Abstract
The anterior belly of the digastric muscle (ABDM) is important in a variety of surgeries including submental lipectomy, rhytidectomy, alteration of the cervicomental angle via muscle resection, the “digastric corset” procedure for submental rejuvenation, the submental artery flap, and reanimation of the mouth after facial nerve palsy. Despite its clinical significance, little information exists regarding the morphometrics of the ABDM or its associated intermediate tendon (IT). This study analyzed a total of 35 intact ABDMs and 43 intact ITs from 23 cadavers. Measurements were taken of the following parameters: muscle belly area, muscle belly length, intermediate tendon length, and intermediate tendon width at mid-tendon. Normative descriptive statistics are included within the report. Males were found to have significantly longer left-sided muscle bellies than 1.) right-sided bellies from males (U=23.0; p=0.044), 2.) left-sided bellies from females (U=19.0; p=0.020), and 3.) right-sided bellies from females (U=12.0; p=0.035). The morphometry, including sexual dimorphism, presented in this report can aid in the surgical planning and execution of numerous operations performed in head and neck, especially digastric muscle transfer surgery.
Keywords: anatomic variation, cosmetic and plastic surgery, maxillofacial surgery, submental artery flap, reconstructive surgery
Introduction
The digastric muscle is typically composed of two muscle bellies joined together by an intermediate tendon (IT).1 The anterior belly of the digastric muscle (ABDM) originates from the digastric fossa to join the IT and the posterior belly of the digastric muscle (PBDM) originates from the mastoid notch of the temporal bone to join the IT. The muscle marks the inferior borders of the submandibular triangle. Likewise, the ABDM marks the lateral border of the submental triangle. Also, the IT, along with the posterior margin of the mylohyoid and the hypoglossal nerve (XII) form the triangle of the lingual artery (Pirogoff’s triangle).2
Although the digastric muscle typically has two bellies joined by an IT, variations are common, particularly with regard to the anterior digastric musculature. Muscle bellies may be supernumerary (double, triple, quadruple), and exist in exotic patterns.3–9 These variant muscles may travel unilaterally or traverse the midline.3–9 Likewise, additional muscle bellies may have attachments that are muscular or tendinous and well-defined, similar to that of a typical IT.3–9
In addition to variation of the ABDM, variations of the IT have been reported, especially with regard to its relationship to the stylohyoid muscle.10,11 The IT may pierce or travel either lateral (superficial) or medial (deep) to the stylohyoid muscle.11 Additionally, the IT may be absent altogether.11
The ABDM and IT have been successfully transferred in order to reanimate the mouth after palsy of the marginal mandibular branch of the facial nerve (MMBFN).12–16 In brief, the muscle transfer procedure involves transection of the IT from the PBDM, splitting the IT, and then tethering the IT to the orbicularis oris ipsilateral to the palsy in order to restore facial symmetry.14–16 Recently, it has been proposed that variant ABDMs could be utilized for the same purpose, in a similar manner, without disturbing the normal musculature.17 However, variant anterior digastric musculature would likely need to have a length comparable to that of normal anterior digastric musculature in order to adequately reach the orbicularis oris.17
Unfortunately, there is a paucity of literature describing the morphometric measurements of the ABDM and the IT of the digastric muscle despite the clinical usefulness, especially with regard to the transfer of the ABDM and IT for the management of MMBFN palsy. Therefore, the aim of this study is to assess the structure of the ABDM and the IT.
Materials and Methods
Twenty-three Caucasian cadavers from the gross anatomical laboratory at West Virginia University School of Medicine, with no remarkable supernumerary anterior digastric musculature identified post-dissection, were selected for the study. The average age-at-death of the population was 82.6 ± 9.8 years (Mean ± SD). The population sample included 11 females with an average age of 81.7 ± 11.6 years (Mean ± SD) and 12 males with an average age of 83.5 ± 8.1 years. Bilateral ABDM bellies were resected from the mandibles complete with their ITs and the anterior-most portions of the PBDMs. The musculature was then positioned alongside digital calipers (Mitutoyo 0–8 in (0–203.2mm) ABSOLUTE™ digimatic caliper series 500, accuracy ± 0.001 in (0.025 mm)) that were locked to a known distance of 10.00 mm. Photographs of the musculature, positioned flush with the calipers, were taken with a digital camera (Canon PowerShot SX50 HS, 12.1 Megapixel). Digital photographs were then assessed with the built-in functions of ImageJ software (NIH) by using the 10.00 mm calibration as a reference. The researcher assessing the photographs was unaware of the sex of the cadavers from whom the ABDMs were sampled. Measurements were taken of the following parameters: muscle belly area, muscle belly length, intermediate tendon length, and intermediate tendon width at mid-tendon. Descriptive and inferential statistics as well as graphical representation of the data were produced using GraphPad Prism software (Version 6).
Results
Musculature from 23 cadavers (11 females and 12 males), with no statistically significant difference in age (U= 59.5; p=0.688) were included in this study; however, due to damages occurring during prior student-performed neck dissections, not all muscle bellies and tendons remained intact. Muscle bellies or tendons that were not intact were excluded from the study. There were a total of 35 intact ABDMs (20 left-sided bellies and 15 right-sided bellies) and 43 intact ITs (22 left-sided and 21 right-sided). Altogether, there were 35 intact ABDMs complete with their own associated tendons. A photograph of resected ABDMs with their ITs can be found in Figure 1. Normative morphometric data collected from intact structures and can be found in Table 1. Left- to right-sided comparison data can be found in Table 2.
Figure 1.
Bilateral anterior digastric muscle bellies resected along with their associated intermediate tendons and initial portions of the posterior bellies.
Table 1.
Normative measurements for the anterior belly of the digastric muscle and the intermediate tendon of the digastric muscle.
| Parameter | Side(s) | N | Minimum | Maximum | Range | Mean ± SD | SEM |
|---|---|---|---|---|---|---|---|
| Belly Length (mm) | L | 20 | 28.2 | 60.9 | 32.7 | 40.6 ± 7.9 | 1.8 |
| R | 15 | 24.8 | 48.1 | 23.3 | 36.2 ± 5.8 | 1.5 | |
| L & R | 35 | 24.8 | 60.9 | 36.1 | 38.7 ± 7.4 | 1.2 | |
| Belly Area (mm2) | L | 20 | 291.0 | 799.0 | 508.0 | 489.0 ± 153.6 | 34.3 |
| R | 15 | 351.5 | 655.0 | 303.5 | 478.5 ± 101.2 | 26.1 | |
| L & R | 35 | 291.0 | 799.0 | 508.0 | 484.5 ± 132.0 | 22.3 | |
| Tendon Length (mm) | L | 22 | 23.9 | 48.1 | 24.2 | 40.6 ± 5.8 | 1.2 |
| R | 21 | 31.0 | 59.8 | 28.8 | 42.6 ± 7.8 | 1.7 | |
| L & R | 43 | 23.9 | 59.8 | 35.9 | 41.6 ± 6.8 | 1.0 | |
| Tendon Width (mm) | L | 22 | 2.95 | 4.89 | 1.94 | 3.58 ± 0.50 | 0.11 |
| R | 21 | 2.11 | 5.52 | 3.41 | 3.71 ± 0.84 | 0.18 | |
| L & R | 43 | 2.11 | 5.52 | 3.41 | 3.64 ± 0.68 | 0.10 | |
| Belly + Tendon Length (mm) | L | 20 | 62.0 | 102.6 | 40.6 | 80.8 ± 9.3 | 2.1 |
| R | 15 | 68.0 | 96.1 | 28.1 | 80.7 ± 9.7 | 2.5 | |
| L & R | 35 | 62.0 | 102.6 | 40.6 | 80.8 ± 9.4 | 1.6 | |
| Belly to Tendon Length Ratio (Belly Length/Tendon Length) | L | 20 | 0.63 | 1.59 | 0.96 | 1.04 ± 0.28 | 0.06 |
| R | 15 | 0.57 | 1.42 | 0.84 | 0.84 ± 0.22 | 0.06 | |
| L & R | 35 | 0.57 | 1.59 | 1.02 | 0.95 ± 0.27 | 0.05 |
Table 2.
Comparison between paired right and left anterior digastric muscle bellies and intermediate tendons via Wilcoxon’s paired-samples signed-rank test.
| Pair | W | P |
|---|---|---|
| L vs R Muscle Belly Length | −69 | 0.0497* |
| L vs R Muscle Belly Area | 26 | 0.4887 |
| L vs R Tendon Length | 66 | 0.2305 |
| L vs R Tendon Width | −26 | 0.6409 |
| L vs R Belly & Tendon Length | −4 | 0.9341 |
| L vs R Belly to Tendon Ratio | −74 | 0.0353* |
statistically significant (p < 0.05)
Anterior Belly of the Digastric Muscle
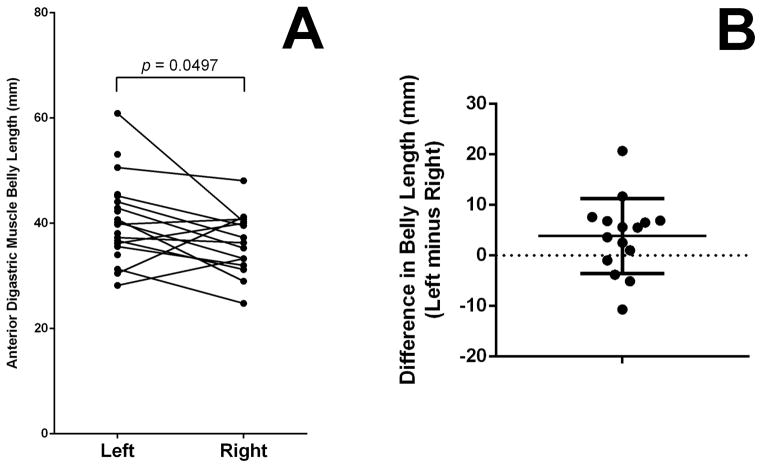
The average muscle belly length and area, determined from all intact samples (n=35) was 38.7 ± 7.4 mm and 484.5 ± 132.0 mm2 (Mean ± SD), respectively. There was no significant difference between the left- and right-sided muscle belly lengths (U=100.5, p=0.1011). Likewise, there was no statistically significant difference between left- and right-sided muscle areas (U=150, p>0.9999). However, Wilcoxon’s matched-pairs signed rank test revealed a significant difference between the lengths of paired (15 intact pairs) left- and right-sided ABDM muscle bellies (W=−69, p=0.0497) (Figure 2A). The left ABDM was longer than the right ABDM in 11 of the 15 pairs (73.3%) (Figure 2B). Wilcoxon’s matched-pairs signed rank test revealed no significant different between left- and right-sided areas (W=26, p=0.4887).
Figure 2.
Comparison of paired left- and right-sided anterior digastric muscle belly lengths. A: Left sided-muscle bellies tended to be longer than their associated right-sided muscle bellies. B: Differences between left-sided and right-sided muscle bellies tended to be positive, indicating longer left-sided bellies. The right-sided muscle belly was longer than that of the left side in four of the 15 muscle belly pairs (26.6%) as indicated by the four dots below the dotted line at zero.
Intermediate Tendon of the Digastric Muscle
The minimum and maximum lengths of the IT tendons were 23.9 mm and 59.8 mm, respectively. The minimum and maximum tendon widths were 2.11 mm and 5.52 mm. The average IT length and width at mid-tendon, determined from all intact samples (n=43) was 41.6 ± 6.8 mm and 3.64 ± 0.68 mm (Mean ± SD), respectively. There was no significant difference between the between the left- and right-sided IT lengths (U=221.5, p=0.824) nor between the left- and right-sided widths (U=216, p=0.7228). Likewise, Wilcoxon’s signed-ranks test did not reveal a significant difference between paired (20 intact pairs) right and left IT lengths (W=66, p=0.2305) nor widths (W=−26, p=0.6409).
Muscle Belly and Tendon Relationships
When muscle belly and tendon lengths were added, the minimum total length was 62.0 mm and the maximum total length was 102.6 mm. The average ABDM with IT length was 80.8 ± 9.4 mm (Mean ± SD). There were no correlations between left-sided belly and tendon lengths (rs=−0.20, p=0.394), right-sided belly and tendon lengths (rs=−0.05, p=0.860), or belly and tendon lengths, in general (rs=−0.18, p=0.294). The average ratio between muscle belly length and IT length was 0.95 ± 0.27 (Mean ± SD) among the 35 intact bellies with tendons, which is consistent with the similar aforementioned averages of muscle belly length (38.7 ± 7.4 mm) and IT length (41.6 ± 6.8 mm). Muscle belly length to IT tendon length ratios of paired left and right sides were found to be significantly different (W=−74, p=0.0353) (Figure 3A) (Table 2). The left-sided belly-to-tendon ratio was larger than that of the right-sided ratio in all but four of the 15 paired samples (Figure 3B). Similarly, with regard to the total unpaired sampling of digastric musculature, the left-sided (N=20) belly-to-tendon ratio was significantly larger than that of the right-sided (N=15) belly-to-tendon ratio (U=80, p=0.0189) (Figure 4).
Figure 3.
Comparison of paired left- and right-sided anterior digastric muscle belly to intermediate tendon (IT) lengths. A: Left-sided muscle belly length to IT length ratio was significantly different than that of the right side (W=−74, p=0.0353). In 11 of the 15 pairs, the left-sided belly-to-tendon ratio was larger. A ratio larger than 1.0 indicates a muscle belly length larger than that of the intermediate tendon length. On the left side, 9 ratios were greater than 1.0 and 11 ratios were less than 1.0; therefore, 45% of left sides had muscle bellies longer than their tendons. On the right side, only 2 out of 15 ratios were greater than 1.0; therefore, only 13.3% of right sides had muscle bellies longer than their tendons. B: The difference between the left- and right-sided belly-to-tendon ratios tended to be positive (left side ratio larger than paired right side ratio). On average, the left-side ratio was 0.16 ± 0.26 (Mean ± SD) larger than that of the right side.
Figure 4.
Comparison of unpaired left- and right-sided anterior digastric muscle belly to intermediate tendon length ratios. A ratio larger than 1.0 indicates a muscle belly length larger than that of the intermediate tendon length. Because the average ratio (n=20) on the left side was greater than 1.0, the left-sided muscle bellies tended to be longer than their associated intermediate tendons. Conversely, because the average ratio (n=15) on the right side was less than 1.0, the right-sided muscle bellies tended to be shorter than their associated intermediate tendon.
Sexual Dimorphism
Male muscle belly length was an average of 36.7 ± 6.6 mm (Mean ± SD), whereas female muscle belly length was 35.4 ± 4.9 mm. When grouping left- and right-sided ABDMs together, there was no significant difference between male and female ABDM muscle lengths (U=25.5; p=0.86). However, males were found to have significantly longer left-sided muscle bellies than 1.) right-sided bellies from males (U=23.0; p=0.044), 2.) left-sided bellies from females (U=19.0; p=0.020), and 3.) right-sided bellies from females (U=12.0; p=0.035) (Figure 5). However, no significant differences were found between male and female muscle belly areas (U=13.0; p=0.099), tendon lengths (U=44.0; p=0.477), tendon widths (U=41.5; p=0.374), belly-plus-tendon length (U=26.0; p=0.906), nor belly-to-tendon-ratio (U=26.0; p=0.906).
Figure 5.
Left- and right-sided anterior belly of digastric muscle (ABDM) length - sexual dimorphism. Male left ABDM lengths were significantly longer than those of male right ABDMs and both left- and right-sided ABDMs from females. The average length of the male left ABDM was 44.2 ± 2.5 mm (Mean ± SEM) compared to male right ABDM averaging 36.7 ± 2.2 mm, the female left ABDM averaging 36.3 ± 1.6 mm, and the female right ABDM measuring 35.4 ± 2.0 mm.
Discussion
The ABDM has clinical importance among a variety of surgical procedures including submental lipectomy, rhytidectomy, and alteration of the cervicomental angle via partial resection of the muscle belly.5,18 Likewise, tethering the ABDMs together has been successfully performed in the “digastric corset” procedure for submental rejuvenation.19,20 With regard to reconstructive surgery, the ABDMs have been included in the submental artery flap procedure despite controversy.21 The submental artery flap has been utilized for reconstruction of the lower and mid face, pharynx, palate, oral cavity, eye socket, nose, columella, tongue, and temporal bone defects as well as the prevertebral space during spine surgery.22–36 Additionally, the ABDM and its IT have been successfully transferred in order to reanimate the mouth after palsy of the MMBFN.12–16
In addition to the significance of the ABDM in surgery, an understanding of the musculature is generally important in order to differentiate the muscle bellies from surrounding structures such as variant anterior digastric bellies, submental lymph nodes, muscular asymmetries due to trigeminal nerve lesions, and space occupying lesions.3,11,37–39 Similarly, the ABDM size, specifically the area that it occupies, may be important in botulinum toxin type A injection into ABDM musculature for the correction of post-traumatic anterior open bite.40,41 Despite the clinical importance of the ABDM, few studies have assessed normative morphology of the musculature. This report currently provides the most complete morphometric data regarding the anterior digastric muscle belly and the intermediate tendon of the digastric muscle.
Anterior Belly of the Digastric Muscle Transfer Surgery
Transfer of the ABDM was first introduced by Edgerton in 1965.12,13 The procedure included: 1.) freeing the ABDM from the mandible; 2.) grafting fascia lata to the muscle; 3.) splitting the fascia lata into two tails; 4.) looping the graft through the paralyzed musculature; and 5.) suturing the graft to the orbicularis oris.13 Edgerton also posited an alternative where the fascia is sutured directly to the digastric tendon without freeing the muscle from the mandible.13 Since Edgerton’s first reports, a number of other accounts have documented the successful use of the digastric transfer; however, these procedures varied from those of Edgerton.12–16 Several research groups have utilized a procedure in which the digastric tendon is mobilized and tethered to the orbicularis oris, without the use of the fascia lata.14–16
Conley et al14 mentioned that, “[i]t is important to include all the subdigastric tendon in the transferred specimen, because it supplies over half the length of the transposed segment and permits some liberties in fabrication at its point of insertion into the lower lip.” The present study documents that, on the left side, the muscle belly tends to be longer than the tendon whereas, on the right side, the tendon tends to be longer than the muscle belly. So, the data presented in this study partly disagrees with comment from Conley et al14 that the tendon makes over half the length of the transferred specimen. However, with regard to the inclusion of the entirety of the tendon to be included in the transfer, other authors concur with Conley et al.14 For example, Terzis and Kalantarian15 likewise have noted the importance of including the whole extent of the digastric tendon. Tan16 not only included the entirety of the intermediate tendon in his procedure, but also included the initial portion of the posterior belly in the transfer surgery. Therefore, the length of the IT and the length of the ABDM complete with the IT are clinically relevant measurements.
The average ABDM length from the general population of this study was determined to be 38.7 ± 7.4 mm. With regard to sexual differences, males tended to have longer left-sided muscle bellies than right-sided muscle bellies and muscle bellies from either side of females. However, there were no differences between the belly-plus-tendon lengths between the sexes. Assuming that the distances between the digastric fossae of the mandibles, to the hyoids, and then to the mastoid notch of the temporal bones is equidistant among the left and right sides of males, then PBDMs on the left side would likely be shorter than those of the right side, according to the data of this study. Future studies should pay attention to potential asymmetry in the lengths of the PBDMs in males because the PBDMs are important surgical landmarks.42
The average length of the 43 tendons measured in this study was 41.6 ± 6.8 mm (Mean ± SD), with a minimum length of 23.9 mm and a maximum length of 59.8 mm. The total length of the ABDM complete with its tendon averaged 80.8 ± 9.4 mm (Mean ± SD), with a minimum and maximum length of 60.2 mm and 102.6 mm, respectively. Therefore, surgeons should be aware of the wide range of IT and ABDM with IT lengths. No absence of the IT was noted in this study; however, De-Ary-Pires et al11 have reported that the digastric muscle may not have an IT present. Therefore, it is important for surgeons to be aware that tendon length is, on average, 41.6 mm long but may be as long as 59.8 mm or absent altogether. Intermediate tendon length may affect: 1.) the possibility for transfer, in general, 2.) the decision of insertion locations at the orbicularis oris, and 3.) the decision of whether or not to mobilize the ABDM muscle belly from its mandibular attachment site – a component of the protocol utilized by Terzis and Kalantarian15.
During reconstructive procedures using the ABDM and its IT, several surgeons report splitting the IT longitudinally into strips.14–16 Conley et al14 reported splitting the tendon for 1 cm, in order to form two tendinous strips. Terzis and Kalantarian15 split the tendon into four separate strips, the exact length of which determined by viewing preoperative videos. Tan16 spit the tendon into three slips, suturing the lateral slip near the commissure, the medial slip at the midline of the lip, and the middle slip in between the lateral and medial slip. Because, the aforementioned reports were not in agreement with regard to the number of tendinous slips produced from an individual IT, it warrants discussion about tendon width.
This study documented that the average width of the intermediate tendon was 3.64 ± 0.68 mm (Mean ± SD). However, there was a broad range of IT widths, from a minimum of 2.11 mm to a maximum width of 5.52 mm. Therefore, the assessment of the tendon width may guide the surgeon’s decision to divide the IT into two, three, or four slips in the manner of Conley et al14, Tan16, or Terzis and Kalantarian15, respectively. Upon dividing the average width of the IT determined from this study (3.64 mm) by the number of intended slips, one may calculate the width of the individual slips, assuming the slips are equal in size. For example, if the IT were divided into two equal slips, the width of each slip would average 1.82 mm/slip. Dividing the tendon in three or four equal slips would yield average widths of 1.21 mm/slip and 0.91 mm/slip, respectively.
It was recently suggested that variant anterior digastric musculature could be transferred in a manner similar to procedures utilizing normal ABDM musculature without disturbing “normal”, or non-variant, anatomy.17 The variant anterior digastric muscle transfer procedure would, however, require a variant with a structure as long as that required of a normal ABDM transfer.17 According to the data from this report, the average length of the ABDM along with its IT is approximately 8 cm (80.8 ± 9.4 mm; Mean ± SD). Variant digastric musculature may be measured and assessed alongside the normative data presented in this report in order to screen for potential variant ABDM transfer candidates.
This study provides a comprehensive review of the use of the anterior belly of the digastric muscle and its associated intermediate tendon in a variety of head and neck surgeries in the submental region, including submental lipectomy, rhytidectomy, alteration of the cervicomental angle via muscle resection, the “digastric corset” procedure for submental rejuvenation, the submental artery flap, and reanimation of the mouth after facial nerve palsy. Moreover, this paper provides comprehensive morphometric data concerning the muscle belly area, muscle belly length, intermediate tendon length, and intermediate tendon width at mid-tendon. These measurements will assist clinicians in the planning and execution of these reconstructive surgeries to achieve the desired outcome of both the surgeon and the patient.
Acknowledgments
The work was supported by two West Liberty University Faculty Development Grants in addition to grant funding from the West Virginia IDeA Network for Biomedical Research Excellence [P20GM103434], and NIH-NIAID [5K22AI087703]. The authors would like to thank: 1) Dr. Richard Dey, Chair of the Department of Neurobiology and Anatomy at West Virginia University School of Medicine, 2) the West Virginia Anatomical Board, and 3) the individuals who donated their bodies for the advancement of education and research. Without their support, this project could not have been completed.
References
- 1.Standring S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 40. New York: Churchill-Livingston/Elsevier; 2008. p. 600. [Google Scholar]
- 2.Homze EJ, Harn SD, Bavitz BJ. Extraoral ligation of the lingual artery: an anatomic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:321–324. doi: 10.1016/s1079-2104(97)90236-5. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SG, Lufkin RB. Anomalies of digastric muscles: CT and MR demonstration. J Comput Assist Tomogr. 1987;11:422–425. doi: 10.1097/00004728-198705000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Sargon MF, Onderoğlu S, Sürücü HS, et al. Anatomic study of complex anomalies of the digastric muscle and review of the literature. Okajimas Folia Anat Jpn. 1999;75:305–313. doi: 10.2535/ofaj1936.75.6_305. [DOI] [PubMed] [Google Scholar]
- 5.Ozgur Z, Govsa F, Ozgur T. Bilateral quadrification of the anterior digastric muscles with variations of the median accessory digastric muscles. J Craniofac Surg. 2007;18:773–775. doi: 10.1097/scs.0b013e318068ff09. [DOI] [PubMed] [Google Scholar]
- 6.Ozgur Z, Govsa F, Celik S, et al. An unreported anatomical finding: unusual insertions of the stylohyoid and digastric muscles. Surg Radiol Anat. 2010;32:513–517. doi: 10.1007/s00276-009-0553-4. [DOI] [PubMed] [Google Scholar]
- 7.Zdilla MJ, Soloninka HJ, Lambert HW. A fractal anterior digastric: a case report with surgical implications. Int J Anat Var. 2014;7:106–108. doi: 10.1093/jscr/rju131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zdilla MJ, Soloninka HJ, Lambert HW. Unilateral duplication of the anterior digastric muscle belly: a case report with implications for surgeries of the submental region. J Surg Case Rep. 2014;12:1–2. doi: 10.1093/jscr/rju131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey JA, Call Z, Peterson K, et al. Weave pattern of accessory heads to the anterior digastric muscle. Surg Radiol Anat. 2015;37(8):1001–1004. doi: 10.1007/s00276-014-1401-8. [DOI] [PubMed] [Google Scholar]
- 10.Mori M. Statistics on the musculature of the Japanese. Okajimas Fol Anat Jap. 1964;40:195–300. doi: 10.2535/ofaj1936.40.3_195. [DOI] [PubMed] [Google Scholar]
- 11.De-Ary-Pires B, Ary-Pires R, Pires-Neto MA. The human digastric muscle: patterns and variations with clinical and surgical correlations. Ann Anat. 2003;185(5):471–479. doi: 10.1016/S0940-9602(03)80110-3. [DOI] [PubMed] [Google Scholar]
- 12.Edgerton MT. Digastric muscle transfer to correct deformity of the lower lip resulting from paralysis of the marginal branch of the facial nerve. Presented at the Meeting of the American Society of Plastic and Reconstructive Surgeons; Philadelphia. October, 1965. [Google Scholar]
- 13.Edgerton MT. Surgical correction of facial paralysis: a plea for better reconstructions. Ann Surg. 1967;165:985–998. doi: 10.1097/00000658-196706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conley J, Baker DC, Selfe RW. Paralysis of the mandibular branch of the facial nerve. Plast Reconstr Surg. 1982;70:569–577. doi: 10.1097/00006534-198211000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Terzis JK, Kalantarian B. Microsurgical strategies in 74 patients for restoration of dynamic depressor muscle mechanism: a neglected target in facial reanimation. Plast Reconstr Surg. 2000;105:1917–1931. doi: 10.1097/00006534-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Tan ST. Anterior belly of digastric muscle transfer: A useful technique in head and neck surgery. Head Neck. 2002;24:947–954. doi: 10.1002/hed.10150. [DOI] [PubMed] [Google Scholar]
- 17.Zdilla MJ. Variant anterior digastric muscle transfer for marginal mandibular branch of facial nerve palsy. Plast Reconstr Surg Glob Open. 2014;2:e110. doi: 10.1097/GOX.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connell BF, Shamoun JM. The significance of digastric muscle contouring for rejuvenation of the submental area of the face. Plast Reconstr Surg. 1997;99:1586–1590. [PubMed] [Google Scholar]
- 19.Labbé D, Giot JP, Kaluzinski E. Submental area rejuvenation by digastric corset: anatomical study and clinical application in 20 cases. Aesthetic Plast Surg. 2013;37:222–31. doi: 10.1007/s00266-013-0083-7. [DOI] [PubMed] [Google Scholar]
- 20.Labbé D, Giot JP. Open Neck Contouring. Clin Plast Surg. 2014;41:57–63. doi: 10.1016/j.cps.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Zdilla MJ. Exclusion of musculature from the submental flap: a contingency plan for facial nerve palsy. Plast Reconstr Surg Glob Open. 2014;2:e266. doi: 10.1097/GOX.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curran AJ, Neligan P, Gullane PJ. Submental artery island flap. Laryngoscope. 1997;107:1545–1549. doi: 10.1097/00005537-199711000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz M, Menderes A, Barutçu A. Submental artery island flap for reconstruction of the lower and mid face. Ann Plast Surg. 1997;39:30–35. doi: 10.1097/00000637-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Kim JT, Kim SK, Koshima I, et al. An anatomic study and clinical applications of the reversed submental perforator-based island flap. Plast Reconstr Surg. 2002;109:2204–2210. doi: 10.1097/00006534-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Demir Z, Kurtay A, Sahin U, et al. Hair-bearing submental artery island flap for reconstruction of mustache and beard. Plast Reconstr Surg. 2003;112:423–429. doi: 10.1097/01.PRS.0000070520.96829.85. [DOI] [PubMed] [Google Scholar]
- 26.Genden EM, Buchbinder D, Urken ML. The submental island flap for palatal reconstruction: a novel technique. J Oral Maxillofac Surg. 2004;62:387–390. doi: 10.1016/j.joms.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Demir Z, Velidedeoğlu H, Celebioğlu S. Repair of pharyngocutaneous fistulas with the submental artery island flap. Plast Reconstr Surg. 2005;115:38–44. [PubMed] [Google Scholar]
- 28.Karaçal N, Ambarcioglu O, Topal U, et al. Reverse-flow submental artery flap for periorbital soft tissue and socket reconstruction. Head Neck. 2006;28:40–45. doi: 10.1002/hed.20313. [DOI] [PubMed] [Google Scholar]
- 29.Tan O, Kiroglu AF, Atik B, et al. Reconstruction of the columella using the prefabricated reverse flow submental flap: A case report. Head Neck. 2006;28:653–657. doi: 10.1002/hed.20395. [DOI] [PubMed] [Google Scholar]
- 30.Tan O, Atik B, Parmaksizoglu D. Soft-tissue augmentation of the middle and lower face using the deepithelialized submental flap. Plast Reconstr Surg. 2007;119:873–879. doi: 10.1097/01.prs.0000252002.76466.cf. [DOI] [PubMed] [Google Scholar]
- 31.Sebastian P, Thomas S, Varghese BT, et al. The submental island flap for reconstruction of intraoral defects in oral cancer patients. Oral Oncol. 2008;44:1014–1018. doi: 10.1016/j.oraloncology.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Amin AA, Sakkary MA, Khalil AA, et al. The submental flap for oral cavity reconstruction: extended indications and technical refinements. Head Neck Oncol. 2011;3:51. doi: 10.1186/1758-3284-3-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahpeyma A, Khajehahmadi S, Nakhaei M. Submental artery island flap in reconstruction of hard palate after wide surgical resection of verruccous carcinoma, two case reports. Iran J Otorhinolaryngol. 2013;25:177–181. [PMC free article] [PubMed] [Google Scholar]
- 34.Abboud O, Shedid D, Ayad T. Reconstruction of the prevertebral space with a submental flap: a novel application. J Plast Reconstr Aesthet Surg. 2013;66:1763–1765. doi: 10.1016/j.bjps.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 35.Miller C, Hanley JC, Gernon TJ, et al. The submental island flap for reconstruction of temporal bone defects. Otol Neurotol. 2015;36:879–885. doi: 10.1097/MAO.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 36.Hanna TC, Lubek JE. The hybrid submental flap for tongue reconstruction. J Oral Maxillofac Surg. 2015;73(9):1876. doi: 10.1016/j.joms.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Muraki AS, Mancuso AA, Harnsberger HR, et al. CT of the oropharynx, tongue base, and the floor of the mouth: normal anatomy and range of variations, and applications in staging carcinoma. Radiology. 1983;148:725–731. doi: 10.1148/radiology.148.3.6878693. [DOI] [PubMed] [Google Scholar]
- 38.Guelfguat M, Nurbhai N, Solounias N. Median accessory digastric muscle: radiological and surgical correlation. Clin Anat. 2001;14:42–46. doi: 10.1002/1098-2353(200101)14:1<42::AID-CA1007>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 39.Robbins KT, Clayman G, Levine PA, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128:751–758. doi: 10.1001/archotol.128.7.751. [DOI] [PubMed] [Google Scholar]
- 40.Seok H, Park YT, Kim SG, et al. Correction of post-traumatic anterior open bite by injection of botulinum toxin type A into the anterior belly of the digastric muscle: case report. J Korean Assoc Oral Maxillofac Surg. 2013;39:188–192. doi: 10.5125/jkaoms.2013.39.4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zdilla MJ. Screening for variations of anterior digastric musculature prior to correction of post-traumatic anterior open bite by injection of botulinum toxin type A: a technical note. J Korean Assoc Oral Maxillofac Surg. 2015;41:165–167. doi: 10.5125/jkaoms.2015.41.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ankolekar VH, Souza AD, Alva R, et al. Posterior belly of the digastric muscle: an important landmark for various head and neck surgeries. Arch Clin Exp Surg. 2015;4:79–82. [Google Scholar]