Abstract
Despite recent increases of psychosocial programs for pediatric chronic illness, few studies have explored their economic benefits. This study investigated the costs–benefits of a family systems-based, psychosocial intervention for pediatric chronic illness (MEND: Mastering Each New Direction). A quasi-prospective study compared the 12-month pre–post direct and indirect costs of 20 families. The total cost for program was estimated to $5,320. Families incurred $15,249 less in direct and $15,627 less in indirect costs after MEND. On average, medical expenses reduced by 86% in direct and indirect costs, for a cost–benefit ratio of 0.17. Therefore, for every dollar spent on the program, families and their third payers saved approximately $5.74. Implications for healthcare policy and reimbursements are discussed.
Approximately 27% of children in the United States have a chronic illness or condition, and one in fifteen have multiple chronic illnesses (Anderson, 2010). Chronic illnesses/conditions are exceedingly expensive to both patients and society. Nearly 86% of all medical costs in the United States are attributable to chronic conditions, and individuals with chronic illnesses incur up to two and a half times the medical expenses than those without a chronic illness (Gerteis et al., 2010). Furthermore, a large portion of these costs may be associated with the indirect effects of poor medical regime adherence and unaddressed social and psychological factors (Aitken & Valkova, 2013; Modi et al., 2012). To this end, limited adherence to a treatment regimen is estimated to cost $100 to $300 billion in healthcare costs in the United States annually (Aitken & Valkova, 2013). Given the importance of behavioral factors in chronic illness management, psychosocial interventions are a promising approach to more effectively reduce poor adherence and the accompanying progression of the illness, as well as reduce the overall medical costs associated with a chronic illness (Modi et al., 2012).
In the case of pediatric chronic illness, disease management and adherence present an even more critical issue as these children with CI often expect a lifelong need to manage their condition and are furthermore at a crucial age developmentally to make adherence changes that may influence the way they view their illness and manage it for the rest of their lives (Dashiff, Bartolucci, Wallander, & Abdullatif, 2005). Although there are many factors associated with medical adherence for pediatric chronic illness (Aitken & Valkova, 2013; Modi et al., 2012), there is sufficient evidence connecting adherence to developmental changes (such as gaining independence in adolescence), psychological factors (such as self-efficacy), and parent–child relationships (dysfunction in the home, etc.; Dashiff et al., 2005; Fiese & Everhart, 2006; La Greca et al., 1995; Laird, Pettit, Bates, & Dodge, 2003). There is also growing evidence linking physiological stress responses to worsening prognoses (Wood et al., 2008; Woods & McWey, 2012). While normative developmental milestones such as gaining independence from parents and gaining social support from peers is important to pediatric patients, these tasks of adolescence can be compromised or delayed due to the demands of a chronic illness. The consequences associated with these delays often increase the stress and negative emotions associated with the illness and tend to further compromise physical health and the ability to adhere to treatment recommendations (Dashiff et al., 2005; Fiese & Everhart, 2006; La Greca et al., 1995; Laird et al., 2003).
Benefits and Barriers of Family-Based Psychosocial Interventions for Chronic Illness
Preliminary research supports both the economic and clinical usefulness of psychosocial interventions for pediatric and adolescent chronic illness. Psychosocial interventions have been shown to reduce medical expenses by upwards of 20%, mainly by reducing patients’ lengths of stay and other direct medical costs offsets (Chiles, Lambert, & Hatch, 1999). In terms of improved adherence, Graves, Roberts, Rapoff, and Boyer (2010) reviewed 71 studies and found moderate to strong effect sizes for psychosocial interventions (Cohen’s d = 0.50 to 1.44), depending on the intervention and design of the studies. Given the wide range of effects, Graves et al. (2010) argue that, to be successful, psychosocial programs should include multiple components including education and behavioral interventions.
Benefits
While meta-analyses reveal that a wide variety of psychosocial interventions (cognitive behavioral, group therapy, and family therapy) impact quality of life and adherence immediately after completion of the intervention, only multidimensional programs that include the family systems demonstrate sustained improvements long after the program has ended (Eccleston, Palermo, Fisher, & Law, 2012; Ellis et al., 2012). This aligns with the work by McBroom and Enriquez (2009) who examined family centered treatments for type I diabetes. They concluded that, while family centered approaches produced effects similar to individual approaches such as CBT, programs that incorporated the family might provide a better degree of sustainability. This conclusion has also been supported by additional empirical evidence regarding health-related quality of life (HRQL) within chronically ill populations (Ellis et al., 2012; Santos, Crespo, Silva, & Canavarro, 2012; Scholten et al., 2013; Woods & McWey, 2012). Therefore, when considering outcomes of family-based treatments, one should not only consider reduction in costs and improvements in patient health but also the added benefit of sustainability.
One explanation for the sustainable effect of family interventions comes from the work of Wood and colleagues and their development of the biobehavioral family model (BBFM; Wood, 1993; Wood, Klebba, & Miller, 2000). The BBFM is a biopsychosocial approach used to conceptualize the complex interdependent ecological levels of influence on chronic illness. BBFM is derived from Minuchin’s structural family therapy model, which originally highlighted the relationship between disease activity and family dynamic patterns (Minuchin et al., 1975). The BBFM is also a systemic and ecological process-oriented model that assumes that family members’ emotional regulation is interdependent, collectively shared, and influenced by family systems dynamics (Wood et al., 2008). The BBFM proposes that the family emotional climate, parent–child relationships, and biobehavioral reactivity (physiological and psychological processes for emotion regulation) influence the child’s health (Wood et al., 2008). Thus, the BBFM offers a family relational stress model of disease that suggests that high levels of stress within these family systems results in maladaptive responses to stress and high levels of disease activity within the individual. Within this model, the concept of biobehavioral reactivity is a critical element that links the psychological and emotional processes of both the child and the family to the child’s disease process. Moreover several studies provide empirical evidence for the BBFM in chronic diseases such as inflammatory bowel disease (Wood et al., 1989) and asthma (Wood et al., 2000, 2008; Woods & McWey, 2012).
Barriers
Although it seems clear that family systems approaches offer a more sustainable effect, two related issues have historically limited the implementation of family-based, multidimensional models for pediatric chronic illness. First, the most effective and sustainable approaches are fairly intensive (multidimensional and integrative of individual, family and community stakeholders; Eccleston et al., 2015; Ellis et al., 2012; Graves et al., 2010; AUTHORS, 2014, 2015) and thus somewhat costly to implement. As a result, reluctance by third-party payers to reimburse these interventions often creates a barrier to the adoption of these promising interventions. Specifically, as many of these interventions are conducted through behavioral health referrals (Modi et al., 2012), families are reimbursed only if there is a comorbid diagnosable mental health condition. While comorbid Axis I mental health conditions are common in the population, children with psychological disorders are by no means the only type of patient that can benefit from these programs (Dashiff et al., 2005; Fiese & Everhart, 2006; La Greca et al., 1995; Laird et al., 2003; Modi et al., 2012).
The second barrier that arises is that psychosocial programs not limited to behavioral health referrals are typically offered in community-based settings within the pediatric practice itself (Eccleston et al., 2015). These types of programs are traditionally less intensive and not multidimensional (Distelberg, Williams-Reade, Tapanes, montgomery & Pandit, 2014). As such, the optimal outcome would be to have a multidimensional, intensive program that benefits from direct medical referral and medical reimbursement rates regardless of mental illness comorbidity.
In addition, while family systems-based approaches to chronic illness can be costly and involve a higher level of involvement of the family and mental–physical health team, the actual financial impact is often seen as delayed and long term making it less attractive to insurance carriers who often experience high client turn over. Nevertheless, a noted by Finney and Monahan (1996) and Holder, Longabaugh, Miller, and Rubonis (1991) who have successfully demonstrated that the cost of the treatment does not necessarily equate to the quality and sustainability of the outcome, we must consider not only the immediate postprogram effect, but also the long-term sustainability in assessing the true cost–benefit of a program. Given the literature regarding family systems approaches, this may very well mean that family systems approaches might cost more to implement, but they might also provide a longer-term benefit that greatly outweighs the initial cost.
Likewise, a recent wave of research looking specifically at the cost–benefit of marriage and family therapy (MFT) versus other types of mental health treatment or individual approaches (Crane & Payne, 2011; Morgan & Crane, 2010) has shown a benefit to the systemic lens and use of family-level intervention in MFT. Specifically, in comparison with other approaches, the effect of MFT seems to sustain long after the intervention, as measured by reductions in future needs for service, recidivism, and rehospitalization rates. Finally, the MFT field has long argued that cost– benefit research for family systems-based programs is crucial to the proliferation of the program, as well as the field as a whole (Christenson & Crane, 2014; Pinsof & Wynn, 1995; Sprenkle, 2012). In this case, showing the cost effectiveness and cost–benefit of family systems approaches is a useful way to introduce these programs into the larger mental and physical health fields and build the empirical base for the program.
Although there have been a number of economic studies for issues like alcohol and substance use dependency (Morgan & Crane, 2010), there has been little evidence of the economic benefit of family systems-based psychosocial interventions for chronic illness specifically. As aforementioned, although some research has provided preliminary evidence as to the indirect benefits of psychosocial interventions (Chiles et al., 1999; Graves et al., 2010), there lacks a direct cost–benefit analysis of a program’s impact on health costs of pediatric chronic illness. In addition, these investigations do not include family systems-based approaches, and they rely on proxy measures such as quality of health/life rather than direct financial costs.
This study therefore evaluated a small sample of families that received a family systems-based psychosocial program for pediatric chronic illness (Distelberg et al., 2014) to determine the cost–benefit ratio of program cost versus savings. To accomplish this, we accessed families’ medical expenses pre- and postparticipation in the program for the purpose of constructing and evaluating both the direct health costs and the indirect costs associated with parental missed days of work and child care needs associated with the severity of the child’s illness.
Mastering Each New Direction
Mastering Each New Direction (MEND; Distelberg et al., 2014; Tapanes, Distelberg, Williams-Reade & Montgomery, 2015) is a principle-based intensive outpatient psychosocial intervention for children with a chronic illness/condition. MEND is delivered in a behavioral health medical center, and as such, utilizes a multidisciplinary team. Families are referred to the MEND program when the patient’s general or specialty care physician is concerned that the prescribed treatment protocol is not having its desired effect, most often due to suspected adherence issues. In addition, there are often confounding psychosocial variables that are beyond the scope of the medical team’s purview and/or believed to be impacting the child’s medical regime adherence and prognosis. Currently, referrals to the MEND program come directly from either a psychiatrist, or more commonly, the child’s specialty physician. The MEND team has marketed the program through institution grand round presentations and individual department presentations, often with nursing and social work professionals as the target market for referrals. There are no specific criteria for referrals by a physician, other than their clinical judgment, but third-party reimbursement is often contingent upon a comorbid chronic illness mal-adherence and a diagnosable mental health condition.
The MEND program is most often paid for by the family’s third-party payer who often requires an Axis I diagnosis before approval of reimbursement, although they occasionally approve Axis II or V codes. When a family’s insurance refuses to cover the program’s expenses, the MEND program is able to access a small amount of funding in the form of scholarships. This scholarship program was initially funded by a local philanthropy donation. On average, 12 families receive this funding annually.
Upon referral, the child receives an initial psychiatric evaluation and ongoing psychiatric monitoring throughout the program. The family then begins MEND, progressing through the phases of the program (Distelberg et al., 2014; Tapanes et al., 2015). The MEND team works as a multidisciplinary team, inclusive of the referring physician, an outpatient psychiatrist, marriage and family therapists, psychologists and the child’s academic community. MEND is an intensive outpatient program that meets three times weekly. Each session is 3.5 hr in length. The first two hours are spent in individual, family, and peer group therapy, and the remaining hour and a half varies from multifamily groups, art, and occupational therapy processes, based on the weekday.
The MEND model assumes a complex interdependent relationship between physical illness, physiological stress, and family systems dynamics. More specifically, the MEND program relies on a biopsychosocial conceptual framework of chronic illness that assumes that the patient’s condition is directly influenced by physiological stress. This stress is hypothesized to reduce the patient’s cognitive functioning, thereby further limiting the adolescent’s ability to manage his or her complex treatment regimen (Distelberg et al., 2014; Tapanes et al., 2015). Additionally, the MEND program uses a family systems perspective to underscore that the illness must be understood for its recursive impact on both the child and family systems. Although some families handle the stress of chronic illness successfully, many others are unable and their relationship functioning declines, which results in additional stress. These family systems are those that exhibit treatment mal-adherence and are eventually referred to the program. Therefore, MEND targets stress at varying levels, including the internal physiological state of the child, the parent–child relationship, and within the larger family systems and social context. MEND also places a significant focus on helping the child develop age-appropriate peer relationships. All of this is accomplished through peer and multifamily groups, individual and family therapy interventions.
Mastering Each New Direction is a family systems approach that highly values the involvement of the family in the intervention. Although families are not required to participate in the intervention, most family members do participate fully in the interventions, including parents and siblings. Lack of family participation is understood by the therapy team as a limitation and symptomatic of underlying system maladaptive functioning. Because of this, the team will address a lack of family involvement from a systemic therapeutic stance. In practice, families are involved in the initial intake process, as well as graduation and discharge, but also more frequently, in regular family therapy sessions and weekly multifamily groups.
Preliminary evidence of the program has shown significant effects in multiple domains (Distelberg et al., 2014). These initial investigations found that the MEND program improved the child’s HRQL by more than 40% and the parents and family’s quality of life by more than 55%. HRQL covers a wide range of factors including physical, emotional, social, school, cognitive, and psychosocial functioning. Families also reported reductions in anxiety, conflict, and worry. Furthermore, the program was seen to reduce the number of missed days of school by more than 63% and missed days of work for parents by more than 77%.
METHODS
Participants
The study design was approved by the (Loma Linda University) institutional review board. All families who participated in the MEND program were eligible to participate in this study if their medical expenses were incurred within the (Loma Linda University) health system. This assured that the children’s medical expenses would be accessible through the child’s medical record.
As seen in Figure 1, 56 families were referred to the MEND program and completed the informed consent process for this study. Twenty-four families met the inclusion criteria noted above and had received at least 12 months of medical care within the (Loma Linda University Health Behavioral Medicine Center) medical system. Twenty of these 24 families completed all 21 session of the MEND program and were included in the analysis below. The initial founders of the program conducted all sessions within this study, and therefore, the services delivered held strictly to the approach described in previous papers (Distelberg et al., 2014; Tanpanes et al., 2015).
Figure 1.
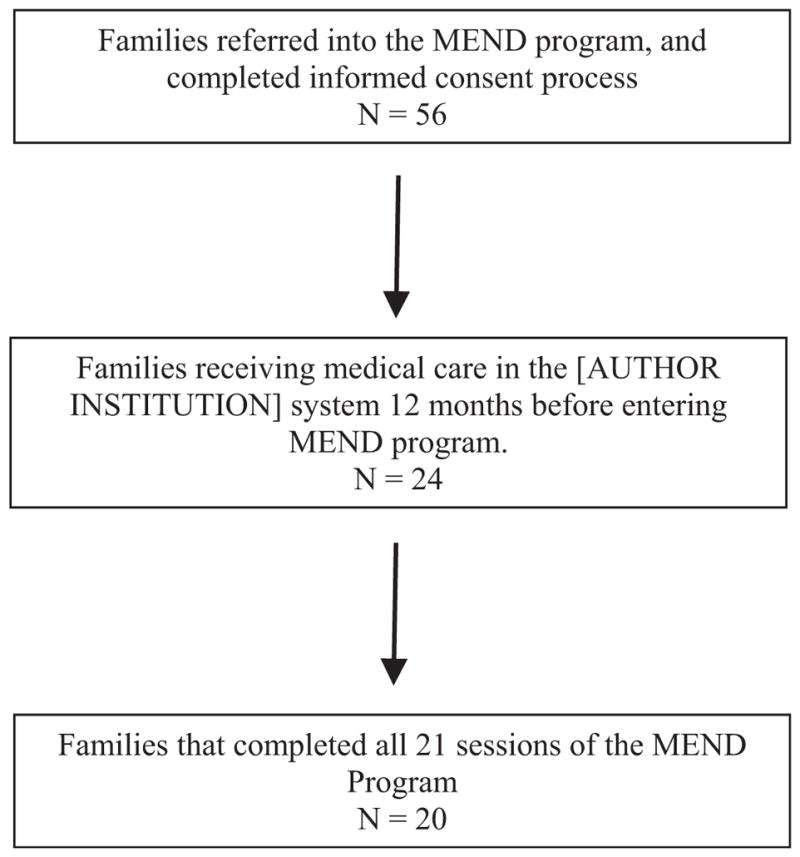
Sample referral and recruitment process.
The total sample offered a diverse makeup of chronic illnesses including kidney disease, type I diabetes, chronic pain conditions, and organ failure/transplants. This sample was also diverse in regard to patient ethnicity and parental education. Please see Table 1 for sample demographics.
Table 1.
Characteristics of Child and Parent Participants (N = 20)
| Gender, N (%) | |
| Male | 3 (17.65) |
| Female | 17 (85.00) |
| Age, M(SD) | 15.2 (0.45) |
| Chronic Illness Type, N (%) | |
| Kidney Disease/Organ Transplant | 4 (18.18) |
| Type 1 Diabetes | 4 (18.18) |
| Chronic Pain | 3 (13.64) |
| Heart Transplant | 2 (9.09) |
| Ulcerative Colitis | 2 (9.09) |
| Seizures | 2 (9.09) |
| Chiari Malformation | 1 (4.55) |
| Asthma | 1 (4.55) |
| Cancer | 1 (4.55) |
| Ethnicity, N (%) | |
| Black, Non-Hispanic | 3 (15.00) |
| Asian or Pacific Islander | 1 (5.00) |
| White, Non-Hispanic | 6 (30.00) |
| Hispanic White | 9 (45.00) |
| Native American or Alaskan Native | 1 (5.00) |
| Mother’s Education, N (%) | |
| Less than High School | 2 (10.00) |
| High School Graduate | 6 (30.00) |
| College Graduate | 8 (40.00) |
| More than College | 3 (15.00) |
| Father’s Education, N (%) | |
| Less than High School | 2 (10.00) |
| High School Graduate | 13 (65.00) |
| College Graduate | 2 (10.00) |
| More than College | 3 (15.00) |
Measures
Direct cost–benefits due to medical expenses
Parents provided the research team with access to their child’s medical record. This access to the electronic medical record was used to identify the child’s medical expenses 12 months prior to beginning MEND (pre-MEND) as well as 12 months after the child graduated (post-MEND). The (Loma Linda University Health) finance department validated medical expense data. These costs included hospitalizations, physician visits, ER visits, and reflect gross (or total) costs (i.e., the total costs billed to the third-party payer, co-pays, and other costs not billed to the third-party payer). The medical expense data was then associated with the family’s survey data.
Upon review of the medical expenses, two expense data points were excluded from the study. In one case, a child was referred to the MEND program under the assumption that she would be eligible for a kidney transplant only after completing the MEND program. In other words, she had been removed from the transplant eligibility list until she successfully completed the program. After successfully graduating from the MEND program, the child in question underwent a $210,000 transplant surgery. This cost was removed from the analysis given that it was both deemed an outlier in comparison with average annual expenses (M = $20,9249, SD = $27,973) and that it reflected a successful outcome rather than a worsening of health postprogram. The other case involved a child who incurred a $28,000 emergency room expense related to influenza prior to entering the MEND program. As the influenza was deemed unrelated to the child’s heart transplant, it was also removed. All other expenses within these cases were retained.
Demographic variables
Upon entering the MEND program, the child and one parent completed a battery of surveys. Included in this survey packet was a demographic survey filled out by the parent, which included education level, ethnicity, and age.
Indirect costs–benefits
Parents also completed surveys that included questions about the number of days, within the last month that parents missed work, and that the child needed additional caregiver support at home. These surveys were collected when the family began the program and upon graduation. From this survey, we computed an average number of days parents missed work in a month pre- and post-MEND, as well as the average number of days a caregiver was needed (Table 3). We also computed the annual number of days by multiplying the 1-month average by 12. To estimate the dollar value associated with missed work and caregiver needs, we calculated a dollar per day value of missed days of work based on the median household income for the area ($54,000; divided by 250 working days a year; U.S. Census Bureau, 2015) and the median income of a caregiver in the area ($24,810; divided by 250 working days a year; Child Care Aware of California and America, 2015; see Table 4 for a summary of this process). We then summed up and compared patients’ direct and indirect costs prior to entering MEND and 12 months after the program.
Table 3.
Repeated-Measures Analysis of Variance of Indirect and Auxiliary Costs Pre- and Post-Mastering Each New Direction (MEND; N = 20)
| Pre-MEND
|
Post-MEND
|
3 Months after
|
|||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| Indirect Costs–Benefits | |||||||
| Missed Days of Work (1 month) | 5.00 | 7.91 | 0.50 | 1.07 | 0.38 | 1.06 | F(2, 40) = 4.94, p < .01, η2 = .38 |
| Days a Caregiver is Needed (1 month) | 10.20 | 12.64 | 7.07 | 11.58 | 3.13 | 7.94 | F(2, 40) = 6.31, p < .01, η2 = .25 |
| Auxiliary Measures | |||||||
| Number of Hospital Stays (1 year) | 1.95 | 2.41 | 1.58 | 2.52 | 1.37 | 4.11 | F(2, 40) = 3.14, p < .05, η2 = .14 |
| Number of ER visits (1 year) | 2.42 | 2.07 | 1.42 | 2.81 | 0.42 | 0.51 | F(2, 40) = 7.11, p < .05, η2 = .40 |
| Missed days of school (1 month) | 12.53 | 12.11 | 1.60 | 3.02 | 1.73 | 3.15 | F(2, 40) = 20.92, p < .001, η2 = .54 |
Note. Means (M) and standard deviations (SD) represented in the table are based on nontransformed raw data. The ANOVA F and effect size are based on the natural log-transformed data.
Table 4.
Summary of Monetization of Indirect Costs (N = 20)
| Items | Benefit in 1 month | Benefit after 12 months | Estimated cost per unit | Total annual benefit per patient |
|---|---|---|---|---|
| Reduced number of missed days of work | 4.50 | 54.00 | $216.36a | $11,683.44 |
| Reduced number of days a caregiver is needed | 3.13 | 37.56 | $95.42b | $3,583.98 |
| Indirect benefit subtotal | $15,267.42 | |||
| Direct benefit subtotal | $15,248.00 | |||
| Total benefit | $30,515 |
Note.
Estimated daily wage based San Bernardino median household income of $54,090 from census 2013 data. Per day wage = 54.090/250 working days per year.
Estimated daily caregiver costs based on annual cost of child care from the National Association of Child Care Aware of America (2015) (median annual wage = $24,810.
Auxiliary costs–benefits
The parent survey also included the number of self-reported hospital admissions and Emergency Department (e.g., ER) visits and the number of days that the child had missed school, within the last 30 days. We measured these auxiliary variables to provide additional context to the economic benefits of the program, but do not include these variables within the economic analysis.
MEND program costs
Program costs are defined as the cost to the parents and their third-party payer. In this case, the program cost is $5,320 per family/patient. This cost was reported by (Loma Linda University Health finance department) and is based on a session cost of $246.19, multiplied by 21 sessions of MEND (the average number of sessions required in the MEND program). It also includes a onetime cost of $150 for laboratory tests for stress biomarkers. The session cost is based on the following: $140.33 for staff/labor (e.g., therapists, psychiatrists, nursing, case management), $93.55 for administrative overhead, and $12.31 for other costs (professional fees, supplies, etc.). In other words, this per session fee incorporates the following items: (a) initial and ongoing psychiatric and nurse evaluation, monitoring, and case consultation; (b) three and a half hours of psychosocial interventions of MEND with MEND therapists (inclusive of one on one, family, and multifamily therapy session); (c) case consultation by the MEND therapist with the school, physician, or specialty medical team; and (d) administrative overhead and expenses (all the costs associated with implementing the program including room, lighting, staff salaries, etc.).
Data Analysis
We examined all variables for univariate normality and for the existence of outliers. In all cases, except for the one noted above, there were no outliers for the direct medical costs, and all conformed to univariate normality (each variable was measured to have a skewness and kurtosis value between ± 2.00). For the indirect and auxiliary variables, some measurement points resulted in skewness (skewness >2.0, <4.0). Therefore, we used a natural log transformation of the indirect and auxiliary variables before testing the change over time with repeated-measures ANOVA. All analyses with these variables were performed with the natural log transformation and without the transformation. No differences were noted in the significance of the tests. Therefore, we report the natural log transformation test results as the transformed variable conform to ANOVA univariate assumptions.
We began first by evaluating the direct, indirect, and auxiliary variables for central tendency and changes over time. For direct medical costs, we used a paired-sample t-test to evaluate whether the medical expenses decreased pre- and postprogram. Next, we evaluated whether there were changes in the indirect and auxiliary variables over time. We then applied the resulting measured difference of the indirect variables from this analysis to the cost–benefit analysis.
We performed two cost–benefit analyses from the perspective of the patient and their payers. The first was a direct medical cost analysis. The benefit (denominator) in this analysis was the decrease in medical expenses (post-MEND cost–Pre-MEND cost). The numerator was the cost of the MEND program. The second cost–benefit analysis included indirect benefits within the denominator. Specifically, the benefit was associated with changes in the indirect variables (missed work and caregiver days).
RESULTS
Direct Benefits–Cost Due to Medical Expenses
Overall, there was a significant reduction in medical expenses post-MEND. MEND families entered the program with an average of $20,924 (SD = $27,973) of incurred medical expenses annually (Table 2). After MEND, the families’ annual medical expenses averaged $5,675 (SD = $7,543), a reduction of $15,249 (SD = $28,800) or 73% annually, t(19) = 2.18, p = .03.
Table 2.
Results of the Repeated-Measures T-Tests of Medical Record Expenses Pre- and Post-Mastering Each New Direction (MEND; N = 20)
| Pre-MEND
|
Post-MEND
|
Change between Pre- and Post-MEND
|
||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | Q1–Q3 | M | SD | Q1–Q3 | M | SD | t(df) |
| $20,924 | $27,973 | $2,906–24,270 | $5,675 | $7,543 | $411–6,899 | −$15,248 | $28,800 | 2.18* (16) |
Note.
p < 0.05.
Indirect and Auxiliary Benefits
As detailed in Table 3 below, there was a significant reduction in the indirect costs. In Table 3, the median and SD data reported are based on the nontransformed data, and the ANOVA F statistics as well as effect size measures are based on the natural log transformation of the raw data. Parents self-reported fewer missed days of work and days needing a caregiver. Specifically, the number of missed days of work decreased from an average of 5.00 days per month (median = 0, IQR = 0|6) to an average of 0.38 days a month (median = 0, IQR = 0|1.0) 3 months post-MEND. In addition, the families decreased their need for a caregiver from 10.20 days a month (median = 4.5, IQR = 0|30) to 3.13 days a month (median = 0.0, IQR = 0|2). The auxiliary measures showed similar reductions with decreases in missed days of school (pre-MEND: median = 7, IQR = 3|20, post-MEND: median = 0, IQR = 0|2), hospital visits (pre-MEND: median = 1, IQR = 0|3, post-MEND: median = 0, IQR = 0|1), and ER visits (pre-MEND: median = 2, IQR = 1|3, post-MEND: median = 0.0, IQR = 0|1).
Cost–Benefit Analysis: Direct Healthcare Costs
The total cost of participating in the program was $5,320 per patient or $106,400 for the 20 patients, reflecting the cost of MEND to the parents and their payers. As noted above, participation in the program was associated with a reduction of $15,248 in medical expenses per patient over 12 months. This translates into a direct cost–benefit ratio of $5,320/$15,249, or 0.35. Stated otherwise, for every $1 spent on the program there is a $2.87 saving ($15,248/$5,320) of direct medical expenses.
Cost–Benefit Analysis: Direct Medical Expenses and Indirect Benefits
In addition to the above direct benefits (e.g., reduction in medical expenses), participation in the program was associated with indirect benefits such as missing less work, and less need for caregivers. For example, there was an average reduction of 4.50 fewer days of missed work. Using the area-adjusted median income, we estimated that the annual benefit for missing less work to be equal to $11,683 annually. Inclusive of the annual cost for caregiver needs, the total annual indirect benefit = $15,267 per patient, bringing the total benefit of MEND to $30,516 ($15,267 + $15,249). Given this total benefit, the cost–benefit ratio for participating in the program was calculated to be $5,320/$30,515 = 0.17, or $5.74 of savings for every dollar spent in the first 12 months after the MEND program (Table 4).
DISCUSSION
With an emergent literature suggesting the effectiveness of family systems-based psychosocial intervention for pediatric chronic illness, we sought to also evaluate their possible economic benefit. The results of the cost–benefit analysis indicate that MEND has a significant economic benefit for families and their third-party payers for whom the medical “treatment as usual” is not sufficient. In this study, we found a cost–benefit of .35 in reduced direct medical expenses. In other words, for every dollar spent on the psychosocial program, we were able to show a savings of $2.87 in direct medical expenses such as hospitalizations, physician appointments, associated treatment, and tests. These direct costs–benefits pertain mostly to the third-party payers as the direct costs focused only on billable medical expenses pre- and post-MEND. When taking into consideration the additional indirect costs of illness, such as missed work and need for caregiving, the cost–benefit ratio improved further to .17. Therefore, the combined benefit to the family and their third-party payer is significant. In this case, for every dollar spent on the program, families and their third-party payers saved an average of $5.74.
As mentioned above, prior psychosocial intervention for chronic illness, cost–benefit studies have estimated a 20% reduction in costs for psychosocial interventions in chronic illness (Chiles et al., 1999). Even without the inclusion of the important indirect costs to the families, we found a 73% reduction in medical expenses over 12 months. This is a larger reduction than noted in the other studies, and speaks to an important role of programs like MEND in more complex medical cases or cases that might be considered more costly and higher service utilizers. For example, in the meta-analysis by Chiles et al. (1999), studies using behavioral interventions for surgery had an average effect size (Cohen’s d) of 0.60–0.81. This level of effect was higher than any other type of population receiving psychosocial interventions and is a comparable effect size to the gains seen in our study. Together, this study and others of its kind suggest that the largest economic benefit of psychosocial interventions exists in populations where chronic illnesses are severe and likely to be associated with costly medical expenses. This might also provide some guidance in identifying populations that might benefit from programs like MEND.
Another strength of this study is that it offers a realistic evaluation of costs. Previous studies have relied on either reduced hospital stays (Chiles et al., 1999) or improved HRQL (Graves et al., 2010). While beneficial, these studies might underestimate the true cost associated with more auxiliary expenses, such medical office visits, missing work or requiring additional caregivers. This study provided indirect costs and tracked the costs longitudinally, creating a more reliable benchmark for generalization to other families.
Finally, this study provides added support for the inclusion of psychosocial intervention reimbursement. Currently, robust family systems-based programs such as MEND require a preceding mental health (or Axis I) diagnosis before most third-party payers will reimburse the family for program costs. This tends to limit many patients’ access to these programs. The results of this study, in combination with prior research (Chiles et al., 1999; Graves et al., 2010), provide strong support for reimbursement regardless of comorbid psychiatric illness. Furthermore, one might argue that programs like MEND could serve as prevention for Axis I health issues in CI children.
We also agree with Modi et al. (2012) that lower income families, due to underinsurance and economic strain, have the least access to programs like these. Given the strain of socioeconomic stress in concert with a chronically ill child, it is likely that low-income families would benefit the most from family-based programs. This study provides support for the inclusion of programs like MEND in lower income-providing insurance programs, such as Medicaid. Such providers could now consider reimbursing multidimensional and multidisciplinary approaches over lower cost mainstream approaches, being aware that the cost savings of intensive family approaches are both significant and long lasting.
While there are significant strengths to this study, there are also a number of limitations. First, this study is based on 20 families. Although the study sample included a wide range of illnesses and demographic presentations, it is possible that larger sample size studies would find different results. Additionally, there is a possibility that not all family medical expenses were accessed in this study. This study relied on medical records within the (Loma Linda University) health system, but it is important to note that a family may have incurred additional expenses outside of the (Loma Linda University Health system). Although we verified that each child’s primary physician was within the (Loma Linda University) health system, a family may nonetheless have used an outside pharmacy or used emergency services in another city or state or had a “second opinion” visit with a physician outside of the system. Although highly unlikely, given the population, we cannot fully rule this out. Future studies will need to replicate these findings within a larger sample and with more control over out-of-system medical expenses. Also important to consider in future study would be the inclusion of a randomized control group for comparison of effects. A future study with a comparable control or treatment as usual group design, would be beneficial in illuminating potential confounding variables, concerns regarding regression to the mean, and other third variable situations. However, our findings are consistent with prior research making our inferences plausible.
In conclusion, this study was the first economic evaluation of a family systems-based approach called MEND. This study found that participation in the MEND program was associated with a significant economic benefit to both families and third-party payers. For every dollar spent on the program, families and their third-party payers saved about $2.87 in direct medical expenses and $5.74 when additional indirect costs were considered. These findings provide support for the inclusion of multidimensional, family-based psychological intervention reimbursement by insurance providers as well as financial benefit for the families of CI children.
Footnotes
Funding source: None.
References
- Aitken M, Valkova S. Avoidable costs in U.S. health care: The $200 billion opportunity from using medicines more responsibly. IMS Institute for Healthcare Informatics. 2013 Retrieved September 17, 2015 from: http://www.drugstorenews.com/sites/drugstorenews.com/files/Avoidable%20Costs%20in%20Healthcare.pdf.
- Anderson G. Chronic care: Making the case for ongoing care. Princeton, NJ: Robert Wood Johnson Foundation; 2010. [Google Scholar]
- Child Care Aware of America. Childcare in the state of California. Child Care Aware of America. 2015 Retrieved September 17, 2015, from: http://usa.childcareaware.org/advocacy-public-policy/resources/reports-and-research/statefactsheets.
- Chiles J, Lambert M, Hatch A. The impact of psychological interventions on medical cost offset: A meta-analytic review. Clinical Psychology: Science and Practice. 1999;6(2):204–220. [Google Scholar]
- Christenson JD, Crane DR. Integrating costs into marriage and family therapy research. In: Miller RB, Johnson LN, editors. Advanced methods in family therapy research: A focus on validity and change. New York, NY: Routledge; 2014. pp. 420–436. [Google Scholar]
- Crane RD, Payne SH. Individual versus family psychotherapy in managed care: Comparing the costs of treatment by the mental health professions. Journal of Marital and Family Therapy. 2011;37:273–289. doi: 10.1111/j.1752-0606.2009.00170.x. [DOI] [PubMed] [Google Scholar]
- Dashiff C, Bartolucci A, Wallander J, Abdullatif H. The relationship of family structure, maternal employment, and family conflict with self-care adherence of adolescents with Type 1 Diabetes. Families, Systems, & Health. 2005;23(1):66–79. [Google Scholar]
- Distelberg B, Williams-Reade J, Tapanes D, Montgomery S, Pandit M. Evaluation of a family systems approach to managing pediatric chronic illness: Managing Each New Direction (MEND) Family Process. 2014;53(2):194–213. doi: 10.1111/famp.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Palermo TM, Fisher E, Law E. Psychological interventions for parents of children and adolescents with chronic illness. The Cochrane Library. 2012;4:1–172. doi: 10.1002/14651858.CD009660.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D, Naar-King S, Chen X, Moltz K, Cunningham P, Idalski-Carcone A. Multisystemic therapy compared to telephone support for youth with poorly controlled diabetes: Findings from a randomized controlled trial. Annals of Behavioral Medicine. 2012;44:207–215. doi: 10.1007/s12160-012-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiese BH, Everhart RS. Medical adherence and childhood chronic illness: Family daily management skills and emotional climate as emerging contributors. Current Opinion in Pediatrics. 2006;18:551–557. doi: 10.1097/01.mop.0000245357.68207.9b. [DOI] [PubMed] [Google Scholar]
- Finney JW, Monahan SC. The cost-effectiveness of treatment for alcoholism: A second approximation. Journal of Studies on Alcohol. 1996;57:229–243. doi: 10.15288/jsa.1996.57.229. [DOI] [PubMed] [Google Scholar]
- Gerteis J, Izrael D, Deitz D, LeRoy L, Ricciardi R, Miller T, et al. Multiple chronic conditions chartbook: 2010 medical expenditure panel survey data. Agency for Healthcare Research and Quality. 2010;14-0038:1–52. Retrieved September 1, 2015 from: http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/preventionchronic-care/decision/mcc/mccchartbook.pdf. [Google Scholar]
- Graves MM, Roberts MC, Rapoff M, Boyer A. The efficacy of adherence interventions for chronically ill children: A meta-analytic review. Journal of Pediatric Psychology. 2010;35:368–382. doi: 10.1093/jpepsy/jsp072. [DOI] [PubMed] [Google Scholar]
- Holder H, Longabaugh R, Miller WR, Rubonis AV. The cost effectiveness of treatment for alcoholism: A first approximation. Journal of Studies on Alcohol. 1991;52:517–540. doi: 10.15288/jsa.1991.52.517. [DOI] [PubMed] [Google Scholar]
- La Greca A, Auslander W, Greco P, Spetter D, Fisher E, Santiago J. I get by with a little help from my family and friends: Adolescents’ support for diabetes care. Journal of Pediatric Psychology. 1995;20:449–476. doi: 10.1093/jpepsy/20.4.449. [DOI] [PubMed] [Google Scholar]
- Laird R, Pettit G, Bates J, Dodge K. Parents’ monitoring-relevant knowledge and adolescents’ delinquent behavior: Evidence of correlated developmental changes and reciprocal influences. Child Development. 2003;74:752–768. doi: 10.1111/1467-8624.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBroom LA, Enriquez M. Review of family-centered interventions to enhance the health outcomes of children with type 1 diabetes. The Diabetes Educator. 2009;35:428–438. doi: 10.1177/0145721709332814. [DOI] [PubMed] [Google Scholar]
- Minuchin S, Baker L, Rosman BL, Liebman R, Milman L, Todd TC. A conceptual model of psychosomatic illness in children: Family organization and family therapy. Archives of General Psychiatry. 1975;32:1031–1038. doi: 10.1001/archpsyc.1975.01760260095008. [DOI] [PubMed] [Google Scholar]
- Modi AC, Pai AL, Hommel KA, Hood KK, Cortina S, Hilliard ME, et al. Pediatric self-management: A framework for research, practice, and policy. Pediatrics. 2012;129:e473–e485. doi: 10.1542/peds.2011-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TB, Crane R. Cost-effectiveness of family-based substance abuse treatment. Journal of Marital and Family Therapy. 2010;36:486–498. doi: 10.1111/j.1752-0606.2010.00195.x. [DOI] [PubMed] [Google Scholar]
- Pinsof W, Wynn L. Family therapy effectiveness, current research & theory. Journal of Marital and Family Therapy. 1995;21:585–613. [Google Scholar]
- Santos S, Crespo C, Silva N, Canavarro MC. Quality of life and adjustment in youths with asthma: The contributions of family rituals and the family environment. Family Process. 2012;51:557–569. doi: 10.1111/j.1545-5300.2012.01416.x. [DOI] [PubMed] [Google Scholar]
- Scholten L, Willemen AM, Last BF, Maurice-Stam H, van Dijk EM, Ensink E, et al. Efficacy of psychosocial group intervention for children with chronic illness and their parents. Pediatrics. 2013;131:e1196–e1203. doi: 10.1542/peds.2012-2222. [DOI] [PubMed] [Google Scholar]
- Sprenkle D. Intervention research in couple and family therapy: A methodological and substantive review and an introduction to the special issue. Journal of Marital and Family Therapy. 2012;38(1):3–29. doi: 10.1111/j.1752-0606.2011.00271.x. [DOI] [PubMed] [Google Scholar]
- Tapanes D, Distelberg B, Williams-Reade J, Montgomery S. MEND biopsychosocial intervention for pediatric chronic illness. Journal of Family Psychotherapy. 2015;26(1):3–8. doi: 10.1080/08975353.2015. [DOI] [Google Scholar]
- U.S. Census Bureau. American Community survey: 5-year estimates. 2015 Retrieved September 17, 2015, from: http://www.census.gov/data/developers/data-sets/acs-survey-5-year-data.html.
- Wood BL. Beyond the “psychosomatic family”: A biobehavioral family model of pediatric illness. Family Process. 1993;32:261–278. doi: 10.1111/j.1545-5300.1993.00261.x. [DOI] [PubMed] [Google Scholar]
- Wood BL, Klebba KB, Miller BD. Evolving the biobehavioral family model: The fit of attachment. Family Process. 2000;39:319–344. doi: 10.1111/j.1545-5300.2000.39305.x. [DOI] [PubMed] [Google Scholar]
- Wood B, Lim J, Miller BD, Chean P, Zwetsch T, Ramesh S, Simmens S. Testing the biobehavioral family model in pediatric asthma: Pathways of effect. Family Process. 2008;47:21–40. doi: 10.111/j.1545-5300.00237x. [DOI] [PubMed] [Google Scholar]
- Woods SB, McWey LM. A biopsychosocial approach to asthma in adolescents encountering child protective services. Journal of Pediatric Psychology. 2012;37:404–413. doi: 10.1093/jpepsy/jsr104. [DOI] [PubMed] [Google Scholar]
- Wood B, Watkins JB, Boyle JT, Nogueira J, Zimand E, Carroll L. The “psychosomatic family” model: An empirical and theoretical and empirical analysis. Family Process. 1989;28:399–417. doi: 10.1111/j.1545-5300.1989.00399.x. [DOI] [PubMed] [Google Scholar]


