In the Syrian hamster, calbindin-expressing SCN neurons form a spatially segregated subgroup densely packed into the central region of the core compartment. The mouse SCN contains both calbindin and calretinin neurons, with greatest expression within the core compartment. However, neither population forms a dense subnucleus. In contrast, calretinin neurons in the rat appear in the SCN shell rather than the SCN core. Another example of SCN variability across species is in neuropeptide Y (NPY) expression. The rodent SCN does not produce NPY, but the human SCN does (Moore 1991). The functional consequence of species variation in SCN chemoarchitecture warrants further study given its potential influence on SCN function.
In addition to differences in neuropeptide expression, SCN neurons can be distinguished based on their response to afferent input. For instance, light exposure during the night typically increases SCN electrical activity and gene expression (i.e., C-fos, Period), but does so only in a subset of neurons (Aggelopoulos and Meissl 2000; Brown, et al. 2011; Hamada, et al. 2001; Jiao, et al. 1999; Meijer, et al. 1986; Nakamura, et al. 2004; Schwartz, et al. 2000). Of those SCN neurons directly affected by retinal stimulation, some respond with sustained increases in electrical activity, some respond with phasic responses, and others are inhibited. Further, photoresponsive SCN neurons may be distinguished based on the source of retinal input they receive (Brown et al. 2011). Tract tracing studies suggest that there is a denser retinal projection to the SCN core than the SCN shell (Abrahamson and Moore 2001; Lokshin, et al. 2015), which maps onto functional differences in photic response. After light exposure, the SCN core displays changes in gene expression that precede those in the SCN shell (Dardente, et al. 2002; Kuhlman, et al. 2003; Nagano, et al. 2003; Silver, et al. 1996; Yan and Okamura 2002; Yan and Silver 2002, 2004). Regional differences in photic responsiveness have functional consequences that can influence how the network adjusts following changes in environmental lighting conditions. For instance, after simulated travel across time zones, the SCN core re-entrains faster than the SCN shell (Albus, et al. 2005; Davidson, et al. 2009; Nagano et al. 2003; Nakamura, et al. 2005; Rohling, et al. 2011; Sellix, et al. 2012), and this form of light-driven desynchrony is thought to contribute to symptoms of jetlag. Moreover, regional patterning of afferent projections to the SCN is not unique to the retina, with inputs from the thalamus, pretectum, and median raphe also being densest in the SCN core (Figure 3, for a review, see Morin and Allen 2006). Further, the SCN shell may receive input from a distinct set of structures, at least in the rat (Leak and Moore 2001; Moga and Moore 1997). Future studies should test whether this is consistent across mammalian species given the potential implications of the SCN shell receiving inputs from these particular structures (i.e., cortex, limbic structures, the basal forebrain, brainstem, and other hypothalamic nuclei).
SCN neurons can also be distinguished based on efferent projections. The SCN communicates with downstream targets using both synaptic and humoral signals (Mohawk and Takahashi 2011). In the case of synaptic connections, the SCN innervates structures in the hypothalamus, thalamus, and forebrain (Kalsbeek and Buijs 2002; Watts 1991), and these projections may originate from specific SCN regions. With regard to outputs from SCN neuronal subclasses, those originating from AVP and VIP neurons have been most extensively studied. AVP and VIP neurons often project to the same target, but the relative patterning of innervation can be target-specific (Abrahamson and Moore 2001; Kalsbeek and Buijs 2002; Watts and Swanson 1987). For instance, both AVP and VIP neurons in the murine SCN project to the paraventricular nucleus and the dorsomedial nucleus of the hypothalamus, but each target contains more AVP fibers than VIP fibers (Abrahamson and Moore 2001). The functional roles of projections from distinct SCN regions are far from fully understood. Thus far, research indicates that both SCN compartments influence rhythms in downstream targets, yet there are differences in the role of outputs from different SCN regions (Butler, et al. 2012; Evans et al. 2015; Kalsbeek, et al. 2010; Lee, et al. 2009; Schwartz, et al. 2009; Smarr, et al. 2012; Wotus, et al. 2013; Yan, et al. 2005; Zhou and Cheng 2005). One emerging theme is that the SCN shell appears to set the phase of downstream tissues; however, both SCN shell and core neurons provide signals to downstream tissues that can influence their rhythms. The efferent projection patterns of distinct SCN subclasses should be revisited in future work given recent advances in cell-type specific tract tracing (Callaway and Luo 2015). A deeper understanding of this issue may provide insight into the functional role of outputs from different SCN subclasses.
Lastly, there is a rich body of work describing regional differences in SCN function. As mentioned above, SCN neurons display regional differences in the phase of electrical and molecular activity, which can be detected even within a chemically defined subclass (Evans et al. 2011; Hamada et al. 2004; Kalsbeek, et al. 2006; Yoshikawa, et al. 2015). Although the majority of SCN neurons tend to exhibit clustered phases of electrical and molecular activity, anti-phase rhythms are expressed by subgroups of SCN neurons whose chemical identity remains ill defined (King, et al. 2003; Lee, et al. 2003; Nakamura, et al. 2001). In addition, SCN neurons from distinct regions or peptidergic subclasses can exhibit different period length (Myung et al. 2012; Nakamura et al. 2001; Noguchi and Watanabe 2008; Shinohara, et al. 1995). Further, daily patterns of electrical activity can differ markedly among neuronal subgroups (Belle, et al. 2009; Jiao et al. 1999; Jobst and Allen 2002; Saeb-Parsy and Dyball 2003; Shibata, et al. 1984). This suggests that SCN neurons can oscillate with different properties, but the presence of cellular rhythms may not be universal among SCN neurons. Many studies examining the SCN network have found that photoresponsive neurons within the SCN core display a less rhythmic or arrhythmic phenotype (Hamada et al. 2001; Jiao et al. 1999; Jobst and Allen 2002). However, recent work indicates that cellular rhythmicity is stochastic, not restricted to neurons of a particular peptidergic subgroup, and sustained by network interactions (Webb, et al. 2009). This provides new insight in that it suggests that there is no single subclass of “pacemaker” neuron in the SCN network and that cellular rhythmicity is strongly influenced by network connectivity rather than chemical phenotype.
In summary, SCN neurons in different regions of the network can be distinguished in many ways. The most common model of SCN organization highlights functional distinctions between its shell and core compartments. In this scheme, the SCN core contains first order neurons that receive and process photic input. SCN core neurons display strong rhythms in light-induced responses, but electrical and molecular rhythms of these neurons may be low amplitude or non-existent.
Nevertheless, input transmitted from SCN core neurons is important for coordinating neurons in the rest of the network. On the other hand, neurons in the SCN shell are strongly rhythmic and play a key role in transmitting daily outputs to downstream structures. Overall, there is utility in this linear model, but some key aspects of SCN circuitry remain unexplained. For example, if SCN core neurons are not different from SCN shell neurons when studied in isolation, what accounts for their differences when they are embedded in the network? If specific subclasses of SCN core neurons do indeed lack a strong molecular oscillator, how do they maintain circadian rhythms in photic responsiveness? Presumably, these observations reflect that molecular and photoresponsive rhythms of SCN core neurons are influenced by intercellular communication, but the relevant signals remain undefined. Additional work investigating SCN signaling is expected to expand understanding of its circuitry.
SCN coupling mechanisms
SCN neurons communicate through multiple mechanisms and signaling factors (Aton and Herzog 2005; Michel and Colwell 2001; van den Pol and Dudek 1993). An important role of synaptic signaling is based on observations that SCN neurons desynchronize when cultured with tetrodotoxin (TTX) to block Na+ dependent action potentials (Yamaguchi et al. 2003). However, there is also evidence that the SCN network can use other forms of coupling that do not depend on synaptic communication. For instance, SCN timekeeping can be maintained in the absence of Na+-dependent action potentials and Ca+2-dependent synaptic transmission (Bouskila and Dudek 1993; Dudek, et al. 1993; Earnest, et al. 1991; Schwartz 1991; Schwartz, et al. 1987; Shibata and Moore 1993). Further, circadian rhythms are maintained in some species in vivo under environmental conditions that severely compromise neuronal activity (Grahn, et al. 1994; Menaker 1961). Further, the SCN displays metabolic rhythms at an embryonic age (Reppert 1992; Shibata and Moore 1987) that precedes the completion of synaptogenesis (Bedont and Blackshaw 2015). Collectively, this suggests that synaptic transmission is not the exclusive means by which SCN neurons can communicate with one another. Interestingly, functional studies indicate that SCN neurons can communicate through paracrine signaling (Maywood, et al. 2011). In this work, co-cultured SCN slices were able to influence the rhythmic properties of one another even though they were unable to establish cross-slice synaptic connections. One potential mechanism driving this effect may be the non-synaptic release of SCN neuropeptides from axons, dendrites and somata (Castel, et al. 1996). While it remains unclear which specific SCN neuropeptides are released at these sites, this form of communication may influence the function of neuronal networks (Ludwig and Leng 2006; van den Pol 2012). In the section below, I will review recent insight into the roles of different SCN coupling factors. In particular, much has been learned about the ways in which VIP, GABA, and AVP signaling influence SCN function. Nevertheless, it remains a challenge to fully map the neurochemical, temporal, and spatial properties of SCN circuits.
Vasoactive Intestinal Polypeptide (VIP)
Over the past decade, clear evidence has emerged that indicates VIP is important for synchronizing SCN neurons (Vosko, et al. 2007). As described above, VIP is produced by a subset of SCN neurons located within the ventral SCN core, with functional evidence suggesting that there are at least two subclasses of VIP neurons in the rat (Kawamoto, et al. 2003). VIP is expected to have pervasive effects because VIP+ fibers innervate nearly all SCN regions (Card, et al. 1981; Card and Moore 1984), and most SCN neurons express the VIP receptor, VPAC2 (An, et al. 2012; Kalamatianos, et al. 2004b; Kallo, et al. 2004b). The influence of VIP signaling is expected to fluctuate daily since VIP and its receptor are expressed rhythmically in the SCN in vivo and in vitro (Cagampang, et al. 1998; Dardente, et al. 2004; Duncan, et al. 1995; Glazer and Gozes 1994; Shinohara et al. 1995; Takahashi, et al. 1989). The VPAC2 receptor is a Gαs coupled receptor that activates adenylyl cyclase, cAMP, PKA, and CRE-dependent transcription (Figure 4A, Couvineau and Laburthe 2011; Harmar, et al. 1998). Given that Period genes are activated by CREB, this suggests that VIP will alter cellular rhythms in SCN neurons. As expected, VIP signaling alters SCN electrical and molecular activity in vivo and in vitro (Cutler, et al. 2003; Irwin and Allen 2010; Itri and Colwell 2003; Kudo, et al. 2013; Nielsen, et al. 2002; Pakhotin, et al. 2006; Piggins, et al. 1995). Moreover, it can phase shift other SCN neuropeptide rhythms, such as AVP release (Watanabe, et al. 2000). Consistent with VPAC2 being a Gαs coupled-receptor, the effects of VIP on SCN electrical and molecular rhythms depend on PKA signaling (An, et al. 2011; Meyer-Spasche and Piggins 2004; Nielsen, et al. 2002). However, VIP-induced effects also require activation of other intracellular cascades (i.e., mitogen-activated protein kinase, phospholipase C), which indicates that other signaling mechanisms may be involved (Figure 4A).
Figure 4.
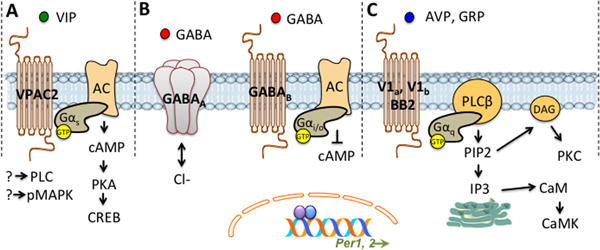
Intercellular signaling mechanisms that alter cellular activity and clock gene expression within SCN neurons. A. SCN neurons express the VPAC2 receptor for VIP, which is a G-protein coupled receptor with seven transmembrane domains. Upon VIP binding to VPAC2, Gαs activates adenylyl cyclase, cAMP, PKA, and CRE-dependent transcription. B. Within the SCN both GABAA receptors and GABAB receptors are expressed. GABA binding to the ionotropic GABAA receptor allows for influx or efflux of Cl-, depending on [Cl-]i. In contrast, the metabotropic GABAB receptor is coupled to Gαi/o, which inhibits adenylyl cyclase. C. The receptors for both AVP and GRP are Gαq coupled receptors that stimulate phospholipase C (PLCβ), which in turn activates phosphatidylinositol 4,5-bisphosphate (PIP2) to cause diacyl glycerol (DAG)-mediated activation of protein kinase C (PKC) and inositol triphosphate (IP3)-induced release of intracellular calcium stores. Intracellular release of calcium activates Calmodulin (CaM), which stimulates Ca2+/ calmodulin-dependent protein kinase (CaMK). Due to the expression of CRE-elements in Period genes, changes in the intracellular signaling cascades illustrates in A-C can alter the function of the molecular clock.
Interest in the role of VIP signaling was especially piqued when it was discovered that the loss of VIP signaling is able to compromise circadian rhythmicity. When studied under constant dark conditions, the majority of VIP and VPAC2 knockout mice display either arrhythmic locomotor patterns or low amplitude rhythms with altered period (Aton et al. 2005; Ciarleglio, et al. 2009; Colwell, et al. 2003; Harmar, et al. 2002). Arrhythmic VIP deficient mice also display loss of rhythms in SCN electrical activity and gene expression due to lack of network synchrony and a decrease in the number of SCN neurons able to maintain viable cellular rhythms (Aton, et al. 2005; Ciarleglio, et al. 2009; Brown, et al. 2007; Hughes, et al. 2008; Maywood, et al. 2006). Importantly, SCN cellular rhythms and synchrony can be rescued in VIP knockout slices by daily application of a VPAC2 agonist (Aton et al. 2005). Loss of cellular rhythms and synchrony likewise occurs in wildtype SCN slices exposed to a VPAC2 antagonist (Brown et al. 2007; Evans et al. 2013), which suggests that effects of VIP/VPAC2 deletion are not due to developmental abnormalities. Further, VIP signaling has important consequences for spatiotemporal arrangements of the SCN network, which involves intercellular communication triggered by Gαq signaling in VIP neurons (Brancaccio et al. 2013). Collectively, this work indicates that VIP is a local coupling factor that amplifies and entrains SCN neurons that are weak intrinsic oscillators. These effects are very important for maintaining cellular rhythms and synchronized network activity within the SCN network.
In addition to serving as a local coupling factor, VIP signaling also influences other processes. In particular, VIP is involved in photic signaling (An, et al. 2013; Colwell et al. 2003; Dragich, et al. 2010; Hughes, et al. 2004; Kuhlman et al. 2003; Lucassen, et al. 2012; Shen, et al. 2000), and light influences VIP expression in the SCN (Duncan et al. 1995; Francl, et al. 2010; Isobe and Nishino 1998; Shinohara and Inouye 1995; Smith and Canal 2009). Deficits in VIP signaling cause abnormal responses to light, with one result being an unusual responsiveness to light during the day. Furthermore, loss of VIP causes disruptions in a wide variety of physiological processes, including cardiovascular function, metabolism, and reproduction (Bechtold, et al. 2008; Hannibal, et al. 2011; Loh, et al. 2014; Schroeder, et al. 2011; Sheward, et al. 2010; Shimizu, et al. 1996). This indicates that VIP signaling is important for maintaining endocrine function, which could be due to its role in the SCN network itself and/or its role as an output signal to downstream tissues (Egli, et al. 2004; Fahrenkrug, et al. 2012; Gerhold, et al. 2001; Gerhold and Wise 2006; Kallo et al. 2004b; Kalsbeek, et al. 1993; Loh, et al. 2011; van der Beek, et al. 1993). Also, age- and sex-related differences in VIP expression may influence changes in the strength and robustness of circadian function (Kallo, et al. 2004a; Kawakami, et al. 1997; Krajnak, et al. 1998a, b; Mahoney, et al. 2009; Zhou, et al. 1995). Thus, VIP signaling is an important modulator whose absence has widespread consequences for behavior and physiology. There continues to be a need to define the properties and mechanisms of VIP signaling to better understand how it regulates clock function at the cellular, network, and systems levels. For instance, recent work demonstrates that VIP can desynchronize the phase of SCN neurons if given at the wrong time or at high doses (An et al. 2013; Ananthasubramaniam, et al. 2014). This suggests the likelihood that VIP expression is tightly regulated within the SCN network. Of interest, 4E-BP1 has been identified recently as a molecular repressor of Vip translation (Cao, et al. 2013). Future studies are expected to provide additional insight into the temporal and spatial features of VIP signaling, as well as modulatory mechanisms.
γ-aminobutyric acid (GABA)
Since it was first reported that nearly all SCN neurons express GABA, it has been suspected that this neurotransmitter regulates SCN communication. The SCN displays a high density of GABA terminals and cells (Abrahamson and Moore 2001; Castel and Morris 2000; Decavel and Van den Pol 1990; Huhman, et al. 1996; Moore and Speh 1993; Okamura, et al. 1989; van den Pol and Tsujimoto 1985). Receptors for GABA include both ionotropic GABAA receptors and metabotropic GABAB receptors coupled to Gαi/o (Figure 4B, Bormann 2000), with both being expressed in the SCN (Belenky, et al. 2003; Belenky, et al. 2008; Gao, et al. 1995; Naum, et al. 2001; O’Hara, et al. 1995). As with other SCN factors, daily rhythms in GABA signaling are evident in the SCN, and these rhythms can be modulated by light exposure (Aguilar-Roblero, et al. 1993; Huhman et al. 1996; Itri, et al. 2004; Naum et al. 2001). Consistent with this anatomical work, SCN neurons are sensitive to GABA signaling (Biggs and Prosser 1998; Cardinali and Golombek 1998; Ehlen, et al. 2008; Gillespie, et al. 1997; Gillespie, et al. 1999; Jiang, et al. 1997b; Liou, et al. 1990; Mason, et al. 1991; Mintz, et al. 2002; Strecker, et al. 1997). Collectively, these studies indicate that GABA regulates SCN neuronal activity, modulates photic signaling, and serves as an output signal to downstream tissues (Wang, et al. 2003). For modulation of photic signaling, an important role has been demonstrated for both GABAA and GABAB receptors (Belenky et al. 2003; Biggs and Prosser 1998; Ehlen and Paul 2009; Gillespie et al. 1997; Novak, et al. 2004). In contrast, GABAA receptor signaling is thought to be the strongest local regulator of SCN network activity (Albus et al. 2005; Fan, et al. 2015; Kim and Dudek 1992; Shimura, et al. 1996; Strecker et al. 1997). Over the last two decades, work has revealed surprising ways in which GABA can modulate SCN function.
One of the first surprises discovered about GABA signaling in the SCN concerns the polarity of responses to this neurotransmitter. GABA is typically defined as an inhibitory neurotransmitter; however, GABA can depolarize and increase intracellular calcium concentration in SCN neurons (Choi, et al. 2008; Irwin and Allen 2009; Wagner, et al. 1997). Although excitatory GABAergic responses are not always observed (Bos and Mirmiran 1993; Dudek et al. 1993; Gribkoff, et al. 1999), this may stem at least in part from spatial or temporal differences in recordings performed in different laboratories (Alamilla, et al. 2014; Albus et al. 2005; Choi et al. 2008; De Jeu and Pennartz 2002; DeWoskin, et al. 2015; Ikeda, et al. 2013). Indeed, the SCN displays regional differences in the expression of the chloride co-transporters (Belenky, et al. 2010; Belenky et al. 2008; Choi et al. 2008) that determine chloride reversal potential (Vogt 2015). Adding to this complexity, it has been demonstrated recently that the polarity of GABA responses in the SCN is influenced by the duration of daily light exposure (Farajnia, et al. 2014; Myung et al. 2015). This may have great relevance for the ability of the SCN to serve as an annual calendar, and firmly places the SCN on the growing list of mature neural networks that exhibit plasticity in the polarity of GABA responses (Marty and Llano 2005).
Given that the nature of SCN responses to GABA remain unclear, it should come as little surprise that the precise role of GABA in SCN coupling has proven difficult to define. Early work demonstrated that dissociated SCN neurons exposed to GABA will synchronize their electrical rhythms via GABAA signaling (Liu and Reppert 2000; Shirakawa, et al. 2000). Additional studies further indicated that GABAA signaling is involved in the transfer of resetting information from the SCN core to the SCN shell (Albus et al. 2005; Han, et al. 2012). However, GABA receptor antagonists do not desynchronize SCN neurons in vitro (Aton, et al. 2006). These results came as a surprise because they run counter to the hypothesis that GABA acts as a coupling factor. Recent work has provided some insight into these conflicting findings. GABAA signaling does influence SCN coupling, but surprisingly it destabilizes phase relationships of SCN neurons (DeWoskin et al. 2015; Evans et al. 2013; Freeman, et al. 2013; Myung et al. 2015). Normally, this effect of GABAA signaling is hard to detect because VIP signaling is a potent synchronizing agent. But in the absence of VIP, SCN neurons desynchronize due to GABAA signaling (Evans et al. 2013; Freeman et al. 2013). In fact, antagonism of GABAA signaling will “fix” a VIP KO slice and prevent desynchrony from occurring. This indicates that the loss of synchrony that emerges during VIP deficiency is not due to “passive” desynchrony caused by cellular period differences but rather “active” desynchronizing responses elicited by GABA signaling. This provides us with a new view into SCN circuitry because it indicates that some SCN signaling mechanisms promote synchronization, while others may cause desynchronization. This also reveals that SCN coupling factors may be arranged into pairs that oppose one another’s effects. Building on this theme of interactive coupling mechanisms, GABAA signaling can interact with VIP signaling in either an antagonistic or cooperative manner depending on the state of the network (Evans et al. 2013), which may account for earlier work demonstrating synchronizing effects (Albus et al. 2005; Han, et al. 2012;Liu and Reppert 2000; Shirakawa, et al. 2000). Together with previous work, this strongly suggests that the functional role of a coupling mechanism can change based on the experience, history, or age of the animal (Bedont, et al. 2014; Evans et al. 2013; Wang, et al. 2014).
Arginine vasopressin (AVP)
Although AVP has been traditionally viewed more as an SCN output signal, a more direct role in network synchronization has been suggested recently. AVP is rhythmically expressed both in vivo and in vitro (Cassone et al. 1988; Dardente et al. 2004; Mahoney et al. 2009; Miller, et al. 2006; Noguchi and Watanabe 2008; Shinohara, et al. 1998; Sukhov, et al. 1993; Van der Veen, et al. 2005; Yoshikawa et al. 2015). Of three AVP receptors, V1a (V1) and V1b (V3) are expressed within the SCN (Kalamatianos, et al. 2004a; Li, et al. 2009), which are both Gαq coupled receptors that stimulate phospholipase C to cause DAG-mediated activation of PKC and IP3-induced mobilization of intracellular calcium (Figure 4C, Maybauer, et al. 2008). Although AVP was one of the first SCN neuropeptides to be discovered, it was deemed not necessary for circadian rhythms based on work in rats with a spontaneous loss of function mutation (Boer, et al. 1998; Groblewski, et al. 1981). Further work, however, revealed that these rats display lower amplitude rhythms of sleep, melatonin release, and corticosterone (Brown and Nunez 1989; Schroder, et al. 1988; Wideman, et al. 2000), and this led to the suggestion that AVP was mostly an important output signal (Jin, et al. 1999; Tousson and Meissl 2004). In support of a network role, however, AVP neurons project locally within the SCN itself (Castel, et al. 1990; Romijn, et al. 1997) and exogenous AVP is able to regulate the cellular activity of SCN neurons (Ingram, et al. 1998; Liou and Albers 1989; Mihai, et al. 1994). However, it was unclear whether these responses actually produced functional consequences because AVP application in vitro or in vivo does not phase shift SCN rhythms. Nevertheless, recent work has revealed that AVP is able to influence the function of the SCN network. V1a and V1b receptor knockout mice display a pronounced circadian phenotype in that they are resistant to jetlag and re-entrain almost instantly following a shift in the light:dark cycle (Yamaguchi, et al. 2013). Wild type mice receiving AVP receptor antagonists directed to the SCN likewise shift quickly, thus discounting a developmental basis to this effect. Rapid recovery following simulated jetlag is also observed in mice lacking Bmal1 in AVP neurons, which can be reversed by SCN-specific rescue of molecular clock function (Mieda, et al. 2015). Collectively, this work suggests that AVP signaling is involved in setting the pace of re-entrainment. It has been proposed that loss of AVP signaling causes a change in SCN coupling that allows the entire network to shift rapidly to the new time zone. Consistent with this, application of AVP can synchronize SCN neurons collected from mice deficient in VIP signaling (Maywood et al. 2011). Further work testing the specific role of AVP signaling in SCN coupling is warranted.
Gastrin releasing peptide (GRP)
The potential role of GRP in SCN coupling remains to be determined, although there is clear evidence that GRP signaling is involved in photic processing. GRP is rhythmically expressed in the SCN and modulated by light (Dardente et al. 2004; Lee et al. 2013; Shinohara, et al. 1993). The GRP receptor, also known as the bombesin 2 (BB2) receptor, is likewise rhythmically expressed within the SCN in a manner influenced by light exposure (Karatsoreos, et al. 2006). Like the V1a receptor for AVP, BB2 is a Gαq coupled receptor (Figure 4C, Jensen, et al. 2008). GRP neurons within the SCN receive retinal input that induces cellular responses (Dardente et al. 2002; Lesauter, et al. 2011). Further, GRP can phase shift SCN rhythms in vitro and locomotor rhythms in vivo in a pattern similar to those induced by light and VIP (Aida, et al. 2002; Antle, et al. 2005a; Biello 2009; Gamble, et al. 2007; Gillespie et al. 1997; McArthur, et al. 2000; Piggins et al. 1995; Piggins, et al. 2005). These effects of GRP are dependent on BB2 receptor signaling, CREB-dependent transcription, clock gene activation, and changes in fast delayed rectifier potassium currents (Aida et al. 2002; Gamble et al. 2007; Gamble, et al. 2011; Piggins et al. 2005). However, the role of GRP may not be limited to photic signaling because GRP can enhance cellular rhythms in SCN slices collected from mice deficient in VIP signaling (Brown et al. 2005; Maywood et al. 2011; Maywood et al. 2006). Further, cellular rhythmicity is attenuated by a BB2 receptor antagonist, but only when applied in the absence of VIP signaling (Brown et al. 2005). Together with the work on GABA described above, this suggests that VIP is a very strong modulator of SCN cellular rhythms that may mask the effects of other signaling mechanisms.
Other neuroactive substances
In addition to these well-studied subgroups, there are likely novel subclasses of SCN neurons that play a role in network coupling. The SCN produces dozens of signaling factors, and the number of recognized SCN peptides continues to increase (Lee et al. 2015; Lee et al. 2013; van den Pol and Tsujimoto 1985). A recent study demonstrated that SCN network function is regulated by Neuromedin S, which is an SCN peptide produced by both AVP and VIP neurons (Lee et al. 2015). Also, a forward peptidomics screen identified little SAS as a novel peptide produced in the SCN core that relays photic signals independent of VIP- or GRP-dependent signaling (Atkins et al. 2010). Another interesting development is that intra-SCN glutamatergic signaling may play a role in coupling the left and right SCN (Michel, et al. 2013), and yet glutamate has not been detected in the SCN (Strecker et al. 1997). Communication between the left and right SCN likely differs from that coupling neurons within each SCN (Bouskila and Dudek 1993), and additional studies may shed new light on mechanisms mediating this coupling. Overall, this work highlights that the SCN remains a complex structure, and it is likely that additional coupling signals will be identified in the future.
Non-synaptic interactions
One early hypothesis posited a role of local electric field effects produced by changes in the membrane potential of adjacent cells. Ephaptic interactions produced by electrical field potentials in dendrites can synchronize electrical activity in the mammalian neocortex, but it is unlikely that this mechanism couples oscillators within the SCN given its non-laminar organization (Van den Pol 1980; van Esseveldt, et al. 2000). Nevertheless, functional and anatomical evidence suggests that SCN neurons can communicate through non-synaptic release of neuropeptides (Castel et al. 1996; Maywood et al. 2011). Moreover, electrotonic communication through low-resistance gap junctions may influence SCN coupling (Colwell 2000; Jiang, et al. 1997a; Jobst, et al. 2004; Long, et al. 2005). Gap junctions are channels that allow the exchange of small molecules between neurons and/or glia in close apposition (Bennett, et al. 1991; Connors and Long 2004; Rash, et al. 2000; Rash, et al. 2001). Gap junctions can be found in both SCN neurons and glia, with the diffusion of labeled molecules (i.e., dye coupling) occurring mostly between homotypic cells located in the same SCN compartment (Colwell 2000; Jiang et al. 1997a). Studies using paired intracellular recordings and dye coupling suggest that electrical coupling varies as a function of circadian phase (Colwell 2000; Long, et al. 2005), which suggests that communication through gap junctions is not a passive process but one that is actively regulated in a dynamic fashion. Further evidence that gap junctions likely influence SCN function stems from pharmacological experiments demonstrating that SCN electrical rhythms become broader, arrhythmic, or bimodal after octanol or halothane application (Prosser, et al. 1994; Shinohara, et al. 2000a; Shinohara, et al. 2000b; Shirakawa, et al. 2001). These specific changes in waveform are thought to reflect changes in SCN phase relationships caused by altered coupling, although this has yet to be tested directly. Further, the cellular location of these gap junctions remains unclear since these pharmacological inhibitors would be expected to affect both neurons and glia, as well as producing off-target effects. A specific role for neuronal gap junctions is indicated by work demonstrating that SCN electrical coupling is dependent on expression of the neuron-specific gap junction protein connexin36 (Long, et al. 2005). Signaling through these gap junctions is thought to infleunce circadian behavior because mice lacking connexin36 display reduced amplitude of locomotor rhythms under constant darkness (Long, et al. 2005). Another factor that may modulate circadian behavior via gap junction signaling is the expression of the polysialylated form of neural cell adhesion molecule (Glass, et al. 2003; Lee, et al. 1995), although this protein is also involved in synaptic transmission. This work suggests that gap junctions influence SCN function, but additional studies are needed to delineate their precise role.
Astrocyte
While most circadian research has focused on the functional properties of SCN neurons, the SCN also contains glial cells (Guldner 1983). SCN astrocytes can be detected by labeling glial fibrillary acidic protein (GFAP), which displays a daily rhythm that is modulated by light (Becquet, et al. 2008; Canal, et al. 2009; Gerics, et al. 2006; Lavialle and Serviere 1993; Lindley, et al. 2008; Moriya, et al. 2000). Like other cell types, astrocytes are intrinsic oscillators that display daily rhythms in metabolic function (Burkeen, et al. 2011; Lavialle and Serviere 1993; Schwartz and Gainer 1977; van den Pol, et al. 1992; Womac, et al. 2009) and clock gene/protein expression (Cheng, et al. 2009; Duhart, et al. 2013; Prolo, et al. 2005; van den Pol et al. 1992; Yagita, et al. 2010). The available evidence points to several ways in which SCN glia and neurons interact (Jackson 2011); however, the precise role of glia in the SCN network is not well characterized. In general, astrocytes provide physical support, release pro-survival factors, influence synaptic clearance, and produce gliotransmitters that interact with presynaptic and postsynaptic receptors (Faissner, et al. 2010). Anatomical and functional evidence suggests that SCN astrocytes may influence light-induced resetting by regulating glutamate release from retinal terminals (Girardet, et al. 2010; Lavaille and Serviere 1995; Lavialle, et al. 2001; Moriya et al. 2000; Tamada, et al. 1998; van den Pol et al. 1992). Interestingly, VIP and AVP neurons in the SCN core and shell compartments display differences in daily rhythms of glial coverage of dendrites, with higher coverage of VIP and AVP neurons during the night and day, respectively (Becquet et al. 2008). This suggests that SCN glia may differentially regulate signaling to these two subpopulations across the circadian cycle. Consistent with a glial role in SCN photic responses and/or interneuronal coupling, mice that have a mutation in GFAP have altered locomotor activity rhythms in LL (Moriya et al. 2000). More direct evidence for glial involvement in SCN coupling is provided by work demonstrating that SCN electrical rhythms become bimodal in the presence of the glial metabolism antagonist, fluorocitrate (Prosser et al. 1994). These changes in the waveform of SCN electrical rhythms are thought to be due to altered communication among SCN neuronal subpopulations (Wang et al. 2014), which could be tested further with real-time imaging techniques. Communication between SCN neurons and astrocytes is likely bi-directional since damped rhythms in astrocytes are enhanced by co-culture with SCN explants, but not cortical explants (Prolo et al. 2005). Further, VIP influences the phase and amplitude of astrocyte rhythms in a dose-dependent manner (Marpegan, et al. 2009). Collectively, this work suggests that SCN astrocytes and neurons interact with one another, and future studies are expected to clarify the functional consequences of this relationship.
Conclusions
In many ways, mapping the circuitry of the SCN remains a challenge. Defining the specific role of any given signaling mechanism can be complicated given that multiple processes interact and modulate one another (Haas, et al. 2011; Itri et al. 2004; Shinohara et al. 2000b; Wang et al. 2014). In addition, it remains a challenge to separate the role of a given neuromodulator in mediating intra-network coupling from its role in processing input and transmitting output. Despite the difficulty in addressing these issues, it remains critical to address how SCN cells integrate the various signals provided by other cell types in the network. The combinatorial effects of SCN coupling factors have yet to be systemically investigated, although some work has addressed this issue in the context of photic signaling (Albers, et al. 1991; Piggins et al. 1995). It is likely that future technological advances will prove critical for achieving a deeper understanding of SCN circuitry. Going forward, it will be important to define the precise cellular location of receptors for putative coupling factors. Further, whether these receptors are co-expressed spatially and temporally on specific subclasses of SCN neurons should be addressed in future work. Given the potential for rhythms in each component of SCN signaling (e.g., signal release, receptor expression, physical connectivity, astrocyte function), advanced techniques for imaging cellular connections and responses will continue to be essential for making progress in this area.
Acknowledgments
Funding
I acknowledge support from the NIH (R01NS091234) and the Whitehall Foundation (2014-12-65).
Footnotes
Declaration of Interest
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Abraham U, Granada AE, Westermark PO, Heine M, Kramer A, Herzel H. Coupling governs entrainment range of circadian clocks. Mol Syst Biol. 2010;6:438. doi: 10.1038/msb.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: Retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- Aggelopoulos NC, Meissl H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol. 2000;523(Pt 1):211–222. doi: 10.1111/j.1469-7793.2000.t01-1-00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Roblero R, Verduzco-Carbajal L, Rodriguez C, Mendez-Franco J, Moran J, de la Mora MP. Circadian rhythmicity in the GABAergic system in the suprachiasmatic nuclei of the rat. Neurosci Lett. 1993;157:199–202. doi: 10.1016/0304-3940(93)90736-5. [DOI] [PubMed] [Google Scholar]
- Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S. Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol Pharmacol. 2002;61:26–34. doi: 10.1124/mol.61.1.26. [DOI] [PubMed] [Google Scholar]
- Alamilla J, Perez-Burgos A, Quinto D, Aguilar-Roblero R. Circadian modulation of the Cl(−) equilibrium potential in the rat suprachiasmatic nuclei. Biomed Res Int. 2014;2014:424982. doi: 10.1155/2014/424982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Liou SY, Stopa EG, Zoeller RT. Interaction of colocalized neuropeptides: Functional significance in the circadian timing system. J Neurosci. 1991;11:846–851. doi: 10.1523/JNEUROSCI.11-03-00846.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Current Biology. 2005;15:886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- An S, Harang R, Meeker K, Granados-Fuentes D, Tsai CA, Mazuski C, Kim J, Doyle FJ, 3rd, Petzold LR, Herzog ED. A neuropeptide speeds circadian entrainment by reducing intercellular synchrony. Proc Natl Acad Sci U S A. 2013;110:E4355–4361. doi: 10.1073/pnas.1307088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Irwin RP, Allen CN, Tsai CA, Herzog ED. Vasoactive intestinal polypeptide requires parallel changes in adenylate cyclase and phospholipase C to entrain circadian rhythms to a predictable phase. J Neurophysiol. 2011 doi: 10.1152/jn.00966.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Tsai C, Ronecker J, Bayly A, Herzog ED. Spatiotemporal distribution of vasoactive intestinal polypeptide receptor 2 in mouse suprachiasmatic nucleus. J Comp Neurol. 2012;520:2730–2741. doi: 10.1002/cne.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthasubramaniam B, Herzog ED, Herzel H. Timing of neuropeptide coupling determines synchrony and entrainment in the mammalian circadian clock. PLoS Comput Biol. 2014;10:e1003565. doi: 10.1371/journal.pcbi.1003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Kriegsfeld LJ, Silver R. Signaling within the master clock of the brain: Localized activation of mitogen-activated protein kinase by gastrin-releasing peptide. J Neurosci. 2005a;25:2447–2454. doi: 10.1523/JNEUROSCI.4696-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, LeSauter J, Silver R. Neurogenesis and ontogeny of specific cell phenotypes within the hamster suprachiasmatic nucleus. Brain Res Dev Brain Res. 2005b;157:8–18. doi: 10.1016/j.devbrainres.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Silver R. Orchestrating time: Arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Atkins N, Jr, Mitchell JW, Romanova EV, Morgan DJ, Cominski TP, Ecker JL, Pintar JE, Sweedler JV, Gillette MU. Circadian integration of glutamatergic signals by little SAAS in novel suprachiasmatic circuits. PLoS One. 2010;5:e12612. doi: 10.1371/journal.pone.0012612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Herzog ED. Come together, right…now: Synchronization of rhythms in a mammalian circadian clock. Neuron. 2005;48:531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Huettner JE, Straume M, Herzog ED. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci U S A. 2006;103:19188–19193. doi: 10.1073/pnas.0607466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold DA, Brown TM, Luckman SM, Piggins HD. Metabolic rhythm abnormalities in mice lacking VIP-VPAC2 signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R344–351. doi: 10.1152/ajpregu.00667.2007. [DOI] [PubMed] [Google Scholar]
- Becquet D, Girardet C, Guillaumond F, Francois-Bellan AM, Bosler O. Ultrastructural plasticity in the rat suprachiasmatic nucleus. Possible involvement in clock entrainment. Glia. 2008;56:294–305. doi: 10.1002/glia.20613. [DOI] [PubMed] [Google Scholar]
- Bedont JL, Blackshaw S. Constructing the suprachiasmatic nucleus: A watchmaker’s perspective on the central clockworks. Front Syst Neurosci. 2015;9:74. doi: 10.3389/fnsys.2015.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedont JL, LeGates TA, Slat EA, Byerly MS, Wang H, Hu J, Rupp AC, Qian J, Wong GW, Herzog ED, et al. Lhx1 controls terminal differentiation and circadian function of the suprachiasmatic nucleus. Cell Rep. 2014;7:609–622. doi: 10.1016/j.celrep.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Sagiv N, Fritschy JM, Yarom Y. Presynaptic and postsynaptic GABAA receptors in rat suprachiasmatic nucleus. Neuroscience. 2003;118:909–923. doi: 10.1016/s0306-4522(03)00062-9. [DOI] [PubMed] [Google Scholar]
- Belenky MA, Sollars PJ, Mount DB, Alper SL, Yarom Y, Pickard GE. Cell-type specific distribution of chloride transporters in the rat suprachiasmatic nucleus. Neuroscience. 2010;165:1519–1537. doi: 10.1016/j.neuroscience.2009.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of gamma-aminobutyric acid and gamma-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. J Comp Neurol. 2008;506:708–732. doi: 10.1002/cne.21553. [DOI] [PubMed] [Google Scholar]
- Belle MD, Diekman CO, Forger DB, Piggins HD. Daily electrical silencing in the mammalian circadian clock. Science. 2009;326:281–284. doi: 10.1126/science.1169657. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Saez JC. Gap junctions: New tools, new answers, new questions. Neuron. 1991;6:305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Biello SM. Circadian clock resetting in the mouse changes with age. Age (Dordr) 2009;31:293–303. doi: 10.1007/s11357-009-9102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs KR, Prosser RA. GABAB receptor stimulation phase-shifts the mammalian circadian clock in vitro. Brain Res. 1998;807:250–254. doi: 10.1016/s0006-8993(98)00820-8. [DOI] [PubMed] [Google Scholar]
- Boer GJ, van Esseveldt LE, Rietveld WJ. Cellular requirements of suprachiasmatic nucleus transplants for restoration of circadian rhythm. Chronobiol Int. 1998;15:551–566. doi: 10.3109/07420529808998707. [DOI] [PubMed] [Google Scholar]
- Bormann J. The ‘ABC’ of GABA receptors. Trends Pharmacol Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- Bos NP, Mirmiran M. Effects of excitatory and inhibitory amino acids on neuronal discharges in the cultured suprachiasmatic nucleus. Brain Res Bull. 1993;31:67–72. doi: 10.1016/0361-9230(93)90012-z. [DOI] [PubMed] [Google Scholar]
- Bouskila Y, Dudek FE. Neuronal synchronization without calcium-dependent synaptic transmission in the hypothalamus. Proc Natl Acad Sci U S A. 1993;90:3207–3210. doi: 10.1073/pnas.90.8.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaccio M, Maywood ES, Chesham JE, Loudon AS, Hastings MH. A Gq-Ca2+ axis controls circuit-level encoding of circadian time in the suprachiasmatic nucleus. Neuron. 2013;78:714–728. doi: 10.1016/j.neuron.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MH, Nunez AA. Vasopressin-deficient rats show a reduced amplitude of the circadian sleep rhythm. Physiol Behav. 1989;46:759–762. doi: 10.1016/0031-9384(89)90364-8. [DOI] [PubMed] [Google Scholar]
- Brown TM, Colwell CS, Waschek JA, Piggins HD. Disrupted neuronal activity rhythms in the suprachiasmatic nuclei of vasoactive intestinal polypeptide-deficient mice. J Neurophysiol. 2007;97:2553–2558. doi: 10.1152/jn.01206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Hughes AT, Piggins HD. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling. J Neurosci. 2005;25:11155–11164. doi: 10.1523/JNEUROSCI.3821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Piggins HD. Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol. 2007;82:229–255. doi: 10.1016/j.pneurobio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Brown TM, Piggins HD. Spatiotemporal heterogeneity in the electrical activity of suprachiasmatic nuclei neurons and their response to photoperiod. J Biol Rhythms. 2009;24:44–54. doi: 10.1177/0748730408327918. [DOI] [PubMed] [Google Scholar]
- Brown TM, Wynne J, Piggins HD, Lucas RJ. Multiple hypothalamic cell populations encoding distinct visual information. J Physiol. 2011;589:1173–1194. doi: 10.1113/jphysiol.2010.199877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Takahashi JS. Molecular components of the mammalian circadian clock. Handb Exp Pharmacol. 2013:3–27. doi: 10.1007/978-3-642-25950-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkeen JF, Womac AD, Earnest DJ, Zoran MJ. Mitochondrial calcium signaling mediates rhythmic extracellular ATP accumulation in suprachiasmatic nucleus astrocytes. J Neurosci. 2011;31:8432–8440. doi: 10.1523/JNEUROSCI.6576-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Rainbow MN, Rodriguez E, Lyon SM, Silver R. Twelve-hour days in the brain and behavior of split hamsters. Eur J Neurosci. 2012;36:2556–2566. doi: 10.1111/j.1460-9568.2012.08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagampang FRA, Sheward WJ, Harmar AJ, Piggins HD, Coen CW. Circadian changes in the expression of vasoactive intestinal peptide 2 receptor mRNA in the rat suprachiasmatic nuclei. Molecular Brain Research. 1998;54:108–112. doi: 10.1016/s0169-328x(97)00327-6. [DOI] [PubMed] [Google Scholar]
- Callaway EM, Luo L. Monosynaptic Circuit Tracing with Glycoprotein-Deleted Rabies Viruses. J Neurosci. 2015;35:8979–8985. doi: 10.1523/JNEUROSCI.0409-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal MM, Mohammed NM, Rodriguez JJ. Early programming of astrocyte organization in the mouse suprachiasmatic nuclei by light. Chronobiol Int. 2009;26:1545–1558. doi: 10.3109/07420520903398542. [DOI] [PubMed] [Google Scholar]
- Cao R, Robinson B, Xu H, Gkogkas C, Khoutorsky A, Alain T, Yanagiya A, Nevarko T, Liu AC, Amir S, et al. Translational control of entrainment and synchrony of the suprachiasmatic circadian clock by mTOR/4E-BP1 signaling. Neuron. 2013;79:712–724. doi: 10.1016/j.neuron.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Brecha N, Karten HJ, Moore RY. Immunocytochemical localization of vasoactive intestinal polypeptide-containing cells and processes in the suprachiasmatic nucleus of the rat: Light and electron microscopic analysis. J Neurosci. 1981;1:1289–1303. doi: 10.1523/JNEUROSCI.01-11-01289.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Moore RY. The suprachiasmatic nucleus of the golden hamster: Immunohistochemical analysis of cell and fiber distribution. Neuroscience. 1984;13:415–431. doi: 10.1016/0306-4522(84)90240-9. [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Golombek DA. The rhythmic GABAergic system. Neurochem Res. 1998;23:607–614. doi: 10.1023/a:1022426519297. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Speh JC, Card JP, Moore RY. Comparative anatomy of the mammalian hypothalamic suprachiasmatic nucleus. J Biol Rhythms. 1988;3:71–91. doi: 10.1177/074873048800300106. [DOI] [PubMed] [Google Scholar]
- Castel M, Feinstein N, Cohen S, Harari N. Vasopressinergic innervation of the mouse suprachiasmatic nucleus: An immuno-electron microscopic analysis. J Comp Neurol. 1990;298:172–187. doi: 10.1002/cne.902980204. [DOI] [PubMed] [Google Scholar]
- Castel M, Morris J, Belenky M. Non-synaptic and dendritic exocytosis from dense-cored vesicles in the suprachiasmatic nucleus. Neuroreport. 1996;7:543–547. doi: 10.1097/00001756-199601310-00040. [DOI] [PubMed] [Google Scholar]
- Castel M, Morris JF. Morphological heterogeneity of the GABAergic network in the suprachiasmatic nucleus, the brain’s circadian pacemaker. J Anat. 2000;196(Pt 1):1–13. doi: 10.1046/j.1469-7580.2000.19610001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HY, Alvarez-Saavedra M, Dziema H, Choi YS, Li A, Obrietan K. Segregation of expression of mPeriod gene homologs in neurons and glia: Possible divergent roles of mPeriod1 and mPeriod2 in the brain. Hum Mol Genet. 2009;18:3110–3124. doi: 10.1093/hmg/ddp252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Lee CJ, Schroeder A, Kim YS, Jung SH, Kim JS, do Kim Y, Son EJ, Han HC, Hong SK, et al. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci. 2008;28:5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio CM, Gamble KL, Axley JC, Strauss BR, Cohen JY, Colwell CS, McMahon DG. Population encoding by circadian clock neurons organizes circadian behavior. J Neurosci. 2009;29:1670–1676. doi: 10.1523/JNEUROSCI.3801-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. Rhythmic coupling among cells in the suprachiasmatic nucleus. J Neurobiol. 2000;43:379–388. doi: 10.1002/1097-4695(20000615)43:4<379::aid-neu6>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Couvineau A, Laburthe M. VPAC receptors: Structure, molecular pharmacology and interaction with accessory proteins. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, Marston HM, Harmar AJ, Piggins HD. The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. Eur J Neurosci. 2003;17:197–204. doi: 10.1046/j.1460-9568.2003.02425.x. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–130. discussion 130–132. [PubMed] [Google Scholar]
- Dardente H, Menet JS, Challet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res. 2004;124:143–151. doi: 10.1016/j.molbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Dardente H, Poirel VJ, Klosen P, Pevet P, Masson-Pevet M. Per and neuropeptide expression in the rat suprachiasmatic nuclei: Compartmentalization and differential cellular induction by light. Brain Res. 2002;958:261–271. doi: 10.1016/s0006-8993(02)03563-1. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Castanon-Cervantes O, Leise TL, Molyneux PC, Harrington ME. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur J Neurosci. 2009;29:171–180. doi: 10.1111/j.1460-9568.2008.06534.x. [DOI] [PubMed] [Google Scholar]
- De Jeu M, Pennartz C. Circadian modulation of GABA function in the rat suprachiasmatic nucleus: Excitatory effects during the night phase. J Neurophysiol. 2002;87:834–844. doi: 10.1152/jn.00241.2001. [DOI] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN. GABA: A dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- DeWoskin D, Myung J, Belle MD, Piggins HD, Takumi T, Forger DB. Distinct roles for GABA across multiple timescales in mammalian circadian timekeeping. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1420753112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragich JM, Loh DH, Wang LM, Vosko AM, Kudo T, Nakamura TJ, Odom IH, Tateyama S, Hagopian A, Waschek JA, et al. The role of the neuropeptides PACAP and VIP in the photic regulation of gene expression in the suprachiasmatic nucleus. Eur J Neurosci. 2010;31:864–875. doi: 10.1111/j.1460-9568.2010.07119.x. [DOI] [PubMed] [Google Scholar]
- Drouyer E, LeSauter J, Hernandez AL, Silver R. Specializations of gastrin-releasing peptide cells of the mouse suprachiasmatic nucleus. J Comp Neurol. 2010;518:1249–1263. doi: 10.1002/cne.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE, Kim YI, Bouskila Y. Electrophysiology of the suprachiasmatic nucleus: Synaptic transmission, membrane properties, and neuronal synchronization. J Biol Rhythms. 1993;8(Suppl):S33–37. [PubMed] [Google Scholar]
- Duhart JM, Leone MJ, Paladino N, Evans JA, Castanon-Cervantes O, Davidson AJ, Golombek DA. Suprachiasmatic astrocytes modulate the circadian clock in response to TNF-alpha. J Immunol. 2013;191:4656–4664. doi: 10.4049/jimmunol.1300450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Cheng X, Heller KS. Photoperiodic exposure and time of day modulate the expression of arginine vasopressin mRNA and vasoactive intestinal peptide mRNA in the suprachiasmatic nuclei of Siberian hamsters. Brain Res Mol Brain Res. 1995;32:181–186. doi: 10.1016/0169-328x(95)00072-z. [DOI] [PubMed] [Google Scholar]
- Earnest DJ, Digiorgio SM, Sladek CD. Effects of tetrodotoxin on the circadian pacemaker mechanism in suprachiasmatic explants in vitro. Brain Res Bull. 1991;26:677–682. doi: 10.1016/0361-9230(91)90160-l. [DOI] [PubMed] [Google Scholar]
- Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: Action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology. 2004;145:3386–3394. doi: 10.1210/en.2003-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlen JC, Novak CM, Karom MC, Gamble KL, Albers HE. Interactions of GABAA receptor activation and light on Period mRNA expression in the suprachiasmatic nucleus. J Biol Rhythms. 2008;23:16–25. doi: 10.1177/0748730407310785. [DOI] [PubMed] [Google Scholar]
- Ehlen JC, Paul KN. Regulation of light’s action in the mammalian circadian clock: Role of the extrasynaptic GABAA receptor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1606–1612. doi: 10.1152/ajpregu.90878.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki R, Kuroda S, Ono D, Hasan MT, Ueda T, Honma S, Honma K. Topological specificity and hierarchical network of the circadian calcium rhythm in the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 2012;109:21498–21503. doi: 10.1073/pnas.1214415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Gorman MR. In synch but not in step: Circadian clock circuits regulating plasticity in daily rhythms. Neuroscience. doi: 10.1016/j.neuroscience.2016.01.072. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Leise TL, Castanon-Cervantes O, Davidson AJ. Intrinsic regulation of spatiotemporal organization within the suprachiasmatic nucleus. PLoS One. 2011;6:e15869. doi: 10.1371/journal.pone.0015869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Leise TL, Castanon-Cervantes O, Davidson AJ. Dynamic interactions mediated by nonredundant signaling mechanisms couple circadian clock neurons. Neuron. 2013;80:973–983. doi: 10.1016/j.neuron.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Pan H, Liu AC, Welsh DK. Cry1−/− circadian rhythmicity depends on SCN intercellular coupling. J Biol Rhythms. 2012;27:443–452. doi: 10.1177/0748730412461246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Suen TC, Callif BL, Mitchell AS, Castanon-Cervantes O, Baker KM, Kloehn I, Baba K, Teubner BJ, Ehlen JC, et al. Shell neurons of the master circadian clock coordinate the phase of tissue clocks throughout the brain and body. BMC Biol. 2015;13:43. doi: 10.1186/s12915-015-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J, Georg B, Hannibal J, Jorgensen HL. Altered rhythm of adrenal clock genes, StAR and serum corticosterone in VIP receptor 2-deficient mice. J Mol Neurosci. 2012;48:584–596. doi: 10.1007/s12031-012-9804-7. [DOI] [PubMed] [Google Scholar]
- Faissner A, Pyka M, Geissler M, Sobik T, Frischknecht R, Gundelfinger ED, Seidenbecher C. Contributions of astrocytes to synapse formation and maturation - Potential functions of the perisynaptic extracellular matrix. Brain Res Rev. 2010;63:26–38. doi: 10.1016/j.brainresrev.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Fan J, Zeng H, Olson DP, Huber KM, Gibson JR, Takahashi JS. Vasoactive intestinal polypeptide (VIP)-expressing neurons in the suprachiasmatic nucleus provide sparse GABAergic outputs to local neurons with circadian regulation occurring distal to the opening of postsynaptic GABAA ionotropic receptors. J Neurosci. 2015;35:1905–1920. doi: 10.1523/JNEUROSCI.2661-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, van Westering TL, Meijer JH, Michel S. Seasonal induction of GABAergic excitation in the central mammalian clock. Proc Natl Acad Sci U S A. 2014;111:9627–9632. doi: 10.1073/pnas.1319820111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnell YF, Shende VR, Neuendorff N, Allen GC, Earnest DJ. Immortalized cell lines for real-time analysis of circadian pacemaker and peripheral oscillator properties. Eur J Neurosci. 2011;33:1533–1540. doi: 10.1111/j.1460-9568.2011.07629.x. [DOI] [PubMed] [Google Scholar]
- Francl JM, Kaur G, Glass JD. Regulation of vasoactive intestinal polypeptide release in the suprachiasmatic nucleus circadian clock. Neuroreport. 2010;21:1055–1059. doi: 10.1097/WNR.0b013e32833fcba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GM, Jr, Krock RM, Aton SJ, Thaben P, Herzog ED. GABA networks destabilize genetic oscillations in the circadian pacemaker. Neuron. 2013;78:799–806. doi: 10.1016/j.neuron.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble KL, Allen GC, Zhou T, McMahon DG. Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J Neurosci. 2007;27:12078–12087. doi: 10.1523/JNEUROSCI.1109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble KL, Kudo T, Colwell CS, McMahon DG. Gastrin-releasing peptide modulates fast delayed rectifier potassium current in Per1-expressing SCN neurons. J Biol Rhythms. 2011;26:99–106. doi: 10.1177/0748730410396678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Fritschy JM, Moore RY. GABAA-receptor subunit composition in the circadian timing system. Brain Res. 1995;700:142–156. doi: 10.1016/0006-8993(95)00944-l. [DOI] [PubMed] [Google Scholar]
- Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: Comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–2843. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- Geoghegan D, Carter DA. A novel site of adult doublecortin expression: Neuropeptide neurons within the suprachiasmatic nucleus circadian clock. BMC Neurosci. 2008;9:2. doi: 10.1186/1471-2202-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhold LM, Horvath TL, Freeman ME. Vasoactive intestinal peptide fibers innervate neuroendocrine dopaminergic neurons. Brain Res. 2001;919:48–56. doi: 10.1016/s0006-8993(01)02993-6. [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Wise PM. Vasoactive intestinal polypeptide regulates dynamic changes in astrocyte morphometry: Impact on gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:2197–2202. doi: 10.1210/en.2005-1262. [DOI] [PubMed] [Google Scholar]
- Gerics B, Szalay F, Hajos F. Glial fibrillary acidic protein immunoreactivity in the rat suprachiasmatic nucleus: Circadian changes and their seasonal dependence. J Anat. 2006;209:231–237. doi: 10.1111/j.1469-7580.2006.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Mintz EM, Marvel CL, Huhman KL, Albers HE. GABA(A) and GABA(B) agonists and antagonists alter the phase-shifting effects of light when microinjected into the suprachiasmatic region. Brain Research. 1997;759:181–189. doi: 10.1016/s0006-8993(97)00235-7. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Van Der Beek EM, Mintz EM, Mickley NC, Jasnow AM, Huhman KL, Albers HE. GABAergic regulation of light-induced c-Fos immunoreactivity within the suprachiasmatic nucleus. J Comp Neurol. 1999;411:683–692. [PubMed] [Google Scholar]
- Girardet C, Becquet D, Blanchard MP, Francois-Bellan AM, Bosler O. Neuroglial and synaptic rearrangements associated with photic entrainment of the circadian clock in the suprachiasmatic nucleus. Eur J Neurosci. 2010;32:2133–2142. doi: 10.1111/j.1460-9568.2010.07520.x. [DOI] [PubMed] [Google Scholar]
- Glass JD, Watanabe M, Fedorkova L, Shen H, Ungers G, Rutishauser U. Dynamic regulation of polysialylated neural cell adhesion molecule in the suprachiasmatic nucleus. Neuroscience. 2003;117:203–211. doi: 10.1016/s0306-4522(02)00817-5. [DOI] [PubMed] [Google Scholar]
- Glazer R, Gozes I. Diurnal oscillation in vasoactive intestinal peptide gene expression independent of environmental light entraining. Brain Res. 1994;644:164–167. doi: 10.1016/0006-8993(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Grahn DA, Miller JD, Houng VS, Heller HC. Persistence of circadian rhythmicity in hibernating ground squirrels. Am J Physiol. 1994;266:R1251–1258. doi: 10.1152/ajpregu.1994.266.4.R1251. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, Park WK, Strecker GJ, de Jeu MT, Pennartz CM, Dudek FE. A reexamination of the role of GABA in the mammalian suprachiasmatic nucleus. J Biol Rhythms. 1999;14:126–130. doi: 10.1177/074873099129000515. [DOI] [PubMed] [Google Scholar]
- Groblewski TA, Nunez AA, Gold RM. Circadian rhythms in vasopressin deficient rats. Brain Res Bull. 1981;6:125–130. doi: 10.1016/s0361-9230(81)80036-6. [DOI] [PubMed] [Google Scholar]
- Guldner FH. Numbers of neurons and astroglial cells in the suprachiasmatic nucleus of male and female rats. Exp Brain Res. 1983;50:373–376. doi: 10.1007/BF00239203. [DOI] [PubMed] [Google Scholar]
- Haas JS, Zavala B, Landisman CE. Activity-dependent long-term depression of electrical synapses. Science. 2011;334:389–393. doi: 10.1126/science.1207502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, Antle MC, Silver R. Temporal and spatial expression patterns of canonical clock genes and clock-controlled genes in the suprachiasmatic nucleus. Eur J Neurosci. 2004;19:1741–1748. doi: 10.1111/j.1460-9568.2004.03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Venuti JM, Silver R. Expression of Period genes: Rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci. 2001;21:7742–7750. doi: 10.1523/JNEUROSCI.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Yu FH, Schwartz MD, Linton JD, Bosma MM, Hurley JB, Catterall WA, de la Iglesia HO. Na(V)1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms. Proc Natl Acad Sci U S A. 2012;109:E368–377. doi: 10.1073/pnas.1115729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Hsiung HM, Fahrenkrug J. Temporal phasing of locomotor activity, heart rate rhythmicity, and core body temperature is disrupted in VIP receptor 2-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2011;300:R519–530. doi: 10.1152/ajpregu.00599.2010. [DOI] [PubMed] [Google Scholar]
- Harmar AJ. An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei. J Neuroendocrinol. 2003;15:335–338. doi: 10.1046/j.1365-2826.2003.01005.x. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, et al. The VPAC (2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Hazlerigg DG, Ebling FJ, Johnston JD. Photoperiod differentially regulates gene expression rhythms in the rostral and caudal SCN. Curr Biol. 2005;15:R449–450. doi: 10.1016/j.cub.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: A reliable clock from less reliable neurons. J Biol Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- Honma S, Shirakawa T, Katsuno Y, Namihira M, Honma K. Circadian periods of single suprachiasmatic neurons in rats. Neurosci Lett. 1998;250:157–160. doi: 10.1016/s0304-3940(98)00464-9. [DOI] [PubMed] [Google Scholar]
- Hughes AT, Fahey B, Cutler DJ, Coogan AN, Piggins HD. Aberrant gating of photic input to the suprachiasmatic circadian pacemaker of mice lacking the VPAC2 receptor. J Neurosci. 2004;24:3522–3526. doi: 10.1523/JNEUROSCI.5345-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AT, Guilding C, Lennox L, Samuels RE, McMahon DG, Piggins HD. Live imaging of altered Period1 expression in the suprachiasmatic nuclei of Vipr2−/− mice. J Neurochem. 2008;106:1646–1657. doi: 10.1111/j.1471-4159.2008.05520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Hennessey AC, Albers HE. Rhythms of glutamic acid decarboxylase mRNA in the suprachiasmatic nucleus. J Biol Rhythms. 1996;11:311–316. doi: 10.1177/074873049601100404. [DOI] [PubMed] [Google Scholar]
- Hundahl CA, Fahrenkrug J, Hay-Schmidt A, Georg B, Faltoft B, Hannibal J. Circadian behaviour in neuroglobin deficient mice. PLoS One. 2012;7:e34462. doi: 10.1371/journal.pone.0034462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Kumagai H, Skach A, Sato M, Yanagisawa M. Modulation of circadian glucocorticoid oscillation via adrenal opioid-CXCR7 signaling alters emotional behavior. Cell. 2013;155:1323–1336. doi: 10.1016/j.cell.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Honma S, Ono D, Tanahashi Y, Honma K. Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc Natl Acad Sci U S A. 2007;104:7664–7669. doi: 10.1073/pnas.0607713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram CD, Ciobanu R, Coculescu IL, Tanasescu R, Coculescu M, Mihai R. Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus. Prog Brain Res. 1998;119:351–364. doi: 10.1016/s0079-6123(08)61580-0. [DOI] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network. Eur J Neurosci. 2009;30:1462–1475. doi: 10.1111/j.1460-9568.2009.06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. Neuropeptide-mediated calcium signaling in the suprachiasmatic nucleus network. Eur J Neurosci. 2010;32:1497–1506. doi: 10.1111/j.1460-9568.2010.07411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. Simultaneous electrophysiological recording and calcium imaging of suprachiasmatic nucleus neurons. J Vis Exp. 2013:50794. doi: 10.3791/50794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y, Nishino H. AVP rhythm in the suprachiasmatic nucleus in relation to locomotor activity under constant light. Peptides. 1998;19:827–832. doi: 10.1016/s0196-9781(98)00021-7. [DOI] [PubMed] [Google Scholar]
- Itri J, Colwell CS. Regulation of inhibitory synaptic transmission by vasoactive intestinal peptide (VIP) in the mouse suprachiasmatic nucleus. J Neurophysiol. 2003;90:1589–1597. doi: 10.1152/jn.00332.2003. [DOI] [PubMed] [Google Scholar]
- Itri J, Michel S, Waschek JA, Colwell CS. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J Neurophysiol. 2004;92:311–319. doi: 10.1152/jn.01078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson FR. Glial cell modulation of circadian rhythms. Glia. 2011;59:1341–1350. doi: 10.1002/glia.21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagota A, de la Iglesia HO, Schwartz WJ. Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nat Neurosci. 2000;3:372–376. doi: 10.1038/73943. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z-G, Yang Y-Q, Allen CN. Tracer and electrical coupling of rat suprachiasmatic nucleus neurons. Neuroscience. 1997a;77:1059–1066. doi: 10.1016/s0306-4522(96)00539-8. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Yang Y, Liu ZP, Allen CN. Membrane properties and synaptic inputs of suprachiasmatic nucleus neurons in rat brain slices. J Physiol. 1997b;499(Pt 1):141–159. doi: 10.1113/jphysiol.1997.sp021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao YY, Lee TM, Rusak B. Photic responses of suprachiasmatic area neurons in diurnal degus (Octodon degus) and nocturnal rats (Rattus norvegicus) Brain Res. 1999;817:93–103. doi: 10.1016/s0006-8993(98)01218-9. [DOI] [PubMed] [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- Jobst EE, Allen CN. Calbindin neurons in the hamster suprachiasmatic nucleus do not exhibit a circadian variation in spontaneous firing rate. Eur J Neurosci. 2002;16:2469–2474. doi: 10.1046/j.1460-9568.2002.02309.x. [DOI] [PubMed] [Google Scholar]
- Jobst EE, Robinson DW, Allen CN. Potential pathways for intercellular communication within the calbindin subnucleus of the hamster suprachiasmatic nucleus. Neuroscience. 2004;123:87–99. doi: 10.1016/j.neuroscience.2003.08.059. [DOI] [PubMed] [Google Scholar]
- Kalamatianos T, Kallo I, Coen CW. Ageing and the diurnal expression of the mRNAs for vasopressin and for the V1a and V1b vasopressin receptors in the suprachiasmatic nucleus of male rats. J Neuroendocrinol. 2004a;16:493–501. doi: 10.1111/j.1365-2826.2004.01196.x. [DOI] [PubMed] [Google Scholar]
- Kalamatianos T, Kallo I, Piggins HD, Coen CW. Expression of VIP and/or PACAP receptor mRNA in peptide synthesizing cells within the suprachiasmatic nucleus of the rat and in its efferent target sites. J Comp Neurol. 2004b;475:19–35. doi: 10.1002/cne.20168. [DOI] [PubMed] [Google Scholar]
- Kallo I, Kalamatianos T, Piggins HD, Coen CW. Ageing and the diurnal expression of mRNAs for vasoactive intestinal peptide and for the VPAC2 and PAC1 receptors in the suprachiasmatic nucleus of male rats. J Neuroendocrinol. 2004a;16:758–766. doi: 10.1111/j.1365-2826.2004.01232.x. [DOI] [PubMed] [Google Scholar]
- Kallo I, Kalamatianos T, Wiltshire N, Shen S, Sheward WJ, Harmar AJ, Coen CW. Transgenic approach reveals expression of the VPAC2 receptor in phenotypically defined neurons in the mouse suprachiasmatic nucleus and in its efferent target sites. Eur J Neurosci. 2004b;19:2201–2211. doi: 10.1111/j.0953-816X.2004.03335.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: Coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Hofman MA, Swaab DF, Buijs RM. Vasopressin and the output of the hypothalamic biological clock. J Neuroendocrinol. 2010;22:362–372. doi: 10.1111/j.1365-2826.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Perreau-Lenz S, Buijs RM. A network of (autonomic) clock outputs. Chronobiol Int. 2006;23:521–535. doi: 10.1080/07420520600651073. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Teclemariam-Mesbah R, Pevet P. Efferent projections of the suprachiasmatic nucleus in the golden hamster (Mesocricetus auratus) J Comp Neurol. 1993;332:293–314. doi: 10.1002/cne.903320304. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Romeo RD, McEwen BS, Silver R. Diurnal regulation of the gastrin-releasing peptide receptor in the mouse circadian clock. European Journal of Neuroscience. 2006;23:1047–1053. doi: 10.1111/j.1460-9568.2006.04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami F, Okamura H, Tamada Y, Maebayashi Y, Fukui K, Ibata Y. Loss of day-night differences in VIP mRNA levels in the suprachiasmatic nucleus of aged rats. Neurosci Lett. 1997;222:99–102. doi: 10.1016/s0304-3940(97)13355-9. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Nagano M, Kanda F, Chihara K, Shigeyoshi Y, Okamura H. Two types of VIP neuronal components in rat suprachiasmatic nucleus. J Neurosci Res. 2003;74:852–857. doi: 10.1002/jnr.10751. [DOI] [PubMed] [Google Scholar]
- Kim YI, Dudek FE. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: Inhibitory synaptic mechanisms. J Physiol. 1992;458:247–260. doi: 10.1113/jphysiol.1992.sp019416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King VM, Chahad-Ehlers S, Shen S, Harmar AJ, Maywood ES, Hastings MH. A hVIPR transgene as a novel tool for the analysis of circadian function in the mouse suprachiasmatic nucleus. Eur J Neurosci. 2003;17:822–832. [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind’s Clock. New York: University Oxford Press; 1991. [Google Scholar]
- Ko CH, Yamada YR, Welsh DK, Buhr ED, Liu AC, Zhang EE, Ralph MR, Kay SA, Forger DB, Takahashi JS. Emergence of noise-induced oscillations in the central circadian pacemaker. PLoS Biol. 2010;8:e1000513. doi: 10.1371/journal.pbio.1000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutkia P, Schurgin S, Berry J, Breu J, Lee BS, Klibanski A, Grinspoon S. Reciprocal changes in endogenous ghrelin and growth hormone during fasting in healthy women. Am J Physiol Endocrinol Metab. 2005;289:E814–822. doi: 10.1152/ajpendo.00093.2005. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998a;18:4767–4774. doi: 10.1523/JNEUROSCI.18-12-04767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Sex differences in the daily rhythm of vasoactive intestinal polypeptide but not arginine vasopressin messenger ribonucleic acid in the suprachiasmatic nuclei. Endocrinology. 1998b;139:4189–4196. doi: 10.1210/endo.139.10.6259. [DOI] [PubMed] [Google Scholar]
- Kudo T, Tahara Y, Gamble KL, McMahon DG, Block GD, Colwell CS. Vasoactive intestinal peptide produces long-lasting changes in neural activity in the suprachiasmatic nucleus. J Neurophysiol. 2013;110:1097–1106. doi: 10.1152/jn.00114.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ. Biological Rhythms Workshop IB: Neurophysiology of SCN pacemaker function. Cold Spring Harb Symp Quant Biol. 2007;72:21–33. doi: 10.1101/sqb.2007.72.061. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Quintero JE, McMahon DG. GFP fluorescence reports Period 1 circadian gene regulation in the mammalian biological clock. Neuroreport. 2000;11:1479–1482. [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci. 2003;23:1441–1450. doi: 10.1523/JNEUROSCI.23-04-01441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavaille M, Serviere J. Developmental study in the circadian clock of the golden hamster: A putative role of astrocytes. Developmental Brain Research. 1995;86:275–282. doi: 10.1016/0165-3806(95)00039-g. [DOI] [PubMed] [Google Scholar]
- Lavialle M, Begue A, Papillon C, Vilaplana J. Modifications of retinal afferent activity induce changes in astroglial plasticity in the hamster circadian clock. Glia. 2001;34:88–100. doi: 10.1002/glia.1044. [DOI] [PubMed] [Google Scholar]
- Lavialle M, Serviere J. Circadian fluctuations in GFAP distribution in the Syrian hamster suprachiasmatic nucleus. Neuroreport. 1993;4:1243–1246. doi: 10.1097/00001756-199309000-00008. [DOI] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol. 2001;433:312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- Lee HS, Nelms JL, Nguyen M, Silver R, Lehman MN. The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nat Neurosci. 2003;6:111–112. doi: 10.1038/nn1006. [DOI] [PubMed] [Google Scholar]
- Lee IT, Chang AS, Manandhar M, Shan Y, Fan J, Izumo M, Ikeda Y, Motoike T, Dixon S, Seinfeld JE, et al. Neuromedin s-producing neurons act as essential pacemakers in the suprachiasmatic nucleus to couple clock neurons and dictate circadian rhythms. Neuron. 2015;85:1086–1102. doi: 10.1016/j.neuron.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Zamdborg L, Southey BR, Atkins N, Jr, Mitchell JW, Li M, Gillette MU, Kelleher NL, Sweedler JV. Quantitative peptidomics for discovery of circadian-related peptides from the rat suprachiasmatic nucleus. J Proteome Res. 2013;12:585–593. doi: 10.1021/pr300605p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ML, Swanson BE, de la Iglesia HO. Circadian timing of REM sleep is coupled to an oscillator within the dorsomedial suprachiasmatic nucleus. Curr Biol. 2009;19:848–852. doi: 10.1016/j.cub.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Watanabe M, Glass JD. Photoperiod affects the expression of neural cell adhesion molecule and polysialic acid in the hypothalamus of the Siberian hamster. Brain Res. 1995;690:64–72. doi: 10.1016/0006-8993(95)00588-h. [DOI] [PubMed] [Google Scholar]
- Leise TL, Wang CW, Gitis PJ, Welsh DK. Persistent cell-autonomous circadian oscillations in fibroblasts revealed by six-week single-cell imaging of PER2::LUC bioluminescence. PLoS One. 2012;7:e33334. doi: 10.1371/journal.pone.0033334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesauter J, Silver R, Cloues R, Witkovsky P. Light exposure induces short- and long-term changes in the excitability of retinorecipient neurons in suprachiasmatic nucleus. J Neurophysiol. 2011;106:576–588. doi: 10.1152/jn.00060.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JD, Burton KJ, Zhang C, Hu SB, Zhou QY. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am J Physiol Regul Integr Comp Physiol. 2009;296:R824–830. doi: 10.1152/ajpregu.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley J, Deurveilher S, Rusak B, Semba K. Transforming growth factor-alpha and glial fibrillary acidic protein in the hamster circadian system: Daily profile and cellular localization. Brain Res. 2008;1197:94–105. doi: 10.1016/j.brainres.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Liou SY, Albers HE. Single unit response of suprachiasmatic neurons to arginine vasopressin (AVP) is mediated by a V1-like receptor in the hamster. Brain Res. 1989;477:336–343. doi: 10.1016/0006-8993(89)91424-8. [DOI] [PubMed] [Google Scholar]
- Liou SY, Shibata S, Albers HE, Ueki S. Effects of GABA and anxiolytics on the single unit discharge of suprachiasmatic neurons in rat hypothalamic slices. Brain Res Bull. 1990;25:103–107. doi: 10.1016/0361-9230(90)90259-3. [DOI] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron. 2000;25:123–128. doi: 10.1016/s0896-6273(00)80876-4. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: Period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Locke JC, Westermark PO, Kramer A, Herzel H. Global parameter search reveals design principles of the mammalian circadian clock. BMC Syst Biol. 2008;2:22. doi: 10.1186/1752-0509-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh DH, Dragich JM, Kudo T, Schroeder AM, Nakamura TJ, Waschek JA, Block GD, Colwell CS. Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs. J Biol Rhythms. 2011;26:200–209. doi: 10.1177/0748730411401740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh DH, Kuljis DA, Azuma L, Wu Y, Truong D, Wang HB, Colwell CS. Disrupted reproduction, estrous cycle, and circadian rhythms in female mice deficient in vasoactive intestinal peptide. J Biol Rhythms. 2014;29:355–369. doi: 10.1177/0748730414549767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokshin M, LeSauter J, Silver R. Selective distribution of retinal input to mouse SCN revealed in analysis of sagittal sections. J Biol Rhythms. 2015;30:251–257. doi: 10.1177/0748730415584058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Jutras MJ, Connors BW, Burwell RD. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci. 2005;8:61–66. doi: 10.1038/nn1361. [DOI] [PubMed] [Google Scholar]
- Low-Zeddies SS, Takahashi JS. Chimera analysis of the Clock mutation in mice shows that complex cellular integration determines circadian behavior. Cell. 2001;105:25–42. doi: 10.1016/s0092-8674(01)00294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen EA, van Diepen HC, Houben T, Michel S, Colwell CS, Meijer JH. Role of vasoactive intestinal peptide in seasonal encoding by the suprachiasmatic nucleus clock. Eur J Neurosci. 2012;35:1466–1474. doi: 10.1111/j.1460-9568.2012.08054.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Mackey SR. Biological Rhythms Workshop IA: Molecular basis of rhythms generation. Cold Spring Harb Symp Quant Biol. 2007;72:7–19. doi: 10.1101/sqb.2007.72.060. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, Ramanathan C, Hagenauer MH, Thompson RC, Smale L, Lee T. Daily rhythms and sex differences in vasoactive intestinal polypeptide, VIPR2 receptor and arginine vasopressin mRNA in the suprachiasmatic nucleus of a diurnal rodent. Arvicanthis niloticus Eur J Neurosci. 2009;30:1537–1543. doi: 10.1111/j.1460-9568.2009.06936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Krall TJ, Herzog ED. Vasoactive intestinal polypeptide entrains circadian rhythms in astrocytes. J Biol Rhythms. 2009;24:135–143. doi: 10.1177/0748730409332042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A, Llano I. Excitatory effects of GABA in established brain networks. Trends Neurosci. 2005;28:284–289. doi: 10.1016/j.tins.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Mason R, Biello SM, Harrington ME. The effects of GABA and benzodiazepines on neurones in the suprachiasmatic nucleus (SCN) of Syrian hamsters. Brain Res. 1991;552:53–57. doi: 10.1016/0006-8993(91)90659-j. [DOI] [PubMed] [Google Scholar]
- Maybauer MO, Maybauer DM, Enkhbaatar P, Traber DL. Physiology of the vasopressin receptors. Best Pract Res Clin Anaesthesiol. 2008;22:253–263. doi: 10.1016/j.bpa.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci U S A. 2011;108:14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Drynan L, Chesham JE, Edwards MD, Dardente H, Fustin JM, Hazlerigg DG, O’Neill JS, Codner GF, Smyllie NJ, et al. Analysis of core circadian feedback loop in suprachiasmatic nucleus of mCry1-luc transgenic reporter mouse. Proc Natl Acad Sci U S A. 2013;110:9547–9552. doi: 10.1073/pnas.1220894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- McArthur AJ, Coogan AN, Ajpru S, Sugden D, Biello SM, Piggins HD. Gastrin-releasing peptide phase-shifts suprachiasmatic nuclei neuronal rhythms in vitro. J Neurosci. 2000;20:5496–5502. doi: 10.1523/JNEUROSCI.20-14-05496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer JH, Colwell CS, Rohling JH, Houben T, Michel S. Dynamic neuronal network organization of the circadian clock and possible deterioration in disease. Prog Brain Res. 2012;199:143–162. doi: 10.1016/B978-0-444-59427-3.00009-5. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Groos GA, Rusak B. Luminance coding in a circadian pacemaker: The suprachiasmatic nucleus of the rat and the hamster. Brain Res. 1986;382:109–118. doi: 10.1016/0006-8993(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Menaker M. The free running period of the bat clock; seasonal variations at low body temperature. J Cell Comp Physiol. 1961;57:81–86. doi: 10.1002/jcp.1030570204. [DOI] [PubMed] [Google Scholar]
- Meyer-Spasche A, Piggins HD. Vasoactive intestinal polypeptide phase-advances the rat suprachiasmatic nuclei circadian pacemaker in vitro via protein kinase A and mitogen-activated protein kinase. Neurosci Lett. 2004;358:91–94. doi: 10.1016/j.neulet.2003.12.114. [DOI] [PubMed] [Google Scholar]
- Michel S, Colwell CS. Cellular communication and coupling within the suprachiasmatic nucleus. Chronobiol Int. 2001;18:579–600. doi: 10.1081/cbi-100106074. [DOI] [PubMed] [Google Scholar]
- Michel S, Marek R, Vanderleest HT, Vansteensel MJ, Schwartz WJ, Colwell CS, Meijer JH. Mechanism of bilateral communication in the suprachiasmatic nucleus. Eur J Neurosci. 2013;37:964–971. doi: 10.1111/ejn.12109. [DOI] [PubMed] [Google Scholar]
- Mieda M, Ono D, Hasegawa E, Okamoto H, Honma K, Honma S, Sakurai T. Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron. 2015;85:1103–1116. doi: 10.1016/j.neuron.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Mihai R, Juss TS, Ingram CD. Suppression of suprachiasmatic nucleus neurone activity with a vasopressin receptor antagonist: Possible role for endogenous vasopressin in circadian activity cycles in vitro. Neurosci Lett. 1994;179:95–99. doi: 10.1016/0304-3940(94)90943-1. [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod. 2006;75:778–784. doi: 10.1095/biolreprod.106.052845. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Jasnow AM, Gillespie CF, Huhman KL, Albers HE. GABA interacts with photic signaling in the suprachiasmatic nucleus to regulate circadian phase shifts. Neuroscience. 2002;109:773–778. doi: 10.1016/s0306-4522(01)00519-x. [DOI] [PubMed] [Google Scholar]
- Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol. 1997;389:508–534. doi: 10.1002/(sici)1096-9861(19971222)389:3<508::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Takahashi JS. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci. 2011;34:349–358. doi: 10.1016/j.tins.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY. Disorders of circadian function and the human circadian timing system. In: K DC, M RY, R SM, editors. Suprachiasmatic Nucleus: the Mind’s Clock. New York: University Oxford Press; 1991. [Google Scholar]
- Moore RY, Silver R. Suprachiasmatic nucleus organization. Chronobiol Int. 1998;15:475–487. doi: 10.3109/07420529808998703. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett. 1993;150:112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Leak RK. Suprachiasmatic nucleus organization. Cell Tissue Res. 2002;309:89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- Morin LP. SCN organization reconsidered. J Biol Rhythms. 2007;22:3–13. doi: 10.1177/0748730406296749. [DOI] [PubMed] [Google Scholar]
- Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Moriya T, Yoshinobu Y, Kouzu Y, Katoh A, Gomi H, Ikeda M, Yoshioka T, Itohara S, Shibata S. Involvement of glial fibrillary acidic protein (GFAP) expressed in astroglial cells in circadian rhythm under constant lighting conditions in mice. J Neurosci Res. 2000;60:212–218. doi: 10.1002/(SICI)1097-4547(20000415)60:2<212::AID-JNR10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Myung J, Hong S, DeWoskin D, De Schutter E, Forger DB, Takumi T. GABA-mediated repulsive coupling between circadian clock neurons in the SCN encodes seasonal time. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1421200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung J, Hong S, Hatanaka F, Nakajima Y, De Schutter E, Takumi T. Period coding of Bmal1 oscillators in the suprachiasmatic nucleus. J Neurosci. 2012;32:8900–8918. doi: 10.1523/JNEUROSCI.5586-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Adachi A, Nakahama K, Nakamura T, Tamada M, Meyer-Bernstein E, Sehgal A, Shigeyoshi Y. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J Neurosci. 2003;23:6141–6151. doi: 10.1523/JNEUROSCI.23-14-06141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: Cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Naito E, Watanabe T, Tei H, Yoshimura T, Ebihara S. Reorganization of the suprachiasmatic nucleus coding for day length. J Biol Rhythms. 2008;23:140–149. doi: 10.1177/0748730408314572. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Fujimura K, Ebihara S, Shinohara K. Light response of the neuronal firing activity in the suprachiasmatic nucleus of mice. Neurosci Lett. 2004;371:244–248. doi: 10.1016/j.neulet.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Nakamura W, Honma S, Shirakawa T, Honma K. Regional pacemakers composed of multiple oscillator neurons in the rat suprachiasmatic nucleus. Eur J Neurosci. 2001;14:666–674. doi: 10.1046/j.0953-816x.2001.01684.x. [DOI] [PubMed] [Google Scholar]
- Nakamura W, Honma S, Shirakawa T, Honma K. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat Neurosci. 2002;5:399–400. doi: 10.1038/nn843. [DOI] [PubMed] [Google Scholar]
- Nakamura W, Yamazaki S, Takasu NN, Mishima K, Block GD. Differential response of Period1 expression within the suprachiasmatic nucleus. J Neurosci. 2005;25:5481–5487. doi: 10.1523/JNEUROSCI.0889-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: Maintenance of a meal-related pattern. Eur J Endocrinol. 2005;152:845–850. doi: 10.1530/eje.1.01919. [DOI] [PubMed] [Google Scholar]
- Naum OG, Fernanda Rubio M, Golombek DA. Rhythmic variation in gamma-aminobutyric acid(A)-receptor subunit composition in the circadian system and median eminence of Syrian hamsters. Neurosci Lett. 2001;310:178–182. doi: 10.1016/s0304-3940(01)02129-2. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Hannibal J, Fahrenkrug J. Vasoactive intestinal polypeptide induces per1 and per2 gene expression in the rat suprachiasmatic nucleus late at night. Eur J Neurosci. 2002;15:570–574. doi: 10.1046/j.0953-816x.2001.01882.x. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Michihata T, Nakamura W, Takumi T, Shimizu R, Yamamoto M, Ikeda M, Ohmiya Y, Nakajima Y. Dual-color luciferase mouse directly demonstrates coupled expression of two clock genes. Biochemistry. 2010;49:8053–8061. doi: 10.1021/bi100545h. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Watanabe K. Regional differences in circadian period within the suprachiasmatic nucleus. Brain Res. 2008;1239:119–126. doi: 10.1016/j.brainres.2008.08.082. [DOI] [PubMed] [Google Scholar]
- Novak CM, Ehlen JC, Huhman KL, Albers HE. GABA(B) receptor activation in the suprachiasmatic nucleus of diurnal and nocturnal rodents. Brain Res Bull. 2004;63:531–535. doi: 10.1016/j.brainresbull.2004.05.001. [DOI] [PubMed] [Google Scholar]
- O’Hara BF, Andretic R, Heller HC, Carter DB, Kilduff TS. GABAA, GABAC, and NMDA receptor subunit expression in the suprachiasmatic nucleus and other brain regions. Brain Res Mol Brain Res. 1995;28:239–250. doi: 10.1016/0169-328x(94)00212-w. [DOI] [PubMed] [Google Scholar]
- Okamura H, Berod A, Julien JF, Geffard M, Kitahama K, Mallet J, Bobillier P. Demonstration of GABAergic cell bodies in the suprachiasmatic nucleus: In situ hybridization of glutamic acid decarboxylase (GAD) mRNA and immunocytochemistry of GAD and GABA. Neurosci Lett. 1989;102:131–136. doi: 10.1016/0304-3940(89)90067-0. [DOI] [PubMed] [Google Scholar]
- Pakhotin P, Harmar AJ, Verkhratsky A, Piggins H. VIP receptors control excitability of suprachiasmatic nuclei neurones. Pflugers Arch. 2006;452:7–15. doi: 10.1007/s00424-005-0003-z. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Antle MC, Rusak B. Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci. 1995;15:5612–5622. doi: 10.1523/JNEUROSCI.15-08-05612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggins HD, Goguen D, Rusak B. Gastrin-releasing peptide induces c-Fos in the hamster suprachiasmatic nucleus. Neurosci Lett. 2005;384:205–210. doi: 10.1016/j.neulet.2005.03.072. [DOI] [PubMed] [Google Scholar]
- Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. 2005;25:404–408. doi: 10.1523/JNEUROSCI.4133-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Edgar DM, Heller HC, Miller JD. A possible glial role in the mammalian circadian clock. Brain Res. 1994;643:296–301. doi: 10.1016/0006-8993(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Quintero JE, Kuhlman SJ, McMahon DG. The biological clock nucleus: A multiphasic oscillator network regulated by light. J Neurosci. 2003;23:8070–8076. doi: 10.1523/JNEUROSCI.23-22-08070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Staines WA, Yasumura T, Patel D, Furman CS, Stelmack GL, Nagy JI. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin-36 but not connexin-32 or connexin-43. Proc Natl Acad Sci U S A. 2000;97:7573–7578. doi: 10.1073/pnas.97.13.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2001;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert S. Pre-natal development of a hypothalamic biological clock. In: Swabb D, Hofman M, Mirmiran M, Ravid R, van Leeuwen F, editors. Progress in Brain Reserach. B.V.: Elsevier Science Pulishers; 1992. pp. 119–132. [DOI] [PubMed] [Google Scholar]
- Rohling J, Meijer JH, VanderLeest HT, Admiraal J. Phase differences between SCN neurons and their role in photoperiodic encoding; a simulation of ensemble patterns using recorded single unit electrical activity patterns. J Physiol Paris. 2006;100:261–270. doi: 10.1016/j.jphysparis.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Rohling JH, Vanderleest HT, Michel S, Vansteensel MJ, Meijer JH. Phase resetting of the mammalian circadian clock relies on a rapid shift of a small population of pacemaker neurons. PLoS One. 2011;6:e25437. doi: 10.1371/journal.pone.0025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn HJ, Sluiter AA, Pool CW, Wortel J, Buijs RM. Evidence from confocal fluorescence microscopy for a dense, reciprocal innervation between AVP-, somatostatin-, VIP/PHI-, GRP-, and VIP/PHI/GRP-immunoreactive neurons in the rat suprachiasmatic nucleus. Eur J Neurosci. 1997;9:2613–2623. doi: 10.1111/j.1460-9568.1997.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Russell W, Harrison RF, Smith N, Darzy K, Shalet S, Weetman AP, Ross RJ. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab. 2008;93:2300–2306. doi: 10.1210/jc.2007-2674. [DOI] [PubMed] [Google Scholar]
- Saeb-Parsy K, Dyball RE. Defined cell groups in the rat suprachiasmatic nucleus have different day/night rhythms of single-unit activity in vivo. J Biol Rhythms. 2003;18:26–42. doi: 10.1177/0748730402239674. [DOI] [PubMed] [Google Scholar]
- Schaap J, Albus H, VanderLeest HT, Eilers PH, Detari L, Meijer JH. Heterogeneity of rhythmic suprachiasmatic nucleus neurons: Implications for circadian waveform and photoperiodic encoding. Proc Natl Acad Sci U S A. 2003;100:15994–15999. doi: 10.1073/pnas.2436298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder H, Stehle J, Henschel M. Twenty-four-hour pineal melatonin synthesis in the vasopressin-deficient Brattleboro rat. Brain Res. 1988;459:328–332. doi: 10.1016/0006-8993(88)90648-8. [DOI] [PubMed] [Google Scholar]
- Schroeder A, Loh DH, Jordan MC, Roos KP, Colwell CS. Circadian regulation of cardiovascular function: A role for vasoactive intestinal peptide. Am J Physiol Heart Circ Physiol. 2011;300:H241–250. doi: 10.1152/ajpheart.00190.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MD, Wotus C, Liu T, Friesen WO, Borjigin J, Oda GA, de la Iglesia HO. Dissociation of circadian and light inhibition of melatonin release through forced desynchronization in the rat. Proc Natl Acad Sci U S A. 2009;106:17540–17545. doi: 10.1073/pnas.0906382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz WJ. Further evaluation of the tetrodotoxin-resistant circadian pacemaker in the suprachiasmatic nuclei. J Biol Rhythms. 1991;6:149–158. doi: 10.1177/074873049100600205. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Carpino A, Jr, de la Iglesia HO, Baler R, Klein DC, Nakabeppu Y, Aronin N. Differential regulation of fos family genes in the ventrolateral and dorsomedial subdivisions of the rat suprachiasmatic nucleus. Neuroscience. 2000;98:535–547. doi: 10.1016/s0306-4522(00)00140-8. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Gainer H. Suprachiasmatic nucleus: Use of 14C-labeled deoxyglucose uptake as a functional marker. Science. 1977;197:1089–1091. doi: 10.1126/science.887940. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Gross RA, Morton MT. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci U S A. 1987;84:1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellix MT, Evans JA, Leise TL, Castanon-Cervantes O, Hill DD, DeLisser P, Block GD, Menaker M, Davidson AJ. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci. 2012;32:16193–16202. doi: 10.1523/JNEUROSCI.3559-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Spratt C, Sheward WJ, Kallo I, West K, Morrison CF, Coen CW, Marston HM, Harmar AJ. Overexpression of the human VPAC2 receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc Natl Acad Sci U S A. 2000;97:11575–11580. doi: 10.1073/pnas.97.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheward WJ, Naylor E, Knowles-Barley S, Armstrong JD, Brooker GA, Seckl JR, Turek FW, Holmes MC, Zee PC, Harmar AJ. Circadian control of mouse heart rate and blood pressure by the suprachiasmatic nuclei: Behavioral effects are more significant than direct outputs. PLoS One. 2010;5:e9783. doi: 10.1371/journal.pone.0009783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Liou S, Ueki S, Oomura Y. Influence of environmental light-dark cycle and enucleation on activity of suprachiasmatic neurons in slice preparations. Brain Res. 1984;302:75–81. doi: 10.1016/0006-8993(84)91286-1. [DOI] [PubMed] [Google Scholar]
- Shibata S, Moore RY. Development of neuronal activity in the rat suprachiasmatic nucleus. Brain Res. 1987;431:311–315. doi: 10.1016/0165-3806(87)90220-3. [DOI] [PubMed] [Google Scholar]
- Shibata S, Moore RY. Tetrodotoxin does not affect circadian rhythms in neuronal activity and metabolism in rodent suprachiasmatic nucleus in vitro. Brain Res. 1993;606:259–266. doi: 10.1016/0006-8993(93)90993-w. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Nagai K, Nakagawa H. An immunotoxin, anti-VIP antibody-ricin A chain conjugate eliminates neurons in the hypothalamic suprachiasmatic nucleus selectively and abolishes the circadian rhythm of water intake. Brain Res Bull. 1996;41:369–378. doi: 10.1016/s0361-9230(96)00070-6. [DOI] [PubMed] [Google Scholar]
- Shimura M, Harata N, Tamai M, Akaike N. Allosteric modulation of GABAA receptors in acutely dissociated neurons of the suprachiasmatic nucleus. Am J Physiol. 1996;270:C1726–1734. doi: 10.1152/ajpcell.1996.270.6.C1726. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Mitushima D, Kimura F. Effects of gap junction blocker on vasopressin and vasoactive intestinal polypeptide rhythms in the rat suprachiasmatic nucleus in vitro. Neurosci Res. 2000a;38:43–47. doi: 10.1016/s0168-0102(00)00141-3. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Hiruma H, Funabashi T, Kimura F. GABAergic modulation of gap junction communication in slice cultures of the rat suprachiasmatic nucleus. Neuroscience. 2000b;96:591–596. doi: 10.1016/s0306-4522(99)00556-4. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. Two distinct oscillators in the rat suprachiasmatic nucleus in vitro. Proc Natl Acad Sci U S A. 1995;92:7396–7400. doi: 10.1073/pnas.92.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. Circadian release of amino acids in the suprachiasmatic nucleus in vitro. Neuroreport. 1998;9:137–140. doi: 10.1097/00001756-199801050-00027. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Inouye ST. Photic information coded by vasoactive intestinal polypeptide and neuropeptide Y. Neurosci Biobehav Rev. 1995;19:349–352. doi: 10.1016/0149-7634(94)00048-6. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Isobe Y, Inouye ST. Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: Daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J Neurosci. 1993;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa T, Honma S, Honma K. Multiple oscillators in the suprachiasmatic nucleus. Chronobiol Int. 2001;18:371–387. doi: 10.1081/cbi-100103962. [DOI] [PubMed] [Google Scholar]
- Shirakawa T, Honma S, Katsuno Y, Oguchi H, Honma KI. Synchronization of circadian firing rhythms in cultured rat suprachiasmatic neurons. Eur J Neurosci. 2000;12:2833–2838. doi: 10.1046/j.1460-9568.2000.00170.x. [DOI] [PubMed] [Google Scholar]
- Silver R, Romero MT, Besmer HR, Leak R, Nunez JM, LeSauter J. Calbindin-D28K cells in the hamster SCN express light-induced Fos. Neuroreport. 1996;7:1224–1228. doi: 10.1097/00001756-199604260-00026. [DOI] [PubMed] [Google Scholar]
- Smarr BL, Morris E, de la Iglesia HO. The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology. 2012;153:2839–2850. doi: 10.1210/en.2011-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Canal MM. Expression of circadian neuropeptides in the hypothalamus of adult mice is affected by postnatal light experience. J Neuroendocrinol. 2009;21:946–953. doi: 10.1111/j.1365-2826.2009.01914.x. [DOI] [PubMed] [Google Scholar]
- Strecker GJ, Wuarin JP, Dudek FE. GABAA-mediated local synaptic pathways connect neurons in the rat suprachiasmatic nucleus. J Neurophysiol. 1997;78:2217–2220. doi: 10.1152/jn.1997.78.4.2217. [DOI] [PubMed] [Google Scholar]
- Sukhov RR, Walker LC, Rance NE, Price DL, Young WS., 3rd Vasopressin and oxytocin gene expression in the human hypothalamus. J Comp Neurol. 1993;337:295–306. doi: 10.1002/cne.903370210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Kipnis DM, Daughaday WH. Growth hormone secretion during sleep. J Clin Invest. 1968;47:2079–2090. doi: 10.1172/JCI105893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Okamura H, Yanaihara N, Hamada S, Fujita S, Ibata Y. Vasoactive intestinal peptide immunoreactive neurons in the rat suprachiasmatic nucleus demonstrate diurnal variation. Brain Res. 1989;497:374–377. doi: 10.1016/0006-8993(89)90283-7. [DOI] [PubMed] [Google Scholar]
- Tamada Y, Tanaka M, Munekawa K, Hayashi S, Okamura H, Kubo T, Hisa Y, Ibata Y. Neuron-glia interaction in the suprachiasmatic nucleus: A double labeling light and electron microscopic immunocytochemical study in the rat. Brain Res Bull. 1998;45:281–287. doi: 10.1016/s0361-9230(97)00403-6. [DOI] [PubMed] [Google Scholar]
- Tousson E, Meissl H. Suprachiasmatic nuclei grafts restore the circadian rhythm in the paraventricular nucleus of the hypothalamus. J Neurosci. 2004;24:2983–2988. doi: 10.1523/JNEUROSCI.5044-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: Intrinsic anatomy. J Comp Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Dudek FE. Cellular communication in the circadian clock, the suprachiasmatic nucleus. Neuroscience. 1993;56:793–811. doi: 10.1016/0306-4522(93)90128-3. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Finkbeiner SM, Cornell-Bell AH. Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. J Neurosci. 1992;12:2648–2664. doi: 10.1523/JNEUROSCI.12-07-02648.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: Immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R, Buijs RM. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J Neuroendocrinol. 1993;5:137–144. doi: 10.1111/j.1365-2826.1993.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Van der Veen DR, Castillo MR, Van der Zee EA, Jansen K, Gerkema MP, Bult-Ito A. Circadian dynamics of vasopressin in mouse selection lines: Translation and release in the SCN. Brain Res. 2005;1060:16–25. doi: 10.1016/j.brainres.2005.07.068. [DOI] [PubMed] [Google Scholar]
- van Esseveldt KE, Lehman MN, Boer GJ. The suprachiasmatic nucleus and the circadian time-keeping system revisited. Brain Res Brain Res Rev. 2000;33:34–77. doi: 10.1016/s0165-0173(00)00025-4. [DOI] [PubMed] [Google Scholar]
- Vogt K. Diversity in GABAergic signaling. Adv Pharmacol. 2015;73:203–222. doi: 10.1016/bs.apha.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152:165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature. 1997;387:598–603. doi: 10.1038/42468. [DOI] [PubMed] [Google Scholar]
- Wang D, Cui LN, Renaud LP. Pre- and postsynaptic GABA(B) receptors modulate rapid neurotransmission from suprachiasmatic nucleus to parvocellular hypothalamic paraventricular nucleus neurons. Neuroscience. 2003;118:49–58. doi: 10.1016/s0306-4522(02)00906-5. [DOI] [PubMed] [Google Scholar]
- Wang MH, Chen N, Wang JH. The coupling features of electrical synapses modulate neuronal synchrony in hypothalamic superachiasmatic nucleus. Brain Res. 2014;1550:9–17. doi: 10.1016/j.brainres.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Vanecek J, Yamaoka S. In vitro entrainment of the circadian rhythm of vasopressin-releasing cells in suprachiasmatic nucleus by vasoactive intestinal polypeptide. Brain Res. 2000;877:361–366. doi: 10.1016/s0006-8993(00)02724-4. [DOI] [PubMed] [Google Scholar]
- Watts AG. The efferent projections of the suprachiasmatic nucleus: Anatomical insights into the control of circadian rhythms. In: K DC, M RY, R SM, editors. Suprachiasmatic Nucleus: the Mind’s Clock. New York: University Oxford Press; 1991. pp. 77–106. [Google Scholar]
- Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- Weaver DR. The suprachiasmatic nucleus: A 25-year retrospective. J Biol Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci U S A. 2009;106:16493–16498. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman CH, Murphy HM, Nadzam GR. Vasopressin deficiency provides evidence for separate circadian oscillators of activity and temperature. Peptides. 2000;21:811–816. doi: 10.1016/s0196-9781(00)00213-8. [DOI] [PubMed] [Google Scholar]
- Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ. Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci. 2009;30:869–876. doi: 10.1111/j.1460-9568.2009.06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotus C, Lilley TR, Neal AS, Suleiman NL, Schmuck SC, Smarr BL, Fischer BJ, de la Iglesia HO. Forced desynchrony reveals independent contributions of suprachiasmatic oscillators to the daily plasma corticosterone rhythm in male rats. PLoS One. 2013;8:e68793. doi: 10.1371/journal.pone.0068793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita K, Yamanaka I, Emoto N, Kawakami K, Shimada S. Real-time monitoring of circadian clock oscillations in primary cultures of mammalian cells using Tol2 transposon-mediated gene transfer strategy. BMC Biotechnol. 2010;10:3. doi: 10.1186/1472-6750-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, Fustin JM, Yamazaki F, Mizuguchi N, Zhang J, et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science. 2013;342:85–90. doi: 10.1126/science.1238599. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yan L, Foley NC, Bobula JM, Kriegsfeld LJ, Silver R. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J Neurosci. 2005;25:9017–9026. doi: 10.1523/JNEUROSCI.2538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Karatsoreos I, Lesauter J, Welsh DK, Kay S, Foley D, Silver R. Exploring spatiotemporal organization of SCN circuits. Cold Spring Harb Symp Quant Biol. 2007;72:527–541. doi: 10.1101/sqb.2007.72.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Okamura H. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur J Neurosci. 2002;15:1153–1162. doi: 10.1046/j.1460-9568.2002.01955.x. [DOI] [PubMed] [Google Scholar]
- Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. European Journal of Neuroscience. 2002;16:1531–1540. doi: 10.1046/j.1460-9568.2002.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Silver R. Resetting the brain clock: Time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur J Neurosci. 2004;19:1105–1109. doi: 10.1111/j.1460-9568.2004.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Nakajima Y, Yamada Y, Enoki R, Watanabe K, Yamazaki M, Sakimura K, Honma S, Honma K. Spatiotemporal profiles of arginine vasopressin transcription in cultured suprachiasmatic nucleus. Eur J Neurosci. 2015;42:2678–2689. doi: 10.1111/ejn.13061. [DOI] [PubMed] [Google Scholar]
- Zhang EE, Kay SA. Clocks not winding down: Unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11:764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging. 1995;16:571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Cheng MY. Prokineticin 2 and circadian clock output. FEBS J. 2005;272:5703–5709. doi: 10.1111/j.1742-4658.2005.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


