Abstract
Noxious pH triggers pungent taste and nocifensive behavior. While the mechanisms underlying acidic pH sensation has been extensively characterized, little is known about how animals sense alkaline pH in the environment. TMC genes encode a family of evolutionarily conserved membrane proteins, whose functions are largely unknown. Here, we characterize C. elegans TMC-1 which was suggested to form a Na+-sensitive channel mediating salt chemosensation. Interestingly, we find that TMC-1 is required for worms to avoid noxious alkaline environment. Alkaline pH evokes an inward current in nociceptive neurons, which is primarily mediated by TMC-1 and to a lesser extent by the TRP channel OSM-9. However, unlike OSM-9 which is sensitive to both acidic and alkaline pH, TMC-1 is only required for alkali-activated current, revealing a specificity for alkaline sensation. Ectopic expression of TMC-1 confers alkaline sensitivity to alkali-insensitive cells. Our results identify an unexpected role for TMCs in alkaline sensation and nociception.
Introduction
The sense of pH is critical for the quality and survival of life (Holzer, 2009; Liman et al., 2014). Noxious pH produces pungent taste and also induces pain (Holzer, 2009; Liman et al., 2014). Animals have evolved a number of proton-activated channels (e.g. ASICs and TRP channels) and GPCRs to detect acidic pH (Holzer, 2009; Wemmie et al., 2006). By contrast, much less is known about how animals sense alkaline pH in the environment. For example, sensory neurons, such as trigeminal neurons, are excited by a wide range of external alkaline pH (Bryant, 2005); however, the underlying receptors/channels have not been identified. A couple of TRP channels (i.e. TRPV1 and TRPA1) are known to be activated by intracellular alkalization but not by external alkaline pH. These channels, however, can also be activated by acidic pH (de la Roche et al., 2013; Tominaga et al., 1998), and thus do not exhibit specificity towards alkali. Some other channels, such as the constitutive-active sperm channel CatSper1 and the leaky K+ channels TASK/TALK, are potentiated rather than activated by alkaline pH (Kirichok et al., 2006). Apparently, unknown alkali-activated channels, which sense external alkali insults, must be present but remain to be identified.
TMCs (transmembrane channel-like) form a novel family of channel-like proteins conserved from worms to humans (Keresztes et al., 2003; Kurima et al., 2003). The mammalian genome encodes eight TMC genes, among which TMC1 and 2 are essential for hearing (Kawashima et al., 2011; Pan et al., 2013). However, their exact role in auditory transduction is unclear (Beurg et al., 2014; Kawashima et al., 2015; Pan et al., 2013), and it has not been possible to functionally express them in heterologous systems (Kawashima et al., 2015). The other six TMC genes remain uncharacterized. As such, little is known about the function and regulation of these channel-like proteins.
The C. elegans genome encodes nearly all major classes of receptors and ion channels, offering an excellent genetic model for the study of their function and regulation in vivo (Bargmann, 1998). Two TMC family genes, tmc-1 and tmc-2, are present in C. elegans, of which tmc-1 has been suggested to encode a Na+-sensitive channel required for salt chemosensation and dietary signaling (Chatzigeorgiou et al., 2013; Zhang et al., 2015). We have characterized tmc-1 gene in the current study, and found that it is required for worms to sense noxious alkaline environment mediated by the ASH nociceptive neurons. Alkaline pH evokes an inward current in ASH, which primarily depends on TMC-1 and to a lesser extent on the TRPV channel OSM-9. While OSM-9/TRPV is sensitive to both acidic and basic pH, TMC-1 demonstrates a specificity towards alkali, as it is required for alkali- but not acid-triggered behavioral response as well as electrical current in ASH. Importantly, ectopic expression of TMC-1 is sufficient to confer alkaline sensitivity to alkali-insensitive cells by promoting alkali-activated calcium transients and non-selective cation conductance. Our results identify an unexpected role of TMC-1 in alkaline sensation, demonstrate it as an essential component of a new type of alkali-activated cation channel, and also uncover a new function for TMC family genes in nociception.
Results
Requirement of G protein signaling for TRPV/OSM-9 channel activation in ASH neurons
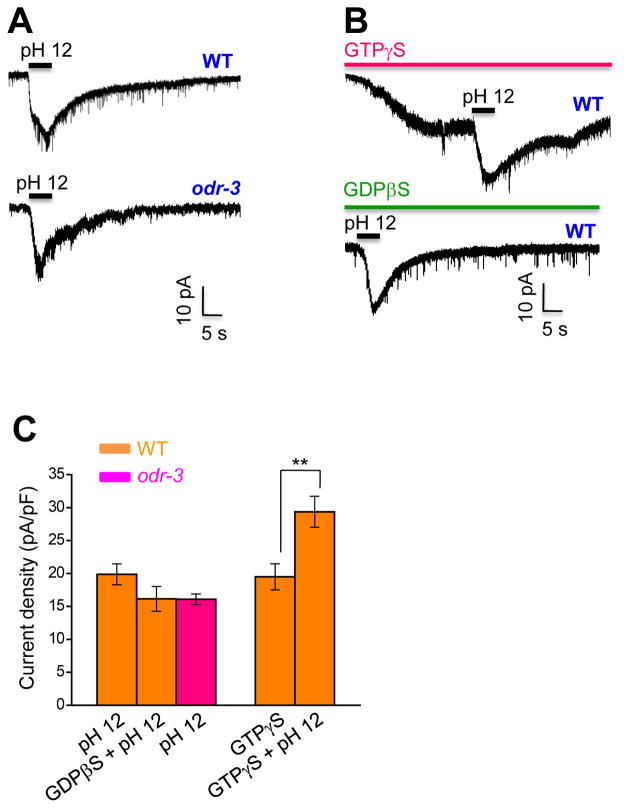
As TMC-1 is expressed in ASH (Chatzigeorgiou et al., 2013), a pair of polymodal nociceptive neurons, we first decided to gather some basic understandings of these neurons by characterizing their responses to noxious cues through electrophysiology. Previous work by calcium imaging showed that ASH neurons respond to a multitude of noxious stimuli such as high osmolarity and various pungent chemicals (Hilliard et al., 2005). We first tested high osmolarity and found that perfusion of glycerol-containing high osmotic solution to the nose tip, where the sensory endings of ASH neurons reside, evoked a robust inward current (Figure S1A). Consistent with previous calcium imaging results (Hilliard et al., 2005), we found that this current required OSM-9, a TRPV channel (Figure S1A and S1D). This current also depended on ODR-3 (Figure S1A and S1D), which is a Gα protein acting in sensory neurons including ASH (Roayaie et al., 1998). This supports the notion that osmosensation in ASH is mediated by G protein signaling, leading to the activation of the transduction channel OSM-9.
To provide direct evidence, we dialyzed GTPγS into ASH, a chemical that constitutively activates G protein signaling by locking G proteins in an active state. We found that it induced an inward current, showing that activation of G protein signaling was sufficient to stimulate ASH (Figure S1B and S1D). GTPγS-induced currents cannot be further enhanced by perfusion of high osmotic solution (glycerol), suggesting that the two may act in the same pathway (Figure S1B and S1D). Similarly, GTPγS-induced currents were absent in osm-9 and odr-3 mutant backgrounds (Figure S1C and S1D), indicating that these currents were mediated by ODR-3 signaling-dependent activation of OSM-9. We also performed the converse experiment by testing the effect of GDPβS which inhibits G protein signaling by locking G proteins in an inactive state. Blocking G protein signaling with GDPβS prevented ASH neurons from being activated by high osmolarity (Figure S1B and S1D). Thus, G protein signaling is necessary for high osmolarity-induced activation of OSM-9 in ASH neurons. Similar results were also obtained with some pungent chemicals such as octanol (unpublished observations, X.W. and X.Z.S.X.). These data suggest that the TRP channel OSM-9 can be activated through G protein signaling.
Alkaline pH induces an inward current in ASH neurons independently of G protein signaling
As TRPV channels are also known to be directly activated by noxious stimuli (Caterina et al., 1997), we sought to search for such a stimulus for OSM-9. A prior study demonstrated by calcium imaging that alkaline pH can excite ASH neurons through OSM-9 (Sassa et al., 2013). Alkaline pH can also activate the mammalian TRP channels TRPV1 and TRPA1 (Dhaka et al., 2009; Fujita et al., 2008). We thus tested how ASH neurons react to alkaline pH by patch-clamp. Perfusion of alkaline pH solution to the nose tip activated an inward current in ASH neurons (Figure 1A and 1C). Unlike high osmolarity, alkaline pH activated an inward current even in the presence of GTPγS (Figure 1B and 1C), suggesting that alkali can excite ASH independently of G protein signaling. Additional evidence came from GDPβS which failed to block alkali-activated currents in ASH neurons (Figure 1B and 1C), though it completely abolished glycerol-evoked currents in these neurons (Figure S1B and S1D). Similarly, alkali-activated currents were nearly normal in odr-3 mutant worms (Figure 1A and 1C), yet glycerol-evoked currents were absent in this mutant (Figure S1A and S1D). Much to our surprise, alkali-activated currents remained in ASH of osm-9 mutant worms (see below), in which glycerol failed to elicit any current (Figure S1A and S1D). These observations demonstrate that alkali can excite ASH neurons independently of G protein signaling and OSM-9/TRPV channel.
Figure 1. Alkaline sensation does not require G protein signaling.
(A) Alkaline pH evokes an inward current in ASH of wild-type, as well as odr-3(n2150) mutant worms. Bath solution (pH 12) was perfused towards the nose tip where ASH sensory endings are localized. Voltage: −60 mV.
(B) Alkali-activated currents are independent of G protein signaling. Recordings were done as described in (A). Alkaline pH further induced an inward current in the presence of GTPγS (100 μM), while glycerol cannot further evoke a current under the same recording condition (see Figure S1B). GDPβS (500 μM) cannot block alkali-activated current, while it can block glycerol-evoked current under the same recording condition (see Figure S1B). GTPγS and GDPβS were included in the pipette solution and dialyzed into the cell after the formation of whole-cell configuration. Voltage: −60 mV.
(C) Bar graph summarizing the data described in (A–B). n≥8. Error bars: SEM. **p<0.005 (t test).
Alkaline pH-induced avoidance behavior requires TMC-1 and OSM-9/TRPV
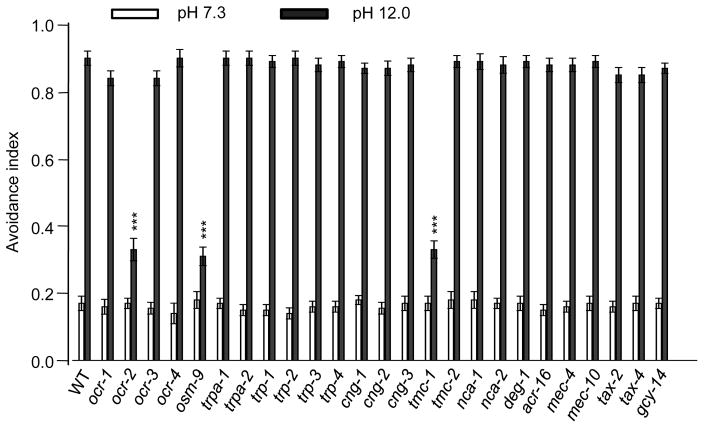
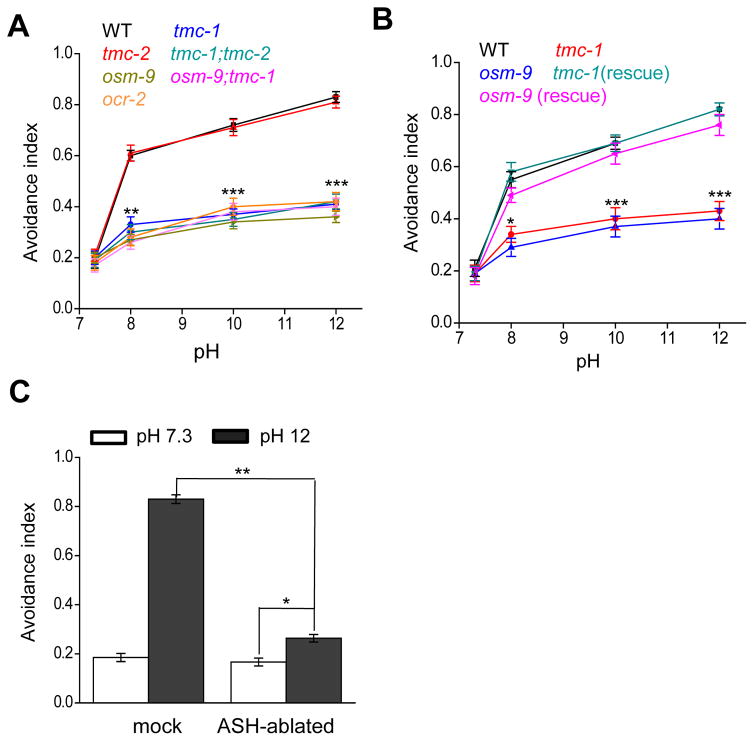
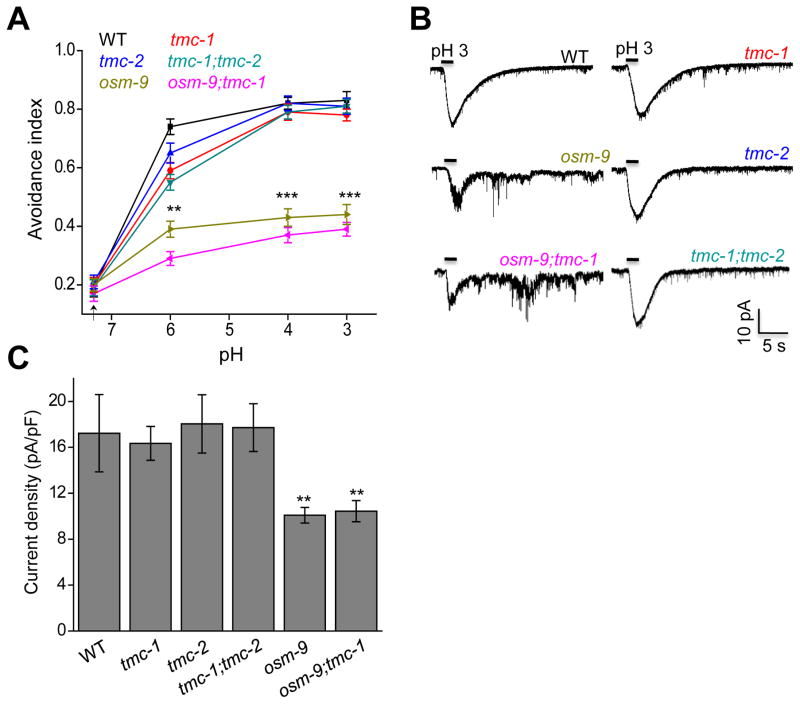
What mediates alkali-activated currents in ASH neurons? To address this question, we screened a select collection of ion channel mutants for defects in alkaline pH-induced avoidance behavior, including TRP, ENaC/DEG, TMC, and CNG family of channels (Figure 2). Wild-type worms began to avoid alkaline solution at pH 8.0 (Figure 3A). The response rate became saturated at pH 11 (Figure S2). As such, we focused on pH 12 for further analysis. As a positive control, osm-9 mutant worms, which are known to be defective in this behavior (Sassa et al., 2013), showed a strong defect (Figure 2 and 3A–B). Notably, we found that tmc-1 mutant worms were also severely defective in this avoidance behavior (Figure 2 and 3A–B). By contrast, loss of tmc-2 had no notable effect (Figure 2 and 3A–B). The guanylate cyclase GCY-14, which mediates alkali-induced attractive responses (Murayama et al., 2013), did not have a notable role in this avoidance behavior (Figure 2). Transgenic expression of wild-type tmc-1 and osm-9 cDNA in ASH neurons was sufficient to rescue the behavioral phenotype (Figure 3B), suggesting that they act in ASH to mediate alkaline pH-induced avoidance behavior. In support of this view, laser ablation of ASH neurons led to a strong defect in this avoidance behavior (Figure 3C), which is consistent with previous results (Sassa and Maruyama, 2013). Notably, ASH-ablated worms retained a residual response to alkaline pH, indicating the presence of additional alkali-sensitive neurons, though these neurons probably only play a rather minor role (Figure 3C). This may also explain why the tmc-1;osm-9 double mutant did not exhibit a more severe behavioral phenotype than single mutants (Figure 3A). These results identify both TMC-1 and OSM-9 as important players in mediating alkaline sensation. Loss of OCR-2, another TRPV channel subunit that forms a heteromeric channel with OSM-9 (Tobin et al., 2002), also gave rise to a similar phenotype (Figure 2 and 3A–B). For simplicity, we decided to focus on TMC-1 and OSM-9 for further characterizations.
Figure 2. A genetic screen identifies an important role for TMC-1 and TRPV channels in alkaline pH-induced avoidance behavior.
A select collection of ion channel mutants were examined for defects in noxious alkaline pH induced avoidance behavioral response. n=20. Error bars: SEM. ***p<0.0005 (ANOVA with Dunnett’s test).
Figure 3. Alkaline pH-induced avoidance behavior requires TMC-1 and OSM-9/TRPV.
(A) Alkaline pH-induced avoidance behavior requires TMC-1 and OSM-9 but not TMC-2. tmc-1(ok1859), osm-9(ky10), and ocr-2(ak47) but not tmc-2(ok1302) mutant worms were severely defective in this behavior. The basal response rate resulted from spontaneous reversals. n=20. Error bars: SEM. **p<0.005, ***p<0.0005 (ANOVA with Dunnett’s test).
(B) TMC-1 and OSM-9 act in ASH neurons to mediate alkaline sensation. Transgenic expression of wild-type tmc-1 and osm-9 cDNA in ASH under the sra-6 promoter rescued the avoidance behavior. n=20. Error bars: SEM. *p<0.05, ***p<0.0005 (ANOVA with Dunnett’s test).
(C) ASH neurons are the primary sensory neurons mediating alkaline pH-induced avoidance behavior. Laser ablation of ASH led to a severe defect in the behavior. n≥20. Error bars: SEM. *p<0.05, **p<0.005 (ANOVA with Dunnett’s test).
TMC-1 plays a critical role in mediating alkali-activated currents in ASH neurons
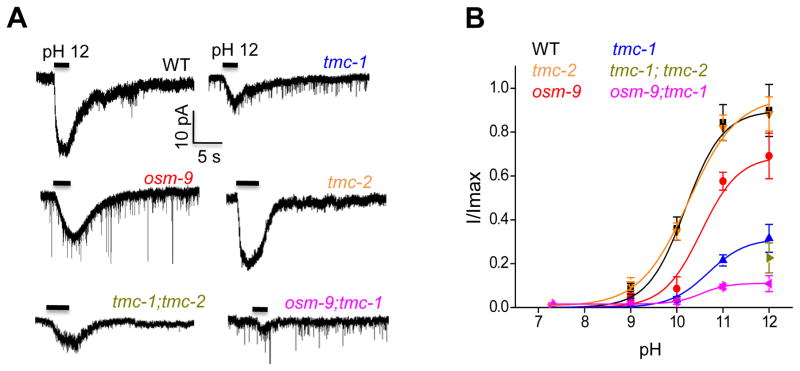
We then assessed how tmc-1 and osm-9 genes may affect alkali-activated currents in ASH neurons by whole-cell recording. Unlike the severe behavioral deficit, mutations in osm-9 only led to a moderate reduction in alkali-activated currents in ASH (Figure 4A–B). This could be explained by the relative low resolution of the behavioral assay, in which case a mild deficit in neuronal activity might lead to a severe phenotype in behavioral output. Alternatively but not mutually exclusively, OSM-9 might also act in additional neurons to mediate alkaline pH-induced avoidance behavior. By contrast, alkali-activated currents in ASH were severely diminished in tmc-1 mutant worms (Figure 4A–B), revealing an important role for TMC-1 in mediating this type of currents. Transgenic expression of wild-type tmc-1 cDNA in ASH rescued alkali-activated currents in tmc-1 mutant worms (Figure S3A–B). Consistent with the behavioral data, no defect was detected in tmc-2 mutant worms (Figure 4A–B), and no additive effect was observed in tmc-1;tmc-2 double mutant worms (Figure 4A–B), indicating the lack of a role for TMC-2 in alkali-activated currents in ASH neurons. Notably, in tmc-1;osm-9 double mutant worms, very little if any current was detected (Figure 4A–B), suggesting that TMC-1 and OSM-9 together are required for alkali-activated currents in ASH neurons. These data demonstrate that though TMC-1 and OSM-9 both contribute to alkali-activated currents in ASH neurons, TMC-1 apparently plays a more important role.
Figure 4. TMC-1 plays a critical role in mediating alkali-activated currents in ASH neurons.
(A) Alkali-activated currents in ASH are severely diminished in tmc-1(ok1859) mutant and nearly absent in tmc-1(ok1859); osm-9(ky10) double mutant worms. Recordings were done as described in Figure 1A. Shown are sample traces. Voltage: −60 mV.
(B) Bar graph summarizing the data in (A). n≥8. Error bars: SEM.
TMC-1 is expressed in some other sensory neurons in the amphid, such as ADL and ADF which also send their sensory endings to the nose tip (Chatzigeorgiou et al., 2013). Like ASH, ADL is also considered a nociceptive neuron (Troemel et al., 1995). However, perfusion of alkaline pH solution to the nose tip evoked very little if any current in ADL and ADF (Figure S3C and S3E). This is consistent with the view that ASH is the primary sensory neuron detecting noxious alkaline pH and mediating avoidance responses to such noxious environment in the worm nose. Interestingly, perfusion of alkaline pH solution towards the cell body of ADL and ADF evoked robust currents (Figure S3D–E). These alkali-activated currents were severely diminished in tmc-1 mutant worms but were only slightly reduced in osm-9 mutants (Figure S3D–E), which are very similar to those in ASH neurons evoked at the nose tip. The finding that we can detect alkali-activated currents in ADL and ADF soma rather than their sensory endings suggests that TMC-1 is indeed functionally expressed in these neurons but probably not dedicated to detecting alkaline pH in the environment. This is also consistent with the notion that TMC-1 may have other functions (Zhang et al., 2015). Future effort is needed to determine the physiological roles of TMC-1 in these neurons.
TMC-1 is not required for acidic pH-induced avoidance behavior or electrical currents in ASH
As TRPV channels can be activated by both acidic and basic pH (Dhaka et al., 2009; Tominaga et al., 1998), we asked whether this is also the case for TMC-1. We first tested low pH-induced avoidance behavior. In line with previous work (Hilliard et al., 2004), we found that worms showed a robust avoidance response to acidic pH, which saturated at pH 3–4 (Figure 5A). Interestingly, while osm-9 mutant worms exhibited a deficit in this behavior, tmc mutants did not (Figure 5A). In addition, tmc-1;osm-9 double mutant displayed a behavioral phenotype similar to that of osm-9 single mutant (Figure 5A), indicating the lack of a role for TMC-1 in sensing acidic pH.
Figure 5. TMC-1 is not involved in mediating acidic pH-activated currents in ASH neurons.
(A) Acidic pH induces avoidance behavior that depends on OSM-9 but not TMC-1. osm-9(ky10) but not tmc-1(ok1859) or tmc-2(ok1302) mutant worms showed a defect in this behavior. The basal response rate resulted from spontaneous reversals. n=20. Error bars: SEM. **p<0.005, ***p<0.0005 (ANOVA with Dunnett’s test).
(B) Acidic pH evokes an inward current in ASH neurons and this current is reduced in osm-9 mutant but remained normal in tmc mutants. Bath solution (pH 3) was perfused towards the nose tip where ASH sensory endings are localized. Shown are sample traces. Voltage: −60 mV.
(C) Bar graph summarizing the data in (B). n≥8. Error bars: SEM. **p<0.005 (ANOVA with Dunnett’s test).
We also examined acidic pH-activated currents in ASH neurons by whole-cell recording, and obtained results similar to those collected by behavioral assays (Figure 5B–C). We conclude that while OSM-9 contributes to both acid- and alkali-activated currents in ASH, TMC-1 appears to be specifically involved in mediating alkali-evoked responses.
NaCl-evoked responses in ASH neurons require OSM-9 and G protein signaling
TMC-1 has been suggested to form a Na+-sensitive cation channel in ASH neurons, mediating salt chemosensation in C. elegans (Chatzigeorgiou et al., 2013). We thus tested the possibility whether TMC-1 can be activated by both NaCl and alkali. As previously reported (Chatzigeorgiou et al., 2013), we also found that C. elegans can detect high concentrations of NaCl, as well as Na-gluconate by initiating reversals (Figure S4A and S5A). However, we found that such a behavioral response did not require TMC-1 or TMC-2 (Figure S4A and S5A). We tested a number of experimental conditions using different types of buffers to dissolve NaCl, including the commonly used M9 and S basal buffers, and the one described previously (Chatzigeorgiou et al., 2013), as well as other types of buffers (Figure S4A, S5B–D). No significant defect was detected under these conditions (Figure S4A, S5B–D). A slight behavioral deficit was observed only when one concentration of NaCl was dissolved in pure water (Figure S4D). Thus, Na+-induced behavioral responses do not require TMCs under most conditions.
High concentrations of NaCl increase osmolarity. As OSM-9 channel mediates osmosensation, we tested osm-9 mutant worms. Loss of OSM-9 also gave rise to a strong defect in NaCl- and Na-gluconate-induced behavioral responses (Figure S4A and S5A–E). As osmotic activation of OSM-9 is mediated by G protein signaling, this prompted us to test odr-3 mutants. Indeed, worms lacking ODR-3 were severely defective in responding to NaCl (Figure S4A and S5D–E), suggesting that Na+-induced behavioral response is primarily mediated by G protein signaling-dependent activation of the TRPV channel OSM-9.
To provide additional evidence, we recorded ASH neurons for their responses to NaCl by calcium imaging. We applied the condition described previously by perfusing bath solution containing 500 mM NaCl or Na-gluconate towards the cilia of ASH (Chatzigeorgiou et al., 2013), and found that it evoked calcium transients in ASH (Figure S4B–C and S5F–I). However, such calcium transients did not depend on TMC-1 or TMC-2, but instead required OSM-9 and ODR-3 (Figure S4B–C and S5F–I). To provide further evidence, we directly recorded ASH neurons for their electrical responses to NaCl by patch-clamp. Bath solution containing 500 mM NaCl evoked an inward current in ASH (Figure S4D). This current was severely defective in osm-9 and odr-3 mutant worms, but remained normal in worms lacking tmc-1 and tmc-2 (Figure S4D–E). We thus conclude that NaCl-evoked calcium and electric responses in ASH neurons require OSM-9 and G protein signaling rather than TMCs.
Ectopic expression of TMC-1 can confer alkaline sensitivity to alkali-insensitive cells
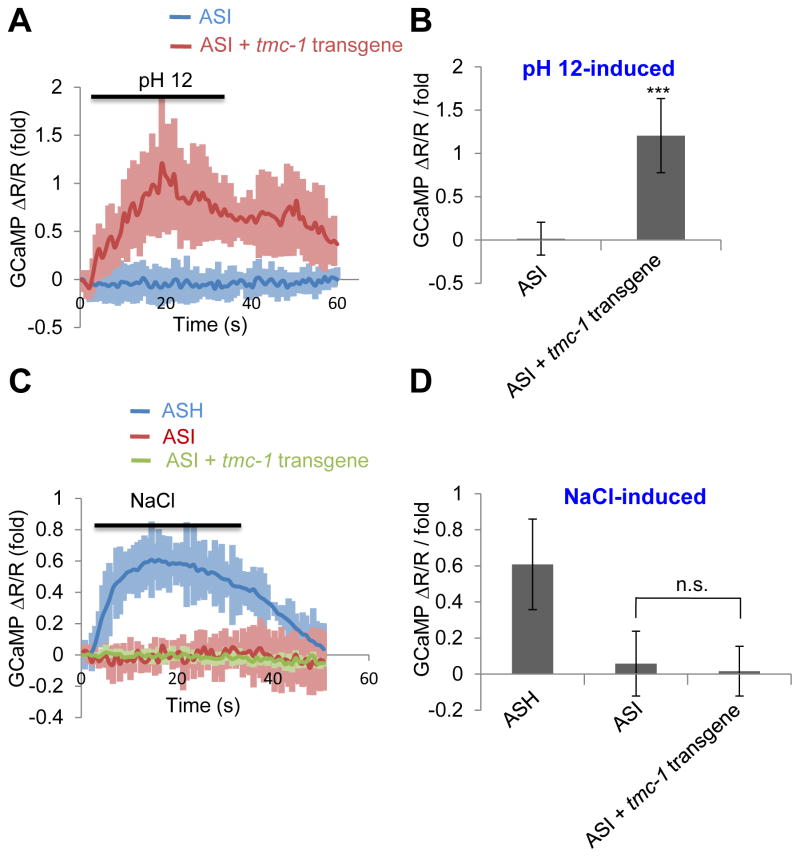
Having demonstrated that TMC-1 is necessary for alkaline sensitivity, we then wondered if TMC-1 is also sufficient to mediate such sensitivity. However, similar to the case with mammalian TMCs (Kawashima et al., 2011; Kawashima et al., 2015), we found that C. elegans TMC-1 was also trapped intracelluarly when transfected into cell lines such as HEK293 and CHO cells (unpublished observations, X.W. and X.Z.S.X.). In ASH neurons, TMC-1 is localized to the plasma membrane in the sensory cilia (Chatzigeorgiou et al., 2013), suggesting that mammalian cells may lack some factors needed to transport TMC-1 to the cell surface. To overcome this difficulty, we sought to ectopically express TMC-1 in worm cells. ASI neurons came to our attention, as calcium imaging revealed that alkaline pH did not induce calcium transients in these cells (Figure 6A–B). We then expressed tmc-1 as a transgene in ASI neurons, and found that alkaline pH evoked calcium transients in these neurons (Figure 6A–B). As a control, bath solution containing 500 mM NaCl failed to trigger a notable calcium response in ASI of wild-type or tmc-1 transgenic animals, though it was able to evoke such a response in ASH neurons (Figure 6C–D). Thus, ectopic expression of TMC-1 can confer alkaline sensitivity to alkali-insensitive cells.
Figure 6. Ectopic expression of TMC-1 can confer alkaline sensitivity to alkali-insensitive cells.
(A–B) Ectopic expression of TMC-1 in ASI confers alkaline sensitivity to these neurons revealed by calcium imaging. ASH was labeled with GCaMP6 and DsRed (internal reference) which were co-expressed as a transgene using the sra-6 promoter. Bath solution (pH 12) was perfused towards the nose tip using a microfluidic system. As ASH neurons are alkali-sensitive and are also functionally connected to ASI neurons (Guo et al., 2015), imaging experiments were performed on worms with ASH ablated using a laser microbeam. tmc-1 cDNA was expressed in ASI under the sra-6 promoter. Shown in (A) are calcium imaging traces. Shadows along the traces denote error bars. Bar graph in (B) summarizes the data. n≥8. Error bars: SEM. ***p<0.0005 (t test).
(C–D) TMC-1 ectopically expressed in ASI does not promote NaCl sensitivity. Bath solution containing 500 mM NaCl was perfused towards the nose tip. As no notable response was detected in ASI in response to NaCl (500 mM), we imaged ASH of wild-type worms as a positive control, and observed NaCl-induced calcium transients. (C) Calcium imaging traces. (D) Bar graph. n≥8. Error bars: SEM. n.s.: not significant (t test).
Ectopic expression of TMC-1 is sufficient to promote alkali-activated currents
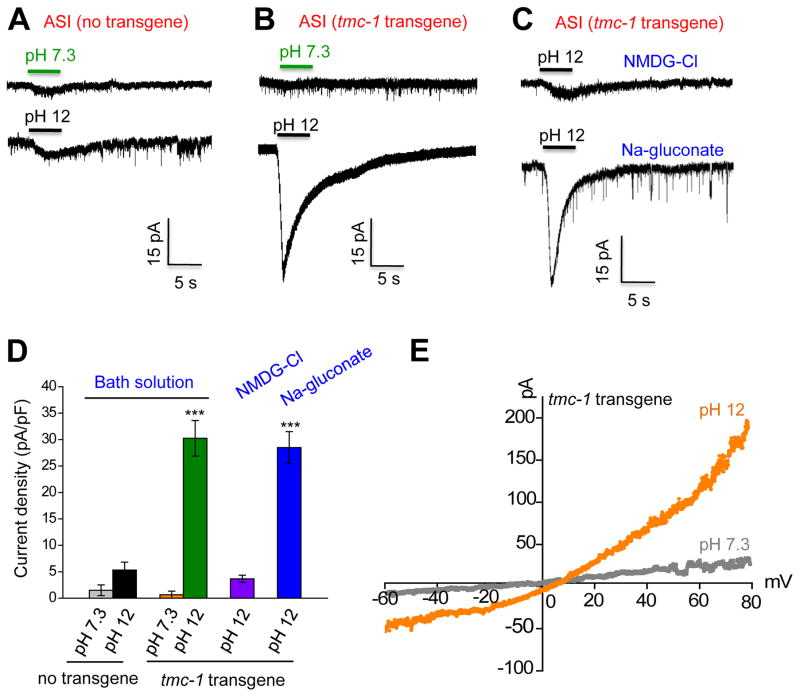
To provide further evidence, we recorded ASI neurons by patch-clamp. As was the case with calcium imaging, alkaline pH evoked little if any current in ASI neurons (Figure 7A and 7D). In animals carrying a tmc-1 transgene in ASI neurons, alkaline pH then elicited a robust inward current in these cells (Figure 7B and 7D). By contrast, the same tmc-1 transgene did not promote acidic pH-evoked currents in ASI neurons, demonstrating a specificity of TMC-1 towards alkaline pH (Figure S6A–B). We also recorded the activity of ASI in response to 500 mM NaCl (Figure S6C–D). Though this high concentration of NaCl induced a small current in ASI neurons, the current density was similar between wild-type and tmc-1 transgenic animals, indicating that this current was endogenous to ASI (Figure S6C–D). Alkali-activated inward current in TMC-1-expressing ASI neurons was nearly eliminated in NMDG-Cl solution but remained normal in Na-gluconate solution (Figure 7C–D), indicating that the current was primarily carried by a cation channel. Notably, this current displayed a nearly linear I–V relationship (slightly outwardly rectifying) with a reversal potential close to zero (Figure 7E), suggesting that it was carried by a non-selective cation channel. These results demonstrate that ectopic expression of TMC-1 is sufficient to promote alkali-activated currents.
Figure 7. Ectopic expression of TMC-1 promotes alkali-activated currents.
(A–B) Ectopic expression of TMC-1 in ASI promotes alkali-activated currents. tmc-1 cDNA was expressed in ASI as a transgene with the sra-6 promoter. As ASH neurons express robust alkali-activated currents and are functionally connected to ASI (Guo et al., 2015), we mechanically removed ASH neurons prior to recording ASI. Little if any alkali-activated currents were detected in ASI neurons of worms carrying no tmc-1 transgene (A). By contrast, robust alkali-activated currents were detected in ASI carrying a tmc-1 transgene (B). Shown are sample traces. Voltage: −60 mV.
(C) Alkali-activated currents are primarily carried by cations. Little alkali-activated current was detected in bath solution containing NMDG-Cl. By contrast, a robust alkali-activated current was observed in bath solution containing Na-gluconate. Voltage: −60 mV.
(D) Bar graph summarizing the data in (A–C). n≥9. Error bars: SEM. ***p<0.0005 (ANOVA with Dunnett’s test).
(E) I–V relations of alkali-activated currents in ASI of worms carrying a tmc-1 transgene. A voltage ramp (−60 to +80 mV; 200 ms) was applied to ASI.
Discussion
Environmental pH is closely monitored by animals through taste and somatosensation (Holzer, 2009; Liman et al., 2014). Acidic pH induces sour taste and pain (Holzer, 2009; Liman et al., 2014). Similarly, alkaline pH also induces gustatory responses and nociception. For example, it has been known for decades that application of alkaline solutions of pH 11-12.5 to the tongue induces profound taste responses in mammals, though the cellular and molecular nature of such taste responses is not well understood (Liljestrand and Zotterman, 1956). As worms may encounter noxious pH environment in the wild (e.g. alkali soils), alkaline sensation would provide a protective mechanism for these animals to avoid such harsh environment. While the molecular mechanisms by which animals detect acidic pH have been extensively characterized, little effort has been directed to understand how animals sense alkali in the environment. In the current study, we showed that TMC-1 plays a key role in alkaline sensation in C. elegans. Specifically, it is required for alkaline pH-triggered nocifensive behavior. Calcium imaging experiments show that ectopic expression of TMC-1 can confer alkaline sensitivity to alkali-insensitive cells, suggesting that it has the potential to function as an alkali sensor to detect noxious alkaline pH insults in the environment. In addition, TMC-1 is both necessary and sufficient to mediate alkali-activated currents, indicating that it is an essential component of a novel type of alkali-activated channel.
TMC-1 has been suggested to form a Na+-selective channel activated by high concentrations of NaCl (Chatzigeorgiou et al., 2013). It has also been demonstrated to be critical for salt chemosensory behavior (Chatzigeorgiou et al., 2013). It is a bit surprising that we did not detect a major role for TMC-1 in sensing high concentrations of Na+ or salt chemosensory behavior. On the other hand, we did identify a critical role for the TRPV channel OSM-9 in NaCl sensation. Notably, the G protein ODR-3 is also required for NaCl sensation by ASH neurons, suggesting that the NaCl sensor in ASH is probably a GPCR that is coupled to the OSM-9/TRPV channel through G protein signaling. Alternatively, as high concentrations of NaCl yield high osmolarity, the responses may result from osmosensation, which also requires both OSM-9 and G protein signaling. Finally, some unknown Na+-sensitive channel(s) may carry such currents. Further work is required to distinguish these possibilities.
Unlike high osmolarity- and salt-evoked currents, alkali-activated currents in ASH do not require G protein signaling. This is consistent with the model that TMC-1-dependent currents might be directly activated by alkali. Notably, unlike TRP channels, TMC-1 is specifically required for alkali- but not proton-activated currents, demonstrating a specificity towards alkali. Ectopic expression of TMC-1 can promote alkali-evoked currents, suggesting that TMC-1 has the potential to form an alkali-activated channel. These currents are primarily carried by cations and exhibit a reversal potential close to zero, indicating that TMC-1-dependent channel is a non-selective cation channel. Nevertheless, it should be noted that TMC-1 may be incapable of forming an alkali-activated channel on its own and might instead function as an auxiliary subunit of a channel complex. Future effort is needed to address this question; and given the difficulty of expressing TMCs in heterologous cells, it may be necessary to consider alternative approaches, for example, functional reconstitution of purified TMC proteins in vitro in a lipid bilayer.
TMC-1 is a member of the TMC family of channel-like proteins, which are evolutionarily conserved from worms to humans (Keresztes et al., 2003; Kurima et al., 2003). The function and regulation of TMCs are poorly understood. Mammalian TMC1 and TMC2 are enriched in hair cells and are required for hearing, but their exact role in auditory transduction is not well understood (Beurg et al., 2014; Pan et al., 2013). Little is known about the other six mammalian TMC genes. As they are not restricted to the auditory system and are more widely expressed in different tissues, they may regulate other forms of physiological processes (Keresztes et al., 2003; Kurima et al., 2003). Our finding that C. elegans TMC-1 plays a critical role in sensing noxious alkaline environment uncovers a new function for TMCs. We suggest that some mammalian TMCs may also function in alkaline sensation and/or other forms of nociception.
Experimental Procedures
Strains
Wild type: N2. TQ1764: xuEx631[Psra-6::DsRed + Pstr-3::yfp2]. TQ5709: osm-9(ky10) null allele. TQ600: odr-3(n2150) null allele. TQ3367: tmc-1(ok1859) deletion allele, x4 outcrossed. TQ3368: tmc-2(ok1302) deletion allele, x4 outcrossed. TQ3369: tmc-1(ok1859); tmc-2(ok1302) x4 outcrossed. TQ548: ocr-2(ak47) deletion allele. TQ5664: osm-9(ky10); xuEx631[Psra-6::DsRed + Pstr-3::yfp2]. TQ5918: odr-3(n2150); xuEx631 x4 outcrossed. TQ6111: tmc-1(ok1859); xuEx631. TQ6112: tmc-2(ok1302); xuEx631. TQ6113: tmc-1(ok1859); tmc-2(ok1302); xuEx631. TQ6122: tmc-1(ok1859); osm-9(ky10); xuEx631. TQ5855: ocr-2(ak47); xuEx631. TQ6382: tmc-1(ok1859); xuEx2201[Psra-6::tmc-1(cDNA)::sl2::CFP]; xuEx631. TQ6835: osm-9(ky10); xuEx2205[Psra-6::osm-9(cDNA)::sl2::CFP]; xuEx631. TQ5856: xuEx1978[Psra-6::Gcamp6(f) + Psra-6::DsRed]. TQ6021: osm-9(ky10); xuEx1978. TQ6114: tmc-1(ok1859); xuEx1978. TQ6115: tmc-2(ok1302); xuEx1978. TQ6857: odr-3(n2150); xuEx1978. TQ6116: tmc-1(ok1859); tmc-2(ok1302); xuEx1978. TQ6125: tmc-1(ok1859); osm-9(ky10); xuEx1978. TQ574: ocr-1(ok132) deletion allele. TQ575: ocr-3(ok1559) deletion allele. TQ564: ocr-4(vs137) insertion/deletion allele. TQ223: trpa-1(ok999) deletion allele. TQ946: trpa-2(tm3085) deletion allele. TQ17: trp-1(ok323) deletion allele. TQ194: trp-2(sy691) deletion allele. TQ51: trp-3(sy693) deletion allele. TQ296: trp-4(sy695) deletion allele. TQ5729: cng-1(jh111) insertion/deletion allele. TQ6003: cng-2(tm4267) insertion/deletion allele. TQ5730: cng-3(jh113) deletion allele. TQ1763: nca-1(e625) missense allele. TQ1825: nca-2(gk5) deletion allele. TQ536: deg-1(u506) missense allele. TQ275: acr-16(ok789) deletion allele. TQ2126: mec-4(u253) deletion allele. TQ1243: mec-4(e1611) gain-of-function allele. TQ356: mec-10(e1515) missense allele. TQ634: tax-2(p691) missense allele. TQ86: tax-4(p678) nonsense allele. JN1194: gcy-14(pe1102) insertion/deletion allele. TQ7180: xuEx2625[Psrh-220::gfp]. TQ7181: xuEx2625; tmc-1(ok1859). TQ7259: xuEx2625; osm-9(ky10). TQ7258: xuEx2625[Psrh-220::gfp]; osm-9(ky10); tmc-1(ok1859). TQ6483: xuEx2304[Ptph-1(BC)::sl2::mcherry2 + Punc-122::sl2::GFP]. TQ7182: xuEx2304; tmc-1(ok1859). TQ7261: xuEx2304; osm-9(ky10). TQ7260: xuEx2304; tmc-1(ok1859); osm-9(ky10).
Behavioral assays
Avoidance behavior was performed using a drop test assay at 20 °C as previously described (Mellem et al., 2002; Piggott et al., 2011). Briefly, a small drop of solution was placed on the path of a forward-moving animal. If the animal stops forward movement and also initiates a reversal that lasts at least half a head swing, it is scored as a positive response. OP50 was not included on the assay plate (NGM plate). In general, M9 buffer was used as the vehicle for the tests, though S-basal and other types of buffers were also used in some cases (e.g. Figure S4 and S5). The pH of the solutions was adjusted to designated values using HCl (to acidic pH) and Tris base followed by NaOH (to alkaline pH), and the solutions were made and used freshly at room temperature to avoid precipitation. To make NaCl solutions, varying amounts of NaCl were dissolved in M9, S basal, standard bath solution (see below), water, or the same buffer described previously in (Chatzigeorgiou et al., 2013): 1 mM MgSO4, 1 mM CaCl2, and 5 mM KPO4. The final concentration values of NaCl shown in Figures S4 and S5 include both newly added NaCl and that pre-exiting in the original buffers. To make Na-gluconate solutions, NaCl was replaced with Na-gluconate. Each animal was tested five times and a response rate was tabulated for each animal.
Calcium imaging
Calcium imaging was performed using a microfluidic system as previously described (Chronis et al., 2007). Day 1 adult worms were used for imaging. Images were acquired on an Olympus upright microscope (BX51WI) with MetaFluor (Molecular Devices, Inc.). G-CaMP and DsRed fluorescence was excited at 484 nm and 535 nm, respectively, and the latter was included as an internal reference. As ASH neurons are intrinsically light-sensitive (Ward et al., 2008), we pre-exposed the animal to light for 2 min to establish a baseline before stimulus application. We quantified the peak fold change in the ratio of G-CaMP/DsRed fluorescence. Standard bath solution (in mM): 145 NaCl, 2.5 KCl, 1 CaCl2, 1 MgCl2, 20 glucose, and 5 HEPES (320 mOsm; pH adjusted to 7.3). We also used the same bath solution for calcium imaging as described previously in (Chatzigeorgiou et al., 2013): 1 mM MgSO4, 1 mM CaCl2, and 5 mM KPO4. To make alkaline solution, the pH value of standard bath solution was adjusted with Tris base to 9 and then to 12 with NaOH, and the osmolarity of the solution was also adjusted to 320 mOsm. To make NaCl solution, the final concentration of NaCl was adjusted to 500 mM. To make Na-gluconate solution, NaCl was replaced with Na-gluconate. In some cases where we tested salt-induced calcium transients (Figure S3 and S4), the osmolarity of bath solution used to pre-incubate the worm nose was adjusted to the same level of the stimulus-solution that contained 500 mM NaCl or Na-gluconate. In doing so, worms would not detect a change in osmolarity, and the only difference would be salt.
Electrophysiology
Patch-clamp recordings were performed as previously described on an Olympus upright microscope with an EPC-10 amplifier (Kang et al., 2010; Li et al., 2011; Li et al., 2014). Worms were dissected on a sylgard-coated coverglass. Standard bath solution (in mM): 145 NaCl, 2.5 KCl, 1 CaCl2, 1 MgCl2, 20 glucose, and 5 HEPES (320 mOsm; pH adjusted to 7.3). To make alkaline pH bath solutions, we used Tris base to adjust the pH to 9 and then to 12 with NaOH, and the osmolarity of the alkaline solution was also adjusted to 320 mOsm. This solution was made and used freshly to avoid precipitation. NMDG-Cl and Na-gluconate bath solution was made by replacing NaCl with the same concentration of NMDG-Cl and Na-gluconate, respectively. Normal pipette solution: 115 K-gluconate, 15 KCl, 1 MgCl2, 10 HEPES, 0.25 CaCl2, 20 sucrose, 5 BAPTA, 5 Na2ATP and 0.5 NaGTP. To derive I–V relations, 115 mM CsCl was used to replace K-gluconate in the pipette solution, and 20 mM TEA and 5 mM 4-AP were also included to block voltage-gated potassium channels. To identify ASH and ASI neurons for recording, we labeled them with DsRed and YFP fluorescence markers expressed from a transgene xuEx631[Psra-6::DsRed + Pstr-3::yfp2]. To record ASH currents, we perfused the solution towards the nose tip where the sensory endings of ASH are localized. To record ASI neurons ectopically expressing TMC-1, we perfused alkaline pH solution towards the cell body, as this induced more robust currents. It is possible that the ectopically-expressed TMC-1 was enriched in the cell body. A similar phenomenon was observed with ADL and ADF neuron recordings, in which case we detected robust currents when stimulating the cell body rather than the nose tip. Series resistance and membrane capacitance were both compensated during recording.
Supplementary Material
Acknowledgments
We thank Eleni Gourgou, Daphne Bazopoulou, and Nikos Chronis for assistance on setting up the microfluidic system, and other lab members for technical assistance. Some strains were obtained from the CGC and Knockout Consortiums in the U.S.A. and Japan. This work was supported by the NSFC (31130028, 31225011 and 31420103909 to J.L.), the Program of Introducing Talents of Discipline to the Universities from the Ministry of Education (B08029 to J.L.), the Ministry of Science and Technology of China (2012CB51800 to J.L.), and the NIGMS (X.Z.S.X.).
Footnotes
Author Contributions
X.W. performed patch-clamp recordings and behavioral experiments and analyzed the data. G.L. carried out molecular biology, genetics, and calcium imaging experiments and analyzed the data. J.L. provided technical assistance on patch-clamp. X.W., G.L., J.L. and X.Z.S.X. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Beurg M, Kim KX, Fettiplace R. Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. The Journal of general physiology. 2014;144:55–69. doi: 10.1085/jgp.201411173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant BP. Mechanisms of somatosensory neuronal sensitivity to alkaline pH. Chemical senses. 2005;30(Suppl 1):i196–197. doi: 10.1093/chemse/bjh182. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chatzigeorgiou M, Bang S, Hwang SW, Schafer WR. tmc-1 encodes a sodium-sensitive channel required for salt chemosensation in C. elegans. Nature. 2013;494:95–99. doi: 10.1038/nature11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis N, Zimmer M, Bargmann CI. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods. 2007;4:727–731. doi: 10.1038/nmeth1075. [DOI] [PubMed] [Google Scholar]
- de la Roche J, Eberhardt MJ, Klinger AB, Stanslowsky N, Wegner F, Koppert W, Reeh PW, Lampert A, Fischer MJ, Leffler A. The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. The Journal of biological chemistry. 2013;288:20280–20292. doi: 10.1074/jbc.M113.479337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, Patapoutian A. TRPV1 is activated by both acidic and basic pH. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:153–158. doi: 10.1523/JNEUROSCI.4901-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita F, Uchida K, Moriyama T, Shima A, Shibasaki K, Inada H, Sokabe T, Tominaga M. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. The Journal of clinical investigation. 2008;118:4049–4057. doi: 10.1172/JCI35957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Wu TH, Song YX, Ge MH, Su CM, Niu WP, Li LL, Xu ZJ, Ge CL, Al-Mhanawi MT, et al. Reciprocal inhibition between sensory ASH and ASI neurons modulates nociception and avoidance in Caenorhabditis elegans. Nature communications. 2015;6:5655. doi: 10.1038/ncomms6655. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. Embo J. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Bergamasco C, Arbucci S, Plasterk RH, Bazzicalupo P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. Embo J. 2004;23:1101–1111. doi: 10.1038/sj.emboj.7600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Acid-sensitive ion channels and receptors. Handbook of experimental pharmacology. 2009:283–332. doi: 10.1007/978-3-540-79090-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZS. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Geleoc GS, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, et al. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. The Journal of clinical investigation. 2011;121:4796–4809. doi: 10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Kurima K, Pan B, Griffith AJ, Holt JR. Transmembrane channel-like (TMC) genes are required for auditory and vestibular mechanosensation. Pflugers Archiv: European journal of physiology. 2015;467:85–94. doi: 10.1007/s00424-014-1582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes G, Mutai H, Heller S. TMC and EVER genes belong to a larger novel family, the TMC gene family encoding transmembrane proteins. BMC genomics. 2003;4:24. doi: 10.1186/1471-2164-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- Kurima K, Yang Y, Sorber K, Griffith AJ. Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis. Genomics. 2003;82:300–308. doi: 10.1016/s0888-7543(03)00154-x. [DOI] [PubMed] [Google Scholar]
- Li W, Kang L, Piggott BJ, Feng Z, Xu XZS. The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nature communications. 2011;2:315. doi: 10.1038/ncomms1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu J, Zheng M, Xu XZ. Encoding of both analog- and digital-like behavioral outputs by one C. elegans interneuron. Cell. 2014;159:751–765. doi: 10.1016/j.cell.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljestrand G, Zotterman Y. The alkaline taste. Acta physiologica Scandinavica. 1956;35:380–389. doi: 10.1111/j.1748-1716.1955.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Liman ER, Zhang YV, Montell C. Peripheral coding of taste. Neuron. 2014;81:984–1000. doi: 10.1016/j.neuron.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellem JE, Brockie PJ, Zheng Y, Madsen DM, Maricq AV. Decoding of polymodal sensory stimuli by postsynaptic glutamate receptors in C. elegans. Neuron. 2002;36:933–944. doi: 10.1016/s0896-6273(02)01088-7. [DOI] [PubMed] [Google Scholar]
- Murayama T, Takayama J, Fujiwara M, Maruyama IN. Environmental alkalinity sensing mediated by the transmembrane guanylyl cyclase GCY-14 in C. elegans. Current biology: CB. 2013;23:1007–1012. doi: 10.1016/j.cub.2013.04.052. [DOI] [PubMed] [Google Scholar]
- Pan B, Geleoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron. 2013;79:504–515. doi: 10.1016/j.neuron.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott BJ, Liu J, Feng Z, Wescott SA, Xu XZS. The neural circuits and synaptic mechanisms underlying motor initiation in C. elegans. Cell. 2011;147:922–933. doi: 10.1016/j.cell.2011.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roayaie K, Crump JG, Sagasti A, Bargmann CI. The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron. 1998;20:55–67. doi: 10.1016/s0896-6273(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Sassa T, Maruyama IN. A G-protein alpha subunit, GOA-1, plays a role in C. elegans avoidance behavior of strongly alkaline pH. Communicative & integrative biology. 2013;6:e26668. doi: 10.4161/cib.26668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa T, Murayama T, Maruyama IN. Strongly alkaline pH avoidance mediated by ASH sensory neurons in C. elegans. Neuroscience letters. 2013;555:248–252. doi: 10.1016/j.neulet.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83:207–218. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- Ward A, Liu J, Feng Z, Xu XZ. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nature Neurosci. 2008;11:916–922. doi: 10.1038/nn.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends in neurosciences. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gualberto DG, Guo X, Correa P, Jee C, Garcia LR. TMC-1 attenuates C. elegans development and sexual behaviour in a chemically defined food environment. Nature communications. 2015;6:6345. doi: 10.1038/ncomms7345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.