SUMMARY
AMP-activated protein kinase (AMPK) plays an important role in regulating food intake. The downstream AMPK substrates and neurobiological mechanisms responsible for this, however, are ill-defined. Agouti-related peptide (AgRP)-expressing neurons in the arcuate nucleus regulate hunger. Their firing increases with fasting, and once engaged they cause feeding. AgRP neuron activity is regulated by state-dependent synaptic plasticity; fasting increases dendritic spines and excitatory synaptic activity, feeding does the opposite. The signaling mechanisms underlying this, however, are also unknown. Using neuron-specific approaches to measure and manipulate kinase activity specifically within AgRP neurons, we establish that fasting increases AMPK activity in AgRP neurons, that increased AMPK activity in AgRP neurons is both necessary and sufficient for fasting-induced spinogenesis and excitatory synaptic activity, and that the AMPK phosphorylation target mediating this plasticity is p21-activated kinase. This provides a signaling and neurobiological basis for both AMPK regulation of energy balance and AgRP neuron state-dependent plasticity.
INTRODUCTION
AMP-activated protein kinase (AMPK) is an evolutionarily conserved serine/threonine kinase stimulated by both decreased cellular energy status and increased calcium (Hardie et al., 2012). In the hypothalamus, it is inhibited by leptin (Andersson et al., 2004; Dagon et al., 2012; Minokoshi et al., 2004) and activated by fasting (Minokoshi et al., 2004), ghrelin (Andersson et al., 2004; Andrews et al., 2008; Lopez et al., 2008), and neuronal activity (Hawley et al., 2005; Kawashima et al., 2012). Notably, manipulation of AMPK activity in the hypothalamus affects energy balance (Andersson et al., 2004; Claret et al., 2007; Minokoshi et al., 2004). However, the neurobiological mechanism and downstream AMPK target responsible for these effects are not known.
In this context, hypothalamic agouti-related peptide (AgRP)-expressing neurons, and their excitatory synaptic inputs, are of interest. AgRP neurons are activated by fasting (Takahashi and Cone, 2005), and once engaged, they induce intense hunger and reduce energy expenditure (Aponte et al., 2011; Gropp et al., 2005; Krashes et al., 2011; Luquet et al., 2005). Chemogenetic activation or inhibition of the excitatory neuronal drive to AgRP neurons stimulates/inhibits hunger, respectively (Krashes et al., 2014). Indeed, synaptic plasticity of these excitatory afferents is an important control point. Fasting, ghrelin and low leptin increases excitatory synapses, dendritic spines and excitatory synaptic activity in AgRP neurons (Liu et al., 2012; Pinto et al., 2004; Yang et al., 2011), and this fasting-induced plasticity, which requires NMDA receptors on AgRP neurons, contributes importantly to activation (Liu et al., 2012).
AMPK in AgRP neurons could trigger this plasticity because a) it is activated in the hypothalamus by fasting and by ghrelin, although it is not known if this occurs specifically in AgRP neurons, b) when stimulated pharmacologically in isolated neurons, brain slices, or in vivo in mice, it increases AgRP neuronal activity (Kohno et al., 2008; Kohno et al., 2011) and excitatory input to AgRP neurons (Yang et al., 2011), although the later was reported to be mediated by AMPK in the presynaptic neurons, and c) of significant interest, p21-activated kinase (PAK), a known inducer of spinogenesis and excitatory synaptic plasticity (Hayashi et al., 2004; Kreis and Barnier, 2009; Penzes et al., 2003), was recently identified in an unbiased chemical genetic screen in cultured cells as a novel AMPK substrate (Banko et al., 2011). In the present study, we use neuron-specific approaches to test the following two hypotheses: 1) a postsynaptic AMPK → PAK pathway drives state-dependent excitatory synaptic plasticity in AgRP neurons, and 2) the plasticity brought about by this AMPK → PAK pathway accounts for effects of AMPK on energy balance.
RESULTS
Fasting Increases AMPK Activity in AgRP neurons
AMPK activity in the hypothalamus, including the arcuate nucleus (ARC), is higher in fasted versus refed mice (Minokoshi et al., 2004). However, since AgRP neurons are just one of many subpopulations of neurons in the ARC, and since the other neurons have opposite or unrelated functions, it is unknown if fasting increases AMPK activity specifically in AgRP neurons. To monitor activity selectively in AgRP neurons, we constructed a cre-dependent adeno-associated virus (AAV) expressing FLAG-tagged α2 AMPK (Figure 1A) and stereotaxically injected it into the arcuate nucleus of Agrp-IRES-Cre mice (Figure 1B). Mice were then studied in the fed or fasted state (food was removed at 9 AM and assays were performed 24 hrs later). AgRP neuron-specific α2 AMPK was then immunoprecipitated (Figure 1C) and assayed for kinase activity as described previously (Dagon et al., 2012; Minokoshi et al., 2004). Of note, AMPK activity was increased more than two-fold in AgRP neurons of fasted versus fed mice (Figure 1D). Thus, marked fasting-feeding regulation of AMPK occurs specifically in AgRP neurons.
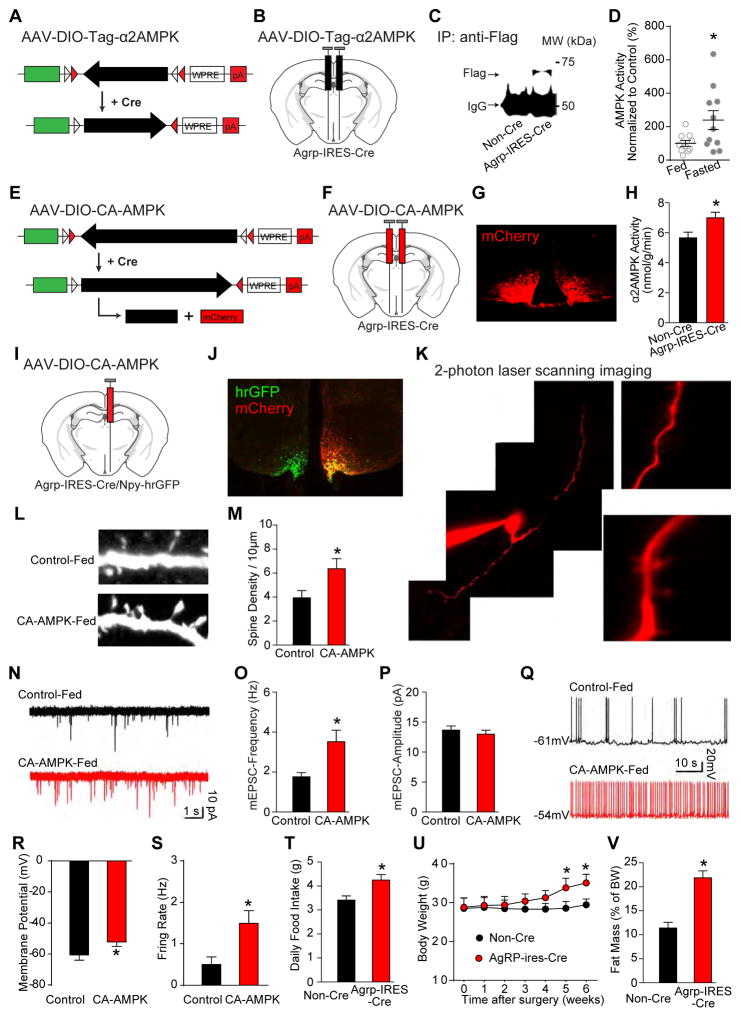
Figure 1. AMPK stimulates excitatory synaptogenesis in AgRP neurons.
(A–D) Schematics of AAV-DIO-Tag-α2AMPK (A) and stereotaxic injection (B), immunoprecipitation from arcuate lysates of fed mice (C), and AgRP neuron α2AMPK activity immunoprecipitated with the anti-Flag antibody from the arcuate nucleus of fed and fasted Agrp-IRES-Cre mice (D) (nfed=10 and nfasted=11).
(E–H) Schematics of constitutively active AAV-DIO-CA-AMPK (E) and stereotaxic injection (F), immunofluorescence of mCherry (G), and arcuate α2AMPK activity immunoprecipitated with anti-α2AMPK antibody from ad libitum fed mice (H) (n=8).
(I–S) Following unilateral injection of AAV-DIO-CA-AMPK (I), immunofluorescence (J), example of two-photon imaging of an AgRP neuron (K), examples and summary of dendritic spines (L and M), mEPSCs (N–P), and firing properties (Q–S) are shown (n=10 neurons from 3 mice).
(T–V) Following bilateral injection of AAV-DIO-CA-AMPK, daily food intake (T), body weight (U), and body fat mass (V) (n=8).
Data are mean ± SEM and * indicates p<0.05 with unpaired two-tailed student’s t-test.
Stimulation of AMPK Activity in AgRP neurons Drives Plasticity
To stimulate AMPK selectively in AgRP neurons, we constructed and stereotaxically injected cre-dependent AAV co-expressing mCherry and a constitutively active (CA) mutant (H150R) of the γ1 subunit of AMPK (Minokoshi et al., 2004) into the arcuate nucleus of Agrp-IRES-Cre mice (Figure 1E, 1F). We chose this mutant over constitutively active truncated α2 AMPK lacking the autoinhibitory domain to preserve the normal subcellular localization of activated AMPK. Expression occurred in a pattern consistent with AgRP neurons (Figure 1G) and increased α2 AMPK activity in the arcuate nucleus (Figure 1H). To assess effects of AMPK activation on synaptic plasticity, we injected AAV-DIO-CA-AMPK unilaterally into the arcuate nucleus of Agrp-IRES-Cre, Npy-hrGFP mice (Figure 1I) and then assessed various parameters, within the same mice in the ad libitum fed state, in CA-AMPK-expressing (mCherry+, hrGFP+) versus “control” non-expressing (hrGFP+) AgRP neurons (Figure 1J). As AgRP neurons co-express neuropeptide Y (NPY), the Npy-hrGFP BAC transgene allows visualization of AgRP neurons (van den Pol et al., 2009). We employed 2-photon laser scanning microscopy combined with whole-cell patch clamp electrophysiology (Kozorovitskiy et al., 2012) to analyze synaptic plasticity of AgRP neurons (Figure 1K). CA-AMPK expression in fed mice increased dendritic spines (Figure 1L, 1M) and the frequency of mEPSCs (Figure 1N, 1O) but not their amplitude (Figure 1P). CA-AMPK expression also activated AgRP neurons as judged by their depolarization (Figure 1Q, 1R) and increased firing rate (Figure 1Q, 1S). Furthermore, in animals bilaterally injected with AAV-DIO-CA-AMPK, the amount of food eaten (Figure 1T), body weight (Figure 1U), and body fat (Figure 1V) also increased. Thus, activation of AMPK in AgRP neurons increases dendritic spines and excitatory synaptic transmission, AgRP neuron firing rate, and consequently hunger.
Inhibition of AMPK Activity in AgRP Neurons Blocks Fasting-Induced Plasticity
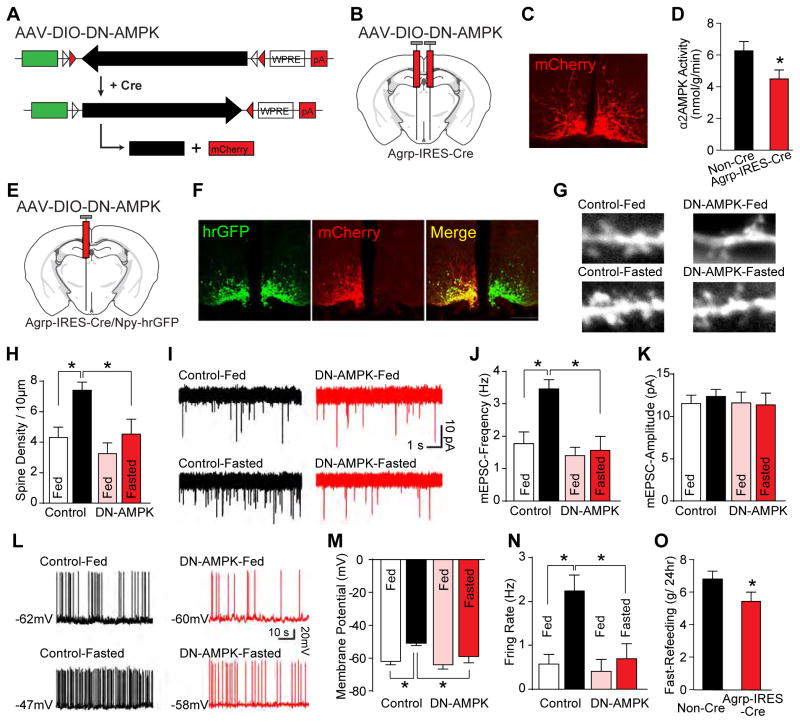
To inhibit AMPK activity selectively in AgRP neurons, we next constructed and stereotaxically injected cre-dependent AAV co-expressing mCherry and dominant negative (DN) kinase dead (K45R) α2 AMPK (Minokoshi et al., 2004) into the arcuate nucleus of Agrp-IRES-Cre mice (Figure 2A, 2B). Expression occurred in a pattern consistent with AgRP neurons (Figure 2C) and lowered total α2 AMPK activity in the arcuate nucleus (Figure 2D) where AgRP neurons are located. Of note, DN-AMPK expression did not cause death of AgRP neurons (Figure S1). To assess effects of AMPK inhibition on synaptic plasticity, we injected AAV-DIO-DN-AMPK unilaterally into the arcuate nucleus of Agrp-IRES-Cre, Npy-hrGFP mice (Figure 2E) and then assessed various parameters, within the same mice, in DN-AMPK-expressing (mCherry+, hrGFP+) versus “control” non-expressing (hrGFP+) AgRP neurons (Figure 2F). In control AgRP neurons, as previously observed (Liu et al., 2012), fasting increased dendritic spines (Figure 2G, 2H) and the frequency of mEPSCs (Figure 2I, 2J), but not their amplitude (Figure 2K). Fasting also activated control AgRP neurons as judged by their depolarization (Figure 2L, 2M) and increased firing rate (Figure 2L, 2N). Notably, these effects of fasting on both synaptic plasticity and activation of AgRP neurons were absent in DN-AMPK-expressing AgRP neurons (Figure 2G–2N). Also, in animals bilaterally injected with AAV-DIO-DN-AMPK, the amount of food eaten following 24-hr fasting was reduced (Figure 2O). Thus, activation of AMPK in AgRP neurons is both sufficient (CA-AMPK studies, Figure 1) and necessary (DN-AMPK studies, Figure 2) for fasting-induced effects on plasticity, AgRP neuron activation, and consequently hunger.
Figure 2. AMPK is required for fasting-induced synaptic plasticity in AgRP neurons.
(A–D) Schematics of dominant negative AAV-DIO-DN-AMPK (A) and stereotaxic injection (B), immunofluorescence of mCherry (C), and arcuate α2AMPK kinase activity from ad libitum fed mice (D) (n=8).
(E–N) Following unilateral injection of AAV-DIO-DN-AMPK (E), immunofluorescence (F), examples and summary of dendritic spines (G and H), mEPSCs (I–K), and firing properties (L–N) are shown (nfed=9 and nfasted=11 neurons from 3 mice per group) in fed or fasted mice.
(O) Following bilateral injection of AAV-DIO-DN-AMPK, food eaten following 24-hr fasting (n=8).
Data are mean ± SEM and * indicates p<0.05 with unpaired two-tailed student’s t-test (D and O) and with unpaired one-way ANOVA test (H, J, K, M, and N).
AMPK Phosphorylates PAK2 and Regulates its Activity in Neurons
We then considered AMPK targets that could regulate synaptic plasticity. A recent unbiased screen for α2 AMPK substrates identified p21-activated protein kinase (specifically the PAK2 isoform) (Banko et al., 2011), a known post-synaptic driver of excitatory synaptic plasticity (Hayashi et al., 2004; Kreis and Barnier, 2009; Penzes et al., 2003). PAKs are serine/threonine kinases regulated by GTPases of the Rac1 and Cdc42 family (Bokoch, 2003). The group 1 members of PAKs (PAKs 1, 2 and 3) are typified by a common N-terminal autoinhibitory domain (AID) and are highly homologous throughout (Bokoch, 2003). AMPK phosphorylates serine 20 of PAK2, and this appears to be necessary for AMPK-induced phosphorylation of the PAK2 substrate, myosin regulatory light chain (MRLC) (Banko et al., 2011). Of note, a phosphorylation site mapping program (http://scansite.mit.edu) strongly suggests that AMPK also phosphorylates PAK1 (on serine-21), but likely not PAK3 (on serine-20), which lacks an AMPK phosphorylation consensus motif (Banko et al., 2011). We performed RT-PCR on disassociated, single AgRP neurons and detected Pak1, Pak2 and Pak3 mRNAs, respectively, in 100%, 50% and 90% of AgRP neurons (Figure 3A). We focused our efforts on PAK2 because of the availability of reagents that readily detect its serine-20 phosphorylation, and prior work establishing that it is a downstream target of AMPK (Banko et al., 2011).
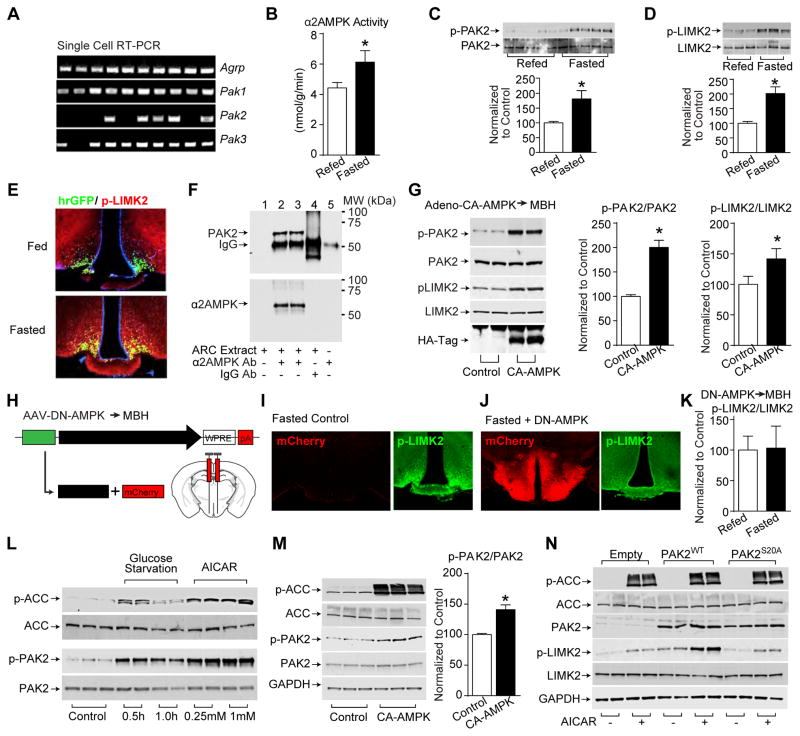
Figure 3. AMPK phosphorylates and stimulates PAK signaling.
(A) Single cell RT-PCR in AgRP neurons
(B–D) Arcuate α2AMPK activity (B) and total and phosphorylated PAK2 (Ser20) (C) and LIMK2 (Thr505) (D) in arcuate lysates from fasted and 6-hr refed wildtype mice (nrefed=9 and nfasted=8).
(E) Immunofluorescence of arcuate p-Thr505LIMK2 from fed and 24-hr fasted Npy-hrGFP mice.
(F) Immunoprecipitation of PAK2 and α2AMPK from arcuate lysates of fed wildtype mice.
(G) Phosphorylation of PAK2 (Ser20) and LIMK2 (Thr505) in the arcuate of fed wildtype mice following bilateral injection of HAtag-CA-AMPK adenovirus (n=5).
(H–K) Schematics of cre-independent AAV-DN-AMPK and stereotaxic injection into mediobasal hypothalamus (MBH) (H), immunofluorescence of mCherry (red) and p-Thr505LIMK2 (green) from fasted non-viral infected control mice (I) and AAV-DN-AMPK injected mice (J), and the ratio of total and phosphorylated LIMK2 (Thr505) in the arcuate lysates as detected with western blot from fasted and 6-hr refed mice following AAV-DN-AMPK injection (K)(n=8).
(L–N) Total and phosphorylated ACC (Ser79), PAK2 (Ser20) and LIMK2 (Thr505) in GT1-7 cells following glucose starvation or AICAR treatment (L), or transfection of CA-AMPK (n=9) (M), or transfection of PAK2WT and PAK2S20A mutants with 1 mM AICAR treatment (N). Proteins are normalized to GAPDH.
Data are mean ± SEM and * indicates p<0.05 with unpaired two-tailed student’s t-test.
In the arcuate nucleus, fasting, which increases AMPK activity (Figure 3B), increased serine-20 phosphorylation of PAK2 (Figure 3C), and also threonine-508/505 phosphorylation of the PAK target, LIM kinase 2 (LIMK2) (Figure 3D). Of note, this fasting-induced increase in LIMK phosphorylation occurred specifically in AgRP neurons (Figure 3E). Importantly, PAK2 co-precipitates with α2 AMPK from protein lysates of the arcuate nucleus indicating that the two interact in cells within the arcuate nucleus (Figure 3F). We next injected into the mediobasal hypothalamus an adenovirus expressing, independently of cre, HA-tagged CA-γ1 AMPK (Minokoshi et al., 2004). As shown in Figure 3G, CA-γ1 AMPK in the hypothalamus increased phosphorylation of PAK2 and LIMK2. We further constructed an AAV viral vector expressing DN-AMPK and mCherry independently of cre and similarly injected it into the mediobasal hypothalamus (Figure 3H). As shown in Figure 3I–K, hypothalamic expression of DN-AMPK significantly attenuated fasting-induced phosphorylation of LIMK2, as evidenced by either immunofluorescence (Figure 3I, 3J) or western blotting (Figure 3K). Thus, increased AMPK activity is required for fasting-induced phosphorylation of the major PAK target, LIMK2. Using the immortalized hypothalamic cell line, GT1-7 (Mellon et al., 1990), we confirmed that two known activators of AMPK, reduced energy state (glucose starvation) and a cell permeable AMP analogue (AICAR), increased serine-20 phosphorylation of PAK2 (Figure 3L) and phosphorylation of acetyl CoA carboxylase on the well-known AMPK phosphorylation site. Likewise, expression of CA-AMPK also increased serine-20 phosphorylation (Figure 3M). Finally, AMPK activation by AICAR increased phosphorylation of the PAK2 target, LIMK2 (Figure 3N, “Empty” lanes), and overexpression of wild-type PAK2 greatly augmented this effect (Figure 3N, PAK2WT lanes). Importantly, this augmentation was not seen following overexpression of a phospho-defective S20A mutant of PAK2 (Figure 3N, PAK2S20A lanes). In total, these studies and those of Banko et al. (Banko et al., 2011) demonstrate that AMPK phosphorylates serine 20 on PAK2, that this is associated with increased phosphorylation of the PAK2 targets LIMK2 (this study) and MRLC (Banko et al., 2011), and that the ability of serine 20 to be phosphorylated by AMPK is necessary for AMPK-induced increased activity of PAK2 on LIMK2 (this study) and MRLC (Banko et al., 2011). Furthermore, our study demonstrates that AMPK regulation of PAK2 occurs in neurons. Of note, given the sequence homology between PAK1 and PAK2, such AMPK regulation may also occur for PAK1, which was not assessed in the current study due to unavailability of antibodies against serine-21 phosphorylated PAK.
Inhibition of PAKs Blocks Fasting- and AMPK-Mediated Plasticity in AgRP Neurons
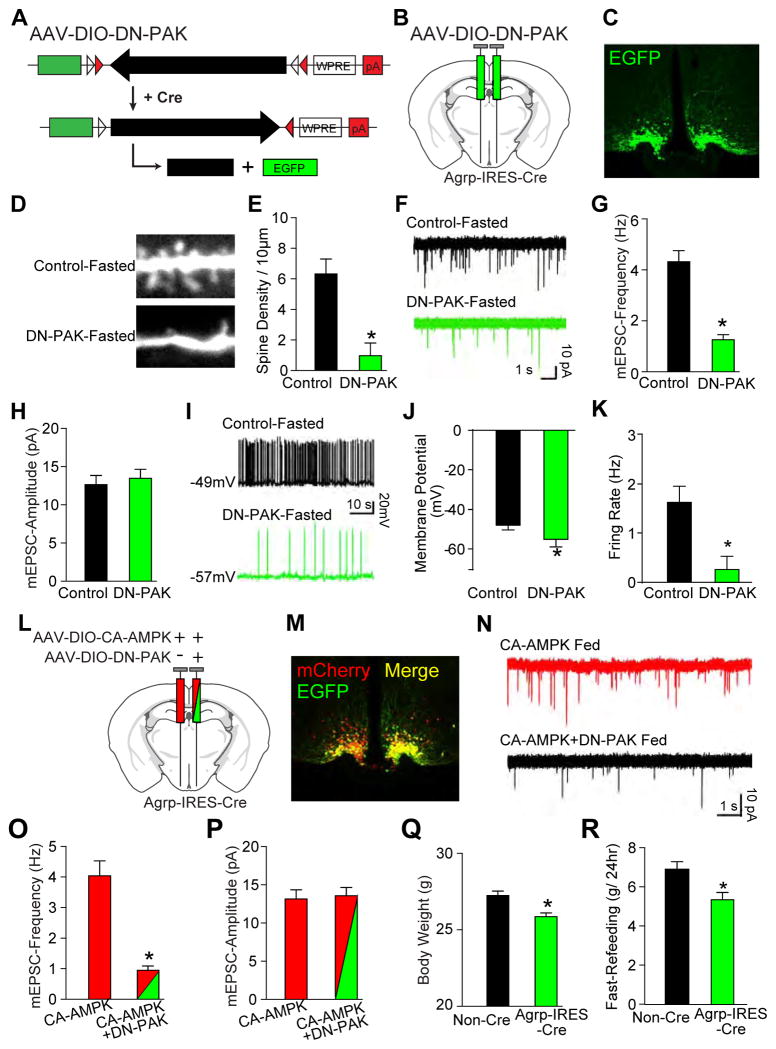
Since all three PAKs are expressed in AgRP neurons (Figure 3A) and since PAK1, in addition to PAK2, could mediate the effects of AMPK on synaptic plasticity in AgRP neurons, we generated a cre-dependent AAV co-expressing EGFP and the autoinhibitory domain (AID) of PAK1(DN-PAK). Of note, overexpressed DN-PAK binds to the catalytic domain of all three group 1 PAKs, preventing their activation (Hayashi et al., 2004). Hence, DN-PAK will inhibit all three PAKs in AgRP neurons. This DN-PAK virus was then stereotaxically injected into the arcuate nucleus of Agrp-IRES-Cre mice (Figure 4A, 4B). Expression of AAV-DIO-DN-PAK, as indicated by EGFP fluorescence, occurred in a pattern consistent with AgRP neurons (Figure 4C). To assess effects of PAK inhibition on synaptic plasticity, we injected AAV-DIO-DN-PAK into the arcuate nucleus of Agrp-IRES-Cre mice and assessed various parameters, in the fasted state, in DN-PAK-expressing neurons. Control AgRP neurons for these studies were from uninjected fasted Npy-hrGFP mice. Of note, PAK inhibition of AgRP neurons in fasted mice decreased dendritic spines (Figure 4D, 4E) and greatly reduced the frequency of mEPSCs (Figure 4F, 4G), but not their amplitude (Figure 4H). In addition, PAK inhibition decreased the activity of AgRP neurons as judged by their hyperpolarization (Figure 4I, 4J) and decreased firing rate (Figure 4K). To determine if PAK activity was required for AMPK’s effects on plasticity, we injected one side of the ARC with AAV-DIO-CA-AMPK alone and the other with a 1:1 mix of both AAV-DIO-CA-AMPK and AAV-DIO-DN-PAK (Figure 4L, 4M). Importantly, in ad libitum fed Agrp-IRES-Cre mice, the ability of CA-AMPK to increase mEPSC frequency (Figure 4O left bar, CA-AMPK alone, as previously seen in Figure 1O) was blocked by simultaneous inhibition of PAK (Figure 4O right bar, CA-AMPK + DN-PAK). Finally, in Agrp-IRES-Cre mice bilaterally injected with AAV-DIO-DN-PAK, body weight (Figure 4Q) and the amount of food eaten following a fast was significantly reduced (Figure 4R). These studies demonstrate that activation of group 1 PAKs is required for the stimulatory effects of AMPK on excitatory synaptic plasticity.
Figure 4. PAK is required for fasting- and AMPK-stimulated synaptic plasticity.
(A–C) Schematics of dominant negative AAV-DIO-DN-PAK (A) and stereotaxic injection (B), and immunofluorescence of EGFP (C) in Agrp-IRES-Cre mice.
(D–K) Examples and summary of dendritic spines (D and E), mEPSCs (F–H), and firing properties (I–K) in 24-hr fasted Npy-hrGFP control and AAV-DIO-DN-PAK virus-injected Agrp-IRES-Cre mice.
(L–P) Schematic of AAV-DIO-CA-AMPK and AAV-DIO-DN-PAK viral injection (L), immunofluorescence (M), and example and summery of mEPSCs (N–P).
(Q and R) Body weight (Q) and food eaten following 24-hr fasting (R) from mice bilaterally injected with AAV-DIO-DN-PAK (n=8).
Data are mean ± SEM (n=10 neurons from 3 mice per group in E, G, H, J, K, O, and P) and * indicates p<0.05 with unpaired two-tailed student’s t-test.
DISCUSSION
In the present study, we demonstrate the following: 1) AMPK activity in AgRP neurons is increased by fasting; 2) this is both necessary and sufficient for fasting-induced spinogenesis and excitatory synaptic plasticity; 3) in neurons AMPK phosphorylates PAK and leads to increased phosphorylation of a downstream substrate of PAK (LIMK2); and 4) this activation of PAK by AMPK mediates fasting- and also AMPK-mediated excitatory plasticity. Upregulation of synaptic activity by this AMPK → PAK pathway is likely consequential because chemogenetic activation of the excitatory neuronal inputs to AgRP neurons drives hunger (Krashes et al., 2014), and NMDAR deletion in AgRP neurons, which prevents fasting-induced synaptic plasticity, reduces hunger (Liu et al., 2012). Furthermore, stimulation of excitatory neurotransmission in AgRP neurons by CA-AMPK promotes hunger. Conversely, inhibition of glutamatergic neurotransmission by DN-AMPK or DN-PAK suppresses hunger. Thus, regulation of synaptic plasticity by the AMPK → PAK pathway in AgRP neurons is important in controlling hunger. In total, these findings establish a signaling (AMPK → PAK) and neurobiological basis (postsynaptic regulation of glutamatergic neurotransmission in AgRP neurons) for AMPK regulation of energy balance.
A prior study concluded that a site of action by which AMPK regulates state-dependent plasticity is presynaptic, i.e. within the excitatory afferent axon terminals (Yang et al., 2011). There are, however, differences between the two studies that are worth noting. First, the prior study largely examined ghrelin-stimulated plasticity while our study focused on fasting-induced plasticity. Second, the means of altering AMPK and timescales for observing effects are different; the prior study used AMPK pharmacologic activators (AICAR and ZMP) and an inhibitor (compound C) and looked at effects following addition of these drugs to brain slices, while our study used genetic tools (CA-AMPK and DN-AMPK) delivered directly to postsynaptic AgRP neurons in vivo and then looked at effects ex vivo. Third, the prior study inferred a presynaptic role for AMPK by excluding a postsynaptic role, while our study directly tested and demonstrated a postsynaptic role for AMPK. As our study focused on postsynaptic AMPK and did not address the role of presynaptic AMPK, our findings do not exclude an additional presynaptic site of action. That said, we believe postsynaptic regulation of plasticity is important for the following reasons: a) postsynaptic NMDA receptors on AgRP neurons are required for fasting-induced plasticity (Liu et al., 2012), b) PAK, a known postsynaptic regulator of spinogenesis and excitatory synaptic plasticity(Kreis and Barnier, 2009), is phosphorylated and activated by AMPK ((Banko et al., 2011) and the present study), and c) by direct genetic manipulation of AMPK in postsynaptic AgRP neurons, we demonstrate that postsynaptic AMPK is both necessary and sufficient for fasting-induced plasticity.
How then does fasting activate AMPK in AgRP neurons? While AMPK is regulated by cellular energy status (Hardie et al., 2012), this would seem to be an unlikely regulator in this scenario. Alternatively, intracellular calcium, which is known to drive synaptic plasticity (Bloodgood and Sabatini, 2007), could be responsible. Prior studies have established that increased calcium and subsequent activation of CAMKKβ, an upstream AMPK-kinase, can increase AMPK activity (Anderson et al., 2008; Hardie et al., 2012; Hawley et al., 2005; Kawashima et al., 2012; Mairet-Coello et al., 2013). In neurons, intracellular calcium is increased by NMDA receptor activation, neuronal firing and ghrelin, and these three manipulations have been shown to activate AMPK via CAMKKβ (Anderson et al., 2008; Andersson et al., 2004; Andrews et al., 2008; Hardie et al., 2012; Lopez et al., 2008; Yang et al., 2011). In this context it is of interest that AgRP neurons abundantly express the receptor for the fasting-induced hormone ghrelin (Willesen et al., 1999; Zigman et al., 2006), and that fasting-induced synaptic plasticity in AgRP neurons requires functional NMDA receptors on AgRP neurons (Liu et al., 2012). With regards to the source of glutamate which would activate these NMDA receptors, we have found that AgRP neurons receive strong excitatory drive from the paraventricular nucleus (PVH) and that this input is important in activating AgRP neurons and causing hunger (Krashes et al., 2014). Taken together, this leads to the hypothesis that increased calcium, secondary to elevated ghrelin, NMDA receptor action and increased neuronal firing, activates CaMKKβ and its downstream target AMPK, and that this is responsible for fasting-induced plasticity in AgRP neurons. Given the widespread expression of NMDA receptors, CaMKKβ, AMPK and PAK, it is tempting to speculate that the AMPK → PAK → plasticity pathway reported here will operate in circuits both within and also beyond the hypothalamus. If true, this would have important implications for many processes where plasticity plays a key regulatory role, one example being learning and memory.
Finally, it is possible that other targets in addition to PAK may be involved in AMPK-mediated synaptic plasticity. In this light, mitochondrial homeostasis and perhaps also mitochondrial distribution are of interest since they can affect neuronal activity (Dietrich et al., 2013; Li et al., 2004; Schneeberger et al., 2013) and can be regulated by AMPK (Toyama et al., 2016). If such pathways do indeed play a role, they appear to require PAK as PAK inhibition prevents AMPK-mediated synaptic plasticity (Figure 4O).
EXPERIMENTAL PROCEDURES
AAV viral expression
AAV viruses were packaged at BCH Viral Core or UNC Viral Core and stereotaxically injected into the arcuate nucleus of Agrp-IRES-Cre mice. See Supplemental Information for the detail.
Electrophysiology and two-photon imaging
Whole-cell patch-clamp recordings were obtained from fluorescent protein-identified AgRP neurons in acute coronal slices. Cells were filled with Alexa Fluor594 (10–20μM) and imaged using a home-built two-photon laser-scanning microscope (810–840 nm). See Supplemental Information for the detail.
Supplementary Material
Acknowledgments
We thank members of the Lowell, Sabatini, Kahn, and Kong laboratories for helpful discussions and comments on the manuscript; K. Deisseroth for AAV backbones; C.B. Saper, Tufts CNR Imaging Core, and BIDMC FNL Neuron-Nutrition Core for imaging service; Boston Children’s Hospital viral core (supported by NEI grant 5P30EY012196-17) and UNC viral core for AAV virus packaging; and P. Mellon for providing GT1-7 cell line. This work was supported by the following grants: to B.B.L.: NIDDK R01 DK096010, R01 DK089044, R01 DK071051, R01 DK075632, R37 DK053477, BNORC Transgenic Core P30 DK046200 and BADERC Transgenic Core P30 DK57521; to B.L.S.: NINDS NS046579; to B.B.K.: NIDDK R01 DK098002 and BADERC Metabolic Physiology Core P30 DK57521; to D.K.: NIDDK K01 DK094943, R01 DK108797, BNORC P&F P30 DK046200, AHA SDG 13SDG14620005, Charles Hood Foundation Grant, and BIDMC-FNL Core grant; to J.N.C.: AHA Postdoctoral Fellowship 14POST20100011.
Footnotes
Supplemental Information includes full description of Experimental Procedures and one Figure and can be found with this report online at
AUTHOR CONTRIBUTIONS
D.K. and B.B.L. conceived the project. D.K., Y.D., B.B.K., B.L.S., and B.B.L. designed the experiments and analyzed data. D.K. constructed AAVs, performed electrophysiology and multiphoton imaging. Y.D. performed biochemistry studies. J.N.C. performed single cell gene expression. Y.G., Z.Y., P.A, X.Y., and K.W. assisted in experiments. D.K. and B.B.L. prepared the manuscript with contributions from Y.D., B.B.K, and B.L.S..
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7:377–388. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko MR, Allen JJ, Schaffer BE, Wilker EW, Tsou P, White JL, Villen J, Wang B, Kim SR, Sakamoto K, et al. Chemical genetic screen for AMPKalpha2 substrates uncovers a network of proteins involved in mitosis. Mol Cell. 2011;44:878–892. doi: 10.1016/j.molcel.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Ca(2+) signaling in dendritic spines. Curr Opin Neurobiol. 2007;17:345–351. doi: 10.1016/j.conb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake. Cell Metab. 2012;16:104–112. doi: 10.1016/j.cmet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Liu ZW, Horvath TL. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell. 2013;155:188–199. doi: 10.1016/j.cell.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Choi SY, Rao BS, Jung HY, Lee HK, Zhang D, Chattarji S, Kirkwood A, Tonegawa S. Altered cortical synaptic morphology and impaired memory consolidation in forebrain-specific dominant-negative PAK transgenic mice. Neuron. 2004;42:773–787. doi: 10.1016/j.neuron.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kawashima J, Alquier T, Tsuji Y, Peroni OD, Kahn BB. Ca2+/calmodulin-dependent protein kinase kinase is not involved in hypothalamic AMP-activated protein kinase activation by neuroglucopenia. PLoS One. 2012;7:e36335. doi: 10.1371/journal.pone.0036335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno D, Sone H, Minokoshi Y, Yada T. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem Biophys Res Commun. 2008;366:388–392. doi: 10.1016/j.bbrc.2007.11.166. [DOI] [PubMed] [Google Scholar]
- Kohno D, Sone H, Tanaka S, Kurita H, Gantulga D, Yada T. AMP-activated protein kinase activates neuropeptide Y neurons in the hypothalamic arcuate nucleus to increase food intake in rats. Neurosci Lett. 2011;499:194–198. doi: 10.1016/j.neulet.2011.05.060. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Saunders A, Johnson CA, Lowell BB, Sabatini BL. Recurrent network activity drives striatal synaptogenesis. Nature. 2012;485:646–650. doi: 10.1038/nature11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis P, Barnier JV. PAK signalling in neuronal physiology. Cell Signal. 2009;21:384–393. doi: 10.1016/j.cellsig.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Lage R, Saha AK, Perez-Tilve D, Vazquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodriguez-Cuenca S, et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008;7:389–399. doi: 10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Mairet-Coello G, Courchet J, Pieraut S, Courchet V, Maximov A, Polleux F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Abeta oligomers through Tau phosphorylation. Neuron. 2013;78:94–108. doi: 10.1016/j.neuron.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5:1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Schneeberger M, Dietrich MO, Sebastian D, Imbernon M, Castano C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodriguez IC, Bortolozzi A, et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- Toyama EQ, Herzig S, Courchet J, Lewis TL, Jr, Loson OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351:275–281. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Yao Y, Fu LY, Foo K, Huang H, Coppari R, Lowell BB, Broberger C. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29:4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flipflop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.