Abstract
Background
Renal ischemia-reperfusion (RIR) injury, commonly caused by major surgery and shock, leads to acute kidney injury, and is associated with high morbidity and mortality. Cold-inducible RNA-binding protein (CIRP), a cold shock protein, has been recently identified as a damage-associated molecular pattern (DAMP). We hypothesized that CIRP exacerbates severity of injury in RIR.
Methods
Renal ischemia was induced in 8-week-old male C57BL/6 wild-type (WT) mice and Cirp−/− mice via bilateral clamping of renal pedicles for 30 min, followed by reperfusion for 5 h or 24 h and harvest of blood and renal tissue for analysis. Anti-CIRP antibody or non-immunized IgG was injected intravenously (10 mg/kg body weight) at time of reperfusion.
Results
After RIR, Cirp−/− mice demonstrated a reduction of BUN and creatinine of 53% and 60%, respectively, compared to WT mice. Serum IL-6 levels were significantly reduced 70% in Cirp−/− mice compared to WT mice after RIR. Levels of nitrotyrosine, an oxidatively-modified protein marker, and cyclooxygenase-2, an inflammatory mediator, were also significantly decreased in the kidneys of the Cirp−/− mice compared to WT mice after RIR. Renal caspase-3 activity was decreased in Cirp−/− mice compared to WT mice after RIR, which corresponded to the reduction of apoptotic cells determined by TUNEL assay. Injection of neutralizing anti-CIRP antibody into WT mice led to an 82% reduction in BUN compared to the vehicle after RIR.
Conclusions
Deficiency of CIRP results in less renal injury after RIR by attenuating inflammation and oxidative stress. Furthermore, blockade of CIRP shows a protective effect, indicating CIRP as a target in the treatment of RIR.
Keywords: Acute kidney injury, cold shock protein, inflammation, apoptosis, neutrophils, oxidative stress
INTRODUCTION
Renal ischemia-reperfusion (RIR) injury, commonly caused by major surgery and shock, leads to acute kidney injury (AKI), and is associated with high morbidity and mortality. Critically ill patients with AKI are four times more likely to die in the hospital, with an estimated mortality rate reported between 30–70%, particularly higher when associated with stage 2 or 3 AKI.1–5 For those who survive AKI, many patients progress to chronic kidney disease (CKD) and some eventually to end-stage renal disease (ESRD). These patients are also twice as likely to be discharged to a short- or long-term care facility instead of home, which adds to healthcare costs.1, 3, 6 Treatment is currently limited to prevention and supportive care, though advancements to understanding the pathophysiology of ischemic AKI have allowed for experimental research to explore novel therapies.
In ischemia, deprivation of oxygen and nutrient delivery to cells lead to accumulation of waste products of metabolism in the tissue.1, 7 In the kidneys, anoxic cell injury develops in the tubular epithelium and can lead to either apoptosis or necrosis (acute tubular necrosis [ATN]).1 This leads to a release of damage associated molecules patterns (DAMPs) recognized by pathogen recognition receptors leading to a sterile inflammatory response despite the absence of pathogens.8, 9 Innate immune cells such as neutrophils and macrophages become activated, which in turn causes further cellular injury of the renal epithelium through the release of cytokines and reactive oxygen species.10, 11
Cold-inducible RNA-binding protein (CIRP), a 172-amino-acid protein, has been recently identified as a DAMP.12 It is constitutively expressed at low levels in various tissues, and normally functions as an RNA chaperone protein during translation.12, 13 CIRP is upregulated and secreted from the cell under conditions of stress such as hypothermia, hypoxia, UV radiation.14–16 Administration of rmCIRP to healthy animals results in an increase of serum organ injury markers and proinflammatory cytokines.12
In our study, we employed a mouse model of RIR and showed that CIRP levels rise in renal tissue after ischemic and reperfusion injury. This was associated with increased levels of organ injury, inflammation, apoptosis, and oxidative stress. Administration of a neutralizing anti-CIRP antibody led to reduction of organ injury. Together, the data supports our hypothesis that CIRP exacerbates severity of injury in RIR.
MATERIALS AND METHODS
Animal model of RIR
Adult male C57BL/6 wild-type (WT) mice and CIRP knockout (Cirp−/−) mice (20–25 g) were selected for either a sham or RIR group, totaling 4 groups. The animals were induced with 2.5% inhalational isoflurane, then their abdomens prepped with 10% povidone-iodine wash. A midline incision was performed, and bowel was displaced to reveal bilateral renal hila. Microvascular clips were applied to each renal pedicle for 30 mins; after removal, the abdomen was closed with a running 6-0 nylon suture, and a 500 μl bolus of normal saline was given subcutaneously. Reperfusion was allowed for 5 h or 24 h; animals were then harvested for blood and renal tissue. Sham animals underwent laparotomy without renal ischemia. All experiments were performed in accordance with the guidelines for the use of experimental animals by the National Institutes of Health (Bethesda, MD) and were approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research.
Western blot analysis
Renal tissue was homogenized in lysis buffer (10 mM Tris-HCl, pH 7.5; 120 mM NaCl; 1% NP-40; 1% sodium deoxycholate; and 0.1% sodium dodecyl sulfate) containing protease inhibitor (Roche Diagnostics; Indianapolis, IN). Protein concentrations were determined by Bio-Rad protein assay reagent (Hercules, CA). Total lysate was electrophoresed on SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were then blocked with 0.1% casein in 0.2× PBS, and then incubated with anti-CIRP, nitrotyrosine, or β-actin (Sigma-Aldrich, St. Louis, MO) primary antibodies. After washing, the membranes were incubated with a fluorescently-labeled secondary antibody (LI-COR; Lincoln, NE). The Odyssey image system (LI-COR) was used to scan the membranes, and the Odyssey densitometric software used to measure band intensities.
Analysis of serum organ injury markers and interleukin 6
Blood samples were centrifuged at 2,000g for 15 min to collect serum and then either analyzed for injury parameters immediately, or stored at −80°C. Blood urea nitrogen (BUN), and creatinine were measured by using commercial assay kits according to the manufacturer’s instructions (Pointe Scientific; Lincoln Park, MI). IL-6 levels were determine by an enzyme-linked immunosorbent assay (ELISA) kit specific for mouse IL-6 (BD Biosciences; San Diego, CA).
Quantitative real-time PCR analysis
Total RNA was extracted from renal tissue using TRIzol (Invitrogen; Carlsbad, CA) and reverse-transcribed into cDNA using murine leukemia virus reverse transcriptase (Applied Biosystems; Foster City, CA). The PCR reaction was performed in 25 μl of final volume containing 0.08 μmol of forward and reverse primer, cDNA, and 12.5 μl SYBR Green PCR Master Mix (Applied Biosystems). The thermal profile used by the Applied Biosystems 7300 real-time PCR machine was: 50°C for 2 min, 95°C for 10 min, 45 cycles of 95°C for 15 seconds, and 60°C for 1 min. Mouse β-actin was used for normalization. Relative expression of mRNA was represented as fold change in comparison to the sham group. The primer sequences are: IL-6: Forward: CCGGAGAGGAGACTTCACAG, Reverse: CAGAATTGCCATTGCACAAC; COX-2: Forward: CTCAGCCAGGCAGCAAATC, Reverse: ACATTCCCCACGGTTTTGAC; β-actin: Forward: CGTGAAAAGATGACCCAGATCA, Reverse: TGGTACGACCAGAGGCATACAG.
Histological evaluation of renal injury and immunohistochemistry
Renal tissue was fixed in 10% formalin and embedded in paraffin. Tissue was sectioned into 5 μm slices and stained with hematoxylin and eosin (H&E). Using light microscopy, the level of injury was assessed in a blinded fashion in the following categories: tubular cell injury, tubular cell detachment, loss of brush border, tubular simplification, and cast formation. Scores ranged from 0 (0% injury), 1 (<10%), 2 (10–25%), 3 (25–50%), 4 (50–75%), 5 (>75%), for a maximal score of 25. Scores were averaged for each sample over 10 randomly selected fields.
For Gr-1 immunohistochemistry, paraffin-embedded renal tissues were dewaxed in xylene and rehydrated in serial ethanol washes. The slides were heated in 0.92% citric acid buffer (Vector Laboratories; Burlingame, CA) at 95°C for 30 min. After cooling, the slides were incubated with 2% H2O2/60% methanol and blocked in normal rabbit serum/Tris-buffered saline. The anti-Gr-1 antibody (Abcam; Cambridge, MA) was applied and incubated overnight at 4°C. Vectastain ABC reagent and a DAB kit (Vector Laboratories) were used to detect the immunohistochemical reaction. Slides were counterstained with hematoxylin and examined under a phase contrast Eclipse Ti-S light microscope (Nikon; Melville, NY).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
The presence of apoptotic cells was measured by in situ labeling of DNA fragmentation using a TUNEL assay. An In Situ Cell Death Detection kit (Roche Diagnostics; Indianapolis, IN) was used. Slides were counterstained with 4′,6-diamidino-2′-phenylindole dihydrochloride (DAPI).
Measurement of caspase-3 activity
Renal tissue was homogenized in lysis buffer (10 mM HEPES, pH 7.4; 5 mM MgCl; 1 mM DTT; 1% Triton X-100; 2 mM EGTA; 2 mM EDTA) containing protease inhibitor (Roche Diagnostics; Indianapolis, IN). Protein concentrations were determined by Bio-Rad protein assay reagent (Hercules, CA). Caspase-3 substrate peptide (DEVD-AMC) was added to an assay buffer (20 mM HEPES, pH 7.4; 5 mM DTT, 2 mM EDTA, 0.1% CHAPS). Assay buffer and sample were mixed on a 96 well plate. After a 60–90 min incubation at 37°C in the dark, a fluorometric reader was used to measure excitation (range 365–380 nm) and emission (range 430–460 nm). The results were divided by the protein concentration and expressed as fold change.
Administration of anti-CIRP antibody
Another group of adult male C57BL/6 wild-type (WT) mice (20–25 g) were given neutralizing anti-CIRP antibody or non-immunized IgG intravenously (10 mg/kg body weight [BW]) at time of reperfusion after ischemia as described above. The anti-CIRP antibody was generated as antisera against purified recombinant murine CIRP from rabbits. The IgG fraction was isolated from rabbit serum using immobilized immunopure proteins A and G chromatography as described previously.12
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) and compared via one way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) test for multiple group comparisons. Significance was considered if p < 0.05 between the experimental groups. Reductions in serum and tissue parameters are calculated from (WT RIR-KO RIR)/WT RIR and expressed as a percentage.
RESULTS
CIRP expression is increased after RIR
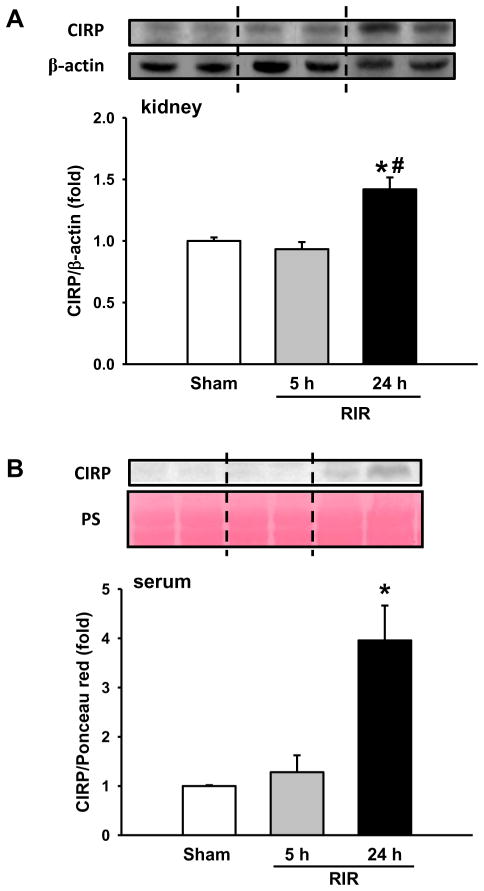
We first examined the expression of CIRP in the kidneys and serum after RIR by western blot. In renal tissue, CIRP was not changed at 5 h after RIR, but increased by 42% at 24 h after RIR, compared to sham (Figure 1A). In serum, CIRP was barely detected at 5 h after RIR, but increased 4.0-fold at 24 h after RIR in comparison to the sham (Figure 1B).
Figure 1. CIRP expression after RIR.
(A) Renal tissue was harvested at 5 and 24 h after 30 min-ischemia. The tissue was lysed and subjected to western blot against CIRP. Histogram shows mean densitometric analysis of protein bands after normalization with β-actin. (B) Western blot against CIRP in serum. n=5–9 mice/group. Data are expressed as mean ± SEM and compared by one-way ANOVA by SNK method. *p<0.05 vs WT sham; # p<0.05 vs WT vehicle.
Renal injury markers and inflammation are attenuated in Cirp−/− mice after RIR
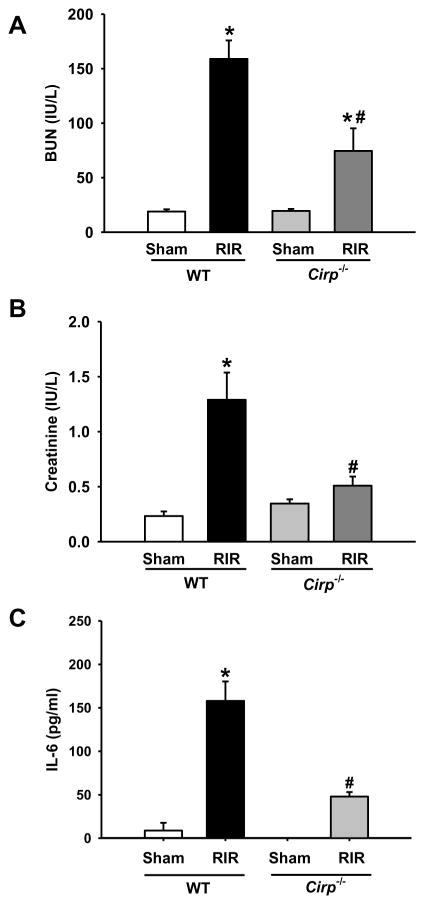
After observing an increase in the level of CIRP expression in the kidneys and in the circulation, we then investigated the contribution of CIRP in renal damage by comparing the WT and Cirp−/− mice after RIR. At 24 h after RIR, the levels of BUN and creatinine in the Cirp−/− mice were 53% and 60% lower than those in WT mice, respectively (Figure 2A and B). Serum IL-6 levels were also significantly reduced by 70% in Cirp−/− mice compared to those in WT mice after RIR (Figure 2C).
Figure 2. Alterations in the levels of serum renal injury markers and cytokine after RIR.
Serum was collected 24 h after 30 min-ischemia for measuring levels of (A) BUN and (B) creatinine. n=5–9 mice/group. Serum was also measured for levels of (C) IL-6 by ELISA. Sham, n=3–4 mice/group; RIR, n=5–6 mice/group. Data are expressed as mean ± SEM and compared by one-way ANOVA by SNK method. *p<0.05 vs WT sham; # p<0.05 vs WT vehicle.
Histologic architecture of the kidneys is less damaged in Cirp−/− mice after RIR
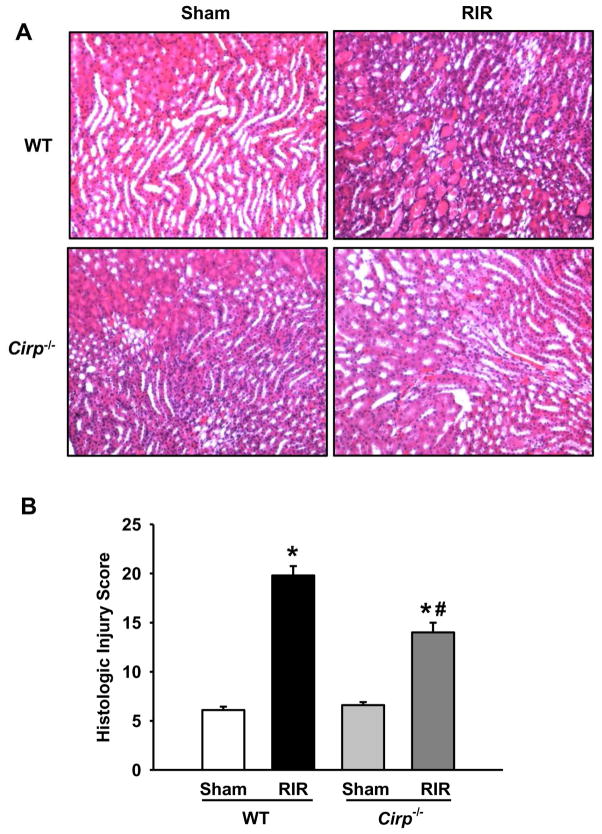
We next examined the integrity of renal structure in WT and Cirp−/− mice after RIR by histological evaluation. As shown in Figure 3A, both WT and Cirp−/− sham tissue showed normal architecture by H&E staining, while the kidneys in both RIR groups exihibited tubular cell injury, tubular cell detachment, loss of brush border, tubular simplification, and cast formation. Quantification using a histologic injury score indicated the severity of renal injury in the Cirp−/− RIR group to be 15 out of a possible 25 points, while the WT RIR group had a score of 20 (Figure 3B).
Figure 3. Histological changes in WT and Cirp−/− kidneys after RIR.
Renal tissue from sham WT and Cirp−/− mice and WT and Cirp−/− mice after RIR were harvested and subjected to histological analysis with hematoxylin-eosin staining. (A) Representative images were chosen from each group out of 10 randomly selected fields. Magnification 200×. (B) Histologic injury score measuring severity of tubular cell injury, tubular cell detachment, loss of brush border, tubular simplification, and cast formation on scale of 1–5 per category, as described in Materials and Methods. n=5–9 mice/group. Data are expressed as mean ± SEM and compared by one-way ANOVA by SNK method. *p<0.05 vs WT sham; # p<0.05 vs WT vehicle.
Apoptosis is decreased in Cirp−/− mice after RIR
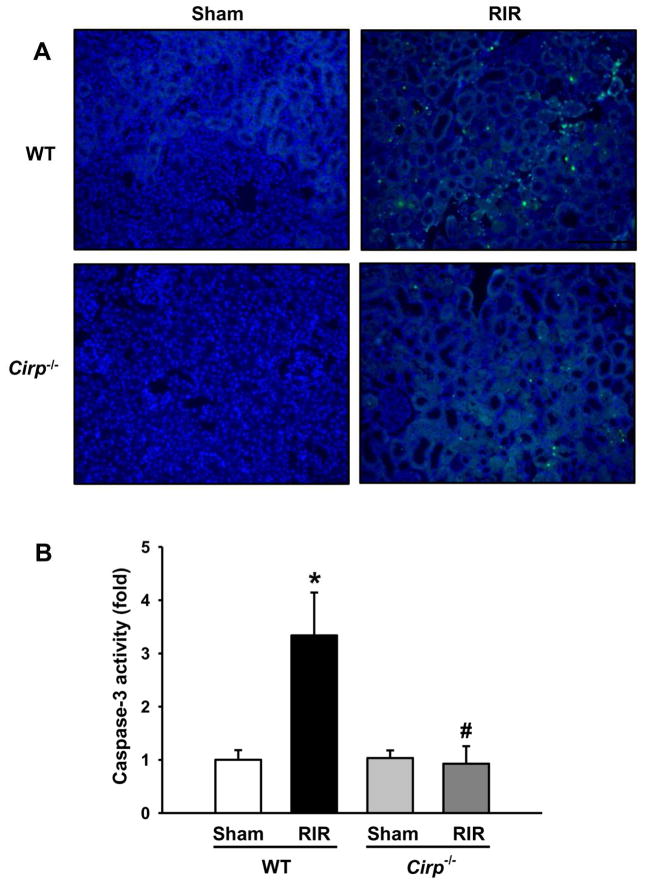
To assess the effect of CIRP expression on apoptosis, we performed a terminal deoxynucleotidyl transferase dUTP nick end labeling assay (TUNEL) on renal tissue in both WT and Cirp−/− mice after RIR. WT mice showed prominent areas of TUNEL-positive staining at 24 h after RIR, while, there were fewer of these apoptotic cells in the Cirp−/− mice (Figure 4A). To further validate the occcurence of apoptosis in renal tissue, we measured the caspase-3 enzymatic activity. There was a marked increase in caspase-3 activity after RIR in WT mice (Figure 4B). In contrast, the casapse-3 activity in the kidneys of Cirp−/− mice after RIR was similar to their sham groups (Figure 4B).
Figure 4. Apoptosis in the kidneys after RIR.
Renal tissue from WT and Cirp−/− mice were harvested 24 h after 30 min-ischemia to evaluate for apoptosis. (A) Representative images of TUNEL staining (green fluorescence) and nuclear counterstaining (blue fluorescence). Magnification 200×. (B) Renal caspase-3 activity was determined spectrophotometrically. Sham, n=4 mice/group; RIR, n=6–7 mice/group. Data are expressed as means ± SEM and compared by one-way ANOVA by SNK method. *p<0.05 vs WT sham; # p<0.05 vs WT vehicle.
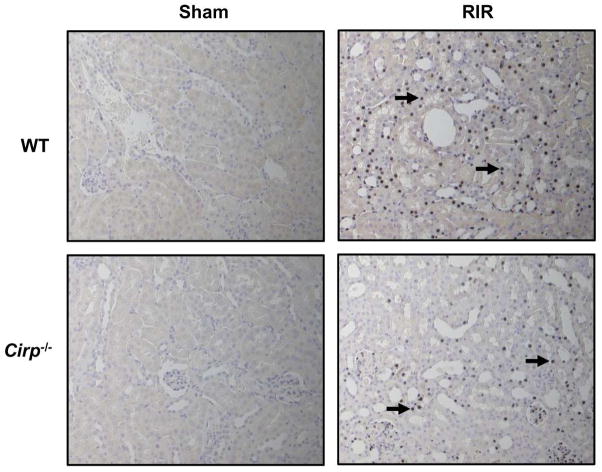
Neutrophil infiltration is reduced in Cirp−/− mice after RIR
After RIR, inflammatory mediators lead to increased neutrophil infiltration. We performed immunohistochemistry for Gr-1, a myeloid differentiation marker expressed in neutrophils in peripheral organs. Renal tissue in WT mice after RIR had a marked increase in Gr-1 staining, while it was undetectable in the sham group (Figure 5, black arrows). In the Cirp−/− mice, there was less Gr-1 expression, compared to the WT mice after RIR (Figure 5, black arrows).
Figure 5. Neutrophil infiltration after renal I/R.
Renal tissue from sham WT and Cirp−/− mice and WT and Cirp−/− mice after RIR were harvested and subjected to immunohistochemistry. Renal tissue was immunostained for Gr-1, a marker for neutrophil infiltration. Representative images shown for (A) WT sham, (B) WT RIR, (C) Cirp−/− sham, and (D) Cirp−/− RIR. Black arrows in (B) and (D) indicate representative staining for Gr-1. Magnification 200×.
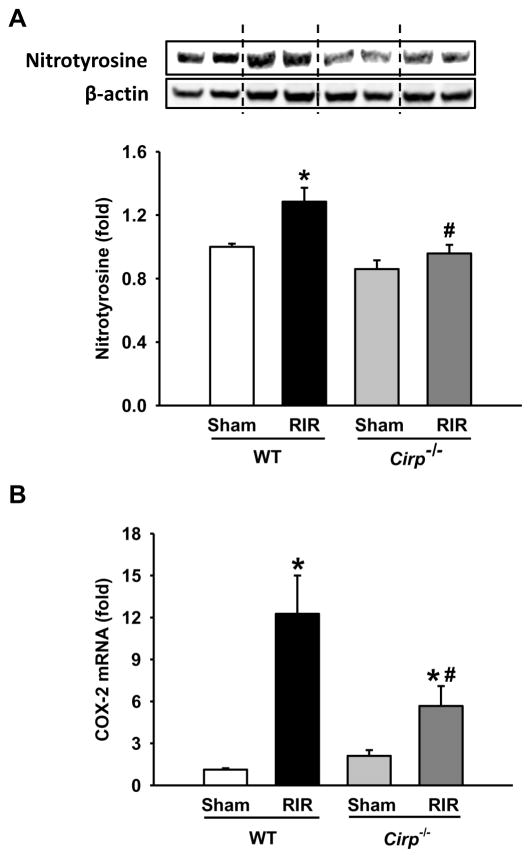
Oxidative stress is descreased in Cirp−/− mice after RIR
Generation of oxidative stress is an important factor to cause tissue damage in IR. The levels of nitrotyrosine, an oxidatively-modified protein marker, was significantly elevated in the kidneys of the WT mice at 24 h after RIR, compared to the WT sham (Figure 6A). In contrast, the elevation of nitrotyrosine was diminished in the kidneys of the Cirp−/− mice after RIR when compared to their sham group (Figure 6A). We also determined the expression of COX-2, a mediator of oxidative stress, in the kidneys. The mRNA levels of COX-2 were increased in both WT and Cirp−/− mice after RIR (Figure 6B). However, there was a 54% reduction of COX-2 expression in Cirp−/− mice in comparison to the WT mice after RIR (Figure 6B).
Figure 6. Change in the levels of oxidatively-modified protein markers after RIR.
Renal tissue from sham WT and Cirp−/− mice and WT and Cirp−/− mice after RIR were harvested. Tissue was lysed and subjected to western blot against (A) nitrotyrosine protein and β-actin. Histogram shows mean densitometric analysis of band intensities normalized to β-actin levels. Levels of (B) COX-2 mRNA were determined by real-time PCR. Sham, n=3–4 mice/group; RIR, n=4–7 mice/group. Data are expressed as means ± SEM and compared by one-way ANOVA by SNK method. *p<0.05 vs WT sham; # p<0.05 vs WT vehicle.
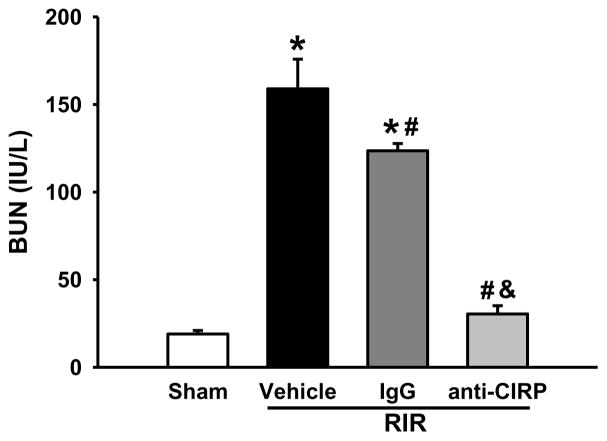
Blockage of CIRP restores renal function in WT mice after RIR
To further verify the detrimental role of CIRP in RIR-induced injury, we administered a neutralizing anti-CIRP antibody to WT mice after RIR. At 24 h after RIR, the BUN levels in anti-CIRP antibody-treated mice were significantly reduced by 81% in comparison to the vehicle-treated mice (Figure 7). Injection of non-immunized IgG as a control only led to a 22% reduction of BUN levels compared to the vehicle (Figure 7).
Figure 7. Alterations in serum organ injury marker BUN with anti-CIRP antibody after RIR.
Mice were injected with anti-CIRP antibody or an IgG control after 30 min-ischemia time. Serum was collected 24 h later and assayed for levels of BUN. n=5–9 mice/group. Data are expressed as means ± SEM and compared by one-way ANOVA by SNK method. *p<0.05 vs sham; # p<0.05 vs vehicle; & p<0.05 vs IgG.
DISCUSSION
CIRP has been shown to function as a DAMP in hemorrhagic shock and sepsis.12 Its expression increases in mice and humans after hemorrhage, and is released from macrophages under hypoxic conditions.12 Addition of recombinant CIRP induces levels of TNF-α and high-mobility group box 1(HMGB1) in both in vitro and in vivo conditions, which contribute to downstream inflammation. In our study, we have demonstrated that after RIR, CIRP expression is increased in the kidneys and circulation, which can be due to the induction of transcription and secretion from the cell, as well as through passive release from necrotic cells. The induction of CIRP has also been observed in the liver and heart starting at 2.5 h and 4 h, respectively, after reperfusion in our previous hemorrhage model.12 While CIRP levels in the kidneys after RIR did not increase at 5 h, there was a significant increase by 24 h. Such a discrepancy can be due to differences in severity of the models, as well as from differences in the organs. In the hemorrhagic shock model, the mean arterial pressure of animals was maintained at 25–30 mmHg for 90 min causing widespread systemic injury, whereas in this study, the animals undergo 30 min of renal ischemia.
To assess renal injury, we assayed for serum BUN and creatinine, which are the most frequently used clinical markers of renal failure. Histological examination of renal tissue reflected our finding that Cirp−/− mice after RIR showed less induction of BUN and creatinine. It is known that ischemia leads to shedding of tubular epithelial cells resulting in reduced cellular integrity.1 Ischemic injury leads to loss of cell junctions and polarity due to mislocalization of adhesion molecules and other membrane proteins such as the Na+K+-ATPase and β-integrins.1 Similar tubular epithelial injury has been reported in other studies that demonstrate severe acute tubular damage in sections of the RIR group.17, 18 We have demonstrated that Cirp−/− mice exhibit less tubular epithelial injury after RIR on histology corresponding to lower levels of serum injury markers BUN and creatinine. This is consistent with previous studies in which Cirp−/− mice show less organ injury after hemorrhage12 and reduced brain infarct size after ischemic stroke19 compared to WT mice.
Inflammatory responses significantly contribute to overall renal damage in AKI.20, 21 Cytokines like TNF-α and IL-6 have been shown to play a key role in promoting an inflammatory response through signal transduction pathways leading to the induction of secondary inflammatory mediators.22 The primary mechanism through which the kidney becomes inflamed involves pattern recognition receptors (PRRs), of which toll-like receptors (TLRs) is a well-known and well-studied member.23 We have previously shown that CIRP interacts with the TLR4-MD2 complex, and activates the nuclear factor κB pathway (NF-κB).12, 24 TLR4 has also previously been studied in a RIR model, and was found to be upregulated after ischemic injury.25 The downstream effects of TLR4 binding lead to increased IL-6 that drives neutrophil accumulation and cellular apoptosis. In Cirp−/− mice, there was a reduction of inflammatory cytokine IL-6 associated with attenuated RIR-induced kidney tubular damage.
Apoptosis was first shown to occur after RIR by Schumer et al. when DNA cleavage and nuclear condensation was demonstrated.26 TUNEL staining is often used to identify apoptotic cells, though TUNEL positivity is not limited to apoptosis; it also labels cells that undergo regulated necrosis.27 We found a significant level of TUNEL-positive cells in both WT and Cirp−/− mice, with qualitatively more cells seen in the WT group. To differentiate apoptotic cells from those that have undergone necrosis, we also assayed for caspase-3 enzymatic activity. This showed high levels of apoptosis in the WT IR mice, but interestingly, near sham levels of enzymatic activity in the Cirp−/− IR group. This difference is likely due to the samples being taken at 24 hours, which is after the time when caspase-3 activity predominates the caspase cascade.28 While we were still able to detect activity in the WT presumably due much higher activity, the Cirp−/− IR tissue had already declined to near baseline levels.
RIR injury is characterized by inflammatory cell infiltration, initially with neutrophils and later with macrophages and lymphocytes.29 Neutrophil accumulation can be found in the peritubular capillary network of the outer medulla as early as 30 minutes after IR, and while neutrophils are known effectors of the inflammatory cascade, its role in RIR pathogenesis has only recently been clarified.30–32 Some early reports demonstrated a reduction in AKI with treatments inhibiting neutrophil infiltration or activity.33, 34 More recently, using in vivo labeling, Awad et al. showed that neutrophils accumulated in the kidneys following IR and transmigrated into the renal interstitium, which led to increased vascular permeability and loss of tubular epithelial and endothelial cell integrity.31 This is consistent with our finding of reduced neutrophil infiltration in the Cirp−/− kidneys compared to the WT kidneys, and shows overall reduced injury.
Another hallmark of RIR pathophysiology is the generation of reactive oxygen species.35 The formation of free radicals furthers renal tissue injury via peroxidation of membrane lipids, and oxidative damage of proteins and DNA, which contributes to apoptosis and cell death. A number of different targets of free radical scavenging and antioxidants have been identified as protective in RIR.36 Notably, inhibition of COX-2 with celecoxib or rofecoxib has been shown to reduce RIR injury.37, 38 Another study looked at COX-2 inhibition in a model of hepatic IR, and also found celecoxib to reduce oxidative stress.39 The authors in the first paper suggest that COX-2 may function as a source of reactive oxygen species, and its inhibition is therefore beneficial.37 Its reduced levels in the Cirp−/− mice, along with reduced levels of oxidatively modified protein marker, nitrotyrosine, correspond to lessened injury after IR.
Finally, we showed that administration of a neutralizing anti-CIRP antibody led to reduction of renal injury. This is consistent with our previous study of CIRP in hepatic IR, in which levels of liver injury markers AST, ALT, LDH and proinflammatory cytokine IL-6 were decreased in animals given anti-CIRP antibody.40 The mechanism by which antibody administration is able to reduce injury is likely via interference with CIRP binding to TLR4, as described earlier. TLR4 has been shown to play an important role in hepatic IR-induced inflammation.41 Additionally, we’ve shown that CIRP drives production of HMGB1, another major DAMP which also binds the TLR4-MD2 complex, and has a known role in hepatic IR injury.12, 41 Targeting HMGB1 with neutralizing antibodies has been shown to significantly decrease organ damage after both hepatic and renal IR models.42 It may be beneficial then to target both DAMPs for more comprehensive protection in preventing renal injury. Also, unlike with small chemical compounds as treatment, there is less concern for toxicity with antibody administration. Rather, the main concern is generation of immunogenicity, especially seen with long-term use.
We have also observed a modest, but significant improvement in reducing BUN levels with control IgG administration after RIR. Intravenous immunoglobulin (IVIg) is an FDA-approved therapeutic modality in the treatment of a wide variety of diseases, including autoimmune or inflammatory conditions.43 One of the mechanisms for using IVIg to treat Kawasaki’s disease is to decrease the production of proinflammatory cytokines and chemokines.44 It has also been demonstrated that administering 2 g/kg of IVIg protects the animal brain from ischemic stroke,45 while 10 mg/kg IgG is applied in this study.
Many knockout and inhibition studies often include an arm with augmentation to further prove the action of their protein of interest. We have previously published that administration of CIRP causes more inflammation and injury in an in vitro model.12 We chose not to perform this in our study since we know that significant inflammation and injury would follow RIR. It would then be difficult to see a difference in a CIRP augmented group since it is reasonable to believe that a threshold in inflammatory mediators may be reached. We believed that we have shown with statistically significant findings that the CIRP knockout mice show improvement based on several parameters, and that our inhibition data is concordant. It would be difficult to show further augmentation above the high levels of inflammation already seen in the WT RIR.
In summary, RIR causes increased expression of CIRP to be released from the kidney and circulate into the serum. Cirp−/− animals demonstrate less inflammation, neutrophil infiltration, apoptosis, and oxidative damage at 24 h after ischemia. Whether CIRP plays a role in affecting long-term recovery or survival after RIR needs to further investigation since, in this study, we are focused only on the acute phase. Nevertheless, blockade of CIRP has shown to be helpful in reducing renal injury and may be helpful as a therapeutic option in preventing acute kidney injury.
Acknowledgments
This work was supported in part by National Institutes of Health grant R01HL076179 and R01GM053008 (to PW).
LIST OF ABBREVIATIONS
- RIR
renal ischemia-reperfusion
- AKI
acute kidney injury
- CKD
chronic kidney disease
- ESRD
end-stage renal disease
- ATN
acute tubular necrosis
- BUN
urea nitrogen
- CIRP
cold-inducible RNA-binding protein
- DAMP
damage-associated molecular pattern
- PRR
pattern recognition receptors
- WT
wild-type
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- BW
body weight
- PBS
phosphate-buffered saline
- SEM
standard error of the mean
- SNK
Student-Newman-Keuls
- IL
interleukin
- HMGB1
high-mobility group box 1
Footnotes
Presented at the American College of Surgeons 101st Annual Clinical Congress, Scientific Forum, Chicago, IL, October 2015.
P.W. is an inventor of the pending PCT application WO 2010/120726 A1 entitled “Treatment of inflammatory diseases by inhibiting cold-inducible RNA-binding protein (CIRP).” This patent application covers the fundamental concept of using CIRP inhibitors for the treatment of inflammatory diseases.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–21. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–73. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munshi R, Hsu C, Himmelfarb J. Advances in understanding ischemic acute kidney injury. BMC Med. 2011;9:11. doi: 10.1186/1741-7015-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian H, Sun T, Hao D, Wang T, Li Z, Han S, et al. The optimal timing of continuous renal replacement therapy for patients with sepsis-induced acute kidney injury. Int Urol Nephrol. 2014;46:2009–14. doi: 10.1007/s11255-014-0747-5. [DOI] [PubMed] [Google Scholar]
- 5.Lopes JA, Jorge S, Resina C, Santos C, Pereira A, Neves J, et al. Acute kidney injury in patients with sepsis: a contemporary analysis. Int J Infect Dis. 2009;13:176–81. doi: 10.1016/j.ijid.2008.05.1231. [DOI] [PubMed] [Google Scholar]
- 6.Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 7.Le Dorze M, Legrand M, Payen D, Ince C. The role of the microcirculation in acute kidney injury. Curr Opin Crit Care. 2009;15:503–8. doi: 10.1097/MCC.0b013e328332f6cf. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of Leukocyte Biology. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 9.Ward PA. An endogenous factor mediates shock-induced injury. Nature Medicine. 2013;19:1368–9. doi: 10.1038/nm.3387. [DOI] [PubMed] [Google Scholar]
- 10.Akcay A, Nguyen Q, Edelstein CL. Mediators of Inflammation in Acute Kidney Injury. Mediators of Inflammation. 2009:12. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emlet DR, Shaw AD, Kellum JA. Sepsis-Associated AKI: Epithelial Cell Dysfunction. Seminars in Nephrology. 2015;35:85–95. doi: 10.1016/j.semnephrol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Qiang X, Yang WL, Wu R, Zhou M, Jacob A, Dong W, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med. 2013;19:1489–95. doi: 10.1038/nm.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. Journal of Cell Biology. 1997;137:899–908. doi: 10.1083/jcb.137.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikh MS, Carrier F, Papathanasiou MA, Hollander MC, Zhan QM, Yu K, et al. Identification of several human homologs of hamster DNA damage-inducible transcripts - Cloning and characterization of a novel UV-inducible cDNA that codes for a putative RNA-binding protein. Journal of Biological Chemistry. 1997;272:26720–6. doi: 10.1074/jbc.272.42.26720. [DOI] [PubMed] [Google Scholar]
- 15.Wellmann S, Buhrer C, Moderegger E, Zelmer A, Kirschner R, Koehne P, et al. Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. Journal of Cell Science. 2004;117:1785–94. doi: 10.1242/jcs.01026. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama H, Higashitsuji H, Yokoi H, Itoh K, Danno S, Matsuda T, et al. Cloning and characterization of human CIRP (cold-inducible RNA-binding protein) cDNA and chromosomal assignment of the gene. Gene. 1997;204:115–20. doi: 10.1016/s0378-1119(97)00530-1. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu S, Saito M, Kinoshita Y, Ohmasa F, Dimitriadis F, Shomori K, et al. Nicorandil ameliorates ischaemia-reperfusion injury in the rat kidney. British Journal of Pharmacology. 2011;163:272–82. doi: 10.1111/j.1476-5381.2011.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadek EM, Afifi NM, Elfattah LIA, Mohsen MAA-E. Histological study on effect of mesenchymal stem cell therapy on experimental renal injury induced by ischemia/reperfusion in male albino rat. International journal of stem cells. 2013;6:55–66. doi: 10.15283/ijsc.2013.6.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou M, Yang WL, Ji Y, Qiang X, Wang P. Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia. Biochim Biophys Acta. 2014;1840:2253–61. doi: 10.1016/j.bbagen.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nature Reviews Nephrology. 2015;11:88–101. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 21.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen LY, et al. Maladaptive role of IL-6 in ischemic acute renal failure. Journal of the American Society of Nephrology. 2005;16:3315–25. doi: 10.1681/ASN.2003090757. [DOI] [PubMed] [Google Scholar]
- 22.Furuichi K, Wada T, Yokoyama H, Kobayashi K. Role of cytokines and chemokines in renal ischemia-reperfusion injury. Drug News & Perspectives. 2002;15:477–82. doi: 10.1358/dnp.2002.15.8.840067. [DOI] [PubMed] [Google Scholar]
- 23.Leemans JC, Kors L, Anders HJ, Florquin S. Pattern recognition receptors and the inflammasome in kidney disease. Nature Reviews Nephrology. 2014;10:398–414. doi: 10.1038/nrneph.2014.91. [DOI] [PubMed] [Google Scholar]
- 24.Brochu C, Cabrita MA, Melanson BD, Hamill JD, Lau R, Pratt MAC, et al. NF-kappa B-Dependent Role for Cold-Inducible RNA Binding Protein in Regulating Interleukin 1 beta. Plos One. 2013:8. doi: 10.1371/journal.pone.0057426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfs T, Buurman WA, van Schadewijk A, de Vries B, Daemen M, Hiemstra PS, et al. In vivo expression of toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. Journal of Immunology. 2002;168:1286–93. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 26.Schumer M, Colombel MC, Sawczuk IS, Gobe G, Connor J, Otoole KM, et al. Morphological, Biochemical, And Molecular Evidence Of Apoptosis During The Reperfusion Phase After Brief Periods Of Renal Ischemia. American Journal of Pathology. 1992;140:831–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Linkermann A, Chen GC, Dong GE, Kunzendorf U, Krautwald S, Dong Z. Regulated Cell Death in AKI. Journal of the American Society of Nephrology. 2014;25:2689–701. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh JG, Logue SE, Luethi AU, Martin SJ. Caspase-1 Promiscuity Is Counterbalanced by Rapid Inactivation of Processed Enzyme. Journal of Biological Chemistry. 2011;286:32513–24. doi: 10.1074/jbc.M111.225862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menke J, Sollinger D, Schamberger B, Heemann U, Lutz J. The effect of ischemia/reperfusion on the kidney graft. Current Opinion in Organ Transplantation. 2014;19:395–400. doi: 10.1097/MOT.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Huang L, Sung S-SJ, Vergis AL, Rosin DL, Rose CE, Jr, et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney International. 2008;74:1526–37. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, et al. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney International. 2009;75:689–98. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolisetty S, Agarwal A. Neutrophils in acute kidney injury: not neutral any more. Kidney International. 2009;75:674–6. doi: 10.1038/ki.2008.689. [DOI] [PubMed] [Google Scholar]
- 33.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clinical Immunology. 2009;130:41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly KJ, Williams WW, Colvin RB, Meehan SM, Springer TA, GutierrezRamos JC, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. Journal of Clinical Investigation. 1996;97:1056–63. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, et al. Oxidative and nitrosative stress in acute renal ischemia. American Journal of Physiology-Renal Physiology. 2001;281:F948–F57. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 36.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4:20–7. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Senbel AM, AbdelMoneim L, Omar AG. Celecoxib modulates nitric oxide and reactive oxygen species in kidney ischemia/reperfusion injury and rat aorta model of hypoxia/reoxygenation. Vascular Pharmacology. 2014;62:24–31. doi: 10.1016/j.vph.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Feitoza CQ, Camara NOS, Pinheiro HS, Goncalves GM, Cenedeze MA, Pacheco-Silva A, et al. Cyclooxygenase 1 and/or 2 blockade ameliorates the renal tissue damage triggered by ischemia and reperfusion injury. International Immunopharmacology. 2005;5:79–84. doi: 10.1016/j.intimp.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 39.Ozturk H, Gezici A. The effect of celecoxib, a selective COX-2 inhibitor, on liver ischemia/reperfusion-induced oxidative stress in rats. Hepatology Research. 2006;34:76–83. doi: 10.1016/j.hepres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Godwin A, Yang WL, Sharma A, Khader A, Wang ZM, Zhang FM, et al. Blocking Cold-Inducible Rna-Binding Protein Protects Liver From Ischemia-Reperfusion Injury. Shock. 2015;43:24–30. doi: 10.1097/SHK.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4-dependent reactive oxygen species production and calcium-mediated signaling. Journal of Experimental Medicine. 2007;204:2913–23. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. Journal of Experimental Medicine. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gelfand EW. Mechanisms of Disease Intravenous Immune Globulin in Autoimmune and Inflammatory Diseases. New England Journal of Medicine. 2012;367:2015–25. doi: 10.1056/NEJMra1009433. [DOI] [PubMed] [Google Scholar]
- 44.Gupta M, Noel GJ, Schaefer M, Friedman D, Bussel J, Johann-Liang R. Cytokine modulation with immune gamma-globulin in peripheral blood of normal children and its implications in Kawasaki disease treatment. J Clin Immunol. 2001;21:193–9. doi: 10.1023/a:1011039216251. [DOI] [PubMed] [Google Scholar]
- 45.Arumugam TV, Tang SC, Lathia JD, Cheng A, Mughal MR, Chigurupati S, et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci U S A. 2007;104:14104–9. doi: 10.1073/pnas.0700506104. [DOI] [PMC free article] [PubMed] [Google Scholar]