Abstract
mTOR (mammalian target of rapamycin) is one of the most important signaling molecules in mammalian cells which regulates an array of cellular events, ranging from cell metabolism to cell proliferation. Based on the association of mTOR with the core component proteins, such as Raptor or Rictor, mTOR can become the mTORC1 (mammalian target of rapamycin complex 1) or mTORC2, respectively. Studies have shown that during the epithelial cycle of spermatogenesis, mTORC1 promotes remodeling and restructuring of the blood-testis barrier (BTB) in vitro and in vivo, making the Sertoli cell tight junction (TJ)-permeability barrier “leaky”; whereas mTORC2 promotes BTB integrity, making the Sertoli cell TJ-barrier “tighter”. These contrasting effects, coupled with the spatiotemporal expression of the core signaling proteins at the BTB that confer the respective functions of mTORC1 vs. mTORC2 thus provide a unique mechanism to modulate BTB dynamics, allowing or disallowing the transport of biomolecules and also preleptotene spermatocytes across the immunological barrier. More importantly, studies have shown that these changes to BTB dynamics conferred by mTORC1 and mTORC2 are mediated by changes in the organization of the actin microfilament networks at the BTB, and involve gap junction (GJ) intercellular communication. Since GJ has recently been shown to be crucial to reboot spermatogenesis and meiosis following toxicant-induced aspermatogenesis, these findings thus provide new insightful information regarding the integration of mTOR and GJ to regulate spermatogenesis.
Keywords: testis, spermatogenesis, mTOR, blood-testis barrier, ectoplasmic specialization, actin microfilaments, gap junction
Introduction
mTOR (mammalian target of rapamycin) is a well conserved Ser/Thr protein kinase, originally identified in yeasts with the aid of the antibiotic rapamycin which specifically inhibits its intrinsic protein kinase activity, and is subsequently found in virtually all mammalian and non-mammalian cells known to regulate cellular energy status (for reviews, see (Laplante and Sabatini, 2012; Shimobayashi and Hall, 2014; Bockaert and Marin, 2015). The polypeptide sequence of mTOR is composed of several functioinal domains as noted in Figure 1. It is also highly expressed in Sertoli and germ cells in the testis (for a review, see (Mok et al., 2013a)). Studies on mTOR have been growing exponentially in recent years since mTOR is the target of chemotherapy by disrupting tumor growth and metastasis by intervening tumor cell energy status using inhibitors specific to mTOR per se or signaling proteins up- or down-stream of mTOR (for reviews, see (Sheppard et al., 2012; Francipane and Lagasse, 2015; Zenardi et al., 2015)). Besides, mTOR is also the target in the action of antidepressant drugs since mTOR is involved in synaptic plasticity in neurons through mTOR signaling pathway (for a review, see (Ignacio et al., 2015)).
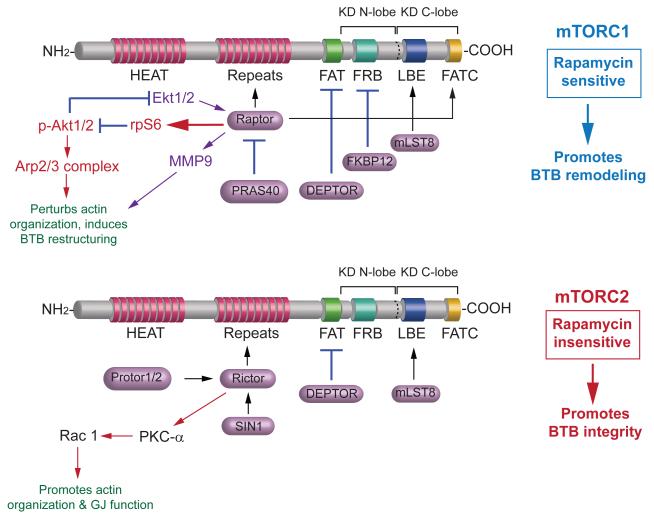
Figure 1. Functional domains of mTOR and the binding partners that confer mTORC1 and mTORC2 signaling complexes.
mTOR per se is a Ser/Thr protein kinase as noted by the two lobes KD called the KD N-lobe and the KD C-lobe. It has 20 tandem HEAT repeat domains near its N-terminus, to be followed by FAT domain, FRB domain, LBE domain and FATC domain at its C-terminus. The critical binding partner to create mTORC1 is Raptor, which together with mLST8, PRAS40, DEPTOR and FKBP12 that bind to the corresponding domains in mTOR constitute the rapamycin sensitive mTORC1. The critical binding partner to create mTORC2 is Rictor, which together with SIN1, mLST8, DEPTOR and Protor1/2 which bind to the corresponding domains in mTOR constitute the rapamycin insensitive mTORC2. Based on recent studies in the testis, the signaling proteins and pathways that modulate Sertoli cell BTB dynamics are also shown (see text for details). It is noted that the signaling pathways depicted herein are highly simplified and based on recent studies in the testis, readers should seek additional information in recent reviews from other investigators in the field, which are cited in text. Abbreviations used: Akt; transforming retrovirus Akt8, an onocogene, also known as PKB; Arp2/3 complex, actin-related protein 2/3 complex; ATM, Ataxia telangiectasia mutated containing Ser/Thr protein kinase intrinsic activity; C-lobe, C-terminal lobe; DEP, Dishevelled, Egl-10 and Pleckstrin; DEPTOR, DEP domain-containing mTOR-interacting protein; EF3, elongating factor 3; FAT domain, FRAP, ATM, TRAP domain; FATC, FAT domain at the C-terminus; FKBP12, FK506/rapamycin-binding protein; HEAT, Huntington, EF3, PP2A, TOR1; FRAP, FKBP rapamycin associated protein; FRB, FKBP132 rapamycin binding; KD, kinase domain; LBE, binding site for mLST8; mLST8, mammalian lethal with SEC thirteen 8, also known as mTOR associated protein LST8 homolog; N-lobe, N-terminal lobe; PKB, protein kinase B; PKC-α, protein kinase C-α; PP2A, protein phosphatase 2A; PRAS40, proline-rich Akt/PKB substrate 40 kDa; Protor1/2, protein observed with Rictor 1 and 2; Rac1, Ras-related C3 botulinum toxin substrate 1, a small GTPase; Raptor, regulatory-associated protein of mTOR; Rictor, rapamycin-insensitive companion of mTOR, a scaffolding protein; rpS6, ribosomal protein S6; SAPK, stress-activated protein kinase; SIN1, SAPK-interacting protein 1; TOR1, yeast kinase target of rapamycin 1; TRAP, thrombospondin-related anonymous protein.
mTOR exists in two functionally and structurally distinct forms, known as mTORC1 (mammalian target of rapamycin complex 1) or mTORC2, depending on the core component proteins that structurally associate with mTOR, a 289 kDa protein in mammalian cells, including Sertoli cells in the rat testis (Mok et al., 2012b) (Figure 1). The core components of mTORC1 are: mTOR, Raptor (regulatory-associated protein of mTOR), mLST8 (mammalian lethal with SEC thirteen 8, also known as mTOR associated protein LST8 homolog), PRAS40 (proline-rich Akt/PKB substrate 40 kDa), DEPTOR (DEP (Dishevelled, Egl-10 and Pleckstrin) domain-containing mTOR-interacting protein) and FKBP12 (FK506/rapamycin-binding protein), which is sensitive to rapamycin (Figure 1). The core components of mTORC2 are: mTOR, Rictor (rapamycin-insensitive companion of mTOR, a scaffolding protein), SIN1 (SAPK (stress-activated protein kinase)-interacting protein 1), mLST8, DEPTOR and Protor1/2 (protein observed with Rictor 1 and 2), which is insensitive to rapamycin (Figure 1). Thus, depending on the association of the crucial binding partner of either Raptor or Rictor, this creates the mTORC1 or mTORC2 signaling complex, respectively.
mTORC1 can be activated by growth factors (e.g., insulin, IGF-1), nutrients (e.g., amino acids), or energy status of the cell (e.g., high ATP/AMP ratio) through PI3-K (phosphoinositide 3-kinase) and Akt/PKB upstream (for reviews, see (Mok et al., 2013a; Huang and Fingar, 2014)) Once the mTORC1 signaling complex is activated, it exerts its effects via ribosomal protein S6K (S6 protein kinase, also known as p70 S6K, containing two protein kinases of S6K1 and S6K2) (for reviews, see (Long et al., 2004; Huang and Fingar, 2014; Tavares et al., 2015)), rpS6 (ribosomal protein S6) (for a review, see (Meyuhas, 2015)) or 4E-BP (4E-Binding Protein 1) (for reviews, see (Korets et al., 2011; Mok et al., 2013a)) downstream via phosphorylation to modulate anabolic metabolism and other cellular functions. Earlier reports including recent studies using global mTOR ‘omics’ including ribosome profiling, phosphoproteomics, transcriptomics and metabolomics have identified an extensive network of signaling proteins, adaptors and pathways that are involved in mediating the signaling function of mTORC1 both up- and down-stream (for a review, see (Shimobayashi and Hall, 2014)). For instance, non-classical cues that modulate mTOR upstream have been shown to include inputs from Hippo, Notch and Wnt signaling pathways (for a review, see (Shimobayashi and Hall, 2014)). Two recent reports have shown that rpS6, a downstream signaling molecule of mTORC1, works in concert with Akt1/2 and Arp2/3/N-WASP (Mok et al., 2015), and also Akt1/2, Erk1/2 and MMP-9 (Mok et al., 2014) to modulate actin microfilament organization at the blood-testis barrier (BTB) (Figure 1). While these findings illustrate the complexity of mTORC1 signaling, the mTORC1 signaling complex exerts its effects through well-defined pathways to modulate different cellular functions. Herein, we discuss some of the latest findings regarding the role of mTORC1 in spermatogenesis, in particular BTB dynamics.
For mTORC2, its regulation is poorly understood. It is currently known that only growth factors stimulate mTORC2 kinase activity through PI3-K-dependent mTORC2-ribosome association based on a study in yeast (Zinzalla et al., 2011). mTORC2 exerts its effects downstream through PKC-α and/or SGK1 (serum- and glucocorticoid-induced protein kinase 1) (for a review, see (Mok et al., 2013a)). Studies in the testis, however, have shown that mTORC2 exerts its effects via PKC-α downstream, but also involve gap junction (GJ) communication function (Mok et al., 2013b) (Figure 1). Furthermore, the two mTOR complexes are now known to have contrasting effects on the Sertoli cell TJ-permeability barrier function. For instance, the knockdown of Rictor (the essential component of mTORC2) in Sertoli cells cultured in vitro with an established TJ-barrier by RNAi perturbs Sertoli cell TJ-permeability function by making it “leaky” (Mok et al., 2013b), and this observation has also been reproduced in a study in vivo by silencing Rictor in the testis using Rictor-specific siRNA duplexes which also leads to a “leaky” BTB when examined by a functional assay (Mok et al., 2013b). On the other hand, the knockdown of rpS6 (the downstream signaling protein of mTORC1) in Sertoli cells cultured in vitro with an established TJ-barrier (or treatment of Sertoli cells with rapamycin) promotes the Sertoli cell TJ-barrier function by making it “tighter”, consistent with analysis of the testis in vivo following rpS6 knockdown using rpS6-specific shRNA (Mok et al., 2012b). In short, in the normal testis, mTORC2 is used to promote the Sertoli cell TJ-barrier function to confer BTB integrity, whereas mTORC1 promotes Sertoli TJ-barrier restructuring/remodeling/disruption, making the BTB leaky. This thus provides a unique and novel mechanism to efficiently support the transport of preleptotene spermatocytes connected in clones across the BTB to be discussed herein. The antagonistic effects of mTORC1 and mTORC2 on BTB dynamics could possibly be used to regulate the in-flow of nutrients and biomolecules into the adluminal compartment to modulate meiosis I/II and post-meiotic spermatid development.
Although mTORC1 and mTORC2 are independent signaling complexes utilizing distinctive up-and also down-stream signaling molecules, these two pathways are also inter-connected, and interact with each other. Following activation of these two mTOR signaling complexes by growth factors and amino acids (Figure 2), they also share a common upstream regulator called the TSC1/2 complex (tuberous sclerosis complex composed of TSC1, TSC2, and Tre2-Bub2-Cdc16-1 domain family member 7 (TBC1D7)), which promotes mTORC1 but suppresses mTORC2 intrinsic activity (for reviews, see (Li et al., 2004; Mok et al., 2013a; Dibble and Cantley, 2015)). Furthermore, S6K1, the downstream activator (and substrate) of mTORC1, can also phosphorylate Rictor, the critical binding partner of mTORC2, by inhibiting the catalytic activity of mTORC2 on PKB which is the upstream regulator of mTORC1 (for reviews, see (Magnuson et al., 2012; Mok et al., 2013a)), thereby forming a negative feedback regulatory loop (Figure 2). The finding that rictor knockdown by RNAi that inactivates mTORC2 can perturb the GJ function has opened an interesting avenue of research to probe the regulatory function of mTOR. Since this observation supports the notion that an inactivation (or activation) of GJ through mTORC2 can modulate intercellular communication, mTORC2 can effectively modulate the transport of biomolecules, including small regulatory miRNAs, across GJ to regulate Sertoli and/or germ cell function in the seminiferous epithelium. As such, coordinated changes involving multiple biomolecules can take place across the seminiferous epithelium in response to changes in stages of the epithelial cycle.
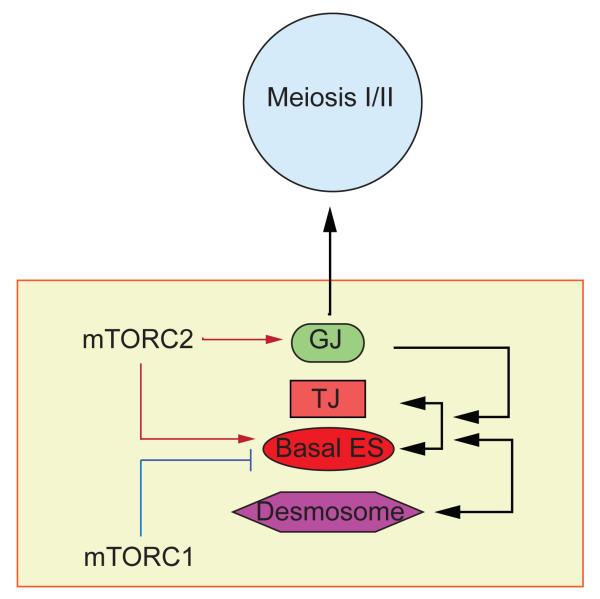
Figure 2. A schematic drawing illustrates the functional relationship of mTORC1 and mTORC2 on BTB dynamics.
As discussed in text, mTORC1 promotes BTB remodeling whereas mTORC2 promotes BTB integrity through their actions on the organization of actin microfilaments at the basal ES/BTB, in which mTORC2 likely promotes unbundling of actin microfilaments whereas mTORC2 promotes bundling of actin microfilaments. The antagonistic actions of two these signaling complexes thus provide an efficient mechanism to confer restructuring of the BTB during the epithelial cycle of spermatogenesis, in particular at stage VII-VIII of the cycle when preleptotene spermatocytes are being transported across the immunological barrier. Furthermore, the effect of mTORC2 on gap junction function can also modulate meiosis as discussed in text.
mTOR: mTORC1 and mTORC2 and BTB dynamics – a mechanistic insight
The notion that mTOR is involved in BTB function stems from the initial observation that using an antibody specific to mTOR, this Ser/Thr protein kinase is highly expressed in the seminiferous epithelium, and it is localized at the BTB (Mok et al., 2013b). A recent report has shown that mTOR found in human Sertoli cells also regulates glucose consumption and redox balance (Jesus et al., 2015). Furthermore, the expression of mTOR at the BTB is stage-specific, being highest at stages VI-IX (Mok et al., 2013b), at the stages when BTB undergoes remodeling and/or restructuring to accommodate the transport of preleptotene spermatocytes across the immunological barrier, which takes place at stage VIII of the epithelial cycle (Parvinen, 1982; Xiao et al., 2014). Furthermore, while rpS6, the downstream signaling molecule of mTOC1, is expressed at the BTB in all stages of the epithelial cycle from I-XIV in the rat testis; however, the expression of its activated form, p-rpS6, at the BTB is stage-specifically, almost restrictively and predominantly expressed at the BTB at stages VIII-IX, coinciding with the stages when the BTB undergoes remodeling (Mok et al., 2012b). p-rpS6 also co-localizes with F-actin at the BTB at stages VIII-IX (Mok et al., 2012b). More importantly, p-rpS6-Ser235/236 and p-rpS6-Ser240/244, the two activated forms of p-rpS6, are also highly expressed at the BTB in the seminiferous epithelium of adult rat testes but are limited only to stage VIII-IX tubules (Mok et al., 2014), co-localized with ZO-1, F-actin but also Eps8 (Mok et al., 2014). Collectively, these findings illustrate the likely involvement of mTORC1 through p-rspS6 downstream to modulate the actin microfilament bundles at the basal ES/BTB to promote BTB remodeling, making the barrier “leaky” (Figure 2). On the other hand, Rictor, the component protein of mTOR that creates the mTORC2 signaling complex, is also expressed at the BTB, and co-localizes with ZO-1, occludin and F-actin (Mok et al., 2013b), supporting the notion that mTORC2 complex can likely modulate BTB dynamics through F-actin organization at the basal ES/BTB (Figures 1, 2). It is of interest to note that the expression of Rictor is considerably down-regulated at the BTB in stages VIII-IX tubules (Mok et al., 2013b), illustrating that it may have contrasting effects on the BTB function vs. mTORC1 based on their differential spatiotemporal expression at the BTB. In short, mTORC2 likely promotes Sertoli cell BTB integrity so that its downregulation at the BTB at stages VIII-IX of the epithelial cycle is necessary to facilitate BTB remodeling/restructuring at these stages.
mTORC1 and BTB function – a mechanistic insight
rpS6 (ribosomal protein S6) is one of 33 proteins, which together with a molecule of 18S rRNA, create the small 40S ribosomal subunit to regulate protein translation (for reviews, see (Meyuhas, 2008, 2015)). It is now established that rpS6 is a well conserved protein among vertebrates, invertebrates, fungi and plants by interacting with the m7GpppG 5′-cap-binding protein complex required for translation initiation, which also serves as a platform for regulatory convergence for signal transduction pathways that controls translation initiation in response to cell growth and proliferation cues (for a review, see (Ruvinsky and Meyuhas, 2006)). rpS6 is activated via inducible phosphorylation on a cluster of 5 Ser residues near its C-terminus at: Ser-235, -236, -240, -244 and -247 (Krieg et al., 1988), which takes place sequentially, beginning with Ser-236, to be followed by Ser-235, Ser-240, Ser-244 and Ser-247 (Martin-Perez and Thomas, 1983; Wettenhall et al., 1992). It has been reported that modification of the four Ser residues at residues 235, 236, 240 and 244 from the N-terminus, such as via phosphorylation considerably up-regulates rpS6 cap binding activity to initiate protein translation, but the function of the S-247 site is not known, which has earlier shown to be a target of the casein kinase 1 (CK1) protein kinase (Hutchinson et al., 2011). Thus, we have used a constitutively active quadruple phosphomimetic mutant of rpS6 of rpS6-S235E/-S236E and rpS6-S240E/-S244E by mutating these four Ser residues to Glu (glutaminc acid) residues, which was then cloned into the pCI-neo mammalian expression vector for its overexpression in Sertoli cells vs. the rpS6-WT (wild type) and pCI-neo empty vector which serve as controls (Mok et al., 2014). The overexpression of this constitutively active rpS6 quadruple phosphomimetic mutant is capable of perturbing the Sertoli cell TJ-barrier function, even more potent than the rpS6 WT (Mok et al., 2014), illustrating that rpS6 promotes Sertoli cell BTB remodeling/restructuring in normal physiological conditions. These findings are also consistent with an earlier study in which a knockdown of rpS6 in Sertoli cell epithelium in vitro by RNAi promotes the Sertoli cell TJ-barrier, making it tighter (Mok et al., 2012b). Using this rpS6 mutant for its overexpression in Sertoli cells in conjunction with inhibitors and RNAi, it is now known that rpS6 exerts its disruptive effects on the TJ-barrier by promoting MMP-9 (matrix metalloprotease 9), which in turn induces proteolysis-mediated downregulation of TJ-proteins (e.g., occludin, claudin-11) (Mok et al., 2014). Furthermore, this rpS6 mutant exerts its effects through a down-regulation of p-Akt1/2 (also known as PKB), suggesting p-Akt1/2 is the likely downstream signaling molecule of rpS6. The involvement of p-AKT1/2 in p-rpS6-mediated effects on the Sertoli cell TJ function is confirmed by the simultaneous knockdown of Akt1 and Akt2 by RNAi since an inactivation of Akt1/2 mimics the phenotypes following overexpression of rpS8 mutant on the Sertoli cell TJ-barrier function by perturbing the TJ-permeability and by disrupting the proper distribution of TJ-proteins at the Sertoli cell-cell interface(Mok et al., 2014), confirming that mTORC1 perturbs the Sertoli cell BTB function through the p-rpS6-Akt1/2-MMP-9 pathway. A subsequent study has expanded this earlier report that this mTOC1-rpS6-Akt1/2-MMP-9 signaling pathway also modulates F-actin organization at the basal ES/BTB via its effects to recruit and/or activate Arp3 (actin related protein 3, which together with Arp2 forms the Arp2/3 complex, known to induce branched actin polymerization, effectively converting bundled actin microfilaments into a branched network) at or near the Sertoli cell-cell interface (Mok et al., 2015). This thus causes de-bundling and truncation of actin microfilaments to de-stabilize the Sertoli cell TJ-barrier, leading to mis-localization of TJ proteins (e.g., occludin and claudin-11) at the Sertoli cell-cell interface, thereby perturbing the TJ-barrier function. In short, mTORC1 promotes BTB remodeling/restructuring in the rat testis.
mTORC2 and BTB function – a mechanistic insight
mTORC2, in contrast to mTORC1 which promotes BTB remodeling/restructuring to make the barrier leaky (Mok et al., 2012b; Mok et al., 2014; Mok et al., 2015), is known to promote BTB integrity by making the Sertoli cell TJ-permeability barrier tighter, since mTORC2 disruption by silencing Rictor (the core component of mTORC2 complex) can perturb the Sertoli cell TJ-barrier function (Mok et al., 2013b). This disruptive effect on the Sertoli cell TJ-barrier function following Rictor knockdown in vitro is found to be related to changes in the organization of actin microfilaments in Sertoli cells in which microfilaments no longer stretch across the entire Sertoli cell cytosol, becoming partially truncated and branched (Mok et al., 2013b). Since TJ- (e.g., occludin/ZO-1) and basal ES (e.g., N-cadherin/ß-catenin) proteins utilize F-actin for their attachment, a disruptive organization of F-actin thus impedes adhesion of these proteins (Mok et al., 2013b). Indeed, the structural association of either ZO-1 or α -catenin with actin is significantly reduced following Rictor knockdown (Mok et al., 2013b), supporting the notion that mTORC2, similar to mTORC1, exerts its effects to modulate the Sertoli cell TJ-barrier function through F-actin organization at the basal ES/BTB. More importantly, these findings in vitro have been confirmed in a study in vivo by silencing Rictor in the testis using Rictor-specific siRNA duplexes vs. non-targeting negative control siRNA duplexes, in which Rictor knockdown also perturbs BTB integrity, making the barrier leaky through changes in the organization of F-actin, such that occludin and ZO-1 are no longer retained at the BTB, destabilizing the BTB (Mok et al., 2013b). Furthermore, these findings have been confirmed in a recent report using a Sertoli cell-specific Rictor KO mouse model in which the loss of Rictor in Sertoli cells leads to a disorganization of F-actin in the testis (Dong et al., 2015), analogous to the in vivo Rictor knockdown rat testis (Mok et al., 2013b). Collectively, these finding illustrate that the antagonistic effects of mTORC1 and mTORC2 that promote BTB remodeling and integrity, respectively, are mediated through changes in the organization actin microfilaments at the basal ES. This thus provides a unique mechanism to fine-tune the organization of F-actin network to support germ cell movement and other cellular events pertinent to the epithelial cycle of spermatogenesis.
mTORC2 and gap junction
In a study that examines the effects of mTORC2 on the Sertoli cell BTB function, Rictor knockdown by RNAi not only perturbs the TJ-barrier function through changes in F-actin organization that impede the localization of TJ-protein complexes (e.g., occludin/ZO-1) at the Sertoli cell-cell interface, the expression of the gap junction (GJ) protein connexin43 (Cx43) is considerably down-regulated and its localization at the Sertoli cell cortical zone is also grossly disrupted (Mok et al., 2013b). Interestingly, GJ communication function is also perturbed based on a functional dye-transfer assay following Rictor knockdown (Mok et al., 2013b). Since Sertoli cells are known to express at least 10 different connexins including the testis-specific Cx33 besides Cx43 (Risley, 2000; Pointis et al., 2010), this finding thus suggests that the loss of mTORC1 function following Rictor knockdown perturbs the general function of connexins in Sertoli cells instead of Cx43 alone. In this context, it is of interest to note that Cx43 is a uniquely important GJ protein in the testis (for a review, see (Pointis et al., 2011)). For instance, Sertoli cell-specific KO of Cx43 leads to infertility in male mice in which the population of spermatogonia is reduced, and they fail to differentiate and develop into spermatocytes, leading to meiotic arrest (Brehm et al., 2007), illustrating other connexins cannot supersede the loss function of Cx43. Also, Sertoli cells remain mitotically active in these Sertoli cell-specific Cx43 KO mice, thus, the epithelium is filled with excessive Sertoli cells, which leads to Sertoli cell sloughing in tubules. Detailed morphological analysis of these Sertoli cell-specific Cx43 KO mice shows that GJs are absent in their testes (Carette et al., 2010), illustrating other GJ proteins in the absence of Cx43 fail to assemble into functional GJ channels to support GJ communications. The BTB in these mice also display reduced expression of ZO-1, and mis-localization of ZO-1 and N-cadherin (Carette et al., 2010). These findings are consistent with earlier findings that Cx43 is crucial to maintain BTB function by providing the necessary cross-talk to maintain the collaborative efforts of multiple junctions, such as TJ and basal ES, to confer BTB integrity (Li et al., 2009). Since junctions at the BTB undergo continuous remodeling, which thus requires their constant disassemble and re-assemble, and Cx43 is crucial to the reassembly of a disrupted Sertoli cell TJ-barrier (Li et al., 2010). At present, the mechanism by which mTORC2 regulates GJ function is not known. However, a recent study has shown that a modulation of mTOR-mediated cap-dependent translation can determine the level of at least 4 truncated Cx43 isoforms in human heart cells, which in turn auto-regulate Cx43 intracellular trafficking, contributing to Cx43-dependent GJ communication to regulate synchronous cardiac contraction in the human heart (Smyth and Shaw, 2013). It is not known at present if mTORC2 disruption via a knockdown of Rictor perturbs the mTOR-mediated cap-dependent translation to alter the level of truncated Cx43 isoforms, which in turn modulate the homeostasis of GJ function at the Sertoli cell BTB; or whether it is associated with changes in a specified signaling pathway.
Gap junction induces re-initiation of spermatogenesis and reboots meiosis
Treatment of adult rats with adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole carbohydrazide, a male contraceptive drug known to induce reversible infertility in rats and rabbits, following a single dose of 50 mg/kg b.w. via oral gavage, leads to exfoliation of all classes of germ cells from the testis, initially elongating/elongated spermatids, to be followed by round spermatids and spermatocytes until only spermatogonia are present in the germ cell-depleted tubules throughout the entire testis (for reviews, see (Cheng et al., 2005; Mruk et al., 2008; Cheng, 2014)). However, when adjudin is used at an acute dose such as at 125 or 250 mg/kg b.w. via oral gavage in adult rats, this leads to irreversible aspermatogenesis in which spermatogenesis fails to resume in these rats, and is associated with an irreversible BTB disruption (Mok et al., 2012a). Moreover, the phenotypes of the testis are similar to the Sertoli cell Cx43-specific KO mice since spermatogonia fail to develop into spermatocytes to initiate meiosis (Brehm et al., 2007; Mok et al., 2012), unlike rats treated with a low dose of adjudin at 50 mg/kg b.w. wherein the BTB is transiently disrupted, and spermatogenesis resumes when the disrupted BTB is resealed, and germ cells gradually repopulate the epithelium of virtually all the tubules (Cheng et al., 2001). The only difference between these two models is that the Sertoli cell population is not altered in adjudin treated rats; however, Sertoli cells are actively proliferating and associated with Sertoli cell sloughing in Sertoli cell Cx43-KO mouse testes. In an attempt to better understand the functional relationship between Cx43, BTB integrity and the onset of meiosis, Cx43 is overexpressed in adjudin-treated rats vs. rats treated with adjudin alone or overexpressed with the empty mammalian transfection vector to assess changes in BTB integrity and the initiation of meiosis in these rats (Li et al., 2016). It is noted that overexpression of Cx43 reboots spermatogenesis, in which spermatogonia differentiate into spermatocytes, and meiosis also takes place since round spermatids are found in a considerable number of tubules (Li et al., 2016). More importantly, testes having overexpressed Cx43 display corrective expression of actin barbed end capping and bundling protein Eps8 and also actin nucleation protein formin 1, which in turn re-establishes the disrupted F-actin network in the seminiferous epithelium of adjudin treated rats. These changes are associated with recruitment of TJ- (e.g., occludin, ZO-1) and basal ES- (e.g., N-cadherin, γ-catenin) proteins to the BTB site, which in turn reseals the disrupted BTB caused by adjudin treatment, making the BTB functional again (Li et al., 2016). These changes thus support re-initiation of spermatogenesis. However, round spermatids fail to develop further into elongating and elongated spermatids (Li et al., 2016), illustrating that overexpression of Cx43 alone is not sufficient to support the full spectrum of cellular events of spermatogenesis following an acute dose of adjudin. In this study, however, it is not known whether the mTORC1 signaling pathway is involved. Nonetheless, these findings support the notion that Cx43-based GJ function is crucial to support spermatogenesis, illustrating that the loss of GJ function following Rictor knockdown that impedes mTORC1 signaling can impair multiple cellular events pertinent to spermatogenesis.
Concluding remarks and future perspectives
mTORC1 and mTORC2 are two signaling complexes that have recently been shown to play contrasting roles in modulating the Sertoli cell BTB function based on studies in vitro and in vivo. For instance, activation of mTORC1 promotes BTB remodeling/restructuring by making the barrier “leaky”, whereas mTORC2 promotes BTB integrity by making the barrier tighter. Furthermore, mTORC2 inactivation following Rictor knockdown by RNAi also impedes GJ-mediated cell-cell communication. Since a number of chemical signals can be transported across the GJ-based communication channels, such as Cx43-based GJs, including small regulatory miRNAs (Suzhi et al., 2015; Valiunas et al., 2015) (for a review, see (Higa et al., 2014)). Indeed, studies have shown that both Sertoli and germ cells possess large numbers of endogenous siRNAs (Song et al., 2009; Song et al., 2011). Thus, miRNAs found in these cells can be transported actively between them to tightly regulate the timely expression of genes and/or sets of genes to modulate cellular changes in response to the epithelial cycle of spermatogenesis, such as the cellular events pertinent to BTB restructuring to facilitate the transport of preleptotene spermatocytes across the immunological barrier. Work is needed to better understand the mechanism(s) by which mTORC1 vs. mTORC2 exert their regulatory effects via actin microfilament organization. For instance, does this involve cap-mediated translation? Does this involve the truncated Cx43 isoforms to auto-regulate Cx43 trafficking? It is likely that cap-mediated translation can affect the spatiotemporal expression of actin binding proteins such as the Arp2/3 complex, Eps8, and formin 1, which in turn modulate the organization of actin microfilament bundles at the basal ES/BTB.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NICHD R01 HD056034; U54 HD029990 Project 5 to C.Y.C.)
References
- Bockaert J, Marin P. Mtor in brain physiology and pathologies. Physiol. Rev. 2015;95:1157–1187. doi: 10.1152/physrev.00038.2014. [DOI] [PubMed] [Google Scholar]
- Brehm R, Zeiler M, Ruttinger C, Herde K, Kibschull M, Winterhager E, Willecke K, Guillou F, Lecureuil C, Steger K, Konrad L, Biermann K, Failing K, Bergmann M. A sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am. J. Pathol. 2007;171:19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette D, Weider K, Gilleron J, Giese S, Dompierre J, Bergmann M, Brehm R, Denizot JP, Segretain D, Pointis G. Major involvement of connexin 43 in seminiferous epithelial junction dynamics and male fertility. Dev. Biol. 2010;346:54–67. doi: 10.1016/j.ydbio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Cheng CY. Toxicants target cell junctions in the testis - insights from the indazole-carboxylic acid model. Spermatogenesis. 2014;4:e981485. doi: 10.4161/21565562.2014.981485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD, Silvestrini B, Bonanomi M, Wong CH, Siu MKY, Lee NPY, Mo MY. Af-2364 [1-(2,4-dichlorobenzyl)-1h-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, Saso L, Leone MG, Palmery M, Mruk D. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol. Reprod. 2001;65:449–461. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- Dibble CC, Cantley LC. Regulation of mtorc1 by pi3k signaling. Trends Cell Biol. 2015;25:545–555. doi: 10.1016/j.tcb.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Chen Z, Wang C, Xiong Z, Zhao W, Jia C, Lin J, Lin Y, Yuan W, Zhao AZ, Bai X. Rictor regulates spermatogenesis by controlling sertoli cell cytoskeletal organization and cell polarity in the mouse testis. Endocrinology. 2015;156:4244–4256. doi: 10.1210/en.2015-1217. [DOI] [PubMed] [Google Scholar]
- Francipane MG, Lagasse E. Therapeutic potential of mtor inhibitors for targeting cancer stem cells. Br. J. Clin. Pharmacol. 2015 doi: 10.1111/bcp.12844. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa GS, de Sousa E, Walter LT, Kinjo ER, Resende RR, Kihatra AH. Micrornas in neuronal communication. Mol. Neurobiol. 2014;49:1309–1326. doi: 10.1007/s12035-013-8603-7. [DOI] [PubMed] [Google Scholar]
- Huang K, Fingar DC. Growing knowledge of the mtor signaling network. Semin. Cell Dev. Biol. 2014;36:79–90. doi: 10.1016/j.semcdb.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JA, Shanware NP, Chang H, Tibbetts RS. Regulation of ribosomal protein s6 phosphorylation by casein kinase 1 and protein phosphatase 1. J. Biol. Chem. 2011;286:8688–8696. doi: 10.1074/jbc.M110.141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignacio ZM, Reus GZ, Arent CO, Abelaira HM, Pitcher MR, Quevedo J. New perspectives on the involvement of mtor in depression as well as in the action of antidepressant drugs. Br. J. Clin. Pharmacol. 2015 doi: 10.1111/bcp.12845. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesus TT, Oliveira PF, Silva J, Barros A, Ferreira R, Sousa M, Cheng CY, Silva BM, Alves MG. Mammalian target of rapamycin controls glucose consumption and redox balance in human sertoli cells. Fertil Steril. 2015 doi: 10.1016/j.fertnstert.2015.11.032. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korets SB, Czok S, Blank SV, Curtin JP, Schneider RJ. Targeting the mtor/4e-bp pathway in endometrial cancer. Clin. Cancer Res. 2011;17:7518–7528. doi: 10.1158/1078-0432.CCR-11-1664. [DOI] [PubMed] [Google Scholar]
- Krieg J, Hofsteenge J, Thomas G. Identification of the 40s ribosomal protein s6 phosphorylation sites induced by cycloheximide. J. Biol. Chem. 1988;263:11473–11477. [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. Mtor signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc. Natl. Acad. Sci. USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 is critical to maintain the homeostasis of blood-testis barrier via its effects on tight junction reassembly. Proc. Natl. Acad. Sci. USA. 2010;107:17998–18003. doi: 10.1073/pnas.1007047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Mruk DD, Mok KW, Li MWM, Wong CKC, Lee WM, Han D, Silvestrini B, Cheng CY. Connexin 43 reboots meiosis and reseals blood-testis barrier following toxicant- mediated aspermatogenesis and barrier disruption. FASEB J. 2016 doi: 10.1096/fj.15-276527. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Corradetti MN, Inoki K, Guan KL. Tsc2:Filling the gap in the mtor signaling pathway. Trends Biochem. Sci. 2004;29:32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Long X, Muller F, Avruch J. Tor action in mammalian cells and in caenorhabditis elegans. Curr. Top Microbiol. Immunol. 2004;279:115–138. doi: 10.1007/978-3-642-18930-2_8. [DOI] [PubMed] [Google Scholar]
- Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein s6 kinase (s6k) within mtor signalling networks. Biochem. J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Martin-Perez J, Thomas G. Ordered phosphorylation of 40s ribosomal protein s6 after serum stimulation of quiescent 3t3 cells. Proc. Natl. Acad. Sci. USA. 1983;80:926–930. doi: 10.1073/pnas.80.4.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyuhas O. Physiological roles of ribosomal protein s6: One of its kind. Int. Rev. Cell Mol. Biol. 2008;268:1–37. doi: 10.1016/S1937-6448(08)00801-0. [DOI] [PubMed] [Google Scholar]
- Meyuhas O. Ribosomal protein s6 phosphorylation: Four decades of research. Int. Rev. Cell Mol. Biol. 2015;320:41–73. doi: 10.1016/bs.ircmb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Cheng CY. Regulation of blood-testis barrier (btb) dynamics during spermatogenesis via the “yin” and “yang” effects of mammalian target of rapamycin complex 1 (mtorc1) and mtorc2. Int. Rev. Cell Mol. Biol. 2013a;301:291–358. doi: 10.1016/B978-0-12-407704-1.00006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Lee WM, Cheng CY. Rictor/mtorc2 regulates blood-testis barrier dynamics via its effects on gap junction communications and actin filament network. FASEB J. 2013b;27:1137–1152. doi: 10.1096/fj.12-212977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Cheng CY. Rps6 regulates blood-testis barrier dynamics through akt- mediated effects on mmp-9. J. Cell Sci. 2014;127:4870–4882. doi: 10.1242/jcs.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int. J. Androl. 2012a;35:86–101. doi: 10.1111/j.1365-2605.2011.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Silvestrini B, Cheng CY. Rps6 regulates blood-testis barrier dynamics by affecting f-actin organization and protein recruitment. Endocrinology. 2012b;153:5036–5048. doi: 10.1210/en.2012-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok KW, Chen H, Lee WM, Cheng CY. Rps6 regulates blood-testis barrier dynamics through arp3-mediated actin microfilament organization in rat sertoli cells. An in vitro study. Endocrinology. 2015;156:1900–1913. doi: 10.1210/en.2014-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol. Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocr. Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Pointis G, Gilleron J, Carette D, Segretain D. Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010;365:1607–1620. doi: 10.1098/rstb.2009.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointis G, Gilleron J, Carette D, Segretain D. Testicular connexin 43, a precocious molecular target for the effect of environmental toxicants on male fertility. Spermatogenesis. 2011;1:303–317. doi: 10.4161/spmg.1.4.18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risley M. Connexin gene expression in seminiferous tubules of the sprague-dawley rat. Biol. Reprod. 2000;62:748–754. doi: 10.1095/biolreprod62.3.748. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Meyuhas O. Ribosomal protein s6 phosphorylation: From protein synthesis to cell size. Trends Biochem. Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA. Targeting pi3 kinase/akt/mtor signalilng in cancer. Crit. Rev. Oncog. 2012;17:69–95. doi: 10.1615/critrevoncog.v17.i1.60. [DOI] [PubMed] [Google Scholar]
- Shimobayashi M, Hall MN. Making new contacts: The mtor network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- Smyth JW, Shaw RM. Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell reports. 2013;5:611–618. doi: 10.1016/j.celrep.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Ro S, Michaels JD, Park C, McCarrey JR, Yan W. Many x-linked micrornas escape meiotic sex chromosome inactivation. Nat. Genet. 2009;41:488–493. doi: 10.1038/ng.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Hennig GW, Wu Q, Jose C, Zheng H, Yan W. Male germ cells express abundant endogenous sirnas. Proc. Natl. Acad. Sci. USA. 2011;108:13159–131564. doi: 10.1073/pnas.1108567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzhi Z, Liang T, Yuexia P, Lucy L, Xiaoting H, Yuan Z, Qin W. Gap junctions enhance the antiproliferative effect of microrna-124-3p in glioblastoma cells. J. Cell Physiol. 2015;230:2476–2488. doi: 10.1002/jcp.24982. [DOI] [PubMed] [Google Scholar]
- Tavares MR, Pavan IC, Amaral CL, Meneguello L, Luchessi AD, Simabuco FM. The s6k protein family in health and disease. Life Sci. 2015;131:1–10. doi: 10.1016/j.lfs.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Valiunas V, Wang HZ, Li L, Gordon C, Valiuniene L, Cohen IS, Brink PR. A comparison of two cellular delivery mechanisms for small interfering rna. Physiol. Rep. 2015;3 doi: 10.14814/phy2.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettenhall RE, Erikson E, Maller JL. Ordered multisite phosphorylation of xenopus ribosomal protein s6 by s6 kinase ii. J. Biol. Chem. 1992;267:9021–9027. [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Wong CKC, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology. 2014;29:286–298. doi: 10.1152/physiol.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenardi E, Bregni G, de Braud F, di Cosimo S. Better together: Targeted combination therapies in breast cancer. Semin. Oncol. 2015;42:887–895. doi: 10.1053/j.seminoncol.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mtorc2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]