Abstract
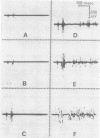
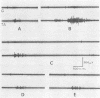
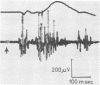
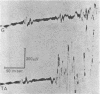
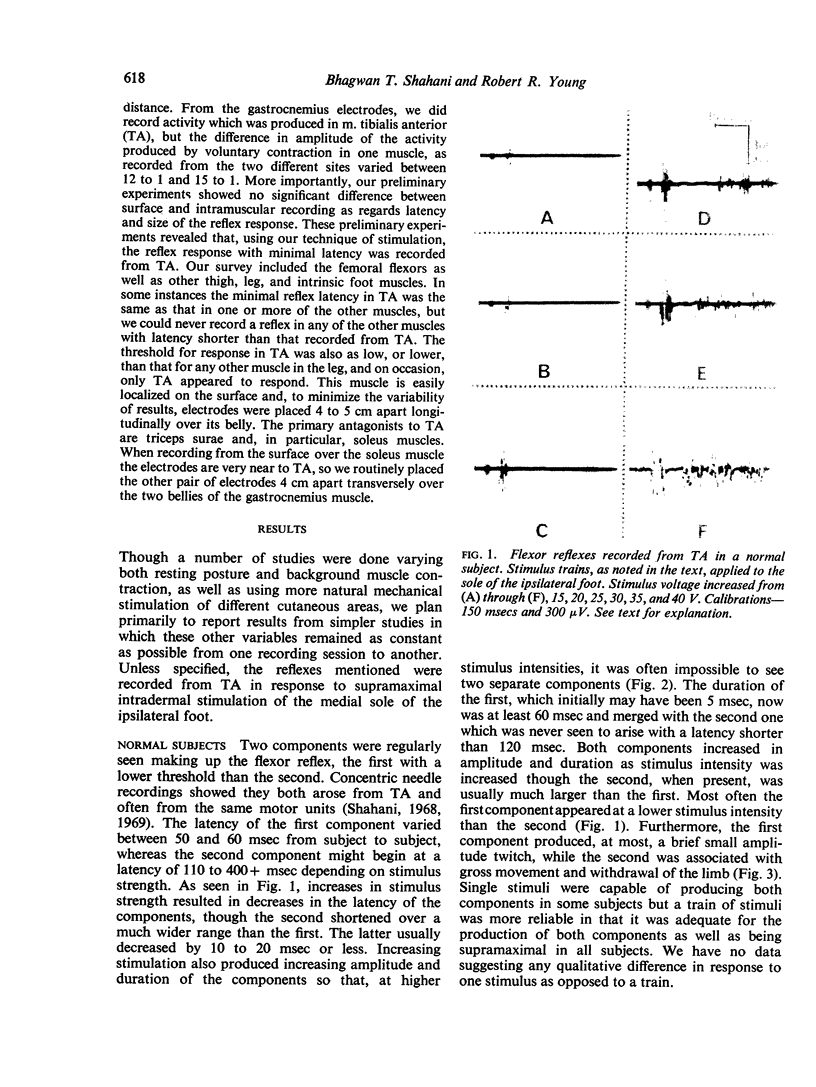
One type of flexor reflex, that recorded from the tibialis anterior muscle in response to electrical stimulation of the sole of the foot, was studied in normal subjects and patients with several neurological disorders. Normally this reflex consists of two components, the second of which is related to the actual withdrawal. The first component, normally of lower threshold, is difficult to evoke in patients with chronic spinal cord or discrete cerebral lesions, whereas it has an unusually low threshold and is very clearly seen in those with Parkinson's disease. In patients with spinal cord disease, the exaggerated flexor reflexes are seen at long latencies after relatively small stimuli. During the early phase of recovery from spinal transection, both components may be seen and are, therefore, spinal in origin. Studies of patients with the sensory neuropathy of Friedreich's ataxia suggest that the afferent fibres responsible for these flexor reflexes are the small myelinated fibres. Recovery curves demonstrate very long-lasting changes in flexor reflex excitability in normal subjects and patients with `spasticity' from spinal lesions. This differs in patients with `spasticity' from lesions rostral to the brain-stem. Examples in man of such physiological phenomena as reciprocal inhibition, local sign, habituation, temporal and spatial summation are discussed.
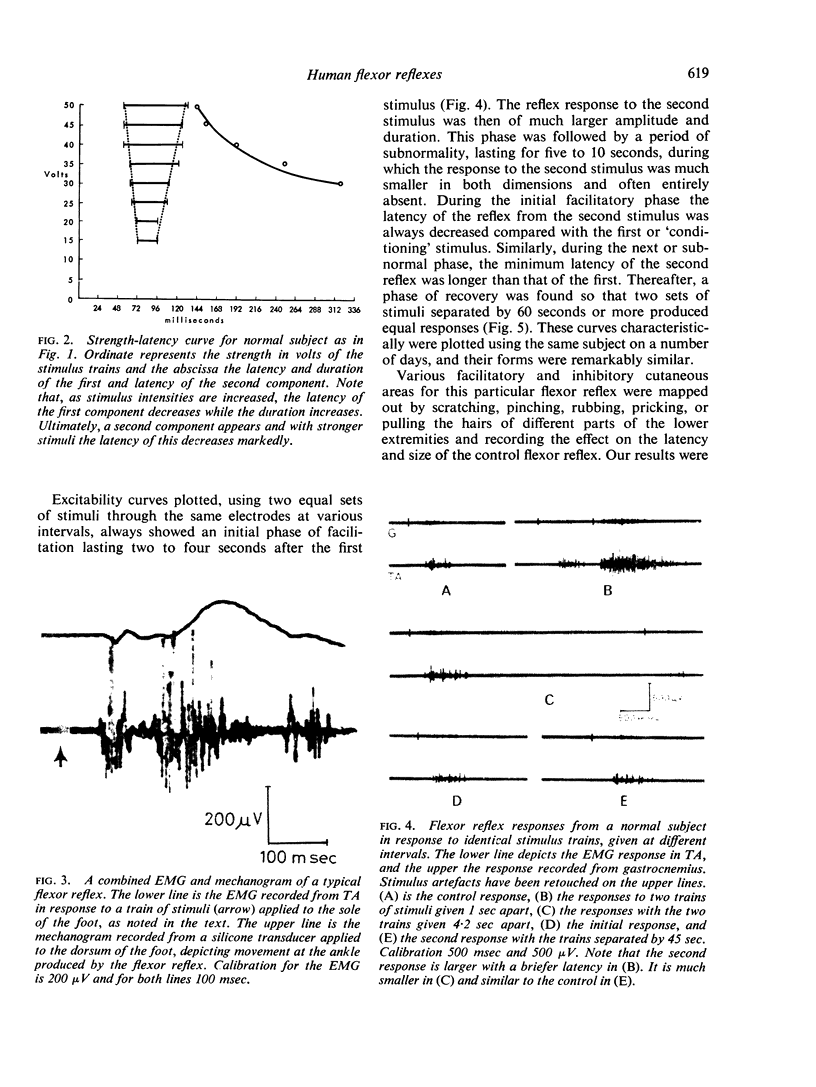
Full text
PDF

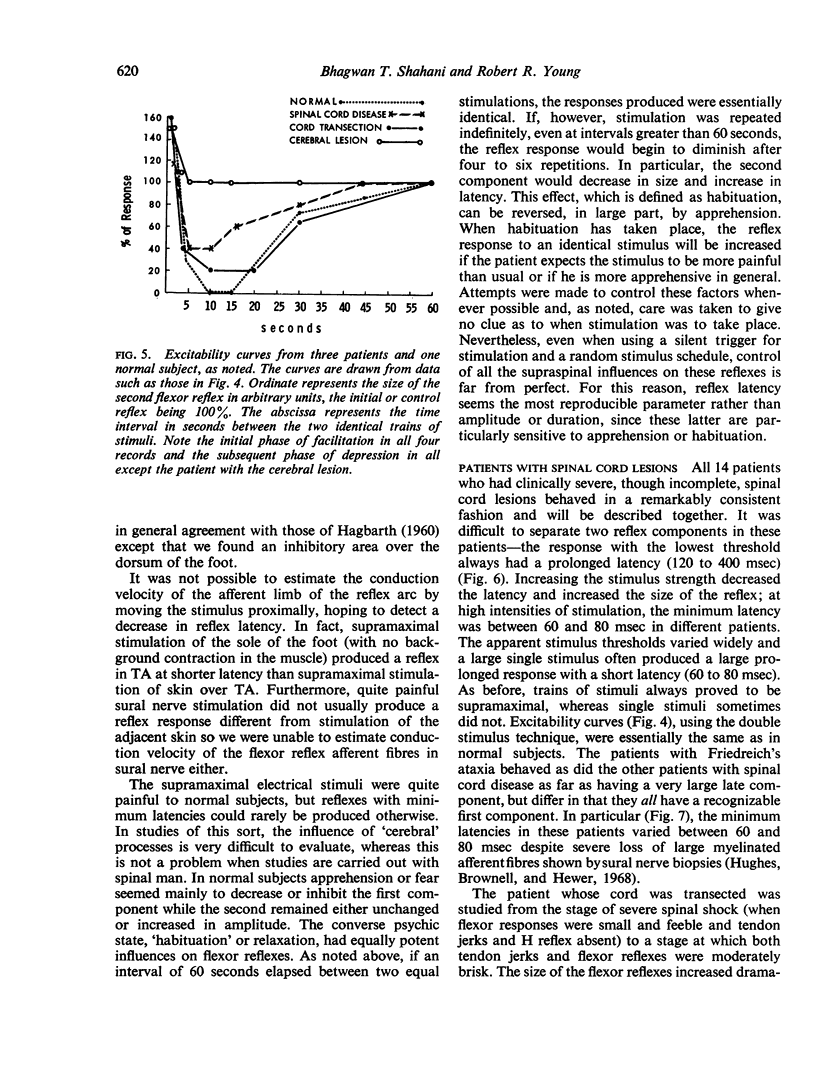






Images in this article
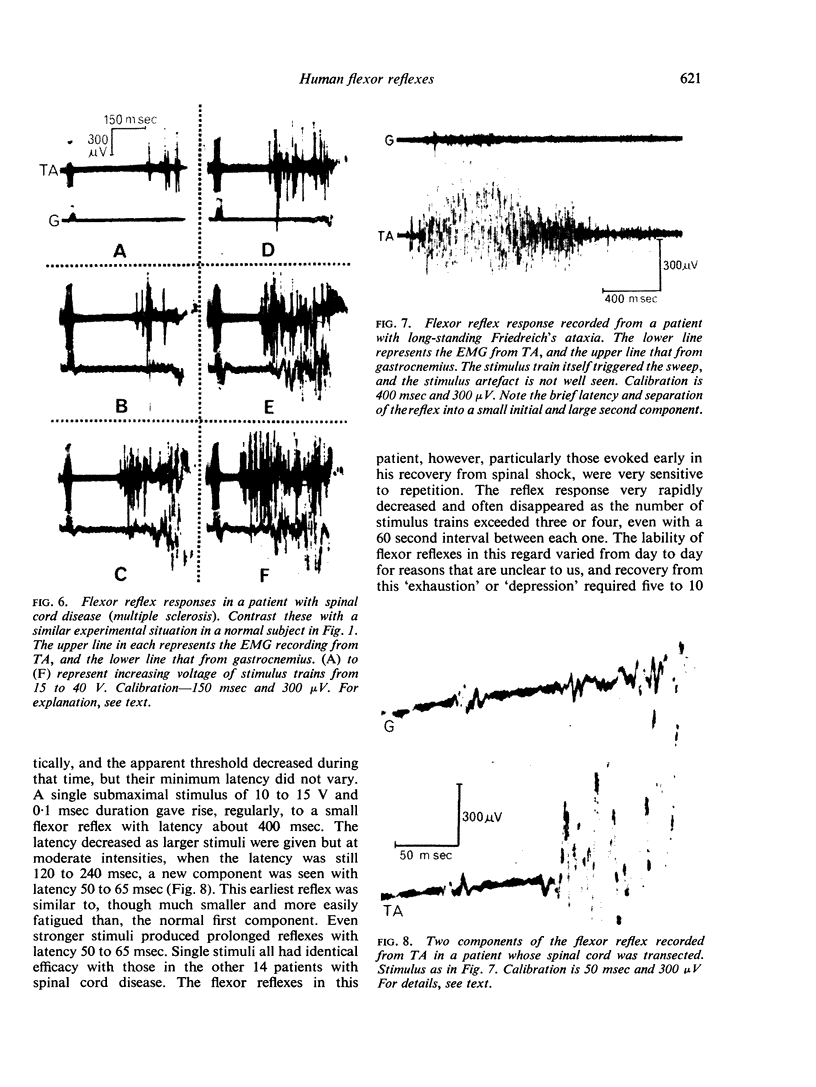
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDEN N. E., JUKES M. G., LUNDBERG A., VYKLICKY L. A NEW SPINAL FLEXOR REFLEX. Nature. 1964 Jun 27;202:1344–1345. doi: 10.1038/2021344b0. [DOI] [PubMed] [Google Scholar]
- BARBEAU A. The pathogenesis of Parkinson's disease: a new hypothesis. Can Med Assoc J. 1962 Oct 13;87:802–807. [PMC free article] [PubMed] [Google Scholar]
- BROOKS C. M., FUORTES M. G. F. Electrical correlates of the spinal flexor reflex. Brain. 1952 Mar;75(1):91–95. doi: 10.1093/brain/75.1.91. [DOI] [PubMed] [Google Scholar]
- COPLAND J. G., DAVIES C. T. A SIMPLE CLINICAL SKIN ELECTRODE. Lancet. 1964 Feb 22;1(7330):416–416. doi: 10.1016/s0140-6736(64)92794-1. [DOI] [PubMed] [Google Scholar]
- Cook W. A., Jr Antagonistic muscles in the production of clonus in man. Neurology. 1967 Aug;17(8 Pt 1):779-81, 796. doi: 10.1212/wnl.17.8.779. [DOI] [PubMed] [Google Scholar]
- Dimitrijević M. R., Nathan P. W. Studies of spasticity in man. 3. Analysis of revlex activity evoked by noxious cutaneous stimulation. Brain. 1968 Jun;91(2):349–368. doi: 10.1093/brain/91.2.349. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Gutrecht J. A., Bastron J. A., Karnes W. E., Dale A. J. Histologic and teased-fiber measurements of sural nerve in disorders of lower motor and primary sensory neurons. Mayo Clin Proc. 1968 Feb;43(2):81–123. [PubMed] [Google Scholar]
- Dyck P. J., Lambert E. H. Lower motor and primary sensory neuron diseases with peroneal muscular atrophy. II. Neurologic, genetic, and electrophysiologic findings in various neuronal degenerations. Arch Neurol. 1968 Jun;18(6):619–625. doi: 10.1001/archneur.1968.00470360041003. [DOI] [PubMed] [Google Scholar]
- HAGBARTH K. E. Spinal withdrawal reflexes in the human lower limbs. J Neurol Neurosurg Psychiatry. 1960 Aug;23:222–227. doi: 10.1136/jnnp.23.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E. Relation between size of neurons and their susceptibility to discharge. Science. 1957 Dec 27;126(3287):1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- Hughes J. T., Brownell B., Hewer R. L. The peripheral sensory pathway in friedreich's ataxia. An examination by light and electron microscopy of the posterior nerve roots, posterior root ganglia, and peripheral sensory nerves in cases of friedreich's ataxia. Brain. 1968;91(4):803–818. doi: 10.1093/brain/91.4.803. [DOI] [PubMed] [Google Scholar]
- KUGELBERG E., EKLUND K., GRIMBY L. An electromyographic study of the nociceptive reflexes of the lower limb. Mechanism of the plantar responses. Brain. 1960 Sep;83:394–410. doi: 10.1093/brain/83.3.394. [DOI] [PubMed] [Google Scholar]
- LANDAU W. M., CLARE M. H. The plantar reflex in man, with special reference to some conditions where the extensor response is unexpectedly absent. Brain. 1959 Sep;82:321–355. doi: 10.1093/brain/82.3.321. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., POWELL T. P. Neural mechanisms subserving cutaneous sensibility, with special reference to the role of afferent inhibition in sensory perception and discrimination. Bull Johns Hopkins Hosp. 1959 Oct;105:201–232. [PubMed] [Google Scholar]
- McLeod J. G. Electrophysiologic and histological studies in patients with Friedreich's ataxia. Electroencephalogr Clin Neurophysiol. 1969 Sep;27(7):723–723. doi: 10.1016/0013-4694(69)91407-2. [DOI] [PubMed] [Google Scholar]
- POWELL T. P., MOUNTCASTLE V. B. Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey: a correlation of findings obtained in a single unit analysis with cytoarchitecture. Bull Johns Hopkins Hosp. 1959 Sep;105:133–162. [PubMed] [Google Scholar]
- SHIMAMURA M., LIVINGSTON R. B. Longitudinal conduction systems serving spinal and brain-stem coordination. J Neurophysiol. 1963 Mar;26:258–272. doi: 10.1152/jn.1963.26.2.258. [DOI] [PubMed] [Google Scholar]
- SHIMAMURA M., MORI S., MATSUSHIMA S., FUJIMORI B. ON THE SPINO-BULBO-SPINAL REFLEX IN DOGS, MONKEYS AND MAN. Jpn J Physiol. 1964 Aug 15;14:411–421. doi: 10.2170/jjphysiol.14.411. [DOI] [PubMed] [Google Scholar]
- Shahani B. Effects of sleep on human reflexes with a double component. J Neurol Neurosurg Psychiatry. 1968 Dec;31(6):574–579. doi: 10.1136/jnnp.31.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahani B. Flexor reflex afferent nerve fibres in man. J Neurol Neurosurg Psychiatry. 1970 Dec;33(6):786–791. doi: 10.1136/jnnp.33.6.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahani B., Young R. R. Normal human flexor reflexes. Electroencephalogr Clin Neurophysiol. 1969 Sep;27(7):725–725. doi: 10.1016/0013-4694(69)91411-4. [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]