Abstract
Background
Emergency department (ED) patients commonly smoke. Current treatment approaches use motivational interviewing, which is effective, but resource-intensive. Mobile health approaches may be more feasible and generalizable.
Objective
To assess the feasibility of an ED-initiated program of tobacco dependence treatment that employs text messaging.
Methods
Smokers age 18 or older were randomized to intervention or control arms. Control subjects received a brochure describing the state smokers’ quitline. Intervention subjects received the brochure, four weeks of nicotine patches and gum, with the initial dose administered in the ED, a referral to the quitline, and enrollment in SmokeFreeTxt, a free SMS-messaging service. SmokeFreeTxt delivered 28 days of messages, 2–5 messages/day. Some messages ask subjects to provide data on mood or craving. Follow-up was conducted by phone call.
Results
60 subjects were enrolled in May 2014. Of all subjects, 33 (55%) were nonwhite; 78% were insured by Medicaid. All intervention subjects used the texting program, with 24/30 (80%) using the program for all 28 days. At one month, 14/30 subjects (47%) in the intervention arm reported abstinence, vs. 3/30 (10%) in the control arm (P=0.003). At three months, the abstinence rates in the intervention and control arms were, respectively, 9/30 (30%) and 4/30 (13%) (P=0.21). Subjects responding to more assessments of mood or craving were more likely to report abstinence at one month.
Conclusion
A texting program, combined with pharmacotherapy and a quitline referral, is feasible and may promote tobacco abstinence in ED smokers. A larger trial is planned to assess these results.
Keywords: smoking cessation, mobile health, texting, tobacco dependence
Introduction
Numerous effective, evidence-based treatments exist to treat tobacco dependence. In general, treatments may be categorized as pharmacologic (nicotine replacement products, bupropion, varenicline) or behavioral (in-person individualized counseling, group counseling, telephone-based counseling). A substantial evidence base exists to support the efficacy of each, with relative risk ratios for long-term quitting of 1.5–3.1, compared to controls.1,2 Approaches that combine medications and counseling are often synergistic.
One limitation of behavioral approaches is that they require the smoker to attend sessions in person, or arrange phone sessions. For many smokers, constraints of employment, childcare, travel time, and expense make attending counseling sessions difficult. Adherence to behavioral approaches is therefore often suboptimal, particularly among low-income smokers. A recent qualitative study of 29 smoker found that 8 (28%) of them missed more than 2 sessions of a scheduled 7–9 session program of telephone or group counseling.3
Newer approaches to behavioral interventions for smokers that are convenient, scalable, and cost-effective often involve mobile health (m-health) interventions using smartphones. Short-message-service texting is one such approach. A recent Cochrane review found that texting programs can result in a long-term quit rate of 9.4%, compared to 5.5% in controls.4 Texting programs are available at no charge (although the end user may be charged for receiving texts based on their cellular plan). Given the near-ubiquity of cell phones, even among the low-income individuals who have disproportionate rates of smoking, m-health approaches are particularly promising as a scalable intervention for smoking.
No study to date has tested the feasibility or efficacy of incorporating texting into the treatment of tobacco dependence initiated in a hospital emergency department (ED). A recent study demonstrated the efficacy of an ED-initiated program that included provision of 6 weeks of nicotine patches and gum, an active referral to a state smokers’ quitline, a post-discharge follow-up call, and a 10–15 minute motivational interview (MI) delivered by a trained research assistant.5
The motivational interview, although effective, is difficult to administer in busy, under-resourced ED environments. Providers can be trained in the technique, but are often overwhelmed by other clinical duties. Hence, our goal was to modify our intervention by using a smartphone-delivered texting program instead of a MI.
The objective of this study was to assess the feasibility of a multicomponent ED-initiated program of tobacco dependence treatment that employs a publicly available text messaging program, and compare it to a control condition. Consistent with other types of feasibility studies, additional goals of this study were to obtain a measure of effect size for a follow-up trial, and to assess willingness of eligible subjects to participate and be randomized, assess study procedures, and follow-up rates and adherence to the intervention.6
Methods
This was a prospective, two-arm randomized pilot clinical trial with blinded outcome assessment of adult patients visiting the emergency department of an urban teaching hospital in the northeastern United States. Research assistants recruiting subjects were not blinded to group assignment.
Subjects aged 18 years and older who spoke English, had access to a cellphone with texting capability, self-reported daily- or some-day tobacco use, lifetime use of at least 100 cigarettes, and were neither physically nor mentally incapacitated were eligible for the study. Standard sociodemographic data were recorded, along with a 2-question screen for depression.7 Subjects were also asked whether they believe they have a tobacco-related illness, and whether their ED visit was related to tobacco. After providing written informed consent, subjects were randomized to one of two arms in a 1:1 fashion.
Subjects randomized to the intervention received a brochure describing the health benefits of quitting, and the phone number for the state smokers’ quitline. In addition, intervention subjects were given 4 weeks of nicotine patches and gum (tailored to daily cigarette consumption), with the first dose of nicotine replacement therapy (NRT) administered in the ED; a referral faxed to the smokers’ quitline; and enrollment by a research assistant in a free, federally supported texting program for tobacco dependence treatment.
Control arm subjects received the smoking cessation brochure, which meets, or exceeds, current standards of care in the ED management of smokers.
The texting program used was SmokeFreeTXT (http://smokefree.gov/smokefreetxt). SmokeFreeTXT was developed by the National Cancer Institute, and is available at no charge to smokers. SmokeFreeTXT consists of a library of 128 messages. Message content employs principles of cognitive behavioral therapy, and attempts to give smokers tips and strategies to remain abstinent. These messages are sent randomly, between the hours of 9 am and 6 pm, on weekends and weekdays. Subjects could receive a maximum of five messages/day. In this pilot study, we used 86 (67%) of SmokeFreeTXT’s library of messages. Some messages were omitted because they were not appropriate for our study (e.g. preparing for the quit date), or extended beyond the life of the study.
Nineteen of the text messages employed principles of ecological momentary assessment (EMA), asking subjects to respond to queries about mood, craving, tobacco use, or contacts with a personal physician.8,9 Mood and craving were assessed with 3-level ordinal scales, respectively: “Good, OK, Bad” and “Low, Medium, High.” Subjects received 0–1 EMA message/day.
All subjects were called at 1 and 3 months after enrollment to assess outcomes. Biochemical verification of tobacco abstinence was not performed. The primary outcomes were self-reported 7-day tobacco abstinence at 1 and 3 months, and were assessed using standard questions (“Have you smoked, even a puff, in the last 7 days?”).10 Both timepoints were considered primary in this pilot study. Secondary endpoints analyzed included associations between EMA responses and tobacco abstinence, and adherence to EMA and abstinence.
Because this was a pilot trial of feasibility, a formal sample size calculation to test efficacy was not performed. Hence, all efficacy data should be considered exploratory and hypothesis-generating. Baseline characteristics are reported with parametric or nonparametric descriptive statistics, as appropriate. All stochastic tests were two-sided. Alpha was set at 0.05. All analyses were performed with SPSS 21.0. The randomization scheme was obtained by using a seed number at www.randomization.com. Group allocation was concealed to the research assistant, but she was not blinded to group assignment after randomization. Telephone follow-up was conducted by research staff who were blinded to group assignment.
All subjects provided written informed consent. The study was approved by the institution’s Human Investigation Committee, and is publicly registered under NCT02081144.
Results
Sixty subjects were enrolled in May, 2014. Clinical descriptors of subjects are provided in Table 1. Treatment groups were comparable with respect to standard demographic variables. Of all subjects, 30 (50%) were female, 27 (45%) were white, mean age was 40 years (SD = 11 years), and insurance coverage of Medicaid, self-pay, or other, respectively, of 78%, 8%, 14%.
Table 1.
Characteristics of 60 pilot study subjects.
| Variables |
|
||
|---|---|---|---|
| Intervention | Control | ||
|
| |||
| Age, years, mean (SD) | 43.9 (11.2) | 36.3 (10.7) | |
|
| |||
| Sex, N (%) | Male | 14 (46.7%) | 16 (53.3%) |
|
| |||
| Ethnicity, N (%) | Hispanic | 5 (16.7%) | 8 (26.7%) |
|
| |||
| Race, N (%) | African-American | 14 (46.7%) | 13 (43.3%) |
| White | 12 (40.0%) | 15 (50.0%) | |
| Other | 4 (13.3%) | 2 (6.7%) | |
|
| |||
| Insurance Status, N (%) | Self-pay/Uninsured | 2 (6.7%) | 3 (10.0%) |
| Medicaid | 24 (80%) | 23 (76.7%) | |
| Other Insurance | 4 (13.3%) | 4 (13.3%) | |
|
| |||
| Cigarettes/day, median (IQR) | 10 (5, 15) | 10 (4.75, 20) | |
|
| |||
| Depression screen (+) | 14 (46.7%) | 17 (56.7%) | |
|
| |||
| Believe has medical illness caused by smoking | Yes | 13 (43.3%) | 9 (30.0%) |
| No | 15 (50.0%) | 20 (66.7%) | |
| Not sure | 2 (6.7%) | 1 (3.3%) | |
|
| |||
| Believe ED visit is related to smoking | Yes | 7 (23.3%) | 8 (26.7%) |
| No | 14 (46.7%) | 20 (66.7%) | |
| Not sure | 9 (30.0%) | 2 (6.7%) | |
All intervention subjects used the texting program, with 24/30 (80%) using the program for all 28 days; 6 subjects (20%) opted out at some point. At 1 month, 14/30 subjects (47%) in the intervention arm reported tobacco abstinence, vs. 3/30 (10%) in the control arm (P=0.003). At 3 months, the self-reported abstinence rates in the intervention and control arms was, respectively, 9/30 (30%) and 4/30 (13%) (P=0.21).
Response to the EMA messages was variable. Twelve of 30 intervention subjects (40%) responded to no messages. The remaining 18 subjects received a total of 342 EMA texts, responding to 83 (24.6%) of them, for a mean of 4.6 responses per subject (range 1–17).
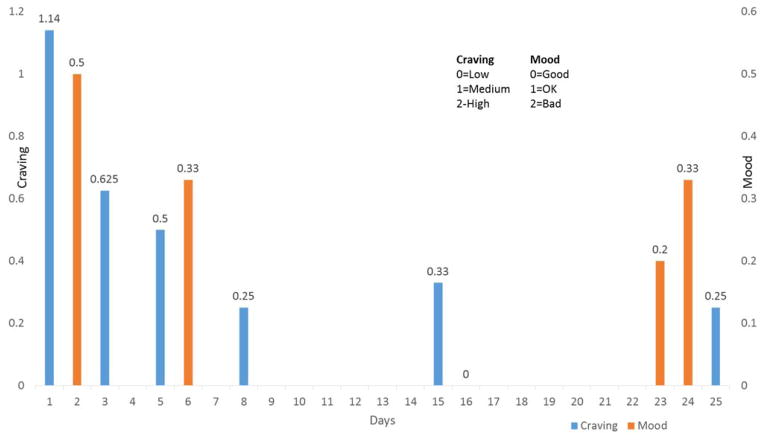
The EMA data in Figure 2 suggest that respondents’ cravings decreased throughout the initial month, with a possible improvement in mood as well. Because of the strong association between cravings, poor mood, and relapse to smoking, these results suggest that the intervention was efficacious in reducing the risk of relapse. However, because of the small number of responses, formal stochastic testing was not performed.
Figure 2.
Mood and craving in intervention subjects.
In an exploratory analysis of secondary outcomes, there was a strong association between responding to the EMA texts and probability of abstinence at one month, as reported in Table 2. Intervention subjects reporting tobacco abstinence at one month responded to a mean of 3.8 texts, versus 0.4 for subjects reporting continued smoking (P=0.001 by t-test). At 3 months, the difference in adherence to text response did not significantly differ between abstinent and nonabstinent subjects: 3.6 vs. 1.3, respectively (P=0.06).
Table 2.
Results of EMA responses in intervention subjects.
| Variable | One month | Three months | ||||
|---|---|---|---|---|---|---|
| Abstinent | Smoking | P value | Abstinent | Smoking | P value | |
| Craving, median (IQR) (0=low; 1=medium; 2=high) | 0.5 (0, 1.08) | 1 (1,2) | 0.14a | 0 (0, 0.37) | 0.5 (9.25, 1.83) | 0.39a |
| Mood, median (IQR) (0=good, 1=OK, 2=bad) | 0 (0, 0.53) | 1 (1,1) | 0.20a | 0.1 (0, 0.35) | 0 (0, 0.83) | 0.61a |
| Number of EMA texts answered, mean (SD) | 3.8 (3.7) | 0.4 (0.6) | 0.001b | 3.6 (4.2) | 1.3 (2.1) | 0.06b |
Mann-Whitney test
t-test
The boldfaced result is statistically significant.
There was no statistically significant association between probability of abstinence at 1 or 3 months, and assessments of mood and craving as noted in Table 2. Again, the number of responses is small.
The proportion of subjects engaging in quitline services for the intervention and control subjects was, respectively, 9/30 (30%) and 1/30 (3%) (P=0.005). Among the 30 intervention subjects, 13 (43%) were successfully contacted by the quitline, of whom 9 (30%) agreed to accept services. Seven subjects accepted a single call, and 2 accepted the 5-call program. Of note, control subjects were able to contact the quitline, but did not receive a facilitated referral like the intervention subjects.
There was no association between subjects’ beliefs about the presence of a tobacco-related illness or their ED visit being related to tobacco, and the likelihood of abstinence.
Discussion
In this brief pilot study, provision of NRT, a quitline referral, and enrollment in a free SMS-texting program was feasible. Recruitment was straightforward, most eligible subjects agreed to participate, and follow-up rates were excellent. ED physicians and nurses were comfortable with study procedures, and referred potential eligible subjects to the research assistant. All subjects in the intervention arm used the texting program, most for its entire 28-day duration.
There appears to be evidence of efficacy in the short-term (1 month) and a trend toward efficacy in the longer-term (3 months), although the study was not powered to detect a difference in tobacco abstinence between groups at either follow-up timepoint.
The association between adherence to EMA texts and 1-month abstinence is intriguing. In clinical trials, greater adherence to therapy, even in control groups,11 is often associated with better outcomes. This has been reported in studies of tobacco dependence treatment as well.12 This likely reflects factors other than the presumed efficacy of the active agent, such as subject motivation and self-efficacy. However, because these are exploratory analyses in a small pilot trial, we cannot infer causality. This finding needs confirmation in a larger trial.
We did not find a statistically significant association between abstinence and assessments of mood and craving, although the direction of the associations favored the intervention. Again, the number of observations was small, and this is an exploratory result as well.
A specific focus of this work was to design an intervention that is broadly generalizable. Past interventions by our group have used trained research associates to deliver brief negotiated interviews (BNI, a variant of motivational interviewing) in the ED.5,13 Although our most recent trial showed reduced smoking at the primary endpoint of 3 months (12.2% vs. 4.9% for controls), the intervention is time- and labor-intensive and costly. Most EDs do not have research staff, of course, although most have access to ancillary healthcare personnel, like respiratory therapists and social workers, who could possibly deliver the BNI. Nonetheless, we wanted to design a behavioral intervention that is broadly generalizable, and that could provide synergy with standard pharmacotherapy. The SmokeFreeTXT program, the universal availability (in North American) of quitlines and the widespread availability of smartphones offers that opportunity.
Because our intervention included administration of nicotine replacement therapies, along with a referral to the state smokers’ quitline, we cannot comment on the possible efficacy of the texting program alone. Future work by our group will employ the Multiple Optimization Strategy approach,14 using a factorial design that will allow us to isolate the effects of individual intervention components, as well as combinations of components.
In addition, at the 3-month follow-up, subjects in the intervention arm were interviewed to assess their views of the feasibility of the texting program, and to comment on the appropriateness, utility, and timing of the texts. The qualitative analysis will be the subject of a separate report.
The follow-up rates in this study were comparable to those in our prior study, in which 85% of subjects were reached by phone at one month, and 80% at three months.5
Limitations
This was a pilot study designed to test the feasibility of the intervention. It was not designed to assess the clinical efficacy of the approach. That will await a future trial.
Because this was a multicomponent intervention, we cannot disaggregate the effects of the individual components, or determine whether there are important interactions between components. A planned future trial will assess these effects.
The study was conducted at a single ED in the northeastern U.S. and so may not be reflective of all smokers. However, we have a racially and ethnically diverse group, and a good mix of payors, suggesting reasonable social and economic diversity of our cohort. Given that our intervention’s components can be delivered anywhere, we believe this intervention is generalizable to other EDs.
Conclusions
A multicomponent strategy of ED-initiated nicotine replacement therapy, proactive quitline referral, and smartphone texting appears feasible. The treatment strategy showed some evidence of efficacy at 1 and 3 months, but the trial was not powered for efficacy. Response to assessments of mood and craving was modest. Adherence to EMA was associated with tobacco abstinence at 1 month, and nearly so at 3 months. These preliminary results suggest that the clinically effective but resource-intensive brief motivational interview may not be needed for ED-initiated tobacco dependence treatment. A formal randomized trial is planned to test the efficacy of the intervention.
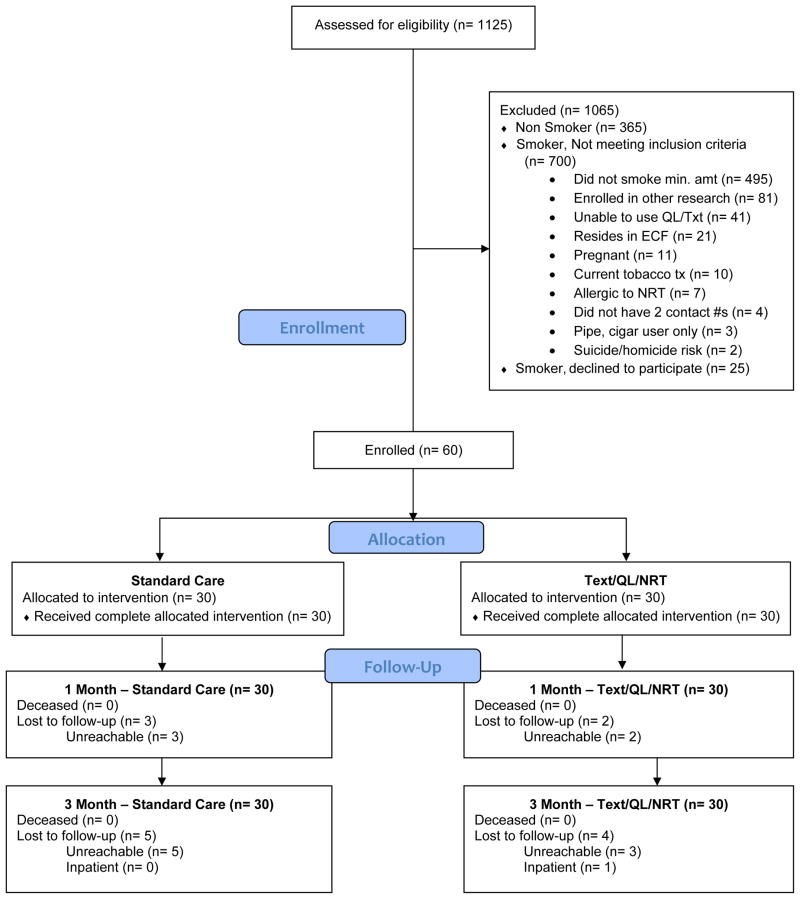
Figure 1.
Flow of subjects through the study.
Acknowledgments
Supported in part by grant R01CA141479 from the National Cancer Institute, National Institutes of Health
We would like to thank Shahina Jain for her expert research assistance, and Erik Augustson, PhD, of the National Cancer Institute for his guidance and support with this project.
Footnotes
Presented in part at the 2015 Annual Meetings of the Society for Research on Nicotine and Tobacco and the Society for Academic Emergency Medicine
References
- 1.Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews. 2015 doi: 10.1002/14651858.CD009670.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- 3.Benson FE, Stronks K, Willemsen MC, Bogaerts NM, Nierkens V. Wanting to attend isn’t just wanting to quit: why some disadvantaged smokers regularly attend smoking cessation behavioural therapy while others do not: a qualitative study. BMC Public Health. 2014;14:1–12. doi: 10.1186/1471-2458-14-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2012 doi: 10.1002/14651858.CD006611.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein SL, D’Onofrio G, Rosner J, et al. Successful Tobacco Dependence Treatment Achieved via Pharmacotherapy and Motivational Interviewing in Low-Income Emergency Department Patients. Ann Emerg Med. 2015;66:140–7. doi: 10.1016/j.annemergmed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Medical Research Methodology. 2010;10:1–7. doi: 10.1186/1471-2288-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whooley MA, Simon GE. Managing depression in medical outpatients. New Engl J Med. 2000;343:1942–50. doi: 10.1056/NEJM200012283432607. [DOI] [PubMed] [Google Scholar]
- 8.Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21:486–97. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson SG, Shiffman S. Using the methods of ecological momentary assessment in substance dependence research--smoking cessation as a case study. Substance Use & Misuse. 2011;46:87–95. doi: 10.3109/10826084.2011.521399. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 11.Horwitz RI, Horwitz SM. Adherence to treatment and health outcomes. Archives Internal Med. 1993;153:1863–8. [PubMed] [Google Scholar]
- 12.Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clinical Therapeutics. 2008;30:1852–8. doi: 10.1016/j.clinthera.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein SL, Bijur P, Cooperman N, et al. A randomized trial of a multicomponent cessation strategy for emergency department smokers. Acad Emerg Med. 2011;18:575–83. doi: 10.1111/j.1553-2712.2011.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins LM, Baker TB, Mermelstein RJ, et al. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011;41:208–26. doi: 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]