Abstract
Objective
Telemedicine networks are beginning to provide an avenue for conducting emergency medicine research, but using telemedicine to recruit participants for clinical trials has not been validated. The goal of this consent study is to determine whether patient comprehension of telemedicine-enabled research informed consent is non-inferior to standard face-to-face research informed consent.
Methods
A prospective, open-label randomized controlled trial was performed in a 60,000-visit Midwestern academic Emergency Department (ED) to test whether telemedicine-enabled research informed consent provided non-inferior comprehension compared with standard consent. This study was conducted as part of a parent clinical trial evaluating the effectiveness of oral chlorhexidine gluconate 0.12% in preventing hospital-acquired pneumonia among adult ED patients with expected hospital admission. Prior to being recruited into the study, potential participants were randomized in a 1:1 allocation ratio to consent by telemedicine versus standard face-to-face consent. Telemedicine connectivity was provided using a commercially available interface (REACH platform, Vidyo Inc., Hackensack, NJ) to an emergency physician located in another part of the ED. Comprehension of research consent (primary outcome) was measured using the modified Quality of Informed Consent (QuIC) instrument, a validated tool for measuring research informed consent comprehension. Parent trial accrual rate and qualitative survey data were secondary outcomes.
Results
One-hundred thirty-one patients were randomized (n = 64, telemedicine), and 101 QuIC surveys were completed. Comprehension of research informed consent using telemedicine was not inferior to face-to-face consent (QuIC scores 74.4 ± 8.1 vs. 74.4 ± 6.9 on a 100-point scale, p = 0.999). Subjective understanding of consent (p=0.194) and parent trial study accrual rates (56% vs. 69%, p = 0.142) were similar.
Conclusion
Telemedicine is non-inferior to face-to-face consent for delivering research informed consent, with no detected differences in comprehension and patient-reported understanding. This consent study will inform design of future telemedicine-enabled clinical trials.
Keywords: Research Methods, Telemedicine, Rural
Introduction
Telemedicine can pair emergency physicians in community hospitals with emergency physicians or specialists in tertiary centers, allowing for tertiary experience in non-tertiary centers 1,2–3. In addition to direct patient care, telemedicine networks are beginning to provide an avenue for expanding research beyond the academic medical center and into the community setting 4, 5. Telemedicine-enabled patient enrollment could allow for easier study recruitment, a broader research participant base, and the ability to study prehospital or early ED care outside of tertiary centers.
However, patients’ understanding of research participation and their rights related to research participation using computer-enabled audio-visual communication to discuss informed consent is still unknown 5–9. High-quality informed consent is a benchmark of ethical research 10; therefore, it is important to evaluate the quality of telemedicine-based informed consent before its utilization in telemedicine-based clinical trials. The objectives of this study are: (1) to determine if participant comprehension of research informed consent is non-inferior using telemedicine-based compared with standard face-to-face (F2F) consent in ED clinical trials, (2) to compare study accrual rates of telemedicine-based and standard research informed consent, and (3) to elicit patient’s perceptions of telemedicine-based informed consent.
Materials and Methods
Study Design
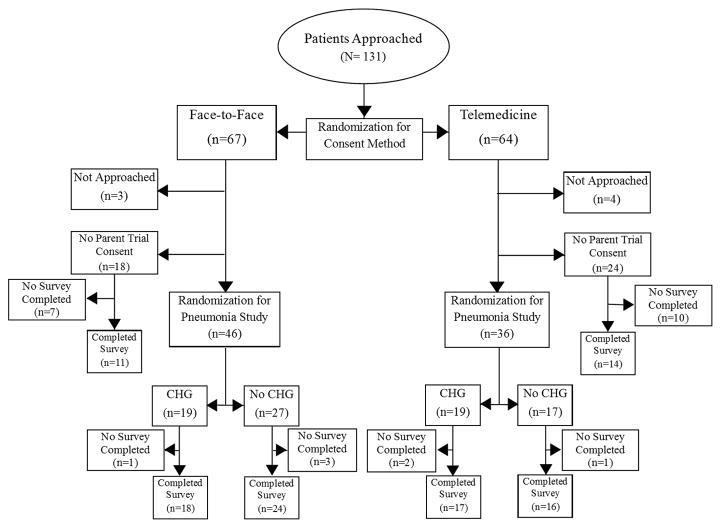
We conducted a prospective, open-label randomized controlled trial to test participant comprehension of telemedicine-enabled informed consent compared with standard F2F consent. The consent study was performed in conjunction with a parent clinical trial to test the effectiveness of oral chlorhexidine gluconate (CHG) 0.12% in preventing hospital-acquired pneumonia in ED patients likely to be admitted to the hospital. The consent study used sequential randomization: potential participants were initially randomized to telemedicine-enabled or F2F consent (prior to being approached for consent for the pneumonia parent trial). After patients were randomized, they were approached using the assigned method, and participants who agreed to participate in the parent pneumonia trial were randomized to chlorhexidine or no treatment. All patients were invited to complete a survey to assess their comprehension of informed consent at the conclusion of the trial (Figure 1). The local institutional review board approved the consent study under waiver of informed consent for the initial randomization, although participants provided prospective informed consent for the pneumonia prevention trial phase of the study. The consent study and parent trial were jointly registered on ClinicalTrials.gov (NCT02541799). Research methods follow and are reported in accordance with CONSORT guidelines 11.
Figure 1.
Study Enrollment Flowchart. CHG= Chlorhexidine Gluconate (see attached TIF file)
Study Setting and Population
Adult (age ≥ 18) patients being treated in a 60,000-visit ED at a Midwestern academic medical center between May 2015 and August 2015 were eligible for participation in the study if they were expected to be admitted to the hospital. Pregnant women, prisoners, patients with an allergy to chlorhexidine documented in the medical record, non-English speakers, and those with impaired ability to provide informed consent were excluded. All participants were being treated in the same ED (e.g., telemedicine was being delivered within a single institution). Participants were drawn from a convenience sample of ED patients between 6:00 and 22:00 due to availability of research staff for enrollment.
Study Protocol
Consent Study Randomization
After patients were initially evaluated by a senior resident or physician assistant, they were randomized to telemedicine-enabled or F2F research consent using a 1:1 allocation ratio. Randomization was conducted using block randomization with block sizes of four, and the code was concealed in sequentially numbered opaque sealed envelopes.
Face-to-Face Consent (Standard)
For those in the standard face-to-face (F2F) arm, care was delivered following usual standard procedures. Following the clinical interaction, a research assistant approached the patient to discuss the pneumonia prevention research study F2F.
Telemedicine-Enabled Consent
For participants randomized to telemedicine-enabled consent, the EM faculty physician met the patient using the telemedicine connection. The telemedicine encounter occurred after initial medical care had been provided by a senior resident or physician’s assistant, but before the patient saw the attending ED physician. Telemedicine was provided using a tablet and a commercially available telemedicine interface (REACH platform, Vidyo Inc., Hackensack, NJ). Both the faculty and the patient were in the same ED, but were in different rooms. Potential participants were not aware the telemedicine physician was onsite until after they had made a decision about participating in the parent trial.
After the faculty physician had completed his evaluation, the research assistant used the telemedicine connection to approach the participant about the study using the same consent script as F2F. A consent document had been placed into the room prior to the interaction, so patients would indicate their consent to participate in the pneumonia trial using the provided consent document. After the telemedicine encounter had concluded and the patient had finished talking with the RA about the parent trial, an EM faculty physician entered the room to conduct an in-person physical examination, solely for the purpose of clinical care. Physicians, physician’s assistants, and residents were in clinical care roles during the study and did not perform any of the research procedures.
Pneumonia Trial (Parent Trial)
The parent trial hypothesis was that a single dose of 0.12% oral chlorhexidine would decrease the incidence of hospital-acquired pneumonia. After consent was obtained, a second envelope was opened to randomize participants to treatment (CHG) or no treatment. No placebo was administered. The participant was notified of his/her allocation, and a study physician or pharmacist administered oral CHG to qualifying patients. The parent trial had the same inclusion and exclusion criteria as the consent study. Pneumonia status was abstracted from the medical records at the time of hospital discharge.
Debriefing
Initially, patients were consented for the parent trial, and the investigators did not immediately disclose that a secondary study was also investigating informed consent. This method is similar to a prior trial performed by the study team on informed consent12. The research participants also did not know that the telemedicine provider was in the same institution as the prospective participant. After conclusion of the pneumonia study, the research assistant re-approached each patient (regardless of whether they consented to participate in the pneumonia study) to disclose that they had been randomized to two different strategies of informed consent.
The debriefing was conducted F2F in both groups. Debriefing for both groups occurred immediately following the conclusion of the remainder of the parent trial procedures. Participants were asked to complete a survey that included the modified Quality of Informed Consent (QuIC) instrument, a validated tool for measuring research informed consent comprehension13. Both groups were approached by the same research assistant (RA) for all interactions using a standard consent document and script. After completion of the survey, study procedures were complete and participants were not contacted for any follow-up.
Survey Scoring
The surveys were scored by the RA using the standard QuIC scoring rubric at the conclusion of the study13. Results were entered into an electronic database by a single research assistant. The database generated a summary score using the methodology previously described. A random sample of 10% of the surveys was scored separately by one of the study investigators to assure accurate transcription into the database (perfect concordance was achieved).
Survey Development
The survey was developed by modifying the prior QuIC survey by consensus of the study investigators. Modifications made to the QuIC document (Figure S1- online appendix) did not alter the original domains of the survey instrument. The original survey instrument was designed for cancer clinical trials; modifications were changes in the language of questions to be appropriate for an ED-based clinical trial. Questions (A6, A7, and A8) from the original instrument did not apply to our ED study population and were deleted, and a qualitative Part C to elucidate opinions on telemedicine was added13. An EM faculty physician, an expert in the bioethics of informed consent, and an epidemiologist reviewed the survey for clarity and consistency with the original QuIC survey. Prior to starting the trial, the RA performed a dry run of survey completion with three ED patients who did not take part in the study. These patients provided feedback to the study team on issues of clarity and consistency, and modifications were made prior to beginning the trial.
Sample Size
The consent study was designed to incorporate an internal pilot study. Based on variance of QuIC scores in a prior study13, a sample size of 100 completed surveys was estimated (non-inferiority design, α=0.05, power=0.9, non-inferiority margin=10). The non-inferiority margin was based on the approximate width of the 95%CI (72.5 – 81.9) for Part A of the QuIC validation study13. An interim analysis was planned after 25 participants were enrolled in the F2F consent group. From this interim analysis (of only the F2F consent patients), the study accrual rate and the standard deviation of the Parts A and B QuIC scores were calculated independently. Based on this interim analysis, the sample size was not modified, but the estimated number of approached patients was set at 130 (to achieve 100 completed surveys, based on the interim analysis consent rate). Because no estimate of efficacy was conducted at this time (telemedicine surveys were not scored in the interim analysis), no sample size adjustment was made for the interim analysis.
Blinding
The scoring of surveys for the interim analysis was performed by an independent RA not affiliated with the study, such that aggregate survey results were not available to the RA conducting the research.
Measures
Comprehension of research informed consent was the primary outcome, and was measured using the modified Quality of Informed Consent (QuIC) instrument 13. Possible scores range from 0 to 100 for both Part A (objective understanding) and Part B (subjective understanding). A higher QuIC score indicates higher quality of research informed consent. Secondary outcomes included parent trial accrual rate, survey completion rates, and qualitative Part C survey responses. An additional series of questions were also included on the survey to elucidate participant opinions about the use of telemedicine for research consent.
Data Analysis
Demographic parameters were compared with the independent samples t-test, the Mann-Whitney U test, or the Chi-squared test, as appropriate. The primary outcomes (QuIC parts A and B) were compared by intention-to-treat analysis using the independent samples t-test (normality was confirmed with graphical inspection). Parent trial accrual rate and survey completion rate were compared using the Chi-squared test. Part C of the survey contained Likert scales, presented as median (IQR), with representative comments. Statistical significance was defined as p < 0.05 using 2-tailed tests and was performed using SPSS v.22 (IBM, Armonk, NY).
Results
During the study period, 131 patients were randomized (n = 64 to the telemedicine group), and 100 surveys were completed (Figure 1). Sixty-one (49%) subjects were male, 62 (50%) subjects lived in non-urban areas [20], and the mean age was 55 (SD 17) years. Groups were well matched with regard to diagnoses, illness severity, and demographic factors (Table 1).
Table 1.
Subject Demographics of Participants Completing Survey
| Usual n=53 | Telemedicine n=47 | Difference (95% CI) | |
|---|---|---|---|
| Age, mean (SD) | 54.7 (14.8) | 54.1 (19.3) | 0.7 (−6.2 – 7.4) |
| Male, n (%) | 25 (47) | 20 (43) | 4.6 (−15.3 – 24.6) |
| Rurality - Urban, n (%) | 25 (47) | 23 (49) | −1.8 (−21.8 – 18.3) |
| Not Admitted, n (%) | |||
| Discharged from ED | 11 (21) | 11 (23) | −2.6 (−19.3 – 14.0) |
| Left AMA | 0 (0) | 1 (2) | −2.1 (−6.1 – 1.8) |
| Admit to ICU, n (%) | 1 (2) | 2 (4) | −2.4 (−9.2 – 4.5) |
| Diagnosis Code, n (%) | |||
| Pulmonary | 9 (14) | 6 (10) | 4.1 (−7.6 – 15.7) |
| Cardiac | 13 (20) | 14 (23) | −3.0 (–17.8 – 11.8) |
| Gastrointestinal | 22 (34) | 18 (30) | −4.4 (−12.4 – 21.1) |
| Infectious | 10 (16) | 8 (13) | −2.3 (−10.3 – 14.9) |
| Neurological/Psychological | 6 (9) | 8 (13) | −4.0 (−15.3 – 7.4) |
| Other | 4 (6) | 6 (10) | −3.8 (−13.5 – 6.0) |
SD= Standard Deviation; AMA= Against Medical Advice; ICU= Intensive Care Unit (Demographics of patients that did not fill out survey are available in online appendix.)
Primary Outcomes
The modified QuIC instrument was completed by 101 participants, and survey completion rates were similar between the two groups. Part A of the survey measured objective understanding of informed consent. Part B measured participants’ subjective impression as to their understanding of the research informed consent process. One incomplete survey was excluded.
No significant differences in objective understanding of research informed consent were observed between participants consented via telemedicine when compared to face-to-face consent (difference 0.0 points, 95%CI −3.0 – 3.0). For subjective consent, there was similarly no significant differences between the two groups’ perceived comprehension of consent (difference 1.8 points, 95%CI −0.4 – 7.1) (Table 2). Telemedicine-based consent scores were non-inferior to F2F scores for Part A and Part B.
Table 2.
Key Outcomes
| Primary Outcomes | Usual n=53 | Telemedicine n=47 | Difference (score) |
|---|---|---|---|
|
Objective Comprehension, mean (SD) QuIC Score- Part A |
74.4 (6.9) | 74.4 (8.1) | 0.0 (−3.0 – 3.0) |
|
Subjective Comprehension, median (IQR) QuIC Score- Part B |
89.3 (16.7–96.4) | 93.8 (83.9–100) | 1.8 (−0.4 – 7.1) 1 |
| Secondary Outcomes | Usual n=67 | Telemedicine n=64 | Difference (%) |
|---|---|---|---|
| Parent Trial Accrual Rate, n (%) | 46 (68.7) | 36 (56.3) | 12.4 (−4.3–29.1) |
| Survey Completion Rate, n (%) | 53 (79.1) | 47 (73.4) | 7.2 (−7.4 – 21.7) |
SD= Standard Deviation; IQR= Interquartile Range
Differences in medians are calculated by a rank-based method, using the user-defined Stata function “cid” published by Patrick Royston, Royal Postgraduate Medical School (March 1998).
Secondary Outcomes
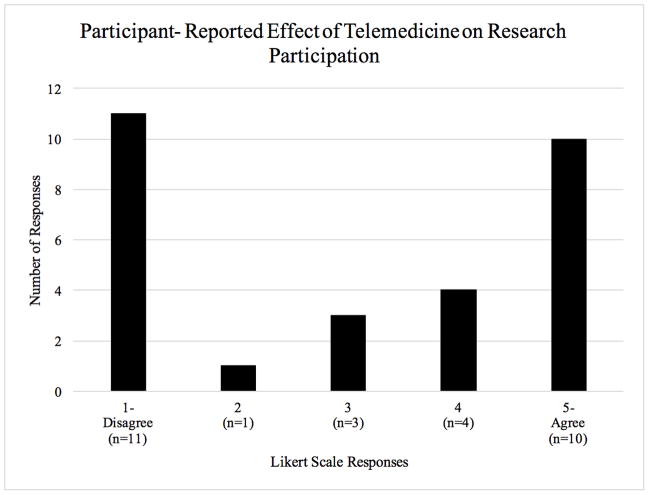
Parent trial accrual rates were measured in the two groups to understand whether the method of approaching potential participants altered their likelihood to consent to participate in a clinical trial (Table 2). There was no statistically significant difference in parent trial accrual rates between participants approached by telemedicine vs. F2F (56% vs. 69%, p = 0.142). Likert scale questions demonstrate participants’ reactions to telemedicine-based research (Figure 2). In Part C of the QuIC post-survey, we asked open-ended responses to identify barriers to participation in telemedicine-based research consent, and no specific barriers were identified. Participants had various opinions when asked to rank the effect of telemedicine on their likelihood to participate in research with a Likert scale (Figure 2). Similar variation was observed for an open-ended question asking participants about their attitudes toward being considered for research by telemedicine.
Figure 2.
QuIC Survey-Part C. n=30. Participants were asked “Telemedicine made me more likely to participate in this trial than I would have been without telemedicine”. Responses utilized a Likert Scale of 1(Disagree) to 5 (Agree). (see attached TIF file)
Parent Trial Results
Twenty-eight participants were excluded, most commonly because they were subsequently discharged without hospital admission or because they subsequently withdrew from the study. No participants in the CHG treatment (n=26) or control groups (n=28) developed pneumonia during their hospital encounter, so no inference could be drawn on the utility of oral CHG in prevention of pneumonia.
Discussion
Telemedicine can connect community EDs to large academic EDs 14, increasing access to specialty care and research participation. Differences in clinical outcomes associated with medical care at high-volume medical centers compared to local, community medical centers have prompted telemedicine-based clinical trials 15. While there have been multiple telemedicine-based ED clinical trials, few have consented patients for research over a telemedicine link 16, 17.
Our consent study is the first to investigate the quality of informed consent using telemedicine. There is the growing need to enroll patients remotely to save time in implementing time-sensitive interventions, reduce the variability of consent, and enhance clinical trial enrollment of rural and remote populations. Our findings support remote enrollment of clinical trial participants by telemedicine, especially for emergency medicine clinical trials.
As expected, many ED clinical trials have “time-to-intervention” requirements, and often patients transferred from community hospitals to academic centers are excluded from study participation because they violate clinical trial protocols’ time window constraints 5. Enrolling patients in local community EDs allows for interventions to be administered more quickly, perhaps even prior to arriving in academic EDs 17.
Participation in clinical trials is known to be affected by structural factors, such as geography and tertiary care center access, and has traditionally resulted in remote and rural populations being underrepresented in clinical research 18–20. In the United States, about 60 million people, or 19.3% of the population, live in a rural area 21. More proportionate representation of rural populations in emergency medicine research could improve research enrollment, exposing rural and non-rural persons more equally to the potential risks and benefits of participation in clinical research. Further, complex interventions that are tested solely in large, academic center clinical trials may not be easily translated or valid in smaller, community-based non-academic EDs 5. Enhancing recruitment in rural, non-academic community setting is important to expand the generalizability of study findings.
Secondary outcomes demonstrating similar study accrual rates and survey completion rates can inform the design of future telemedicine clinical trials in EDs. While our consent study was not powered to detect small differences in study accrual rates, the non-significantly higher accrual in the F2F group is notable, and may be related to reduced enrollment rates. Differences in qualitative participant opinions towards telemedicine-based consent could also warrant further study. One ethical concern we had about using telemedicine to recruit for clinical trials was the potential for coercion in study recruitment (especially when care was being delivered using the same telemedicine link). Lower study accrual rates in the telemedicine-based consent group argue against the possibility of coercion.
Limitations
This study has several limitations. First, although the telemedicine link was used for actual patient care in addition to the clinical trial, participants were communicating with a clinician and research assistant in the same facility. It is possible the artificial nature of this telemedicine relationship failed to capture important aspects of a remote telemedicine encounter.
Second, the study was conducted at a single large academic center. Using a single center with a single research assistant reduced the variability in the consent process for each participant, but it also may reduce the generalizability. Further, the use of a single research assistant opened the possibility for participant feedback to modify the study team’s delivery of informed consent (e.g., perhaps all the telemedicine patients were confused about a particular aspect and required additional time or clarification). Although this is a reasonable criticism, the consent delivered was for an actual clinical trial and this type of feedback is common in recruiting for clinical studies. Further, a standard script was used by the research team to limit variability between the two groups. This was a pragmatic study, and the fact that neither format was clearly superior simply reflects that in the real world, telemedicine-enabled consent can be used (with whatever natural patient-oriented feedback exists) to deliver complete and accurate research information to potential study participants.
Third, although this study was well-powered to detect differences in research comprehension, it was not powered to find differences between the study accrual rates. The absolute difference between the study accrual rates in the two groups, although not statistically significant, may still be important, and investigators should consider this issue of differential study accrual rates during trial design and planning. Finally, some patients were not enrolled after randomization. Because potential participants were identified early in their ED stay, several patients were taken for procedures or diagnostic tests after randomization but before enrollment. Typically, intention-to-treat analyses would be used to account for this weakness, but because these individuals were never approached, we have no data on their comprehension. Fortunately, the number of these lost participants is small enough that it is unlikely that their inclusion could change the conclusions of this study.
Conclusion
Comprehension of telemedicine-based research informed consent is non-inferior to standard face-to-face consent in ED clinical trials. Study accrual rates may be lower for telemedicine-based research consent, but no statistically significant difference was observed in this study. Future work should be conducted with remote patients to understand the influence of being treated by a remote physician in the research consent process. Because patient understanding of telemedicine-enabled research informed consent is non-inferior to standard face-to-face consent, telemedicine could be used for remote enrollment of emergency department-based research participants in the future.
Supplementary Material
Acknowledgments
Funding Sources/Disclosures: This study was supported by the University of Iowa Department of Emergency Medicine and the University of Iowa Carver College of Medicine (NIH training grant #2T35HL007485-36). The University of Iowa Department of Emergency Medicine sponsors a rural emergency telemedicine network.
Dr. Mohr is supported by grants from the Emergency Medicine Foundation and the U.S. Department of Health and Human Services Health Resources and Services Administration.
Footnotes
Prior Presentations: This study was presented at the Society for Academic Emergency Medicine Great Plains Regional Meeting in Minneapolis, MN, September 19, 2015 and received Best Oral Presentation.
Contributor Information
Morgan R. Bobb, Department of Emergency Medicine, University of Iowa Carver College of Medicine.
Paul G. Van Heukelom, Department of Emergency Medicine, University of Iowa Carver College of Medicine.
Brett A. Faine, Department of Emergency Medicine, University of Iowa Carver College of Medicine.
Azeemuddin Ahmed, Department of Emergency Medicine, University of Iowa Carver College of Medicine.
Jeffrey T. Messerly, Department of Emergency Medicine, University of Iowa Carver College of Medicine.
Gregory Bell, Department of Emergency Medicine, University of Iowa Carver College of Medicine.
Karisa K. Harland, Department of Emergency Medicine, University of Iowa Carver College of Medicine.
Christian Simon, Program in Bioethics and Humanities, Department of Internal Medicine, University of Iowa Carver College of Medicine.
Nicholas M. Mohr, Department of Emergency Medicine, Division of Critical Care, Department of Anesthesia, University of Iowa Carver College of Medicine.
References
- 1.Brennan JA, Kealy JA, Gerardi LH, et al. Telemedicine in the emergency department: a randomized controlled trial. J Telemed Telecare. 1999;5:18–22. doi: 10.1258/1357633991932342. [DOI] [PubMed] [Google Scholar]
- 2.Cho J, Chung HS, Choa M, Yoo SK, Kim J. A pilot study of the Tele-Airway Management System in a hospital emergency department. J Telemed Telecare. 2011;17:49–53. doi: 10.1258/jtt.2010.100202. [DOI] [PubMed] [Google Scholar]
- 3.Duong TA, Cordoliani F, Julliard C, et al. Emergency department diagnosis and management of skin diseases with real-time teledermatologic expertise. JAMA Dermatol. 2014;150:743–7. doi: 10.1001/jamadermatol.2013.7792. [DOI] [PubMed] [Google Scholar]
- 4.Marziali E, Serafini JM, McCleary L. A systematic review of practice standards and research ethics in technology-based home health care intervention programs for older adults. J Aging Health. 2005;17:679–96. doi: 10.1177/0898264305281100. [DOI] [PubMed] [Google Scholar]
- 5.Switzer JA, Hall CE, Close B, et al. A telestroke network enhances recruitment into acute stroke clinical trials. Stroke; a journal of cerebral circulation. 2010;41:566–9. doi: 10.1161/STROKEAHA.109.566844. [DOI] [PubMed] [Google Scholar]
- 6.Levine SR, Gorman M. “Telestroke” : the application of telemedicine for stroke. Stroke; a journal of cerebral circulation. 1999;30:464–9. doi: 10.1161/01.str.30.2.464. [DOI] [PubMed] [Google Scholar]
- 7.Chouinard I, Scott RE. Informed consent for videoconsultations in Canada. J Telemed Telecare. 2009;15:171–4. doi: 10.1258/jtt.2008.080905. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan B, Litewka S. Ethical Challenges of Telemedicine and Telehealth. Cambridge Quarterly of Healthcare Ethics. 2008;17:401–16. doi: 10.1017/S0963180108080535. [DOI] [PubMed] [Google Scholar]
- 9.Dreezen I. Telemedicine and Informed Consent. Medicine and Law. 2004;23:541–50. [PubMed] [Google Scholar]
- 10.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? Jama. 2000;283:2701–11. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 11.Schulz KF, Altman Dg, Moher D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leira EC, Ahmed A, Lamb DL, et al. Extending acute trials to remote populations: a pilot study during interhospital helicopter transfer. Stroke; a journal of cerebral circulation. 2009;40:895–901. doi: 10.1161/STROKEAHA.108.530204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: a new measure of understanding among research subjects. J Natl Cancer Inst. 2001;93:139–47. doi: 10.1093/jnci/93.2.139. [DOI] [PubMed] [Google Scholar]
- 14.Capampangan DJ, Wellik KE, Bobrow BJ, et al. Telemedicine versus telephone for remote emergency stroke consultations: a critically appraised topic. Neurologist. 2009;15:163–6. doi: 10.1097/NRL.0b013e3181a4b79c. [DOI] [PubMed] [Google Scholar]
- 15.Leira EC, Hess DC, Torner JC, Adams HP., Jr Rural-urban differences in acute stroke management practices: a modifiable disparity. Arch Neurol. 2008;65:887–91. doi: 10.1001/archneur.65.7.887. [DOI] [PubMed] [Google Scholar]
- 16.Wu TC, Sarraj A, Jacobs A, et al. Telemedicine-guided remote enrollment of patients into an acute stroke trial. Ann Clin Transl Neurol. 2015;2:38–42. doi: 10.1002/acn3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alfredo Caceres J, Greer DM, Goldstein JN, et al. Enrollment of research subjects through telemedicine networks in a multicenter acute intracerebral hemorrhage clinical trial: design and methods. J Vasc Interv Neurol. 2014;7:34–40. [PMC free article] [PubMed] [Google Scholar]
- 18.Leira EC, Ahmed A, Lamb DL, et al. Extending acute trials to remote populations: a pilot study during interhospital helicopter transfer. Stroke; a journal of cerebral circulation. 2009;40:895–901. doi: 10.1161/STROKEAHA.108.530204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baquet CR, Commiskey P, Daniel Mullins C, Mishra SI. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detect Prev. 2006;30:24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanner A, Kim SH, Friedman DB, Foster C, Bergeron CD. Promoting clinical research to medically underserved communities: current practices and perceptions about clinical trial recruiting strategies. Contemp Clin Trials. 2015;41:39–44. doi: 10.1016/j.cct.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 21.State and metropolitan area data book. U.S. Dept. of Commerce, Economics and Statistics Administration, Bureau of the Census; at http://permanent.access.gpo.gov/lps2647/.) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.