Abstract
Successful memory for an image can be supported by retrieval of one’s personal reaction to the image (i.e., internal vividness), as well as retrieval of the specific details of the image itself (i.e., external vividness). Prior research suggests that memory vividness relies on regions within the medial temporal lobe, particularly the hippocampus, but it is unclear whether internal and external vividness are supported by the hippocampus in a similar way. To address this open question, the current study examined hippocampal connectivity associated with enhanced internal and external vividness ratings during retrieval. Participants encoded complex visual images paired with verbal titles. During a scanned retrieval session, they were presented with the titles and asked whether each had been seen with an image during encoding. Following retrieval of each image, participants were asked to rate internal and external vividness. Increased hippocampal activity was associated with higher vividness ratings for both scales, supporting prior evidence implicating the hippocampus in retrieval of memory detail. However, different patterns of hippocampal connectivity related to enhanced external and internal vividness. Further, hippocampal connectivity with medial prefrontal regions was associated with increased ratings of internal vividness, but with decreased ratings of external vividness. These findings suggest that the hippocampus may contribute to increased internal and external vividness via distinct mechanisms and that external and internal vividness of memories should be considered as separable measures.
Keywords: Hippocampal connectivity, memory, internal vividness, external vividness
1. Introduction
The successful retrieval of events from our personal past is central to our everyday well-being, allowing us to reminisce about our past, make decisions about our future, and even formulate our own identity (Bluck, Alea, Habermas, & Rubin, 2005). However, the utility of these memories may be tied to the ability to remember specific details; memories that are less detailed may not be as useful for directing future actions and can lead to inaccurate generalizations (Gilbert & Wilson, 2009). As such, it is critical to understand the cognitive and neural mechanisms that support this sense of vividness during memory retrieval.
Neuroimaging researchers have examined the quality and quantity of memory detail using a number of behavioral measures. The Remember-Know procedure (Tulving, 1985) asks participants to indicate, for each item they classify as “old” on a recognition test, whether their memory is rich in contextual detail (“Remember”) or based on a more general sense of familiarity (“Know”). Another common approach involves using a single likert scale representing a measure of recollection such as confidence (e.g., Yonelinas, 1994), reliving (e.g., Daselaar et al., 2008), or vividness (e.g., Gilboa et al., 2004) for each old item.
For these behavioral measures, high ratings of recollection may be supported by retrieval of any additional contextual information, including details about thoughts and feelings that the participant had at the time of encoding (here, called “internal details”) or details about the event itself (here, called “external details”). There is evidence to believe that retrieval of these two types of details may be supported by distinct mechanisms. Research has shown that task instructions may be utilized to separately manipulate different types of memory details (e.g., Suengas & Johnson, 1988), and that ratings of internal and external details are differentially affected by healthy aging (e.g., Hashtroudi et al., 1990). Studies examining the separate effects of internal and external detail retrieval either utilize two separate likert scales (i.e., “internal vividness” and “external vividness”) or a variation of the Memory Characteristics Questionnaire (Johnson et al., 1988), a questionnaire that includes an overall vividness rating (on a scale from 1-7) as well as more specific questions regarding the extent of perceptual, temporal, spatial, emotional, and conceptual detail recalled during retrieval.
Although behavioral research has clearly demonstrated patterns that distinguish internal and external vividness ratings, it remains an open question to what extent these two characteristics are supported by distinct neural networks during memory search. Episodic memory retrieval is associated with recruitment of a widespread network of regions, including medial-temporal, prefrontal, and parietal regions (see Spaniol et al., 2009). Of these regions, neuroimaging studies have suggested that recruitment of the medial temporal lobe, and in particular, the hippocampus, is related to increased ratings of memory vividness, level of detail, and recollection (e.g., Gilboa et al., 2004; Addis et al., 2004). However, it is unclear whether increased hippocampal recruitment enhances internal and external details using similar or distinct mechanisms. Specifically, hippocampal activity may be associated with corresponding increases in distinct neural regions to selectively enhance retrieval of these two types of details.
In a meta-analysis examining regions associated with increased ratings of subjective recollection, Spaniol and colleagues (2009) found that, in addition to the left hippocampus, subjective recollection was associated with increased activity in the ventromedial prefrontal cortex, precentral gyrus, posterior cingulate, inferior parietal lobe, parahippocampal gyrus, lateral temporal lobes, and fusiform gyrus. However, this meta-analysis was conducted with studies in which participants were able to rely on any contextual details (internal or external) for “remember” or high confidence responses. Therefore, this network is likely based on trials related to high internal vividness and high external vividness, and it is still unknown how internal and external vividness may differentially influence hippocampal connectivity with these particular regions.
One particularly interesting region of interest within this network is the ventromedial prefrontal cortex. Prior research implicates the medial prefrontal cortex (mPFC) in self-referential processing during cognitive tasks (e.g., Cabeza et al., 2004; Gusnard et al., 2001; Kelley et al., 2002; Maguire et al., 2001), suggesting that recruitment of medial prefrontal regions to enhance vividness may be specific to internal, rather than external, details. The mPFC is also a key node within the default mode network, a set of interacting and functionally connected neural regions that is engaged when participants are scanned during rest, and deactivated during task (e.g., Shulman et al., 1997; Mazoyer et al., 2001; Raichle et al., 2001).
It has been suggested that the default mode network supports self-referential processing, including personal memory retrieval (Buckner et al., 2008). In fact, the relation of this network with internal mentation may be so powerful that its suppression may be necessary for disengagement from distracting internal thought to optimize externally-directed cognition (Anticevic et al., 2012). Consistent with this hypothesis, several studies have found that reduced default mode activity is associated with more successful performance in stimulus-driven goal-directed cognitive tasks (e.g., Anticevic et al., 2010; Daselaar et al., 2004). Such an explanation may suggest that hippocampal connectivity to default mode regions would increase as a function of internal vividness, but would be associated with a reduction in external detail retrieval, or decreased external vividness.
The current study examines how internal and external vividness ratings differentially influence hippocampal connectivity during the initial search phase of memory retrieval. Based on research implicating the medial prefrontal cortex in self-referential processing, we hypothesize that internal vividness will be associated with enhanced connectivity between the mPFC and hippocampus. Conversely, the relation between default mode network deactivation and externally-directed task performance suggests that external vividness will be inversely related to mPFC-hippocampal connectivity. Additionally, increased external detail may rely on increased hippocampal connectivity with other regions associated with perceptual and conceptual detail retrieval, such as the precuneus (Gilboa et al., 2004), ventrolateral prefrontal cortex (Badre & Wagner, 2007), and lateral temporal lobes (Binder & Desai, 2011).
2. Methods
2.1 Participants
Data from fifty-nine healthy adults (mean age= 48.17, sd= 20.34, ages 19-85; mean education= 16.60, sd= 2.41; 27 females) are reported. Age and education did not differ across genders (p>.2 for both contrasts) and age was not significantly correlated with education (p= .64). Two additional participants were recruited but not scanned due to contraindications for MRI (ages 50 and 75; both male). Another fourteen participants were scanned, but were excluded from the current analysis due to equipment malfunction (n=1; age=49, edu=16, male), an abnormal structural scan (n=1, age= 49, edu=17, female), excessive motion in the scanner resulting in termination of MR session (n=1, age=56, edu=16, male), voluntary early termination of the MR session (n=1, age=49, edu=14, female), low behavioral performance (i.e., hit rate below.50 or false alarm rate above .50; n=6, mean age= 55.64, sd= 18.12, ages 30-83; mean education= 16.12, sd= 3.49; 2 female), or lack of variability in their vividness ratings (i.e., only providing a single value for all vividness ratings; n=4, mean age= 44.25, sd= 9.00, ages 36-53; mean education= 16.50, sd= 1.00; 2 female). Participants were right-handed native English speakers without psychiatric illness or neurological disorder and were recruited from the greater Boston area. All participants were screened using an extensive cognitive battery to ensure that cognitive performance was in the normal range for participants of all ages. The tests used in this battery are described in more detail in supplementary materials. Although effects of age are not the focus of this report, the relations of age with all cognitive variables are reported in Supplementary Table 1 and explained in the supplementary materials. All participants were paid for their participation and gave written informed consent in accordance with the requirements of the Institutional Review Board at Boston College.
2.2 Materials
Stimuli were 480 pictures (160 positive, 160 negative, and 160 neutral) and the neutral titles used in prior studies from our lab (Ford et al., 2014a, 2014b; Ford & Kensinger, 2014). The 480 title-picture pairs were divided into 4 sets of 120 pictures each (40 positive, 40 negative, and 40 neutral) for counterbalancing purposes. The current analysis focused on responses to neutral title-picture pairs only, and results related to memory for emotional images will not be discussed.
2.3 Procedure
Following instruction and a short practice, participants encoded one set of 120 title-image pairs. Titles (e.g., “Lettuce”) were paired with a positive, negative, or neutral image (e.g., a piece of rotting lettuce with bugs crawling on it as a negative image). In an intentional encoding task (outside of the scanner) participants were given 3 seconds to make a decision regarding the appropriateness of the word as a description of the image (1= poor description, 2= acceptable description, and 3= very good description). After a half-hour delay (M= 34.3 minutes, sd= 7.8), participants took part in a scanned retrieval task. Participants were presented with the 240 titles (120 neutral titles that were studied during the encoding phase and 120 unstudied neutral titles) randomly across 6 retrieval runs of equal length. Participants were given up to 4 seconds to decide whether the word was “old” (i.e., seen previously) or “new” (i.e., not seen previously). The screen was removed following the participant’s button press. Across participants, it was varied which items were studied and which were reserved as foils on the recognition test.
Immediately following an “old” response, 80% of the time, participants were asked to “Elaborate” on the old item (i.e., think about the image presented with the title and the experience with that title and image at encoding) for 5 seconds. To discourage participants from beginning to elaborate during the search phase, and to distinguish activity during search from activity during elaboration, 20% of trials were catch trials; instead of an elaboration phase, the next trial was presented. Following a “new” response, 80% of the time, participants moved on to the next trial. To minimize the likelihood that participants would automatically begin preparing for the next trial after a “new” response, on 20% of the trials, participants were asked to “Imagine” an image that could have accompanied the new item for 5 sec.
Following the elaboration phase, participants were asked to consider how well they were able to remember each item in two separate rating scales, counterbalanced across participants1. Participants were given five seconds each for two scales: 1) On a scale of 1-5, how well did they remember the details of the image associated with the cue word or phrase (“External Vividness”) and 2) On a scale of 1-5, how well did they remember their own personal thoughts and feelings from encoding the title-picture pair (“Internal Vividness”). Following each trial, participants viewed a fixation cross for 0-6 seconds to introduce jitter. A visual schematic of this procedure is presented in Figure 1.
Figure 1.
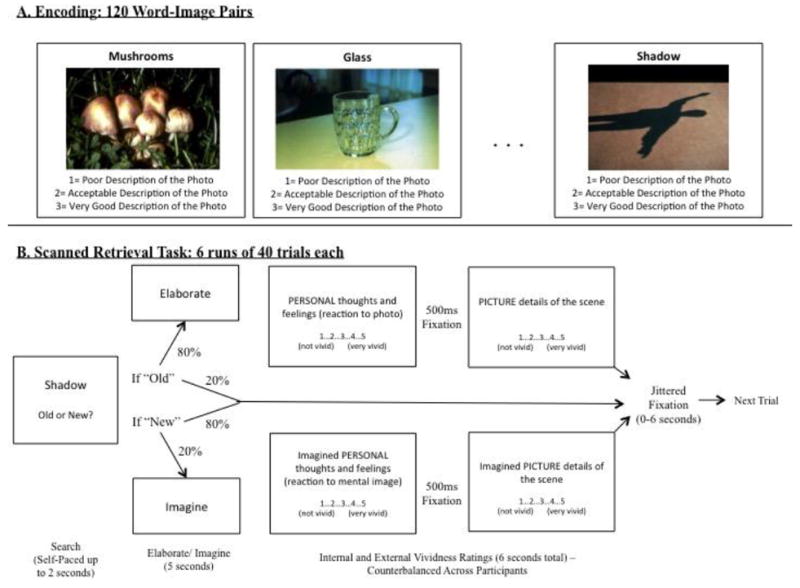
Visual schematic of behavioral methods for A) the encoding task and B) the scanned retrieval task.
After being removed from the scanner, participants were re-presented with the images from the encoding phase. They rated each image’s valence and arousal on a 1-7 scale and indicated which specific emotions they experienced with each image. This portion was self-paced and participants were encouraged to respond based on their initial reaction.
2.4 Data Acquisition
Participants’ heads were stabilized in a Siemens Tim Trio 3 Tesla scanner. A localizing scan and auto-align scout were followed by a high resolution multi-echo T1 structural scan for anatomical visualization (176 1mm slices, TR=2200ms, TE1=1.64ms, TE2= 3.5ms, TE3= 5.36ms, TE4= 7.22ms). Six runs of whole brain, gradient-echo, echo planar images (31 3mm slices aligned along the line between the anterior and posterior commissures, 20% skip, TR=2s, TE=30ms, Flip angle=90) were acquired during memory retrieval using interleaved slice acquisition. A diffusion weighted scan was collected but will not be discussed. Response data were collected using a magnet-safe button response box.
2.5 Preprocessing and Data Analysis
Images were preprocessed and analyzed using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB. Images were co-registered, realigned, normalized (resampled at 3 mm at the segmentation stage and written at 2mm at the normalization stage) and smoothed using a Gaussian 8 mm kernel. The current analysis examined effects of internal and external vividness on recruitment during memory search, modeled as a two-second block beginning at stimulus onset2. This analysis focused on search processes to facilitate comparisons with autobiographical memory studies examining hippocampal recruitment at stimulus onset (e.g., Gilboa et al., 2004; Addis et al., 2004), as well as traditional episodic memory studies that do not distinguish between search and elaboration phases.
The first level fMRI analysis examined the effect of vividness on neural activity during accurate “old” responses to studied items (i.e., “hits”). Neutral hits were modeled as a condition of interest with internal and external vividness as parametric modulators of interest. Positive and negative hits (and associated vividness ratings), incorrect responses and correct “new” responses to positive, negative, and neutral items, although not relevant for the current analysis, were included in each model as separate nuisance variables.
Because SPM automatically orthogonalizes parametric regressors in fixed-effects models, four separate models were generated for each subject to capture the effects of internal and external vividness on recruitment during neutral hits:
Internal vividness only, identifying regions that exhibited a parametric effect of internal vividness on recruitment
External vividness only, identifying regions that exhibited a parametric effect of external vividness on recruitment
Internal vividness followed by external vividness, identifying regions that exhibited a parametric effect of external vividness on recruitment above and beyond the effect of internal vividness
External vividness followed by internal vividness, identifying regions that exhibited a parametric effect of internal vividness on recruitment above and beyond the effect of external vividness
Using these four models, we were able to identify regions in which activity was associated with internal and external vividness with (1 and 2) and without (3 and 4) taking the other variable into account.
The first two fixed-effects analyses above were used in random-effects models designed to examine the regions commonly associated with internal and external vividness. Therefore, individual-subject effects from models 1 and 2 above were included in a single model including internal and external vividness as variables of interest, and age as a covariate of no-interest (to control for potential confounds of age). Conjunction analyses were conducted to identify regions that exhibited significant effects of both internal and external vividness. Conjunction analyses were performed by creating explicit masks of one contrast (e.g., the effect internal vividness) at p<.005 and applying this mask to the second contrast at the same threshold (e.g., the effect of external vividness).
The third and fourth fixed-effects models above were used in a different random-effects analysis, to determine how internal and external vividness may differentially affect neural recruitment. Therefore, at the second-level (random-effects) analysis, a model incorporated individual-subject effects from models 3 and 4 above, in which the effects of internal and external vividness were identified controlling for the other variable. Age was included as a covariate of no-interest, so that the model could identify regions associated with increased or decreased ratings of internal and external vividness, controlling for both the other type of vividness rating and for age. Within this model, two separate contrasts were performed to examine the effects of internal and external vividness, controlling for the effects of the other. Because these analyses were conducted examining the effect of one variable controlling for the other, conjunction analyses were not conducted.
The significance threshold for all analyses was set at p < .005 (uncorrected). Monte Carlo simulations (Slotnick et al., 2003), incorporating the smoothness of the data and run with the normalized voxel size of 2×2×2, determined that a 29-voxel extent corrected results to p < .05. Therefore, we discuss all clusters that reach this threshold. Clusters reaching significance were overlaid on anatomical images from MRICron. For all analyses, reported coordinates reflect the peak activity within active regions. These coordinates were converted from MNI coordinates to Talairach space, localized using the Talairach Client, and confirmed with the Talairach and Tournoux atlas (Talairach & Tournoux, 1988).
In addition to relations between vividness ratings and neural recruitment, the current study examined relations between vividness ratings and hippocampal connectivity utilizing the generalized psychophysiological interactions (gPPI; http://brainmap.wisc.edu/PPI; McLaren et al., 2012) toolbox in SPM8. The gPPI toolbox, which is configured to automatically accommodate multiple task conditions in the same PPI model, compares functional connectivity to a single seed region across tasks. The hippocampal seed region was selected from the conjunction analysis examining common effects of internal and external vividness on recruitment. A peak voxel within this cluster (-28, -10, -12) was used to create volumes of interest (VOIs) for each subject. Specifically, for each subject, a VOI was generated by creating a 6mm sphere around this voxel. Within each subject, the gPPI toolbox was used to estimate functional connectivity across the entire brain with this 6mm VOI in the neutral memory as a function of internal and external vividness, controlling for one another (i.e., from fixed-effects models 3 and 4).
At the group level, contrast files from individual-subject gPPIs were entered into a random-effects model examining internal and external vividness, with age as a continuous variable of no- interest. This model was used to examine regions in which hippocampal connectivity was either enhanced or diminished by increased ratings of internal and external vividness. As with the prior analysis, the significance threshold was set at p < .005 with a 29-voxel extent (correcting results to p < .05).
Two additional analyses were conducted to examine the relation between internal and external vividness effects on connectivity. The first analysis utilized paired-sample t-tests to compare the effects of internal and external vividness in regions of interest. Five-millimeter spheres were created around the peak voxels of regions exhibiting significant effects of either internal or external vividness, and parameter estimates of internal and external vividness effects were extracted from each ROI. Paired-sample t-tests were conducted between internal and external vividness to determine whether the effects were significantly different from one another. A second paired- sample t-test examined whether the strength (or absolute value) of internal and external vividness effects were significantly different from one another. For example, this analysis would test whether a positive effect of internal vividness was stronger than a negative effect of external vividness, or if the effects were statistically equivalent.
Finally, based on observations that regions exhibiting a significant effect of internal vividness also exhibited a significant negative effect of external vividness, inclusive masks of the negative effect of external vividness at p<.005 and p<.05 were applied to the internal vividness contrast to identify extent of overlap in the conjunction. Similar conjunctions were conducted by applying masks of the negative effect of internal vividness at p<.005 and p<.05 to the external vividness contrast.
3. Results
3.1 Behavioral Results
Participants reported significantly greater ratings of external (M= 3.53, SE=.09) compared to internal vividness (M=2.93, SE= .09; F(1,57)= 13.51, p<.001). Vividness ratings were not affected by participant age (p=.97) and the difference between internal and external vividness did not differ across age (p=.19). Not surprisingly, average ratings of internal and external vividness were highly correlated across individuals (r= .45, p.<.001), where individuals who reported high internal vividness ratings also reported high external vividness ratings, as well as within individuals (Mcorr= .51, SD= .27), where items rated as having high internal vividness also had high external vividness.
Participants performed well on the memory task, with a corrected accuracy rate (hits minus false alarms) of .59 (SE= .02; See Table 1 for all behavioral measures). Performance was not related to vividness ratings or response times for vividness ratings (Supplementary Table 2).
Table 1.
Behavioral measures of memory and vividness ratings.
| Measure | Mean | Standard Deviation | Standard Error | |
|---|---|---|---|---|
| Hit Rate | 0.71 | 0.11 | 0.01 | |
| False Alarm Rate | 0.12 | 0.11 | 0.01 | |
| Accuracy (Hits-False Alarm) | 0.59 | 0.12 | 0.02 | |
| d Prime | 2.06 | 0.97 | 0.13 | |
| External Vividness | 3.53 | 0.7 | 0.09 | |
| Internal Vividness | 2.93 | 0.68 | 0.09 | |
| Response Time - Hits | 1793.74 | 336.03 | 43.75 | |
| Response Time - External Vividness | 963.07 | 326.02 | 42.44 | |
| Response Time - Internal Vividness | 1135.22 | 334.70 | 43.57 | |
| Average Frequency of Response | External Vividness = 1 | 1.66 | 2.36 | |
| External Vividness = 2 | 3.02 | 3.05 | ||
| External Vividness = 3 | 5.66 | 3.35 | ||
| External Vividness = 4 | 6.36 | 3.97 | ||
| External Vividness = 5 | 6.17 | 5.77 | ||
| Internal Vividness = 1 | 3.42 | 3.84 | ||
| Internal Vividness = 2 | 5.1 | 3.88 | ||
| Internal Vividness = 3 | 6.73 | 3.7 | ||
| Internal Vividness = 4 | 4.97 | 3.62 | ||
| Internal Vividness = 5 | 2.66 | 3.55 | ||
| Encoding Task Response = 1 | 5.12 | 3.44 | ||
| Encoding Task Response = 2 | 11.88 | 4.89 | ||
| Encoding Task Response = 3 | 21.36 | 6.1 | ||
RT= Response Time (in milliseconds)
3.2 Imaging Results
3.2.1 Vividness-related changes in neural recruitment during memory retrieval
The first analysis examined the effects of internal and external vividness on activity separately, without taking into account the strong relation between these two variables (Figure 2 and Table 2). Both internal and external vividness were associated with increased activity in a widespread network of both medial and lateral regions. Therefore, the conjunction analysis examining regions exhibiting a significant relation with both internal and external vividness ratings revealed a similarly widespread network, including bilateral medial and lateral prefrontal cortex, parietal lobe, and lateral temporal lobe. There were no regions that exhibited decreased activity as a function of internal or external vividness ratings.
Figure 2.
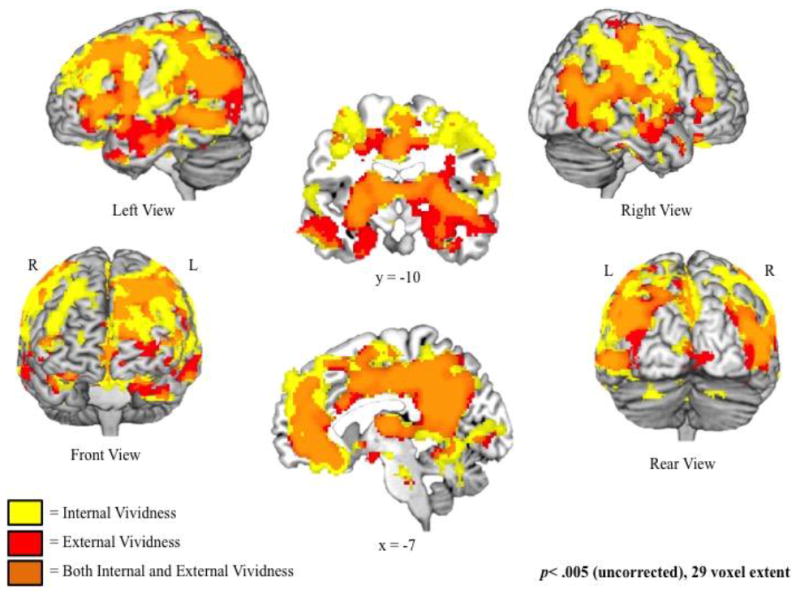
Regions associated with increased ratings of internal (yellow) and external (red) vividness, examined separately (i.e., not controlling for one another). Regions of overlap (orange) exhibit significant increases in activity as a function of both internal and external vividness.
Table 2.
Regions exhibiting a significant relation between neural activity and ratings of internal and external vividness, examined separately.
| Region | Hemisphere | BA | MNI Coordinates
|
t-value | k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Effect of Internal Vividness | |||||||
| Dorsomedial Prefrontal Cortex | L | 8 | -18 | 34 | 48 | 6.42 | 77642 |
| Superior Temporal Gyrus | L | 38 | -42 | 14 | -38 | 3.7 | 105 |
| Middle Temporal Gyrus | R | 21 | 42 | 2 | -38 | 3.3 | 55 |
| Cerebellum | L | na | -10 | -26 | -28 | 3.93 | 179 |
| L | na | -18 | -54 | -40 | 3.33 | 35 | |
| Effect of External Vividness | |||||||
| Posterior Cingulate | L | 23 | -8 | -52 | 24 | 6.01 | 58649 |
| Precentral Gyrus | L | 4 | -14 | -26 | 68 | 3.85 | 196 |
| Superior Temporal Gyrus | R | 38 | 46 | 16 | -24 | 3.19 | 91 |
| Postcentral Gyrus | L | 40 | -62 | -24 | 20 | 2.99 | 38 |
| Cerebellum | L | na | -8 | -26 | -28 | 3.13 | 34 |
| R | na | 10 | -72 | -28 | 2.98 | 46 | |
| Negative Effect of Internal Vividness | |||||||
| No significant clusters | |||||||
| Negative Effect of External Vividness | |||||||
| No significant clusters | |||||||
| Conjunction: Effects of both Internal and External Vividness (both p<.005) | |||||||
| Dorsomedial Prefrontal Cortex | L | 8 | -18 | 34 | 48 | 6.42 | 40515 |
| Ventrolateral Prefrontal Cortex | R | 46 | 44 | 34 | 6 | 3.95 | 258 |
| Anterior Cingulate | R | 32 | 14 | 16 | 26 | 3.75 | 39 |
| Precentral Gyrus | L | 4 | -16 | -28 | 66 | 3.11 | 48 |
| Superior Temporal Gyrus | R | 38 | 40 | 12 | -20 | 4.34 | 45 |
| Superior Temporal Gyrus | L | 38 | -42 | 14 | -38 | 3.7 | 47 |
| Inferior Temporal Gyrus | L | 21 | -64 | -6 | -20 | 3.62 | 303 |
| Inferior Parietal Lobule | L | 40 | -66 | -26 | 24 | 3.76 | 38 |
| Uncus | L | 36 | -28 | -4 | -38 | 3.15 | 117 |
| Cerebellum | R | na | 16 | -70 | -26 | 3.73 | 29 |
Clusters significant at an uncorrected threshold of p<.005, k ≥ 29 voxels
BA= approximate Brodmann Area; L=L, R=R
The second analysis separated the unique effects of internal and external vividness by identifying regions showing a significant relation with each measure, controlling for the other measure (Figure 3 and Table 3). Analyses were conducted examining each unique effect separately, as well as relative to one another (i.e., internal vividness > external vividness and external vividness > internal vividness). Compared to effects of external vividness, ratings of internal vividness were associated with increased recruitment of a ventrolateral region of the prefrontal cortex on the rectal gyrus (BA 11). Relative to internal vividness, ratings of external vividness were associated with increased recruitment of anterior cingulate, lateral prefrontal cortex, lateral and medial temporal lobe, and posterior occipital regions. Analyses examining effects of increased and decreased vividness ratings revealed that these comparisons were driven by positive relations between ratings and neural recruitment, as no regions were identified that exhibited increased activity as a function of decreased internal or external vividness ratings. For example, the increased relation between neural recruitment and external relative to internal vividness was driven by a significant relation between recruitment and increased external vividness ratings.
Figure 3.
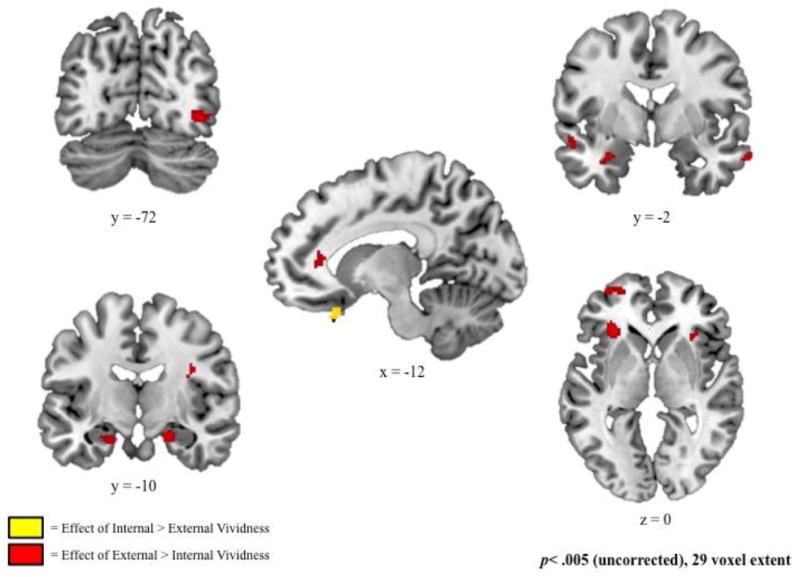
Regions associated with increased ratings of internal relative to external vividness (yellow) and external relative to internal vividness (red), derived from fixed-effects models examining measures of internal and external vividness, controlling for one another.
Table 3.
Regions exhibiting a significant relation between neural activity and ratings of internal and external vividness, controlling for one another.
| Region | Hemisphere | BA | MNI Coordinates
|
t-value | k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Effect of Internal Vividness (Controlling for External) | |||||||
| Frontal Lobe | |||||||
| Ventromedial Prefrontal Cortex | L | 11 | -12 | 24 | -24 | 4.61 | 397 |
| L | 11 | -4 | 36 | -18 | 2.98 | 80 | |
| Dorsomedial Prefrontal Cortex | L | 8 | -18 | 34 | 56 | 4.14 | 231 |
| L | 9 | -10 | 40 | 28 | 3.61 | 48 | |
| L | 9 | -8 | 58 | 44 | 3.47 | 205 | |
| Premotor Cortex | L | 6 | 0 | -6 | 62 | 3.79 | 156 |
| L | 6 | -22 | 0 | 58 | 3.38 | 102 | |
| R | 6 | 34 | -8 | 50 | 3.41 | 154 | |
| Ventrolateral Prefrontal Cortex | L | 45 | -54 | 28 | 8 | 3.5 | 89 |
| Dorsolateral Prefrontal Cortex | L | 8 | -40 | 22 | 52 | 3.48 | 117 |
| R | 8 | 30 | 28 | 48 | 3.05 | 66 | |
| Paracentral Lobule | R | 5 | 20 | -36 | 54 | 3.32 | 39 |
| Anterior Cingulate | R | 24 | 10 | 28 | 12 | 4.13 | 176 |
| R | 32 | 6 | 8 | 42 | 3.18 | 135 | |
| Parietal Lobe | |||||||
| Angular gyrus | L | 39 | -54 | -60 | 22 | 3.93 | 326 |
| Inferior Parietal Lobule | R | 40 | 46 | -36 | 44 | 3.77 | 739 |
| R | 40 | 34 | -56 | 38 | 3.05 | 106 | |
| L | 40 | -48 | -44 | 56 | 3.38 | 36 | |
| Precuneus | R | 7 | 6 | -64 | 50 | 3.76 | 251 |
| Postcentral Gyrus | L | 1 | -68 | -22 | 36 | 3.68 | 121 |
| Supramarginal Gyrus | L | 40 | -38 | -48 | 36 | 3.2 | 86 |
| Temporal Lobe | |||||||
| Superior Temporal Gyrus | L | 22 | -48 | -34 | 2 | 3.51 | 44 |
| L | 22 | -56 | 4 | 6 | 3.4 | 130 | |
| Middle Temporal Gyrus | R | 21 | 64 | -26 | -18 | 3.15 | 31 |
| Other | |||||||
| Posterior Cingulate | L | 31 | -6 | -38 | 42 | 4.13 | 554 |
| Parahippocampal Gyrus | L | 36 | -26 | -20 | -30 | 3.46 | 34 |
| Putamen | L | na | -26 | 6 | 4 | 3.08 | 50 |
| Cerebellum | L | na | -12 | -26 | -30 | 3.47 | 53 |
| L | na | -12 | -52 | -28 | 3.16 | 39 | |
| L | na | -28 | -60 | -32 | 3.06 | 60 | |
| L | na | -28 | -76 | -32 | 4.05 | 63 | |
| R | na | 10 | -60 | -18 | 3.33 | 64 | |
| Effect of External Vividness (Controlling for Internal) | |||||||
| Frontal Lobe | |||||||
| Dorsal Prefrontal Cortex | L | 8 | -22 | 24 | 52 | 3.98 | 639 |
| Precentral Gyrus | L | 6 | -12 | -24 | 70 | 3.83 | 108 |
| Anterior Cingulate | R | 24 | 10 | 38 | 4 | 4.4 | 1943 |
| R | 33 | 6 | 18 | 20 | 3.68 | 193 | |
| R | 24 | 18 | -2 | 38 | 3.39 | 215 | |
| Parietal Lobe | |||||||
| Inferior Parietal Lobule | L | 40 | -56 | -42 | 54 | 3.19 | 31 |
| Temporal Lobe | |||||||
| Superior Temporal Gyrus | L | 13 | -48 | -44 | 16 | 4.34 | 1175 |
| Middle Temporal Gyrus | L | 21 | -56 | -42 | -6 | 3.58 | 236 |
| R | 37 | 48 | -68 | 4 | 3.41 | 319 | |
| R | 20 | 50 | -40 | -10 | 3.19 | 49 | |
| Inferior Temporal Gyrus | R | 21 | 66 | -4 | -22 | 3.44 | 91 |
| Occipital Lobe | |||||||
| Inferior Occipital Gyrus | L | 19 | -38 | -78 | -4 | 3.9 | 254 |
| Lingual Gyrus | R | 18 | 24 | -80 | -4 | 3.69 | 60 |
| Other | |||||||
| Parahippocampal Gyrus | R | 35 | 22 | -34 | -14 | 4.63 | 9664 |
| Posterior Cingulate | L | 31 | -8 | -56 | 24 | 4.11 | 1047 |
| R | 31 | 8 | -40 | 34 | 2.98 | 34 | |
| Cerebellum | R | 10 | -52 | -2 | 2.93 | 37 | |
| Negative Effect of Internal Vividness (Controlling for External) | |||||||
| No significant clusters | |||||||
| Negative Effect of External Vividness (Controlling for Internal) | |||||||
| No significant clusters | |||||||
| Conjunction: Effects of both Internal and External Vividness (both p<.005) | |||||||
| No significant clusters | |||||||
| Effect of Internal > External Vividness | |||||||
| Frontal Lobe | |||||||
| Ventromedial Prefrontal Cortex | L | 11 | -12 | 24 | -26 | 3.23 | 41 |
| Effect of External > Internal Vividness | |||||||
| Frontal Lobe | |||||||
| Ventrolateral Prefrontal Cortex | L | 47 | -30 | 28 | 0 | 3.53 | 122 |
| Anterior Cingulate | L | 24 | -16 | 10 | 34 | 3.41 | 37 |
| L | 32 | -14 | 34 | 10 | 2.98 | 31 | |
| Anterior Prefrontal Cortex | L | 10 | -34 | 58 | 0 | 3.12 | 64 |
| Temporal Lobe | |||||||
| Middle Temporal Gyrus | L | 21 | -54 | 0 | -14 | 3.17 | 40 |
| Inferior Temporal Gyrus | R | 21 | 66 | -4 | -22 | 3.09 | 35 |
| Occipital Lobe | |||||||
| Inferior Occipital Gyrus | R | 19 | 40 | -72 | -6 | 3.47 | 88 |
| Other | |||||||
| Parahippocampal Gyrus | R | 34 | 20 | -10 | -20 | 3.38 | 58 |
| L | 28 | -20 | -10 | -24 | 3.02 | 33 | |
| Amygdala | L | na | -28 | -2 | -22 | 3.13 | 34 |
| Striatum | R | na | 30 | 20 | 14 | 4.23 | 166 |
| Insula | R | 13 | 38 | -8 | 26 | 3.23 | 29 |
Clusters significant at an uncorrected threshold of p<.005, k ≥ 29 voxels
BA= approximate Brodmann Area; L=L, R=R
3.2.2 Vividness-related changes in hippocampal connectivity during memory retrieval
The seed region selected for the current hippocampal connectivity analysis was a 6mm sphere surrounding the peak voxel (-28, -10, -12; See Figure 4 and Figure 5) of a cluster exhibiting significant increases in activity as a function of both internal and external vividness (see Table 2). Importantly, this sphere did not overlap with medial temporal lobe clusters in which the relation between recruitment and external vividness was greater than the relation between recruitment and internal vividness (see Table 3).
Figure 4.
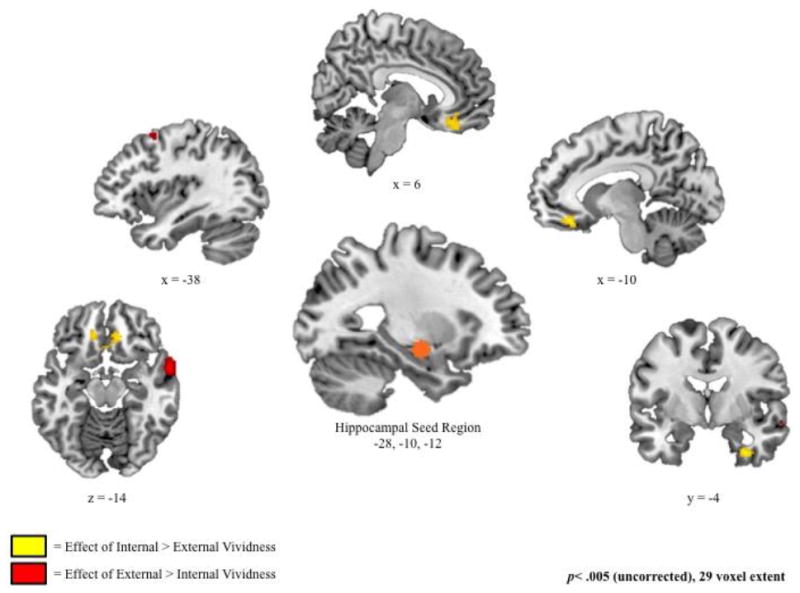
Regions in which hippocampal connectivity was associated with increased ratings of internal relative to external vividness (yellow) and external relative to internal vividness (red), derived from fixed-effects models examining measures of internal and external vividness, controlling for one another.
Figure 5.
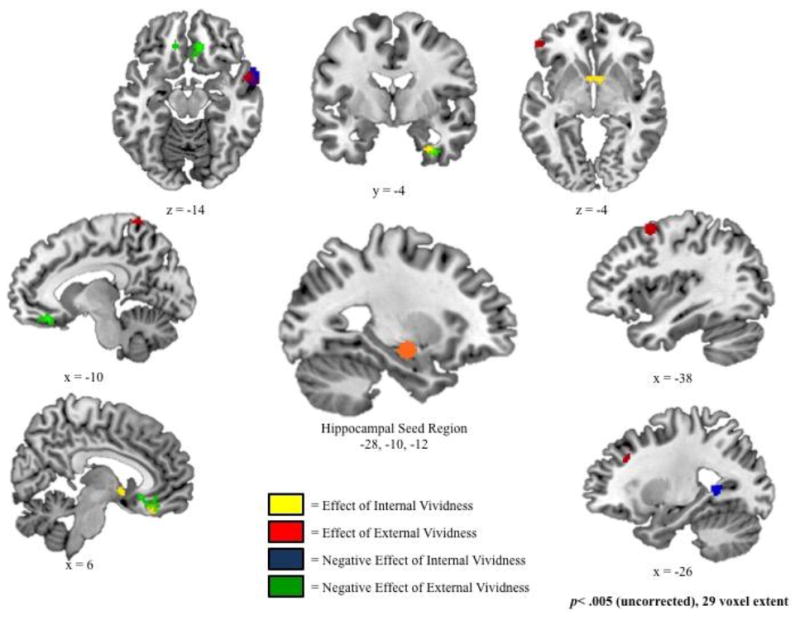
Regions in which hippocampal connectivity was associated with increased ratings of internal vividness (yellow), increased ratings of external vividness (red), decreased ratings of internal vividness (blue), and decreased ratings of external vividness (green). Note the extensive overlap between regions depicted in yellow and in green. Group effects were estimated using fixed-effects models examining measures of internal and external vividness, controlling for one another.
Internal vividness ratings were associated with increased hippocampal connectivity with bilateral ventromedial prefrontal cortex (BA 25 and 11) and right uncus (BA 36; Figure 5 and Table 4). Conjunction analyses revealed significant overlap between these regions and those exhibiting a negative effect of external vividness. In fact, all clusters exhibiting a significant positive relation between internal vividness ratings and hippocampal connectivity at our standard threshold of p<.005 had an overlap of at least 29 voxels with those regions exhibiting a significant negative relation between external vividness and connectivity at a reduced threshold of p<.05. In other words, there were no regions that exclusively exhibited this positive relation between internal vividness ratings and hippocampal connectivity. Similarly, there were no regions that exclusively exhibited a negative relation between external vividness and connectivity. Follow-up t-tests confirmed that the effect of internal vividness was significantly different from the effect of external vividness in all regions, but the strengths of the positive internal effect and the negative external effect were statistically equivalent (p>.05 for all contrasts; see Table 5).
Table 4.
Regions exhibiting significant modulation of hippocampal connectivity as a function of internal and external vividness ratings (controlling for one another).
| Region | Hemisphere | BA | MNI Coordinates
|
t-value | k | Conjunction Analysis | |||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Effect of Internal Vividness | With Negative External Inclusive Mask at p<.005 | With Negative External Inclusive Mask at p<.05 | |||||||
| Ventromedial Prefrontal Cortex | L | 25 | -4 | 8 | -8 | 3.27 | 95 | No Overlap | Overlap in 36 Voxels |
| R | 11 | 6 | 36 | -22 | 2.89 | 52 | Overlap in 34 Voxels | Overlap in 52 Voxels | |
| Uncus | R | 36 | 30 | -4 | -36 | 3.26 | 50 | Overlap in 19 Voxels | Overlap in 48 Voxels |
| Effect of External Vividness | With Negative Internal Inclusive Mask at p<.005 | With Negative Internal Inclusive Mask at p<.05 | |||||||
| Middle Temporal Gyrus | R | 21 | 60 | 4 | -14 | 3.83 | 147 | Overlap in 101 Voxels | Overlap in 141 Voxels |
| Postcentral Gyrus | L | 5 | -10 | -50 | 76 | 3.39 | 111 | No Overlap | Overlap in 20 Voxels |
| Dorsal Prefrontal Cortex | L | 6 | -38 | 10 | 58 | 3.38 | 78 | No Overlap | Overlap in 59 Voxels |
| L | 9 | -30 | 28 | 40 | 2.85 | 55 | No Overlap | No Overlap | |
| Ventrolateral Prefrontal Cortex | L | 47 | -54 | 40 | -4 | 3.23 | 38 | No Overlap | Overlap in 16 Voxels |
| Negative Effect of Internal Vividness | With External Inclusive Mask at p<.005 | With External Inclusive Mask at p<.05 | |||||||
| Middle Temporal Gyrus | R | 21 | 62 | 4 | -14 | 3.57 | 195 | Overlap in 101 Voxels | Overlap in 194 Voxels |
| Lingual Gyrus | L | 19 | -26 | -50 | 6 | 3.33 | 52 | No Overlap | No Overlap |
| Negative Effect of External Vividness | With Internal Inclusive Mask at p<.005 | With Internal Inclusive Mask at p<.05 | |||||||
| Ventromedial Prefrontal Cortex | R | 32 | 12 | 36 | -14 | 3.62 | 261 | Overlap in 34 Voxels | Overlap in 218 Voxels |
| L | 25 | -10 | 34 | -18 | 3.48 | 100 | No Overlap | Overlap in 98 Voxels | |
| Uncus | R | 20 | 34 | -6 | -36 | 3.27 | 50 | Overlap in 19 Voxels | Overlap in 50 Voxels |
Clusters significant at an uncorrected threshold of p<.005, k ≥ 29 voxels
BA= approximate Brodmann Area; L=L, R=R
Overlapping regions reaching voxel threshold of 29 voxels are bolded
Table 5.
Comparison of internal and external vividness effect in regions exhibiting significant modulation of hippocampal connectivity as a function of internal and external ratings.
| Region of Interest | |||
|---|---|---|---|
| Effect of Internal Vividness | Difference from External Vividness Effect | Difference from Strength (absolute value) of External Vividness Effect | |
| Left Ventromedial Prefrontal Cortex (-4, 8, -8) | 0.001 | 0.76 | |
| Right Ventromedial Prefrontal Cortex (6, 36, -22) | 0.004 | 0.13 | |
| Right Uncus (30, -4, -36) | 0.003 | 0.22 | |
| Effect of External Vividness | Difference from Internal Vividness Effect | Difference from Strength (absolute value) of Internal Vividness Effect | |
| Right Middle Temporal Gyrus (60, 4, -14) | <0.0001 | 0.02 | |
| Left Postcentral Gyrus (-10, -50, 76) | 0.006 | 0.02 | |
| Left Dorsal Prefrontal Cortex (-38, 10, 58) | 0.01 | 0.03 | |
| Left Dorsal Prefrontal Cortex (-30, 28, 40) | 0.09 | 0.09 | |
| Left Ventrolateral Prefrontal Cortex (-54, 40, -4) | 0.04 | 0.04 |
Coordinates are presented in MNI format.
Contrasts reaching significance at p<.05 are bolded; trends of p<.1 are in italics.
Increased external vividness ratings related to enhanced hippocampal connectivity with lateral temporal lobe (BA 21), postcentral gyrus (BA 5), dorsal prefrontal cortex (BA 6 and 9), and ventrolateral prefrontal cortex (BA 47; Figure 5 and Table 4). Although there was some overlap in regions showing enhanced connectivity as a function of increased external vividness and decreased internal vividness—specifically in the middle temporal gyrus (BA 21) and, to a lesser extent, in one cluster in the dorsal prefrontal cortex (BA 6)—these networks were also somewhat distinct. Specifically, increased external vividness ratings were associated with enhanced hippocampal connectivity with the postcentral gyrus (BA 5), ventrolateral prefrontal cortex (BA 47), and a second dorsal prefrontal cluster (BA 9) that were not associated with decreased ratings of internal vividness. Follow-up t-tests confirmed that the effect of external vividness in these regions was significantly different from the effect of external vividness in all regions except in BA9, where the difference was trending (p= .09; Table 5). Importantly, the absolute effect of external vividness on connectivity was also stronger than the effect of internal vividness for all regions, supporting the conclusion from the conjunction analysis that these regions were not associated with a strong negative effect of internal vividness,
4. Discussion
The current study was the first to examine the differential effects of hippocampal connectivity during memory search on internal and external vividness ratings. Ratings of internal and external vividness were associated with distinct hippocampal connectivity patterns, with internal ratings associated with connectivity to regions implicated in self-referential processing and external ratings associated with connectivity to regions involved in retrieval of conceptual and episodic contextual details. Further, the current study revealed that hippocampal connectivity patterns can relate to both internal and external vividness, but in opposing directions. Specifically, there was extensive overlap between regions associated with increased internal and decreased external vividness. The opposite set of relations (increased external and decreased internal) showed little overlap, suggesting that the connectivity patterns that enable vivid internal detail interfere more with those that enable vivid external detail than vice-versa.
The divergence of neural networks supporting internal and external vividness ratings as early as the first two seconds of memory demonstrates that search processes, and not just elaboration, are intimately tied to detail retrieval. This early emergence may suggest that event vividness is embedded in the memory trace, directing search processes, or that the way a memory representation is initially accessed has downstream implications for how details are recovered.
4.1 Neural recruitment and connectivity associated with internal vividness ratings
Relative to ratings of external vividness, increased internal vividness was associated with increased recruitment of a single cluster in the ventromedial prefrontal cortex, along the rectal gyrus. Internal vividness ratings were also associated with enhanced hippocampal connectivity with two ventromedial prefrontal clusters. Such enhancement in medial prefrontal regions is consistent with the role of this region in self-referential processing during memory retrieval (e.g., Cabeza et al., 2004; Gusnard et al., 2001), and with extensive research suggesting that the vmPFC and hippocampus are functionally connected in a default mode network that supports such internal operations (Buckner et al., 2008). Although the clusters reported in the current manuscript are more subgenual than those highlighted as the most commonly occurring in meta-analyses of self-referential processing (Denny et al., 2012; Murray et al., 2012), many studies report self-reference effects in this more posterior and ventral region (Kjaer et al., 2002; Meffert et al., 2013; Northoff et al., 2009; Summerfield, Hassabis, & Maguire, 2009). Specifically, studies find a role of this more medial orbital prefrontal region in self-referential processing in emotional contexts or in the interaction of emotional and self-referential processing (Moran et al., 2006; Northoff et al., 2006; Northoff et al., 2009). This interaction account may explain the involvement of the subgenual vmPFC in the reported contrast; given that internal vividness ratings relied on participant judgments of their thoughts and feelings during encoding, it is likely that they were driven by recall of one’s own prior emotions.
Critically, the regions exhibiting enhanced hippocampal connectivity as a function of increased internal vividness ratings exhibited an equivalent enhanced connectivity as a function of decreased external vividness. In other words, the neural interactions that supported retrieval of internal details during retrieval were also associated with impoverished retrieval of external details. Such a pattern is consistent with the proposal that functions associated with regions in the default mode network contrast with externally-oriented goal-directed thought (Buckner et al., 2008), and that suppression of this network may be critical to successful performance on some cognitive tasks (e.g., Anticevic et al., 2010; Daselaar et al., 2004).
4.2 Neural recruitment and connectivity associated with external vividness ratings
Activity in a widespread network of regions was more strongly associated with external vividness ratings compared to internal vividness ratings. Specifically, increased recruitment was identified in lateral and anterior prefrontal regions, lateral temporal lobe, posterior visual regions, and medial temporal lobe. External vividness ratings were associated with increased hippocampal connectivity with a similar set of neural regions, including lateral and dorsal prefrontal regions, lateral temporal lobe, and postcentral gyrus. These findings suggest a widespread network of regions that work together to support retrieval of external event details.
Observed activity and hippocampal connectivity in lateral temporal lobes may be related to their role in category-general conceptual processing (Binder & Desai, 2011). Specifically, Binder and Desai propose a role of this region in the retrieval of conceptual knowledge, including that required for semantic memory, social cognition, episodic memory, scene construction, and self-knowledge. Therefore, increases in hippocampal connectivity to the lateral temporal lobe may reflect a hippocampally-mediated enhancement of knowledge related to external memory details, including semantic memory for image content and scene construction.
External vividness ratings were also associated with enhanced recruitment and hippocampal connectivity in regions within the ventrolateral PFC (vlPFC). Prior research has revealed a critical role of the vlPFC in supporting cognitive processes needed to recall specific event details (e.g., selection, maintenance, reorganization; see Badre & Wagner, 2007; Simons & Spiers, 2003). Further, in their meta-analysis examining neural networks associated with subjective and objective recollection, Spaniol and colleagues (2009) found that objective recollection (i.e., the recall of source or contextual details) was more strongly associated with activation in left vlPFC. These prior findings are consistent with enhanced hippocampal-vlPFC connectivity supporting retrieval of image-related details in the current study.
Importantly, the current study revealed an interesting asymmetry between internal and external vividness: while regions associated with increased internal vividness regions also showed negative external effects, this wasn’t necessarily true of regions associated with increased external vividness. In other words, hippocampal connectivity that supports enhanced retrieval of external details does not necessarily contribute to reductions in internal vividness. Such a finding is important, as it reveals that mechanisms supporting internal and external detail retrieval are not always working in opposition. Rather, there appears to be something unique about regions associated with self-referential processing that leads to a disruption of other forms of processing.
4.3 Limitations and Future Directions
The current analysis examines the relation between hippocampal connectivity and two separate measures of subjective vividness. As research has demonstrated significant distinctions between the neural networks supporting subjective and objective vividness (Spaniol et al., 2009), the differences between internal and external subjective vividness identified in the current study should not be generalized to objective measures. Future work is need to confirm that these patterns and relations extend to one’s objectively-measured ability to recollect personal and event details. Further, as both internal and external vividness ratings can be supported by a large number of different types of details, the current design cannot specify the particular elements supporting these decisions. Objective measures of detail retrieval may be used in the future to pursue this question.
To maximize power and sample size, and to establish a baseline connectivity pattern, the current analysis examined connectivity across a sample of participants ages18-85. Although controlling for age in all analyses minimized any confounding effects of having such a large age range, it is possible that age-related differences exist that are being ignored in this manuscript or that were not controlled for with the linear age covariate (e.g., nonlinear effects of age). As noted previously, age can be associated with changes to reports of internal and external vividness (e.g., Hashtroudi et al., 1990), although this was not the case in the current study (see behavioral results). Further, prior research suggests that older adults may be less able to suppress activity in the default mode network during cognitive tasks (e.g., Grady et al., 2010; Hedden et al., 2009; Lustig et al., 2003). Although this analysis was beyond the scope of the questions addressed in the current manuscript, future analyses will be conducted to determine whether healthy aging influences the relation between connectivity within the default mode network and ratings of internal and external vividness.
In the current study, participants were asked to rate vividness of all “old” responses on two 1-5 scales. These scales were used in our fMRI analyses to examine neural networks associated with internal and external vividness. In this paradigm, “old” guesses and “old” responses with very low familiarity would both receive vividness ratings of “1”, leading to an impure vividness category. This contamination could potentially influence the results reported in the current study, and future studies could incorporate an additional confidence ratings or a “guess” option in addition to the old/new response to avoid this possibility. However, participants in the current study only responded “1” an average of 1.7 times for external vividness and 3.4 times for internal vividness (See Table 1). Therefore, it is unlikely that inclusion of guesses would have a significant influence on the overall analysis.
Summary
The current study examined the unique effects of internal and external vividness on hippocampal connectivity patterns, revealing distinct patterns of connectivity for the two measures. Specifically, internal vividness ratings were associated with connectivity to regions implicated in self-referential processing and external vividness was associated with connectivity to regions involved in the retrieval of conceptual and episodic contextual details. Further, regions exhibiting enhanced hippocampal connectivity as a function of increased internal detail overlapped with those related to decreased external detail, suggesting that the same neural interactions can simultaneously enhance and diminish one’s subjective sense of vividness, depending on the particular aspect being measured. Critically, the fact that this complete overlap was unique to hippocampal connections associated with internal vividness suggests that this dual function may be unique to connectivity between regions of the default mode network rather than all hippocampal interactions.
Supplementary Material
Highlights.
Study examined neural recruitment during retrieval of neutral word-image pairs.
Compared vividness of a) internal thoughts and feelings and b) image details.
Hippocampal activity was associated with internal and external vividness ratings.
Internal vividness was related to increased hippocampal-prefrontal connectivity.
External vividness was related to decreased hippocampal-prefrontal connectivity.
Acknowledgments
The authors would like to thank Katherine Mickley Steinmetz for her assistance designing the current study and Halle Zucker and John Morris for their assistance compiling stimuli, creating presentation scripts, and running participants. Magnetic resonance data were collected at the Harvard Center for Brain Science. We thank the staff there, particularly Tammy Moran and Ross Mair, for their assistance with data collection and quality assurance. This work was supported by a Memory and Cognitive Disorders grant from the McKnight Endowment Fund for Neuroscience (EAK), and NIH grant MH080833 (EAK).
Footnotes
It is possible that the order of vividness ratings could influence how participants recalled images in the search phase. To examine this possibility, all analyses were also conducted with counterbalance order as a controlling variable and the results remained the same.
Memory search varied as a function of response time, potentially introducing additional variance into this model. All analyses were also conducted with response time as a controlling variable and the results remained the same.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluck S, Alea N, Habermas T, Rubin D. A tale of three functions: the self-reported uses of autobiographical memory. Social Cognition. 2005;23:91–117. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC. Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. J Cogn Neurosci. 2004;16:1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cerebral Cortex. 2008;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsney KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24:1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, Kensinger EA. The relation between structural and functional connectivity depends on age and on task goals. Frontiers in Human Neuroscience. 2014;8:307. doi: 10.3389/fnhum.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, Morris JA, Kensinger EA. Neural recruitment and connectivity during emotional memory retrieval across the adult life span. Neurobiology of Aging. 2014a;35(12):2770–2784. doi: 10.1016/j.neurobiolaging.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, Morris JA, Kensinger EA. Effects of emotion and emotional valence on the neural correlates of episodic memory search and elaboration. Journal of Cognitive Neuroscience. 2014b;26:825–839. doi: 10.1162/jocn_a_00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DT, Wilson TD. Why the brain talks to itself: Sources of error in emotional prediction. Phil Trans R Soc B. 2009;364:1335–1341. doi: 10.1098/rstb.2008.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cerebral Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic M, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JA, Churchill N, McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashtroudi S, Johnson MK, Chrosniak LD. Aging and qualitative characteristics of memories for perceived and imagined complex events. Psychology and Aging. 1990;5:119–126. doi: 10.1037//0882-7974.5.1.119. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Beckner JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Foley MA, Suengas AG, Raye CL. Phenomenal characteristics of memories for perceived and imagined autobiographical events. Journal of Experimental Psychology: General. 1988;117:371–376. [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage. 2002;17:1080–1086. [PubMed] [Google Scholar]
- Lustig C, Snyders AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damage of fMRI regional activations and interactions during memory retrieval. Brain. 2001;124:1156–1170. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized from of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert H, Blanken L, Blair KS, White SF, Blair JR. The influence of valence and decision difficulty on self-referential processing. Frontiers in Human Neuroscience. 2013;7:46. doi: 10.3389/fnhum.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton CL, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbane M. Degrees of separation: A quantitative neuroimaging meta-analysis investigating self-specificity and shared activation between self- and other-reflection. Neuroscience & Biobehavioral Reviews. 2012;36:1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, Schneider F, Rotte M, Matthiae C, Tempelman C, Wiebking C, Bermpohl F, Heinzel A, Danos P, Heinze HJ, Bogerts B, Walter M, Panksepp J. Differential parametric modulation of self-relatedness and emotions in different brain regions. Human Brain Mapping. 2009;30:369–382. doi: 10.1002/hbm.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, et al. Common blood flow changes across visual tasks: II.: decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han J, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Suengas AG, Johnson MK. Qualitative effects of rehearsal on memories for perceived and imagined complex events. Journal of Experimental Psychology: General. 1988;117:377–389. [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Cortical midline involvement in autobiographical memory. NeuroImage. 2009;44:1188–1200. doi: 10.1016/j.neuroimage.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychologist. 1985;26:1–12. [Google Scholar]
- Yonelinas A. Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


